Effects of Polydatin on Pentylenetetrazol-Induced Seizures in Zebrafish Larvae
Abstract
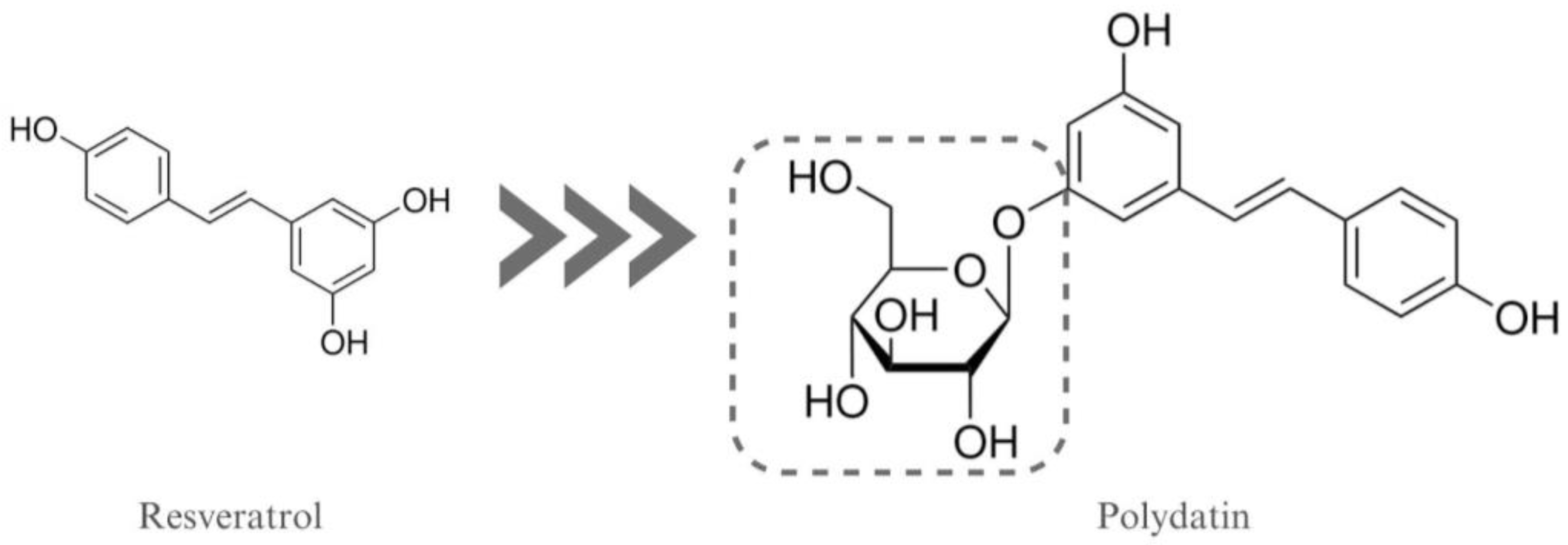
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
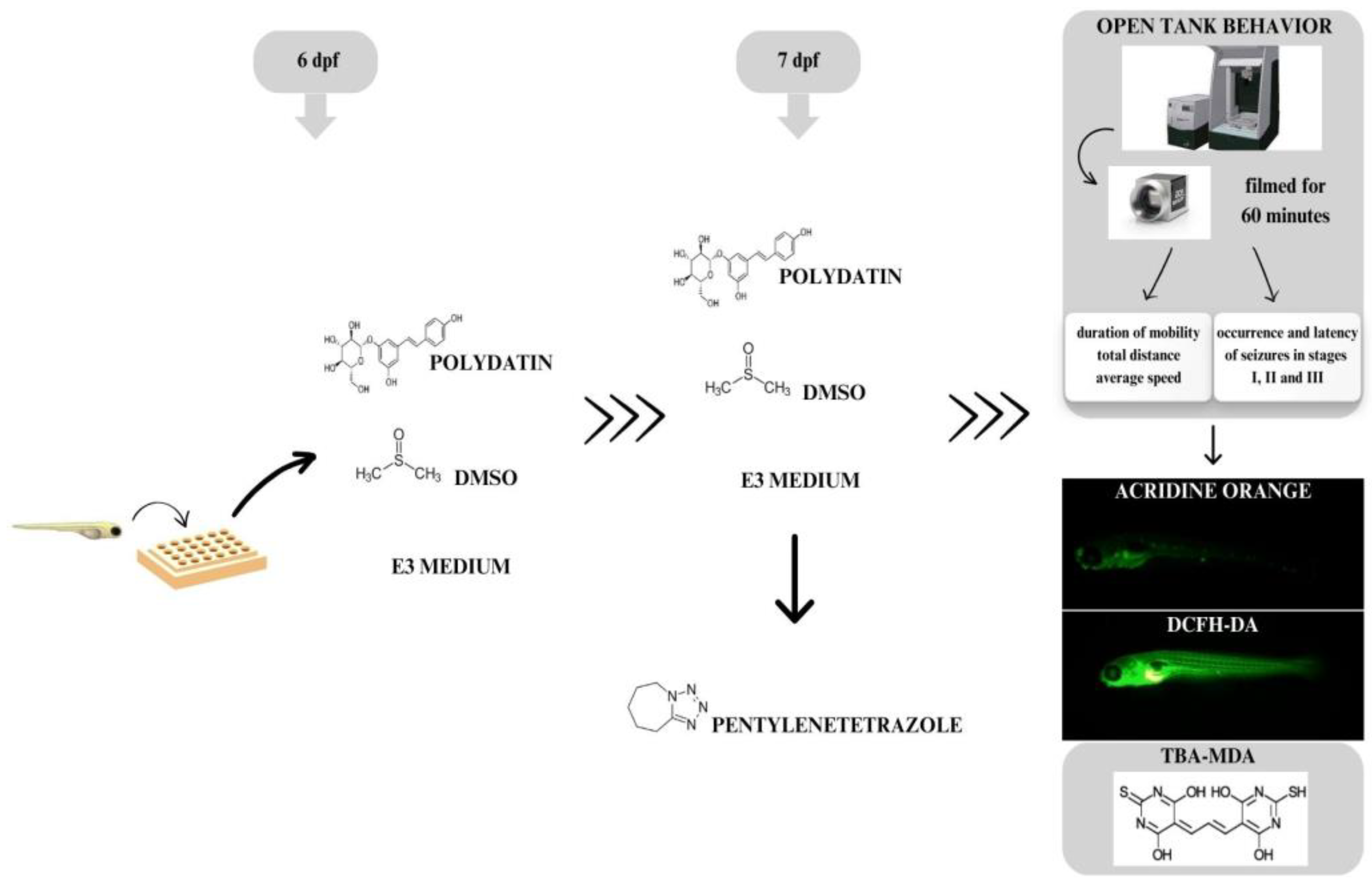
2.3. Experimental Design
2.4. Analyses of Seizure-like Behavior
2.5. Seizure Occurrence and Latency Scoring
2.6. Cell Death Analysis
2.7. Measurement of Reactive Species (RS) Production
2.8. Lipoperoxidation
2.9. Statistical Analysis
3. Results
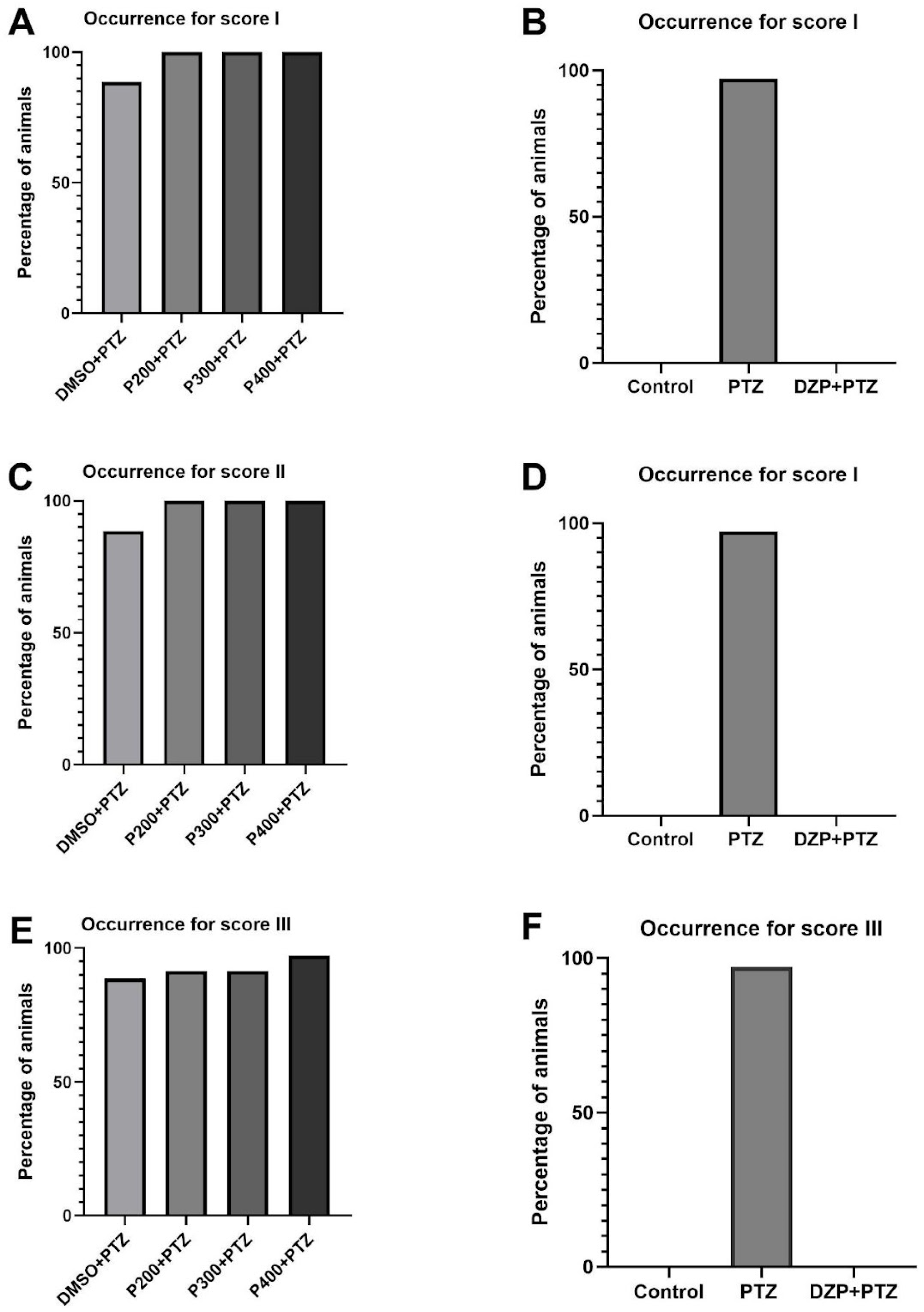
3.1. Effects of Polydatin on Seizure-like Occurrence and Latency
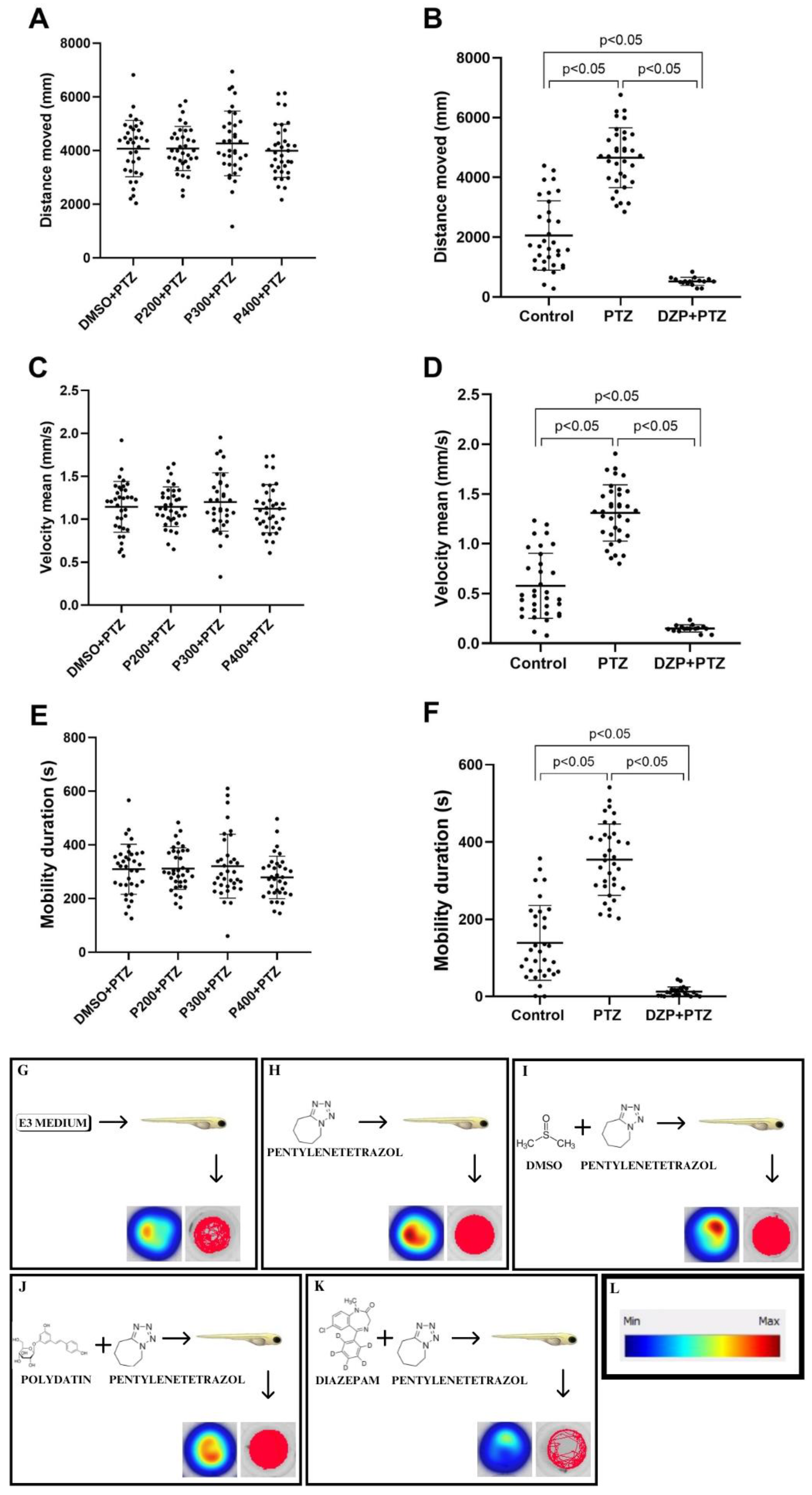
3.2. Effects of Polydatin on Seizure-like Behavior
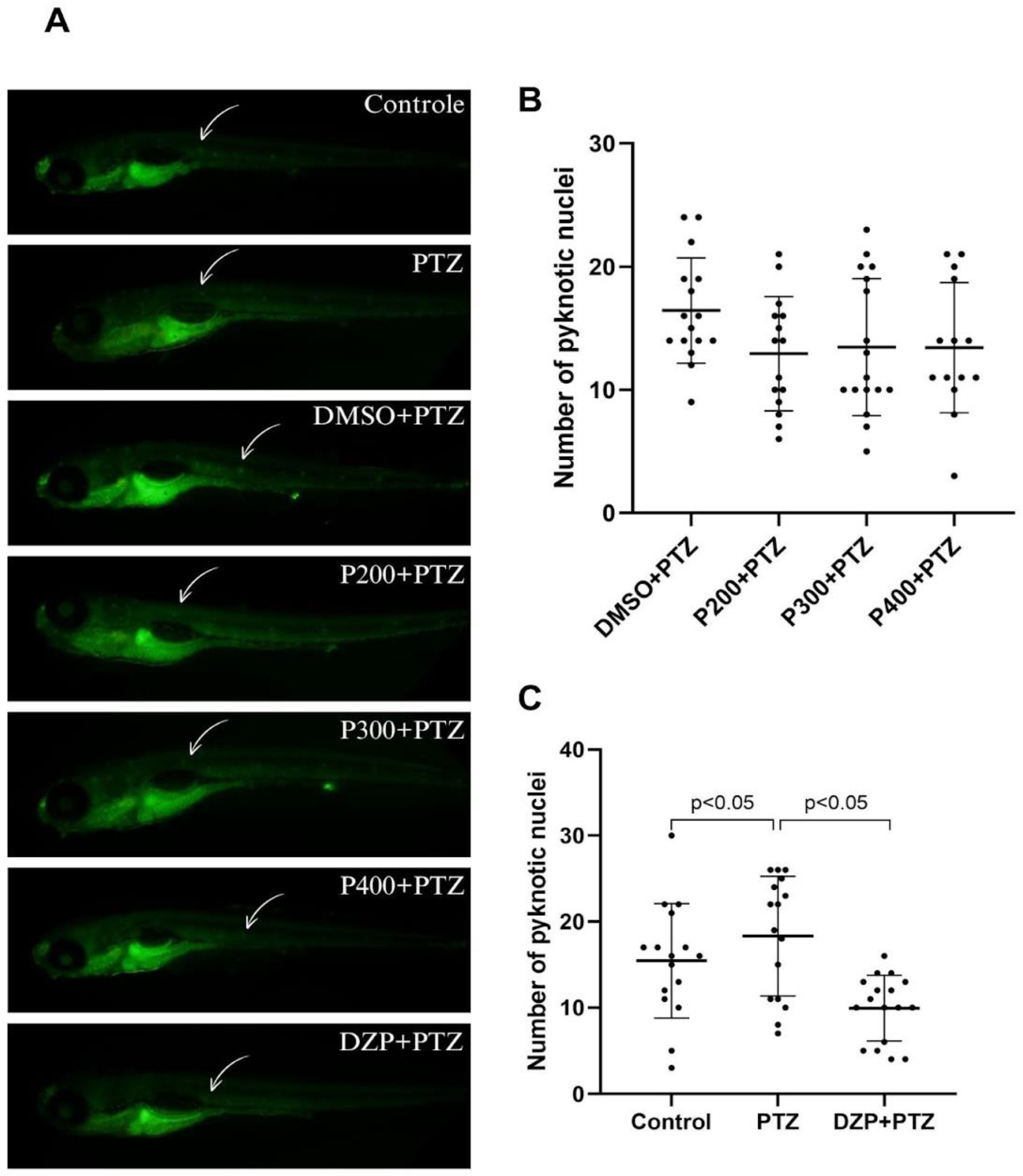
3.3. Effects of Polydatin on Cell Death
3.4. Effect of Polydatin on the Production of RS
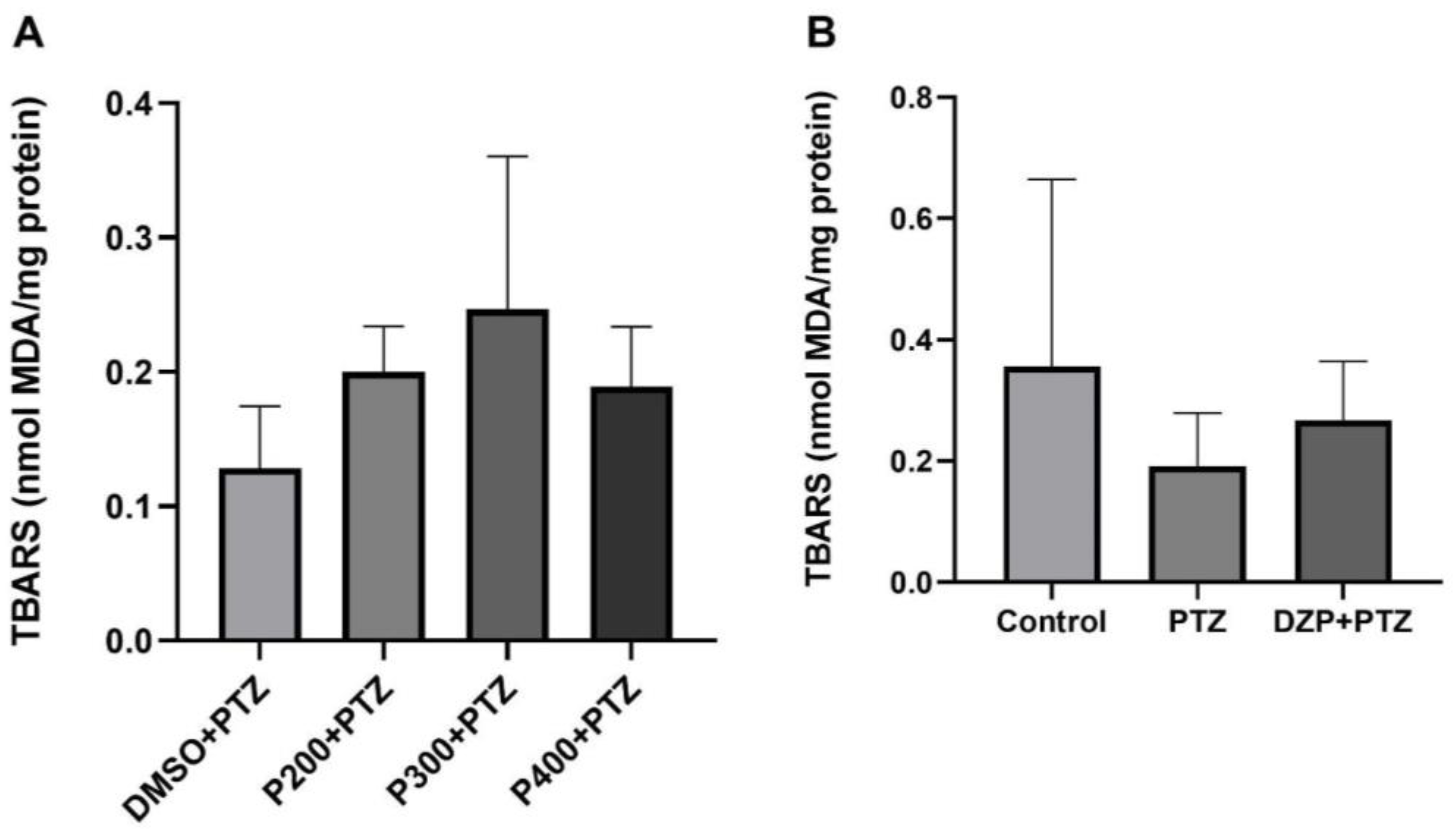
3.5. Effects of Polydatin on Lipid Peroxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Epilepsy. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 9 September 2024).
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A Practical Clinical Definition of Epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Yang, K.; Wang, N.; Yang, L.; Yang, Z.; Xu, L.; Wang, J.; Zhang, L. Advancements in Surgical Therapies for Drug-Resistant Epilepsy: A Paradigm Shift towards Precision Care. Neurol. Ther. 2025, 14, 467–490. [Google Scholar] [CrossRef]
- Karami, A.; Fakhri, S.; Kooshki, L.; Khan, H. Polydatin: Pharmacological Mechanisms, Therapeutic Targets, Biological Activities, and Health Benefits. Molecules 2022, 27, 6474. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- de Sá Coutinho, D.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef]
- Arbo, B.D.; André-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol Derivatives as Potential Treatments for Alzheimer’s and Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 103. [Google Scholar] [CrossRef]
- dos Santos, M.G.; Schimith, L.E.; André-Miral, C.; Muccillo-Baisch, A.L.; Arbo, B.D.; Hort, M.A. Neuroprotective Effects of Resveratrol in In Vivo and In Vitro Experimental Models of Parkinson’s Disease: A Systematic Review. Neurotox. Res. 2022, 40, 319–345. [Google Scholar] [CrossRef]
- dos Santos, M.G.; da Luz, D.B.; de Miranda, F.B.; de Aguiar, R.F.; Siebel, A.M.; Arbo, B.D.; Hort, M.A. Resveratrol and Neuroinflammation: Total-Scale Analysis of the Scientific Literature. Nutraceuticals 2024, 4, 165–180. [Google Scholar] [CrossRef]
- Pallàs, M. Resveratrol in Epilepsy: Preventive or Treatment Opportunities? Front. Biosci. 2014, 19, 1057. [Google Scholar] [CrossRef]
- Long, X.-Y.; Wang, S.; Luo, Z.-W.; Zhang, X.; Xu, H. Comparison of Three Administration Modes for Establishing a Zebrafish Seizure Model Induced by N-Methyl-D-Aspartic Acid. World J. Psychiatry 2020, 10, 150–161. [Google Scholar] [CrossRef]
- Almeida, E.R.; Lima-Rezende, C.A.; Schneider, S.E.; Garbinato, C.; Pedroso, J.; Decui, L.; Aguiar, G.P.S.; Müller, L.G.; Oliveira, J.V.; Siebel, A.M. Micronized Resveratrol Shows Anticonvulsant Properties in Pentylenetetrazole-Induced Seizure Model in Adult Zebrafish. Neurochem. Res. 2021, 46, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Decui, L.; Garbinato, C.L.L.; Schneider, S.E.; Mazon, S.C.; Almeida, E.R.; Aguiar, G.P.S.; Müller, L.G.; Oliveira, J.V.; Siebel, A.M. Micronized Resveratrol Shows Promising Effects in a Seizure Model in Zebrafish and Signalizes an Important Advance in Epilepsy Treatment. Epilepsy Res. 2020, 159, 106243. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Bello, I.; Rivadeneyra-Domínguez, E.; Rodríguez-Landa, J.F. Anticonvulsant Effect of Turmeric and Resveratrol in Lithium/Pilocarpine-Induced Status Epilepticus in Wistar Rats. Molecules 2022, 27, 3835. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Posa, F.; De Maria, S.; Ravagnan, G.; Ballini, A.; Porro, C.; Trotta, T.; Grano, M.; Muzio, L.L.; Mori, G. Polydatin, Natural Precursor of Resveratrol, Promotes Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Med. Sci. 2018, 15, 944–952. [Google Scholar] [CrossRef]
- Du, Q.-H.; Peng, C.; Zhang, H. Polydatin: A Review of Pharmacology and Pharmacokinetics. Pharm. Biol. 2013, 51, 1347–1354. [Google Scholar] [CrossRef]
- Schimith, L.E.; dos Santos, M.G.; Arbo, B.D.; André-Miral, C.; Muccillo-Baisch, A.L.; Hort, M.A. Polydatin as a Therapeutic Alternative for Central Nervous System Disorders: A Systematic Review of Animal Studies. Phytother. Res. 2022, 36, 2852–2877. [Google Scholar] [CrossRef]
- Aleström, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and Husbandry Recommendations. Lab. Anim. 2020, 54, 213–224. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G.; et al. Regular Care and Maintenance of a Zebrafish (Danio rerio) Laboratory: An Introduction. J. Vis. Exp. 2012, 69, e4196. [Google Scholar] [CrossRef]
- ZFIN: Zebrafish Book: Contents. Available online: https://zfin.org/zf_info/zfbook/zfbk.html (accessed on 13 September 2024).
- MCTI. Available online: https://antigo.mctic.gov.br/mctic/opencms/legislacao/outros_atos/resolucoes/Resolucao_CONCEA_n_39_de_20062018.html (accessed on 29 March 2025).
- Schimith, L.E.; Machado da Silva, V.; da Costa-Silva, D.G.; Seregni Monteiro, L.K.; Muccillo-Baisch, A.L.; André-Miral, C.; Hort, M.A. Preclinical Toxicological Assessment of Polydatin in Zebrafish Model. Drug Chem. Toxicol. 2024, 47, 923–932. [Google Scholar] [CrossRef]
- Baraban, S.C.; Taylor, M.R.; Castro, P.A.; Baier, H. Pentylenetetrazole Induced Changes in Zebrafish Behavior, Neural Activity and c-Fos Expression. Neuroscience 2005, 131, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Lanzarin, G.; Venâncio, C.; Félix, L.M.; Monteiro, S. Inflammatory, Oxidative Stress, and Apoptosis Effects in Zebrafish Larvae after Rapid Exposure to a Commercial Glyphosate Formulation. Biomedicines 2021, 9, 1784. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Ding, R.-C.; Yu, Q.; Qiu, C.-Z.; Zhang, H.-Y.; Yin, Z.-J.; Ren, D.-L. Metformin Attenuates Neutrophil Recruitment through the H3K18 Lactylation/Reactive Oxygen Species Pathway in Zebrafish. Antioxidants 2024, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- Oakes, K.D.; Van Der Kraak, G.J. Utility of the TBARS Assay in Detecting Oxidative Stress in White Sucker (Catostomus Commersoni) Populations Exposed to Pulp Mill Effluent. Aquat. Toxicol. 2003, 63, 447–463. [Google Scholar] [CrossRef]
- Liao, Q.; Li, S.; Siu, S.W.I.; Morlighem, J.-É.R.L.; Wong, C.T.T.; Wang, X.; Rádis-Baptista, G.; Lee, S.M.-Y. Novel Neurotoxic Peptides from Protopalythoa Variabilis Virtually Interact with Voltage-Gated Sodium Channel and Display Anti-Epilepsy and Neuroprotective Activities in Zebrafish. Arch. Toxicol. 2019, 93, 189–206. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Franco, V.; French, J.A.; Perucca, E. Challenges in the Clinical Development of New Antiepileptic Drugs. Pharmacol. Res. 2016, 103, 95–104. [Google Scholar] [CrossRef]
- Klein, P.; Kaminski, R.M.; Koepp, M.; Löscher, W. New Epilepsy Therapies in Development. Nat. Rev. Drug Discov. 2024, 23, 682–708. [Google Scholar] [CrossRef]
- Mutanana, N.; Tsvere, M.; Chiweshe, M.K. General Side Effects and Challenges Associated with Anti-Epilepsy Medication: A Review of Related Literature. Afr. J. Prim. Health Care Fam. Med. 2020, 12, 2162. [Google Scholar] [CrossRef]
- Fakhri, S.; Gravandi, M.M.; Abdian, S.; Akkol, E.K.; Farzaei, M.H.; Sobarzo-Sánchez, E. The Neuroprotective Role of Polydatin: Neuropharmacological Mechanisms, Molecular Targets, Therapeutic Potentials, and Clinical Perspective. Molecules 2021, 26, 5985. [Google Scholar] [CrossRef]
- Phukan, B.C.; Roy, R.; Choudhury, S.; Bhattacharya, P.; Borah, A. Neuroprotective Potential of Polydatin in Combating Parkinson’s Disease through the Inhibition of Monoamine Oxidase-B and Catechol-o-Methyl Transferase. Lett. Drug Des. Discov. 2023, 21, 180–188. Available online: http://www.eurekaselect.com (accessed on 12 September 2024). [CrossRef]
- Barros, S.; Coimbra, A.M.; Alves, N.; Pinheiro, M.; Quintana, J.B.; Santos, M.M.; Neuparth, T. Chronic Exposure to Environmentally Relevant Levels of Simvastatin Disrupts Zebrafish Brain Gene Signaling Involved in Energy Metabolism. J. Toxicol. Environ. Health A 2020, 83, 113–125. [Google Scholar] [CrossRef]
- Khan, K.M.; Collier, A.D.; Meshalkina, D.A.; Kysil, E.V.; Khatsko, S.L.; Kolesnikova, T.; Morzherin, Y.Y.; Warnick, J.E.; Kalueff, A.V.; Echevarria, D.J. Zebrafish Models in Neuropsychopharmacology and CNS Drug Discovery. Br. J. Pharmacol. 2017, 174, 1925–1944. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; He, Q.; Zhang, S.; Cui, Y.; Han, L.; Liu, K. Gastrodin Suppresses Pentylenetetrazole-Induced Seizures Progression by Modulating Oxidative Stress in Zebrafish. Neurochem. Res. 2018, 43, 904–917. [Google Scholar] [CrossRef]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef]
- Brustein, E.; Saint-Amant, L.; Buss, R.R.; Chong, M.; McDearmid, J.R.; Drapeau, P. Steps during the Development of the Zebrafish Locomotor Network. J. Physiol. Paris 2003, 97, 77–86. [Google Scholar] [CrossRef]
- Drapeau, P.; Saint-Amant, L.; Buss, R.R.; Chong, M.; McDearmid, J.R.; Brustein, E. Development of the Locomotor Network in Zebrafish. Prog. Neurobiol. 2002, 68, 85–111. [Google Scholar] [CrossRef]
- Farrell, T.C.; Cario, C.L.; Milanese, C.; Vogt, A.; Jeong, J.-H.; Burton, E.A. Evaluation of Spontaneous Propulsive Movement as a Screening Tool to Detect Rescue of Parkinsonism Phenotypes in Zebrafish Models. Neurobiol. Dis. 2011, 44, 9–18. [Google Scholar] [CrossRef]
- Milder, P.C.; Zybura, A.S.; Cummins, T.R.; Marrs, J.A. Neural Activity Correlates With Behavior Effects of Anti-Seizure Drugs Efficacy Using the Zebrafish Pentylenetetrazol Seizure Model. Front. Pharmacol. 2022, 13, 836573. [Google Scholar] [CrossRef]
- Wang, K.; Chen, X.; Liu, J.; Zou, L.-P.; Feng, W.; Cai, L.; Wu, X.; Chen, S. Embryonic Exposure to Ethanol Increases the Susceptibility of Larval Zebrafish to Chemically Induced Seizures. Sci. Rep. 2018, 8, 1845. [Google Scholar] [CrossRef]
- Zheng, Y.-M.; Chen, B.; Jiang, J.-D.; Zhang, J.-P. Syntaxin 1B Mediates Berberine’s Roles in Epilepsy-Like Behavior in a Pentylenetetrazole-Induced Seizure Zebrafish Model. Front. Mol. Neurosci. 2018, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Choo, B.K.M.; Kundap, U.P.; Faudzi, S.M.M.; Abas, F.; Shaikh, M.F.; Samarut, É. Identification of Curcumin Analogues with Anti-Seizure Potential in Vivo Using Chemical and Genetic Zebrafish Larva Seizure Models. Biomed. Pharmacother. 2021, 142, 112035. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, J.S.; Rosani, A.; Saadabadi, A. Diazepam. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Chitolina, R.; Reis, C.G.; Stahlhofer-Buss, T.; Linazzi, A.; Benvenutti, R.; Marcon, M.; Herrmann, A.P.; Piato, A. Effects of N-Acetylcysteine and Acetyl-L-Carnitine on Acute PTZ-Induced Seizures in Larval and Adult Zebrafish. Pharmacol. Rep. 2023, 75, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Paudel, Y.N.; Yang, X.; Ren, Q.; Zhang, S.; Ji, X.; Liu, K.; Jin, M. Schaftoside Suppresses Pentylenetetrazol-Induced Seizures in Zebrafish via Suppressing Apoptosis, Modulating Inflammation, and Oxidative Stress. ACS Chem. Neurosci. 2021, 12, 2542–2552. [Google Scholar] [CrossRef]
- Bulama, I.; Nasiru, S.; Bello, A.; Abbas, A.Y.; Nasiru, J.I.; Saidu, Y.; Chiroma, M.S.; Moklas, M.A.M.; Taib, C.N.M.; Waziri, A.; et al. Antioxidant-based neuroprotective effect of dimethylsulfoxide against induced traumatic brain injury in a rats model. Front. Pharmacol. 2022, 13, 998179. [Google Scholar] [CrossRef]
- Sanmartín-Suárez, C.; Soto-Otero, R.; Sánchez-Sellero, I.; Méndez-Álvarez, E. Antioxidant Properties of Dimethyl Sulfoxide and Its Viability as a Solvent in the Evaluation of Neuroprotective Antioxidants. J. Pharmacol. Toxicol. Methods 2011, 63, 209–215. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Miranda, F.B.; Schimith, L.E.; da Costa Silva, D.G.; de Oliveira Vian, C.; da Luz, D.B.; de Aguiar, R.F.; da Rocha, C.Y.M.; Siebel, A.M.; Oses, J.P.; Hort, M.A. Effects of Polydatin on Pentylenetetrazol-Induced Seizures in Zebrafish Larvae. Future Pharmacol. 2025, 5, 22. https://doi.org/10.3390/futurepharmacol5020022
de Miranda FB, Schimith LE, da Costa Silva DG, de Oliveira Vian C, da Luz DB, de Aguiar RF, da Rocha CYM, Siebel AM, Oses JP, Hort MA. Effects of Polydatin on Pentylenetetrazol-Induced Seizures in Zebrafish Larvae. Future Pharmacology. 2025; 5(2):22. https://doi.org/10.3390/futurepharmacol5020022
Chicago/Turabian Stylede Miranda, Fernanda Barros, Lucia Emanueli Schimith, Dennis Guilherme da Costa Silva, Camila de Oliveira Vian, Diele Bopsin da Luz, Rafael Felipe de Aguiar, Crístian Yan Montana da Rocha, Anna Maria Siebel, Jean Pierre Oses, and Mariana Appel Hort. 2025. "Effects of Polydatin on Pentylenetetrazol-Induced Seizures in Zebrafish Larvae" Future Pharmacology 5, no. 2: 22. https://doi.org/10.3390/futurepharmacol5020022
APA Stylede Miranda, F. B., Schimith, L. E., da Costa Silva, D. G., de Oliveira Vian, C., da Luz, D. B., de Aguiar, R. F., da Rocha, C. Y. M., Siebel, A. M., Oses, J. P., & Hort, M. A. (2025). Effects of Polydatin on Pentylenetetrazol-Induced Seizures in Zebrafish Larvae. Future Pharmacology, 5(2), 22. https://doi.org/10.3390/futurepharmacol5020022







