Antimicrobial Activity of N-Methyl 4-Piperidone-Derived Monoketone Curcuminoids Against Cariogenic Bacteria
Abstract
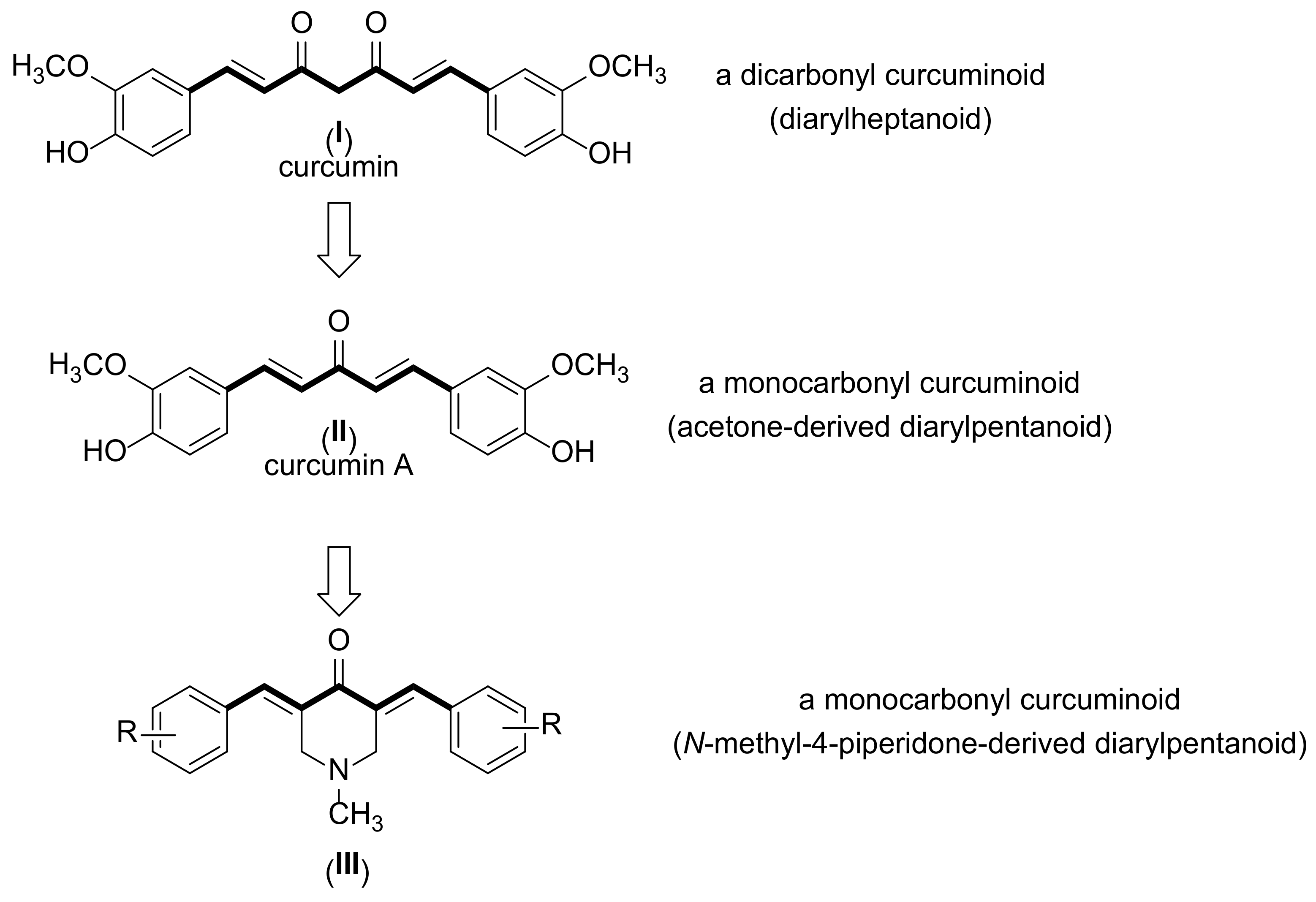
1. Introduction
2. Materials and Methods
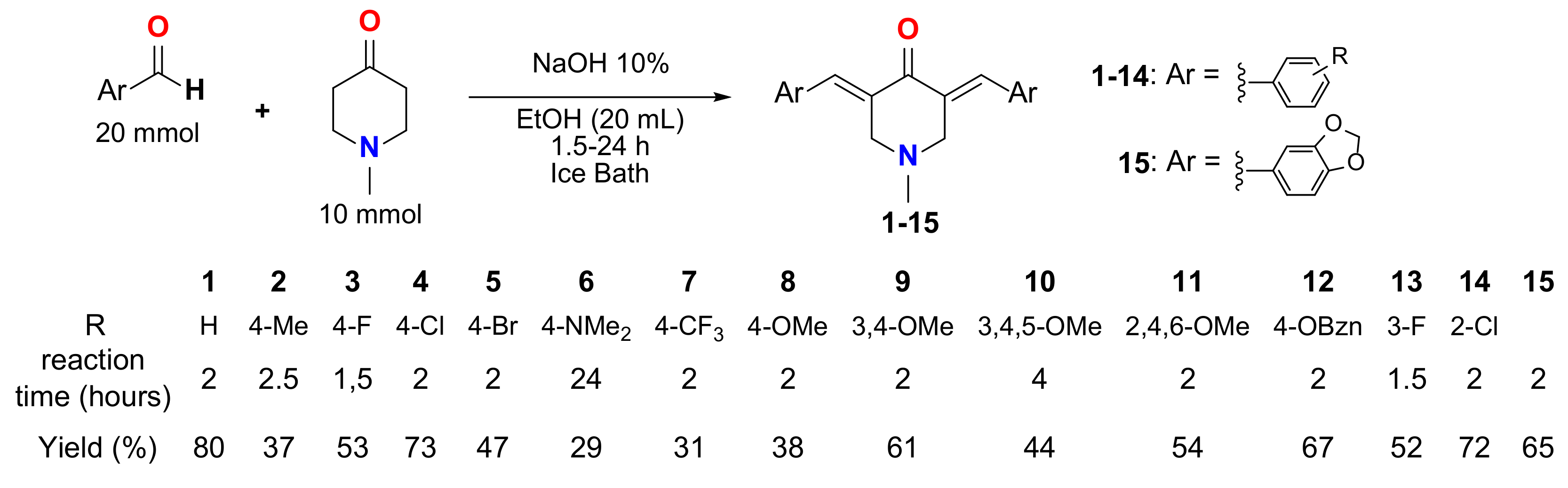
2.1. Synthesis and Structure Elucidation of MKCs
2.2. Antibacterial Activity of Compounds 1–15 Against Cariogenic Bacteria
2.3. Antifungal Activity of Compounds 1–15
3. Results
3.1. Synthesis of Compounds 1–15
3.2. Antimicrobial Activity of MKCs 1–15
4. Discussion
4.1. Synthesis of Compounds 1–15
4.2. Antimicrobial Activity of Compounds 1–15
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHX | Chlorhexidine |
| MKC | MonoKetone Curcuminoid |
| CSC | Claisen–Schimdt Condensation |
| MIC | Minimal Inhibitory Concentration |
| MBC | Minimal Bactericidal Concentration |
| Hex | Hexane |
| EtOAc | Ethyl acetate |
| GC-MS | Gas chromatography-mass spectrometry |
| NMR | Nuclear magnetic resonance |
| APT | Attached Proton Test |
| ATCC | American Type Culture Collection |
| TSB | Tryptic Soy Broth |
| CFU | Colony-Forming Unit |
| CLSI | Clinical and Laboratory Standards Institute |
| DMSO | Dimethyl sulfoxide |
| EtOH | Ethanol |
References
- WHO. Oral Health; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 11 May 2025).
- Liu, R.; Liu, Y.; Yi, J.; Fang, Y.; Guo, Q.; Cheng, L.; He, J.; Li, M. Imbalance of oral microbiome homeostasis: The relationship between microbiota and the occurrence of dental caries. BMC Microbiol. 2025, 25, 46. [Google Scholar] [CrossRef] [PubMed]
- Valm, A.M. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J. Mol. Biol. 2019, 431, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Dong, P.T.; Cen, L.; Bor, B.; Lux, R.; Shi, W.; Yu, Q.; He, X.; Wu, T. Antagonistic interaction between two key endodontic pathogens Enterococcus faecalis and Fusobacterium nucleatum. J. Oral Microbiol. 2023, 15, 2149448. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.C.M.; Bezerra, D.D.S.; Hart-Chu, E.N.S.; Stipp, R.N.; Guedes, S.F.F.; Neves, B.G.; Rodrigues, L.K.A. Quantification and gene expression of Lactobacillus casei group species associated with dentinal lesions in early childhood caries. Saudi Dent. J. 2021, 33, 69–77. [Google Scholar] [CrossRef]
- Zhang, O.L.; Niu, J.Y.; Yu, O.Y.; Mei, M.L.; Jakubovics, N.S.; Chu, C.H. Development of a novel peptide with antimicrobial and mineralising properties for caries management. Pharmaceutics 2023, 15, 2560. [Google Scholar] [CrossRef]
- Oliveira, T.A.S.; Santiago, M.B.; Santos, V.H.P.; Silva, E.O.; Martins, C.H.G.; Crotti, A.E.M. Antibacterial activity of essential oils against oral pathogens. Chem. Biodiv. 2022, 19, e202200097. [Google Scholar] [CrossRef]
- Oliveira, T.A.S.; Silva, J.B.A.; Silva, N.B.S.; Felix, P.C.A.; Dos Santos, D.A.; de Oliveira, A.M.; Martins, C.H.G.; Magalhaes, L.G.; Crotti, A.E.M. Antibacterial and antileishmanial activity of 1,4-dihydropyridine derivatives. Chem. Biodiv. 2025, 22, e202401300. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Pinto, A.; Silva, B.M.D.; Santiago-Junior, J.F.; Sales-Peres, S.H.C. Efficiency of different protocols for oral hygiene combined with the use of chlorhexidine in the prevention of ventilator-associated pneumonia. J. Bras. Pneumol. 2021, 47, e20190286. [Google Scholar] [CrossRef]
- Deus, F.P.; Ouanounou, A. Chlorhexidine in dentistry: Pharmacology, uses, and adverse effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Chang, Y.L.; Chen, Y.L. Deletion of ADA2 Increases antifungal drug susceptibility and virulence in Candida glabrata. Antimicrob. Agents Chemother. 2018, 62, e01924-17. [Google Scholar] [CrossRef]
- Lindberg, E.; Hammarstrom, H.; Ataollahy, N.; Kondori, N. Species distribution and antifungal drug susceptibilities of yeasts isolated from the blood samples of patients with candidemia. Sci. Rep. 2019, 9, 3838. [Google Scholar] [CrossRef]
- Beredaki, M.I.; Arendrup, M.C.; Mouton, J.W.; Meletiadis, J. In vitro pharmacokinetic/pharmacodynamic model data suggest a potential role of new formulations of posaconazole against Candida krusei but not Candida glabrata infections. Int. J. Antimicrob. Agents 2021, 57, 106291. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.D.; Latini, G.; Trilli, I.; Ferrante, L.; Nardelli, P.; Malcangi, G.; Inchingolo, A.M.; Mancini, A.; Palermo, A.; et al. The role of curcumin in oral health and diseases: A systematic review. Antioxidants 2024, 13, 660. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, M.; Yuan, Y. The potential of curcumin-based co-delivery systems for applications in the food industry: Food preservation, freshness monitoring, and functional food. Food Res. Int. 2023, 171, 113070. [Google Scholar] [CrossRef]
- Bērziņa, L.; Mieriņa, I. Antiradical and antioxidant activity of compounds containing 1,3-dicarbonyl moiety: An overview. Molecules 2023, 28, 6203. [Google Scholar] [CrossRef]
- Shi, L.; Qu, Y.; Li, Z.; Fan, B.; Xu, H.; Tang, J. In vitro permeability and bioavailability enhancement of curcumin by nanoemulsion via pulmonary administration. Curr. Drug Deliv. 2019, 16, 751–758. [Google Scholar] [CrossRef]
- Denison, H.J.; Schwikkard, S.L.; Khoder, M.; Kelly, A.F. Review: The chemistry, toxicity and antibacterial activity of curcumin and its analogues. Planta Medica 2023, 90, 47–62. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Anasane, N.; Santos, C.A.D. Curcumin and curcumin-loaded nanoparticles: Antipathogenic and antiparasitic activities. Exp. Rev. Anti-infect. Ther. 2020, 18, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Verma, S.; Fatima, K.; Luqman, S.; Srivastava, S.K.; Khan, F. Pharmacophore & QSAR guided design, synthesis, pharmacokinetics and in vitro evaluation of curcumin analogs for anticancer activity. Curr. Med. Chem. 2024, 31, 620–639. [Google Scholar] [CrossRef] [PubMed]
- Gornicka, J.; Mika, M.; Wroblewska, O.; Siudem, P.; Paradowska, K. Methods to improve the solubility of curcumin from turmeric. Life 2023, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Dytrych, P.; Kejik, Z.; Hajduch, J.; Kaplanek, R.; Vesela, K.; Kucnirova, K.; Skalickova, M.; Venhauerova, A.; Hoskovec, D.; Martasek, P.; et al. Therapeutic potential and limitations of curcumin as antimetastatic agent. Biomed. Pharmacother. 2023, 163, 114758. [Google Scholar] [CrossRef]
- Vieira, T.M.; Dos Santos, I.A.; Silva, T.S.; Martins, C.H.G.; Crotti, A.E.M. Antimicrobial activity of monoketone curcuminoids against cariogenic bacteria. Chem. Biodiv. 2018, 15, e1800216. [Google Scholar] [CrossRef]
- Vieira, T.M.; Tanajura, L.S.; Heleno, V.C.G.; Magalhães, L.G.; Crotti, A.E.M. Monoketone curcuminoids: An updated review of their synthesis and biological activities. Futur. Pharmacol. 2024, 4, 54–77. [Google Scholar] [CrossRef]
- Jonathan, D.R.; DravidaThendral, E.; Priya, M.K.; Shirmila, D.A.; Fathima, A.A.; Yuvashri, R.; Usha, G. Investigations on 3D-structure, properties and antibacterial activity of two new curcumin derivatives. J. Mol. Struc. 2023, 1292, 136063. [Google Scholar] [CrossRef]
- Francisco, K.R.; Monti, L.; Yang, W.; Park, H.; Liu, L.J.; Watkins, K.; Amarasinghe, D.K.; Nalli, M.; Polaquini, C.R.; Regasini, L.O.; et al. Structure-activity relationship of dibenzylideneacetone analogs against the neglected disease pathogen, Trypanosoma brucei. Bioorg. Med. Chem. Lett. 2023, 81, 129123. [Google Scholar] [CrossRef]
- CLSI. Methods for Diluition Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical Laboratory Standard Institute: Wayne, PA, USA, 2018. [Google Scholar]
- CLSI Standard M27–A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. The National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2002.
- Barry, A.L.; Pfaller, M.A.; Brown, S.D.; Espinel-Ingroff, A.; Ghannoum, M.A.; Knapp, C.; Rennie, R.P.; Rex, J.H.; Rinaldi, M.G. Quality control limits for broth microdilution susceptibility tests of ten antifungal agents. J. Clin. Microbiol. 2000, 38, 3457–3459. [Google Scholar] [CrossRef]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef]
- Pati, N.H.; Das, U.; Das, S.; Bandy, B.; Clercq, E.D.; Balzarini, J.; Kawase, M.; Sakagami, H.; Quail, W.J.; Stables, J.P.; et al. The cytotoxic properties and preferential toxicity to tumour cells displayed by some 2,4-bis(benzylidene)-8-methyl-8-azabicyclo[3.2.1] octan-3-ones and 3,5-bis(benzylidene)-1-methyl-4-piperidones. Eur. J. Med. Chem. 2008, 44, 54–62. [Google Scholar] [CrossRef] [PubMed]
- El-Subbagh, H.I.; Abu-Zaid, S.M.; Mahran, M.A.; Badria, F.A.; Al-Obaid, A.M. Synthesis and biological evaluation of certain α,β-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J. Med. Chem. 2000, 43, 2915–2921. [Google Scholar] [CrossRef]
- Eryanti, Y.; Hendra, R.; Herlinda, T.; Zamri, A.; Supratman, U. Synthesis of N-methyl-4-piperidone curcumin analogues and their cytotoxicity activity against T47D cell lines. Indones. J. Chem. 2018, 18, 362–366. [Google Scholar] [CrossRef]
- Yongsheng, J.; Fei, Z.; Hua, Z.; Heyang, Z.; Hongchuan, L. 3,5-Diaryl-Ylidene Piperidone Derivatives and Application Thereof in Preparing Blood Glucose-Reducing and Fat-Reducing Medicine. Application No. CN103626692A, 12 March 2014. [Google Scholar]
- Yadav, B.; Taurin, S.; Rosengren, R.J.; Schumacher, M.; Diederich, M.; Somers-Edgar, T.J.; Larsen, L. Synthesis and cytotoxic potential of heterocyclic cyclohexanone analogues of curcumin. Bioorg. Med. Chem. 2010, 18, 6701–6707. [Google Scholar] [CrossRef]
- Wu, J.; Yali, Z.; Cai, Y.; Wang, J.; Weng, B.; Qinqin, T.; Chen, X.; Pan, Z.; Liang, G.; Yang, S. Discovery and evaluation of piperid-4-one-containing mono-carbonyl analogs of curcumin as anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2013, 21, 3058–3065. [Google Scholar] [CrossRef]
- Makarov, M.V.; Odinets, I.L.; Lyssenko, K.A.; Rybalkina, E.; Kosilkin, I.V.; Antipin, M.Y.; Timofeeva, T.V. N-alkylated 3,5-bis(arylidene)-4-piperidones. Synthetic approaches, X-ray structure and anticancer activity. J. Heterocycl. Chem. 2008, 45, 729–736. [Google Scholar] [CrossRef]
- Castiello, C.; Junghanns, P.; Mergel, A.; Jacob, C.; Ducho, C.; Valente, S.; Rotili, D.; Fioravanti, R.; Zwergel, C.; Mai, A. GreenMedChem: The challenge in the next decade toward eco-friendly compounds and processes in drug design. Green Chem. 2023, 25, 2109–2169. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Rajasekaran, J.J.; Krishnamurthy, H.K.; Bosco, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K. Oral microbiome: A review of its impact on oral and systemic health. Microorganisms 2024, 12, 1797. [Google Scholar] [CrossRef]
- Vieira, T.M.; Barco, J.G.; de Souza, S.L.; Santos, A.L.O.; Daoud, I.; Rahali, S.; Amdouni, N.; Bastos, J.K.; Martins, C.H.G.; Ben Said, R.; et al. In vitro and in silico studies of the antimicrobial activity of prenylated phenylpropanoids of green propolis and their derivatives against oral bacteria. Antibiotics 2024, 13, 787. [Google Scholar] [CrossRef]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef]
- Adamczak, A.; Ozarowski, M.; Karpinski, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Makade, C.S.; Shenoi, P.R.; Bhongade, B.A.; Shingane, S.A.; Ambulkar, P.C.; Shewale, A.M. Estimation of MBC: MIC Ratio of herbal extracts against common endodontic pathogens. J. Pharm. Bioallied Sci. 2024, 16, S1414–S1416. [Google Scholar] [CrossRef]
- Lambert, P.A. Cellular impermeability and uptake of biocides and antibiotics in Gram-positive bacteria and mycobacteria. J. Appl. Microbiol. 2002, 92, 46S–54S. [Google Scholar] [CrossRef]
- Elkanzi, N.A.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of chalcones derivatives and their biological activities: A review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef]
- Wang, C.; Du, W.; Lu, H.; Lan, J.; Liang, K.; Cao, S. A review: Halogenated compounds from marine Actinomycetes. Molecules 2021, 26, 2754. [Google Scholar] [CrossRef]
- Faleye, O.S.; Boya, B.R.; Lee, J.H.; Choi, I.; Lee, J. Halogenated antimicrobial agents to combat drug-resistant pathogens. Pharmacol. Rev. 2023, 76, 90–141. [Google Scholar] [CrossRef]
- Chhillar, A.K.; Arya, P.; Mukherjee, C.; Kumar, P.; Yadav, Y.; Sharma, A.K.; Yadav, V.; Gupta, J.; Dabur, R.; Jha, H.N.; et al. Microwave-assisted synthesis of antimicrobial dihydropyridines and tetrahydropyrimidin-2-ones: Novel compounds against aspergillosis. Bioorg. Med. Chem. 2006, 14, 973–981. [Google Scholar] [CrossRef]
| Streptococcus mitis (ATCC 49456) | Streptococcus mutans (ATCC 25175) | Streptococcus sanguinis (ATCC 10556) | Streptococcus salivarius (ATCC 25975) | Streptococcus sobrinus (ATCC 33478) | Enterococcus faecalis (ATCC 4082) | Lactobacillus paracasei (ATCC 4578) | |
|---|---|---|---|---|---|---|---|
| 1 | 250 (0.86) | 1000 (3.46) | 500 (1.73) | 500 (1.73) | 1000 (3.46) | 2000 (6.92) | 250 (0.86) |
| 1000 (3.46) | 1000 (3.46) | 1000 (3.46) | 1000 (3.46) | 1000 (3.46) | 2000 (6.92) | 1000 (3.46) | |
| 4 | 1 | 2 | 2 | 1 | 1 | 4 | |
| 2 | 500 (1.57) | 1000 (3.15) | 500 (1.57) | 1000 (3.15) | 1000 (3.15) | 2000 (6.30) | 1000 (3.15) |
| 1000 (3.15) | 2000 (6.30) | 2000 (6.30) | 1000 (3.15) | 1000 (3.15) | 2000 (6.30) | 1000 (3.15) | |
| 2 | 2 | 4 | 1 | 1 | 1 | 1 | |
| 3 | 250 (0.77) | 2000 (6.15) | 500 (1.54) | 1000 (3.07) | 1000 (3.07) | 2000 (6.15) | 1000(3.07) |
| 2000 (6.15) | 2000 (6.15) | 2000 (6.15) | 2000 (6.15) | 2000 (6.15) | 2000 (6.15) | 1000(3.07) | |
| 8 | 1 | 4 | 2 | 2 | 1 | 1 | |
| 4 | 500 (1.39) | 2000 (5.58) | 1000 (2.79) | 2000 (5.58) | 2000 (5.58) | 2000 (5.58) | 1000 (2.79) |
| 2000 (5.58) | 2000 (5.58) | 2000 (5.58) | 2000 (5.58) | 2000 (5.58) | 2000 (5.58) | 1000 (2.79) | |
| 4 | 1 | 2 | 1 | 1 | 1 | 1 | |
| 5 | 2000 (4.47) | 2000 (4.47) | 1000 (2.23) | 2000 (4.47) | 2000 (4.47) | 2000 (4.47) | 1000 (2.24) |
| 2000 (4.47) | 2000 (4.47) | 2000 (4.47) | 2000 (4.47) | 2000 (4.47) | 2000 (4.47) | 1000 (2.24) | |
| 1 | 1 | 2 | 1 | 1 | 1 | 1 | |
| 6 | 1000 (2.66) | 2000 (5.33) | 1000 (2.66) | 2000 (5.33) | 2000 (5.33) | 2000 (5.33) | 1000 (2.66) |
| 1000 (2.66) | 2000 (5.33) | 2000 (5.33) | 2000 (5.33) | 2000 (5.33) | 2000 (5.33) | 1000 (2.66) | |
| 1 | 1 | 2 | 1 | 1 | 1 | 1 | |
| 7 | 500 (1.17) | 2000 (4.70) | 1000 (2.35) | 2000 (4.70) | 2000 (4.70) | 2000 (4.70) | 1000 (2.35) |
| 1000 (2.35) | 2000 (4.70) | 2000 (4.70) | 2000 (4.70) | 2000 (4.70) | 2000 (4.70) | 1000 (2.35) | |
| 2 | 1 | 2 | 1 | 1 | 1 | 1 | |
| 8 | 500 (1.43) | 2000 (5.72) | 1000 (2.86) | 2000 (5.72) | 1000 (2.86) | 1000 (2.86) | 500 (1.43) |
| 2000 (5.72) | 2000 (5.72) | 2000 (5.72) | 2000 (5.72) | 2000 (5.72) | 2000 (5.72) | 2000 (5.72) | |
| 4 | 1 | 2 | 1 | 2 | 2 | 4 | |
| 9 | 500 (1.22) | 2000 (4.88) | 1000 (2.44) | 2000 (4.88) | 2000 (4.88) | 2000 (4.88) | 1000 (2.44) |
| 2000 (4.88) | 2000 (4.88) | 2000 (4.88) | 2000 (4.88) | 2000 (4.88) | 2000 (4.88) | 1000 (2.44) | |
| 4 | 1 | 2 | 1 | 1 | 1 | 1 | |
| 10 | 250 (0.53) | 1000 (2.13) | 500 (1.06) | 1000 (2.13) | 1000 (2.13) | 2000 (4.26) | 500 (1.06) |
| 1000 (2.13) | 1000 (2.13) | 2000 (4.26) | 1000 (2.13) | 2000 (4.26) | 2000 (4.26) | 1000 (2.13) | |
| 4 | 1 | 4 | 1 | 2 | 1 | 4 | |
| 11 | 1000 (2.13) | 2000 (4.26) | 1000 (2.13) | 2000 (4.26) | 2000 (4.26) | 2000 (4.26) | 1000 (2.13) |
| 2000 (4.26) | 2000 (4.26) | 2000 (4.26) | 2000 (4.26) | 2000 (4.26) | 2000 (4.26) | 1000 (2.13) | |
| 2 | 1 | 2 | 1 | 1 | 1 | 1 | |
| 12 | 500 (0.99) | 2000 (3.99) | 1000 (1.99) | 2000 (3.99) | 2000 (3.99) | 2000 (3.99) | 1000 (1.99) |
| 1000 (1.99) | 2000 (3.99) | 2000 (3.99) | 2000 (3.99) | 2000 (3.99) | 2000 (3.99) | 1000 (1.99) | |
| 4 | 1 | 2 | 1 | 1 | 1 | 1 | |
| 13 | 250 (0.77) | 500 (1.54) | 250 (0.77) | 1000 (3.07) | 250 (0.77) | 2000 (6.16) | 1000 (3.07) |
| 500 (1.54) | 2000 (6.16) | 2000 (6.16) | 2000 (6.16) | 500 (1.54) | 2000 (6.16) | 1000 (3.07) | |
| 2 | 4 | 8 | 2 | 2 | 1 | 1 | |
| 14 | 1000 (2.79) | 2000 (5.58) | 1000 (2.79) | 2000 (5.58) | 2000 (5.58) | 2000 (5.58) | 1000 (2.79) |
| 2000 (5.58) | 2000 (5.58) | 2000 (5.58) | 2000 (5.58) | 2000 (5.58) | 2000 (5.58) | 1000 (2.79) | |
| 2 | 1 | 2 | 1 | 1 | 1 | 1 | |
| 15 | 500 (1.86) | 2000 (7.43) | 1000 (3.71) | 2000 (7.43) | 2000 (7.43) | 2000 (7.43) | 1000 (3.71) |
| 2000 (7.43) | 2000 (7.43) | 2000 (7.43) | 2000 (7.43) | 2000 (7.43) | 2000 (7.43) | 1000 (3.71) | |
| 4 | 1 | 2 | 1 | 1 | 1 | 1 | |
| CHD | 1.84 (3.65) | 0.92 (1.82) | 3.69 (7.29) | 0.92 (1.82) | 1.84 (3.65) | 3.69 (7.29) | 0.92 (1.82) |
| Compound | Candida albicans (ATCC 4082) | Candida krusei (ATCC 14243) |
|---|---|---|
| 1 | >100 | >100 |
| 2 | >100 | >100 |
| 3 | >100 | >100 |
| 4 | >100 | >100 |
| 5 | >100 | >100 |
| 6 | >100 | >100 |
| 7 | >100 | >100 |
| 8 | >100 | >100 |
| 9 | >100 | >100 |
| 10 | >100 | >100 |
| 11 | >100 | >100 |
| 12 | >100 | >100 |
| 13 | >100 | >100 |
| 14 | >100 | >100 |
| 15 | >100 | >100 |
| fluconazole | 12.5 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, R.H.; Robles, Y.R.; Oliva, I.M.; Santos, A.L.O.; Teixeira, J.G.; Chellegatti, M.A.S.C.; Furtado, N.A.J.C.; Martins, C.H.G.; Nardini, V.; Crotti, A.E.M. Antimicrobial Activity of N-Methyl 4-Piperidone-Derived Monoketone Curcuminoids Against Cariogenic Bacteria. Future Pharmacol. 2025, 5, 23. https://doi.org/10.3390/futurepharmacol5020023
Lima RH, Robles YR, Oliva IM, Santos ALO, Teixeira JG, Chellegatti MASC, Furtado NAJC, Martins CHG, Nardini V, Crotti AEM. Antimicrobial Activity of N-Methyl 4-Piperidone-Derived Monoketone Curcuminoids Against Cariogenic Bacteria. Future Pharmacology. 2025; 5(2):23. https://doi.org/10.3390/futurepharmacol5020023
Chicago/Turabian StyleLima, Richard H., Yan R. Robles, Isabelle M. Oliva, Anna L. O. Santos, Júlia G. Teixeira, Maria A. S. C. Chellegatti, Niege A. J. C. Furtado, Carlos H. G. Martins, Viviani Nardini, and Antônio E. M. Crotti. 2025. "Antimicrobial Activity of N-Methyl 4-Piperidone-Derived Monoketone Curcuminoids Against Cariogenic Bacteria" Future Pharmacology 5, no. 2: 23. https://doi.org/10.3390/futurepharmacol5020023
APA StyleLima, R. H., Robles, Y. R., Oliva, I. M., Santos, A. L. O., Teixeira, J. G., Chellegatti, M. A. S. C., Furtado, N. A. J. C., Martins, C. H. G., Nardini, V., & Crotti, A. E. M. (2025). Antimicrobial Activity of N-Methyl 4-Piperidone-Derived Monoketone Curcuminoids Against Cariogenic Bacteria. Future Pharmacology, 5(2), 23. https://doi.org/10.3390/futurepharmacol5020023









