Abstract
Background/Objectives: Dental caries and candidiasis are major health problems worldwide. Dental caries is caused by cariogenic bacteria, especially those belonging to the Streptococcus genus, whereas candidiasis is caused by Candida species. In this study, the antimicrobial activity of a series of synthetic N-methyl-4-piperidone-derived monoketone curcuminoids (MKCs) against Candida albicans, C. krusei, and a representative panel of cariogenic bacteria was assessed. Methods: Fifteen MKCs were synthesized using an environmentally friendly base-catalyzed Claisen–Schmidt condensation between an aromatic aldehyde (R-PhCHO) and N-methyl-4-piperidone ethanol using NaOH as the catalyst. These compounds were evaluated for their antibacterial activity against a representative panel of cariogenic bacteria, along with their antifungal activity against Candida krusei and C. albicans. The antimicrobial activity was determined based on the Minimum Inhibitory Concentration (MIC) values. Results: Most of the compounds were obtained in about 2 h in yields ranging from 40 to 70%. None of the compounds displayed antifungal activity, even at 100 μg/mL, the highest tested concentration. Similarly, none of the compounds were active against Enterococcus faecalis. On the other hand, compounds 1 (R = H), 10 (R = 3,4,5-OMe), and 13 (R = 3-F) displayed moderate activity against Streptococcus mutans (13), S. salivarus (1), L. paracasei (1 and 10), S. mitis (1, 10, and 13), S. sanguinis (1, 10, and 13), and S. sobrinus (13), with MIC values of 250 μg/mL and 500 μg/mL. The presence of the N-methyl-4-piperidone ring was found to boost the antibacterial activity as compared to the corresponding acetone-derived MKCs. Moreover, the antibacterial activity of compounds 10 and 13 was associated with the presence and position of the fluor atom and the methoxy groups at the aromatic ring. Conclusions: This study contributed to a better understanding of the antimicrobial activity of MKCs, whose data in the literature are still scarce.
1. Introduction
Dental caries is a disease caused by bacteria that is influenced by several factors and affects around 2 billion people worldwide [1]. It is commonly associated with the Streptococcus mutans agent, although studies point out that dental caries is caused by a wide diversity of bacteria, whilst S. mutans alone cannot induce oral infection [2]. Other species within the Streptococcus genus, such as S. sobrinus, S. salivarius, and S. sanguinis, are also implicated in the cariogenic process due to their complementary abilities in adhesion, acid production, and biofilm formation [3]. In addition, Enterococcus faecalis, although primarily associated with endodontic infections, has been linked to the cariogenic process due to its capacity to form resilient biofilms and persist in demineralizing environments typical of carious lesions [4]. Another bacterial group associated with dentinal caries is Lactobacillus casei, known for its acidogenic potential, which contributes to enamel demineralization and the progression of carious lesions [5,6].
This globally affecting disease persists in the demineralization of teeth caused by the acid byproducts formed by cariogenic bacteria. Dietary intake is directly related to the disease progression: frequent sugar intake can boost microbial metabolism, which leads to the adhesion of microbes to the tooth surface and ultimately to teeth demineralization [2,7]. The easiest and most efficient method to prevent dental caries is the mechanical removal of biofilm through brushing and flossing [8,9]. These methods also prevent periodontal diseases, highlighting their importance [10]. When these prevention methods are not performed correctly, chemical treatment is the best way to combat caries [11]. The main agent for the treatment of this infection is chlorhexidine (CHX), whose lipophilic structure prevents the cariogenic agent via the cell wall. However, it has been reported that the use of CHX is related to many adverse effects, such as taste alteration, numbness in the mouth, pain in the application area, and teeth staining [8,12].
The occurrence of infections caused by Candida glabrata has increased significantly. This species, along with C. albicans, C. tropicalis, C. parapsilosis, and C. krusei, is responsible for invasive candidiasis, which has a mortality rate exceeding 70% [13]. C. albicans is the most commonly encountered fungal pathogen associated with candidemia; however, managing infections caused by C. glabrata presents significant difficulties due to its limited susceptibility to antifungal medications [14,15]. C. glabrata quickly develops resistance to fluconazole following exposure to azole antifungals, and instances of multidrug-resistant strains have been documented, resulting in a lack of effective treatment options for this pathogen [16].
Curcumin (I, Figure 1) is a phenolic compound isolated from Curcuma longa rhizomes [17] that finds various applications within the food industry [18]. In addition to its industrial significance, curcumin also displays significant biological and pharmacological potential, such as antioxidant [19], anti-inflammatory [20], antimicrobial [21], antiparasitic [22], and anticancer [23] activities. However, curcumin has poor water solubility, which results in low bioavailability; moreover, because of its hydrophobic character, curcumin is not practically absorbed from the digestive system [24]. These aspects have limited the use of curcumin as a therapeutic agent [25].
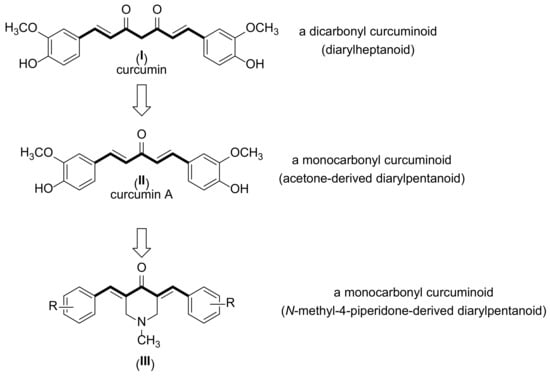
Figure 1.
Chemical structures of curcumin (I), curcumin A (II), and N-methyl-4-piperidone curcuminoids (III).
Monoketone curcuminoids (MKCs) are synthetic monocarbonyl analogs of curcumin. These compounds can be obtained by Claisen–Schmidt condensations from a wide diversity of ketones, including acetone, cyclohexanone, and N-methyl-4-pyrrolidone (Figure 1). Some MKCs have been exploited for their antibacterial activity against cariogenic bacteria [26,27,28]. For example, curcumin A (II), the monocarbonyl analog of curcumin, displays increased activity against S. mutans as compared to curcumin [26]. However, to the best of our knowledge, studies on the antibacterial activity of MKCs against cariogenic bacteria are still scarce [28].
As part of our interest in the biological activities of MKCs [26,29], in this study, we have synthesized 15 N-methyl-4-piperidone-derived MKCs using a base-catalyzed Claisen–Schmidt condensation and evaluated their activities against a representative panel of cariogenic bacteria and two Candida species.
2. Materials and Methods
2.1. Synthesis and Structure Elucidation of MKCs
MKCs 1–15 were synthesized via a base-catalyzed Claisen–Schmidt condensation (CSC) according to a procedure available in the literature [26]. In this methodology, 20 mL of ethanol (EtOH, Synth, Diadema, SP, Brazil) was added to a 200-mL round-bottom flask containing 25 mL of an aqueous sodium hydroxide (NaOH, Synth, Diadema, SP, Brazil)) solution (10%). Next, 20 mmol of an aromatic aldehyde and 10 mmol of N-methyl-4-piperidone (Sigma-Aldrich, St. Louis, MO, USA) were added to the solution and left stirring in an ice bath for 1.5 to 24 h (Scheme 1). The reaction progress was monitored via thin-layer chromatography using Hex:EtOAc 8:2 (v/v) as a solvent. As the reaction ended, a precipitate was formed, and the mixture was filtered off under a vacuum. Finally, the solid product was purified via recrystallization in 95% EtOH and then dried in a vacuum desiccator. All of the compounds were identified based on NMR (1H and 13C) and mass spectrometry data (see Supporting Information) and displayed a purity higher than 95%, as indicated by HPLC analyses.
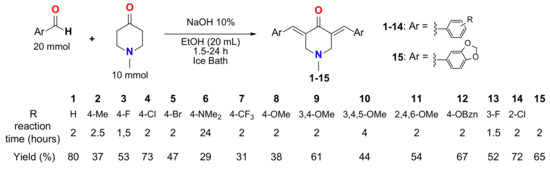
Scheme 1.
Synthesis of compounds 1–15, along with their yields (%) and reaction times (in hours).
2.2. Antibacterial Activity of Compounds 1–15 Against Cariogenic Bacteria
For the evaluation of the antibacterial activity of MKCs 1–15, 7 different bacteria species obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) were selected as follows: Streptococcus mitis (ATCC 49456, isolation not informed), Streptococcus mutans (ATCC 25175, isolated from carious dentin), Streptococcus sanguinis (ATCC 10556, isolated from a patient with subacute bacterial endocarditis), Streptococcus salivarius (ATCC 25975, isolated from saliva), Streptococcus sobrinus (ATCC 33478, isolated from dental plaque), Enterococcus faecalis (ATCC 4082, isolated from the root canal of a pulpless tooth), and Lactobacillus paracasei (ATCC 4578, isolated from the oral cavity). All microorganisms used in this study were part of the culture collection of the Antimicrobial Testing Laboratory (LEA) at the Federal University of Uberlândia (UFU) and were cryopreserved at –80 °C until the beginning of the assays.
The inoculum preparation involved reactivating microorganism strains stored at −20 °C by suspending them in Brain Heart Infusion Broth—BHI (Kasvi, Pinhais, Paraná, BRA). The strains were then streaked onto blood agar (Difco, Detroit, USA) and incubated at 37 °C under appropriate atmospheric conditions for 24 h to verify their purity. Following confirmation, 24-h cultures of the indicator microorganisms were transferred using a sterile platinum loop to tubes containing 5 mL of 0.9% saline solution. The bacterial suspensions were then standardized by adjusting their turbidity to match the reference tube corresponding to the McFarland 0.5 scale [30]. The bacterial suspensions were initially diluted in sterile saline to obtain an inoculum concentration of 1.5 × 107 CFU/mL. Further dilution in tryptic soy broth adjusted the concentration to 2.5 × 106 CFU/mL, with a final concentration of 5 × 105 CFU/mL in the inoculum well, following CLSI guidelines [30].
For the preparation of the MKCs 1–15 solution, 2 mg of the compound was dissolved in 250 μL of dimethylsulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA) using an ultrasonic bath followed by dilution with 1000 μL of BHI Broth (Kasvi). The positive control, an aqueous solution of chlorhexidine (0.1 mg/mL, Sigma-Aldrich, St. Louis, MO, USA), was diluted in BHI to a final concentration of 0.02 mg/mL. Antibacterial activity was assessed across a concentration gradient of chlorhexidine in microplate wells, ranging from 0.115 μg/mL to 59.0 μg/mL. The minimum inhibitory concentration (MIC) was determined using a broth microdilution assay in sterile 96-well microplates. Each well contained a total volume of 100 μL, consisting of BHI, the MKC solution, and the standardized microbial suspension. The samples were tested at concentrations ranging from 0.98 μg/mL to 2000 μg/mL. After incubation, the lowest concentration that inhibited microbial growth, as indicated by the colorimetric change upon the addition of resazurin (Sigma®), was recorded as the MIC. The procedure followed the standardized CLSI methodology [30]. To ascertain the Minimum Bactericidal Concentration (MBC), a 10 μL sample of the inoculum was carefully extracted from each well under aseptic conditions before the addition of resazurin and subsequently cultured on blood agar (Difco, Detroit, MI, USA). The plates were subjected to incubation following the previously outlined procedure. The minimum bactericidal concentration (MBC) was defined as the lowest compound concentration that killed >99.9% of the original bacterial population, resulting in the absence of any visible bacterial growth. The determination of the MIC for each microorganism was performed in triplicate.
2.3. Antifungal Activity of Compounds 1–15
The antifungal tests were conducted using Candida krusei (ATCC 14243) and Candida albicans (ATCC 10231) strains, both of which were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). The values of the MIC of compounds 1–15 were assessed in triplicate by using the microdilution broth method in 96-well microplates following the recommendations of the Clinical and Laboratory Standards Institute (M27-A2) [31]. Samples were initially dissolved in DMSO at a concentration of 1 mg/mL. Subsequently, they were diluted in an RPMI 1640 medium (Gibco-Life Technology, Grand Island, NY, USA) to obtain final concentrations varying from 100 μg/mL down to 0.19 μg/mL. The resulting concentration of DMSO was 5% (v/v), and this solution was used as the negative control. The inoculum was adjusted to achieve a cell concentration of 2.5 × 103 CFU/mL. One non-inoculated well (i.e., free of antimicrobial agent) was also included to ensure medium sterility. Fluconazole (Sigma–Aldrich, St. Louis, MO, USA) was used as the positive control, and its MIC value was also determined to ensure that the antifungal microdilution test was performed appropriately [32]. The microplates were incubated at 35 °C for 48 h. Next, resazurin (Sigma-Aldrich, St. Louis, MO, USA) (30 μL) in an aqueous solution (0.02%) was added to the microplates to indicate microorganism viability [33]. The MIC values were determined as the lowest concentrations of each compound capable of inhibiting microorganism growth. The determination of the MIC for each microorganism was performed in triplicate.
3. Results
3.1. Synthesis of Compounds 1–15
N-methyl-4-piperidone-derived MKCs 1–15 were obtained with reaction times ranging from 1.5 to 24 h, as shown in Table 1. Most of the compounds were obtained as yellow powders in 31–80% yields (see Table 1 and Supporting Information). These compounds were identified based on the comparison of the 1H and 13C NMR and mass spectrometry data with those from the literature [34,35,36,37,38,39,40].

Table 1.
Minimal Inhibitory Concentration (MIC) and Minimal Bactericide Concentration (MBC) values of MKCs 1–15, given in μg/mL, against different cariogenic bacteria. Values between parentheses are given in mM.
3.2. Antimicrobial Activity of MKCs 1–15
All 15 MKCs were tested against a variety of cariogenic bacteria in concentrations ranging from 0.98 μg/mL to 2000 μg/mL. Chlorhexidine, used as the positive control, was tested in concentrations from 0.115 μg/mL to 59.0 μg/mL. The antibacterial activity of these compounds was expressed in terms of their Minimal Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) values, as shown in Table 1.
Most of the compounds displayed MIC values equal to or higher than 1000 μg/mL. However, some compounds displayed MICs of 250 and 500 μg/mL against some cariogenic bacteria. The lowest MIC values were obtained against S. mitis and S. sanguinis, whereas the highest MIC values were obtained against E. faecalis. Compounds 1 and 13 displayed MIC values of 250 and 500 μg/mL against four bacteria, followed by compound 10 (against S. mitis, S. sanguinis, and L. paracasei), 2 and 3 (S. mitis and S. sanguinis), and 8 (S. mitis and L. paracasei). Compounds 4, 7, 9, 12, and 15 displayed MIC values of 500 μg/mL against only S. mitis. On the other hand, compounds 5, 6, 11, and 14 did not exhibit MIC values lower than 1000 μg/mL against any of the tested bacteria.
None of the tested compounds displayed antifungal activity against C. albicans and C. krusei, even at 100 μg/mL, the highest concentration tested (Table 2).

Table 2.
Minimal Inhibitory Concentration (MIC) values of MKCs 1–15, given in μg/mL, against C. albicans ATCC 4082 and C. krusei ATCC 14243.
4. Discussion
4.1. Synthesis of Compounds 1–15
The base-catalyzed Claisen–Schmidt condensation used to obtain compounds 1–15 has been the methodology of choice to synthesize a wide diversity of MKCs [27]. The main advantages of this procedure are the use of a non-toxic solvent (EtOH) and short reaction times (the reaction times did not exceed 4 h, except for compound 6). Additionally, the isolation and purification of MKCs do not require expensive and time-consuming chromatographic steps. These aspects are very interesting from a green chemistry point of view [41,42].
Although all the compounds have been previously reported in the literature [34,35,36,37,38,39,40], data on their antibacterial and antifungal activities are still infrequently exploited [28]. In this study, this goal was achieved using a green chemistry methodology, since the reaction itself can be done with an environmentally friendly synthetic methodology that can easily and quickly afford a library of MKCs for biological assays. Of note, reaction times vary according to the employed aldehyde. The presence of electron-withdrawing groups at the aromatic ring, such as F, Cl, and Br, increases the electrophilicity of the carbonyl of aromatic aldehyde, which consequently increases its susceptibility to the attack of the enolate anion of N-methyl-4-piperidone, thus decreasing the reaction rate. On the other hand, electron-releasing groups such as 3,4,5-OMe and NMe2 decrease the electrophilicity of the carbonyl of aromatic aldehyde, thus increasing the reaction time.
4.2. Antimicrobial Activity of Compounds 1–15
To date, there are only a few reports on the antimicrobial activity of N-methyl-4-piperidone-derived MKCs. For example, Jonathan et al. investigated the antibacterial activity of compound 9 against two Gram-positive (Bacillus subtilis and Staphylococcus aureus) and two Gram-negative (Escherichia coli and Pseudomonas aeruginosa) bacterial strains, which were examined by using the disc diffusion method. The authors found that compound 9 was more active against Gram-negative bacteria than against Gram-positive bacteria and strongly controlled E. coli growth, even at low concentrations [28].
The human oral microbiome comprises more than 700 known bacteria. Among these bacteria, Gram-negative anaerobic bacteria have been reported as putative pathogens in periodontal disease. On the other hand, Gram-positive and facultatively anaerobic streptococci are aetiologically the most important bacteria in dental caries [43]. As part of our ongoing research on novel compounds active against cariogenic bacteria [8,26,44], we evaluated the antimicrobial activity of compounds 1–15 against a representative panel of Gram-positive cariogenic bacteria.
According to the criteria available in the literature, antimicrobial activity can be classified as inactive to very strong/promising based on MIC values [7,20,44,45,46,47]. For values below 100 μg/mL, the molecule activity can be considered promising. For values ranging from 100 to 500 and from 500 to 1000 μg/mL, the activity can be regarded as moderate and weak, respectively [46]. Based on these criteria, it can be concluded that most of the MKCs tested were weakly active or inactive against most of the tested cariogenic bacteria, as they were only able to inhibit growth or kill the cariogenic agents in concentrations higher than 1000 or 2000 μg/mL. Nevertheless, except for compounds 5, 6, 11, and 14, all the compounds displayed moderate activity against at least one bacterium.
Most of the MKCs were moderately active against S. mitis (MIC values of 250 and 500 μg/mL) except for 5, 6, 11, and 14. Indeed, S. mitis was the most sensitive to MKCs 1–15, followed by S. sanguinis. On the other hand, none of the compounds were active against E. faecalis, whereas only compounds 1 and 13 displayed moderate activity against S. mutans and S. sobrinus. These results indicated that E. faecalis, S. mutans, and S. sobrinus were the most resistant to MKCs 1–15.
Compounds that have MBC:MIC ≤ 4 and 4 < MBC:MIC ≤ 32 are considered to display bactericidal (i.e., cell death induction) and bacteriostatic (i.e., cell inhibition) effects, respectively, whereas MBC:MIC > 32 values denote bacterial resistance [48]. Based on these criteria, among the compounds that displayed moderate activity, only the activity of compound 3 against S. mitis (MBC:MIC = 8) and compound 13 against S. sanguinis (MBC:MIC = 8) can be considered to be due to bacteriostatic effects.
Although expressing the MIC values in μg/mL can make the sample solution preparation for the biological assays easier, MIC values in μg/mL can lead to misunderstanding, as they suggest that compounds with very distinct molecular weights displaying the same MIC value are equally effective against a microorganism, pathogen, or cell. Thus, expressing the MIC values in molar subunits, usually mmol/L (or mM), or μmol/L (or μM), can give a clearer idea of the number of molecules of each compound that is responsible for the activity. In this study, we also expressed MIC and MBC values in mM, as shown in Table 1. These values revealed that compound 3 (MIC = 0.77 mM) is more effective than 1 (MIC = 0.86 mM) against S. mitis.
Among the MKCs that displayed moderate activity, compounds 1, 10, and 13 displayed the lowest MIC values. Compound 1 displayed the lowest MIC against S. salivarus (MIC = 1.73 mM) and L. paracasei (MIC = 0.86 mM). On the other hand, compound 10 had the lowest MIC against S. mitis (MIC = 0.43 mM) and the second lowest MIC value against S. mutans (MIC = 2.13 mM), S. sanguinis (MIC = 1.06 mM), S. salivarus (MIC = 2.13 mM), S. sobrinus (MIC = 2.13 mM), and L. paracasei (MIC = 1.06 mM). Finally, compound 13 exhibited the lowest MIC value against S. mutans (MIC = 1.54 mM), S. sanguinis (MIC = 0.77 mM), and S. sobrinus (MIC = 0.77 mM).
The cariogenic bacteria investigated in this study showed different susceptibilities to compounds 1–15, revealing that the different substituents in the aromatic ring may play different roles in the antibacterial activity against each bacterium. For instance, for S. salivarus and L. paracasei, compounds 1 (no substituent at the aromatic ring) and 10 (R = 3,4,5-OMe) displayed the lowest MIC. For S. mitis, the lowest MIC was obtained for the compound, followed by compounds 3 (R = 4-F) and 13 (a 3-F). On the other hand, compound 13 displayed the lowest MIC against S. mutans, S. sanguinis, and S. sobrinus, followed by compound 10.
Vieira et al. investigated the antibacterial activity of dibenzylideneacetone and (1E,4E)-1,5-bis(3,4,5-trimethoxyphenyl)penta-1,4-dien-3-one, the acetone-derived MKC analogs to 1 and 10 [26]. The authors reported that the first compound displayed MIC values equal to or higher than 400 μg/mL (1.70 mM) against all the same cariogenic bacteria investigated in this study, while the latter was inactive in concentrations lower than 400 μg/mL. Similarly, (1E,4E)-1,5-bis(3,4,5-trimethoxyphenyl)penta-1,4-dien-3-one was inactive against the same bacteria (MIC > 400 or 0.96 mM). The higher antibacterial activity of MKCs 1 and 10 compared to their corresponding acetone-derived MKCs indicates that N-methyl-4-piperidone moiety boosts antimicrobial activity.
The cell walls of Gram-positive bacteria are permeable and allow the penetration of hydrophobic antibiotics, which can cross the cell wall by diffusion through the hydrophobic bilayer [49]. In this sense, the lipophilicity of a compound directly affects antibacterial activity because it is associated with its solubility in the lipid bilayers that protect bacterial cell membranes. On the other hand, hydrophilic polar groups may anchor the compound to the bacterial cell wall by increasing the compound’s solubility in the aqueous phase through hydrogen bonds, aligning the non-polar carbon chain into the lipid phase by dispersion forces [44]. Therefore, the lowest MIC values of N-methyl-4-piperidone-derived MKCs compared to acetone-derived MKCs against cariogenic bacteria may be related to the presence of the nitrogen atom in the piperidone ring, which can form hydrogen bonds with the bacterial cell wall and propitiates a better lipophilicity/hydrophilicity balance [44].
Compound 8 (R = 4-OMe) was the most active against E. faecalis, followed by compounds 12 (R = OBzn), 10 (R = 3,4,5-OMe), and 11 (R = 2,4,6-OMe). The presence of aromatic methoxy groups and their electron-releasing mesomeric effects has been previously associated with the antibacterial activity of chalcones (α,β-unsaturated ketones) [50] and curcumin A [26]. However, the fact that compound 10 exhibited the lowest MIC against S. mitis and lower MIC values compared to 8, 9 (3,4-OMe), 10, 11, and 12 against all the other bacteria strongly suggests that antibacterial activity against cariogenic bacteria depends not only on the number of the aromatic ethers but also on their nature and positions. Moreover, the role played by these groups is specific to each bacterium, as previously discussed in this paper.
The antibacterial activity of halogenated compounds against Streptococcus species is well-reported in the literature [51,52]. However, among the halogenated compounds, the fluorinated compounds 13 (R = 3-F) and 3 (R = 4-F) displayed the lowest MIC values, with compound 13 (R = 3-F) being the most active against S. mutans, S. sanguinis, and S. sobrinus. The higher activity of fluorinated compounds as compared to 4, 5, and 14 has been attributed to the high electronegativity and small size of fluorine and its ability to establish weak hydrogen bonds [53]. However, our results revealed that antibacterial activity depends on the position of the fluor atom at the aromatic ring.
5. Conclusions
The CSC reaction was proven to be an efficient, relatively fast, and environmentally friendly methodology to obtain a library of N-methyl-4-piperidone-derived MKCs for further evaluation of their biological activities.
The results of this study revealed that MKCs 1–15 do not display any antifungal activity against C. krusei and C. albicans in concentrations lower than 100 μg/mL. Moreover, these compounds were not active against E. faecalis and displayed weak activity against most of the cariogenic bacteria tested. Among the tested compounds, the moderate activity of compounds 1, 10, and 13 against Streptococcus mutans (13), S. salivarius (1), L. paracasei (1 and 10), S. mitis (1, 10, and 13), S. sanguinis (1, 10, and 13), and S. sobrinus (13), with MIC values between 250 μg/mL and 500 μg/mL, must be highlighted. The antibacterial activity of compounds 10 and 13 was associated with the presence and position of the fluor atom and the methoxy groups at the aromatic ring. The presence of the N-methyl-4-piperidone ring was proven to be a structural feature that boosted antibacterial activity as compared to the corresponding acetone-derived MKCs. Although compounds 1, 10, and 13 cannot be considered as lead compounds, their moderate antibacterial activity, along with their ease of synthesis, makes them attractive to be used as prototypes for the synthesis of derivatives (e.g., containing fluorine and methoxyl groups in the aromatic ring) with improved antimicrobial activity.
In conclusion, this study contributed to a better understanding of the antimicrobial activity of MKCs, whose data in the literature are still scarce.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/futurepharmacol5020023/s1; Figures S1–S60: 1H, 13C, and APT NMR, and mass spectra of compounds 1–15.
Author Contributions
Conceptualization, A.E.M.C.; methodology, A.E.M.C., N.A.J.C.F. and C.H.G.M.; investigation, R.H.L., Y.R.R., I.M.O., V.N., A.L.O.S., J.G.T. and M.A.S.C.C.; formal analysis and supervision, A.E.M.C., V.N., C.H.G.M. and N.A.J.C.F.; writing—original draft preparation, Y.R.R.; writing—review and editing, A.E.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development (CNPq), grant numbers 310648/2022-0 and 301417/2019-9.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
A draft of this manuscript is available on the ChemRxiv repository at https://chemrxiv.org/engage/chemrxiv/article-details/6821ed55927d1c2e663fba69 (accessed on 15 May 2025).
Acknowledgments
Coordination for the Improvement of Higher Education Personnel (CAPES, proc. 88887.893713/2023-00) for the scholarship granted to R.H.L.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CHX | Chlorhexidine |
| MKC | MonoKetone Curcuminoid |
| CSC | Claisen–Schimdt Condensation |
| MIC | Minimal Inhibitory Concentration |
| MBC | Minimal Bactericidal Concentration |
| Hex | Hexane |
| EtOAc | Ethyl acetate |
| GC-MS | Gas chromatography-mass spectrometry |
| NMR | Nuclear magnetic resonance |
| APT | Attached Proton Test |
| ATCC | American Type Culture Collection |
| TSB | Tryptic Soy Broth |
| CFU | Colony-Forming Unit |
| CLSI | Clinical and Laboratory Standards Institute |
| DMSO | Dimethyl sulfoxide |
| EtOH | Ethanol |
References
- WHO. Oral Health; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 11 May 2025).
- Liu, R.; Liu, Y.; Yi, J.; Fang, Y.; Guo, Q.; Cheng, L.; He, J.; Li, M. Imbalance of oral microbiome homeostasis: The relationship between microbiota and the occurrence of dental caries. BMC Microbiol. 2025, 25, 46. [Google Scholar] [CrossRef] [PubMed]
- Valm, A.M. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J. Mol. Biol. 2019, 431, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Dong, P.T.; Cen, L.; Bor, B.; Lux, R.; Shi, W.; Yu, Q.; He, X.; Wu, T. Antagonistic interaction between two key endodontic pathogens Enterococcus faecalis and Fusobacterium nucleatum. J. Oral Microbiol. 2023, 15, 2149448. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.C.M.; Bezerra, D.D.S.; Hart-Chu, E.N.S.; Stipp, R.N.; Guedes, S.F.F.; Neves, B.G.; Rodrigues, L.K.A. Quantification and gene expression of Lactobacillus casei group species associated with dentinal lesions in early childhood caries. Saudi Dent. J. 2021, 33, 69–77. [Google Scholar] [CrossRef]
- Zhang, O.L.; Niu, J.Y.; Yu, O.Y.; Mei, M.L.; Jakubovics, N.S.; Chu, C.H. Development of a novel peptide with antimicrobial and mineralising properties for caries management. Pharmaceutics 2023, 15, 2560. [Google Scholar] [CrossRef]
- Oliveira, T.A.S.; Santiago, M.B.; Santos, V.H.P.; Silva, E.O.; Martins, C.H.G.; Crotti, A.E.M. Antibacterial activity of essential oils against oral pathogens. Chem. Biodiv. 2022, 19, e202200097. [Google Scholar] [CrossRef]
- Oliveira, T.A.S.; Silva, J.B.A.; Silva, N.B.S.; Felix, P.C.A.; Dos Santos, D.A.; de Oliveira, A.M.; Martins, C.H.G.; Magalhaes, L.G.; Crotti, A.E.M. Antibacterial and antileishmanial activity of 1,4-dihydropyridine derivatives. Chem. Biodiv. 2025, 22, e202401300. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Pinto, A.; Silva, B.M.D.; Santiago-Junior, J.F.; Sales-Peres, S.H.C. Efficiency of different protocols for oral hygiene combined with the use of chlorhexidine in the prevention of ventilator-associated pneumonia. J. Bras. Pneumol. 2021, 47, e20190286. [Google Scholar] [CrossRef]
- Deus, F.P.; Ouanounou, A. Chlorhexidine in dentistry: Pharmacology, uses, and adverse effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Chang, Y.L.; Chen, Y.L. Deletion of ADA2 Increases antifungal drug susceptibility and virulence in Candida glabrata. Antimicrob. Agents Chemother. 2018, 62, e01924-17. [Google Scholar] [CrossRef]
- Lindberg, E.; Hammarstrom, H.; Ataollahy, N.; Kondori, N. Species distribution and antifungal drug susceptibilities of yeasts isolated from the blood samples of patients with candidemia. Sci. Rep. 2019, 9, 3838. [Google Scholar] [CrossRef]
- Beredaki, M.I.; Arendrup, M.C.; Mouton, J.W.; Meletiadis, J. In vitro pharmacokinetic/pharmacodynamic model data suggest a potential role of new formulations of posaconazole against Candida krusei but not Candida glabrata infections. Int. J. Antimicrob. Agents 2021, 57, 106291. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.D.; Latini, G.; Trilli, I.; Ferrante, L.; Nardelli, P.; Malcangi, G.; Inchingolo, A.M.; Mancini, A.; Palermo, A.; et al. The role of curcumin in oral health and diseases: A systematic review. Antioxidants 2024, 13, 660. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, M.; Yuan, Y. The potential of curcumin-based co-delivery systems for applications in the food industry: Food preservation, freshness monitoring, and functional food. Food Res. Int. 2023, 171, 113070. [Google Scholar] [CrossRef]
- Bērziņa, L.; Mieriņa, I. Antiradical and antioxidant activity of compounds containing 1,3-dicarbonyl moiety: An overview. Molecules 2023, 28, 6203. [Google Scholar] [CrossRef]
- Shi, L.; Qu, Y.; Li, Z.; Fan, B.; Xu, H.; Tang, J. In vitro permeability and bioavailability enhancement of curcumin by nanoemulsion via pulmonary administration. Curr. Drug Deliv. 2019, 16, 751–758. [Google Scholar] [CrossRef]
- Denison, H.J.; Schwikkard, S.L.; Khoder, M.; Kelly, A.F. Review: The chemistry, toxicity and antibacterial activity of curcumin and its analogues. Planta Medica 2023, 90, 47–62. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Anasane, N.; Santos, C.A.D. Curcumin and curcumin-loaded nanoparticles: Antipathogenic and antiparasitic activities. Exp. Rev. Anti-infect. Ther. 2020, 18, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Verma, S.; Fatima, K.; Luqman, S.; Srivastava, S.K.; Khan, F. Pharmacophore & QSAR guided design, synthesis, pharmacokinetics and in vitro evaluation of curcumin analogs for anticancer activity. Curr. Med. Chem. 2024, 31, 620–639. [Google Scholar] [CrossRef] [PubMed]
- Gornicka, J.; Mika, M.; Wroblewska, O.; Siudem, P.; Paradowska, K. Methods to improve the solubility of curcumin from turmeric. Life 2023, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Dytrych, P.; Kejik, Z.; Hajduch, J.; Kaplanek, R.; Vesela, K.; Kucnirova, K.; Skalickova, M.; Venhauerova, A.; Hoskovec, D.; Martasek, P.; et al. Therapeutic potential and limitations of curcumin as antimetastatic agent. Biomed. Pharmacother. 2023, 163, 114758. [Google Scholar] [CrossRef]
- Vieira, T.M.; Dos Santos, I.A.; Silva, T.S.; Martins, C.H.G.; Crotti, A.E.M. Antimicrobial activity of monoketone curcuminoids against cariogenic bacteria. Chem. Biodiv. 2018, 15, e1800216. [Google Scholar] [CrossRef]
- Vieira, T.M.; Tanajura, L.S.; Heleno, V.C.G.; Magalhães, L.G.; Crotti, A.E.M. Monoketone curcuminoids: An updated review of their synthesis and biological activities. Futur. Pharmacol. 2024, 4, 54–77. [Google Scholar] [CrossRef]
- Jonathan, D.R.; DravidaThendral, E.; Priya, M.K.; Shirmila, D.A.; Fathima, A.A.; Yuvashri, R.; Usha, G. Investigations on 3D-structure, properties and antibacterial activity of two new curcumin derivatives. J. Mol. Struc. 2023, 1292, 136063. [Google Scholar] [CrossRef]
- Francisco, K.R.; Monti, L.; Yang, W.; Park, H.; Liu, L.J.; Watkins, K.; Amarasinghe, D.K.; Nalli, M.; Polaquini, C.R.; Regasini, L.O.; et al. Structure-activity relationship of dibenzylideneacetone analogs against the neglected disease pathogen, Trypanosoma brucei. Bioorg. Med. Chem. Lett. 2023, 81, 129123. [Google Scholar] [CrossRef]
- CLSI. Methods for Diluition Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical Laboratory Standard Institute: Wayne, PA, USA, 2018. [Google Scholar]
- CLSI Standard M27–A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. The National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2002.
- Barry, A.L.; Pfaller, M.A.; Brown, S.D.; Espinel-Ingroff, A.; Ghannoum, M.A.; Knapp, C.; Rennie, R.P.; Rex, J.H.; Rinaldi, M.G. Quality control limits for broth microdilution susceptibility tests of ten antifungal agents. J. Clin. Microbiol. 2000, 38, 3457–3459. [Google Scholar] [CrossRef]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef]
- Pati, N.H.; Das, U.; Das, S.; Bandy, B.; Clercq, E.D.; Balzarini, J.; Kawase, M.; Sakagami, H.; Quail, W.J.; Stables, J.P.; et al. The cytotoxic properties and preferential toxicity to tumour cells displayed by some 2,4-bis(benzylidene)-8-methyl-8-azabicyclo[3.2.1] octan-3-ones and 3,5-bis(benzylidene)-1-methyl-4-piperidones. Eur. J. Med. Chem. 2008, 44, 54–62. [Google Scholar] [CrossRef] [PubMed]
- El-Subbagh, H.I.; Abu-Zaid, S.M.; Mahran, M.A.; Badria, F.A.; Al-Obaid, A.M. Synthesis and biological evaluation of certain α,β-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J. Med. Chem. 2000, 43, 2915–2921. [Google Scholar] [CrossRef]
- Eryanti, Y.; Hendra, R.; Herlinda, T.; Zamri, A.; Supratman, U. Synthesis of N-methyl-4-piperidone curcumin analogues and their cytotoxicity activity against T47D cell lines. Indones. J. Chem. 2018, 18, 362–366. [Google Scholar] [CrossRef]
- Yongsheng, J.; Fei, Z.; Hua, Z.; Heyang, Z.; Hongchuan, L. 3,5-Diaryl-Ylidene Piperidone Derivatives and Application Thereof in Preparing Blood Glucose-Reducing and Fat-Reducing Medicine. Application No. CN103626692A, 12 March 2014. [Google Scholar]
- Yadav, B.; Taurin, S.; Rosengren, R.J.; Schumacher, M.; Diederich, M.; Somers-Edgar, T.J.; Larsen, L. Synthesis and cytotoxic potential of heterocyclic cyclohexanone analogues of curcumin. Bioorg. Med. Chem. 2010, 18, 6701–6707. [Google Scholar] [CrossRef]
- Wu, J.; Yali, Z.; Cai, Y.; Wang, J.; Weng, B.; Qinqin, T.; Chen, X.; Pan, Z.; Liang, G.; Yang, S. Discovery and evaluation of piperid-4-one-containing mono-carbonyl analogs of curcumin as anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2013, 21, 3058–3065. [Google Scholar] [CrossRef]
- Makarov, M.V.; Odinets, I.L.; Lyssenko, K.A.; Rybalkina, E.; Kosilkin, I.V.; Antipin, M.Y.; Timofeeva, T.V. N-alkylated 3,5-bis(arylidene)-4-piperidones. Synthetic approaches, X-ray structure and anticancer activity. J. Heterocycl. Chem. 2008, 45, 729–736. [Google Scholar] [CrossRef]
- Castiello, C.; Junghanns, P.; Mergel, A.; Jacob, C.; Ducho, C.; Valente, S.; Rotili, D.; Fioravanti, R.; Zwergel, C.; Mai, A. GreenMedChem: The challenge in the next decade toward eco-friendly compounds and processes in drug design. Green Chem. 2023, 25, 2109–2169. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Rajasekaran, J.J.; Krishnamurthy, H.K.; Bosco, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K. Oral microbiome: A review of its impact on oral and systemic health. Microorganisms 2024, 12, 1797. [Google Scholar] [CrossRef]
- Vieira, T.M.; Barco, J.G.; de Souza, S.L.; Santos, A.L.O.; Daoud, I.; Rahali, S.; Amdouni, N.; Bastos, J.K.; Martins, C.H.G.; Ben Said, R.; et al. In vitro and in silico studies of the antimicrobial activity of prenylated phenylpropanoids of green propolis and their derivatives against oral bacteria. Antibiotics 2024, 13, 787. [Google Scholar] [CrossRef]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef]
- Adamczak, A.; Ozarowski, M.; Karpinski, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Makade, C.S.; Shenoi, P.R.; Bhongade, B.A.; Shingane, S.A.; Ambulkar, P.C.; Shewale, A.M. Estimation of MBC: MIC Ratio of herbal extracts against common endodontic pathogens. J. Pharm. Bioallied Sci. 2024, 16, S1414–S1416. [Google Scholar] [CrossRef]
- Lambert, P.A. Cellular impermeability and uptake of biocides and antibiotics in Gram-positive bacteria and mycobacteria. J. Appl. Microbiol. 2002, 92, 46S–54S. [Google Scholar] [CrossRef]
- Elkanzi, N.A.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of chalcones derivatives and their biological activities: A review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef]
- Wang, C.; Du, W.; Lu, H.; Lan, J.; Liang, K.; Cao, S. A review: Halogenated compounds from marine Actinomycetes. Molecules 2021, 26, 2754. [Google Scholar] [CrossRef]
- Faleye, O.S.; Boya, B.R.; Lee, J.H.; Choi, I.; Lee, J. Halogenated antimicrobial agents to combat drug-resistant pathogens. Pharmacol. Rev. 2023, 76, 90–141. [Google Scholar] [CrossRef]
- Chhillar, A.K.; Arya, P.; Mukherjee, C.; Kumar, P.; Yadav, Y.; Sharma, A.K.; Yadav, V.; Gupta, J.; Dabur, R.; Jha, H.N.; et al. Microwave-assisted synthesis of antimicrobial dihydropyridines and tetrahydropyrimidin-2-ones: Novel compounds against aspergillosis. Bioorg. Med. Chem. 2006, 14, 973–981. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).