Harnessing the Power of Natural Terpenoid Compounds Against Esophageal Squamous Cell Carcinoma: A Systematic Review
Abstract
1. Introduction
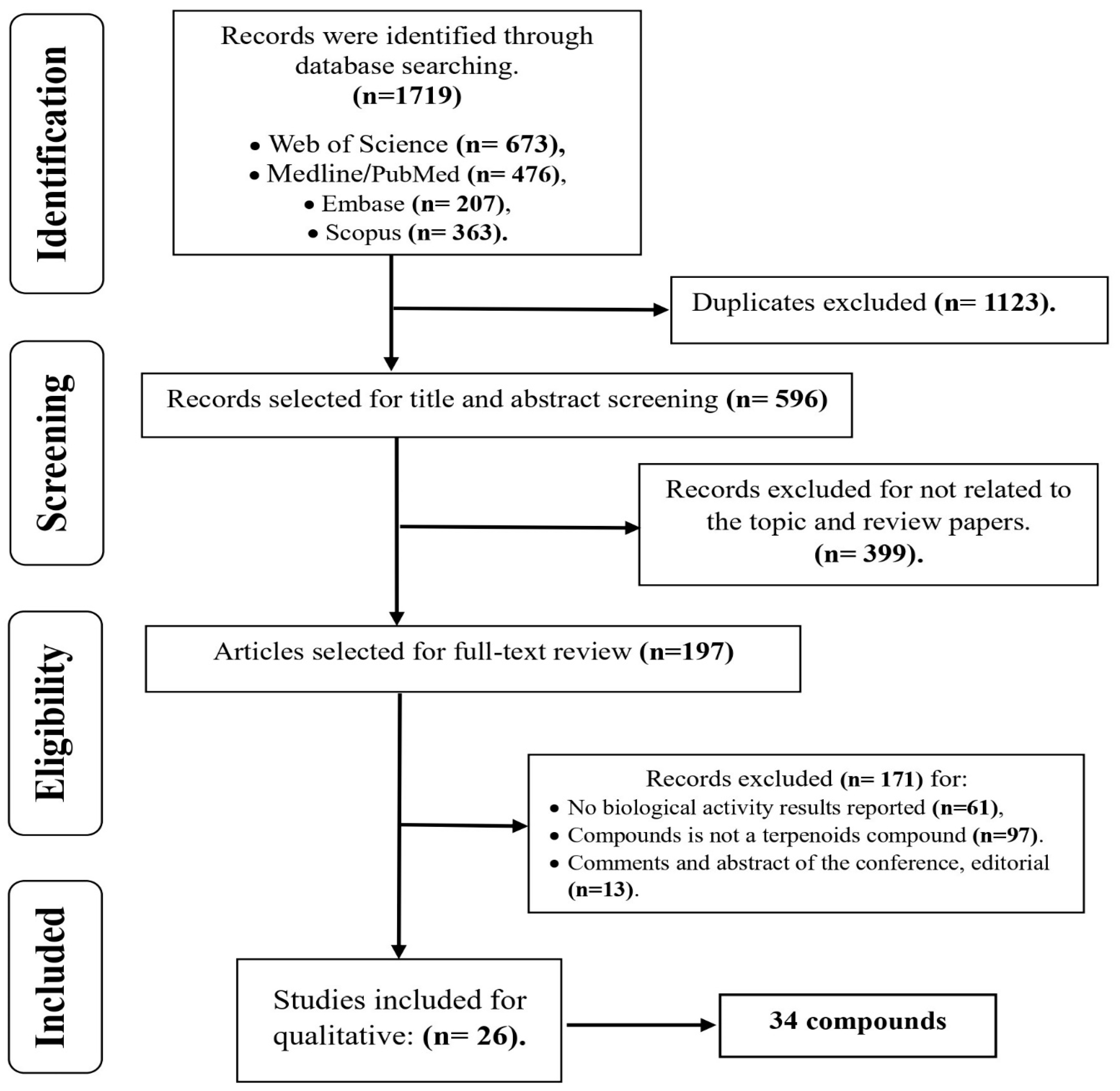
2. Methodology
2.1. Search Protocol and Eligibility Criteria
2.2. Data Extraction and Selection Procedure
2.3. Synthesis Procedure
3. Results
3.1. Synthesis of Research Findings on Terpenes and ESCC
3.2. In Vitro Anti-Esophageal Squamous Cell Carcinoma Potential of Terpenoids
3.2.1. Monoterpenes
3.2.2. Sesquiterpenes
3.2.3. Diterpenes
3.2.4. Triterpenes
3.3. In Vivo Anti-Esophageal Squamous Cell Carcinoma Potential of Terpenoids
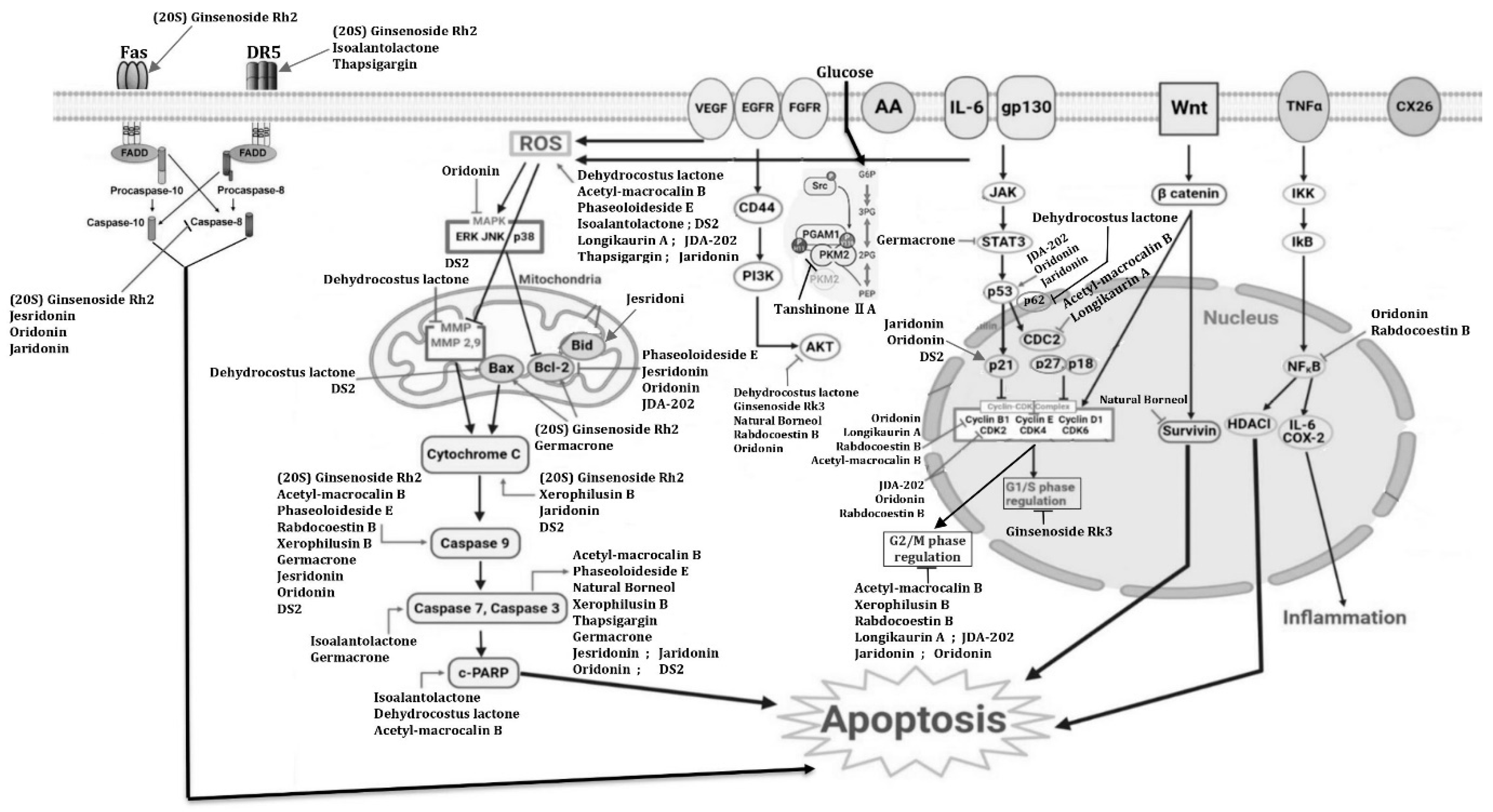
3.4. Mechanism of Action of Terpenoid Compounds on ESCC Cell Lines
4. Discussion
5. Limitations and Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kamsu, G.T.; Chuisseu, D.P.D.; Fodouop, C.S.P.; Feudjio, H.B.L.; Famen, L.C.N.; Kodjio, N.; Sokoudjou, J.B.; Gatsing, D.T. Toxicological profile of the aqueous extract of Tectona grandis L.F. (Verbenaceae) leaves: A medicinal plant used in the treatment of typhoid fever in traditional Cameroonian medicine. J. Toxicol. 2021, 2021, 6646771. [Google Scholar] [CrossRef]
- Wamba, B.E.N.; Ghosh, P.; Mbaveng, A.T.; Bhattacharya, S.; Debarpan, M.; Depanwita, S.; Saunak, M.M.; Kuete, V.; Murmu, N. Botanical from Piper capense Fruit Can Help to Combat the Melanoma as Demonstrated by In Vitro and In Vivo Studies. eCAM 2021, 2021, 8810368. [Google Scholar] [CrossRef]
- Wanjohi, B.K.; Njenga, E.W.; Sudoi, V.; Kipkore, W.K.; Moore, H.L.; Davies, M.I.J. Ecological Knowledge of indigenous plants among the Marakwet Community (Embobut Basin), Elgeyo Marakwet County (Kenya). Ethnobot. Res. Appl. 2020, 20, 1–16. [Google Scholar] [CrossRef]
- Ssenku, J.E.; Okurut, S.A.; Namuli, A.; Kudamba, A.; Tugume, P.; Matovu, P.; Wasige, G.; Kafeero, H.M.; Walusansa, A. Medicinal plant use, conservation, and the associated traditional knowledge in rural communities in Eastern Uganda. Trop. Med. Health 2022, 50, 39. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Cienc. 2019, 91, e20190105. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma, R.N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of terpenes and recent advances in plant protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.M.; Chan, T.F.; Hui, J.H.L. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I.; Negre-Zakharov, F. Plant Volatiles: Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Rosenkranz, M.; Chen, Y.; Zhu, P.; Vlot, A.C. Volatile terpenes—Mediators of plant-to-plant communication. Plant J. 2021, 108, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. The Anti-Inflammatory Properties of Terpenoids from Cannabis. Cannabis Cannabinoid Res. 2018, 3, 282–290. [Google Scholar] [CrossRef]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Kustrin, E.; Gegechkori, V.; Morton, D.W. Anxiolytic Terpenoids and Aromatherapy for Anxiety and Depression. Adv. Exp. Med. Biol. 2020, 1260, 283–296. [Google Scholar]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kamsu, G.T.; Ndebia, E.J. Uncovering risks associated with smoking types and intensities in esophageal cancer within high-prevalence regions in Africa: A comprehensive meta-analysis. Cancer Epidemiol. Biomark. Prev. 2024, 33, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Ndebia, E.J.; Kamsu, G.T. Drinking patterns, alcoholic beverage types, and esophageal cancer risk in Africa: A comprehensive systematic review and meta-analysis. Front. Oncol. 2023, 13, 1310253. [Google Scholar] [CrossRef] [PubMed]
- Ndebia, E.J.; Kamsu, G.T. A comprehensive meta-analysis of dietary and culinary practices on esophageal cancer incidence in the East African corridor. SVU-Int. J. Med. Sci. 2024, 7, 207–222. [Google Scholar] [CrossRef]
- Kamsu, G.T.; Ndebia, E.J. Socioeconomic determinants of esophageal cancer incidence in the East African corridor: A systematic review with meta-analysis. Int. J. Med. Arts. 2024, 6, 4374–4385. [Google Scholar] [CrossRef]
- Ndebia, E.J.; Kamsu, G.T. Natural alkaloids as potential treatments for esophageal squamous-cell carcinoma: A comprehensive review. Gastroenterol. Endosc. 2024, 2, 131–136. [Google Scholar] [CrossRef]
- Kamsu, G.T.; Ndebia, E.J. Usefulness of natural phenolic compounds in the fight against esophageal cancer: A systematic review. Future Pharmacol. 2024, 4, 626–650. [Google Scholar] [CrossRef]
- Matieta, V.Y.; Mbaveng, A.T.; Nouemsi, G.R.S.; Tankeo, S.B.; Kamsu, G.T.; Nayim, P.; Lannang, A.M.; Çelik, İ.; Efferth, T.; Kuete, V. Cytotoxicity, acute and sub-chronic toxicities of the leaves of Bauhinia thonningii (Schumach.) Milne-Redh. (Caesalpiniaceae). BMC Complement. Med. Ther. 2023, 23, 341. [Google Scholar] [CrossRef]
- Megaptche, J.F.; Nago, R.D.T.; Tankeo, S.B.; Matieta, V.Y.; Kamsu, G.T.; Mpetga, J.D.S.; Mbaveng, A.T.; Efferth, T.; Kuete, V. Cytotoxicity, acute and subchronic toxicity of the methanol extract from the fruits of Psorospermun febrifugum Spach (Hypericaceae) in Wistar albino rats. S. Afr. J. Bot. 2025, 178, 424–436. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Kufe, N.C.; Masemola, M.; Chikowore, T.; Kengne, A.P.; Olsson, T.; Goedecke, J.H.; Micklesfield, L.K. Protocol for systematic review and meta-analysis of sex hormones and diabetes risk in ageing men and women of African ancestry. BMJ Open 2019, 9, e024446. [Google Scholar] [CrossRef]
- Hipp, J.; Kuvendjiska, J.; Martini, V.; Hillebrecht, H.C.; Fichtner-Feigl, S.; Diener, M.K. Proximal gastrectomy and double-tract reconstruction vs total gastrectomy in gastric and gastro-esophageal junction cancer patients—A systematic review and meta-analysis protocol (PROSPERO registration number: CRD42021291500). Syst. Rev. 2023, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Antunes, E.M.; Afolayan, A.F.; Chiwakata, M.T.; Fakee, J.; Knott, M.G.; Whibley, C.E.; Hendricks, D.T.; Bolton, J.J.; Beukes, D.R. Identification and in vitro anti-esophageal cancer activity of a series of halogenated monoterpenes isolated from the South African seaweeds Plocamium suhrii and Plocamium cornutum. Phytochemistry 2011, 72, 769–772. [Google Scholar] [CrossRef]
- Meng, X.; Dong, X.; Wang, W.; Yang, L.; Zhang, X.; Li, Y.; Chen, T.; Ma, H.; Qi, D.; Su, J. Natural borneol enhances paclitaxel-induced apoptosis of ESCC cells by inactivation of the PI3K/AKT pathway. J. Food Sci. 2018, 83, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, G.; Zhang, Y.; Hua, P.; Fang, M.; Wu, M.; Liu, T. Isoalantolactone induces apoptosis through reactive oxygen species-dependent upregulation of death receptor 5 in human esophageal cancer cells. Toxicol. Appl. Pharmacol. 2018, 352, 46–58. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, T.; Wang, S.; Bahetjan, Y.; Li, X.; Yang, X. Dehydrocostus lactone inhibits the proliferation of esophageal cancer cells in vivo and in vitro through ROS-mediated apoptosis and autophagy. Food Chem. Toxicol. 2022, 170, 113453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Hao, J.; Guo, K.; Liu, W.; Yao, F.; Wu, Q.; Liu, C.; Wang, Q.; Yang, X. Germacrone inhibits cell proliferation and induces apoptosis in human esophageal squamous cell carcinoma cells. Biomed. Res. Int. 2020, 2020, 7643248. [Google Scholar] [CrossRef]
- Ma, Y.C.; Ke, Y.; Zi, X.; Zhao, F.; Yuan, L.; Zhu, Y.L.; Fan, X.X.; Zhao, N.M.; Li, Q.Y.; Qin, Y.H.; et al. Induction of the mitochondria-mediated apoptosis in human esophageal cancer cells by DS2, a newly synthetic diterpenoid analog, is regulated by Bax and caused by generation of reactive oxygen species. Oncotarget 2016, 7, 86211–86224. [Google Scholar] [CrossRef][Green Version]
- Wang, J.N.; Che, Y.; Yuan, Z.Y.; Lu, Z.L.; Li, Y.; Zhang, Z.R.; Li, N.; Li, R.D.; Wan, J.; Sun, H.D.; et al. Acetyl-macrocalin B suppresses tumor growth in esophageal squamous cell carcinoma and exhibits synergistic anti-cancer effects with the Chk1/2 inhibitor AZD7762. Toxicol. Appl. Pharmacol. 2019, 365, 71–83. [Google Scholar] [CrossRef]
- Wang, C.; Yang, D.; Jiang, L.; Wang, S.; Wang, J.; Zhou, K.; Shi, X.; Chang, L.; Liu, Y.; Ke, Y.; et al. Jesridonin in combination with paclitaxel demonstrates synergistic anti-tumor activity in human esophageal carcinoma cells. Bioorg. Med. Chem. Lett. 2017, 27, 2058–2062. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, L.; Wang, S.; Shi, H.; Wang, J.; Wang, R.; Li, Y.; Dou, Y.; Liu, Y.; Hou, G.; et al. The antitumor activity of the novel compound jesridonin on human esophageal carcinoma cells. PLoS ONE 2015, 10, e0130284. [Google Scholar] [CrossRef]
- Song, M.; Liu, X.; Liu, K.; Zhao, R.; Huang, H.; Shi, Y.; Zhang, M.; Zhou, S.; Xie, H.; Chen, H.; et al. Targeting AKT with oridonin inhibits growth of esophageal squamous cell carcinoma in vitro and patient-derived xenografts in vivo. Mol. Cancer Ther. 2018, 17, 1540–1553. [Google Scholar] [CrossRef]
- Jiang, J.H.; Pi, J.; Jin, H.; Cai, J.Y. Oridonin-induced mitochondria-dependent apoptosis in esophageal cancer cells by inhibiting PI3K/AKT/mTOR and Ras/Raf pathways. J. Cell. Biochem. 2019, 120, 3736–3746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Zhang, F.J.; Li, H.; Liu, Y.; Du, G.Y.; Huang, Y.H. Tanshinone IIA inhibits human esophageal cancer cell growth through miR-122-mediated PKM2 down-regulation. Arch. Biochem. Biophys. 2016, 598, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Wang, J.; Yuan, Z.; Li, Y.; Lu, Z.; Zhang, Z.; Zhang, J.; Wan, J.; Sun, H.; Chen, Z.; et al. The therapeutic effects of longikaurin A, a natural ent-kauranoid, in esophageal squamous cell carcinoma depend on ROS accumulation and JNK/p38 MAPK activation. Toxicol. Lett. 2017, 280, 106–115. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Vagias, C.; Bruyère, C.; Lamoral-Theys, D.; Kiss, R.; Roussis, V. Structure and in vitro antitumor activity evaluation of brominated diterpenes from the red alga Sphaerococcus coronopifolius. Bioorg. Med. Chem. 2010, 18, 1321–1330. [Google Scholar] [CrossRef]
- Ma, Y.C.; Ke, Y.; Zi, X.; Zhao, W.; Shi, X.J.; Liu, H.M. Jaridonin, a novel ent-kaurene diterpenoid from Isodon rubescens, inducing apoptosis via production of reactive oxygen species in esophageal cancer cells. Curr. Cancer Drug Targets 2013, 13, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Fan, C.; Yang, Y.; Di, S.; Hu, W.; Li, T.; Zhu, Y.; Han, J.; Xin, Z.; Wu, G.; et al. Thapsigargin sensitizes human esophageal cancer to TRAIL-induced apoptosis via AMPK activation. Sci. Rep. 2016, 6, 35196. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Che, Y.; Yuan, Z.; Lu, Z.; Li, Y.; Wan, J.; Sun, H.; Chen, Z.; Pu, J.; et al. Rabdocoestin B exhibits antitumor activity by inducing G2/M phase arrest and apoptosis in esophageal squamous cell carcinoma. Cancer Chemother. Pharmacol. 2018, 81, 469–481. [Google Scholar] [CrossRef]
- Silva, V.A.O.; Rosa, M.N.; Martinho, O.; Tanuri, A.; Lima, J.P.; Pianowski, L.F.; Reis, R.M. Modified ingenol semi-synthetic derivatives from Euphorbia tirucalli induce cytotoxicity on a large panel of human cancer cell lines. Investig. New Drugs 2019, 37, 1029–1035. [Google Scholar] [CrossRef]
- Shi, X.J.; Ding, L.; Zhou, W.; Ji, Y.; Wang, J.; Wang, H.; Ma, Y.; Jiang, G.; Tang, K.; Ke, Y.; et al. Pro-apoptotic effects of JDA-202, a novel natural diterpenoid, on esophageal cancer through targeting peroxiredoxin I. Antioxid. Redox Signal. 2017, 27, 73–92. [Google Scholar] [CrossRef]
- Yao, R.; Chen, Z.; Zhou, C.; Luo, M.; Shi, X.; Li, J.; Gao, Y.; Zhou, F.; Pu, J.; Sun, H.; et al. Xerophilusin B induces cell cycle arrest and apoptosis in esophageal squamous cell carcinoma cells and does not cause toxicity in nude mice. J. Nat. Prod. 2015, 78, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Xiong, H.; Shu, G.; Yang, X.; Wang, J.; Zheng, C.; Xiong, W.; Mei, Z. Phaseoloideside E a novel natural triterpenoid identified from Entada phaseoloides, induces apoptosis in Ec-109 esophageal cancer cells through reactive oxygen species generation. J. Pharmacol. Sci. 2013, 122, 163–175. [Google Scholar] [CrossRef]
- Yamai, H.; Sawada, N.; Yoshida, T.; Seike, J.; Takizawa, H.; Kenzaki, K.; Miyoshi, T.; Kondo, K.; Bando, Y.; Ohnishi, Y.; et al. Triterpenes augment the inhibitory effects of anticancer drugs on growth of human esophageal carcinoma cells in vitro and suppress experimental metastasis in vivo. Int. J. Cancer 2009, 125, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.A.O.; Rosa, M.N.; Tansini, A.; Oliveira, R.J.S.; Martinho, O.; Lima, J.P.; Pianowski, L.F.; Reis, R.M. In vitro screening of cytotoxic activity of euphol from Euphorbia tirucalli on a large panel of human cancer-derived cell lines. Exp. Ther. Med. 2018, 16, 557–566. [Google Scholar] [CrossRef]
- Li, H.; Han, C.; Chen, C.; Han, G.; Li, Y. (20S) Ginsenoside Rh2-activated distinct apoptosis pathways in highly and poorly differentiated human esophageal cancer cells. Molecules 2022, 27, 5602. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, A.; Meng, F.; Cao, Q.; Shan, B. Inhibitory effects of lupeal acetate of Cortex periplocae on N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis. Oncol. Lett. 2012, 4, 231–236. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, J.; Fu, R.; Zhu, C.; Fan, D. The ginsenoside Rk3 exerts anti-esophageal cancer activity in vitro and in vivo by mediating apoptosis and autophagy through regulation of the PI3K/Akt/mTOR pathway. PLoS ONE 2019, 14, e0216759. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, S.; Wang, S.; Yang, B.; He, W.; Li, H.; Yang, S.; Wang, G.; Dong, C. Design, synthesis and anticancer activities evaluation of novel pyrazole modified catalpol derivatives. Sci. Rep. 2023, 13, 7756. [Google Scholar] [CrossRef]
- An, J.; An, S.; Choi, M.; Jung, J.H.; Kim, B. Natural products for esophageal cancer therapy: From traditional medicine to modern drug discovery. Int. J. Mol. Sci. 2022, 23, 13558. [Google Scholar] [CrossRef]
- World Health Organization (WHO). National Policy on Traditional Medicine and Regulation of Herbal Medicines; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Wachtel-Galor, S.; Benzie, I.F.F. Herbal medicine: An introduction to its history, usage, regulation, current trends, and research needs. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Kuete, V.; Efferth, T. African flora has the potential to fight multidrug resistance of cancer. BioMed. Res. Int. 2015, 2015, 914813. [Google Scholar] [CrossRef]
- Herman, T.F.; Santos, C. First-Pass Effect; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rourke, J.L.; Sinal, C.J. Biotransformation/metabolism. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 490–502. [Google Scholar]
- Ernstmeyer, K.; Christman, E. Nursing pharmacology. In Chapter 1 Pharmacokinetics & Pharmacodynamics, 2nd ed.; Chippewa Valley Technical College: Eau Claire, WI, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK595006/ (accessed on 28 February 2025).
- Yu, W.; Chen, Y.; Dubrulle, J.; Stossi, F.; Putluri, V.; Sreekumar, A.; Putluri, N.; Baluya, D.; Lai, S.Y.; Sandulache, V.C. Cisplatin generates oxidative stress which is accompanied by rapid shifts in central carbon metabolism. Sci. Rep. 2018, 8, 4306. [Google Scholar] [CrossRef]
- Kumbul, Y.Ç.; Nazıroğlu, M. Paclitaxel promotes oxidative stress-mediated human laryngeal squamous tumor cell death through the stimulation of calcium and zinc signaling pathways: No synergic action of melatonin. Biol. Trace Elem. Res. 2022, 200, 2084–2098. [Google Scholar] [CrossRef] [PubMed]
- Nizami, Z.N.; Aburawi, H.E.; Semlali, A.; Muhammad, K.; Iratni, R. Oxidative stress inducers in cancer therapy: Preclinical and clinical evidence. Antioxidants 2023, 12, 1159. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Selective protection of normal cells from chemotherapy, while killing drug-resistant cancer cells. Oncotarget 2023, 14, 193–206. [Google Scholar] [CrossRef]
- Codony-Servat, J.; Garcia-Albeniz, X.; Pericay, C.; Alonso, V.; Escudero, P.; Fernández-Martos, C.; Gallego, R.; Martínez-Cardús, A.; Martinez-Balibrea, E.; Maurel, J. Soluble FAS in the prediction of benefit from cetuximab and irinotecan for patients with advanced colorectal cancer. Med. Oncol. 2013, 30, 428. [Google Scholar] [CrossRef]
- Yuan, X.; Gajan, A.; Chu, Q.; Xiong, H.; Wu, K.; Wu, G.S. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev. 2018, 37, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Atriya, A.; Majee, C.; Mazumder, R.; Choudhary, A.N.; Salahuddin Mazumder, A.; Dahiya, A.; Priya, N. Insight into the Various Approaches for the Enhancement of Bioavailability and Pharmacological Potency of Terpenoids: A Review. Curr. Pharm. Biotechnol. 2023, 24, 1228–1244. [Google Scholar] [CrossRef]
- Gómez-Favela, M.A.; Santos-Ballardo, D.U.; Bergés-Tiznado, M.E.; Ambriz-Pérez, D.L. Chapter 6—Nanoformulations applied to the delivery of terpenes. In Nanotechnology in Biomedicine; Heredia, J.B., Gutiérrez-Grijalva, E.P., Licea-Claverie, A., Gutierrez-Uribe, J.A., Patra, J.K., Eds.; Phytochemical Nanodelivery Systems as Potential Biopharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 221–256. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Small-Howard, A.L.; Fernández-Arévalo, M.; Martín-Banderas, L. Development of enhanced drug delivery vehicles for three cannabis-based terpenes using poly(lactic-co-glycolic acid) based nanoparticles. Ind. Crops Prod. 2021, 164, 113345. [Google Scholar] [CrossRef]
| Class | Compounds | Structure | Plants of Origin | ESCC Cell Lines | Reference (Country) |
|---|---|---|---|---|---|
| Monoterpenoids | 1Z,3R,4S,5E,7Z)-1-bromo-3,4,8-trichloro-7-(dichloromethyl)-3-methylocta-1,5,7-triene | 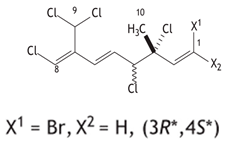 | seaweeds Plocamium suhrii | WHCO1 | [32] (South Africa) |
| (3R,4S)-3,4,6,7- tetrachloro-3,7-dimethyl- octen-1-ene | 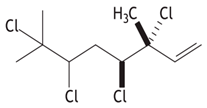 | ||||
| Natural borneol | 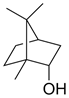 | Cinnamomum spp. | TE-1, TE-13 | [33] (China) | |
| Sesquiterpenoids | Isoalantolactone |  | Inula helenium L. | Eca-109, EC9706, TE-1, TE-13 | [34] (China) |
| Dehydrocostus lactone |  | Saussurea costus F. | Eca-109, KYSE150 | [35] (China) | |
| Germacrone | 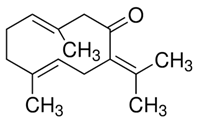 | Saussurea costus | Eca-109, EC9706 | [36] (China) | |
| Thapsigargin | 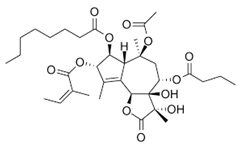 | Thapsia garganica | Eca109, TE12 | [37] (China) | |
| Diterpenoids | Acetyl-macrocalin B |  | Isodon silvatica | KYSE30, KYSE450 | [38] (China) |
| Jesridonin |  | From Oridonin modification | Eca-109 | [39] (China) | |
| Eca-109, EC9706, TE-1 | [40] (China) | ||||
| Oridonin |  | Rabdosia rubescens | KYSE70, KYSE410, KYSE450 | [41] (China) | |
| KYSE-30, KYSE-150, EC9706 | [42] (China) | ||||
| Tanshinone IIA | 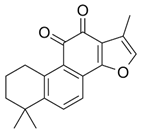 | Salvia miltiorrhiza Bunge. | Eca-109 | [43] (China) | |
| Longikaurin A | 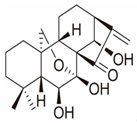 | Isodon ternifolius | KYSE-30, KYSE-450 | [44] (China) | |
| Sphaerococcenol A | 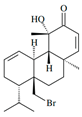 | Sphaerococcus coronopifolius | Apoptosis-resistant OE21 | [45] (Greece) | |
| 14R-hydroxy-13,14-dihydro-sphaerococcenol A | 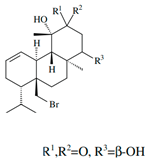 | ||||
| 12S-hydroxy-bromosphaerol | 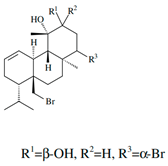 | ||||
| Bromosphaerodiol |  | ||||
| Jaridonin |  | Isodon rubescens | Eca-109, EC9706, EC-1 | [46] (China) | |
| DS2 | 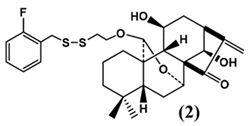 | From Jaridonin modification | EC9706, Eca-109 | [47] (China) | |
| Rabdocoestin B | 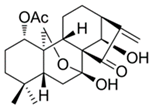 | Isodon serra Maxim. | KYSE30, KYSE450, KYSE70, KYSE150, KYSE180, KYSE410, KYSE510 | [48] (China) | |
| Ingenol A | 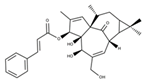 | Ingenol analogues | KYSE30, KYSE70, KYSE270, KYSE410 | [49] (Brazil) | |
| Ingenol B |  | ||||
| Ingenol C | 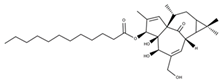 | ||||
| Ingenol-3,20-dibenzoate | 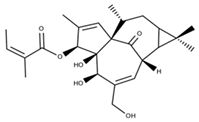 | ||||
| Ingenol-3-angelate |  | ||||
| JDA-202 | 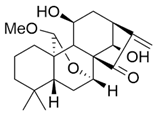 | Isodon rubescens | EC9706, EC109, KYSE-450, HET-1A | [50] (China) | |
| Xerophilusin B | 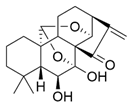 | Isodon xerophilus | KYSE-150, KYSE-450 | [51] (China) | |
| Triterpenoids | Phaseoloideside E | 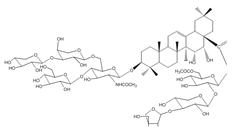 | Entada phaseoloides L. | Eca-109 | [52] (China) |
| Betulinic acid |  | Betula pubescens | YES-1, YES-2, YES-3 | [53] (Japan) | |
| Ursolic acid | 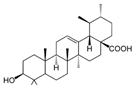 | Prunella vulgaris L. | |||
| Oleanolic acid |  | Olea europaea | |||
| Euphol | 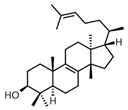 | Euphorbia tirucalli | KYSE30, KYSE70, KYSE270, KYSE410 | [54] (Brazil) | |
| (20S) Ginsenoside Rh2 |  | Panax ginseng Radix Rubra or Red ginseng | Eca109, TE-13 | [55] (China) | |
| Lupeal acetate | 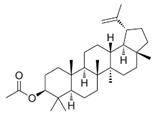 | Cortex periplocae | N-nitrosomethyl-benzylamine-induced rat esophageal tumorigenesis | [56] (China) | |
| Ginsenoside Rk3 | 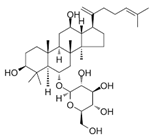 | Panax notoginseng | ECA109, KYSE150 | [57] (China) |
| Compounds | Types of Tests | Anticancer Activities | References |
|---|---|---|---|
| 1Z,3R,4S,5E,7Z)-1-bromo-3,4,8- trichloro-7-(dichloromethyl)-3-methylocta-1,5,7-triene | Antiproliferation assays using the MTT kit | WHCO1 (IC50 = 9.3 µM) | [32] |
| (3R,4S)-3,4,6,7-tetrachloro-3,7- dimethyl-octen-1-ene | WHCO1 (IC50 = 7.9 µM) | ||
| Natural Borneol | Cell viability assays using CCK-8; apoptosis analysis by flow cytometry | TE-1 and TE-13 (no significant activity at 80 μg/mL) | [33] |
| Isoalantolactone | Cell viability assays using CCK-8; apoptosis analysis by flow cytometry; colony formation assay | The 40 μM concentration reduces cell viability by 28.3%), 32.1%, 45%, and 60% for the Eca-109, EC9706, TE-1, and TE-13 cell lines. | [34] |
| Dehydrocostus lactone | Cell viability assay using the MTT kit; wound-healing assay | Eca-109 (IC50 = 10.55 µM) and KYSE150 (IC50 = 8.35 µM) | [35] |
| Germacrone | Cell viability assays using MTT assay; apoptosis analysis by flow cytometry; wound-healing assay | Eca-109 (IC50 = 15.23 μg/mL) and EC9706 (IC50 = 17.19 μg/mL) | [36] |
| Thapsigargin | Cell viability assays using MTT assay; cell matrigel invasion; adhesion analysis and wound-healing assay | At a concentration of 1 µM, cell proliferation is inhibited by 60% and 73.33% for the Eca-109 and TE-12 cell lines. | [37] |
| Acetyl-macrocalin B | Cell viability assays using CCK-8; cell apoptosis analysis by flow cytometry | KYSE30 (IC50 = 1.42 µM) and KYSE450 (IC50 = 1.43 µM) | [38] |
| Jesridonin | Cytotoxicity determined by MTT assay; cell apoptosis analysis by flow cytometry | Concentration of 60 µM, inhibited approximately 76% of the viability of Eca-109 cells and a combination index (CI) with paclitaxel (5 nM) of 0.43 | [39] |
| Cell proliferation assay by MTT assays; clonogenicity assay | Eca-109 (IC50 = 4.1 µM), EC9706 (IC50 = 4.0 µM), KYSE450 (IC50 = 2.0 µM), KYSE750 (IC50 = 16.2 µM), and TE-1 (IC50 = 9.4 µM) | [40] | |
| Oridonin | Cell proliferation assay by MTT assay; cell apoptosis by Annexin V-FITC Kit | 76% to 98% for KYSE70, KYSE410, and KYSE450 lines at a concentration of 20 µmol/mL | [41] |
| Cell proliferation assay | Eca-109 (IC50 = 38.9 µM), EC9706 (IC50 = 23.9 µM), KYSE450 (IC50 = 17.1 µM), KYSE750 (IC50 = 14.3 µM), and TE-1 (IC50 = 8.4 µM) | [40] | |
| Cell proliferation assay by MTT assay; cell apoptosis by Annexin V-FITC Kit | KYSE-150 (IC50 = 28.69 µM), EC9706 (IC50 = 34.43 µM), and KYSE-30 (IC50 = 32.29 µM). | [42] | |
| Cell proliferation assay | The concentration of 4 µM for 48 h had no effect on the proliferation of cell lines EC9706 and Eca-109. | [47] | |
| Tanshinone IIA | Cell viability assays using MTS kit | Eca-109 (IC50 = 1.925 µM) | [43] |
| Longikaurin A | Cell viability assays using CCK-8; colony formation assay; cell apoptosis by Annexin V-FITC Kit | KYSE-30 (IC50 = 1.259 µM) and KYSE-450 (IC50 = 1.370 µM) | [44] |
| Sphaerococcenol A | Cell viability assay using MTT colorimetric assay | OE21 (IC50 = 3.0 µM) | [45] |
| Bromosphaerodiol | OE21 (IC50 = 15 µM) | ||
| DS2 | Cell viability by MTT assay | EC9706 (IC50 = 2.33 µM) and Eca-109 (IC50 = 2.14 µM) | [47] |
| Rabdocoestin B | Cell viability assays using CCK-8; colony formation assays; cell cycle distribution and apoptosis by flow cytometry | KYSE30 (IC50 = 1.56 µM) and KYSE450 (IC50 = 1.94 µM) | [48] |
| Ingenol A | Cell proliferation assay by MTS assay | KYSE30 (IC50 = 15.51 μM), KYSE70 (IC50 = 11.23 μM), KYSE270 (IC50 = 3.38 μM), and KYSE410 (IC50 = 10.78 μM) | [49] |
| Ingenol B | KYSE30 (IC50 = 34.34 μM), KYSE70 (IC50 = 26.53 μM), KYSE270 (IC50 = 7.77 μM), and KYSE410 (IC50 = 19.24 μM) | ||
| Ingenol C | KYSE30 (IC50 = 6.54 μM), KYSE70 (IC50 = 3.58 μM), KYSE270 (IC50 = 1.88 μM), and KYSE410 (IC50 = 3.49 μM) | ||
| Ingenol-3,20-dibenzoate | KYSE30 (IC50 = 41.02 μM), KYSE70 (IC50 = 6.01 μM), KYSE270 (IC50 = 0.10 μM), and KYSE410 (IC50 = 9.26 μM). | ||
| Ingenol-3-angelate | KYSE30 (IC50 = 47.20 μM), KYSE70 (IC50 = 14.72 μM), KYSE270 (IC50 = 4.24 μM), and KYSE410 (IC50 = 24.08 μM) | ||
| JDA-202 | Cell viability by MTT assay; cell apoptosis using the Annexin V-FITC/PI Kit; analyzed by flow cytometry | Eca-109 (IC50 = 8.6 μM), EC9706 (IC50 = 9.4 μM), HET-1A (IC50 = 36.1 μM), and KYSE-450 (IC50 = 26.2 μM) | [50] |
| Xerophilusin B | Cell viability assays using CCK-8; cell apoptosis by Annexin V-FITC Kit | KYSE-140 (IC50 = 2.8 μM), KYSE-150 (IC50 = 1.2 μM), KYSE-450 (IC50 = 1.7 μM), and KYSE-510 (IC50 = 2.6 μM) | [51] |
| Phaseoloideside E | Cell viability assay by MTT assay; cell apoptosis by acridine orange/ethidium bromide (AO/EB) staining and flow cytometry | Eca-109 (IC50 = 25.3 μM) | [52] |
| Betulinic acid | Cell viability assay using CCK-8 | YES-2 (IC50 = 5.09 μM) | [53] |
| Ursolic acid | YES-2(IC50 = 19.1 μM) | ||
| Oleanolic acid | YES-2(IC50 = 119 μM) | ||
| Euphol | Cell proliferation assay by MTS assay | KYSE30 (IC50 = 3.52 μM), KYSE70 (IC50 = 8.77 μM), KYSE270 (IC50 = 10.71 μM), and KYSE410 (IC50 = 4.35 μM) | [54] |
| (20S) Ginsenoside Rh2 | Cell viability assay by MTT assay; cell apoptosis analysis by flow cytometry and Annexin V assay | Eca-109 (IC50 = 2.9 μg/mL) and TE-13 (IC50 = 3.7 μg/mL) | [55] |
| Ginsenoside Rk3 | Cell viability by MTT assay and colony formation assay | The concentration of 200 μM inhibits the proliferation of Eca-109 and KYSE150 lines by 83.8% and 76.8%, respectively. | [57] |
| Compounds | Animal Model | Cell Lines Used for Induction | Administration Route of Compounds | Anticancer Activities | Ref. |
|---|---|---|---|---|---|
| Isoalantolactone | Female BALB/c nude mice | Eca-109 | Intragastrical administration | The 80 mg/kg dose reduces the volume of the artificially induced tumor by more than 50% in 27 days. | [34] |
| Dehydrocostus lactone | Female BALB/c nude mice | Eca-109 | Intraperitoneal injection | The 40 mg/kg dose of lactone decreases the tumor mass by approximately 61%. | [35] |
| Thapsigargin | Mice | Eca-109 | Intraperitoneal injection | The combination of thapsigargin and hrTRAIL (1 mg/kg/60 mg/kg) reduces the volume of the artificially induced tumor by about 87% in 28 days. | [37] |
| Acetyl-macrocalin B | Mice | KYSE30 | Intraperitoneal injection | A dose of 12 mg/kg alone inhibits tumor mass by approximately 38% over 29 days. Acetyl-macrocalin B combined with AZD7762 (12 mg/kg/25 mg/kg) inhibits tumor mass by around 77% over 29 days. | [38] |
| Jesridonin | Nude mice | Eca-109 | / | The combination of paclitaxel and jesridonin (5 mg/kg/10 mg/kg) reduces the volume of the artificially induced tumor by 77.21% in 21 days. | [39] |
| Female BALB/c nude mice | Eca-109 | Vena caudalis injection | A 10 mg/kg dose reduces tumor mass by 45%, while 5-FU reduces it by 44% at a concentration of 12 mg/kg. | [40] | |
| Oridonin | Female SCID mice | ESCC | Oral by gavage | A 40 mg/kg dose reduces the size of the ESCC tumor induced in a PDX model by approximately 35% over 52 days. | [41] |
| Female BALB/c nude mice | KYSE-150 | Intraperitoneal injection | A 10 mg/kg dose reduces tumor mass by about 75% in 14 days. | [42] | |
| Longikaurin A | Female BALB/c nude mice | KYSE-30 | Intraperitoneal injection | A 12 mg/kg dose of longikaurin A inhibits tumor proliferation by about 79% in 20 days. | [44] |
| Rabdocoestin B | Female Athymic nude mice | KYSE30 | Intraperitoneal injection | A 12 mg/kg dose reduces the volume of artificially induced tumors by approximately 60%. | [48] |
| JDA-202 | Male BALB/c nude mice | EC109 | Intravenous injection | After 21 days of treatment, artificially induced tumor volumes are reduced by 61.7%. | [50] |
| Xerophilusin B | Female BALB/c nude mice | KYSE-150 and KYSE-450 | Intraperitoneal injection | A 15 mg/kg dose reduces tumor masses by about 87.5% and 85%, respectively, over 20 days. | [51] |
| Lupeal acetate | F344 rats (Fisher 344 rats) | / | Intramuscularly injection | The incidence of esophageal tumors decreases from 93.3% to 33.3% after 25 weeks. | [56] |
| Ginsenoside Rk3 | Female BALB/c nude mice | KYSE150 | Intraperitoneal injection | A 40 mg/kg dose reduces the volume of artificially induced tumors by 66.2%. | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndebia, E.J.; Kamsu, G.T. Harnessing the Power of Natural Terpenoid Compounds Against Esophageal Squamous Cell Carcinoma: A Systematic Review. Future Pharmacol. 2025, 5, 21. https://doi.org/10.3390/futurepharmacol5020021
Ndebia EJ, Kamsu GT. Harnessing the Power of Natural Terpenoid Compounds Against Esophageal Squamous Cell Carcinoma: A Systematic Review. Future Pharmacology. 2025; 5(2):21. https://doi.org/10.3390/futurepharmacol5020021
Chicago/Turabian StyleNdebia, Eugene Jamot, and Gabriel Tchuente Kamsu. 2025. "Harnessing the Power of Natural Terpenoid Compounds Against Esophageal Squamous Cell Carcinoma: A Systematic Review" Future Pharmacology 5, no. 2: 21. https://doi.org/10.3390/futurepharmacol5020021
APA StyleNdebia, E. J., & Kamsu, G. T. (2025). Harnessing the Power of Natural Terpenoid Compounds Against Esophageal Squamous Cell Carcinoma: A Systematic Review. Future Pharmacology, 5(2), 21. https://doi.org/10.3390/futurepharmacol5020021






