Can Drug-Induced Yawning Serve as a Biomarker for Drug Safety and Effectiveness?
Abstract
1. Introduction
- Compare yawning patterns under both physiological and pharmacological conditions, examining differences in frequency and timing.
- Explore the neurochemical mechanisms involved in yawning, with particular attention to the roles of serotonin, dopamine, and oxytocin.
- Investigate the relationship between DIY and clinical outcomes, including therapeutic efficacy and adverse effects.
- Discuss and propose standardized methods for assessing yawning in clinical and research settings.
2. Materials and Methods
2.1. Literature Search
2.1.1. Systematic Search for Physiological Yawning
2.1.2. Systematic Search for Drug-Induced Yawning
2.2. Databases and Search Terms
2.2.1. Databases
2.2.2. Search Terms and Operators
2.3. Inclusion and Exclusion Criteria
- Peer-reviewed studies published within the last 10 years.
- Clinical and observational studies (case–control, cohort, and randomized controlled trials).
- Studies focusing on human participants.
- Research addressing yawning’s neurobiological mechanisms or pharmacological triggers.
- Studies without direct relevance to yawning or DIY.
- Animal studies, excluded to focus on clinical human applications. Since preclinical findings may not directly translate to human pharmacodynamics, animal models were omitted but may be referenced in the discussion for comparative insights.
- Non-peer-reviewed sources, including gray literature (conference abstracts, dissertations).
- Review articles, editorials, and opinion pieces.
2.4. Study Selection Process
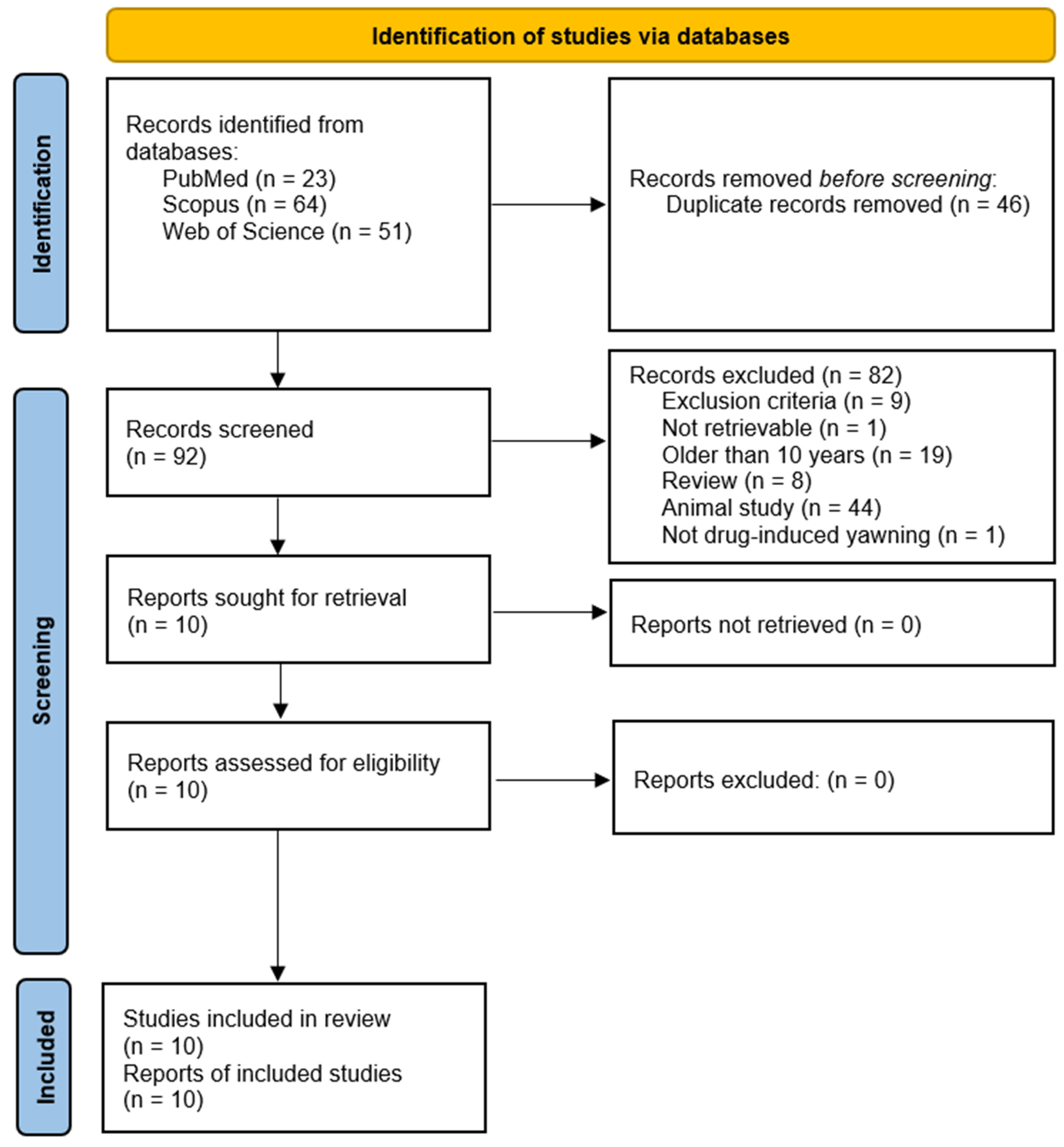
- Identification: A total of 138 records were retrieved (PubMed: 23; Scopus: 64; Web of Science: 51). After duplicate removal, 92 unique articles remained.
- Screening: Titles and abstracts were independently screened by two reviewers based on inclusion/exclusion criteria.
- Full-Text Review: 10 articles were selected for the final analysis.
2.5. Data Extraction and Analysis
2.5.1. Data Extraction
2.5.2. Comparative Analysis
2.5.3. Thematic Analysis
2.6. Ethics
3. Results
3.1. Search Results
3.2. Study Characteristics
3.3. Key Themes and Findings
3.4. Narrative Synthesis of Findings and Reflections
4. Discussion
4.1. Discussion of the Applied Method
4.1.1. Scoping Review Design
4.1.2. Strengths and Limitations
4.1.3. Recommendations for Future Methodology
4.2. Discussion of Findings: Neurobiological and Pharmacological Insights
4.2.1. Neurotransmitter Systems in Yawning
4.2.2. Patterns and Presentation of DIY
4.2.3. Yawning as a Neurobehavioral Feedback Mechanism
4.2.4. Yawning as a Pharmacodynamic Biomarker
4.2.5. Clinical and Translational Potential
4.3. Methodological Considerations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Baenninger, R. On yawning and its functions. Psychon. Bull. Rev. 1997, 4, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mittal, S. Yawning and its physiological significance. Int. J. Appl. Basic. Med. Res. 2013, 3, 11–15. [Google Scholar] [CrossRef]
- Askenasy, J.J. Is yawning an arousal defense reflex? J. Psychol. 1989, 123, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Corey, T.P.; Shoup-Knox, M.L.; Gordis, E.B.; Gallup, G.G., Jr. Changes in Physiology before, during, and after Yawning. Front. Evol. Neurosci. 2011, 3, 7. [Google Scholar] [CrossRef]
- Zilli, I.; Giganti, F.; Uga, V. Yawning and subjective sleepiness in the elderly. J. Sleep Res. 2008, 17, 303–308. [Google Scholar] [CrossRef]
- Doelman, C.J.; Rijken, J.A. Yawning and airway physiology: A scoping review and novel hypothesis. Sleep Breath. 2022, 26, 1561–1572. [Google Scholar] [CrossRef]
- Patatanian, E.; Williams, N.T. Drug-induced yawning—A review. Ann. Pharmacother. 2011, 45, 1297–1301. [Google Scholar] [CrossRef]
- Mogilnicka, E.; Klimek, V. Drugs affecting dopamine neurons and yawning behavior. Pharmacol. Biochem. Behav. 1977, 7, 303–305. [Google Scholar] [CrossRef]
- Lee, Y.; Field, J.M.; Sehgal, A. Circadian Rhythms, Disease and Chronotherapy. J. Biol. Rhythm. 2021, 36, 503–531. [Google Scholar] [CrossRef]
- Blin, O.; Masson, G.; Azulay, J.P.; Fondarai, J.; Serratrice, G. Apomorphine-induced blinking and yawning in healthy volunteers. Br. J. Clin. Pharmacol. 1990, 30, 769–773. [Google Scholar] [CrossRef]
- Beale, M.D.; Murphree, T.M. Excessive yawning and SSRI therapy. Int. J. Neuropsychopharmacol. 2000, 3, 275–276. [Google Scholar] [CrossRef]
- Anagha, K.; Shihabudheen, P.; Uvais, N.A. Side Effect Profiles of Selective Serotonin Reuptake Inhibitors: A Cross-Sectional Study in a Naturalistic Setting. Prim. Care Companion CNS Disord. 2021, 23, 20m02747. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G.; et al. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M.; Collins, G.T.; Paul, N.M.; Grundt, P.; Newman, A.H.; Xu, M.; Grandy, D.K.; Woods, J.H.; Katz, J.L. Yawning and locomotor behavior induced by dopamine receptor agonists in mice and rats. Behav. Pharmacol. 2010, 21, 171–181. [Google Scholar] [CrossRef]
- Melis, M.R.; Succu, S.; Argiolas, A. Dopamine agonists increase nitric oxide production in the paraventricular nucleus of the hypothalamus: Correlation with penile erection and yawning. Eur. J. Neurosci. 1996, 8, 2056–2063. [Google Scholar] [CrossRef]
- Collins, G.T.; Witkin, J.M.; Newman, A.H.; Svensson, K.A.; Grundt, P.; Cao, J.; Woods, J.H. Dopamine agonist-induced yawning in rats: A dopamine D3 receptor-mediated behavior. J. Pharmacol. Exp. Ther. 2005, 314, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Zarrindast, M.R.; Jamshidzadeh, A. Inhibitory effect of morphine on yawning induced by cholinoceptor and dopamine D2 receptor activation in rats. Br. J. Pharmacol. 1992, 105, 675–678. [Google Scholar] [CrossRef]
- Lynch, N.; Lima, J.D.; Spinieli, R.L.; Kaur, S. Opioids, sleep, analgesia and respiratory depression: Their convergence on Mu (μ)-opioid receptors in the parabrachial area. Front. Neurosci. 2023, 17, 1134842. [Google Scholar] [CrossRef]
- Boom, M.; Niesters, M.; Sarton, E.; Aarts, L.; Smith, T.W.; Dahan, A. Non-analgesic effects of opioids: Opioid-induced respiratory depression. Curr. Pharm. Des. 2012, 18, 5994–6004. [Google Scholar] [CrossRef]
- Gallup, A.C.; GallupJr, G.G. Yawning as a Brain Cooling Mechanism: Nasal Breathing and Forehead Cooling Diminish the Incidence of Contagious Yawning. Evol. Psychol. 2007, 5, 147470490700500109. [Google Scholar] [CrossRef]
- Gallup, A.C.; Eldakar, O.T. The thermoregulatory theory of yawning: What we know from over 5 years of research. Front. Neurosci. 2012, 6, 188. [Google Scholar] [CrossRef]
- Massen, J.J.M.; Dusch, K.; Eldakar, O.T.; Gallup, A.C. A thermal window for yawning in humans: Yawning as a brain cooling mechanism. Physiol. Behav. 2014, 130, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Gallup, A.C.; Gallup, G.G., Jr. Yawning and thermoregulation. Physiol. Behav. 2008, 95, 10–16. [Google Scholar] [CrossRef]
- Platek, S.M.; Mohamed, F.B.; Gallup, G.G. Contagious yawning and the brain. Cogn. Brain Res. 2005, 23, 448–452. [Google Scholar] [CrossRef]
- Walusinski, O. Yawning: Unsuspected avenue for a better understanding of arousal and interoception. Med. Hypotheses 2006, 67, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Guggisberg, A.G.; Mathis, J.; Herrmann, U.S.; Hess, C.W. The functional relationship between yawning and vigilance. Behav. Brain Res. 2007, 179, 159–166. [Google Scholar] [CrossRef]
- Dhar, D.K.; Arora, R. The Mystery behind “Yawning”: A Physiological Insight. J. Health Allied Sci. NU 2020, 10, 57–62. [Google Scholar] [CrossRef]
- Sambale, J.; Koehler, U.; Hildebrandt, W.; Kesper, K.; Cassel, W.; Degerli, M.; Korbmacher-Steiner, H.M. Yawning—A review of the literature. Somnologie 2024. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Normoyle, K.P.; Jackson, K.; Spitler, K.; Sharrock, M.F.; Miller, C.M.; Best, C.; Llano, D.; Du, R. Brain temperature and its fundamental properties: A review for clinical neuroscientists. Front. Neurosci. 2014, 8, 307. [Google Scholar] [CrossRef]
- Franzen, A.; Mader, S.; Winter, F. Contagious yawning, empathy, and their relation to prosocial behavior. J. Exp. Psychol. Gen. 2018, 147, 1950–1958. [Google Scholar] [CrossRef]
- Gallup, A.; Church, A.M.; Miller, H.; Risko, E.F.; Kingstone, A. Social Presence Diminishes Contagious Yawning in the Laboratory. Sci. Rep. 2016, 6, 25045. [Google Scholar] [CrossRef] [PubMed]
- Massen, J.J.; Church, A.M.; Gallup, A.C. Auditory Contagious Yawning in Humans: An Investigation into Affiliation and Status Effects. Front. Psychol. 2015, 6, 1735. [Google Scholar] [CrossRef]
- Schürmann, M.; Hesse, M.D.; Stephan, K.E.; Saarela, M.; Zilles, K.; Hari, R.; Fink, G.R. Yearning to yawn: The neural basis of contagious yawning. Neuroimage 2005, 24, 1260–1264. [Google Scholar] [CrossRef]
- Nahab, F.B.; Hattori, N.; Saad, Z.S.; Hallett, M. Contagious yawning and the frontal lobe: An fMRI study. Hum. Brain Mapp. 2009, 30, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.J.; Kim, S.; Saunders, H.; Bachmann, C.; Thompson, J.; Ropar, D.; Jackson, S.R.; Jackson, G.M. A Neural Basis for Contagious Yawning. Curr. Biol. 2017, 27, 2713–2717.E2. [Google Scholar] [CrossRef] [PubMed]
- Gallup, A.C.; Wozny, S. Interspecific Contagious Yawning in Humans. Animals 2022, 12, 1908. [Google Scholar] [CrossRef]
- Heusner, A.P. Yawning and associated phenomena. Physiol. Rev. 1946, 26, 156–168. [Google Scholar] [CrossRef]
- Krestel, H.; Bassetti, C.L.; Walusinski, O. Yawning-Its anatomy, chemistry, role, and pathological considerations. Prog. Neurobiol. 2018, 161, 61–78. [Google Scholar] [CrossRef]
- Walusinski, O. Yawning in diseases. Eur. Neurol. 2009, 62, 180–187. [Google Scholar] [CrossRef]
- Teive, H.A.G.; Munhoz, R.P.; Camargo, C.H.F.; Walusinski, O. Yawning in neurology: A review. Arq. Neuropsiquiatr. 2018, 76, 473–480. [Google Scholar] [CrossRef]
- Walusinski, O. Pathological Yawning, Laughing and Crying. Front. Neurol. Neurosci. 2018, 41, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, A.; Khalil, N.M.; Owbridge, P.; Hakda, M.; Beitverda, Y. Pathological yawning as an ictal seizure manifestation in the elderly. BMJ Case Rep. 2012, 2012, bcr0120125618. [Google Scholar] [CrossRef]
- Goren, J.L.; Friedman, J.H. Yawning as an aura for an L-dopa-induced “on” in Parkinson’s disease. Neurology 1998, 50, 823. [Google Scholar] [CrossRef] [PubMed]
- Güven, B.; Güven, H.; Çomoğlu, S.S. Migraine and Yawning. Headache 2018, 58, 210–216. [Google Scholar] [CrossRef]
- Petrić, D.; Vučić Peitl, M.; Prološčić, J.; Peitl, V. Sertraline Induced Excessive Yawning. Arch. Psychiatry Res. 2019, 55, 85–88. [Google Scholar] [CrossRef]
- Gutiérrez-Alvarez, A.M. Do your patients suffer from excessive yawning? Acta Psychiatr. Scand. 2007, 115, 80–81. [Google Scholar] [CrossRef]
- Argiolas, A.; Melis, M.R. The neuropharmacology of yawning. Eur. J. Pharmacol. 1998, 343, 1–16. [Google Scholar] [CrossRef]
- Qin, C.; Li, J.; Tang, K. The Paraventricular Nucleus of the Hypothalamus: Development, Function, and Human Diseases. Endocrinology 2018, 159, 3458–3472. [Google Scholar] [CrossRef]
- Kubota, N.; Amemiya, S.; Yanagita, S.; Kita, I. Neural pathways from the central nucleus of the amygdala to the paraventricular nucleus of the hypothalamus are involved in induction of yawning behavior due to emotional stress in rats. Behav. Brain Res. 2023, 436, 114091. [Google Scholar] [CrossRef]
- Sato-Suzuki, I.; Kita, I.; Oguri, M.; Arita, H. Stereotyped yawning responses induced by electrical and chemical stimulation of paraventricular nucleus of the rat. J. Neurophysiol. 1998, 80, 2765–2775. [Google Scholar] [CrossRef]
- Cao, Z.; Yung, W.-H.; Ke, Y. Repeated activation of preoptic area-recipient neurons in posterior paraventricular nucleus mediates chronic heat-induced negative emotional valence and hyperarousal states. eLife 2025, 13, RP101302. [Google Scholar] [CrossRef]
- Boggio, P.S.; Wingenbach, T.S.H.; da Silveira Coêlho, M.L.; Comfort, W.E.; Murrins Marques, L.; Alves, M.V.C. (Eds.) Social and Affective Neuroscience of Everyday Human Interaction: From Theory to Methodology; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Urba-Holmgren, R.; Holmgren, B.; Rodriguez, R.; Gonzalez, R.M. Serotonergic modulation of yawning. Pharmacol. Biochem. Behav. 1979, 11, 371–372. [Google Scholar] [CrossRef]
- Zarrindast, M.R.; Poursoltan, M. Interactions of drugs acting on central dopamine receptors and cholinoceptors on yawning responses in the rat induced by apomorphine, bromocriptine or physostigmine. Br. J. Pharmacol. 1989, 96, 843–848. [Google Scholar] [CrossRef]
- Béné, J.; Bastides, M.; Auffret, M.; Gautier, S. Serotonin and yawning: A possible adverse drug reaction during antidepressant therapy. Presse Med. 2014, 43, 1135–1136. [Google Scholar] [CrossRef]
- Nazar, B.P.; Hiluy, J.C.; Mattos, P. Antidepressant induced excessive yawning and indifference. J. Bras. De Psiquiatr. 2015, 64, 82–85. [Google Scholar] [CrossRef]
- Blin, O.; Azulay, J.P.; Masson, G.; Aubrespy, G.; Serratrice, G. Apomorphine-induced yawning in migraine patients: Enhanced responsiveness. Clin. Neuropharmacol. 1991, 14, 91–95. [Google Scholar] [CrossRef]
- Sanna, F.; Argiolas, A.; Melis, M.R. Oxytocin-induced yawning: Sites of action in the brain and interaction with mesolimbic/mesocortical and incertohypothalamic dopaminergic neurons in male rats. Horm. Behav. 2012, 62, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Gallup, A.C.; Church, A.M. The effects of intranasal oxytocin on contagious yawning. Neurosci. Lett. 2015, 607, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J.V., Jr.; Raffa, R.B.; Rosenblatt, M.H. Opioid withdrawal symptoms, a consequence of chronic opioid use and opioid use disorder: Current understanding and approaches to management. J. Clin. Pharm. Ther. 2020, 45, 892–903. [Google Scholar] [CrossRef]
- Dibaj, P.; Brockmann, K.; Gärtner, J. Dopamine-Mediated Yawning-Fatigue Syndrome With Specific Recurrent Initiation and Responsiveness to Opioids. JAMA Neurol. 2020, 77, 254. [Google Scholar] [CrossRef]
- Olmo, M.; González-Barboteo, J.; Moreno, D.; Coma, E.; Serrano, G. Acute opioid withdrawal syndrome from naloxone/naloxegol interaction. BMJ Support. Palliat. Care 2021, 11, 408–410. [Google Scholar] [CrossRef]
- Marraffa, A.; Lekander, M.; Solsjö, P.; Olsson, M.J.; Lasselin, J.; Axelsson, J. Yawning, a thermoregulatory mechanism during fever? A study of yawning frequency and its predictors during experimentally induced sickness. Physiol. Behav. 2017, 182, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Menin, D.; Ballardini, E.; Panebianco, R.; Garani, G.; Borgna-Pignatti, C.; Oster, H.; Dondi, M. Factors affecting yawning frequencies in preterm neonates. PLoS ONE 2022, 17, e0268083. [Google Scholar] [CrossRef] [PubMed]
- Gallup, A.C. On the link between emotional contagion and contagious yawning. Neurosci. Biobehav. Rev. 2021, 121, 18–19. [Google Scholar] [CrossRef]
- Menin, D.; Aureli, T.; Dondi, M. Two forms of yawning modulation in three months old infants during the Face to Face Still Face paradigm. PLoS ONE 2022, 17, e0263510. [Google Scholar] [CrossRef]
- Bergeria, C.L.; Huhn, A.S.; Dunn, K.E. The impact of naturalistic cannabis use on self-reported opioid withdrawal. J. Subst. Abus. Treat. 2020, 113, 108005. [Google Scholar] [CrossRef]
- Dibaj, P.; Seeger, D.; Gärtner, J.; Petzke, F. Follow-Up of a Case of Dopamine-Mediated Yawning-Fatigue-Syndrome Responsive to Opioids, Successful Desensitization via Graded Activity Treatment. Neurol. Int. 2021, 13, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.E.; Bird, H.E.; Bergeria, C.L.; Ware, O.D.; Strain, E.C.; Huhn, A.S. Operational definition of precipitated opioid withdrawal. Front. Psychiatry 2023, 14, 1141980. [Google Scholar] [CrossRef]
- Alóe, F. Yawning. Arq. Neuropsiquiatr. 1994, 52, 273–276. [Google Scholar] [CrossRef]
- Hensch, T.; Blume, A.; Böttger, D.; Sander, C.; Niedermeier, N.; Hegerl, U. Yawning in depression: Worth looking into. Pharmacopsychiatry 2015, 48, 118–120. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, S.; Shirmohammadi, S.; Hariri, B.; Laroche, D.; Martel, L. A yawning measurement method using embedded smart cameras. In Proceedings of the 2013 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Minneapolis, MN, USA, 6–9 May 2013; pp. 1605–1608. [Google Scholar]
- Omidyeganeh, M.; Shirmohammadi, S.; Abtahi, S.; Khurshid, A.; Farhan, M.; Scharcanski, J.; Hariri, B.; Laroche, D.; Martel, L. Yawning Detection Using Embedded Smart Cameras. IEEE Trans. Instrum. Meas. 2016, 65, 570–582. [Google Scholar] [CrossRef]
- Zhang, W.; Murphey, Y.L.; Wang, T.; Xu, Q. Driver yawning detection based on deep convolutional neural learning and robust nose tracking. In Proceedings of the 2015 International Joint Conference on Neural Networks (IJCNN), Killarney, Ireland, 12–17 July 2015; pp. 1–8. [Google Scholar]
- Gupta, N.; Bari, A.K.; Kumar, S.; Garg, D.; Gupta, K.O. Review Paper on Yawning Detection Prediction System for Driver Drowsiness. In Proceedings of the 2021 5th International Conference on Trends in Electronics and Informatics (ICOEI), Tirunelveli, India, 3–5 June 2021; pp. 1–6. [Google Scholar]
- Yang, H.; Liu, L.; Min, W.; Yang, X.; Xiong, X. Driver Yawning Detection Based on Subtle Facial Action Recognition. IEEE Trans. Multimed. 2021, 23, 572–583. [Google Scholar] [CrossRef]
| Neurotransmitter System | Receptor(s) Involved | Yawning Effect | Drugs That Increase Yawning | Drugs That Decrease Yawning | References |
|---|---|---|---|---|---|
| Serotonin (5-HT) | 5-HT2C, 5-HT1A | Increases yawning | SSRIs (e.g., Sertraline, Paroxetine), Buspirone (5-HT1A agonist) | 5-HT2C Antagonists (e.g., Cyproheptadine) | [7,11,12,13,45,47,53,55,56] |
| Dopamine (DA) | D3, D2 | Increases yawning | Dopamine Agonists (e.g., Apomorphine, Pramipexole) | Dopamine Antagonists (e.g., Haloperidol, Chlorpromazine) | [8,10,14,15,16,17,54,57] |
| Oxytocin (OXT) | Oxytocin Receptors | Facilitates yawning | Oxytocin administration (experimental) | Not well-studied | [47,58,59] |
| Opioids (μ-Opioid Receptors) | μ-opioid receptors | Suppresses yawning | Opioid Agonists (e.g., Morphine, Tilidine) | Opioid Antagonists (e.g., Naloxone, Naloxegol) | [17,19,60,61,62] |
| Search # | Query Description | Results |
|---|---|---|
| 1 | Yawning terms | 1492 |
| 2 | Physiological yawning | 1 |
| 3 | Drug-induced yawning | 23 |
| 4 | Cholinergic agents | 99,626 |
| 5 | Oxytocin | 27,398 |
| 6 | Nitric oxide and related compounds | 213,251 |
| 7 | Dopaminergic agents | 170,515 |
| 8 | Serotonergic agents | 139,062 |
| 9 | Opioids | 166,108 |
| 10 | GABAergic agents | 54,959 |
| 11 | Adrenergic agents | 318,762 |
| 12 | #1 + #3 (Yawning + Drug-induced yawning) | 23 |
| 13 | #12 AND #4 (Cholinergic agents) | 5 |
| 14 | #12 AND #5 (Oxytocin) | 4 |
| 15 | #12 AND #6 (Nitric oxide and related) | 3 |
| 16 | #12 AND #7 (Dopaminergic agents) | 11 |
| 17 | #12 AND #8 (Serotonergic agents) | 5 |
| 18 | #12 AND #9 (Opioids) | 14 |
| 19 | #12 AND #10 (GABAergic agents) | 1 |
| 20 | #12 AND #11 (Adrenergic agents) | 1 |
| First Author (Year) | Study Design | Sample Size | Geography | Population | Intervention/Drug | Yawning Patterns | Neurobiological Mechanisms | Drug Responsiveness | Clinical Implications |
|---|---|---|---|---|---|---|---|---|---|
| Béné, J (2014) [55] | Case Study | 1 | France | SSRI therapy patient | Paroxetine (SSRI) | Excessive daytime yawning associated with SSRIs | Serotonin receptor inhibitors | Yawning disappeared after SSRI discontinuation | Unwanted SSRI side effect |
| Gallup, AC (2015) [59] | Experimental | 60 | USA | Undergraduate students (general population) | Oxytocin | Contagious yawning documented via video | Oxytocin receptor involvement | Spontaneous yawning predictive value | Oxytocin ineffective in enhancing contagious yawning |
| Nazar, BP (2015) [56] | Case Study | 2 | Brazil | Patients with major or minor depressive disorder | Various SSRIs | Excessive yawning frequency (25–80 yawns/day) | Serotonin augmentation | Pattern unaffected by sedation or sleep disorders | Switching antidepressants resolved yawning |
| Petrić, D (2019) [45] | Case Study | 1 | Croatia | Psychiatric patient (moderate depressive episode) | Sertraline (SSRI) | Yawning correlated with sertraline dosage | Serotonin involvement confirmed | Discontinuation resolved yawning, maintaining psychiatric stability | Yawning as a side effect, not therapeutic marker |
| Bergeria, CL (2020) [67] | Survey | 200 | USA | Cannabis users | Cannabis | Not described | Not discussed | Cannabis reduced withdrawal symptoms but worsened yawning | Gender differences in cannabis efficacy |
| Dibaj, P (2020) [61] | Case Study | 1 | Germany | Sciatic pain patient | μ-opioid agonist | Moderate exercise-induced yawning, prevented by μ-opioid agonists | μ-opioid receptor activation | Yawning prevented by tilidine | Tilidine effective for yawning-fatigue syndrome |
| Dibaj, P (2021) [68] | Case Study | 1 | Germany | Fatigue syndrome patient | Tilidine (opioid) | Not described | Not discussed | Effective management of yawning-fatigue with tilidine | Increased exercise tolerance without yawning |
| Olmo, M (2021) [62] | Case Study | 1 | Spain | Opioid withdrawal patient | Naloxone, naloxegol | Severe yawning during opioid antagonist use | Not discussed | Yawning indicates acute opioid withdrawal syndrome | Marker for opioid withdrawal |
| Anagha, K (2021) [12] | Observational | 100 | India | SSRI users with depression, anxiety, and related disorders | Sertraline, escitalopram, fluoxetine | Common yawning as a side effect | SSRI-induced yawning noted | Yawning associated with multiple SSRIs | Distinctive side effect of SSRIs |
| Dunn, KE (2023) [69] | Experimental | 106 | USA | Morphine-maintained patients | Naloxone | Yawning linked to withdrawal symptoms | μ-opioid receptor involvement | Naloxone triggered yawning in morphine-maintained patients | Biomarker of opioid receptor antagonist effects |
| First Author (Year) | Sample Size | Yawning Patterns in Drug-Induced Yawning | Neurobiological Mechanisms | Correlation with Drug Responsiveness | Clinical Implications as a Biomarker |
|---|---|---|---|---|---|
| Dunn, KE (2023) [69] | 106 | Within 15 min, ~51% of participants had peak ratings for SOWS, and ~48% for COWS. Symptoms included runny eyes, yawning, sweating, hot flashes, and pupil dilation. | γ-opioid receptor involvement in naloxone-precipitated withdrawal. Yawning identified as a sentinel symptom. | Naloxone administration induced withdrawal symptoms, including yawning, in morphine-maintained patients. | Yawning may indicate opioid receptor antagonist effects in individuals using morphine therapeutically or recreationally. |
| Dibaj, P (2021) [68] | 1 | Yawning pattern not described. | Neurobiological mechanism not discussed. | Yawning-fatigue syndrome successfully treated with tilidine. | Tilidine increased exercise tolerance, reducing yawning and fatigue episodes. |
| Olmo, M (2021) [62] | 1 | Not described. | Not discussed. | Shortly after opioid antagonist administration, yawning appeared alongside severe withdrawal symptoms. | Yawning, in conjunction with opioid withdrawal symptoms, may signal opioid cessation or dosage adjustments. |
| Anagha, K (2021) [12] | 100 | Yawning reported as a common SSRI side effect (47%). Characteristics not described. | SSRI involvement noted. | Yawning occurred in 47.2% of sertraline users, 51.3% of escitalopram users, and 25% of fluoxetine users. | Yawning was listed among common SSRI side effects, alongside somnolence, dry mouth, and fatigue. |
| Dibaj, P (2020) [61] | 1 | Yawning and fatigue occurred during moderate leg exercise but disappeared after μ-opioid agonist treatment. | μ-opioid receptor activation in the paraventricular nucleus inhibited yawning. | Yawning and fatigue prevented by subcutaneous injection of 3.75 mg piritramide. | Tilidine treatment before exercise prevented yawning and fatigue, supporting the role of opioid receptor modulation. |
| Bergeria, CL (2020) [67] | 200 | Not described. | Not discussed. | Some participants (n = 12) reported cannabis worsened opioid withdrawal symptoms, including yawning. | Cannabis may influence opioid withdrawal symptoms differently in men and women, with greater symptom relief in females. |
| Petrić, D (2019) [45] | 1 | Patient reported yawning began shortly after sertraline initiation: 2–3 yawns after 25 mg, 3–4 yawns after 50 mg. | Serotonin’s role in yawning supported by 5-HT2C receptor involvement. | Yawning onset correlated with sertraline dosage. | Yawning was an SSRI side effect rather than a sign of therapeutic efficacy. |
| Gallup, AC (2015) [59] | 60 | Yawning characterized by wide jaw opening, deep inhalation, brief hold, and short exhale. | Oxytocin receptor involvement noted. | A total of 33.3% of participants yawned while watching a video stimulus; spontaneous yawning predicted increased contagious yawning. | Oxytocin did not enhance contagious yawning, suggesting a limited role in yawning regulation. |
| Nazar, BP (2015) [56] | 2 | Yawning frequency increased to 80 yawns/day in one patient and 25 yawns/day in another. Occurred during social and professional settings. | Serotonin increase induced yawning, likely due to dopaminergic reduction in the basal ganglia or frontal lobe dysfunction. | Yawning occurred independently of sedation or sleep disorders in both patients. | Yawning-related distress was resolved by switching medications. |
| Béné, J (2014) [55] | 1 | Abnormal excessive daytime yawning, lasting several seconds and associated with jaw contractures. | Serotonin receptor inhibition. | Patient experienced excessive yawning after starting paroxetine 20 mg/day. | Yawning was an unwanted SSRI side effect, resolving after discontinuation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.R.; Alzaeem, K.; Bejermie, M.; Fofang, C.N.M.; Mohamad, S.; Gazerani, P. Can Drug-Induced Yawning Serve as a Biomarker for Drug Safety and Effectiveness? Future Pharmacol. 2025, 5, 20. https://doi.org/10.3390/futurepharmacol5020020
Ali MR, Alzaeem K, Bejermie M, Fofang CNM, Mohamad S, Gazerani P. Can Drug-Induced Yawning Serve as a Biomarker for Drug Safety and Effectiveness? Future Pharmacology. 2025; 5(2):20. https://doi.org/10.3390/futurepharmacol5020020
Chicago/Turabian StyleAli, Mohammad Rokan, Khaled Alzaeem, Mostafa Bejermie, Cole Ngwachi Mangong Fofang, Siamand Mohamad, and Parisa Gazerani. 2025. "Can Drug-Induced Yawning Serve as a Biomarker for Drug Safety and Effectiveness?" Future Pharmacology 5, no. 2: 20. https://doi.org/10.3390/futurepharmacol5020020
APA StyleAli, M. R., Alzaeem, K., Bejermie, M., Fofang, C. N. M., Mohamad, S., & Gazerani, P. (2025). Can Drug-Induced Yawning Serve as a Biomarker for Drug Safety and Effectiveness? Future Pharmacology, 5(2), 20. https://doi.org/10.3390/futurepharmacol5020020








