Synergistic Antinociceptive Effects of Ketorolac and Ascorbic Acid in a Formalin-Induced Pain Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Pain Model
2.4. Experimental Groups
2.5. Statistical Analysis
3. Results
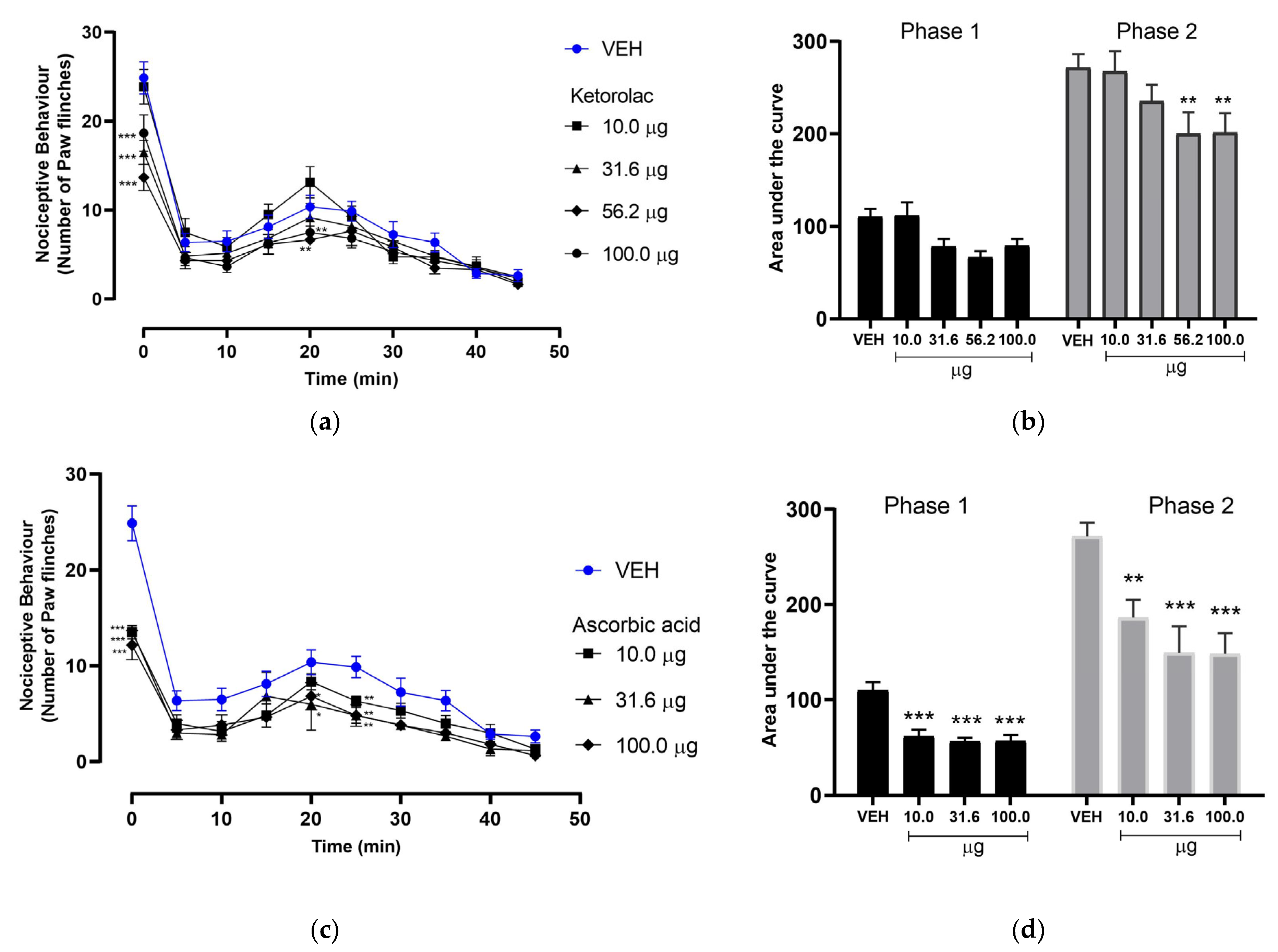
3.1. Effect of Ketorolac on Nociceptive Behavior in the Formalin-Induced Pain Model
3.2. Effect of Ascorbic Acid on Nociceptive Behavior in the Formalin-Induced Pain Model
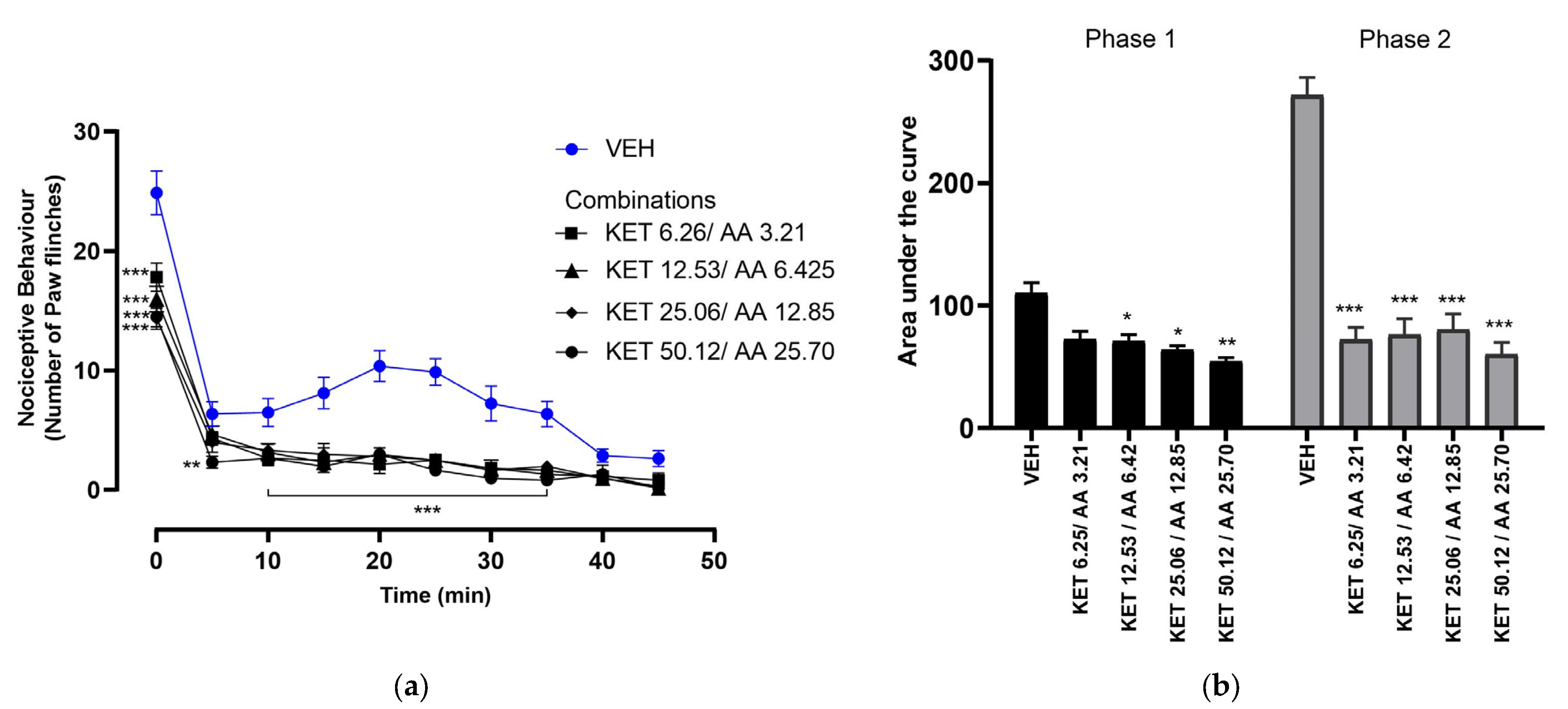
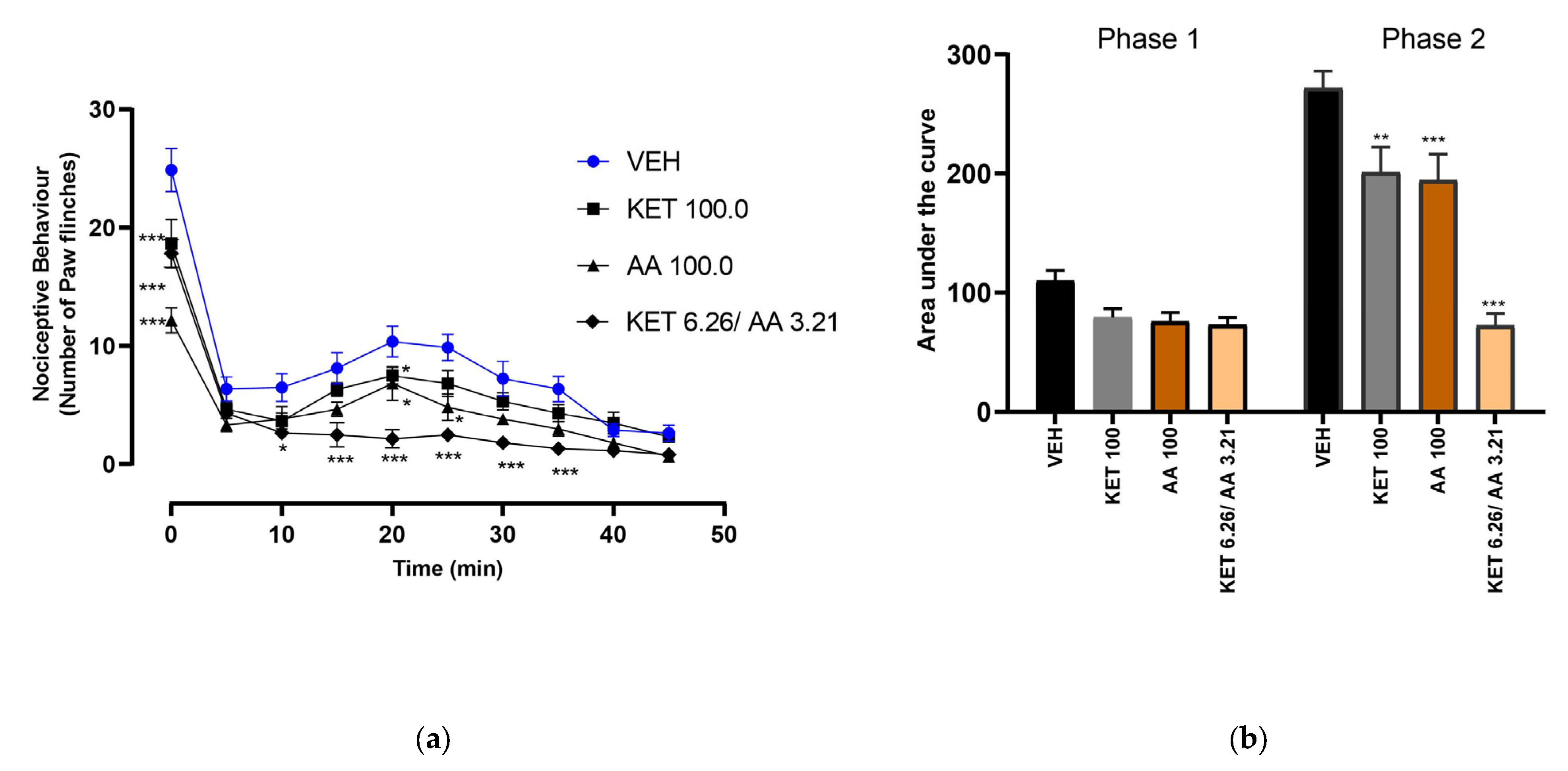
3.3. Dose–Response of Ketorolac and Ascorbic Acid Combinations
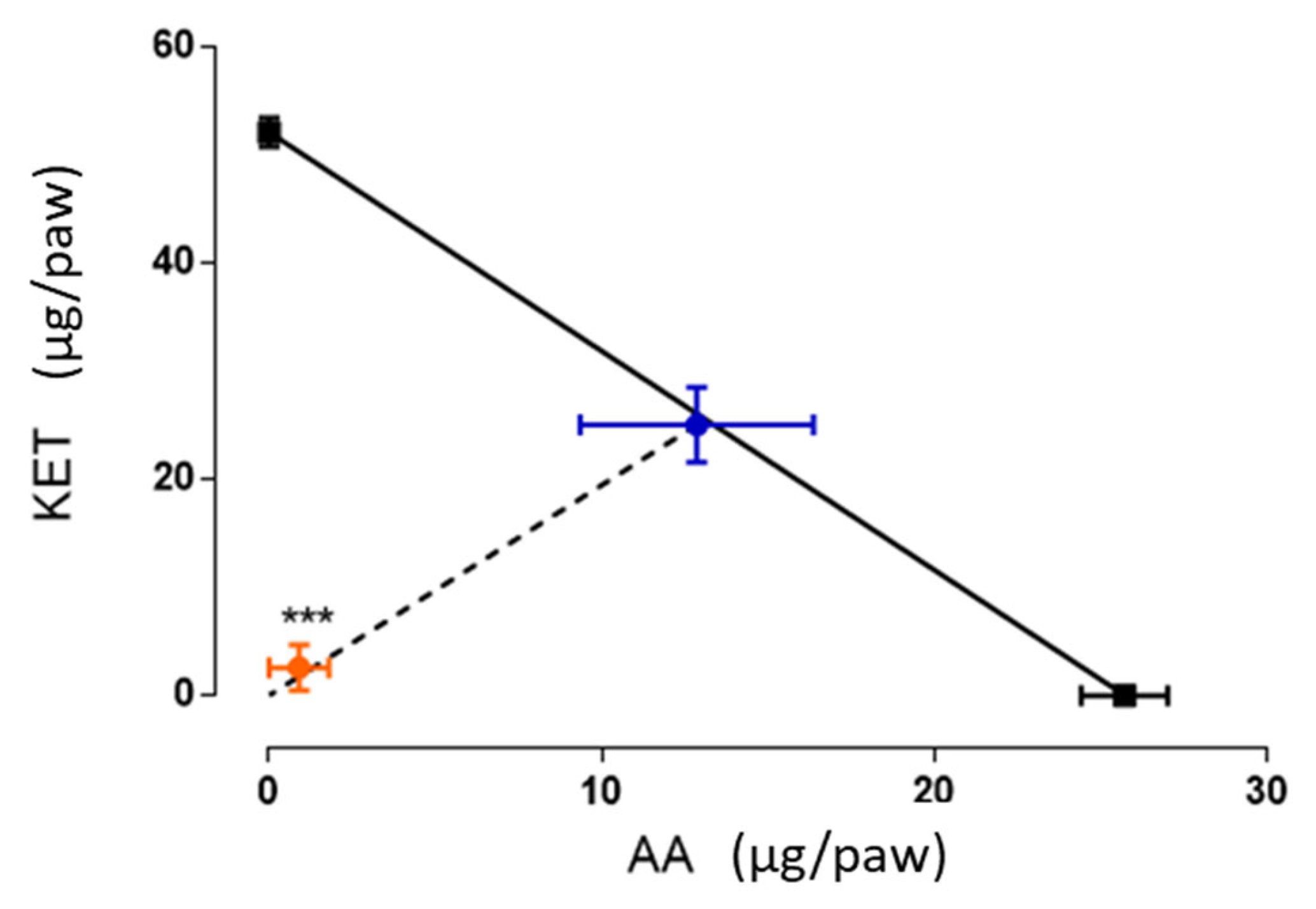
3.4. Isobolographic Analysis of the Antinociceptive Interaction Between the Combination of Ketorolac and Ascorbic Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Wise, E.; Khanna, P.P. The Impact of Gout Guidelines. Curr. Opin. Rheumatol. 2015, 27, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Bokermann, J.; König, H.H.; Hajek, A. Pain: Its Prevalence and Correlates among the Oldest Old. Aging Clin. Exp. Res. 2024, 36, 2. [Google Scholar] [CrossRef] [PubMed]
- Henschke, N.; Kamper, S.J.; Maher, C.G. The Epidemiology and Economic Consequences of Pain. Mayo Clin. Proc. 2015, 90, 139–147. [Google Scholar] [CrossRef]
- Atkinson, T.J.; Fudin, J. Nonsteroidal Antiinflammatory Drugs for Acute and Chronic Pain. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 219–231. [Google Scholar] [CrossRef]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical Pharmacology of Non-Steroidal Anti-Inflammatory Drugs: A Review. Antiinflamm. Antiallergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef]
- Rojas-Aguilar, F.A.; Briones-Aranda, A.; Jaramillo-Morales, O.A.; Romero-Nava, R.; Esquinca-Avilés, H.A.; Espinosa-Juárez, J.V. The Additive Antinociceptive Effect of Resveratrol and Ketorolac in the Formalin Test in Mice. Pharmaceuticals 2023, 16, 1078. [Google Scholar] [CrossRef]
- Beltrán-Montoya, J.J.; Herrerias-Canedo, T.; Arzola-Paniagua, A.; Vadillo-Ortega, F.; Dueñas-Garcia, O.F.; Rico-Olvera, H. A Randomized, Clinical Trial of Ketorolac Tromethamine vs Ketorolac Trometamine plus Complex B Vitamins for Cesarean Delivery Analgesia. Saudi J. Anaesth. 2012, 6, 207–212. [Google Scholar] [CrossRef]
- Rahman, M. Vitamin B12 and Ketorolac on Pain and Inflammation in Long Evans Rats. J. Pain 2017, 18, S3–S4. [Google Scholar] [CrossRef]
- Bai, A.; Abdullah, F.; Kumar, J.; Lal, A.; Abbas, M.; Sandesh, R.; Naz, S.; Shahid, S.; Anees, F.; Memon, S. The Role of Vitamin C in Reducing Pain Associated With Diabetic Neuropathy. Cureus 2021, 13, e15895. [Google Scholar] [CrossRef]
- Ayatollahi, V.; Dehghanpour Farashah, S.; Behdad, S.; Vaziribozorg, S.; Rabbani Anari, M. Effect of Intravenous Vitamin C on Postoperative Pain in Uvulopalatopharyngoplasty with Tonsillectomy. Clin. Otolaryngol. 2017, 42, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Kallenborn-Gerhardt, W.; Geisslinger, G.; Schmidtko, A. Additive Antinociceptive Effects of a Combination of Vitamin C and Vitamin E after Peripheral Nerve Injury. PLoS ONE 2011, 6, e29240. [Google Scholar] [CrossRef]
- Mikirova, N.; Casciari, J.; Rogers, A.; Taylor, P. Effect of High-Dose Intravenous Vitamin C on Inflammation in Cancer Patients. J. Transl. Med. 2012, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.C.; Lin, Y.T.; Chen, K.H.; Wang, L.K.; Chen, J.Y.; Chang, Y.J.; Wu, S.C.; Chiang, M.H.; Sun, C.K. The Effect of Perioperative Vitamin C on Postoperative Analgesic Consumption: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 3109. [Google Scholar] [CrossRef]
- Carr, A.C.; Vissers, M.C.M.; Vissers, M.C.M.; Cook, J.S.; Cook, J.S. Parenteral Vitamin C Relieves Chronic Fatigue and Pain in a Patient with Rheumatoid Arthritis and Mononeuritis Multiplex Secondary to CNS Vasculitis. Case Rep. Clin. Pathol. 2015, 2, 57. [Google Scholar] [CrossRef]
- Klimant, E.; Wright, H.; Rubin, D.; Seely, D.; Markman, M. Intravenous Vitamin C in the Supportive Care of Cancer Patients: A Review and Rational Approach. Curr. Oncol. 2018, 25, 139–148. [Google Scholar] [CrossRef]
- Bayu, P.; Wibisono, J.J. Vitamin C and E Antioxidant Supplementation May Significantly Reduce Pain Symptoms in Endometriosis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2024, 19, e0301867. [Google Scholar] [CrossRef]
- NOM-062-ZOO-1999; Especificaciones Técnicas Para La Producción, Cuidado y Uso de Los Animales de Laboratorio. Diario Oficial de la Feeración: Mexico City, Mexico, 2001. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=762506&fecha=22/08/2001#gsc.tab=0 (accessed on 31 March 2025).
- Zimmermann, M. Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Dubuisson, D.; Dennis, S.G. The Formalin Test: A Quantitative Study of the Analgesic Effects of Morphine, Meperidine, and Brain Stem Stimulation in Rats and Cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef]
- Gibaldi, M. Estimation of Area under the Curve. In Biopharmaceutics and Clinical Pharmacokinetics; Routledge: London, UK, 1991; pp. 377–378. [Google Scholar]
- Tallarida, R.J. The Interaction Index: A Measure of Drug Synergism. Pain 2002, 98, 163–168. [Google Scholar] [CrossRef]
- Tjølsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The Formalin Test: An Evaluation of the Method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.M.; Cater, H.L.; Thakur, M.; Wells, S.; McMahon, S.B. A Refinement to the Formalin Test in Mice. F1000Research 2019, 8, 891. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, A.B.; Yaksh, T. Pharmacology of the Spinal Action of Ketorolac, Morphine, ST-91, U50488H, and L-PIA on the Formalin Test and an Isobolographic Analysis of the NSAID Interaction. Anesthesiology 1993, 79, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen Martínez-Martínez, M.; Parra-Flores, L.I.; del Carmen Baeza-Flores, G.; Torres-López, J.E. Isobolographic Analysis of Antinociceptive Effect of Ketorolac, Indomethacin, and Paracetamol after Simultaneous Peripheral Local and Systemic Administration. Behav. Pharmacol. 2022, 33, 15–22. [Google Scholar] [CrossRef]
- Mroszczak, E.; Combs, D.; Chaplin, M.; Tsina, I.; Tarnowski, T.; Rocha, C.; Tam, Y.; Boyd, A.; Young, J.; Depass, L. Chiral Kinetics and Dynamics of Ketorolac. J. Clin. Pharmacol. 1996, 36, 521–539. [Google Scholar]
- Vadivelu, N.; Gowda, A.M.; Urman, R.D.; Jolly, S.; Kodumudi, V.; Maria, M.; Taylor, R.; Pergolizzi, J.V. Ketorolac Tromethamine—Routes and Clinical Implications. Pain Pract. 2015, 15, 175–193. [Google Scholar]
- Viegas, A.; Manso, J.; Corvo, M.C.; Marques, M.M.B.; Cabrita, E.J. Binding of Ibuprofen, Ketorolac, and Diclofenac to COX-1 and COX-2 Studied by Saturation Transfer Difference NMR. J. Med. Chem. 2011, 54, 8555–8562. [Google Scholar] [CrossRef]
- Rodríguez-Silverio, J.; Sánchez-Mendoza, M.E.; Arrieta-Valencia, J.; Rocha-Gonzalez, H.I.; Flores-Murrieta, F.J. Tizanidine Increases Antinociceptive Effect and Prevents Gastric Damage Induced by Ketorolac in the Rat. Drug Dev. Res. 2013, 74, 38–42. [Google Scholar] [CrossRef]
- Rocha-González, H.I.; Sánchez-Mendoza, M.E.; Cruz-Antonio, L.; Flores-Murrieta, F.J.; Cornelio-Huerta, X.I.; Arrieta, J. Antinociceptive Interaction and Pharmacokinetics of the Combination Treatments of Methyleugenol Plus Diclofenac or Ketorolac. Molecules 2020, 25, 5106. [Google Scholar] [CrossRef]
- Medina-Santillán, R.; Reyes-García, G.; Rocha-González, H.I.; Granados-Soto, V. B Vitamins Increase the Analgesic Effect of Ketorolac in the Formalin Test in the Rat—PubMed. Proc. West. Pharmacol. Soc. 2004, 47, 95–99. [Google Scholar]
- Barreras-Espinoza, I.; Soto-Zambrano, J.A.; Serafín-Higuera, N.; Zapata-Morales, R.; Alonso-Castro, Á.; Bologna-Molina, R.; Granados-Soto, V.; Isiordia-Espinoza, M.A. The Antinociceptive Effect of a Tapentadol-Ketorolac Combination in a Mouse Model of Trigeminal Pain Is Mediated by Opioid Receptors and ATP-Sensitive K+ Channels. Drug Dev. Res. 2017, 78, 63–70. [Google Scholar] [CrossRef] [PubMed]
- López-Muñoz, F.J.; Díaz-Reval, M.I.; Terrón, J.A.; Déciga Campos, M. Analysis of the Analgesic Interactions between Ketorolac and Tramadol during Arthritic Nociception in Rat. Eur. J. Pharmacol. 2004, 484, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Zylinska, L.; Lisek, M.; Guo, F.; Boczek, T. Vitamin C Modes of Action in Calcium-Involved Signaling in the Brain. Antioxidants 2023, 12, 231. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Rosa, K.A.; Gadotti, V.M.; Rosa, A.O.; Rodrigues, A.L.S.; Calixto, J.B.; Santos, A.R.S. Evidence for the Involvement of Glutamatergic System in the Antinociceptive Effect of Ascorbic Acid. Neurosci. Lett. 2005, 381, 185–188. [Google Scholar] [CrossRef]
- Goecks, C.S.B.; Horst, A.; Moraes, M.S.; Scheid, T.; Kolberg, C.; Belló-Klein, A.; Partata, W.A. Assessment of Oxidative Parameters in Rat Spinal Cord After Chronic Constriction of the Sciatic Nerve. Neurochem. Res. 2012, 37, 1952–1958. [Google Scholar] [CrossRef]
- Riffel, A.P.K.; de Souza, J.A.; Santos, M.d.C.Q.; Horst, A.; Scheid, T.; Kolberg, C.; Belló-Klein, A.; Partata, W.A. Systemic Administration of Vitamins C and E Attenuates Nociception Induced by Chronic Constriction Injury of the Sciatic Nerve in Rats. Brain Res. Bull. 2016, 121, 169–177. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, C.K.; Lee, H.C.; Jung, H.; Choi, K.U.; Hong, S.W.; Lim, D.G.; Baek, W.Y.; Kwak, K.H. Antiallodynic Effects of Vitamin C and Vitamin E in Chronic Post-Ischemia Pain Rat Model. Korean J. Anesthesiol. 2013, 65, 442–448. [Google Scholar] [CrossRef]
- Mayland, C.R.; Bennett, M.I.; Allan, K. Vitamin C Deficiency in Cancer Patients. Palliat. Med. 2005, 19, 17–20. [Google Scholar] [CrossRef]
- Hill-Mündel, K.; Schlegl, J.; Biesalski, H.K.; Ehnert, S.; Schröter, S.; Bahrs, C.; Nohr, D.; Nüssler, A.K.; Ihle, C. Preoperative Ascorbic Acid Levels in Proximal Femur Fracture Patients Have No Postoperative Clinical Impact, While Ascorbic Acid Levels upon Discharge Have a Major Effect on Postoperative Outcome. J. Clin. Med. 2019, 9, 66. [Google Scholar] [CrossRef]
| Combination | Dose (µg/paw) | ||
|---|---|---|---|
| Ketorolac | Ascorbic Acid | Total | |
| EC30 + EC30 | 6.26 | 3.21 | 9.48 |
| (EC30 + EC30)/2 | 12.53 | 6.42 | 18.96 |
| (EC30 + EC30)/4 | 25.06 | 12.85 | 37.91 |
| (EC30 + EC30)/8 | 50.12 | 25.70 | 75.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-Juárez, J.V.; Hoover-Lazo, E.F.; Rubio-Trujillo, S.d.J.; de la Torre-Sosa, C.N.; Vega-Cabrera, N.V.; Corzo-Gómez, J.C.; Cruz-Trujillo, R.; Jaramillo-Morales, O.A. Synergistic Antinociceptive Effects of Ketorolac and Ascorbic Acid in a Formalin-Induced Pain Model. Future Pharmacol. 2025, 5, 15. https://doi.org/10.3390/futurepharmacol5020015
Espinosa-Juárez JV, Hoover-Lazo EF, Rubio-Trujillo SdJ, de la Torre-Sosa CN, Vega-Cabrera NV, Corzo-Gómez JC, Cruz-Trujillo R, Jaramillo-Morales OA. Synergistic Antinociceptive Effects of Ketorolac and Ascorbic Acid in a Formalin-Induced Pain Model. Future Pharmacology. 2025; 5(2):15. https://doi.org/10.3390/futurepharmacol5020015
Chicago/Turabian StyleEspinosa-Juárez, Josué Vidal, Erika Florecita Hoover-Lazo, Sergio de Jesús Rubio-Trujillo, Citlaly Natali de la Torre-Sosa, Nereida Violeta Vega-Cabrera, Josselin Carolina Corzo-Gómez, Refugio Cruz-Trujillo, and Osmar Antonio Jaramillo-Morales. 2025. "Synergistic Antinociceptive Effects of Ketorolac and Ascorbic Acid in a Formalin-Induced Pain Model" Future Pharmacology 5, no. 2: 15. https://doi.org/10.3390/futurepharmacol5020015
APA StyleEspinosa-Juárez, J. V., Hoover-Lazo, E. F., Rubio-Trujillo, S. d. J., de la Torre-Sosa, C. N., Vega-Cabrera, N. V., Corzo-Gómez, J. C., Cruz-Trujillo, R., & Jaramillo-Morales, O. A. (2025). Synergistic Antinociceptive Effects of Ketorolac and Ascorbic Acid in a Formalin-Induced Pain Model. Future Pharmacology, 5(2), 15. https://doi.org/10.3390/futurepharmacol5020015






