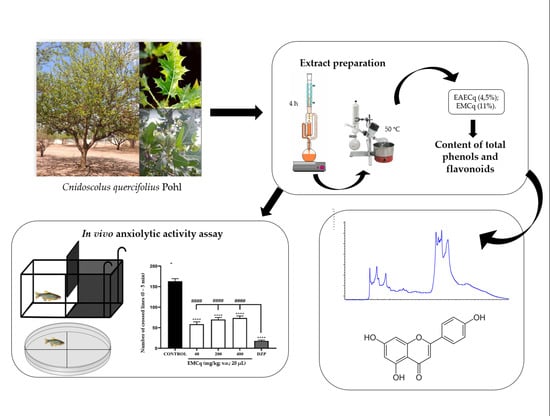
Antioxidant Activity and Anxiolytic Effect of Cnidoscolus quercifolius Pohl Stem Bark Extract in Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Obtaining Extracts
2.2. Total Phenols
2.3. Total Flavonoid
2.4. HPLC/DAD Analysis
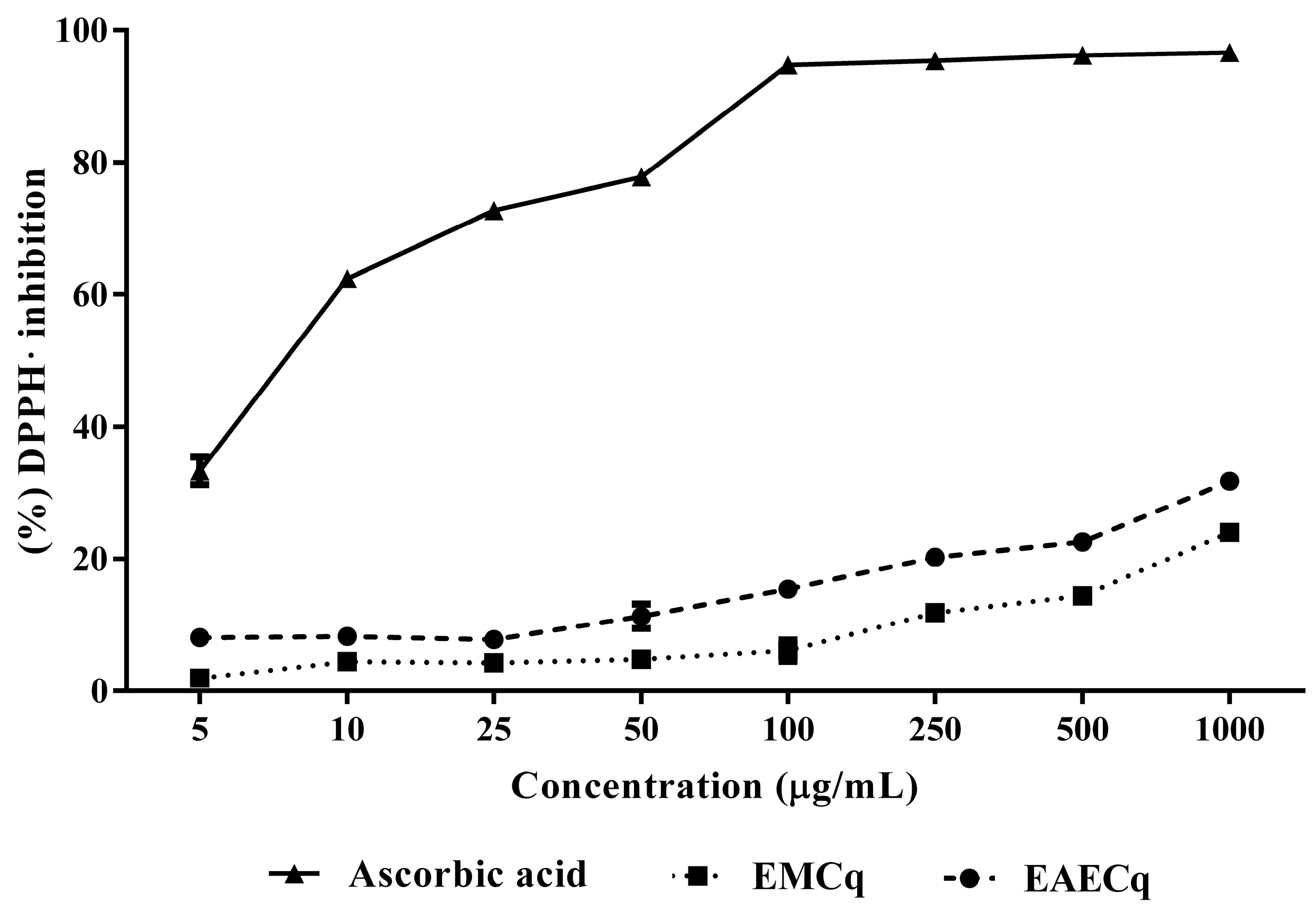
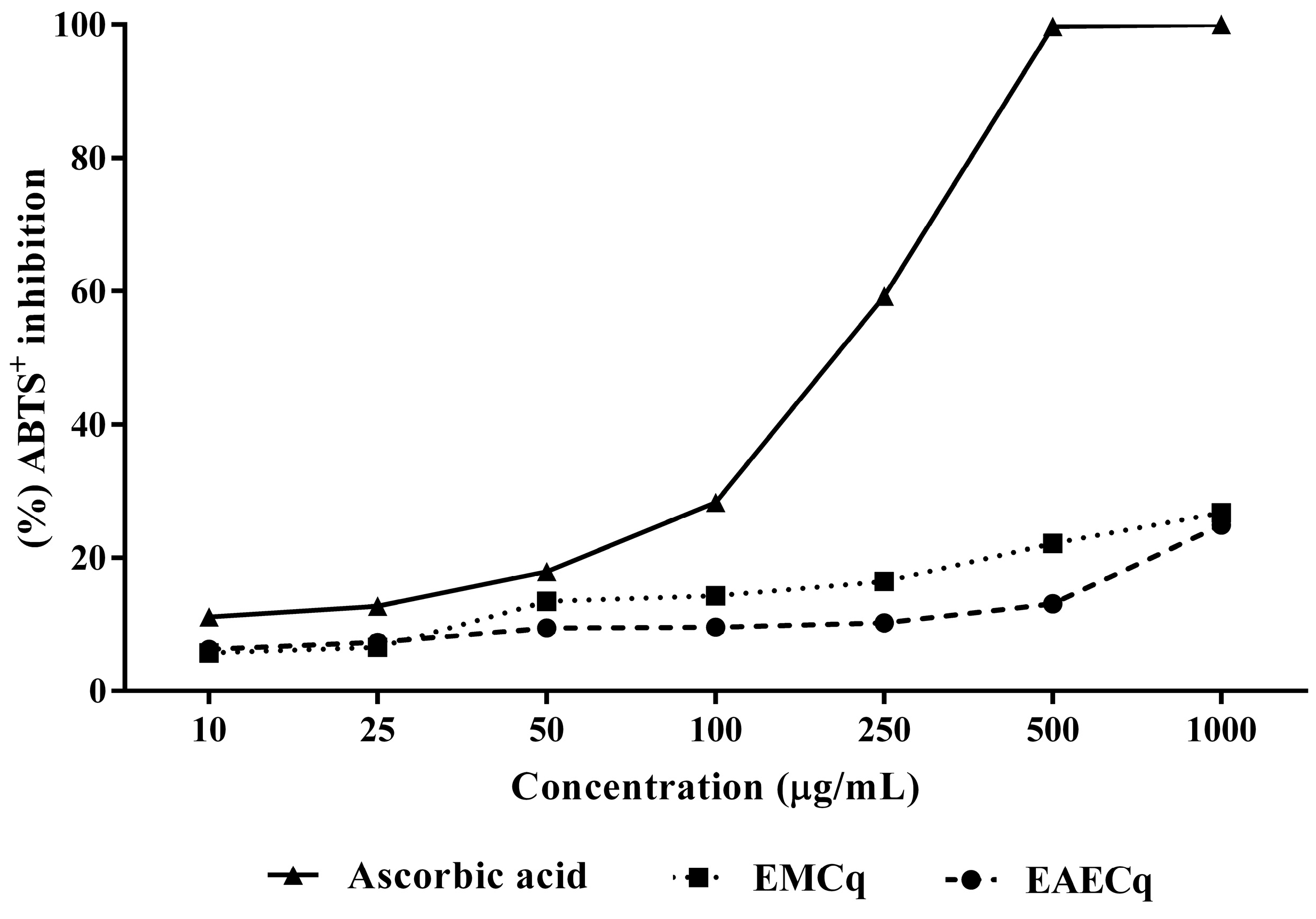
2.5. Antioxidant Activity
2.5.1. ABTS+ Radical Discoloration
2.5.2. DPPH• Radical Scavenging Capacity
2.6. Zebrafish Experimental Model
2.6.1. Animals
2.6.2. Determination of Acute Toxicity 96 h
2.6.3. Assessment of Locomotor Activity (Open Field Test)
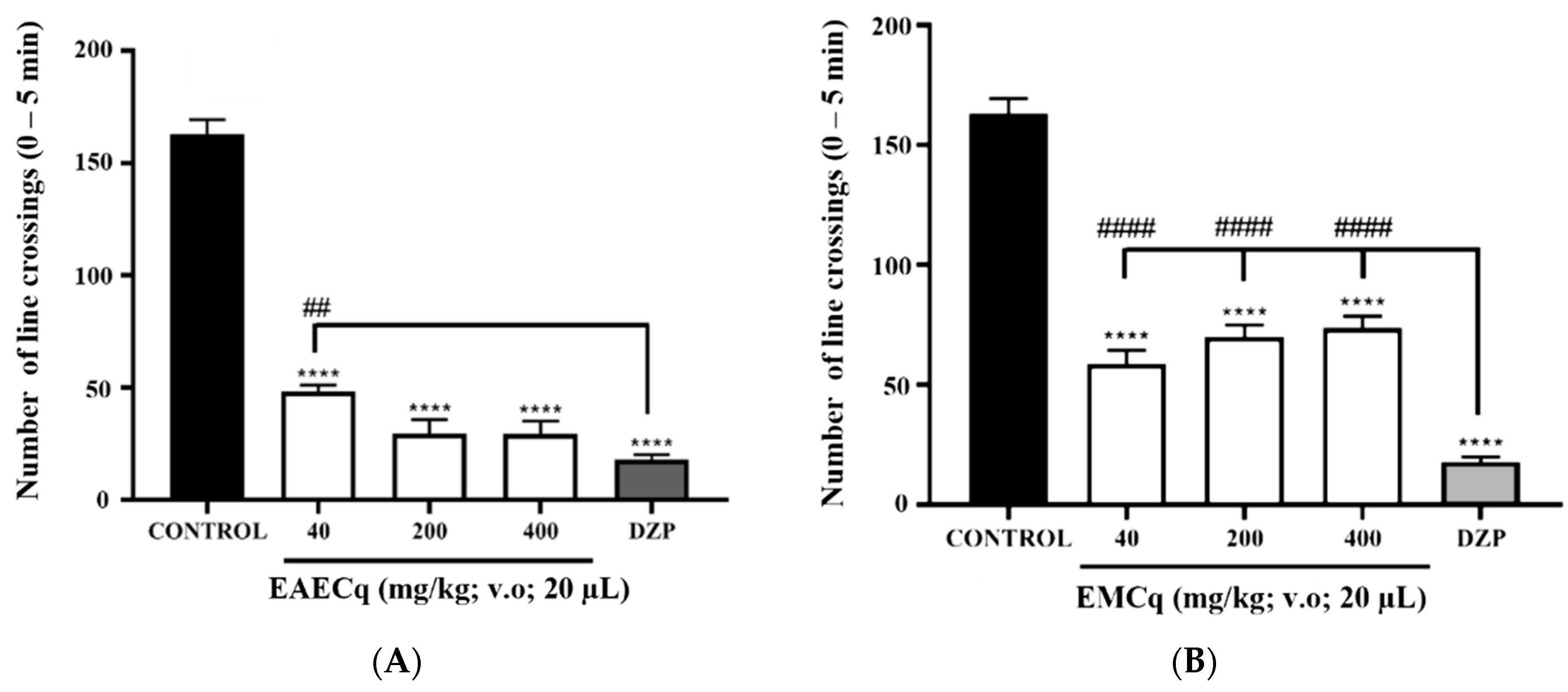
2.6.4. Analysis of Anxiolytic Activity (Light/Dark Test)
2.7. Statistical Analysis
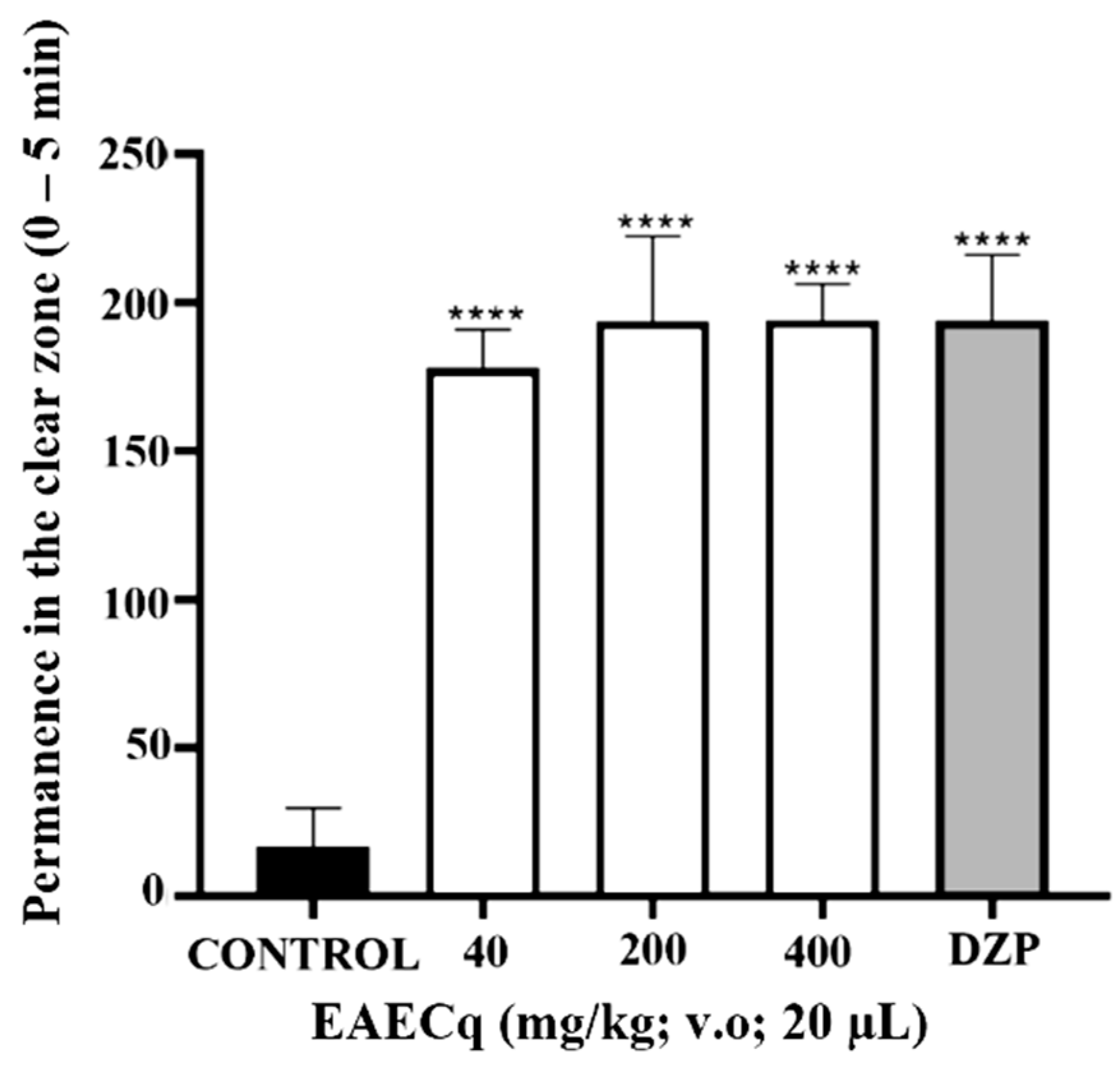
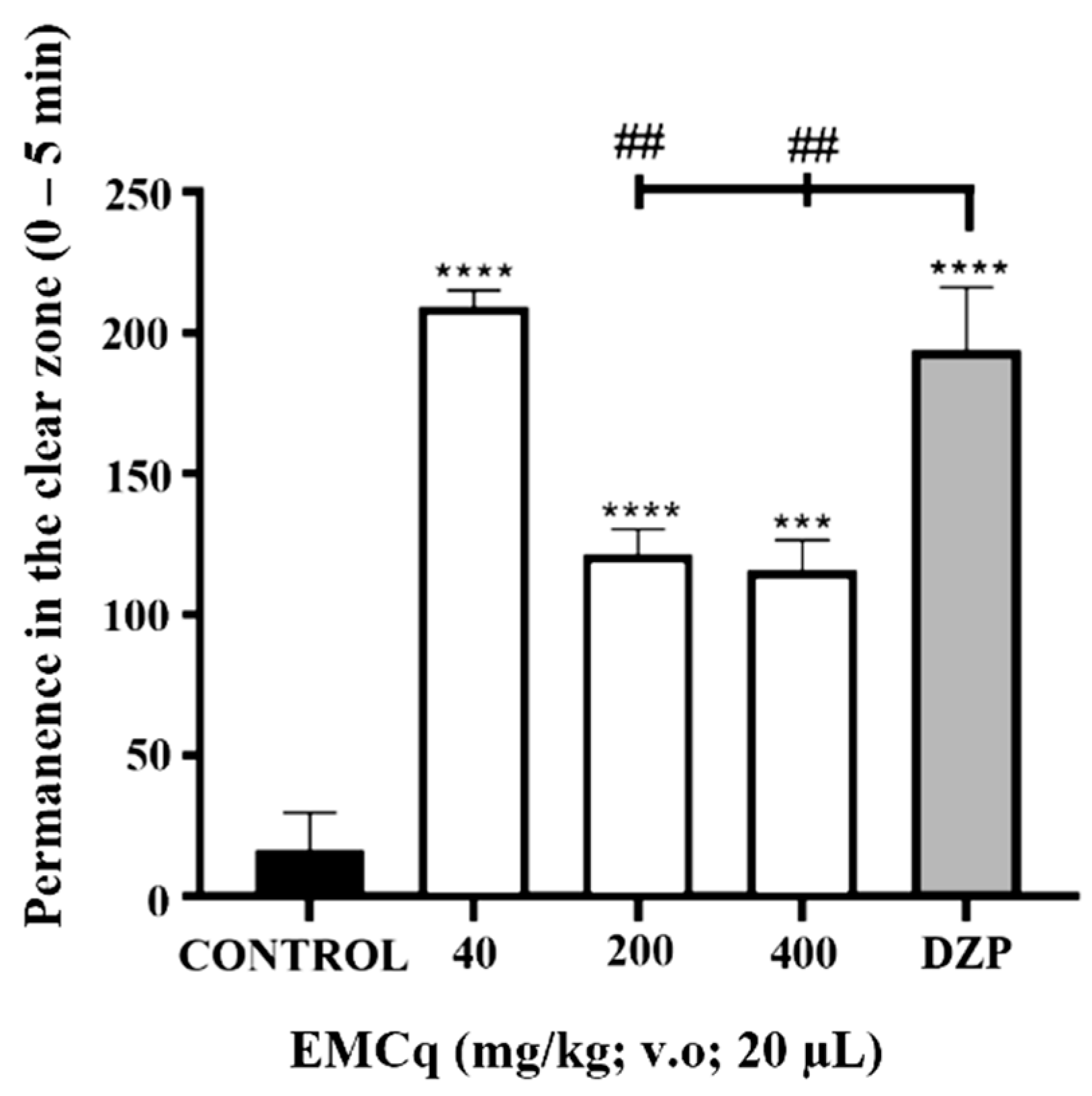
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxic 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Fedoce, A.G.; Ferreira, F.; Bota, R.G.; Bonet-Costa, V.; Sun, P.Y.; Davies, K.J.A. The role of oxidative stress in anxiety disorder: Cause or consequence? Free Radic. Res. 2018, 52, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, D.V.; Silva, D.M.; Magalhães, F.E.A.; Araújo, J.I.F.; Araujo, S.M.B.; Silva Lopes, F.F.; Guedes, M.I.F. Evaluation of antioxidant, toxicological and anxiolytic-like effect of ethanolic extracts of Ziziphus cotinifolia Reissek in adult zebrafish (Danio rerio). Phytomed. Plus 2024, 4, 100504. [Google Scholar] [CrossRef]
- Frota, I.J.; Moura Fé, A.A.C.; Paula, F.T.M.; Gomes, V.E.; Moura, S.E.; Campos, M. Anxiety disorders: History, clinical features, and current classifications. J. Health Biol. Sci. 2022, 10, 1–8. [Google Scholar] [CrossRef]
- Lira, S.M.; Dionísio, A.P.; Holanda, M.O.; Marques, C.G.; Silva, G.S.; Correa, L.C.; Santos, G.B.M.; Abreu, F.A.P.; Magalhães, F.E.A.; Rebouças, E.L.; et al. Metabolic profile of pitaya (Hylocereus polyrhizus (F.A.C. Weber) Britton & Rose) by UPLC-QTOF-MSE and assessment of its toxicity and anxiolytic-like effect in adult zebrafish. Food Res. Int. 2020, 127, 108701. [Google Scholar] [CrossRef]
- Santos, M.F. O Uso da Erythrina Velutina (Mulungu) Como Recurso Terapeutico para os Transtornos de Ansiedade: Uma Revisão Bibliográfica. Master’s Thesis, Pró-Reitoria de Pesquisa, Inovação e Pós-Graduação Coordenação do Curso de Pós-Graduação em Gestão dos Recursos Ambientais do Semiárido, PICUÍ-PB Instituto Federal da Paraíba-IFPB, João Pessoa, Paraíba, Brazil, 2022. [Google Scholar]
- Pereira, A.C.C.; Pereira, M.M.A.; Silva, B.L.L.; Freitas, C.M.; Cruz, C.S.; David, D.B.M.; Santos, D.L.; Delfraro, D.O.; Ura, F.A.C. The aggravation of anxiety disorders in healthcare professionals in the context of COVID-19 pandemic. Braz. J. Health Rev. 2021, 4, 4094–4110. [Google Scholar] [CrossRef]
- Menezes, J.F.S.; Silva, A.M.S.P.; Almeida, E.A.F.; Silva, A.F.; Lima, J.M.B.; Silva, A.W.; Fonseca, A.M. Synthesis and anxiolytic effect of europium metallic complex containing lapachol [Eu (DBM) 3. LAP] in adult zebrafish through serotonergic neurotransmission: In vivo and in silico approach. J. Biomol. Struct. Dyn. 2024, 42, 1280–1292. [Google Scholar] [CrossRef]
- Klüver, N.; König, M.; Ortmann, J.; Massei, R.; Paschke, A.; Kühne, R.; Scholz, S. Fish embryo toxicity test: Identification of compounds with weak toxicity and analysis of behavioral effects to improve prediction of acute toxicity for neurotoxic compounds. Environ. Sci. Technol. 2015, 49, 7002–7011. [Google Scholar] [CrossRef]
- Barboza, J.R.; Pereira, F.A.N.; Leite, J.A.C.; Coutinho, D.F.; Sousa Ribeiro, M.N. Abordagem química e toxicidade em modelo zebrafish de geoprópolis de Melipona fasciculata Smith. Braz. J. Health Rev. 2019, 2, 5582–5594. [Google Scholar] [CrossRef]
- Salim, M.; Saeed, A.; Iqbal, M.; Khan, B.A.; Khan, N.; Rabbani, I.; Rasul, A. Phytochemical screening and evaluation of antioxidant, total phenolic and flavonoid contents in various weed plants associated with wheat crops. Braz. J. Biol. 2022, 82, 256–486. [Google Scholar] [CrossRef]
- Sacramento, V.d.M.; Veloso, P.H.F.; Royo, V.d.A.; Abreu, N.S.; Souto, K.S.F.; Melo Júnior, A.F.d. Métodos Para Determinação de Capacidade Antioxidante; Atena: Ponta Grossa, Parana, Brazil, 2023. [Google Scholar]
- Melo, A.L.D.; Sales, M.F.D. O gênero Cnidoscolus Pohl (Crotonoideae-Euphorbiaceae) no Estado de Pernambuco, Brasil. Acta Bot. Bras. 2008, 22, 806–827. [Google Scholar]
- Albuquerque, U.P.; Medeiros, P.M.; Almeida, A.L.S.; Monteiro, J.M.; Neto, E.M.D.F.L.; Melo, J.G.; Santos, J.P. Medicinal plants of the caatinga (semi-arid) vegetation of NE Brazil: A quantitative approach. J. Ethnopharmacol. 2007, 114, 325–354. [Google Scholar] [CrossRef] [PubMed]
- Agra, M.F.; Silva, K.N.; Lima, J.; Basílio, D.; França, P.F.; Barbosa-Filho, M. Survey of medicinal plants used in the region Northeast of Brazil. Rev. Bras. Farmacogn. 2008, 18, 472–508. [Google Scholar] [CrossRef]
- Gomes, L.M.A.; Andrade, T.M.D.; Silva, J.C.; Lima, J.T.; Quintans-Junior, L.J.; Silva Almeida, J.R.G. Phytochemical screening and anti-inflammatory activity of Cnidoscolus quercifolius (Euphorbiaceae) in mice. Pharmacogn. Res. 2014, 6, 345–349. [Google Scholar] [CrossRef]
- Peixoto Sobrinho, T.J.S. Phytochemical screening and antibacterial activity of four Cnidoscolus species (Euphorbiaceae) against standard strains and clinical isolates. J. Med. Plants Res. 2012, 6, 3742–3748. [Google Scholar] [CrossRef]
- Lira, S.M.; Canabrava, N.V.; Benjamin, S.R.; Silva, J.Y.G.; Viana, D.A.; Lima, C.L.S.; Paredes, P.F.M.; Marques, M.M.M.; Pereira, E.O.; Queiroz, E.A.M.; et al. Evaluation of the toxicity and hypoglycemic effect of the aqueous extracts of Cnidoscolus quercifolius pohl. Braz. J. Med. Biol. Res. 2017, 50, e6361. [Google Scholar] [CrossRef]
- Nonato, C.D.F.A.; Melo, E.V.S.; Camilo, C.J.; Ferreira, M.K.A.; Meneses, J.E.A.; Silva, A.W.; Costa, J.G.M. Antibacterial Activity and Anxiolytic Effect in Adult Zebrafish of Genus Lippia L. Species. Plants 2023, 12, 1675. [Google Scholar] [CrossRef]
- Kosalec, I.; Bakmaz, M.; Pepeljnjak, S.; Vladimir- Knezevic, S. Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharm. 2004, 54, 65–72. [Google Scholar]
- Rufino, M.D.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.D.G.; Pérez-Jimenez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas pela Captura do Radical Livre ABTS; Embrapa Tropical Agroindustry: Fortaleza, Brazil, 2007; Technical Communication, 128. [Google Scholar]
- Rufino, M.D.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.D.G.; Pérez-Jimenez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas pela Captura do Radical Livre DPPH; Embrapa Tropical Agroindustry: Fortaleza, Brazil, 2007; Technical Communication, 127. [Google Scholar]
- OECD. Fish, acute toxicity test. In Guideline for the Testing of Chemicals, 19; OECD: Paris, France, 1992. [Google Scholar]
- Ferreira, M.K.A.; Silva, A.W.; Moura, A.L.S.; Sales, K.V.B.; Marinho, E.M.; Cardoso, J.N.M.; Marinho, M.M.; Bandeira, P.N.; Magalhães, F.E.A.; Marinho, E.S.; et al. Chalcones reverse the anxiety and convulsive behavior of adult zebrafish. Epilepsy Behav. 2021, 117, 107–881. [Google Scholar] [CrossRef]
- Gonçalves, N.G.G.; Araújo, J.I.F.; Magalhães, F.E.A.; Mendes, F.R.S.; Lobo, M.D.P.; Moreira, A.C.O.M.; Moreira, R.A. Protein fraction from Artocarpus altilis pulp exhibits antioxidant properties and reverses anxiety behavior in adult zebrafish via the serotoninergic system. J. Funct. Foods 2020, 66, 103772. [Google Scholar] [CrossRef]
- Gebauer, D.L.; Pagnussat, N.; Piato, Â.L.; Schaefer, I.C.; Bonan, C.D.; Lara, D.R. Effects of anxiolytics in zebrafish: Similarities and differences between benzodiazepines, buspirone and ethanol. Pharm. Bioch. Behav. 2011, 99, 480–486. [Google Scholar] [CrossRef]
- Oliveira Júnior, R.G.; Ferraz, C.A.A.; Pereira, E.C.V.; Sampaio, P.A.; Silva, M.F.S.; Pessoa, C.O.; Rolim, L.A.; da Silva Almeida, J.R.G. Phytochemical analysis and cytotoxic activity of Cnidoscolus quercifolius Pohl (Euphorbiaceae) against prostate (PC3 and PC3-M) and breast (MCF-7) cancer cells. Pharmacogn. Mag. 2019, 15, 24. [Google Scholar] [CrossRef]
- Peixoto Sobrinho, T.J.S. Estudo Químico e Biológico de Espécies do Gênero Cnidoscolus Presentes no Ecossistema Caatinga com Potencial Atividade Terapêutica. Master’s Thesis, Universidade Federal de Pernambuco Centro de Ciências da Saúde, Recife, Brazil, 2011. [Google Scholar]
- Nunes, F.R.S.; Dias, H.M.C.; Cavalcante, G.M. Investigação das atividades antioxidante e antimicrobiana de duas espécies arbóreas ocorrentes no bioma caatinga. Estação Científica (UNIFAP) 2016, 6, 81–90. [Google Scholar] [CrossRef]
- Valares Masa, C.; Sosa Díaz, T.; Alías Gallego, J.C.; Chaves Lobón, N. Quantitative Variation of Flavonoids and Diterpenes in Leaves and Stems of Cistus ladanifer L. at Different Ages. Molecules 2016, 21, 275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Jia, X.; Wang, W.K.; Liu, T.; Huang, S.P.; Yang, M.Y. Growth under elevated air temperature alters secondary metabolites in Robinia pseudoacacia L. seedlings in Cd- and Pb-contaminated soils. Sci. Total Environ. 2016, 565, 586–594. [Google Scholar] [CrossRef]
- Kaczorová, D.; Karalija, E.; Dahija, S.; Bešta-Gajević, R.; Parić, A.; Ćavar Zeljković, S. Influence of extraction solvent on the phenolic profile and bioactivity of two achillea species. Molecules 2021, 26, 1601. [Google Scholar] [CrossRef]
- Zargoosh, Z.; Ghavam, M.; Bacchetta, G.; Tavili, A. Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Sci. Rep. 2019, 9, 16021. [Google Scholar] [CrossRef]
- da Torres, D.S.; Pereira, E.C.V.; Sampaio, P.A.; De Souza, N.A.C.; Ferraz, C.A.A.; De Oliveira, A.P.; Moura, C.A.; Almeida, J.R.G.S.; Rolim-Neto, P.J.; De Oliveira-Júnior, R.G.; et al. Influence of extraction process on flavonoid content from Cnidoscolus quercifolius Pohl (euphorbiaceae) and antioxidant activity. Quim. Nova 2018, 41, 743–747. [Google Scholar] [CrossRef]
- Eduardo, L.G.; Ashley, S.H.G.; Maribel, C.F.; María, G.N.P.; Adolfo, P.M.; Angel, A.V.H. Anti-inflammatory effect of chaya extracts fractions. J. Med. Plants Res. 2023, 17, 218–224. [Google Scholar] [CrossRef]
- Patel, D.; Shukla, S.; Gupta, S. Apigenin and cancer chemoprevention: Progress, potential and promise. Int. J. Oncol. 2007, 30, 233–245. [Google Scholar]
- Pan, X.; Shao, Y.; Wang, F.; Cai, Z.; Liu, S.; Xi, J.; He, R.; Zhao, Y.; Zhuang, R. Protective effect of apigenin magnesium complex on H2O2-induced oxidative stress and inflammatory responses in rat hepatic stellate cells. Pharm. Biol. 2020, 58, 553–560. [Google Scholar] [CrossRef]
- Damasceno, S.S.; Dantas, B.B.; Ribeiro-Filho, J.; Antônio, M.; Araújo, D.; Galberto, M.; da Costa, J. Chemical Properties of Caffeic and Ferulic Acids in Biological System: Implications in Cancer Therapy. A Review. Curr. Pharm. Des. 2017, 23, 3015–3023. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Luo, Q.; Wen, X.; Xiao, M.; Mei, Q. Antioxidant and anti-diabetic effects of caffeic acid in a rat model of diabetes. Trop. J. Pharm. Res. 2020, 19, 1227–1232. [Google Scholar] [CrossRef]
- Rychlicka, M.; Rot, A.; Gliszczyńska, A. Biological properties, health benefits and enzymatic modifications of dietary methoxylated derivatives of cinnamic acid. Foods 2021, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Elbatreek, M.H.; Mahdi, I.; Ouchari, W.; Mahmoud, M.F.; Sobeh, M. Current advances on the therapeutic potential of pinocembrin: An updated review. Biomed. Pharm. 2023, 157, 114032. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.P.C.; Silva, D.M.D.L.; Assis, C.F.; Correia, R.T.P.; Damasceno, K.S.F.D.S.C. Bioactive properties of faveleira (Cnidoscolus quercifolius) seeds, oil and press cake obtained during oilseed processing. PLoS ONE 2017, 12, e8. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Stability of total phenolic concentration and antioxidant capacity of extracts from pomegranate co-products subjected to in vitro digestion. BMC Complement Altern. Med. 2016, 16, 358. [Google Scholar] [CrossRef] [PubMed]
- Furdak, P.; Bartosz, G.; Sadowska-Bartosz, I. Which Constituents Determine the Antioxidant Activity and Cytotoxicity of Garlic? Role of Organosulfur Compounds and Phenolics. Int. J. Mol. Sci. 2024, 25, 8391. [Google Scholar] [CrossRef]
- Rêgo, M.S.A.; Silva, V.C.L.; Maia, C.S.; Teixeira, M.N.; Marinho, M.L.; Lima, E.R. Evaluation of the safety of the hydroalcoholic extract of aerial parts of Plectranthus neochilus schlechter, lamiaceae and Cnidoscolus quercifolius pohl, eupharbiaceae in rodents. Med. Vet. 2018, 12, 82–92. [Google Scholar] [CrossRef]
- Ribeiro, P.P.C.; Sousa, F.C.; Assis, C.F.; Veras, B.O.; Araújo, C.E.P.; Stamford, T.C.M.; Damasceno, K.S.F.S.C. Phenolic profiles of faveleira (Cnidoscolus quercifolius Pohl) seed and press cake extracts: Potential for a new trend in functional food. Braz. J. Food Technol. 2020, 23, e2019315. [Google Scholar] [CrossRef]
- Paredes, P.F.M.; Vasconcelos, F.R.; Paim, R.T.T.; Marques, M.M.M.; Morais, S.M.; Lira, S.M.; Braquehais, I.D.; Vieira, Í.G.P.; Mendes, F.N.P.; Guedes, M.I.F. Screening of Bioactivities and Toxicity of Cnidoscolus quercifolius Pohl. Evid.-Based Compl. Alt. Med. 2016, 2016, 7930563. [Google Scholar] [CrossRef]
- Silva, A.W.; Kueirislene, M.; Ferreira, A.; Rebouças, E.L.; Rogenio, F.; Mendes, S.; Marinho, M.M.; Marinho, E.S.; Magno, A.; Teixeira, R. Anxiolytic-Like Effect of Natural Product 2-hydroxy-3,4,6-trimethoxyacetophenone Isolated from Croton Anisodontus in Adult Zebrash via Serotonergic Neuromodulation Involvement of the 5-ht System. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 2023–2032. [Google Scholar] [CrossRef]
- Öztürk, Y.; Aydin, S.; Beis, R.; Başer, K.H.C.; Berberoǧlu, H. Effects of Hypericum perforatum L. and Hypericum calycinum L. extracts on the central nervous system in mice. Phytomedicine 1996, 3, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Lemos, C.G.; Vale, J.P.C.; Ferreira, M.K.A.; Silva, A.W.; Menezes, J.E.S.A.; Santos, H.S. Avaliação da atividade locomotora e teste de toxicidade do eugenol utilizando zebrafish (Danio rerio) adulto. Ambiente Gestão Desenvolv. 2021, 1. [Google Scholar] [CrossRef]
- Diniz, T.C.; Souza Araújo, C.; Oliveira Junior, R.G.; Raquel, S. Phytochemical screening and central nervous system effects of ethanolic extract of Annona vepretorum (Annonaceae) in mice. J. Med. Plants Res. 2013, 7, 2729–2735.e37. [Google Scholar]
- Gupta, P.; Khobragade, S.; Rajaram, S.; Shingatgeri, V. Assessment of locomotion behavior in adult Zebrafish after acute exposure to different pharmacological reference compounds. Drug Develop. Ther. 2014, 5, 127.e2. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Rokonuzzman, M.; Hossain, M.I.; Ansari, S.A.; Ansari, I.A.; Islam, T.; Al Hasan, M.S.; Mubarak, M.S.; Islam, M.T. Anxiolytic-like Effects by trans-Ferulic Acid Possibly Occur through GABAergic Interaction Pathways. Pharmaceuticals 2023, 16, 1271. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Bhat, Z.A. Apigenin 7-glucoside from Stachys tibetica Vatke and its anxiolytic effect in rats. Phytomedicine 2014, 21, 1010–1014. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaokun, O.O. The Beneficial Role of Apigenin against Cognitive and Neurobehavioural Dysfunction: A Systematic Review of Preclinical Investigations. Biomedicines 2024, 12, 178. [Google Scholar] [CrossRef]
- Adebiyi, O.A.; Adebiyi, O.O.; Adu, T. An appraisal of the interactions between hydroalcholic leaf extract of Cnidoscolous acontifolius and benzodiazepine-GABAA receptor complex. Int. J. Pharma. Biol. Arch. 2012, 3, 4. [Google Scholar]
- Galdino, P.M. Avaliação da Atividade Tipo Antidepressiva do Óleo Essencial das Folhas de Spiranthera odoratissima A. St.-Hil. e de Seu Componente Majoritário, β-cariofileno. Master’s Thesis, Universidade Federal de Goiás, Goiânia, Brazil, 2011. [Google Scholar]
- Lin, Y.S.; Peng, W.H.; Shih, M.F.; Cherng, J.Y. Anxiolytic effect of an extract of Salvia miltiorrhiza Bunge (Danshen) in mice. J. Ethnopharmacol. 2021, 264, 113285. [Google Scholar] [CrossRef]
| Compound | LD (mg/mL) | LQ (mg/mL) | EAECq | EMCq | ||
|---|---|---|---|---|---|---|
| mg/g | % | mg/g | % | |||
| Caffeic acid | 0.0001 | 0.0003 | 0.2122 ± 0.05513 | 0.02122 | nd | nd |
| p-Coumaric acid | 0.0007 | 0.0025 | 0.0506 ± 0.0153 | 0.00506 | nd | nd |
| Ferulic acid | 0.0048 | 0.0162 | nd | nd | nd | nd |
| Cinnamic acid | 0.0009 | 0.0032 | 0.3898 ± 0.05011 | 0.0398 | nd | nd |
| Naringenin | 0.0011 | 0.0036 | nd | nd | nd | nd |
| Pinocembrin | 0.0030 | 0.0102 | 1.5553 ± 0.05658 | 0.15553 | nd | nd |
| Apigenin | 0.0027 | 0.0092 | 0.3940 ± 0.042811 | 0.03940 | 0.1484 ± 0.000057 | 0.01484 |
| Total | 2.6019 | 0.26019 | 0.1484 | 0.01484 | ||
| Sample | Mortality | 96 h LD50 (mg/kg)/IV | |||
|---|---|---|---|---|---|
| NC | D1 | D2 | D3 | ||
| EAECq | 0 | 0 | 1 | 0 | >400 |
| EMCq | 0 | 0 | 0 | 0 | >400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Nascimento, J.B.; da Silva Mendes, J.W.; Viturino, J.J.F.; Inácio da Silva, M.; Pereira da Silva, M.; Duarte Leite, D.O.; Marinho, E.S.; de Menezes, J.E.S.A.; Santos, H.S.d.; da Costa, J.G.M. Antioxidant Activity and Anxiolytic Effect of Cnidoscolus quercifolius Pohl Stem Bark Extract in Zebrafish. Future Pharmacol. 2025, 5, 16. https://doi.org/10.3390/futurepharmacol5020016
do Nascimento JB, da Silva Mendes JW, Viturino JJF, Inácio da Silva M, Pereira da Silva M, Duarte Leite DO, Marinho ES, de Menezes JESA, Santos HSd, da Costa JGM. Antioxidant Activity and Anxiolytic Effect of Cnidoscolus quercifolius Pohl Stem Bark Extract in Zebrafish. Future Pharmacology. 2025; 5(2):16. https://doi.org/10.3390/futurepharmacol5020016
Chicago/Turabian Styledo Nascimento, Joice Barbosa, Johnatan Wellisson da Silva Mendes, José Jonas Ferreira Viturino, Maria Inácio da Silva, Mariana Pereira da Silva, Débora Odília Duarte Leite, Emmanuel Silva Marinho, Jane Eire Silva Alencar de Menezes, Hélcio Silva dos Santos, and José Galberto Martins da Costa. 2025. "Antioxidant Activity and Anxiolytic Effect of Cnidoscolus quercifolius Pohl Stem Bark Extract in Zebrafish" Future Pharmacology 5, no. 2: 16. https://doi.org/10.3390/futurepharmacol5020016
APA Styledo Nascimento, J. B., da Silva Mendes, J. W., Viturino, J. J. F., Inácio da Silva, M., Pereira da Silva, M., Duarte Leite, D. O., Marinho, E. S., de Menezes, J. E. S. A., Santos, H. S. d., & da Costa, J. G. M. (2025). Antioxidant Activity and Anxiolytic Effect of Cnidoscolus quercifolius Pohl Stem Bark Extract in Zebrafish. Future Pharmacology, 5(2), 16. https://doi.org/10.3390/futurepharmacol5020016