Abstract
The aim of this review is to evaluate the therapeutic possibilities of trifluralin and other 2,6-dinitroaniline herbicides by assessing different aspects of trifluralin’s toxicology (including its mitochondrial toxicity), pharmacokinetics, and environmental fate. The particular features of TFL have triggered a wide range of policies about its properties. Is has been banned in some countries and, at the same time, has been proposed as a drug for the cure of parasitic disease by some scientific research articles. The use of this pre-emergence herbicide to control broadleaf weeds and annual grasses is assumed to rely only on its microtubule depolarization or cytoskeleton disassembly abilities (on-target effect), a fact that justifies its inhibition of a wide range of microorganisms (mostly protozoans), sharing a relatively high degree of conservation in tubulin protein sequences with weeds and grasses. Recent studies have confirmed that TFL also affects mitochondrial function (off-target effect), a hypothesis previously suggested in earlier works. Here, we account for the main issues in TFL toxicology, other potential uses of the herbicide outside crops, and its feasibility for use as an antiprotozoal drug.
1. Trifluralin, the Molecule
Trifluralin (TFL) is also known as 2,6-dinitro-N, N-di-n-propyl-a, a, a-trifluoro-p-toluidine, or its IUPAC name aaa-trifluoro-2,6-dinitro-N,N-dipropyl-p-toluidine, CAS number (CAS#) 1582-09-8. Its commercial names vary from one country to another, with the most popular in Western countries being the herbicide sold under the tradename of Treflan (DowElanco). Others are Olitref (Chemol), Tri-4 (Cyanamid), Triflurex (Makhteshim-Agan), Trigard (FCC), Triplen (Sipcam), Tristar (Pan Britanica), and Zeltoxone (Zeneca). Trifluralin is also commercialized in mixtures such as [Trifluralin +] linuron; napropamide; metribuzin; clomazone; tebutam; napropamide: boromoxinil + ioxynil; isoproturon; terbutryn; linuron + trietazine; linuron + neburon; linuron + clomazone; and isoxaben [1].
TFL belongs to a 2. 6-dinitroaniline herbicide class, some of the other well-known members of which include pendimethalin (CAS# 40487-42-1), oryzalyn (CAS# 19044-88-3), ethalfluralin (CAS# 55283-68-6), dinitramine (CAS# 29091-05-2), and prodiamine (CAS# 29091-21-2), among others (Figure 1, Table 1).
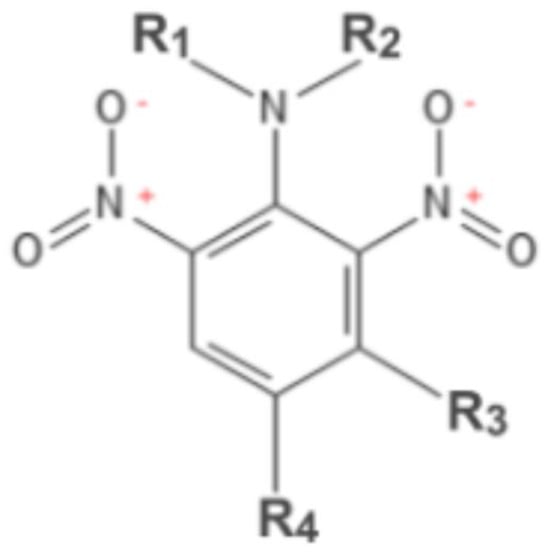
Figure 1.
2,6 dinitroanilines.

Table 1.
Representative 2,6 dinitroaniline herbicides.
Trifluralin’s commercial synthesis, as well as that of other dinitroaniline herbicides, uses 4-Chloro-3,5-dinitrobenzotrifluoride (chloralin) [2,3] as a main intermediate (Figure 2), a compound that has been shown to be hematotoxic [4,5].
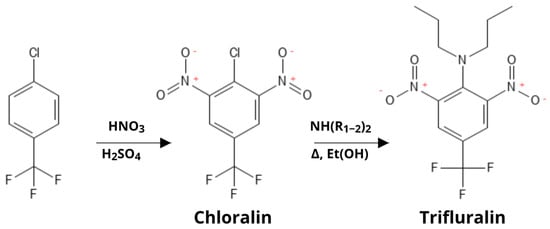
Figure 2.
Synthesis of Trifluralin.
Like other dinitroaniline herbicides, TFL belongs to group 3 herbicides, which are known to inhibit microtubule assembly [6]. Heavily used herbicides like TFL, pendimethalin, and oryzalin disrupt mitosis by the inhibition of spindle microtubules during prometaphase–metaphase transition [7].
TFL is applied to soil after planting and prior to the emergence of plants, which means that this compound is used as a pre-emergence herbicide and soil incorporation markedly enhances its activity. TFL does not directly inhibit germination, but the inhibition of lateral root development and swelling of the root tips are universally recognized morphological effects of TFL. Its main action is exerted towards organs that have plenty of rapidly dividing mitotic cells such as roots and shoots, where active meristems are located. Injury to the top of the shoots is recognized by sudden darkness pigmentation, stunting, and tumescence of the hypocotyl. The results in those affected organs are quite pronounced and lead to the plant’s death, mainly when the young shoot has been exposed to TFL [7,8,9].
2. The Inhibitor: Molecular Mechanisms
The specificity of Trifluralin as a microtubule inhibitor for plants is based on the lack of homology between plants (or plant relative microorganisms) and metazoans. The early studies that allowed TFL to become a worldwide approved herbicide were focused only on its general toxicological aspects, and none of them measured TFL as a microtubule inhibitor [10,11,12,13]. Some of these studies were carried out by the same company that produced TFL [10,11], which always raises suspicions. Other earlier works on TFL showed that plant microtubules stabilized with Taxol (paclitaxel, a microtubule-stabilizing drug) rapidly depolymerized when a dinitroaniline was added. On the contrary, animal microtubules treated with Taxol did not break up upon dinitroaniline exposure, evidencing dinitroaniline specificity towards plant tubulines (at least in the presence of Taxol) [14]. An interesting conundrum about these experiments is that Taxol is believed to interact with β-tubulin, whereas dinitroanilines are claimed to bind α-tubulin. Indeed, some years after those seminal experiments, molecular models for dinitroaniline interactions with plant α-tubulin pointed out a possible binding site for these inhibitors in the molecule. Furthermore, when analyzing plant-related parasites that display resistance to oryzalin due to mutations in the α-tubulin gene, some interesting 3D models for possible docking sites of the drug were generated [15]. Nevertheless, since most α-tubulin mutations affected the “core” of the molecule, several resistance mutations were located in the M or N loops (tubulin regions that mediate the lateral adhesion of protofilaments) [16,17]. These findings fit with previously mentioned data that show dinitroanilines depolymerizing microtubules fixed with Taxol [14], because, in this model, dinitroaniline inhibitors disrupt microtubules by destabilizing protofilaments instead of α-β-Tubulin dimers. Nevertheless, it was partially concluded that these mutations did not directly define a binding site for dinitroanilines, but also based on designed flexible docking simulations, a likely binding site for dinitroanilines beneath the α-tubulin N loop was suggested [18]. Another work using the analysis of surface electrostatic potential also suggested that dinitroanilines bind α-tubulin [19]. This work postulated a docking site for dinitroanilines in between the dimer interface. Thus, both predicted binding sites were different. A third work also predicted docking sites for dinitroanilines on α-tubulin based on software simulations of tubulins from different species [20]. Time and the profuse use of 2,6 dinitroanilines in different countries provide these speculations with the possibility to happen in nature, specifically in plants. Thus, more recent studies have, indeed, shown that α-tubulin mutations in weeds, particularly involving the residues Arg-243-Met (arginine to methionine in position 243) and Val-202-Phe (valine to phenylalanine in position 202), confer resistance to dinitroaniline herbicides (and TFL among them). Interestingly, resistance mutations conferred with Val-202-Phe are accompanied by an enhanced metabolism [21], an off-target effect that is linked to the cytochrome P450 gene and may be linked to a resistant mitochondria too. This unexpected effect of a resistance mutation on fitness is the opposite in Arg-243-Met α-tubulin mutation (which also confers resistance to dinitroanilines), which is nearly lethal to plants [22]. The effect of the reduced fitness of a resistance mutation is more commonly described for tubulin mutations in several organisms, among them Toxoplasma sp. [18,23]. All in all, 48 α-tubulin resistance mutations against dinitroaniline herbicides have been reported, meanwhile, β-tubulin mutations that confer resistance to these herbicides, are to date, only 2 (Supplementary Material, retrieved from the tubulin mutation database: “https://tubulinmutations.bio.uci.edu/ (accessed on 22 January 2025)”) [24]. Interestingly, an early paper in 1991 described how two β-tubulin mutations in Chlamydomonas reinhardtii confer resistance to microtubule-disrupting drugs and dinitroaniline herbicides while enhancing Taxol-stabilizing actions on microtubules [25] (these were mutations non-specific for dinitroaniline resistance). Finally, although important evidence pointing towards a direct interaction of TFL with plant/protozoan α-tubulin is accumulating over time, an important caveat of most of these works is the lack of a real crystallography study for dinitroaniline binding in plants or in plant-related protozoans tubulins/microtubules. Nevertheless, when the most important mutations conferring resistance to 2,6 dinitroanilines are mapped in a 3D model of α-tubulin/β-tubulin/Darpin D1 proteins, we can see that all of them localize into a specific region of the α-tubulin protein (Figure 3a). Interestingly, all of them cluster in a restricted space, delimiting an area or region that is highly likely to contain the 2,6 dinitroanilines binding site (zoomed for details on inset Figure 3b).
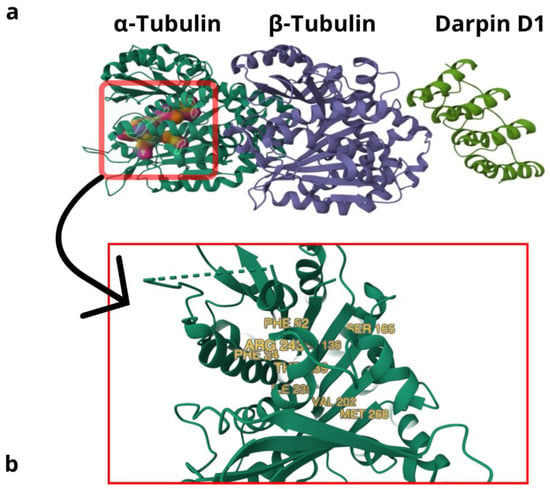
Figure 3.
The 2,6 Dinitroaniline herbicide-resistant mutations mapped in a 3D molecular model of α/βTubulin/Darpin D1 proteins from Tetrahymena thermophila. (a) Three-dimensional model of proteins α-tubulin, β-tubulin, and Darpin D, where most important resistance mutations are clustered in a specific region of α-tubulin. (b) Inset showing a magnification of the area, where you can observe main mutation sites conferring resistance to dinitroaniline herbicides: PHE 24 and PHE52 (T. gondii), LEU 136 (plants: A. aequalis and S. viridis; protists: T. thermophila and T. gondii), SER 165 (T. gondii), VAL 202 (plants: A. aequalis and L. rigidum), ILE 235 (T. gondii), THR 239 (plants: E. indica, S. viridis and L. rigidum; protists: T. gondii), ARG 243 (L. rigidum and T. gondii), and MET 268 (E. indica and T. gondii).
3. Toxicology
A proper introduction to TFL’s toxicological aspects includes a general overview of its metabolism in mammals. Radiocarbonated TFL administered at doses of 1 and 10 mg/kg to rats resulted in the identification of seven metabolites in urine and six in feces, as thoroughly described by Erkog et al. [26]. The main metabolic routes of TFL are N-dealkylation and nitro reduction. Other important ones are hydroxylation, conjugation, and cyclization. This last reaction produces a considerable amount of benzimidazoles, which are also microtubule inhibitors, and its anti-cancer properties have been subject to several appraisals [27,28].
As already stated in the introductory section about the compound, the first studies on TFL’s metabolism came from the same laboratories that manufacture it [10,11]. In the first study, performed by Ely Lilly & Co (Greenfield, IN 46140, USA -nowadays Corteva/DowElanco/DowDuPont-), TFL’s metabolism was studied in rats and dogs. In these studies, they identified four compounds that resulted from an alternative process of N-dealkylation (over one or both alkyl residues) and nitro reduction, predominantly on one nitro group. They found that 100% of the radioactive labeled compound (on the trifluoromethyl carbon) was excreted (78% fecal excretion and 22% urinary) in rats, and the main fecal metabolite only differed from TFL in having one amino instead of a nitro group (nitro reduction). On the other hand, when they marked the N-propyl carbon, they found higher N-dealkylation by a consistent 19% of the radioactivity in expired air (26% in urine and 43% in feces) and the rest probably as part of circulating metabolites of the body. Nevertheless, they concluded that an oral dose of 100 mg/kg was rapidly and completely eliminated by the rat [10]. They performed some complementary experiments on TFL’s solubility in lipids and found that TFL caused orange-colored fat tissue when it was subcutaneously or intraperitoneally administered. They found, thus, that the liposolubility of TFL is a constraint for any administration route other than oral. They claimed to obtain the same results in dogs as those they did for rats.
In the second paper, which is just a personal letter, the writer stated that TFL was not hazardous for vertebrates other than fish (which would increase the issue of possible ecotoxicity). The main arguments against the ecotoxicity possibility for TFL are its non-leaching and its binding to soil properties [11]. With the same concern about freshwater species toxicity, later studies showed that TFL impairs the normal development of amphibians (T. helveticus and P. waltlii) by inhibiting normal cytokinesis and producing chromosome abnormalities [12]. Low TFL concentrations severely harm the gills of freshwater fish [29].
The effects of TFL on mouse liver xenobiotic-metabolizing enzymes (P-450-dependent mono–oxygenases, epoxide hydrolases, and glutathione S-transferases) and carnitine acetyltransferase challenge the vision that TFL is inertly transient in mammalian systems. TFL at a dose of 250 mg/kg injected intraperitoneally over three days was shown to increase the microsomal liver protein fraction, associated with an increase in cytochrome P-450 content and significant increase in aminopyrine N-demethylase activity. At the same, doses of TFL showed a significant increase in cytosolic and microsomal glutathione S-transferases and microsomal-like epoxide hydrolase. A non-significant decrease in carnitine acetyltransferase was also noted at the same dose [30]. It should be noted that, although this high acute dosage did not trigger extreme toxic effects, the chronic effect when exposed to this dose was not evaluated. Among the possible metabolic dysregulation effects caused by dinitronailine herbicides, a recent paper showed in a Wuhan–Zhuhai cohort of patients a positive association with dinitroaniline herbicide blood levels (particularly TFL and pendimethalin) and increased fasting plasma glucose (which, in turn, is correlated with pre-diabetic glucose dysregulation) [31].
A study performed by one of the laboratories involved in the manufacturing of TFL (Hoechst) came in 1992 [32]. This work employed more animals than any other study and different species (rats, mice, dogs, rabbits, and Chinese hamsters) to measure mutagenicity and acute, sub-chronic oral, and chronic and reproductive toxicity. The main findings for sub-chronic oral toxicity was hematoxicity. This consisted of a slight anemia with a decreased red blood cell count, hemoglobin content, and hematocrit value, increased reticulocyte counts (dogs and rats), and the formation of methaemoglobin and Heinz bodies (dogs and mice) for doses ranging from 75 mg/kg body weight/day (dogs) to 485 mg/kg body weight/day (mice). These differences among species were likely due to the different metabolism and alimentary habits of these species, and probably a carnivore like the dog is more sensitive to chemicals due to, evolutionarily, being less exposed to their consumption from vegetables. The LD50 (acute toxicity) for rodents ranged from 1930 to 5000 mg/kg body weight, with LDL0 (Lowest Lethal Dose) ranging from 1600 to 5000 mg/kg body weight; meanwhile, for dogs, both parameters were >10,000 mg/kg body weight. The no-observed-effects levels (NOELs) were 2000 ppm for rats, 400 ppm for mice, and less than 400 ppm for dogs. The sub-chronic toxic effects observed for a dose lower than 200 mg/kg body weight/day were related to an increase in liver weight, meanwhile, chronic effects were observed at doses higher than 800 ppm (which resulted in a marked increase in liver weight and hepatocellular hypertrophy). The developmental toxic dose in rabbits was near to a high sublethal dose. In rats, delayed fetal development and malformations, as well as maternal toxicity, were found at the highest dose (500 mg/kg body weight/day). A dose of 100 mg/kg body weight produced no signs of overt maternal toxicity, but led to the death of all embryos shortly after implantation in one dam and an increase in the number of fetuses with thickened, wavy, or bent ribs. Based on this, the maternal NOEL was 100 mg/kg body weight/day and NOEL for developmental toxicity was 20 mg/kg body weight/day. These numbers were just partially accepted by another study that agreed with a dose of 100 mg/kg body weight/day for maternal NOEL, but disagreed about NOEL for developmental toxicity, considering this to be 100 mg/kg body weight/day rather than 20 mg/kg body weight/day [33]. The authors pointed out that these differences are the result of the use of a different vehicle for oral administration (which is a weak argument in support of such differences).
Cardiotoxic effects of TFL have been proposed based on high creatinine kinase serum levels [34], and concerns about cardiotoxicity are supported by a recent systematic review that determined that exposure to TFL (among other pesticides) is associated with an increased risk for acute myocardial infarction [35]. Since these results are not conclusive, they will be properly discussed in the following sections, with a special emphasis on TFL as a prospective drug.
Increasingly important in recent years has been the off-target toxicity of dinitroaniline herbicides. One reported mode of action of Ethalfluralin is an impairment in mitochondrial membrane potential (ΔΨm) as a putative result of the down-regulation of mitochondrial respiratory complex-related genes, with a consequent decrease in mitochondrial respiration [36]. The same research group showed that pendimethalin also affects mitochondria by reducing the mitochondrial membrane potential with a consequent rise in cytosolic calcium and ER stress [37]. Particularly interesting is that early reports on the off-target effects of 2,6 dinitronilines pointed out mitochondria as one of the main affected intracellular organelles. As measured in plant tissues [38] and on isolated mitochondria [39], decreased Oxidative Phosphorylation (OXPHOS) was a result of the inhibition of multiple electron transport complexes, putatively by partitioning of the herbicide into the inner mitochondrial membrane [40]. In this line, passive calcium efflux from mitochondria as a result of increased membrane permeability has been reported as an effect of TFL in plants and in animals [41,42]. This membrane permeability is associated with a decreased mitochondrial membrane potential (ΔΨm) in immune cells exposed to Pendimethalin [43]. Another work also reported that TFL impairs OXPHOS [44]. Also noteworthy, a recent study linked TFL with Parkinson’s disease and its relevant mitochondrial dysfunction [45]. The mitochondrial effects of TFL (off-target) and the cytoskeletal/microtubular effects (on-target) are illustrated in Figure 4.
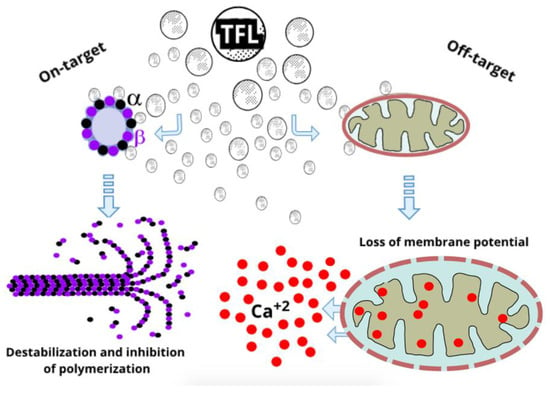
Figure 4.
On-target and off-target effects of Trifluralin. On the left the microtubule disruptive activities of TFL mostly by interaction with α-tubulin subunits. On the right, the effects on mitochondrial membrane potential with the consequent dysregulation of cytosolic calcium (Ca+2) by mitochondrial leakage.
4. Genotoxicity and Oncogenicity
Aside from an early report appearing in 1982 [46] that detected mutagenic effects on mice germ cells treated with a TFL-containing pesticide (Olitref, 26%TFL), the first studies in this area that assessed the effects of pure TFL also came from the laboratories that elaborated it [47,48]. In these reports, they focused on quantitative aspects rather than on a qualitative evaluation of the hazardous effects or on the molecular mechanism. They used the Ames test (that measures the mutagenicity of a substance by its ability to revert auxotrophic mutations in bacteria), which does not assess any aspect related to the action of the herbicide in eukaryotic cells (i.e., mainly anti-microtubule activity during spindle assembly in mitosis/meiosis). They also did not measure it in germ cells. In the sister-chromatid exchange test (SCE) that they performed on Chinese hamsters, TFL was shown to decrease the sister-chromatid exchange ratio. In a mouse lymphoma forward mutation assay, TFL duplicated the mutation frequency of controls at a dose of 15 µg/mL. Nevertheless, they concluded that TFL does not pose a genotoxic hazard to humans and is not carcinogenic in the B6C3F1 mouse model. Some years later, a group studying genotoxicity on human lymphocytes concluded that TFL is able to exert weak cytotoxic and genotoxic effects in cultured human lymphocytes [49]. Some of the authors showed in a later paper that TFL has positive genotoxic effects in a wing spot test of Drosophila melanogaster, results that were reproducible in two different strains [50]. Some other interesting genotoxic evidence (increase in micronuclei formation) in non-mammals has shown that both Treflan® and its active ingredient TFL enhance and increase chromosomal aberrations. These studies show that TFL significantly increases the number of micronuclei events in the peripheral blood erythrocytes of the Nile Tilapia Fish (O. niloticus) at low doses of the herbicide (1 µg/L, over an exposure time of three days). The commercial formulation, Treflan®, causes the same effects at higher doses (5 and 10 µg/L) or longer exposure times (6 to 9 days) [51].
An Environmental Protection Agency (EPA) report on the carcinogenic action of pesticides includes TFL within those that induce thyroid follicular cell tumors in rodents [52]. Since the EPA assessed the relevance of measuring thyroid follicular cell tumors in rodents to determine carcinogenicity in humans, concluding that this method is appropriate [53], the EPA conducted studies to determine carcinogenicity in more than 200 pesticides. Among those 24 pesticides that cause tumors in chronic tests, TFL has been shown to produce significant tumor incidence in rats and mice. The effects of TFL do not remain circumscribed only to thyroid tumors, but also increase the incidence of other types of tumors, like urinary bladder and kidney tumors. Nevertheless, TFL’s mechanism of induction of thyroid tumors is unclear, and a related compound (pendimethalin) has been shown to increase iodide uptake in rats, which was connected to an enhancement in hepatic thyroid hormone metabolism and excretion [52]. The mechanism of induction of thyroid tumors by TFL was studied by the Dow Chemical Company [54], and they detected that a 2-week TFL treatment at a dose of ≈400 mg/kg body weight /day caused a depletion in thyroid hormones that led to thyroid hyperactivity in order to replenish T3 and T4 blood levels (and, thus, homeostasis). This thyroid hyperactivity resulted in cellular hypertrophy and cell proliferation with the development of follicular cell tumors. The authors detected higher levels of biliary T3 and T4 in TFL-treated animals than in controls (negative and positive). Even though they showed a certain degree of depletion in T3 and T4 in the sera of TFL-treated animals, which was coincident with the highest biliary levels of T3 and T4, they did not show how TFL shifted the equilibrium towards an enhanced excretion of thyroid hormones.
5. The Drug
Since the discovery of Chan and Fong that TFL kills intracellular protozoan parasites selectively, without affecting the host cell viability [55], a plethora of studies have been flooding the literature about TFL as a promising antiprotozan drug. Several 2,6 dinitroanilines have been shown to inhibit the replicative stages of protozoan parasites. The first group that proved to be sensitive to dinitroanilines was the class kinetoplastida, which includes Leishmania sp., Trypanosoma brucei, and Trypanosoma cruzi [55,56,57,58,59,60,61,62,63,64,65]. Most seminal works on the inhibition of protists by dinitronilines have been summarized elsewhere [64]. Later studies have shown that dinitroanilines are potent inhibitors of apicomplexan parasites [66,67,68,69] and related protozoans [70]. Many of these works proved the inhibitory efficacy of TFL in both in vitro and in vivo models.
Liposomal and topical formulations against Leishmania sp. could be interesting alternatives for the treatment of visceral leishmaniasis [61,62], but the presence of already mentioned toxic effects make this possibility very unlikely. Perhaps a more plausible treatment could be developed against cutaneous leishmaniasis, but more studies about transdermal penetration and related toxicology must be performed [71]. Based on the aforementioned results, why can TFL not be used for the treatment of cutaneous leishmaniasis? The answer is that the epithelium is one of the most actively dividing tissues among those of the vertebrate body. The actively dividing cells in the epidermis are a conspicuous obstacle to using a mitosis-disrupting agent as topical treatment, with the undesired consequence of a high risk of carcinogenesis. Another important trypanosomiasis is Chagas Disease. Several works have pointed out that TFL [63,65] or other dinitroaniline herbicides [64] could be used against Trypanosoma cruzi, the etiological agent of Chagas disease. In the special case of Chagas, there is a lack of safe pharmaceuticals to treat the chronic phase of the disease [72], which is when the most severe complications involving cardiac compromise arise [73]. An outstanding feature of the cardiomyopathy is myocyte hypertrophy [74], which is one of the causes of chagasic cardiomegaly. Current research about how cardiomegaly has pointed to microtubule accumulation on the cardiomyocyte cytoskeleton as the most likely cause [75,76]. Interestingly enough, microtubule inhibitor drugs used in cancer chemotherapy present relevant levels of cardiotoxicity [77], similarly displayed by TFL in a mice model [34]. The cardiotoxicity inferred for TFL was based on creatine kinase (CK) and lactate dehydrogenase (LDH) activities, both significantly higher than controls at 50 mg/kg body weight/day administered over 30 days. Between the two parameters taken into account to determine cardiotoxicity, just the creatine kinase elevated levels are regarded as a consistently realistic marker of acute myocardial infarction [78]. Anyhow, enhanced LDH activity is a marker of cell destruction that, in this context, could be used indirectly as a secondary indicator of acute myocardial infarction. The explanation for the CK elevated levels were linked to myocarditis in 33% of TFL-treated animals and could be related to a differential cardiotropic concentration of the compound. Interestingly, it was shown that TFL reached its highest concentration in heart, two-fold the concentration detected in the brain, which is rare for a lipophilic compound, but is a very desirable for a compound intended to treat a cardiac disease. Despite its already mentioned several toxic effects in mammals, TFL remained as a potential compound for the treatment of extreme chagasic heart disease cases, until cardiotoxic data showed that TFL is not even suitable for these treatments [36]. It is worth speculating that the cardiac mitochondria can be also affected as a part of the myocardial disfunction caused by TFL. In many cases, the addition of separate and previously independent risk factors, when combined, make TFL inadvisable for use in the treatment of certain protozoal diseases. Furthermore, with the specific characteristics of each disease and the requirement for its treatment, it is difficult to push a compound into pharmacology when it has been already banned as herbicide. Trifluralin was withdrawn from circulation in Sweden in 1990 due to its ability to leach and the potential risk for aquatic organisms; TFL was withdrawn from all stores in Denmark in 1997 because pesticides with a half-life longer than three months are unacceptable for registration there. TFL is not authorized in the Netherlands and was banned in Norway in 1993 [51,79,80].
Software and websites used: chemical structures in figures were partially generated in PubChem Sketcher V2.4 (https://pubchem.ncbi.nlm.nih.gov//edit3/index.html, accessed on 22 January 2025). Figure 3 was generated by using Mol*(WebGL) in RCSB PBD (https://www.rcsb.org, accessed on 22 January 2025) [81] from a protein structure of Tetrahymena thermophila (7PJF, https://doi.org/10.2210/pdb7PJF/pdb, accessed on 22 January 2025) previously crystalized and modeled [82]. Water, ions and ligands were removed from the original 3D model for better visualization of the mutation sites. Figure 2 and Figure 4 were generated by using GravitDesigner version 2020-1.3.4.1.
6. Conclusions
Trifluralin was developed in the 1960s, when the main concerns and regulations for environmental pollution and toxicology were not in vogue. The history of TFL reflects how societies become more conscientious about the dangers of the indiscriminate spreading of hazardous substances in the environment. In this sense, advances in the chemical industry, the availability of new safer herbicides, trends towards a more organic agriculture, and the genetic advances in crop selections will make TFL and its related dinitroanilines useless compounds soon or later. Here, we discussed different aspects of TFL’s toxicology, how it has been shown to exert a wide spectrum of pernicious effects on a wide range of organisms, and especially those relevant off-target effects in mammals. TFL has shown many interesting features as a selective inhibitor of plant microtubules, which also accounts for the inhibition of protozoa with homologous sequences of tubulin proteins (Plasmodium sp., Toxoplasma sp., Cryptosporidium sp., Chlamydomonas sp., Tetrahymena sp., Trypanosoma sp., and Leishmania sp. among those tested). Importantly, to this actions as a microtubule disrupter, non-selective mitochondrial inhibition could be added as an anti-protozoan property to be exploited. Regretfully, the promissory role of TFL as a potential drug has ultimately faced its downfall, since it has been shown repeatedly that its toxicity outweighs its antiprotozoan properties. Whether or not TFL will be part of a future pharmacology will depend on its selective delivery to target parasites, chemical modifications, or substitutions that reduce its toxicity and new safer formulations for specific uses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/futurepharmacol5020014/s1. Tubulin Mutations containing α and β-tubulin mutations conferring resistance to dinitroaniline herbicides was obtained from “The Tubulin Mutation Database” [24] at https://tubulinmutations.bio.uci.edu/ (accessed on the 22 January 2025).
Funding
The elaboration and publication of the present manuscript did not require specific or external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The knowledge and information given in the present work and the points of views are derived from publicly available data, information or knowledge and from the results of validated studies.
Conflicts of Interest
The author declare no conflict of interest.
References
- MacBean, C. (Ed.) The Pesticide Manual, 16th ed.; British Crop Production Council: Hampshire, UK, 2012; ISBN 9781901396867. [Google Scholar]
- Callahan, H.L.; Kelley, C.; Pereira, T.; Grogl, M. Microtubule inhibitors: Structure-activity analyses suggest rational models to identify potentially active compounds. Antimicrob. Agents Chemother. 1996, 40, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Keum, Y.-S.; Li, Q.X. Reduction of nitroaromatic pesticides with zero-valent iron. Chemosphere 2004, 54, 255–263. [Google Scholar] [CrossRef]
- Guastadisegni, C.; Hall, D.; Macrí, A. Hematotoxic effects of 3,5-dinitro-4-chloro-alpha,alpha,alpha-trifluorotoluene, a water contaminant. Ecotoxicol. Environ. Saf. 1986, 12, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Guastadisegni, C.; Mantovani, A.; Ricciardi, C.; Stazi, A.V.; Maffi, D.; Salvati, A.M. Hematotoxic effects in the rat of a toluene dinitro derivative after short-term exposure. Ecotoxicol. Environ. Saf. 1989, 17, 21–29. [Google Scholar] [CrossRef]
- Mallory-Smith, C.A.; Retzinger, E.J. Revised Classification of Herbicides by Site of Action for Weed Resistance Management Strategies. Weed Technol. 2003, 17, 605–619. [Google Scholar] [CrossRef]
- Vaughn, K.C.; Lehnen, L.P. Mitotic Disrupter Herbicides. Weed Sci. 1991, 39, 450–457. [Google Scholar] [CrossRef]
- Parka, S.J.; Soper, O.F. The Physiology and Mode of Action of the Dinitroaniline Herbicides. Weed Sci. 1977, 25, 79–87. [Google Scholar] [CrossRef]
- Appleby, A.P.; Valverde, B.E. Behavior of Dinitroaniline Herbicides in Plants. Weed Technol. 1989, 3, 198–206. [Google Scholar] [CrossRef]
- Emmerson, J.L.; Anderson, R.C. Metabolism of trifluralin in the rat and dog. Toxicol. Appl. Pharmacol. 1966, 9, 84–97. [Google Scholar] [CrossRef]
- Worth, H.M. The toxicologic evaluation of benefin and trifluralin. IMS Ind. Med. Surg. 1968, 37, 545. [Google Scholar]
- Sentein, P. Trifluralin, an inhibitor of the achromatic apparatus which modifies the chromosomes. Arch. D’anatomie Microsc. Morphol. Exp. 1977, 66, 263–277. [Google Scholar]
- Bioassay of trifluralin for possible carcinogenicity. Natl. Cancer Inst. Carcinog. Tech. Rep. Ser. 1978, 34, 1–96.
- Morejohn, L.C.; Bureau, T.E.; Molè-Bajer, J.; Bajer, A.S.; Fosket, D.E. Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro. Planta 1987, 172, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Ortiz, R.; Dominguez, L. Unveiling the Possible Oryzalin-Binding Site in the α-Tubulin of Toxoplasma gondii. ACS Omega 2022, 7, 18434–18442. [Google Scholar] [CrossRef] [PubMed]
- Downing, K.H.; Nogales, E. Tubulin and microtubule structure. Curr. Opin. Cell Biol. 1998, 10, 16–22. [Google Scholar] [CrossRef]
- Nogales, E.; Wolf, S.G.; Downing, K.H. Structure of the alpha beta tubulin dimer by electron crystallography. Nature 1998, 391, 199–203. [Google Scholar] [CrossRef]
- Morrissette, N.S.; Mitra, A.; Sept, D.; Sibley, L.D. Dinitroanilines bind alpha-tubulin to disrupt microtubules. Mol. Biol. Cell 2004, 15, 1960–1968. [Google Scholar] [CrossRef]
- Blume, Y.B.; Nyporko, A.Y.; Yemets, A.I.; Baird, W. V Structural modeling of the interaction of plant alpha-tubulin with dinitroaniline and phosphoroamidate herbicides. Cell Biol. Int. 2003, 27, 171–174. [Google Scholar] [CrossRef]
- Mitra, A.; Sept, D. Binding and interaction of dinitroanilines with apicomplexan and kinetoplastid alpha-tubulin. J. Med. Chem. 2006, 49, 5226–5231. [Google Scholar] [CrossRef]
- Chen, J.; Chu, Z.; Han, H.; Goggin, D.E.; Yu, Q.; Sayer, C.; Powles, S.B. A Val-202-Phe α-tubulin mutation and enhanced metabolism confer dinitroaniline resistance in a single Lolium rigidum population. Pest Manag. Sci. 2020, 76, 645–652. [Google Scholar] [CrossRef]
- Wang, Y.; Han, H.; Chen, J.; Yu, Q.; Vila-Aiub, M.; Beckie, H.J.; Powles, S.B. A dinitroaniline herbicide resistance mutation can be nearly lethal to plants. Pest Manag. Sci. 2022, 78, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, C.; Ganesan, L.; Oak, J.; Tsai, S.; Sept, D.; Morrissette, N.S. Mutations in alpha-tubulin confer dinitroaniline resistance at a cost to microtubule function. Mol. Biol. Cell 2007, 18, 4711–4720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pham, C.L.; Morrissette, N.S. The tubulin mutation database: A resource for the cytoskeleton community. Cytoskeleton 2019, 76, 186–191. [Google Scholar] [CrossRef]
- Schibler, M.J.; Huang, B. The colR4 and colR15 beta-tubulin mutations in Chlamydomonas reinhardtii confer altered sensitivities to microtubule inhibitors and herbicides by enhancing microtubule stability. J. Cell Biol. 1991, 113, 605–614. [Google Scholar] [CrossRef]
- Erkog, F.U.; Menzer, R.E. Metabolism of trifluralin in rats. J. Agric. Food Chem. 1985, 33, 1061–1070. [Google Scholar] [CrossRef]
- Pourgholami, M.H.; Wangoo, K.T.; Morris, D.L. Albendazole-cyclodextrin complex: Enhanced cytotoxicity in ovarian cancer cells. Anticancer Res. 2008, 28, 2775–2779. [Google Scholar] [PubMed]
- Pourgholami, M.H.; Woon, L.; Almajd, R.; Akhter, J.; Bowery, P.; Morris, D.L. In vitro and in vivo suppression of growth of hepatocellular carcinoma cells by albendazole. Cancer Lett. 2001, 165, 43–49. [Google Scholar] [CrossRef]
- Poleksić, V.; Karan, V. Effects of trifluralin on carp: Biochemical and histological evaluation. Ecotoxicol. Environ. Saf. 1999, 43, 213–221. [Google Scholar] [CrossRef]
- Moody, D.E.; Narloch, B.A.; Shull, L.R.; Hammock, B.D. The effect of structurally divergent herbicides on mouse liver xenobiotic-metabolizing enzymes (P-450-dependent mono-oxygenases, epoxide hydrolases and glutathione S-transferases) and carnitine acetyltransferase. Toxicol. Lett. 1991, 59, 175–185. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, X.; Tan, Q.; Liang, R.; Chen, B.; Yu, L.; Wang, M.; Qing, M.; Yang, S.; Li, Y.; et al. Associations of dinitroaniline herbicide exposure, genetic susceptibility, and lifestyle with glucose dysregulation: A gene-environment interaction study from the Wuhan-Zhuhai cohort. Environ. Res. 2024, 262, 119938. [Google Scholar] [CrossRef]
- Ebert, E.; Leist, K.H.; Hack, R.; Ehling, G. Toxicology and hazard potential of trifluralin. Food Chem. Toxicol. 1992, 30, 1031–1044. [Google Scholar] [CrossRef]
- Byrd, R.A.; Markham, J.K.; Emmerson, J.L. Developmental toxicity of dinitroaniline herbicides in rats and rabbits. I. Trifluralin. Fundam. Appl. Toxicol. Off. J. Soc. Toxicol. 1995, 26, 181–190. [Google Scholar] [CrossRef]
- Zaidenberg, A.; Marra, C.; Luong, T.; Gómez, P.; Milani, L.; Villagra, S.; Drut, R. Trifluralin toxicity in a Chagas disease mouse model. Basic Clin. Pharmacol. Toxicol. 2007, 101, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Zago, A.M.; Faria, N.M.X.; Fávero, J.L.; Meucci, R.D.; Woskie, S.; Fassa, A.G. Pesticide exposure and risk of cardiovascular disease: A systematic review. Glob. Public Health 2022, 17, 3944–3966. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.; Lim, W.; Song, G. Ethalfluralin impairs implantation by aggravation of mitochondrial viability and function during early pregnancy. Environ. Pollut. 2022, 307, 119495. [Google Scholar] [CrossRef]
- Ham, J.; Lim, W.; Song, G. Pendimethalin induces apoptosis in testicular cells via hampering ER-mitochondrial function and autophagy. Environ. Pollut. 2021, 278, 116835. [Google Scholar] [CrossRef]
- Moreland, D.E.; Farmer, F.S.; Hussey, G.G. Inhibition of photosynthesis and respiration by substituted 2,6-dinitroaniline herbicides: II. Effects on responses in excised plant tissues and treated seedlings. Pestic. Biochem. Physiol. 1972, 2, 354–363. [Google Scholar] [CrossRef]
- Moreland, D.E.; Farmer, F.S.; Hussey, G.G. Inhibition of photosynthesis and respiration by substituted 2,6-dinitroaniline herbicides: I. Effects on chloroplast and mitochondrial activities. Pestic. Biochem. Physiol. 1972, 2, 342–353. [Google Scholar] [CrossRef]
- Moreland, D.E.; Huber, S.C. Inhibition of photosynthesis and respiration by substituted 2,6-dinitroaniline herbicides: III. Effects on electron transport and membrane properties of isolated mung bean mitochrondria. Pestic. Biochem. Physiol. 1979, 11, 247–257. [Google Scholar] [CrossRef]
- Hertel, C.; Quader, H.; Robinson, D.G.; Marmé, D. Anti-microtubular herbicides and fungicides affect Ca(2+) transport in plant mitochondria. Planta 1980, 149, 336–340. [Google Scholar] [CrossRef]
- Hertel, C.; Quader, H.; Robinson, D.G.; Roos, I.; Carafoli, E.; Marmé, D. Herbicides and fungicides stimulate Ca2+ efflux from rat liver mitochondria. FEBS Lett. 1981, 127, 37–39. [Google Scholar] [CrossRef]
- Ansari, S.M.; Saquib, Q.; Attia, S.M.; Abdel-Salam, E.M.; Alwathnani, H.A.; Faisal, M.; Alatar, A.A.; Al-Khedhairy, A.A.; Musarrat, J. Pendimethalin induces oxidative stress, DNA damage, and mitochondrial dysfunction to trigger apoptosis in human lymphocytes and rat bone-marrow cells. Histochem. Cell Biol. 2018, 149, 127–141. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, B.; Pereira, L.C.; Pazin, M.; Franco-Bernanrdes, M.F.; Dorta, D.J. Do trifluralin and tebuthiuron impair isolated rat liver mitochondria? Pestic. Biochem. Physiol. 2020, 163, 175–184. [Google Scholar] [CrossRef]
- Paul, K.C.; Krolewski, R.C.; Lucumi Moreno, E.; Blank, J.; Holton, K.M.; Ahfeldt, T.; Furlong, M.; Yu, Y.; Cockburn, M.; Thompson, L.K.; et al. A pesticide and iPSC dopaminergic neuron screen identifies and classifies Parkinson-relevant pesticides. Nat. Commun. 2023, 14, 2803. [Google Scholar] [CrossRef] [PubMed]
- Nehéz, M.; Selypes, A.; Páldy, A.; Mazzag, E.; Berencsi, G.; Jármay, K. The effects of five weeks treatment with dinitro-o-cresol- or trifluralin-containing pesticides on the germ cells of male mice. J. Appl. Toxicol. 1982, 2, 179–180. [Google Scholar] [CrossRef]
- Francis, P.C.; Emmerson, J.L.; Adams, E.R.; Owen, N.V. Oncogenicity study of trifluralin in B6C3F1 mice. Food Chem. Toxicol. 1991, 29, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Garriott, M.L.; Adams, E.R.; Probst, G.S.; Emmerson, J.L.; Oberly, T.J.; Kindig, D.E.; Neal, S.B.; Bewsey, B.J.; Rexroat, M.A. Genotoxicity studies on the preemergence herbicide trifluralin. Mutat. Res. 1991, 260, 187–193. [Google Scholar] [CrossRef]
- Ribas, G.; Surrallés, J.; Carbonell, E.; Xamena, N.; Creus, A.; Marcos, R. Genotoxic evaluation of the herbicide trifluralin on human lymphocytes exposed in vitro. Mutat. Res. 1996, 371, 15–21. [Google Scholar] [CrossRef]
- Kaya, B.; Marcos, R.; Yanikoğlu, A.; Creus, A. Evaluation of the genotoxicity of four herbicides in the wing spot test of Drosophila melanogaster using two different strains. Mutat. Res. 2004, 557, 53–62. [Google Scholar] [CrossRef]
- Könen, S.; Cavaş, T. Genotoxicity testing of the herbicide trifluralin and its commercial formulation Treflan using the piscine micronucleus test. Environ. Mol. Mutagen. 2008, 49, 434–438. [Google Scholar] [CrossRef]
- Hurley, P.M. Mode of carcinogenic action of pesticides inducing thyroid follicular cell tumors in rodents. Environ. Health Perspect. 1998, 106, 437–445. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Exposure Factors Handbook (1997, Final Report); U.S. Environmental Protection Agency: Washington, DC, USA, 1997.
- Saghir, S.A.; Charles, G.D.; Bartels, M.J.; Kan, L.H.L.; Dryzga, M.D.; Brzak, K.A.; Clark, A.J. Mechanism of trifluralin-induced thyroid tumors in rats. Toxicol. Lett. 2008, 180, 38–45. [Google Scholar] [CrossRef]
- Chan, M.M.; Fong, D. Inhibition of leishmanias but not host macrophages by the antitubulin herbicide trifluralin. Science 1990, 249, 924–926. [Google Scholar] [CrossRef]
- Chan, M.M.; Grogl, M.; Chen, C.C.; Bienen, E.J.; Fong, D. Herbicides to curb human parasitic infections: In vitro and in vivo effects of trifluralin on the trypanosomatid protozoans. Proc. Natl. Acad. Sci. USA 1993, 90, 5657–5661. [Google Scholar] [CrossRef] [PubMed]
- Werbovetz, K.A.; Sackett, D.L.; Delfín, D.; Bhattacharya, G.; Salem, M.; Obrzut, T.; Rattendi, D.; Bacchi, C. Selective antimicrotubule activity of N1-phenyl-3,5-dinitro-N4,N4-di-n-propylsulfanilamide (GB-II-5) against kinetoplastid parasites. Mol. Pharmacol. 2003, 64, 1325–1333. [Google Scholar] [CrossRef]
- George, T.G.; Johnsamuel, J.; Delfín, D.A.; Yakovich, A.; Mukherjee, M.; Phelps, M.A.; Dalton, J.T.; Sackett, D.L.; Kaiser, M.; Brun, R.; et al. Antikinetoplastid antimitotic activity and metabolic stability of dinitroaniline sulfonamides and benzamides. Bioorg. Med. Chem. 2006, 14, 5699–5710. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, G.; Herman, J.; Delfín, D.; Salem, M.M.; Barszcz, T.; Mollet, M.; Riccio, G.; Brun, R.; Werbovetz, K.A. Synthesis and antitubulin activity of N1- and N4-substituted 3,5-dinitro sulfanilamides against African trypanosomes and Leishmania. J. Med. Chem. 2004, 47, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M.; Tzeng, J.; Emge, T.J.; Ho, C.T.; Fong, D. Structure-function analysis of antimicrotubule dinitroanilines against promastigotes of the parasitic protozoan Leishmania mexicana. Antimicrob. Agents Chemother. 1993, 37, 1909–1913. [Google Scholar] [CrossRef]
- Carvalheiro, M.; Jorge, J.; Eleutério, C.; Pinhal, A.F.; Sousa, A.C.; Morais, J.G.; Cruz, M.E.M. Trifluralin liposomal formulations active against Leishmania donovani infections. Eur. J. Pharm. Biopharm. 2009, 71, 292–296. [Google Scholar] [CrossRef]
- Marques, C.; Carvalheiro, M.; Pereira, M.A.; Jorge, J.; Cruz, M.E.M.; Santos-Gomes, G.M. Efficacy of the liposome trifluralin in the treatment of experimental canine leishmaniosis. Vet. J. 2008, 178, 133–137. [Google Scholar] [CrossRef]
- Zaidenberg, A.; Tournier, H.; Schinella, G.; Marín, G.; Buschiazzo, H. Effects of trifluralin on Trypanosoma cruzi in vitro and in vivo. Pharmacol. Toxicol. 1999, 84, 98–100. [Google Scholar] [CrossRef]
- Traub-Cseko, Y.M.; Ramalho-Ortigão, J.M.; Dantas, A.P.; de Castro, S.L.; Barbosa, H.S.; Downing, K.H. Dinitroaniline herbicides against protozoan parasites: The case of Trypanosoma cruzi. Trends Parasitol. 2001, 17, 136–141. [Google Scholar] [CrossRef]
- Zaidenberg, A.; Luong, T.; Lirussi, D.; Bleiz, J.; Buono, M.B.D.; Quijano, G.; Drut, R.; Kozubsky, L.; Marron, A.; Buschiazzo, H. Treatment of experimental chronic Chagas disease with trifluralin. Basic Clin. Pharmacol. Toxicol. 2006, 98, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Dow, G.S.; Armson, A.; Boddy, M.R.; Itenge, T.; McCarthy, D.; Parkin, J.E.; Thompson, R.C.A.; Reynoldson, J.A. Plasmodium: Assessment of the antimalarial potential of trifluralin and related compounds using a rat model of malaria, Rattus norvegicus. Exp. Parasitol. 2002, 100, 155–160. [Google Scholar] [CrossRef]
- Fennell, B.J.; Naughton, J.A.; Dempsey, E.; Bell, A. Cellular and molecular actions of dinitroaniline and phosphorothioamidate herbicides on Plasmodium falciparum: Tubulin as a specific antimalarial target. Mol. Biochem. Parasitol. 2006, 145, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Naughton, J.A.; Hughes, R.; Bray, P.; Bell, A. Accumulation of the antimalarial microtubule inhibitors trifluralin and vinblastine by Plasmodium falciparum. Biochem. Pharmacol. 2008, 75, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Stokkermans, T.J.; Schwartzman, J.D.; Keenan, K.; Morrissette, N.S.; Tilney, L.G.; Roos, D.S. Inhibition of Toxoplasma gondii replication by dinitroaniline herbicides. Exp. Parasitol. 1996, 84, 355–370. [Google Scholar] [CrossRef]
- Levandowsky, M.; Hauser, D.C.; Glassgold, J.M. Chemosensory responses of a protozoan are modified by antitubulins. J. Bacteriol. 1975, 124, 1037–1038. [Google Scholar] [CrossRef]
- Brand, R.M.; Mueller, C. Transdermal penetration of atrazine, alachlor, and trifluralin: Effect of formulation. Toxicol. Sci. 2002, 68, 18–23. [Google Scholar] [CrossRef]
- Urbina, J.A.; Docampo, R. Specific chemotherapy of Chagas disease: Controversies and advances. Trends Parasitol. 2003, 19, 495–501. [Google Scholar] [CrossRef]
- Bestetti, R.B.; Cardinalli-Neto, A. Sudden cardiac death in Chagas’ heart disease in the contemporary era. Int. J. Cardiol. 2008, 131, 9–17. [Google Scholar] [CrossRef]
- Arnaiz, M.R.; Fichera, L.E.; Postan, M. Cardiac myocyte hypertrophy and proliferating cell nuclear antigen expression in Wistar rats infected with Trypanosoma cruzi. J. Parasitol. 2002, 88, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zile, M.R.; Takahashi, M.; Baicu, C.F.; Bonnema, D.D.; Cabral, F.; Menick, D.R.; Cooper, G., 4th. A direct test of the hypothesis that increased microtubule network density contributes to contractile dysfunction of the hypertrophied heart. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2231–H2241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cooper, G., 4th. Cytoskeletal networks and the regulation of cardiac contractility: Microtubules, hypertrophy, and cardiac dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1003–H1014. [Google Scholar] [CrossRef]
- Simbre, V.C.; Duffy, S.A.; Dadlani, G.H.; Miller, T.L.; Lipshultz, S.E. Cardiotoxicity of cancer chemotherapy: Implications for children. Paediatr. Drugs 2005, 7, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D. Casarett & Doull’s Toxicology: The Basic Science of Poisons, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Giglio, A.; Vommaro, M.L. Dinitroaniline herbicides: A comprehensive review of toxicity and side effects on animal non-target organisms. Environ. Sci. Pollut. Res. Int. 2022, 29, 76687–76711. [Google Scholar] [CrossRef]
- Wallace, D.R. Trifluralin. In Encyclopedia of Toxicology, 4th ed.; Academic Press: Oxford, UK, 2024; pp. 609–613. ISBN 978-0-323-85434-4. [Google Scholar]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Gaillard, N.; Sharma, A.; Abbaali, I.; Liu, T.; Shilliday, F.; Cook, A.D.; Ehrhard, V.; Bangera, M.; Roberts, A.J.; Moores, C.A.; et al. Inhibiting parasite proliferation using a rationally designed anti-tubulin agent. EMBO Mol. Med. 2021, 13, e13818. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).