Interaction of Microcolin Cyanobacterial Lipopeptides with Phosphatidylinositol Transfer Protein (PITP)—Molecular Docking Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protein Structures, Programs and Process
2.2. Protein–Ligand Binding Site Prediction
2.3. Molecular Docking Analysis
3. Results
3.1. PITPα Protein: Topographic Analysis of Drug Binding Site
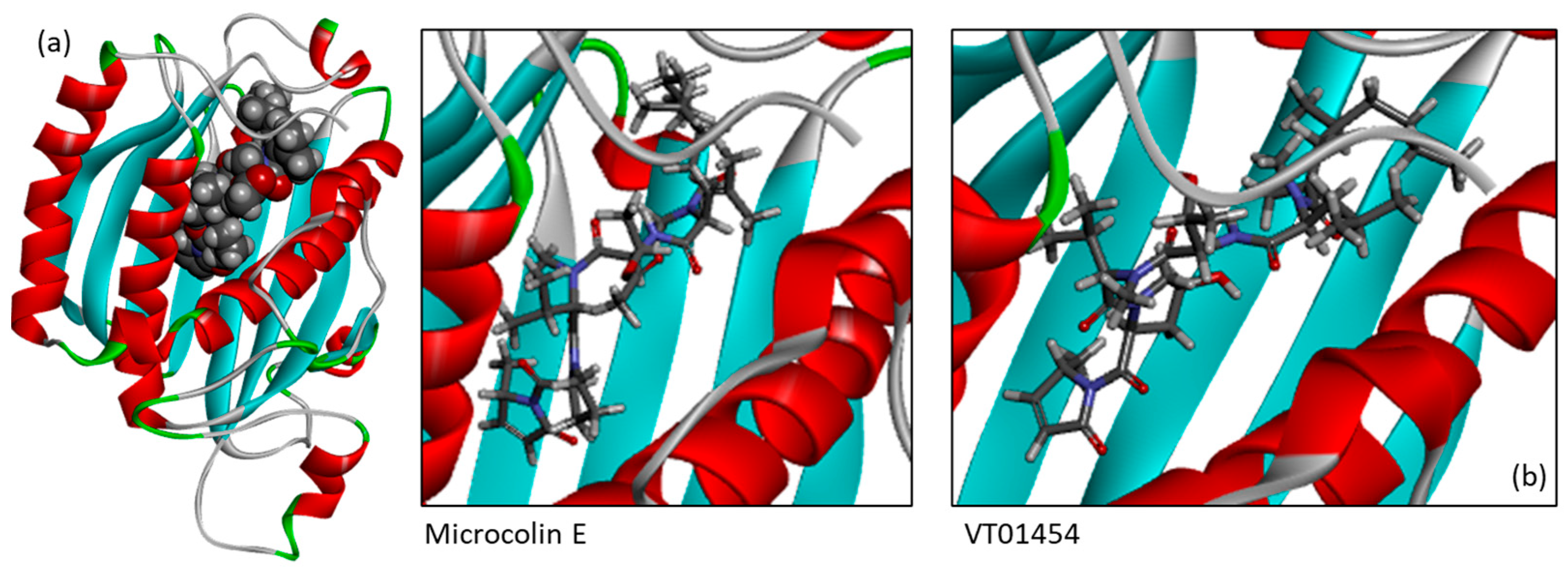
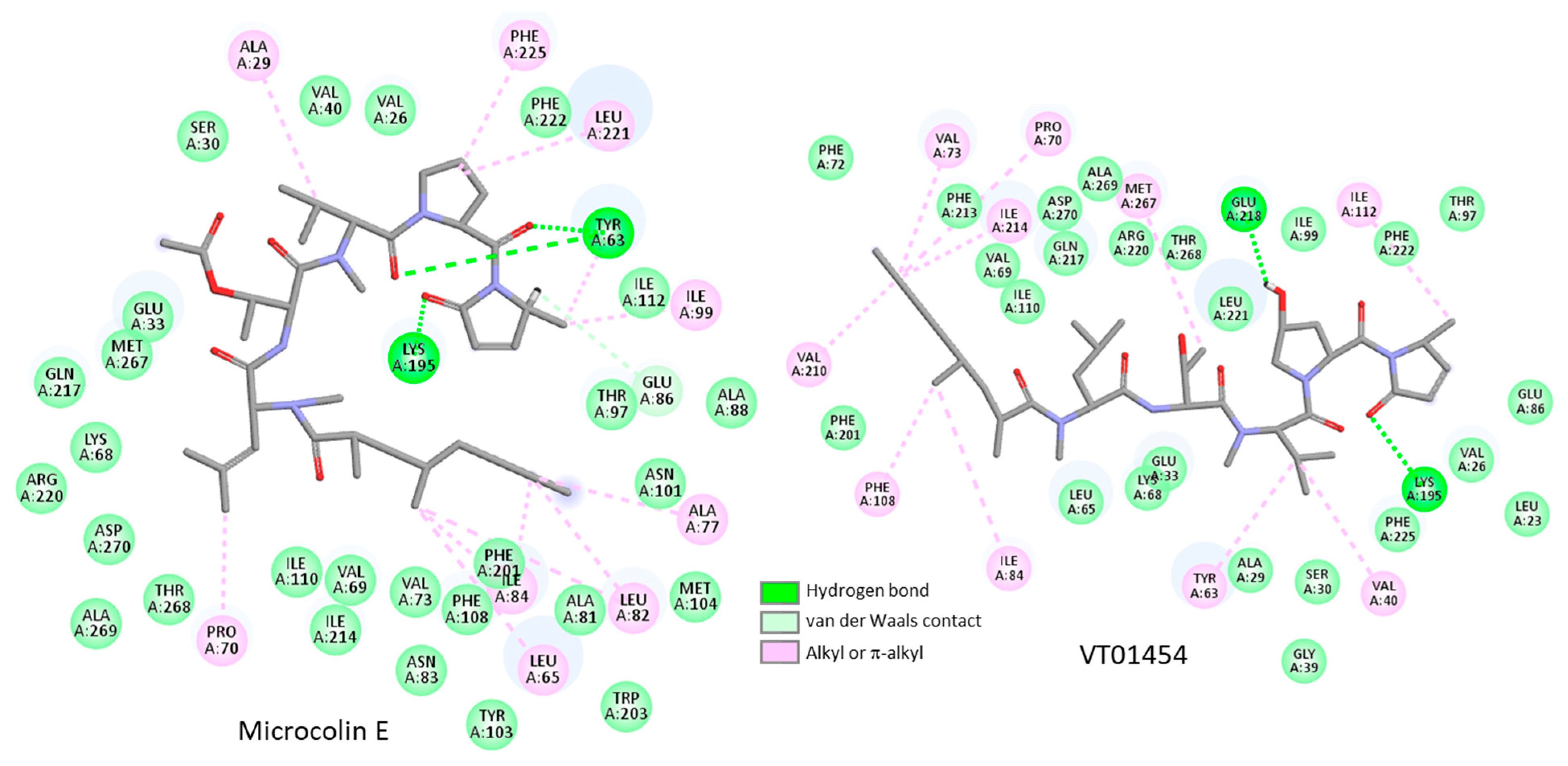
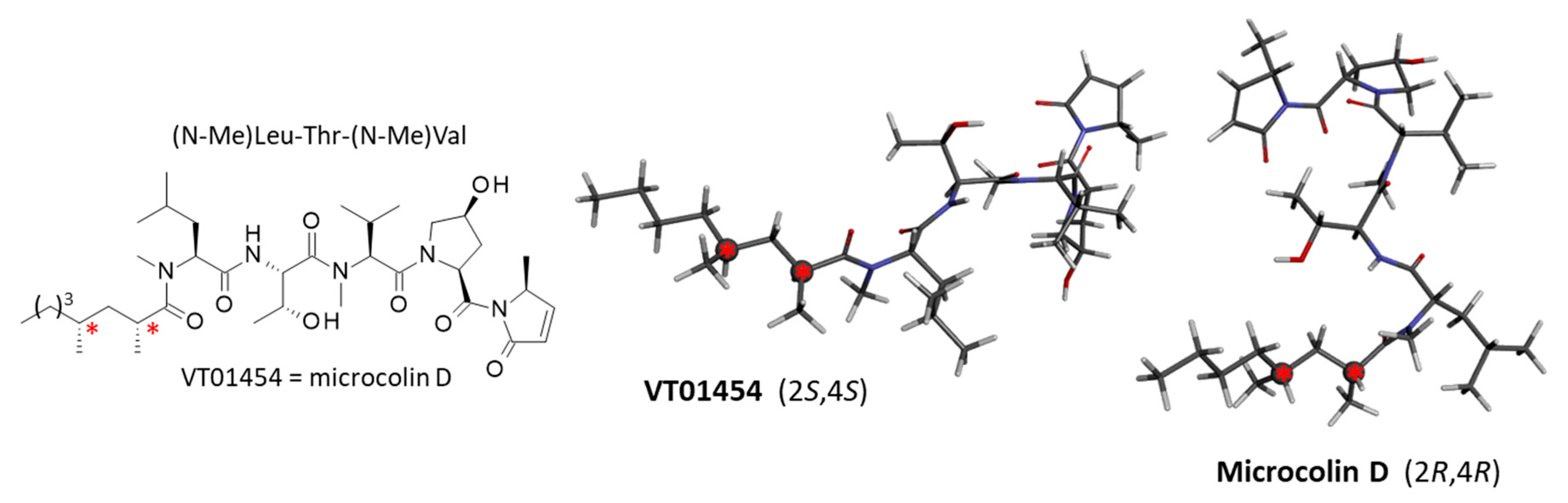
3.2. Binding of Microcolins to PITPα: Docking Study
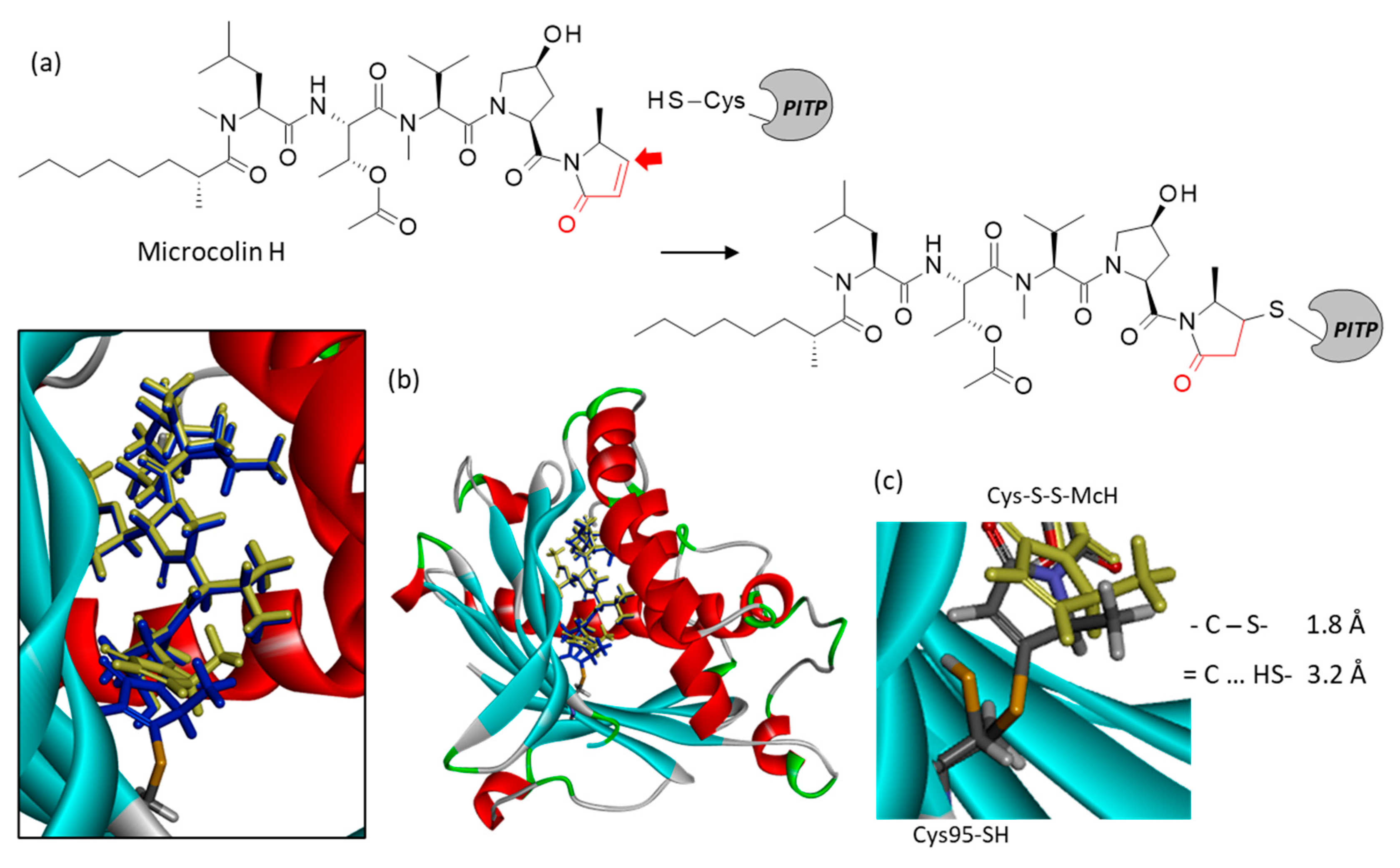
3.3. Covalent Binding of Microcolin H to PITP
3.4. Binding of Related Natural Products to PITPβ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LATS | Large tumor suppressor |
| PITP | Phosphatidylinositol transfer protein |
| TAZ | Transcriptional coactivator with PDZ-binding motif |
| YAP | Yes-associated protein |
References
- Sharp, K.; Arthur, K.E.; Gu, L.; Ross, C.; Harrison, G.; Gunasekera, S.P.; Meickle, T.; Matthew, S.; Luesch, H.; Thacker, R.W.; et al. Phylogenetic and chemical diversity of three chemotypes of bloom-forming lyngbya species (Cyanobacteria: Oscillatoriales) from reefs of southeastern Florida. Appl. Environ. Microbiol. 2009, 75, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.L.; Fulton, H.M.; McGovern, T.W. Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula). Cutis 2024, 113, E38–E40. [Google Scholar] [CrossRef] [PubMed]
- Curren, E.; Leong, S.C.Y. Global phylogeography of toxic cyanobacteria Moorea producens reveals distinct genetic partitioning influenced by Proterozoic glacial cycles. Harmful Algae 2019, 86, 10–19. [Google Scholar] [CrossRef]
- Curren, E.; Leaw, C.P.; Lim, P.T.; Leong, S.C.Y. The toxic cosmopolitan cyanobacteria Moorena producens: Insights into distribution, ecophysiology and toxicity. Environ. Sci. Pollut. Res. Int. 2022, 29, 78178–78206. [Google Scholar] [CrossRef]
- Ngo, T.E.; Ecker, A.; Ryu, B.; Guild, A.; Remmel, A.; Boudreau, P.D.; Alexander, K.L.; Naman, C.B.; Glukhov, E.; Avalon, N.E.; et al. Structure and Biosynthesis of Hectoramide B, a Linear Depsipeptide from Marine Cyanobacterium Moorena producens JHB Discovered via Coculture with Candida albicans. ACS Chem. Biol. 2024, 19, 619–628, Correction in ACS Chem. Biol. 2024, 19, 2593. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Mufti, S.J.; Badiab, A.A.; Shaala, L.A. Anti-Infective Secondary Metabolites of the Marine Cyanobacterium Lyngbya Morphotype between 1979 and 2022. Mar. Drugs 2022, 20, 768. [Google Scholar] [CrossRef]
- Levert, A.; Alvariño, R.; Bornancin, L.; Abou Mansour, E.; Burja, A.M.; Genevière, A.M.; Bonnard, I.; Alonso, E.; Botana, L.; Banaigs, B. Structures and Activities of Tiahuramides A-C, Cyclic Depsipeptides from a Tahitian Collection of the Marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2018, 81, 1301–1310. [Google Scholar] [CrossRef]
- Sweeney-Jones, A.M.; Gagaring, K.; Antonova-Koch, J.; Zhou, H.; Mojib, N.; Soapi, K.; Skolnick, J.; McNamara, C.W.; Kubanek, J. Antimalarial Peptide and Polyketide Natural Products from the Fijian Marine Cyanobacterium Moorea producens. Mar. Drugs 2020, 18, 167. [Google Scholar] [CrossRef]
- Shrestha, S.K.; Min, K.H.; Kim, S.W.; Kim, H.; Gerwick, W.H.; Soh, Y. Kalkitoxin: A Potent Suppressor of Distant Breast Cancer Metastasis. Int. J. Mol. Sci. 2023, 24, 1207. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Satake, M.; Zhang, B.T.; Xiao, Y.Y.; Fukuoka, M.; Uchida, H.; Nagai, H. Neo-Aplysiatoxin A Isolated from Okinawan Cyanobacterium Moorea producens. Molecules 2020, 25, 457. [Google Scholar] [CrossRef]
- Semmouri, I.; Janssen, C.R.; Asselman, J. Health risks associated with the consumption of sea turtles: A review of chelonitoxism incidents and the presumed responsible phycotoxins. Sci. Total Environ. 2024, 954, 176330. [Google Scholar] [CrossRef]
- Pearson, L.A.; Karuso, P.; Neilan, B.A. Structure, biosynthesis and activity of indolactam alkaloids. Alkaloids Chem. Biol. 2024, 92, 1–45. [Google Scholar] [PubMed]
- Yu, H.B.; Glukhov, E.; Li, Y.; Iwasaki, A.; Gerwick, L.; Dorrestein, P.C.; Jiao, B.H.; Gerwick, W.H. Cytotoxic Microcolin Lipopeptides from the Marine Cyanobacterium Moorea producens. J. Nat. Prod. 2019, 82, 2608–2619. [Google Scholar] [CrossRef] [PubMed]
- Koehn, F.E.; Longley, R.E.; Reed, J.K. Microcolins A and B, new immunosuppressive peptides from the blue-green alga Lyngbya majuscula. J. Nat. Prod. 1992, 55, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Longley, R.E.; Koehn, F.E. Antiproliferative and immunosuppressive properties of microcolin A, a marine-derived lipopeptide. Life Sci. 1997, 60, 751–762. [Google Scholar] [CrossRef]
- Zhang, L.H.; Longley, R.E. Induction of apoptosis in mouse thymocytes by microcolin A and its synthetic analog. Life Sci. 1999, 64, 1013–1028. [Google Scholar] [CrossRef]
- Koehn, F.E.; McConnell, O.J.; Longley, R.E.; Sennett, S.H.; Reed, J.K. Analogues of the marine immunosuppressant microcolin A: Preparation and biological activity. J. Med. Chem. 1994, 37, 3181–3186. [Google Scholar] [CrossRef]
- Mandal, A.K.; Hines, J.; Kuramochi, K.; Crews, C.M. Developing microcolin A analogs as biological probes. Bioorg. Med. Chem. Lett. 2005, 15, 4043–4047. [Google Scholar] [CrossRef]
- Li, F.L.; Fu, V.; Liu, G.; Tang, T.; Konradi, A.W.; Peng, X.; Kemper, E.; Cravatt, B.F.; Franklin, J.M.; Wu, Z.; et al. Hippo pathway regulation by phosphatidylinositol transfer protein and phosphoinositides. Nat. Chem. Biol. 2022, 18, 1076–1086. [Google Scholar] [CrossRef]
- Hamaï, A.; Drin, G. Specificity of lipid transfer proteins: An in vitro story. Biochimie 2024, 227, 85–110. [Google Scholar] [CrossRef]
- Allen-Baume, V.; Ségui, B.; Cockcroft, S. Current thoughts on the phosphatidylinositol transfer protein family. FEBS Lett. 2002, 531, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Willis, K.G.; Bankaitis, V.A.; McDermott, M.I. Mammalian START-like phosphatidylinositol transfer proteins—Physiological perspectives and roles in cancer biology. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2024, 1869, 159529. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Jiao, Z.; Yu, F.X. Advances towards potential cancer therapeutics targeting Hippo signaling. Biochem. Soc. Trans. 2024, 52, 2399–2413. [Google Scholar] [CrossRef]
- Hu, D.; Meng, R.Y.; Nguyen, T.V.; Chai, O.H.; Park, B.H.; Lee, J.S.; Kim, S.M. Inhibition of colorectal cancer tumorigenesis by ursolic acid and doxorubicin is mediated by targeting the Akt signaling pathway and activating the Hippo signaling pathway. Mol. Med. Rep. 2023, 27, 11. [Google Scholar] [CrossRef]
- Li, N.; Liu, Y.H.; Wu, J.; Liu, Q.G.; Niu, J.B.; Zhang, Y.; Fu, X.J.; Song, J.; Zhang, S.Y. Strategies that regulate Hippo signaling pathway for novel anticancer therapeutics. Eur. J. Med. Chem. 2024, 276, 116694. [Google Scholar] [CrossRef]
- Koroleva, O.A.; Kurkin, A.V.; Shtil, A.A. The Hippo pathway as an antitumor target: Time to focus on. Expert. Opin. Investig. Drugs 2024, 33, 1177–1185. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, X.; Wang, C.; Zhang, H.; Yi, J.; Wang, K.; Hou, Y.; Ji, P.; Jin, X.; Li, C.; et al. Microcolin H, a novel autophagy inducer, exerts potent antitumour activity by targeting PITPalpha/beta. Signal Transduct. Target. Ther. 2023, 8, 428. [Google Scholar] [CrossRef]
- Yoder, M.D.; Thomas, L.M.; Tremblay, J.M.; Oliver, R.L.; Yarbrough, L.R.; Helmkamp, G.M., Jr. Structure of a multifunctional protein. Mammalian phosphatidylinositol transfer protein complexed with phosphatidylcholine. J. Biol. Chem. 2001, 276, 9246–9252. [Google Scholar] [CrossRef]
- Grabon, A.; Orłowski, A.; Tripathi, A.; Vuorio, J.; Javanainen, M.; Róg, T.; Lönnfors, M.; McDermott, M.I.; Siebert, G.; Somerharju, P.; et al. Dynamics and energetics of the mammalian phosphatidylinositol transfer protein phospholipid exchange cycle. J. Biol. Chem. 2017, 292, 14438–14455. [Google Scholar] [CrossRef]
- Biswas, S.; Mukherjee, P.K.; Harwansh, R.K.; Bannerjee, S.; Bhattacharjee, P. Enhanced bioavailability and hepatoprotectivity of optimized ursolic acid-phospholipid complex. Drug Dev. Ind. Pharm. 2019, 45, 946–958. [Google Scholar] [CrossRef]
- Vordtriede, P.B.; Doan, C.N.; Tremblay, J.M.; Helmkamp, G.M., Jr.; Yoder, M.D. Structure of PITPbeta in complex with phosphatidylcholine: Comparison of structure and lipid transfer to other PITP isoforms. Biochemistry 2005, 44, 14760–14771. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Tirado-Rives, J. Molecular modeling of organic and biomolecular systems using BOSS and MCPRO. J. Comput. Chem. 2005, 26, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Schroeder, M. LIGSITEcsc: Predicting ligand binding sites using the Connolly surface and degree of conservation. BMC Struct. Biol. 2006, 6, 19. [Google Scholar] [CrossRef]
- Dundas, J.; Ouyang, Z.; Tseng, J.; Binkowski, A.; Turpaz, Y.; Liang, J. CASTp: Computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res. 2006, 34, W116–W118. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, Y.; Zhang, L. Exploring the computational methods for protein-ligand binding site prediction. Comput. Struct. Biotechnol. J. 2020, 18, 417–426. [Google Scholar] [CrossRef]
- Prymula, K.; Jadczyk, T.; Roterman, I. Catalytic residues in hydrolases: Analysis of methods designed for ligand-binding site prediction. J. Comput. Aided Mol. Des. 2011, 25, 117–133. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Meziane-Tani, M.; Lagant, P.; Semmoud, A.; Vergoten, G. The SPASIBA force field for chondroitin sulfate: Vibrational analysis of D-glucuronic and N-acetyl-D-galactosamine 4-sulfate sodium salts. J. Phys. Chem. A 2006, 110, 11359–11370. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert. Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Lagant, P.; Nolde, D.; Stote, R.; Vergoten, G.; Karplus, M. Increasing normal modes analysis accuracy: The SPASIBA spectroscopic force field introduced into the CHARMM program. J. Phys. Chem. A 2004, 108, 4019–4029. [Google Scholar] [CrossRef]
- Vergoten, G.; Mazur, I.; Lagant, P.; Michalski, J.C.; Zanetta, J.P. The SPASIBA force field as an essential tool for studying the structure and dynamics of saccharides. Biochimie 2003, 85, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Tirado-Rives, J. Monte Carlo versus Molecular Dynamics for conformational sampling. J. Phys. Chem. 1996, 100, 14508–14513. [Google Scholar] [CrossRef]
- Kim, Y.J.; Pemberton, J.G.; Eisenreichova, A.; Mandal, A.; Koukalova, A.; Rohilla, P.; Sohn, M.; Konradi, A.W.; Tang, T.T.; Boura, E.; et al. Non-vesicular phosphatidylinositol transfer plays critical roles in defining organelle lipid composition. EMBO J. 2024, 43, 2035–2061. [Google Scholar] [CrossRef]
- Moore, R.E.; Entzeroth, M. Majusculamide D and deoxymajusculamide D, two cytotoxins from Lyngbya majuscula. Phytochemistry 1988, 27, 3101–3103. [Google Scholar] [CrossRef]
- Caro-Diaz, E.J.E.; Valeriote, F.A.; Gerwick, W.H. Highly Convergent Total Synthesis and Assignment of Absolute Configuration of Majusculamide D, a Potent and Selective Cytotoxic Metabolite from Moorea sp. Org. Lett. 2019, 21, 793–796, Correction in Org. Lett. 2020, 22, 6220. [Google Scholar] [CrossRef]
- Zhao, X.; Xi, X.; Zhang, M.; Lv, M.; Zhang, X.; Lu, Y.; Wang, L.; Chen, Y. Structure-Activity Relationship Study of Majusculamide D: Overcoming Metabolic Instability and Severe Toxicity with a Fluoro Analogue. Mar. Drugs 2024, 22, 537. [Google Scholar] [CrossRef]
- Zhao, X.; Lv, M.; Xi, X.; Lu, Y.; Wang, L.; Chen, Y. Synthesis of Anti-Pancreatic Cancer Natural Product Majusculamide D and Analogues Reveals a Preliminary Structure-Activity Relationships. Chin. J. Chem. 2024, 42, 605–610. [Google Scholar] [CrossRef]
- Matos, Â.P.; Saldanha-Corrêa, F.M.P.; Gomes, R.D.S.; Hurtado, G.R. Exploring microalgal and cyanobacterial metabolites with antiprotozoal activity against Leishmania and Trypanosoma parasites. Acta Trop. 2024, 251, 107116. [Google Scholar] [CrossRef]
- McPhail, K.L.; Correa, J.; Linington, R.G.; Gonzalez, J.; Ortega-Barría, E.; Capson, T.L.; Gerwick, W.H. Antimalarial linear lipopeptides from a Panamanian strain of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2007, 70, 984–988. [Google Scholar] [CrossRef]
- Shrestha, S.K.; Kim, S.W.; Soh, Y. Kalkitoxin attenuates calcification of vascular smooth muscle cells via RUNX-2 signaling pathways. J. Vet. Sci. 2023, 24, e69. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, M.; Shrestha, S.K.; Kim, H.; Gerwick, W.H.; Soh, Y. Kalkitoxin Reduces Osteoclast Formation and Resorption and Protects against Inflammatory Bone Loss. Int. J. Mol. Sci. 2021, 22, 2303. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, W.; Li, L.; Sun, X.; Song, S.; Xu, Q.; Zhang, L.; Wei, B.G.; Deng, X. Structure Determinants of Lagunamide A for Anticancer Activity and Its Molecular Mechanism of Mitochondrial Apoptosis. Mol. Pharm. 2016, 13, 3756–3763. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Mostert, D.; Orgler, C.; Andler, O.; Zischka, H.; Kazmaier, U.; Vollmar, A.M.; Braig, S.; Sieber, S.A.; Zahler, S. Thermal Proteome Profiling Reveals Insight to Antiproliferative and Pro-Apoptotic Effects of Lagunamide A in the Modulation of DNA Damage Repair. Chembiochem 2024, 25, e202400024. [Google Scholar] [CrossRef]
- Luo, D.; Ratnayake, R.; Atanasova, K.R.; Paul, V.J.; Luesch, H. Targeted and functional genomics approaches to the mechanism of action of lagunamide D, a mitochondrial cytotoxin from marine cyanobacteria. Biochem. Pharmacol. 2023, 213, 115608. [Google Scholar] [CrossRef]
- Huang, W.; Ren, R.G.; Dong, H.Q.; Wei, B.G.; Lin, G.Q. Diverse synthesis of marine cyclic depsipeptide lagunamide A and its analogues. J. Org. Chem. 2013, 78, 10747–10762. [Google Scholar] [CrossRef]
- Zhao, L.; Thorsheim, C.L.; Suzuki, A.; Stalker, T.J.; Min, S.H.; Krishnaswamy, S.; Cockcroft, S.; Anderson, K.E.; Weiderhold, B.; Abrams, C.S. Individual phosphatidylinositol transfer proteins have distinct functions that do not involve lipid transfer activity. Blood Adv. 2023, 7, 4233–4246. [Google Scholar] [CrossRef]
- Nile, A.H.; Tripathi, A.; Yuan, P.; Mousley, C.J.; Suresh, S.; Wallace, I.M.; Shah, S.D.; Pohlhaus, D.T.; Temple, B.; Nislow, C.; et al. PITPs as targets for selectively interfering with phosphoinositide signaling in cells. Nat. Chem. Biol. 2014, 10, 76–84. [Google Scholar] [CrossRef]
- Decicco, C.P.; Grover, P. Total Asymmetric Synthesis of the Potent Immunosuppressive Marine Natural Product Microcolin A. J. Org. Chem. 1996, 61, 3534–3541. [Google Scholar] [CrossRef]
- Andrus, M.B.; Li, W.; Keyes, R.F. Synthesis of Microcolin B, a Potent New Immunosuppressant Using an Efficient Mixed Imide Formation Reaction. J. Org. Chem. 1997, 62, 5542–5549. [Google Scholar] [CrossRef]
- Mattern, R.H.; Gunasekera, S.; McConnell, O. Synthetic studies of microcolin B. Tetrahedron 1996, 52, 425–434. [Google Scholar] [CrossRef]
- Mattern, R.H.; Gunasekera, S.P.; McConnell, O.J. Synthesis of microcolin analogs using trimethylsilylated lactamset. Tetrahedron Lett. 1997, 38, 2197–2200. [Google Scholar] [CrossRef]
- Ye, Z.B.; Chen, J.; Meng, W.H.; Huang, P.Q. N-Benzyloxymalimide for an easy access to 5-alkyl-3-pyrrolin-2-ones: Asymmetric synthesis of the mixed imide substructure of the potent immunosuppressant microcolin B. Tetrahedron Asymmetry 2010, 21, 895–902. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Menakha, M. Tasiamide-B a new cyanobacterial compound for treating skin cancer. Biomed. Prev. Nutr. 2014, 4, 355–358. [Google Scholar] [CrossRef]
- Robles-Bañuelos, B.; Durán-Riveroll, L.M.; Rangel-López, E.; Pérez-López, H.I.; González-Maya, L. Marine Cyanobacteria as Sources of Lead Anticancer Compounds: A Review of Families of Metabolites with Cytotoxic, Antiproliferative, and Antineoplastic Effects. Molecules 2022, 27, 4814. [Google Scholar] [CrossRef]
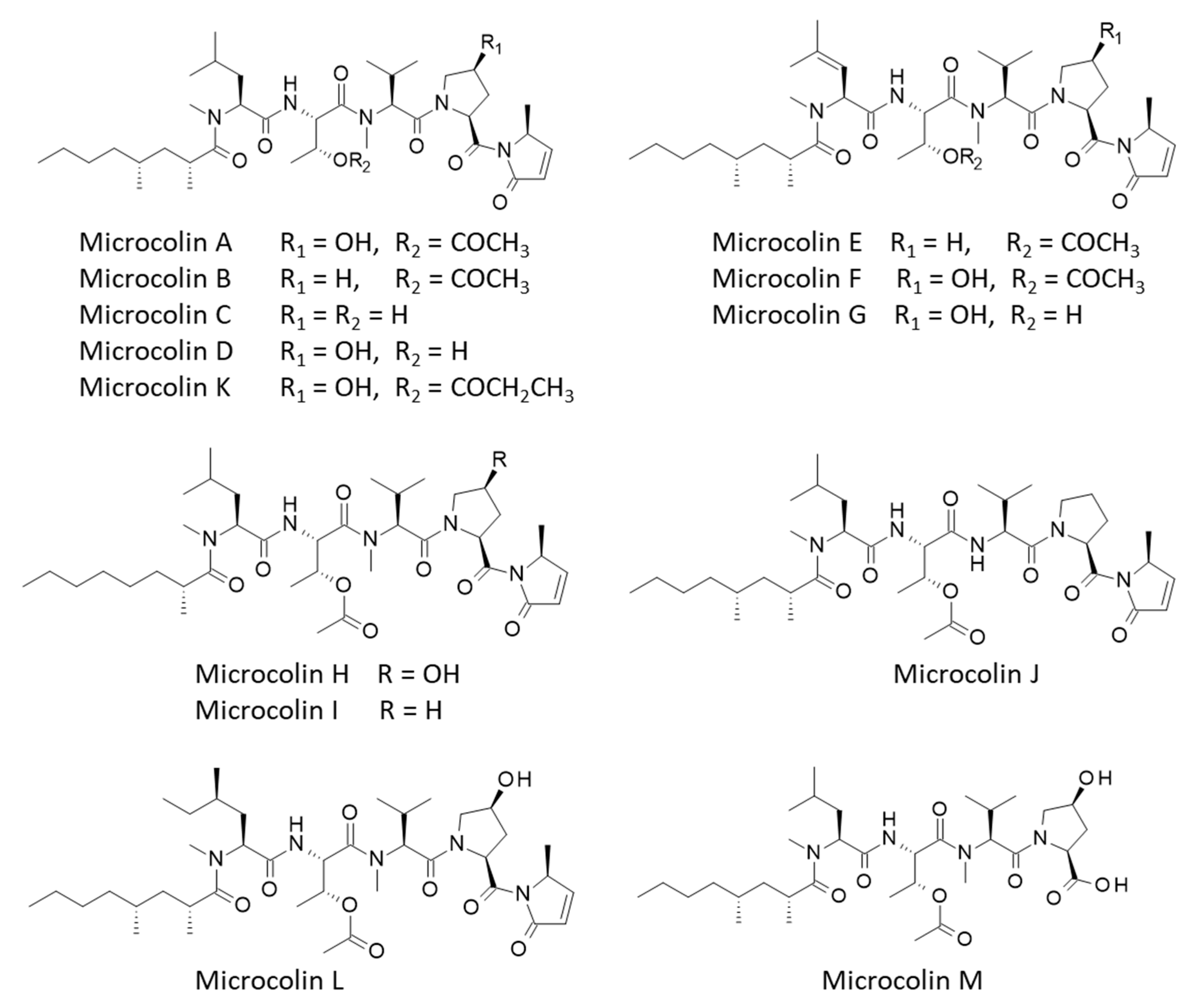
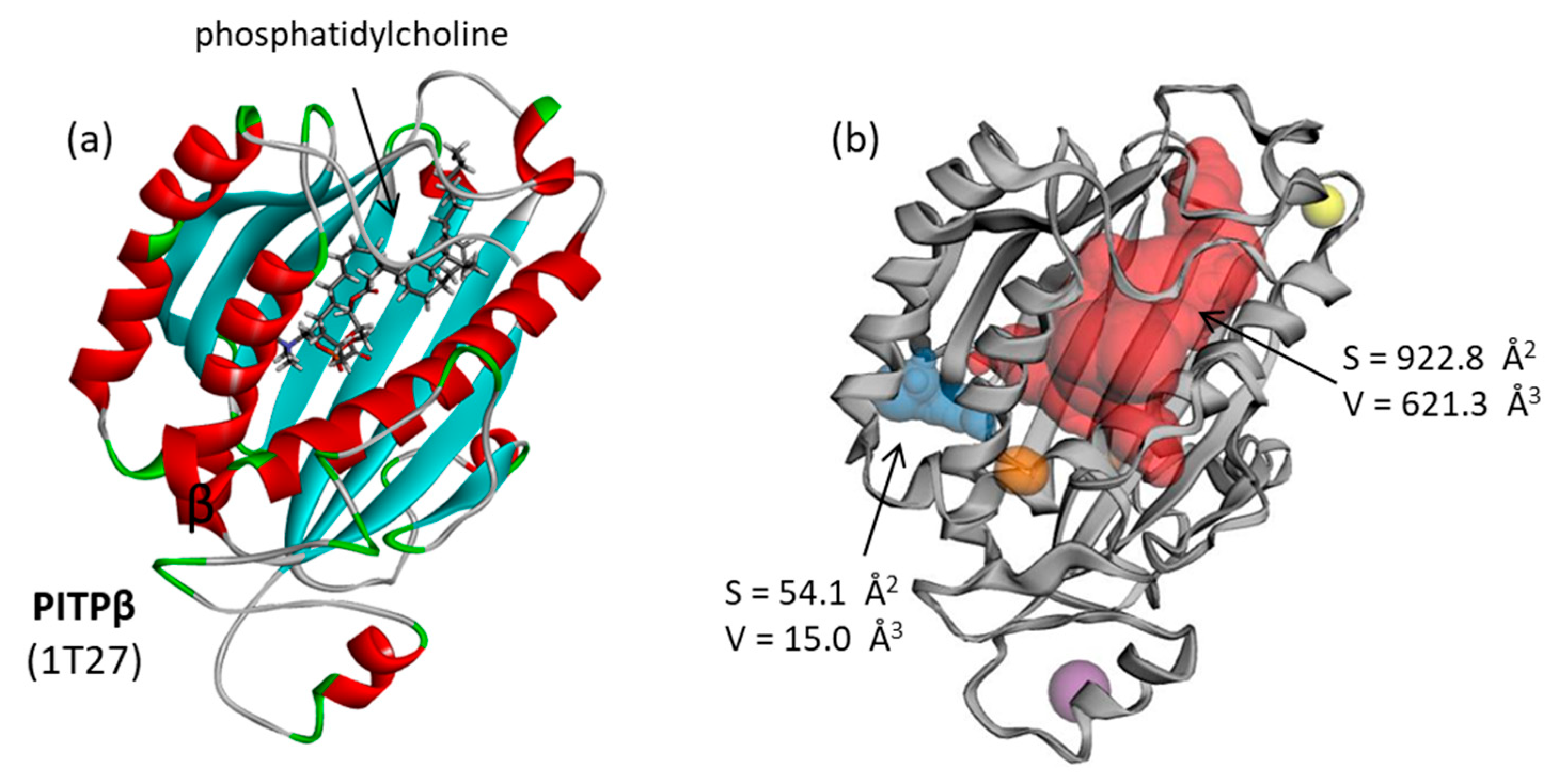
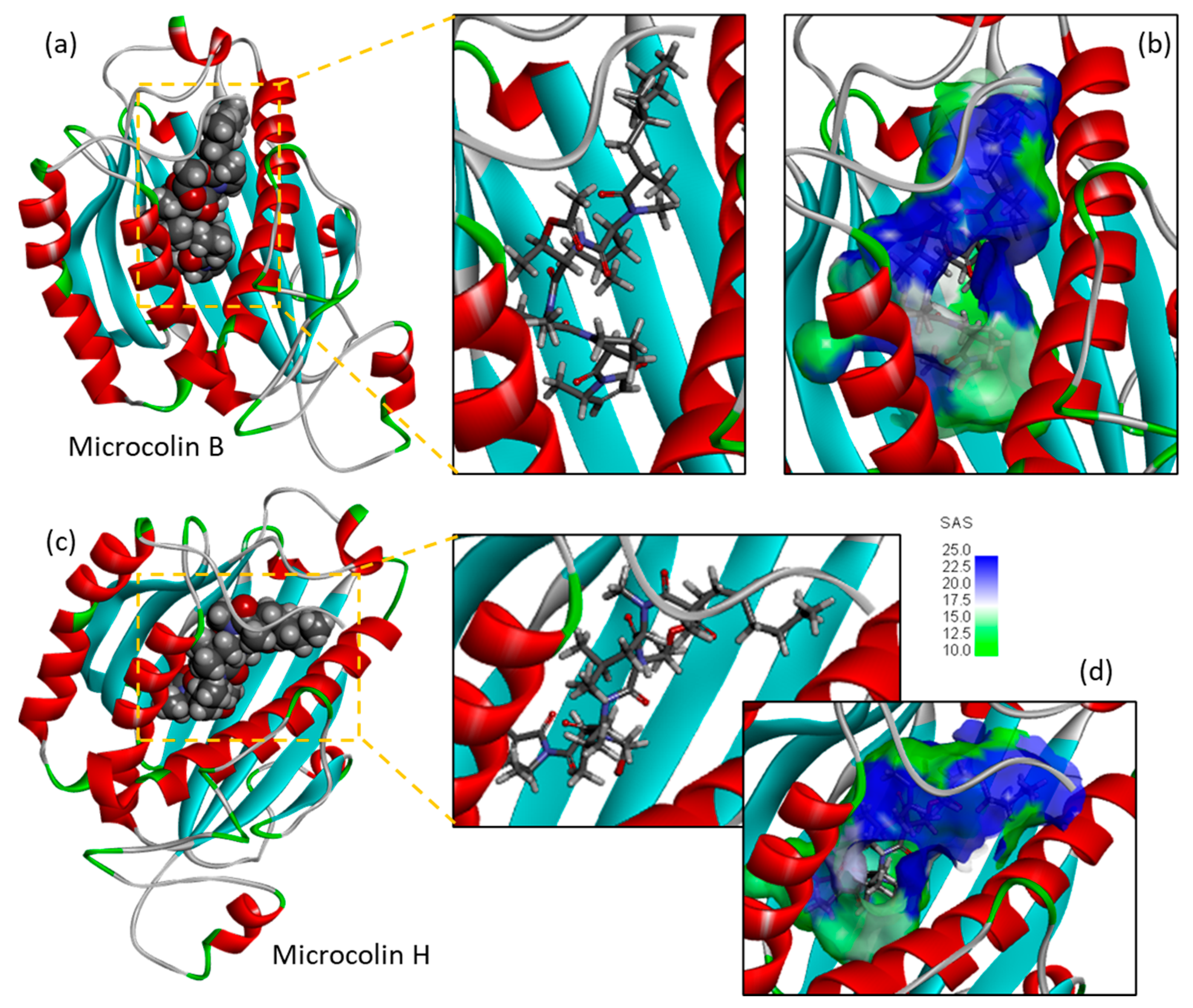
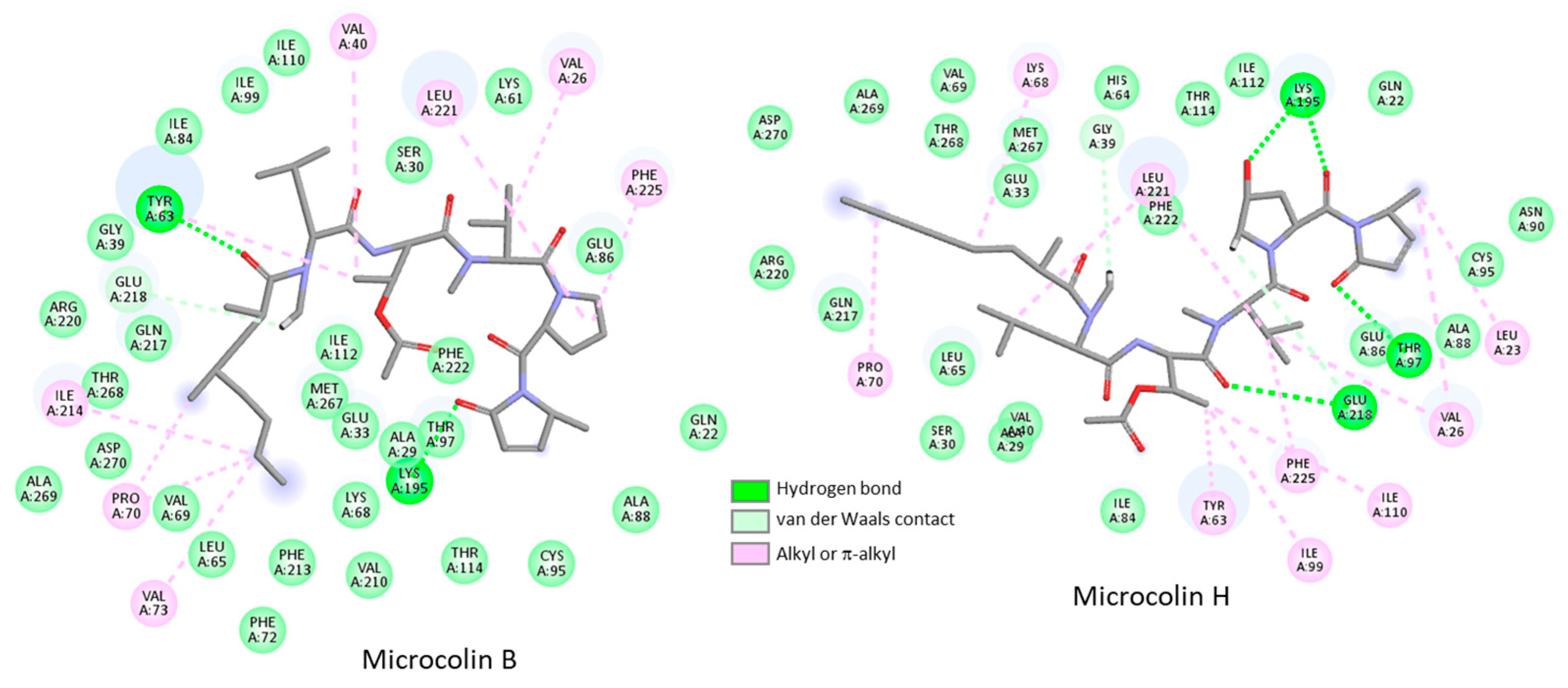

| Compounds | ΔE (kcal/mol) | ΔG (kcal/mol) |
|---|---|---|
| Phosphatidylcholine | −163.80 | −44.95 |
| Microcolin A | −103.10 | −40.35 |
| Microcolin B | −107.35 | −34.55 |
| Microcolin C | −96.40 | −43.20 |
| Microcolin D | −98.50 | −40.00 |
| Microcolin E | −116.60 | −39.70 |
| Microcolin F | −98.20 | −37.70 |
| Microcolin G | −100.60 | −36.85 |
| Microcolin H | −107.20 | −32.80 |
| Microcolin I | −98.15 | −35.20 |
| Microcolin J | −97.60 | −39.75 |
| Microcolin K | −94.60 | −36.80 |
| Microcolin L | −100.70 | −32.30 |
| Microcolin M | −99.40 | −33.80 |
| Majusculamide D | −109.65 | −39.30 |
| Deoxy-majuscul. D | −107.70 | −41.20 |
| VT01454 | −111.70 | −43.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, C.; Vergoten, G. Interaction of Microcolin Cyanobacterial Lipopeptides with Phosphatidylinositol Transfer Protein (PITP)—Molecular Docking Analysis. Future Pharmacol. 2025, 5, 13. https://doi.org/10.3390/futurepharmacol5010013
Bailly C, Vergoten G. Interaction of Microcolin Cyanobacterial Lipopeptides with Phosphatidylinositol Transfer Protein (PITP)—Molecular Docking Analysis. Future Pharmacology. 2025; 5(1):13. https://doi.org/10.3390/futurepharmacol5010013
Chicago/Turabian StyleBailly, Christian, and Gérard Vergoten. 2025. "Interaction of Microcolin Cyanobacterial Lipopeptides with Phosphatidylinositol Transfer Protein (PITP)—Molecular Docking Analysis" Future Pharmacology 5, no. 1: 13. https://doi.org/10.3390/futurepharmacol5010013
APA StyleBailly, C., & Vergoten, G. (2025). Interaction of Microcolin Cyanobacterial Lipopeptides with Phosphatidylinositol Transfer Protein (PITP)—Molecular Docking Analysis. Future Pharmacology, 5(1), 13. https://doi.org/10.3390/futurepharmacol5010013







