Effects of Sinensetin, Eupatilin, and Jaceosidin on Human Melanogenesis: A Pilot Study
Abstract
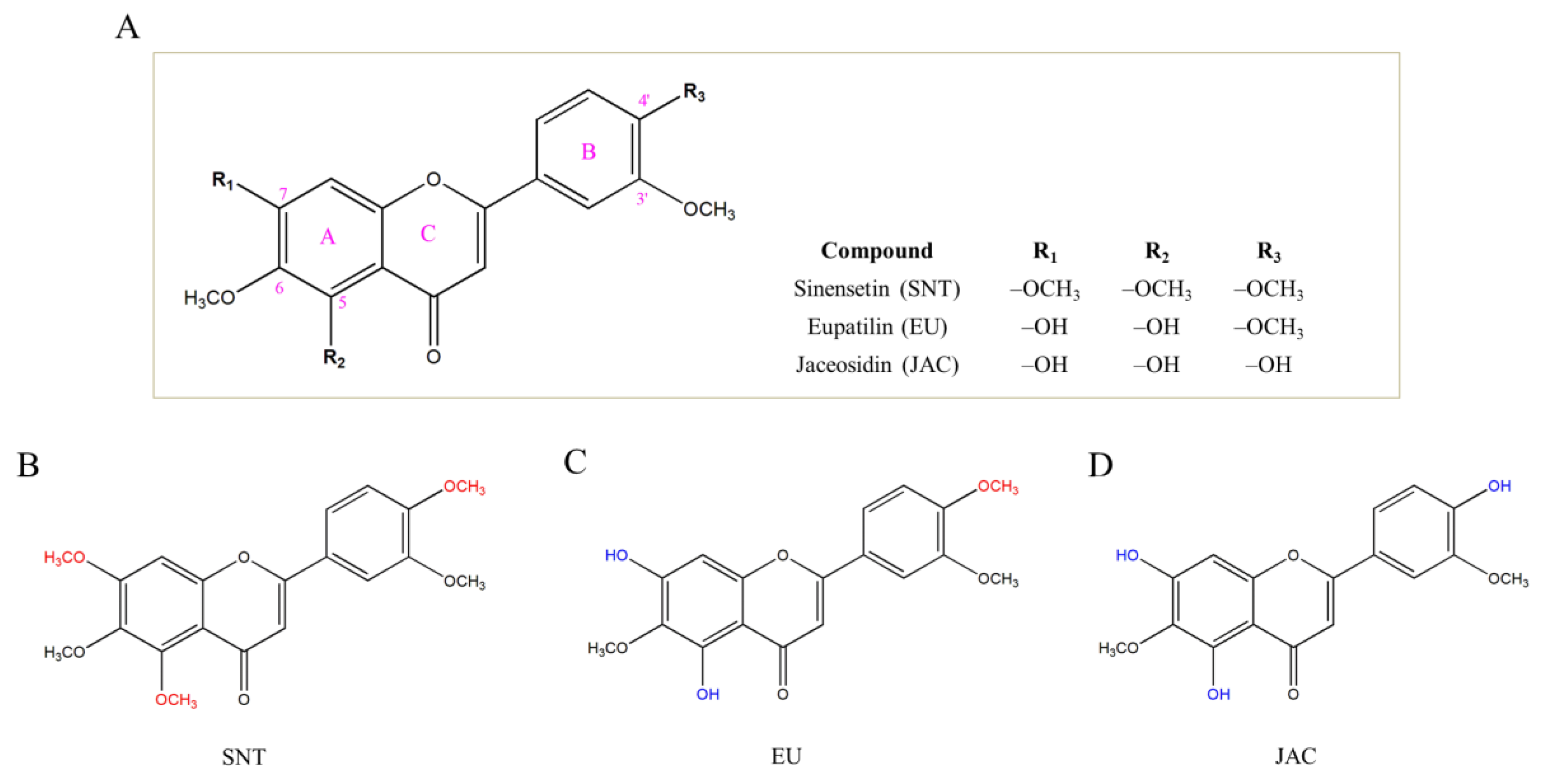
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell-Free TYR Activity
2.3. Pyrocatechol Violet (PV) Copper Chelation Assay
2.4. Cell Culture
2.5. Cytotoxicity Assay
2.6. Cellular Melanin Assay
2.7. Cellular TYR Activity
2.8. ELISA for TYR and MITF Protein
2.9. Recovery Experiment for Melanogenesis
2.10. Statistical Analysis
3. Results
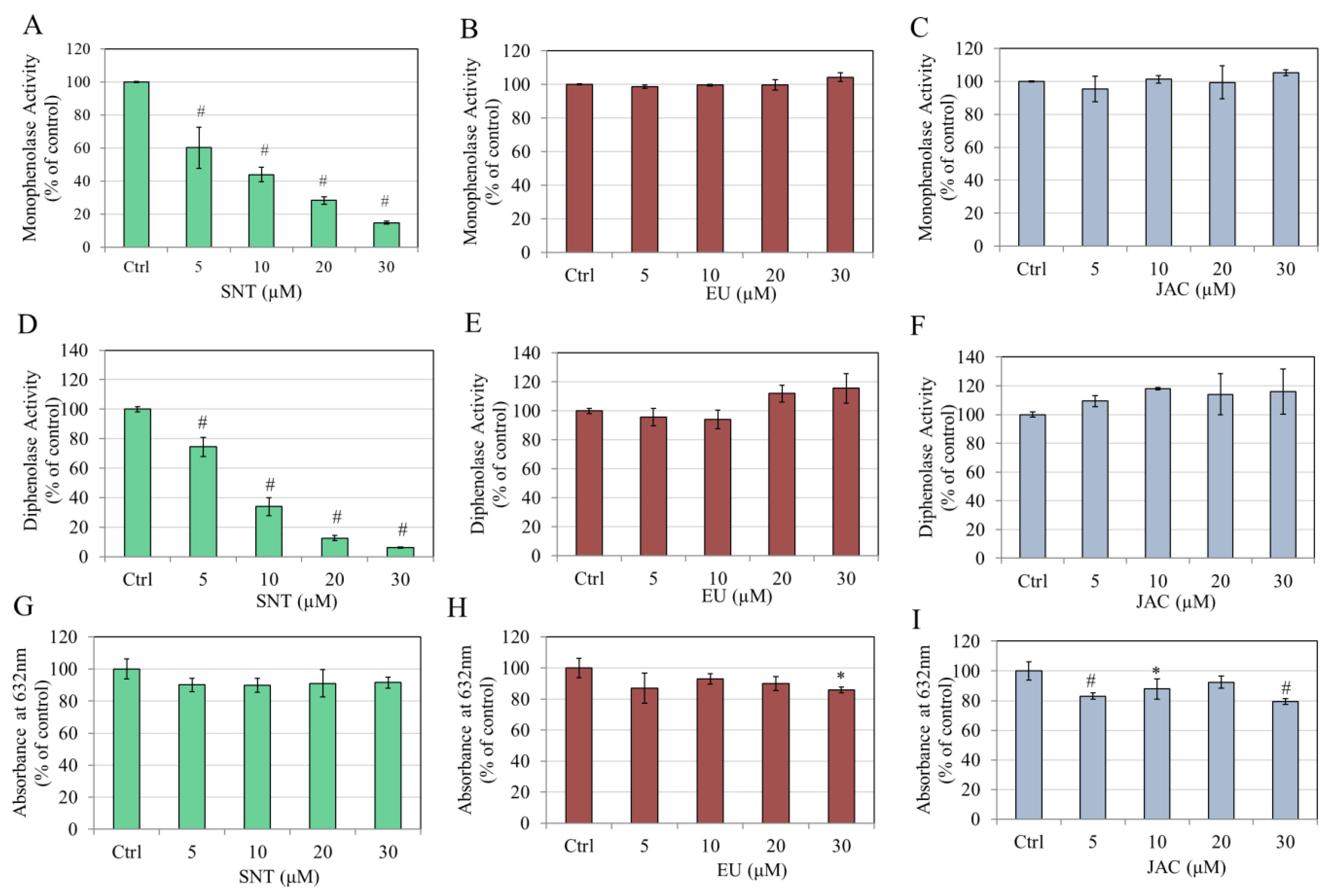
3.1. Direct Effects of Flavones on Mushroom TYR Activity and Copper Chelation
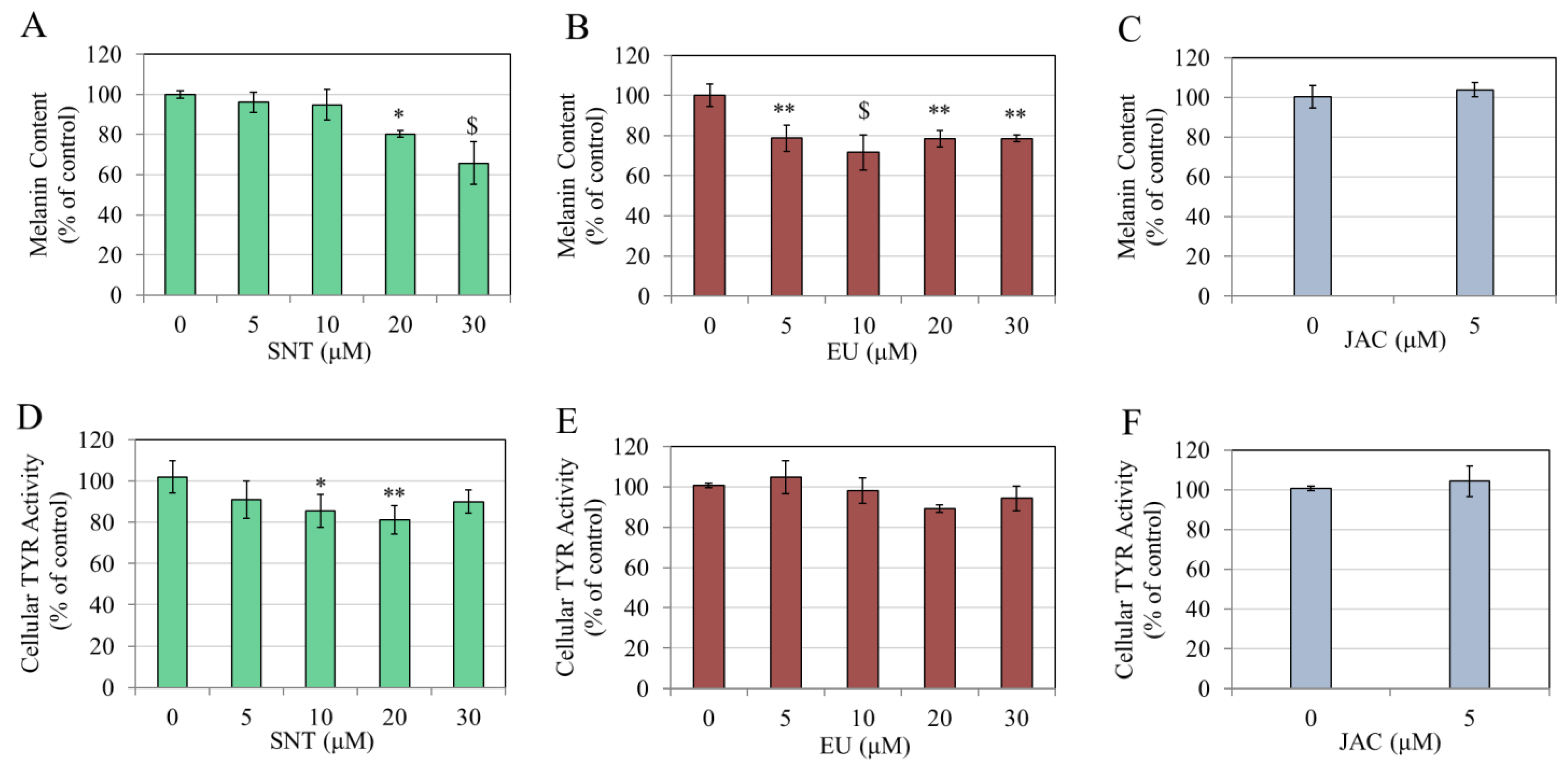
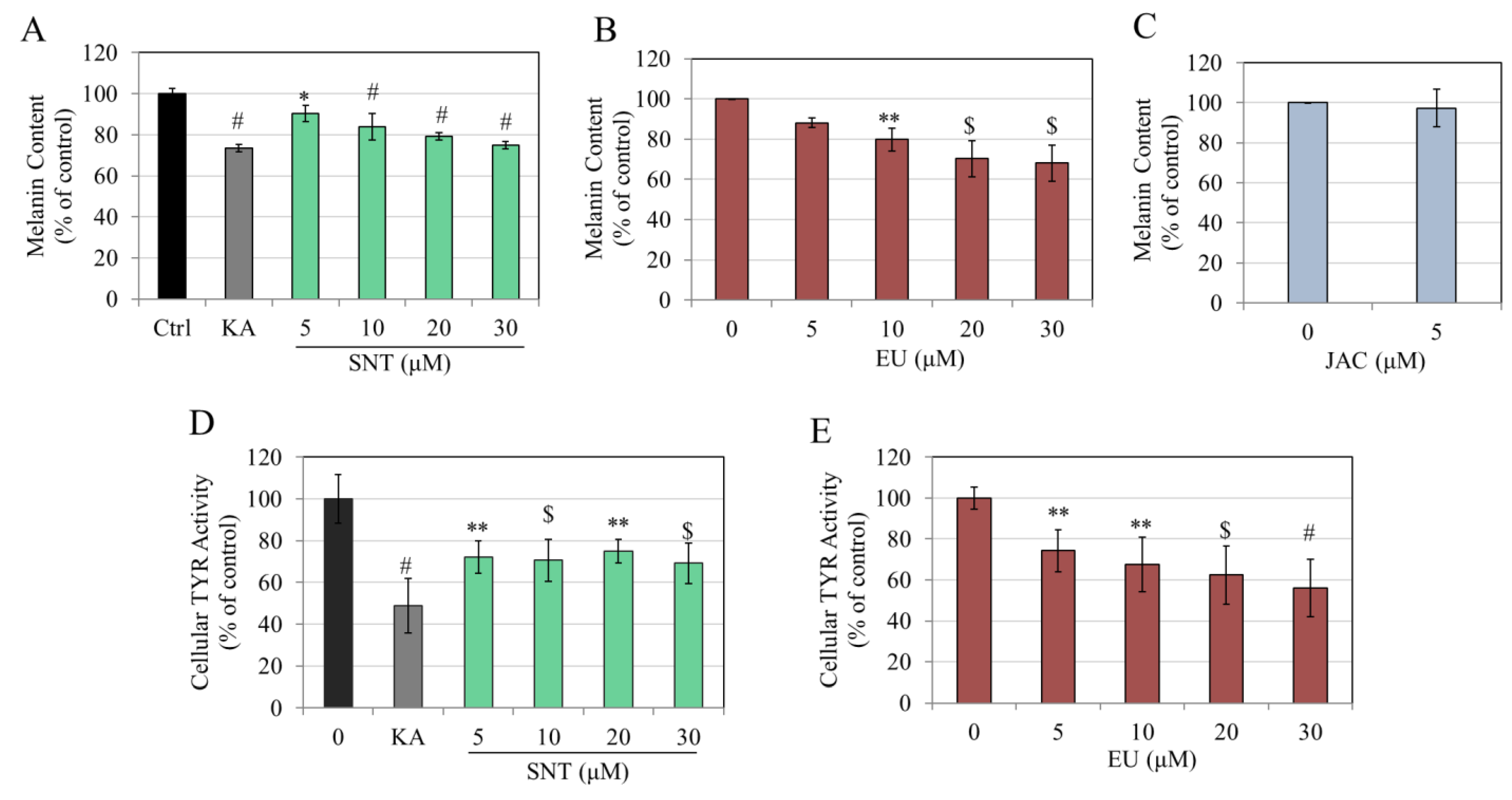
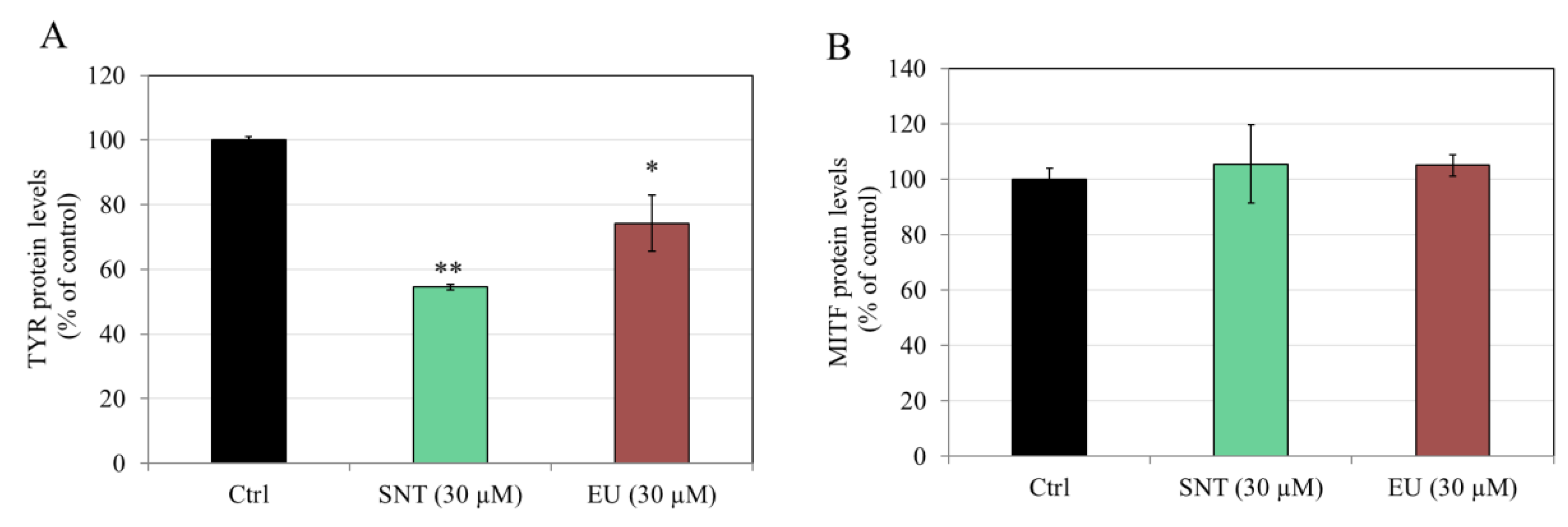
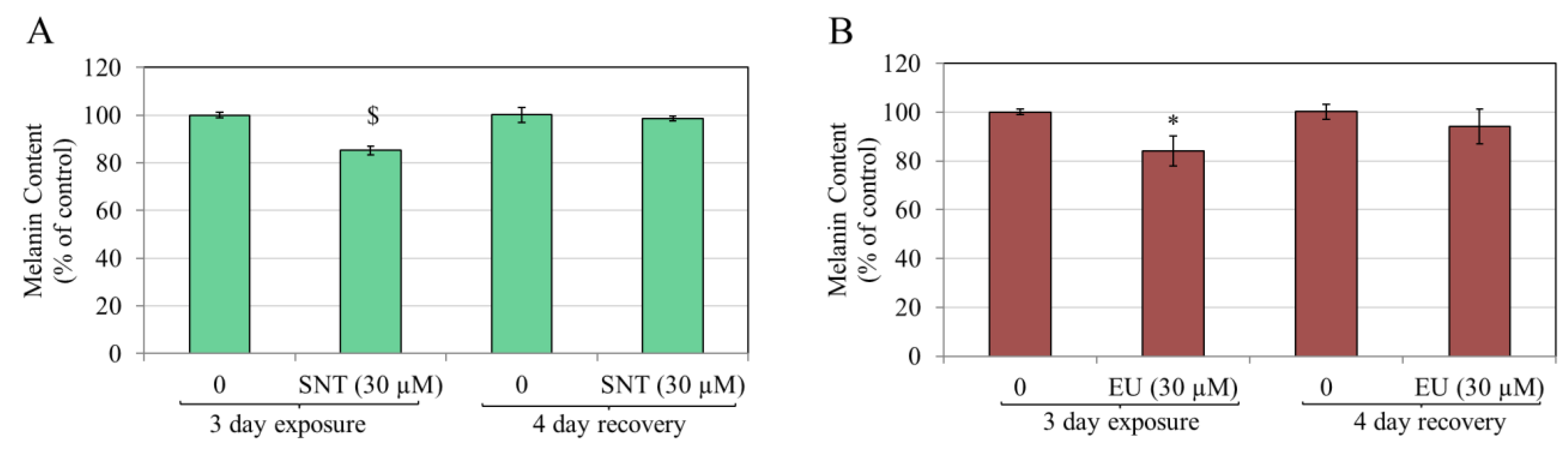
3.2. Effects of Flavones in MNT-1 Human Melanoma Cells
3.3. Effects of Flavones in Darkly Pigmented Primary Human Melanocytes (HEMn-DP Cells)
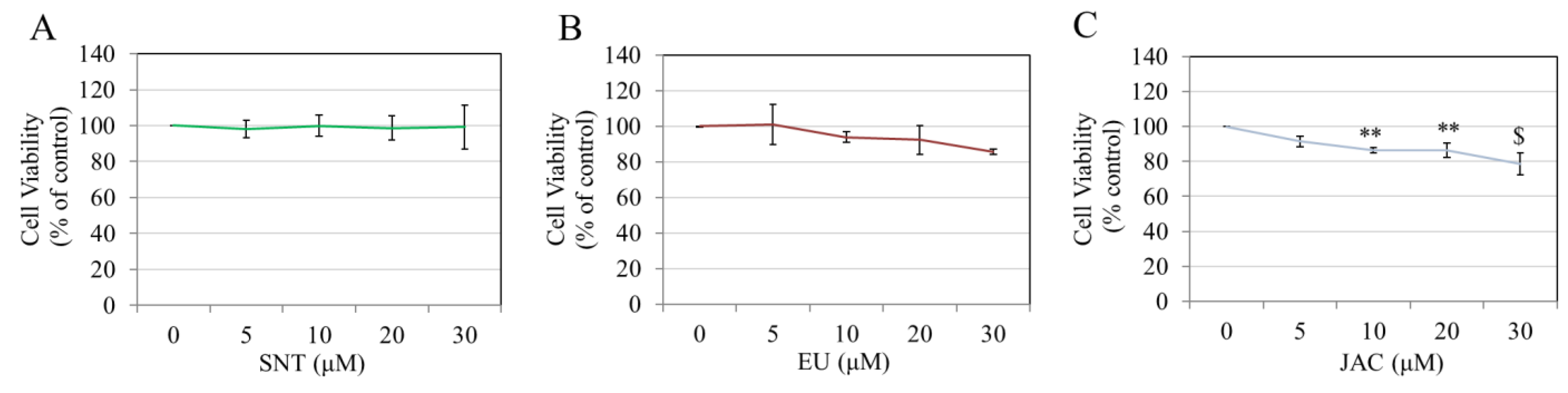
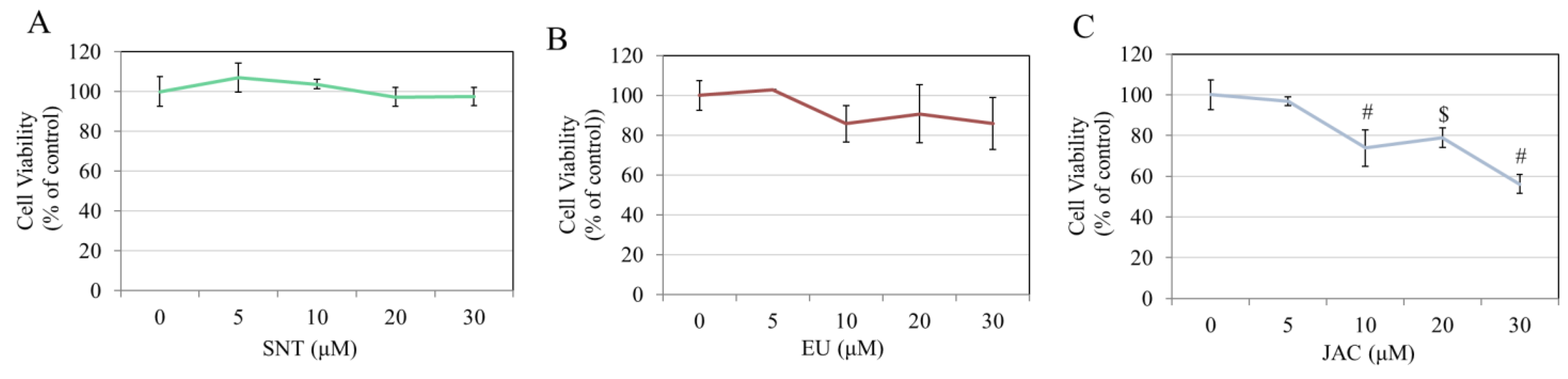
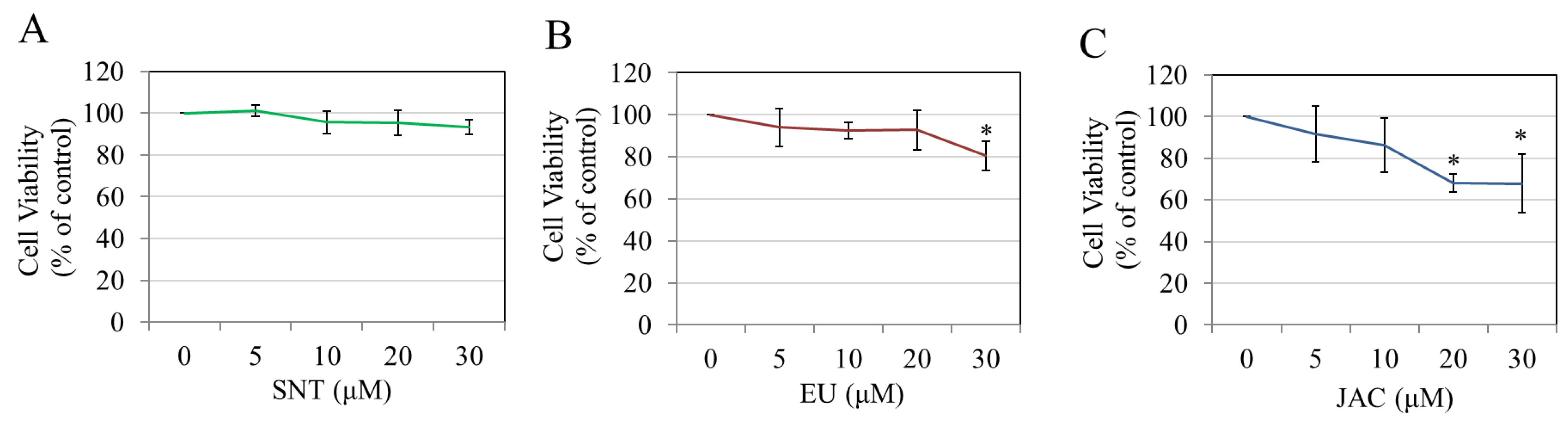
3.4. Effects of Flavones on Keratinocyte Viability
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Rigopoulos, D.; Gregoriou, S.; Katsambas, A. Hyperpigmentation and melasma. J. Cosmet. Dermatol. 2007, 6, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Grimes, P.E. Disorders of pigmentation: Global issues of major cosmetic concern. West. J. Med. 1998, 169, 226–227. [Google Scholar]
- Kanavy, H.E.; Gerstenblith, M.R. Ultraviolet radiation and melanoma. Semin. Cutan. Med. Surg. 2011, 30, 222–228. [Google Scholar] [CrossRef]
- Vashi, N.A.; Wirya, S.A.; Inyang, M.; Kundu, R.V. Facial hyperpigmentation in skin of color: Special considerations and treatment. Am. J. Clin. Dermatol. 2017, 18, 215–230. [Google Scholar] [CrossRef]
- Gaskell, M.; McLuckie, K.I.; Farmer, P.B. Genotoxicity of the benzene metabolites para-benzoquinone and hydroquinone. Chem.-Biol. Interact. 2005, 153, 267–270. [Google Scholar] [CrossRef]
- Hirose, M.; Imai, T.; Mitsumori, K. Carcinogenicity of kojic acid in rodents. JSM Mycotoxins 2004, 2003, 59–67. [Google Scholar] [CrossRef]
- Nakagawa, M.; Kawai, K.; Kawai, K. Contact allergy to kojic acid in skin care products. Contact Dermat. 1995, 32, 9–13. [Google Scholar] [CrossRef]
- Bae-Harboe, Y.-S.C.; Park, H.-Y. Tyrosinase: A central regulatory protein for cutaneous pigmentation. J. Investig. Dermatol. 2012, 132, 2678–2680. [Google Scholar] [CrossRef]
- Vachtenheim, J.; Borovanský, J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef]
- Peng, X.; Ma, Y.; Yan, C.; Wei, X.; Zhang, L.; Jiang, H.; Ma, Y.; Zhang, S.; Xing, M.; Gao, Y. Mechanism, formulation, and efficacy evaluation of natural products for skin pigmentation treatment. Pharmaceutics 2024, 16, 1022. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Fang, Z.; Zhang, P. The melanin inhibitory effect of plants and phytochemicals: A systematic review. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 107, 154449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, J.; Cao, J.; Wang, D.; Liu, C.; Yang, R.; Li, X.; Sun, C. Antioxidant capacity, anticancer ability and flavonoids composition of 35 citrus (Citrus reticulata Blanco) varieties. Molecules 2017, 22, 1114. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A. Fourier transform infrared spectroscopic analysis of the polymethoxylated flavone content of orange oil residues. J. Agric. Food Chem. 2006, 54, 3215–3218. [Google Scholar] [CrossRef]
- Nakanishi, M.; Hino, M.; Yoshimura, M.; Amakura, Y.; Nomoto, H. Identification of sinensetin and nobiletin as major antitrypanosomal factors in a citrus cultivar. Exp. Parasitol. 2019, 200, 24–29. [Google Scholar] [CrossRef]
- Li, S.; Lo, C.-Y.; Ho, C.-T. Hydroxylated polymethoxyflavones and methylated flavonoids in sweet orange (Citrus sinensis) peel. J. Agric. Food Chem. 2006, 54, 4176–4185. [Google Scholar] [CrossRef]
- Haggag, E.; Mahmoud, I.; Abou-Moustafa, E.; Mabry, T. Flavonoids from the leaves of Citrus aurantium (sour orange) and Citrus sinensis (sweet orange). Asian J. Chem. 1999, 11, 707–714. [Google Scholar]
- Hossain, M.A.; Rahman, S.M. Isolation and characterisation of flavonoids from the leaves of medicinal plant Orthosiphon stamineus. Arab. J. Chem. 2015, 8, 218–221. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ismail, Z. Quantification and enrichment of sinensetin in the leaves of Orthosiphon stamineus. Arab. J. Chem. 2016, 9, S1338–S1341. [Google Scholar] [CrossRef]
- Kang, S.I.; Shin, H.S.; Kim, S.J. Sinensetin enhances adipogenesis and lipolysis by increasing cyclic adenosine monophosphate levels in 3T3-L1 adipocytes. Biol. Pharm. Bull. 2015, 38, 552–558. [Google Scholar] [CrossRef]
- Kang, S.I.; Shin, H.S.; Ko, H.C.; Kim, S.J. Effects of sinensetin on lipid metabolism in mature 3T3-L1 adipocytes. Phytother. Res. PTR 2013, 27, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Lam, I.K.; Alex, D.; Wang, Y.H.; Liu, P.; Liu, A.L.; Du, G.H.; Lee, S.M. In vitro and in vivo structure and activity relationship analysis of polymethoxylated flavonoids: Identifying sinensetin as a novel antiangiogenesis agent. Mol. Nutr. Food Res. 2012, 56, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ji, G.; Cao, A.; Shi, J.; Shi, H.; Xie, J.; Wu, D. Effects of sinensetin on proliferation and apoptosis of human gastric cancer AGS cells. Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica 2011, 36, 790–794. [Google Scholar]
- Shin, H.S.; Kang, S.I.; Yoon, S.A.; Ko, H.C.; Kim, S.J. Sinensetin attenuates LPS-induced inflammation by regulating the protein level of IkappaB-alpha. Biosci. Biotechnol. Biochem. 2012, 76, 847–849. [Google Scholar] [CrossRef]
- Laavola, M.; Nieminen, R.; Yam, M.F.; Sadikun, A.; Asmawi, M.Z.; Basir, R.; Welling, J.; Vapaatalo, H.; Korhonen, R.; Moilanen, E. Flavonoids eupatorin and sinensetin present in Orthosiphon stamineus leaves inhibit inflammatory gene expression and STAT1 activation. Planta Med. 2012, 78, 779–786. [Google Scholar] [CrossRef]
- Sun, K.J. Quantitative analysis of eupatilin and jaceosidin in Artemisia herba. Korean J. Crop Sci. 2004, 49, 452–456. [Google Scholar]
- Malhotra, H.; Ashri, A.; Singla, R.K.; Gautam, R.K. Eupatilin: Sources, extraction, derivatives, and pharmacological activity. In Handbook of Dietary Flavonoids; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–50. [Google Scholar]
- Ryoo, S.-B.; Oh, H.-K.; Yu, S.A.; Moon, S.H.; Choe, E.K.; Oh, T.Y.; Park, K.J. The Effects of Eupatilin (Stillen) on Motility of Human Lower Gastrointestinal Tracts. Korean J. Physiol. Pharmacol. 2014, 18, 383–390. [Google Scholar] [CrossRef]
- Lee, B.E.; Park, S.J.; Kim, G.H.; Joo, D.C.; Lee, M.W. Anti-inflammatory effects of eupatilin on Helicobacter pylori CagA-induced gastric inflammation. PLoS ONE 2024, 19, e0313251. [Google Scholar] [CrossRef]
- Giangaspero, A.; Ponti, C.; Pollastro, F.; Del Favero, G.; Della Loggia, R.; Tubaro, A.; Appendino, G.; Sosa, S. Topical anti-inflammatory activity of eupatilin, a lipophilic flavonoid from mountain wormwood (Artemisia umbelliformis Lam.). J. Agric. Food Chem. 2009, 57, 7726–7730. [Google Scholar] [CrossRef]
- Du, L.; Chen, J.; Xing, Y.Q. Eupatilin prevents H2O2-induced oxidative stress and apoptosis in human retinal pigment epithelial cells. Biomed. Pharmacother. 2017, 85, 136–140. [Google Scholar] [CrossRef]
- Bai, D.; Cheng, X.; Li, Q.; Zhang, B.; Zhang, Y.; Lu, F.; Sun, T.; Hao, J. Eupatilin inhibits keratinocyte proliferation and ameliorates imiquimod-induced psoriasis-like skin lesions in mice via the p38 MAPK/NF-κB signaling pathway. Immunopharmacol. Immunotoxicol. 2023, 45, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Min, Q.; Zhao, X.; Li, L.; Zhao, G.; Dong, J. Eupatilin attenuates doxorubicin-induced cardiotoxicity by activating the PI3K-AKT signaling pathway in mice. Mol. Cell. Biochem. 2024, 479, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Gaire, B.P.; Cho, K.S.; Jeon, S.J.; Kwon, O.W.; Jang, D.S.; Kim, S.Y.; Ryu, J.H.; Choi, J.W. Eupatilin exerts neuroprotective effects in mice with transient focal cerebral ischemia by reducing microglial activation. PLoS ONE 2017, 12, e0171479. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Phan, P.T.; Hong, J.G.; Kim, D.H.; Kim, J.M.; Park, S.J.; Liu, X.; Han, J.E.; Park, H.; Choi, J.W.; et al. The neuroprotective effect of eupatilin against ischemia/reperfusion-induced delayed neuronal damage in mice. Eur. J. Pharmacol. 2012, 689, 104–110. [Google Scholar] [CrossRef]
- Hong, L.; Yang, C. Eupatilin ameliorates postmenopausal osteoporosis via elevating microRNA-211-5p and repressing Janus kinase 2/Signal transducer and activator of transcription 3 pathway. Mol. Cell. Biochem. 2024, 479, 2471–2481. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, H.; Li, M.; Yang, Y.; Sun, L. Anticancer effect of eupatilin on glioma cells through inhibition of the Notch-1 signaling pathway. Mol. Med. Rep. 2016, 13, 1141–1146. [Google Scholar] [CrossRef]
- Park, B.B.; Yoon, J.; Kim, E.; Choi, J.; Won, Y.; Choi, J.; Lee, Y.Y. Inhibitory effects of eupatilin on tumor invasion of human gastric cancer MKN-1 cells. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2013, 34, 875–885. [Google Scholar] [CrossRef]
- Kim, M.J.; Han, J.M.; Jin, Y.Y.; Baek, N.I.; Bang, M.H.; Chung, H.G.; Choi, M.S.; Lee, K.T.; Sok, D.E.; Jeong, T.S. In vitro antioxidant and anti-inflammatory activities of Jaceosidin from Artemisia princeps Pampanini cv. Sajabal. Arch. Pharmacal Res. 2008, 31, 429–437. [Google Scholar] [CrossRef]
- Nam, Y.; Choi, M.; Hwang, H.; Lee, M.G.; Kwon, B.M.; Lee, W.H.; Suk, K. Natural flavone jaceosidin is a neuroinflammation inhibitor. Phytother. Res. 2013, 27, 404–411. [Google Scholar] [CrossRef]
- Clavin, M.; Gorzalczany, S.; Macho, A.; Munoz, E.; Ferraro, G.; Acevedo, C.; Martino, V. Anti-inflammatory activity of flavonoids from Eupatorium arnottianum. J. Ethnopharmacol. 2007, 112, 585–589. [Google Scholar] [CrossRef]
- Khan, M.; Yu, B.; Rasul, A.; Al Shawi, A.; Yi, F.; Yang, H.; Ma, T. Jaceosidin induces apoptosis in U87 glioblastoma cells through G2/M phase arrest. Evid.-Based Complement. Altern. Med. 2011, 2012, 703034. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Son, Y.O.; Lee, S.A.; Jeon, Y.M.; Lee, J.C. Quercetin inhibits α-MSH-stimulated melanogenesis in B16F10 melanoma cells. Phytother. Res. 2011, 25, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Takekoshi, S.; Nagata, H.; Kitatani, K. Flavonoids enhance melanogenesis in human melanoma cells. Tokai J. Exp. Clin. Med. 2014, 39, 116–121. [Google Scholar] [PubMed]
- An, S.M.; Kim, H.J.; Kim, J.E.; Boo, Y.C. Flavonoids, taxifolin and luteolin attenuate cellular melanogenesis despite increasing tyrosinase protein levels. Phytother. Res. 2008, 22, 1200–1207. [Google Scholar] [CrossRef]
- Nagata, H.; Takekoshi, S.; Takeyama, R.; Homma, T.; Yoshiyuki Osamura, R. Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and in normal human melanocytes. Pigment. Cell Res. 2004, 17, 66–73. [Google Scholar] [CrossRef]
- Yoon, H.S.; Kim, I.-J. Nobiletin induces differentiation of murine B16/F10 melanoma cells. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 353–361. [Google Scholar] [CrossRef]
- Kim, H.J.; Yonezawa, T.; Teruya, T.; Woo, J.T.; Cha, B.Y. Nobiletin, a polymethoxy flavonoid, reduced endothelin-1 plus SCF-induced pigmentation in human melanocytes. Photochem. Photobiol. 2015, 91, 379–386. [Google Scholar] [CrossRef]
- Guo, C.; Shan, Y.; Yang, Z.; Zhang, L.; Ling, W.; Liang, Y.; Ouyang, Z.; Zhong, B.; Zhang, J. Chemical composition, antioxidant, antibacterial, and tyrosinase inhibition activity of extracts from Newhall navel orange (Citrus sinensis Osbeck cv. Newhall) peel. J. Sci. Food Agric. 2020, 100, 2664–2674. [Google Scholar] [CrossRef]
- Yoon, H.S.; Ko, H.-C.; Kim, S.-J.; Kim, S.S.; Choi, Y.H.; An, H.J.; Lee, N.H.; Hyun, C.-G. Stimulatory effects of a 5, 6, 7, 3′, 4′-pentamethoxyflavone, sinensetin, on melanogenesis in B16/F10 murine melanoma cells. Lat. Am. J. Pharm. 2015, 34, 1087–1092. [Google Scholar]
- Zengin, G.; Nilofar; Yildiztugay, E.; Bouyahya, A.; Cavusoglu, H.; Gevrenova, R.; Zheleva-Dimitrova, D. A Comparative study on UHPLC-HRMS profiles and biological activities of Inula sarana different extracts and its beta-cyclodextrin complex: Effective insights for novel applications. Antioxidants 2023, 12, 1842. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Huang, Z.; Zhang, Y.; Lu, Y.; Zhou, Y. Evaluation of whitening effects and identification of potentially active compounds based on untargeted metabolomic analysis in different chrysanthemum cultivar extracts. Antioxidants 2024, 13, 1557. [Google Scholar] [CrossRef] [PubMed]
- Okhundedaev, B.S.; Bacher, M.; Mukhamatkhanova, R.F.; Shamyanov, I.J.; Zengin, G.; Böhmdorfer, S.; Mamadalieva, N.Z.; Rosenau, T. Flavone glucosides from Artemisia juncea. Nat. Prod. Res. 2019, 33, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Johnson, F.; Simon, S.R. Novel chemically modified curcumin (CMC) derivatives inhibit tyrosinase activity and melanin synthesis in B16f10 mouse melanoma cells. Biomolecules 2021, 11, 674. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Comparative study of curcumin and its hydrogenated metabolites, tetrahydrocurcumin, hexahydrocurcumin, and octahydrocurcumin, on melanogenesis in B16F10 and MNT-1 cells. Cosmetics 2021, 8, 4. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Cmt-308, a nonantimicrobial chemically-modified tetracycline, exhibits anti-melanogenic activity by suppression of melanosome export. Biomedicines 2020, 8, 411. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Asoprisnil, a selective progesterone receptor modulator (SPRM), inhibits melanosome export in B16F10 cells and HEMn-DP melanocytes. Molecules 2020, 25, 3581. [Google Scholar] [CrossRef]
- ATCC. MNT-1, CRL-3450 TM. Available online: https://www.atcc.org/products/crl-3450 (accessed on 20 February 2025).
- Human Epidermal Melanocytes, Neonatal, Darkly Pigmented Donor, (HEMn-DP). Available online: https://www.thermofisher.com/order/catalog/product/C2025C (accessed on 24 December 2024).
- AddexBio. HaCaT Cells, Catalog #:T0020001. Available online: https://addexbio.com/productdetail?pid=117 (accessed on 20 February 2025).
- ATCC. B16-F10, CRL-6475 TM. Available online: https://www.atcc.org/products/crl-6475 (accessed on 20 February 2025).
- O’brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Novel Chemically Modified Curcumin (CMC) analogs exhibit anti-melanogenic activity in primary human melanocytes. Int. J. Mol. Sci. 2021, 22, 6043. [Google Scholar] [CrossRef]
- Takeyama, R.; Takekoshi, S.; Nagata, H.; Yoshiyuki Osamura, R.; Kawana, S. Quercetin-induced melanogenesis in a reconstituted three-dimensional human epidermal model. J. Mol. Histol. 2004, 35, 157–165. [Google Scholar] [CrossRef]
- Horibe, I.; Satoh, Y.; Shiota, Y.; Kumagai, A.; Horike, N.; Takemori, H.; Uesato, S.; Sugie, S.; Obata, K.; Kawahara, H.; et al. Induction of melanogenesis by 4′-O-methylated flavonoids in B16F10 melanoma cells. J. Nat. Med. 2013, 67, 705–710. [Google Scholar] [CrossRef]
- Kumagai, A.; Horike, N.; Satoh, Y.; Uebi, T.; Sasaki, T.; Itoh, Y.; Hirata, Y.; Uchio-Yamada, K.; Kitagawa, K.; Uesato, S.; et al. A potent inhibitor of SIK2, 3, 3′, 7-trihydroxy-4′-methoxyflavon (4′-O-methylfisetin), promotes melanogenesis in B16F10 melanoma cells. PLoS ONE 2011, 6, e26148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sakamoto, K. Citric acid promoted melanin synthesis in B16F10 mouse melanoma cells, but inhibited it in human epidermal melanocytes and HMV-II melanoma cells via the GSK3β/β-catenin signaling pathway. PLoS ONE 2020, 15, e0243565. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Yoshizaki, F. Nobiletin as a tyrosinase inhibitor from the peel of Citrus fruit. Biol. Pharm. Bull. 2002, 25, 806–808. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Y.; Tao, L.; Tao, X.; Su, X.; Wei, D. Tyrosinase inhibitory effects and inhibition mechanisms of nobiletin and hesperidin from citrus peel crude extracts. J. Enzym. Inhib. Med. Chem. 2007, 22, 83–90. [Google Scholar] [CrossRef]
- Yoshizaki, N.; Hashizume, R.; Masaki, H. A polymethoxyflavone mixture extracted from orange peels, mainly containing nobiletin, 3, 3′, 4′, 5, 6, 7, 8-heptamethoxyflavone and tangeretin, suppresses melanogenesis through the acidification of cell organelles, including melanosomes. J. Dermatol. Sci. 2017, 88, 78–84. [Google Scholar] [CrossRef]
- Yoon, H.S.; Ko, H.-C.; Kim, S.S.; Park, K.J.; An, H.J.; Choi, Y.H.; Kim, S.-J.; Lee, N.-H.; Hyun, C.-G. Tangeretin triggers melanogenesis through the activation of melanogenic signaling proteins and sustained extracellular signal-regulated kinase in B16/F10 murine melanoma cells. Nat. Prod. Commun. 2015, 10, 389–392. [Google Scholar] [CrossRef]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Owis, A.I. Citrus polymethoxyflavones: Biofunctional molecules of therapeutic interest. Stud. Nat. Prod. Chem. 2018, 59, 509–530. [Google Scholar]
- Jamaluddin, A.; Yusof, N.; Abdul Rahman, S.; Pilus, N. Effect of Aspergilus oryzae-fermented broken rice, brewers’ rice and rice bran on melanogenesis in highly pigmented human melanoma, MNT-1. Food Res. 2023, 6, 81–89. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Y.; Wang, W.; Zhang, J.; Yin, J.; Le, T.; Xue, J.; Engelhardt, U.H.; Jiang, H. Kojic acid showed consistent inhibitory activity on tyrosinase from mushroom and in cultured B16F10 cells compared with arbutins. Antioxidants 2022, 11, 502. [Google Scholar] [CrossRef]
- Kim, D.-S.; Kim, S.-Y.; Chung, J.-H.; Kim, K.-H.; Eun, H.-C.; Park, K.-C. Delayed ERK activation by ceramide reduces melanin synthesis in human melanocytes. Cell. Signal. 2002, 14, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Yoshimori, A.; Ogawa, H.; Shirai, Y.; Abe, H.; Kamiya, T.; Tanuma, S.-i. The structural differences between mushroom and human tyrosinase cleared by investigating the inhibitory activities of stilbenes. J. Mol. Struct. 2023, 1272, 134180. [Google Scholar] [CrossRef]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Hearing, V.J.; Röhm, K.-H.; Kolbe, L. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Hyun, C.-G. Mechanistic Insights into the Stimulatory Effect of Melanogenesis of 4-Methylcoumarin Derivatives in B16F10 Melanoma Cells. Int. J. Mol. Sci. 2024, 25, 12421. [Google Scholar] [CrossRef]
- Alsantali, R.I.; Mughal, E.U.; Naeem, N.; Alsharif, M.A.; Sadiq, A.; Ali, A.; Jassas, R.S.; Javed, Q.; Javid, A.; Sumrra, S.H.; et al. Flavone-based hydrazones as new tyrosinase inhibitors: Synthetic imines with emerging biological potential, SAR, molecular docking and drug-likeness studies. J. Mol. Struct. 2022, 1251, 131933. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Chae, J.-B.; Lee, S.G.; Min, K.; Kwon, T.K.; Nam, J.-O. Apoptotic effect of jaceosidin on MCF-7 human breast cancer cells through modulation of ERK and p38 MAPK pathways. Nat. Prod. Res. 2021, 35, 6049–6053. [Google Scholar] [CrossRef]
- Lv, W.; Sheng, X.; Chen, T.; Xu, Q.; Xie, X. Jaceosidin induces apoptosis in human ovary cancer cells through mitochondrial pathway. J. Biomed. Biotechnol. 2008, 2008, 394802. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.-M.; Tang, Y.-J.; Cao, J.-L.; Hou, W.-S.; Wang, A.-Q.; Wang, C.; Jin, C.-H. Jaceosidin induces apoptosis and inhibits migration in AGS gastric cancer cells by regulating ROS-mediated signaling pathways. Redox Rep. 2024, 29, 2313366. [Google Scholar] [CrossRef]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Influence of membrane lipid composition on flavonoid–membrane interactions: Implications on their biological activity. Prog. Lipid Res. 2015, 58, 1–13. [Google Scholar] [CrossRef]
- Tsuchiya, H. Structure-dependent membrane interaction of flavonoids associated with their bioactivity. Food Chem. 2010, 120, 1089–1096. [Google Scholar] [CrossRef]
- Jeong, J.-M.; Choi, C.-H.; Kang, S.-K.; Lee, I.-H.; Lee, J.-Y.; Jung, H. Antioxidant and chemosensitizing effects of flavonoids with hydroxy and/or methoxy groups and structure-activity relationship. J. Pharm. Pharm. Sci. 2007, 10, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Kanadaswami, C.; Lee, L.-T.; Lee, P.-P.H.; Hwang, J.-J.; Ke, F.-C.; Huang, Y.-T.; Lee, M.-T. The antitumor activities of flavonoids. Vivo 2005, 19, 895–909. [Google Scholar]
- Ko, H.-H.; Chiang, Y.-C.; Tsai, M.-H.; Liang, C.-J.; Hsu, L.-F.; Li, S.-Y.; Wang, M.-C.; Yen, F.-L.; Lee, C.-W. Eupafolin, a skin whitening flavonoid isolated from Phyla nodiflora, downregulated melanogenesis: Role of MAPK and Akt pathways. J. Ethnopharmacol. 2014, 151, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Ahn, S.; Chang, H.; Cho, N.S.; Joo, K.; Lee, B.G.; Chang, I.; Hwang, J.S. Influence of N-glycan processing disruption on tyrosinase and melanin synthesis in HM3KO melanoma cells. Exp. Dermatol. 2007, 16, 110–117. [Google Scholar] [CrossRef]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Investig. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef]
- Mikami, M.; Sonoki, T.; Ito, M.; Funasaka, Y.; Suzuki, T.; Katagata, Y. Glycosylation of tyrosinase is a determinant of melanin production in cultured melanoma cells. Mol. Med. Rep. 2013, 8, 818–822. [Google Scholar] [CrossRef]
- Bin, B.H.; Seo, J.; Yang, S.H.; Lee, E.; Choi, H.; Kim, K.H.; Cho, E.G.; Lee, T.R. Novel inhibitory effect of the antidiabetic drug voglibose on melanogenesis. Exp. Dermatol. 2013, 22, 541–546. [Google Scholar] [CrossRef]
- Liu, D.; Cao, X.; Kong, Y.; Mu, T.; Liu, J. Inhibitory mechanism of sinensetin on α-glucosidase and non-enzymatic glycation: Insights from spectroscopy and molecular docking analyses. Int. J. Biol. Macromol. 2021, 166, 259–267. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Siddiqui, M.J.; Ang, L.F.; Sadikun, A.; Chan, S.H.; Tan, S.C.; Asmawi, M.Z.; Yam, M.F. Potent alpha-glucosidase and alpha-amylase inhibitory activities of standardized 50% ethanolic extracts and sinensetin from Orthosiphon stamineus Benth as anti-diabetic mechanism. BMC Complement. Altern. Med. 2012, 12, 176. [Google Scholar] [CrossRef]
- Shah, S.W.A.; Ghias, M.; Shoaib, M.; Ali, N.; Shah, I.; Umar, M.N.; Shah, S.M.M.; Shah, S.M.H.; Khan, W.; Khan, S.; et al. Antidiabetic potential of flavonoids from Artemisia macrocephalla Jaquem in streptozotocin-induced diabetic rats: Pharmacological and biochemical approach. Pak. J. Pharm. Sci. 2019, 32, 2865–2871. [Google Scholar]
- Yao, X.; Xu, X.; Fan, G.; Qiao, Y.; Cao, S.; Pan, S. Determination of synergistic effects of polymethoxylated flavone extracts of Jinchen orange peels (Citrus sinensis Osberk) with amino acids and organic acids using chemiluminescence. Eur. Food Res. Technol. 2009, 229, 743–750. [Google Scholar] [CrossRef]
- Manap, A.S.A.; Lum, Y.K.; Ong, L.H.; Tang, Y.-Q.; Gew, L.T.; Chia, A.Y.Y. Perspective approaches on melanogenesis inhibition. Dermatol. Sin. 2021, 39, 1–12. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.K.; Subedi, L.; Jeong, M.; Park, Y.U.; Kim, C.Y.; Kim, H.; Kim, S.Y. Gomisin N inhibits melanogenesis through regulating the PI3K/Akt and MAPK/ERK signaling pathways in melanocytes. Int. J. Mol. Sci. 2017, 18, 471. [Google Scholar] [CrossRef]
- Kim, E.S.; Shin, J.H.; Seok, S.H.; Kim, J.B.; Chang, H.; Park, S.J.; Jo, Y.K.; Choi, E.S.; Park, J.-S.; Yeom, M.H.; et al. Autophagy mediates anti-melanogenic activity of 3′-ODI in B16F1 melanoma cells. Biochem. Biophys. Res. Commun. 2013, 442, 165–170. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Gowrisankar, Y.V.; Wang, L.-W.; Zhang, Y.-Z.; Chen, X.-Z.; Huang, P.-J.; Yen, H.-R.; Yang, H.-L. The in vitro and in vivo depigmenting activity of pterostilbene through induction of autophagy in melanocytes and inhibition of UVA-irradiated α-MSH in keratinocytes via Nrf2-mediated antioxidant pathways. Redox Biol. 2021, 44, 102007. [Google Scholar] [CrossRef]
- Park, H.J.; Jo, D.S.; Choi, H.; Bae, J.-E.; Park, N.Y.; Kim, J.B.; Choi, J.Y.; Kim, Y.H.; Oh, G.S.; Chang, J.H.; et al. Melasolv induces melanosome autophagy to inhibit pigmentation in B16F1 cells. PLoS ONE 2020, 15, e0239019. [Google Scholar] [CrossRef]
- Cho, Y.H.; Park, J.E.; Lee, J.S. Tranexamic acid inhibits melanogenesis by activating the autophagy system in cultured melanoma cells. J. Dermatol. Sci. 2017, 88, 96–102. [Google Scholar] [CrossRef]
- Lee, M.; Yang, C.; Song, G.; Lim, W. Eupatilin impacts on the progression of colon cancer by mitochondria dysfunction and oxidative stress. Antioxidants 2021, 10, 957. [Google Scholar] [CrossRef]
- Lou, Y.; Wu, J.; Liang, J.; Yang, C.; Wang, K.; Wang, J.; Guo, X. Eupatilin protects chondrocytes from apoptosis via activating sestrin2-dependent autophagy. Int. Immunopharmacol. 2019, 75, 105748. [Google Scholar] [CrossRef]
- Kong, Z.; Lv, W.; Wang, Y.; Huang, Y.; Che, K.; Nan, H.; Xin, Y.; Wang, J.; Chen, J.; Wang, Y.; et al. Sinensetin ameliorates high glucose-induced diabetic nephropathy via enhancing autophagy in vitro and in vivo. J. Biochem. Mol. Toxicol. 2023, 37, e23445. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.-J.; Deng, Z.-B.; Liu, J.-N.; Qiu, J.-J.; Guo, L.; Feng, P.-P.; Sui, J.-R.; Chen, D.-P.; Guo, H.-S. Enhancement of epithelial cell autophagy induced by sinensetin alleviates epithelial barrier dysfunction in colitis. Pharmacol. Res. 2019, 148, 104461. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Piras, F.; Pollastro, F.; Sogos, V.; Appendino, G.; Nieddu, M. Comparative evaluation of anticancer activity of natural methoxylated flavones xanthomicrol and eupatilin in A375 skin melanoma cells. Life 2024, 14, 304. [Google Scholar] [CrossRef] [PubMed]
- Shawi, A.; Rasul, A.; Khan, M.; Iqbal, F.; Tonghui, M. Eupatilin: A flavonoid compound isolated from the artemisia plant, induces apoptosis and G2/M phase cell cycle arrest in human melanoma A375 cells. Afr. J. Pharm. Pharmacol. 2011, 5, 582–588. [Google Scholar] [CrossRef]
- Manthey, J.A.; Guthrie, N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002, 50, 5837–5843. [Google Scholar] [CrossRef]
- Brozyna, A.; Jozwicki, W.; Roszkowski, K.; Filipiak, J.; Slominski, A. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget 2016, 7, 17844–17853. [Google Scholar] [CrossRef]
- Slominski, A.; Zbytek, B.; Slominski, R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer 2009, 124, 1470–1477. [Google Scholar] [CrossRef]
- Brożyna, A.A.; VanMiddlesworth, L.; Slominski, A.T. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int. J. Cancer 2008, 123, 1448–1456. [Google Scholar] [CrossRef]
- Chu, C.-N.; Hu, K.-C.; Wu, R.S.-C.; Bau, D.-T. Radiation-irritated skin and hyperpigmentation may impact the quality of life of breast cancer patients after whole breast radiotherapy. BMC Cancer 2021, 21, 330. [Google Scholar] [CrossRef]
- Boissy, R.E.; Visscher, M.; DeLong, M.A. DeoxyArbutin: A novel reversible tyrosinase inhibitor with effective in vivo skin lightening potency. Exp. Dermatol. 2005, 14, 601–608. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.J.; Lee, J.Y.; Park, Y.M. Topical application of eupatilin ameliorates atopic dermatitis-like skin lesions in NC/Nga mice. Ann. Dermatol. 2017, 29, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Suhaimi, S.; Hasham, R.; Rosli, N. Effects of formulation parameters on particle size and polydispersity index of Orthosiphon stamineus loaded nanostructured lipid carrier. J. Adv. Res. Appl. Sci. Eng. Technol. 2015, 1, 36–39. [Google Scholar]
- Hayden, P.J.; Bachelor, M.; Ayehunie, S.; Letasiova, S.; Kaluzhny, Y.; Klausner, M.; Kandárová, H. Application of MatTek in vitro reconstructed human skin models for safety, efficacy screening, and basic preclinical research. Appl. Vitr. Toxicol. 2015, 1, 226–233. [Google Scholar] [CrossRef]
- Goenka, S. Cyclocurcumin, a minor curcuminoid, is a novel candidate for hypopigmentary skin disorders with melanogenesis-stimulating capacity. Drugs Drug Candidates 2024, 3, 410–436. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Organogold drug Auranofin exhibits anti-melanogenic activity in B16F10 and MNT-1 melanoma cells. Arch. Dermatol. Res. 2020, 312, 213–221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goenka, S. Effects of Sinensetin, Eupatilin, and Jaceosidin on Human Melanogenesis: A Pilot Study. Future Pharmacol. 2025, 5, 12. https://doi.org/10.3390/futurepharmacol5010012
Goenka S. Effects of Sinensetin, Eupatilin, and Jaceosidin on Human Melanogenesis: A Pilot Study. Future Pharmacology. 2025; 5(1):12. https://doi.org/10.3390/futurepharmacol5010012
Chicago/Turabian StyleGoenka, Shilpi. 2025. "Effects of Sinensetin, Eupatilin, and Jaceosidin on Human Melanogenesis: A Pilot Study" Future Pharmacology 5, no. 1: 12. https://doi.org/10.3390/futurepharmacol5010012
APA StyleGoenka, S. (2025). Effects of Sinensetin, Eupatilin, and Jaceosidin on Human Melanogenesis: A Pilot Study. Future Pharmacology, 5(1), 12. https://doi.org/10.3390/futurepharmacol5010012






