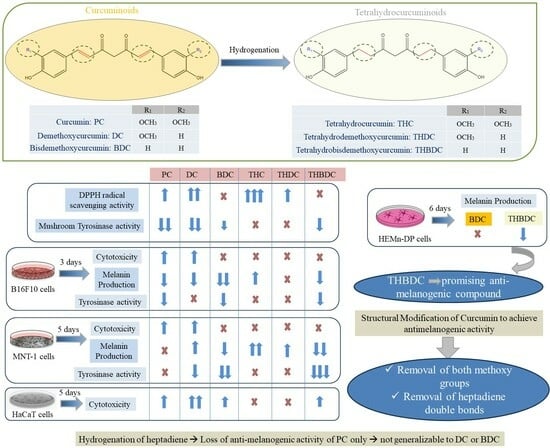
Comparative Study of the Effects of Curcuminoids and Tetrahydrocurcuminoids on Melanogenesis: Role of the Methoxy Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mushroom Tyrosinase Activity
2.3. DPPH Radical Scavenging Assay
2.4. Cell Culture
2.5. MTS Cell Viability Assay
2.6. Estimation of Cellular Melanin Content
2.7. Intracellular Tyrosinase Activity
2.8. Statistical Analysis
3. Results
3.1. Effect of Compounds on Mushroom Tyrosinase Activity and Antioxidant Activity in a Cell-Free System
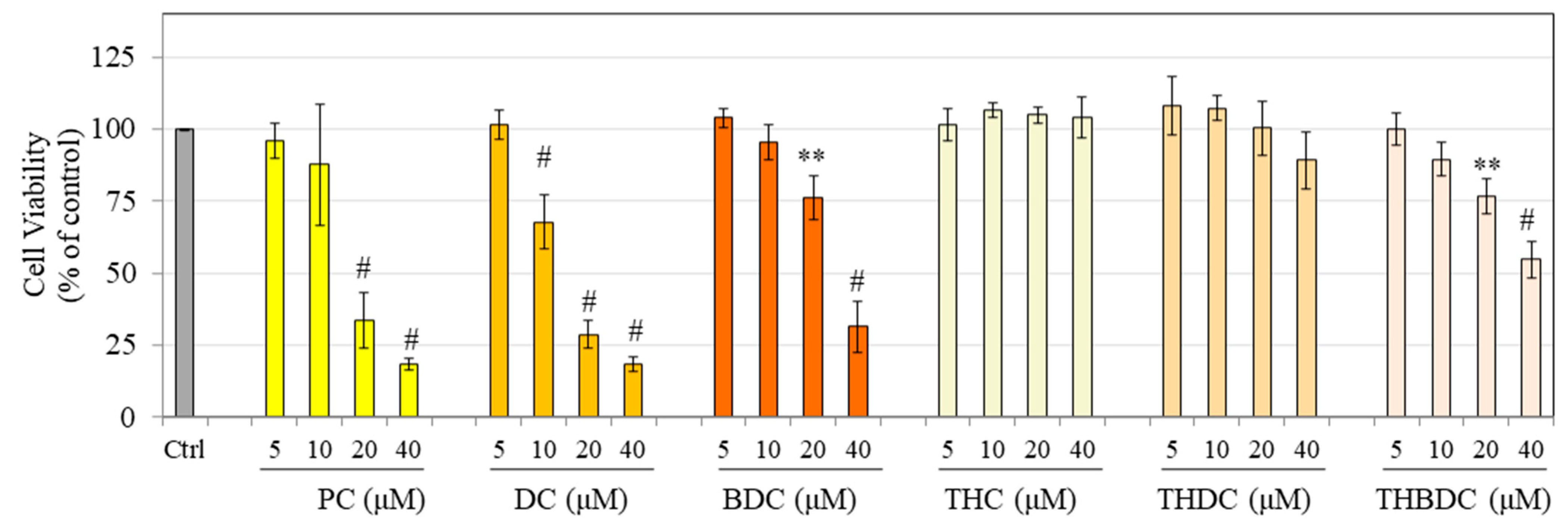
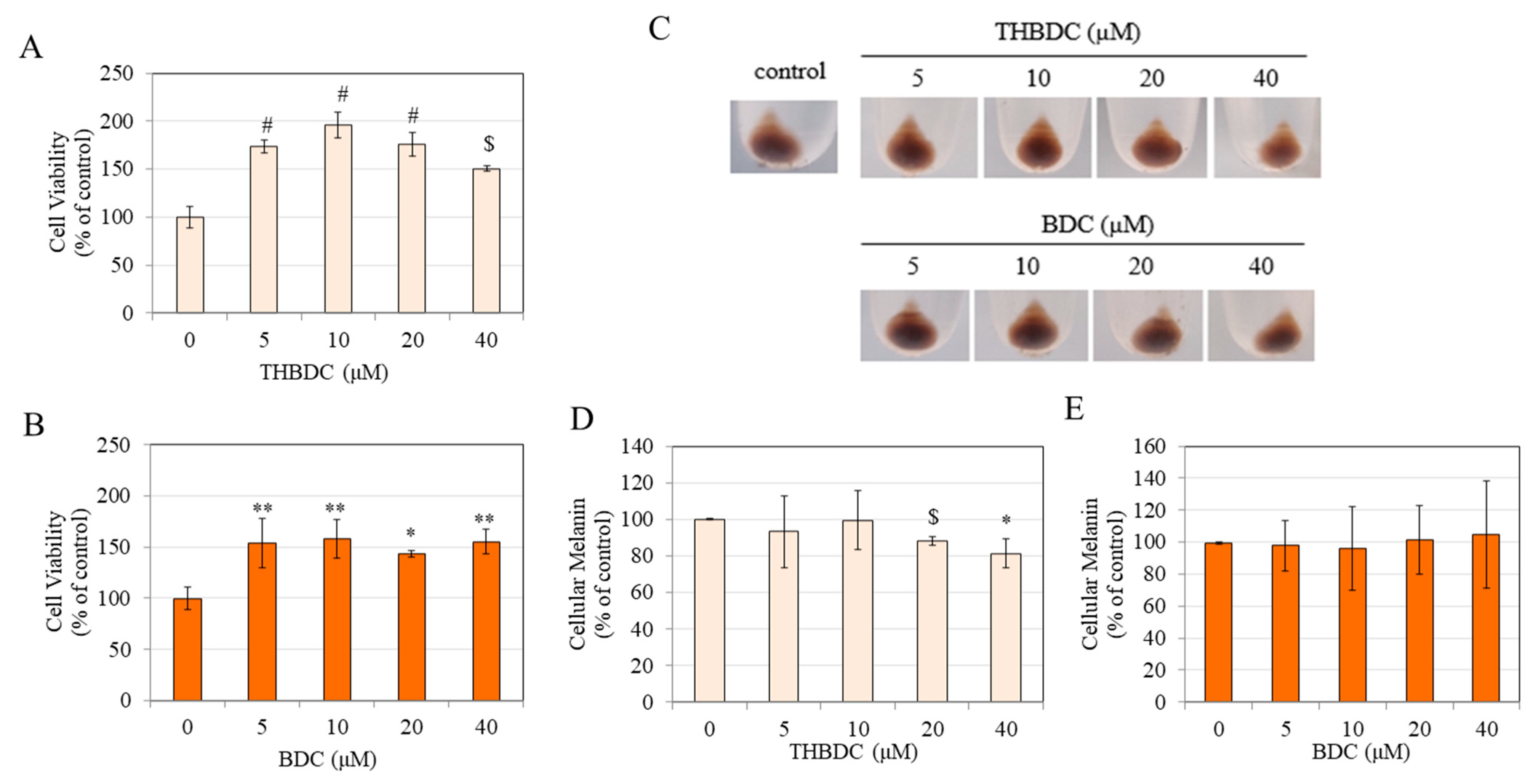
3.2. Effect of Compounds on Viability and Melanin Production in B16F10 Cells
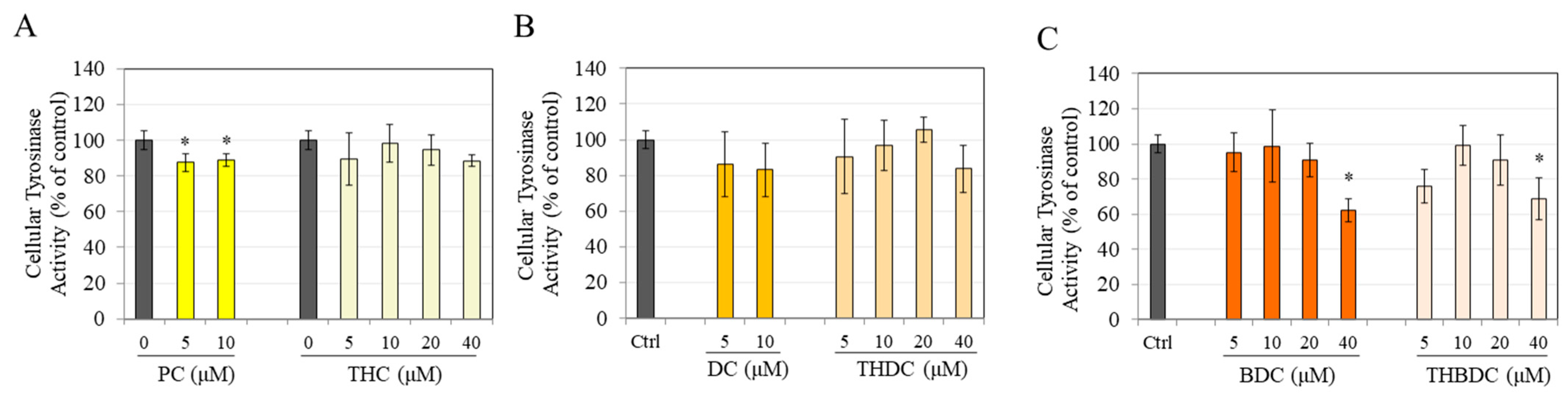
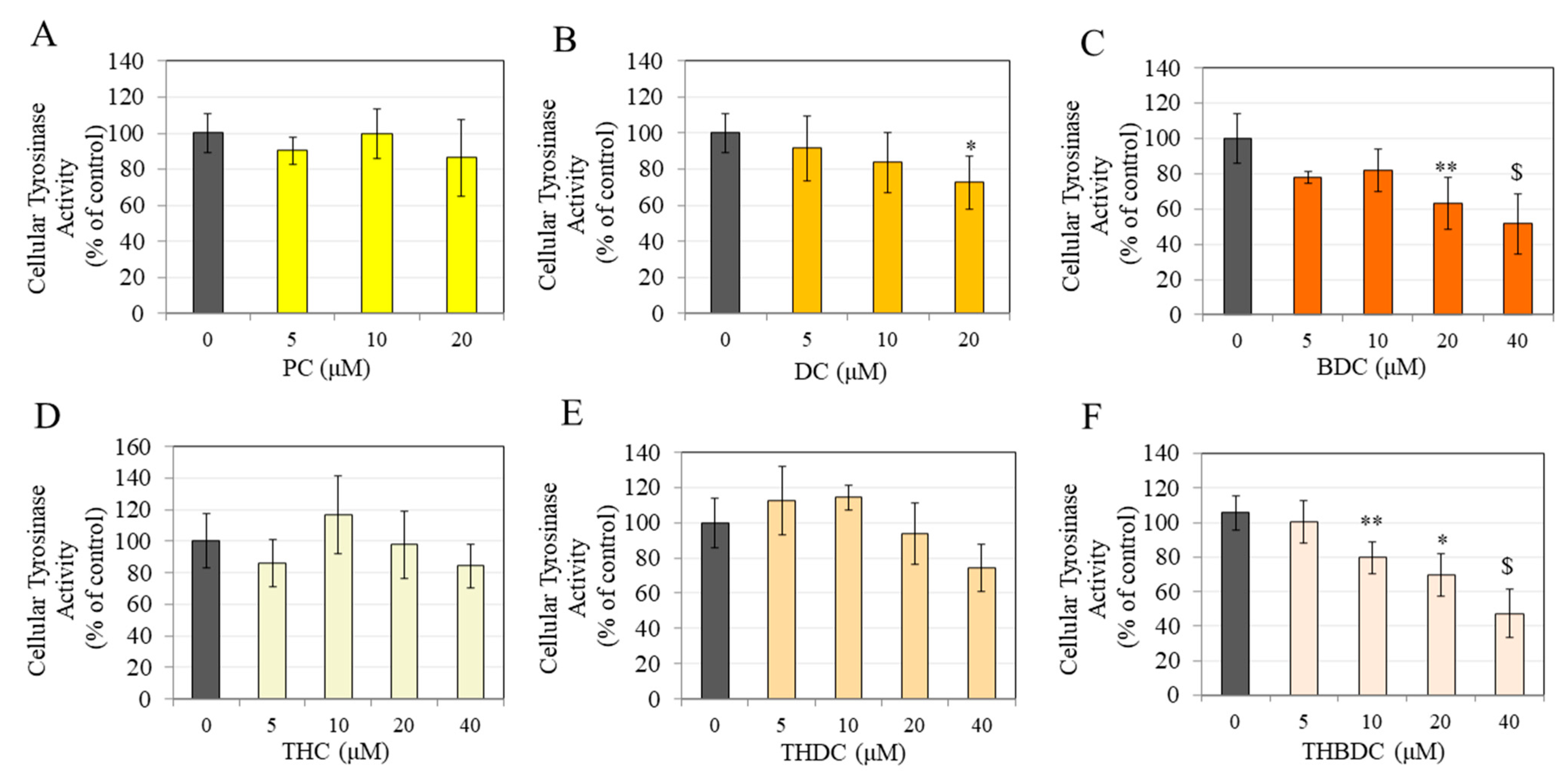
3.3. Effect of Compounds on Tyrosinase Activity in B16F10 cells
3.4. Effect of Compounds on Viability and Melanin Production in MNT-1 Cells
3.5. Effect of Compounds on MNT-1 Cell Morphology
3.6. Effect of Compounds on Tyrosinase Activity in MNT-1 Cells
3.7. Effect of Compounds on HaCaT Cell Viability
3.8. Effect of Compounds on HEMn-DP Cells
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, R.F.; Ko, D.; Friedman, B.J.; Lim, H.W.; Mohammad, T.F. Disorders of hyperpigmentation. Part I. Pathogenesis and clinical features of common pigmentary disorders. J. Am. Acad. Dermatol. 2023, 88, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Choubey, V.; Devadasan, S. Hyperpigmentary skin disorders. In Atlas of Dermatology, Dermatopathology and Venereology: Inflammatory Dermatoses; Springer: Berlin/Heidelberg, Germany, 2022; pp. 223–241. [Google Scholar]
- Rzepka, Z.; Buszman, E.; Beberok, A.; Wrześniok, D. From tyrosine to melanin: Signaling pathways and factors regulating melanogenesis. Adv. Hyg. Exp. Med. 2016, 70, 695–708. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.; Bae, H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Masum, M.N.; Yamauchi, K.; Mitsunaga, T. Tyrosinase inhibitors from natural and synthetic sources as skin-lightening agents. Rev. Agric. Sci. 2019, 7, 41–58. [Google Scholar] [CrossRef]
- Chang, T.-S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef]
- Saba, E.; Kim, S.-H.; Lee, Y.Y.; Park, C.-K.; Oh, J.-W.; Kim, T.-H.; Kim, H.-K.; Roh, S.-S.; Rhee, M.H. Korean Red Ginseng extract ameliorates melanogenesis in humans and induces antiphotoaging effects in ultraviolet B–irradiated hairless mice. J. Ginseng Res. 2020, 44, 496–505. [Google Scholar] [CrossRef]
- Lee, C.-S.; Nam, G.; Bae, I.-H.; Park, J. Whitening efficacy of ginsenoside F1 through inhibition of melanin transfer in cocultured human melanocytes–keratinocytes and three-dimensional human skin equivalent. J. Ginseng Res. 2019, 43, 300. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef]
- Karthik Varma, A.C.; Jude, S.; Varghese, B.A.; Kuttappan, S.; Amalraj, A. Curcuma longa. In Herbs, Spices and Their Roles in Nutraceuticals and Functional Foods; Elsevier: Amsterdam, The Netherlands, 2023; pp. 15–30. [Google Scholar]
- Majeed, M.; Bani, S.; Pandey, A.; Ibrahim, A.M.; Thazhathidath, S. Assessment of Safety Profile of Activated Curcumin C3 Complex (AC 3®), Enriched Extract of Bisdemethoxycurcumin from the Rhizomes of Curcuma longa. J. Toxicol. 2023, 2023, 3729399. [Google Scholar] [CrossRef]
- Fadus, M.C.; Lau, C.; Bikhchandani, J.; Lynch, H.T. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. J. Tradit. Complement. Med. 2017, 7, 339–346. [Google Scholar] [CrossRef]
- Mir, R.H.; Mir, P.A.; Shah, A.J.; Banday, N.; Sabreen, S.; Maqbool, M.; Jan, R.; Shafi, N.; Masoodi, M.H. Curcumin as a privileged scaffold molecule for various biological targets in drug development. Stud. Nat. Prod. Chem. 2022, 73, 405–434. [Google Scholar]
- Hatamipour, M.; Johnston, T.P.; Sahebkar, A. One molecule, many targets and numerous effects: The pleiotropy of curcumin lies in its chemical structure. Curr. Pharm. Des. 2018, 24, 2129–2136. [Google Scholar] [CrossRef]
- Singh, P.; Bhooshan Pandey, K.; Ibrahim Rizvi, S. Curcumin: The yellow molecule with pleiotropic biological effects. Lett. Drug Des. Discov. 2016, 13, 170–177. [Google Scholar] [CrossRef]
- Somparn, P.; Phisalaphong, C.; Nakornchai, S.; Unchern, S.; Morales, N.P. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol. Pharm. Bull. 2007, 30, 74–78. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, A.R.; Chung, H.Y.; Han, S.Y.; Kim, B.S.; Choi, J.S. In vitro peroxynitrite scavenging activity of diarylheptanoids from Curcuma longa. Phytother. Res. 2003, 17, 481–484. [Google Scholar] [CrossRef]
- Yodkeeree, S.; Chaiwangyen, W.; Garbisa, S.; Limtrakul, P. Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit cancer cell invasion through the down-regulation of MMPs and uPA. J. Nutr. Biochem. 2009, 20, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-J.; Hong, Y.-A.; Lin, K.-Y.; Hua, Y.-W.; Kuo, C.-J.; Hu, A.; Shih, T.-L.; Chen, H.-P. Efficient photodynamic killing of Gram-positive bacteria by synthetic curcuminoids. Int. J. Mol. Sci. 2020, 21, 9024. [Google Scholar] [CrossRef] [PubMed]
- Kumar Ray, U.; Nowduri, A.; Babu Korupolu, R.; Boju, S.; Kumar, S.; TSSSundaram, D.; Sekhara Rao Nethinti, C. Controlled Catalytic Reduction in Synthesising Pure Tetrahydrocurcumin. Asian J. Chem. Sci. 2022, 11, 46–53. [Google Scholar]
- Osawa, T.; Sugiyama, Y.; Inayoshi, M.; Kawakishi, S. Antioxidative activity of tetrahydrocurcuminoids. Biosci. Biotechnol. Biochem. 1995, 59, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.C.; Lee, M.F.; Li, C.T.; Chang, K.H.; Pan, M.H. Effects of curcumin metabolites on human colon adenocarcinoma cells. FASEB J. 2016, 30, 691.14. [Google Scholar] [CrossRef]
- Nimiya, Y.; Wang, W.; Du, Z.; Sukamtoh, E.; Zhu, J.; Decker, E.; Zhang, G. Redox modulation of curcumin stability: Redox active antioxidants increase chemical stability of curcumin. Mol. Nutr. Food Res. 2016, 60, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Saradhi, U.V.; Ling, Y.; Wang, J.; Chiu, M.; Schwartz, E.B.; Fuchs, J.R.; Chan, K.K.; Liu, Z. A liquid chromatography–tandem mass spectrometric method for quantification of curcuminoids in cell medium and mouse plasma. J. Chromatogr. B 2010, 878, 3045–3051. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and chemical stability of curcumin in aqueous solutions and emulsions: Impact of pH, temperature, and molecular environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.N.; Luis, P.B.; Ashley, R.E.; Osheroff, N.; Schneider, C. Oxidative transformation of demethoxy-and bisdemethoxycurcumin: Products, mechanism of formation, and poisoning of human topoisomerase IIα. Chem. Res. Toxicol. 2015, 28, 989–996. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Kimura, Y. Effects of a turmeric extract (Curcuma longa) on chronic ultraviolet B irradiation-induced skin damage in melanin-possessing hairless mice. Phytomedicine 2009, 16, 1137–1143. [Google Scholar] [CrossRef]
- Firmansyah, D.; Sumiwi, S.A.; Saptarini, N.M.; Levita, J. Curcuma longa extract inhibits the activity of mushroom tyrosinase and the growth of murine skin cancer B16F10 cells. J. Herbmed Pharmacol. 2022, 12, 153–158. [Google Scholar] [CrossRef]
- Jang, J.Y.; Lee, J.H.; Jeong, S.Y.; Chung, K.T.; Choi, Y.H.; Choi, B.T. Partially purified Curcuma longa inhibits alpha-melanocyte-stimulating hormone-stimulated melanogenesis through extracellular signal-regulated kinase or Akt activation-mediated signalling in B16F10 cells. Exp. Dermatol. 2009, 18, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.; Deng, D.; Robinson, L.; Raleigh, P. Topical turmeric extract in a moisturizing cream formula reduces the appearance of facial spots and fine lines and wrinkles on human facial skin. J. Am. Acad. Dermatol. 2010, 62, 01591–01596. [Google Scholar]
- Lee, J.-H.; Jang, J.-Y.; Park, C.; Kim, B.-W.; Choi, Y.-H.; Choi, B.-T. Curcumin suppresses α-melanocyte stimulating hormone-stimulated melanogenesis in B16F10 cells. Int. J. Mol. Med. 2010, 26, 101–106. [Google Scholar] [PubMed]
- Tu, C.X.; Lin, M.; Lu, S.S.; Qi, X.Y.; Zhang, R.X.; Zhang, Y.Y. Curcumin inhibits melanogenesis in human melanocytes. Phytother. Res. 2012, 26, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Novel Chemically Modified Curcumin (CMC) analogs exhibit anti-melanogenic activity in primary human melanocytes. Int. J. Mol. Sci. 2021, 22, 6043. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Comparative study of curcumin and its hydrogenated metabolites, tetrahydrocurcumin, hexahydrocurcumin, and octahydrocurcumin, on melanogenesis in B16F10 and MNT-1 cells. Cosmetics 2021, 8, 4. [Google Scholar] [CrossRef]
- Goenka, S.; Johnson, F.; Simon, S.R. Novel chemically modified curcumin (CMC) derivatives inhibit tyrosinase activity and melanin synthesis in B16f10 mouse melanoma cells. Biomolecules 2021, 11, 674. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Kim, K.; Kim, C.; Lee, S.-E. Antimelanogenic Effects of Curcumin and Its Dimethoxy Derivatives: Mechanistic Investigation Using B16F10 Melanoma Cells and Zebrafish (Danio rerio) Embryos. Foods 2023, 12, 926. [Google Scholar] [CrossRef]
- Majeed, M.; Pineda, R.; Chan, G.; Gabriel, T.; Dayrit, J.; Pelayo, C.; Prakash, L. A randomized, doubleblind, placebo-controlled, comparative study the safety and efficacy of 0.25% tetrahydrocurcumin (turmeric) cream as depigment agent against 4% hydroquinone cream. HPC Today 2010, 3, 44–46. [Google Scholar]
- Majeed, M.; Badmaev, V. Cross-Regulin Composition of Tumeric-Derived Tetrahydrocurcuminoids for Skin Lightening and Protection Against UVB Rays. U.S. Patent 6,653,327, 25 November 2003. [Google Scholar]
- Candau, D. Self-Tanning Composition Containing a Tetrahydrocurcuminoid and a Self-Tanning Agent. U.S. Patent 6,875,426, 5 April 2005. [Google Scholar]
- Ku, B.; Kim, D.; Choi, E.-M. Tetrahydrocurcumin Inhibits α-MSH-induced Melanogenesis via GSK3β Activation in B16F10 Melanoma Cells. Toxicol. Environ. Health Sci. 2019, 11, 210–218. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Gangwar, M.; Mondal, S.C.; Jana, S. Protective effects of tetrahydrocurcumin (THC) on fibroblast and melanoma cell lines in vitro: It’s implication for wound healing. J. Food Sci. Technol. 2017, 54, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.K.; Subedi, L.; Jeong, M.; Park, Y.U.; Kim, C.Y.; Kim, H.; Kim, S.Y. Gomisin N inhibits melanogenesis through regulating the PI3K/Akt and MAPK/ERK signaling pathways in melanocytes. Int. J. Mol. Sci. 2017, 18, 471. [Google Scholar] [CrossRef]
- Zhou, S.; Sakamoto, K. Citric acid promoted melanin synthesis in B16F10 mouse melanoma cells, but inhibited it in human epidermal melanocytes and HMV-II melanoma cells via the GSK3β/β-catenin signaling pathway. PLoS ONE 2020, 15, e0243565. [Google Scholar] [CrossRef]
- Nagata, H.; Takekoshi, S.; Takeyama, R.; Homma, T.; Yoshiyuki Osamura, R. Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and in normal human melanocytes. Pigment Cell Res. 2004, 17, 66–73. [Google Scholar] [CrossRef]
- Yang, Y.M.; Son, Y.O.; Lee, S.A.; Jeon, Y.M.; Lee, J.C. Quercetin inhibits α-MSH-stimulated melanogenesis in B16F10 melanoma cells. Phytother. Res. 2011, 25, 1166–1173. [Google Scholar] [CrossRef]
- Goenka, S. In Vitro Evaluation of Dental Resin Monomers, Triethylene Glycol Dimethacrylate (TEGDMA), and 2-Hydroxyethyl Methacrylate (HEMA) in Primary Human Melanocytes: A Pilot Study. Oral 2023, 3, 353–371. [Google Scholar] [CrossRef]
- Goenka, S.; Nagabhushanam, K.; Majeed, M.; Simon, S.R. Calebin-A, a Curcuminoid analog inhibits α-MSH-induced melanogenesis in B16F10 mouse melanoma cells. Cosmetics 2019, 6, 51. [Google Scholar] [CrossRef]
- Goenka, S. Novel Hydrogenated Derivatives of Chemically Modified Curcumin CMC2. 24 Are Potent Inhibitors of Melanogenesis in an In Vitro Model: Influence of Degree of Hydrogenation. Life 2023, 13, 1373. [Google Scholar] [CrossRef] [PubMed]
- Morales, N.P.; Sirijaroonwong, S.; Yamanont, P.; Phisalaphong, C. Electron paramagnetic resonance study of the free radical scavenging capacity of curcumin and its demethoxy and hydrogenated derivatives. Biol. Pharm. Bull. 2015, 38, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Barclay, L.R.C.; Vinqvist, M.R.; Mukai, K.; Goto, H.; Hashimoto, Y.; Tokunaga, A.; Uno, H. On the antioxidant mechanism of curcumin: Classical methods are needed to determine antioxidant mechanism and activity. Org. Lett. 2000, 2, 2841–2843. [Google Scholar] [CrossRef] [PubMed]
- Sreejayan, N.; Rao, M. Free radical scavenging activity of curcuminoids. Arzneim.-Forsch. 1996, 46, 169–171. [Google Scholar]
- Foti, M.; Ruberto, G. Kinetic solvent effects on phenolic antioxidants determined by spectrophotometric measurements. J. Agric. Food Chem. 2001, 49, 342–348. [Google Scholar] [CrossRef]
- de Heer, M.I.; Mulder, P.; Korth, H.-G.; Ingold, K.U.; Lusztyk, J. Hydrogen atom abstraction kinetics from intramolecularly hydrogen bonded ubiquinol-0 and other (poly) methoxy phenols. J. Am. Chem. Soc. 2000, 122, 2355–2360. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Kawakishi, S.; Osawa, T. Involvement of the β-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin. Biochem. Pharmacol. 1996, 52, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Girst, G.; Ötvös, S.B.; Fülöp, F.; Balogh, G.T.; Hunyadi, A. Pharmacokinetics-driven evaluation of the antioxidant activity of curcuminoids and their major reduced metabolites—A medicinal chemistry approach. Molecules 2021, 26, 3542. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.N.A.W.; Zulkifli, R.M.; Ahmad, F.; Yunus, M.A.C. Antioxidant and tyrosinase inhibition activities of Eurycoma longifolia and Swietenia macrophylla. J. Appl. Pharm. Sci. 2015, 5, 006–010. [Google Scholar] [CrossRef][Green Version]
- Mazlan, N.A.; Mediani, A.; Abas, F.; Ahmad, S.; Shaari, K.; Khamis, S.; Lajis, N. Antioxidant, antityrosinase, anticholinesterase, and nitric oxide inhibition activities of three Malaysian Macaranga species. Sci. World J. 2013, 2013, 312741. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kang, K.S.; Yokozawa, T. The anti-melanogenic effect of pycnogenol by its anti-oxidative actions. Food Chem. Toxicol. 2008, 46, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Lim, Y.Y.; Wong, L.; Lianto, F.S.; Wong, S.; Lim, K.; Joe, C.; Lim, T. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008, 109, 477–483. [Google Scholar] [CrossRef]
- Goenka, S. Effects of a Standardized Hydrogenated Extract of Curcumin (CurowhiteTM) on Melanogenesis: A Pilot Study. Nutraceuticals 2023, 3, 421–437. [Google Scholar] [CrossRef]
- Liu, R.H.; Finley, J. Potential cell culture models for antioxidant research. J. Agric. Food Chem. 2005, 53, 4311–4314. [Google Scholar] [CrossRef]
- Frankel, E.N.; Meyer, A.S. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000, 80, 1925–1941. [Google Scholar] [CrossRef]
- Lo, C.-Y.; Liu, P.-L.; Lin, L.-C.; Chen, Y.-T.; Hseu, Y.-C.; Wen, Z.-H.; Wang, H.-M. Antimelanoma and antityrosinase from Alpinia galangal constituents. Sci. World J. 2013, 2013, 186505. [Google Scholar] [CrossRef] [PubMed]
- Tima, S.; Ichikawa, H.; Ampasavate, C.; Okonogi, S.; Anuchapreeda, S. Inhibitory effect of turmeric curcuminoids on FLT3 expression and cell cycle arrest in the FLT3-overexpressing EoL-1 leukemic cell line. J. Nat. Prod. 2014, 77, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Riadh, D.; Sakamoto, K. Grape extract promoted α-msh-induced melanogenesis in b16f10 melanoma cells, which was inverse to resveratrol. Molecules 2021, 26, 5959. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Morita, M.; Ichikawa, C.; Takahashi, H.; Toriyama, M. Depigmenting mechanisms of all-trans retinoic acid and retinol on B16 melanoma cells. Biosci. Biotechnol. Biochem. 2008, 72, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Firmansyah, D.; Sumiwi, S.; Saptarini, N.; Levita, J. Molecular interaction of curcumin, demethoxycurcumin, bisdemethoxycurcumin, and turmerone of Curcuma longa with tyrosinase and tyrosinase-related protein-1. Rasayan J. Chem. 2021, 14, 2298–2303. [Google Scholar] [CrossRef]
- Qiao, X.; Ye, L.; Lu, J.; Pan, C.; Fei, Q.; Zhu, Y.; Li, H.; Lin, H.; Ge, R.-S.; Wang, Y. Curcumin analogues exert potent inhibition on human and rat gonadal 3β-hydroxysteroid dehydrogenases as potential therapeutic agents: Structure-activity relationship and in silico docking. J. Enzym. Inhib. Med. Chem. 2023, 38, 2205052. [Google Scholar] [CrossRef] [PubMed]
- Sprous, D.; Palmer, R.; Swanson, J.; Lawless, M. QSAR in the pharmaceutical research setting: QSAR models for broad, large problems. Curr. Top. Med. Chem. 2010, 10, 619–637. [Google Scholar] [CrossRef]
- Shehzadi, S.A.; Saeed, A.; Perveen, F.; Channar, P.A.; Arshad, I.; Abbas, Q.; Kalsoom, S.; Yousaf, S.; Simpson, J. Identification of two novel thiazolidin-2-imines as tyrosinase inhibitors: Synthesis, crystal structure, molecular docking and DFT studies. Heliyon 2022, 8, e10098. [Google Scholar] [CrossRef]
- Patil, S.; Sistla, S.; Jadhav, J. Interaction of small molecules with human tyrosinase: A surface plasmon resonance and molecular docking study. Int. J. Biol. Macromol. 2016, 92, 1123–1129. [Google Scholar] [CrossRef]
- González, Y.; Mojica-Flores, R.; Moreno-Labrador, D.; Pecchio, M.; Rao, K.J.; Ahumedo-Monterrosa, M.; Fernández, P.L.; Larionov, O.V.; Lakey-Beitia, J. Tetrahydrocurcumin Derivatives Enhanced the Anti-Inflammatory Activity of Curcumin: Synthesis, Biological Evaluation, and Structure–Activity Relationship Analysis. Molecules 2023, 28, 7787. [Google Scholar] [CrossRef]
- Alvarado, H.-L.; Limón, D.; Calpena-Campmany, A.-C.; Mallandrich, M.; Rodríguez-Cid, L.; Aliaga-Alcalde, N.; González-Campo, A.; Pérez-García, L. Intrinsic Permeation and Anti-Inflammatory Evaluation of Curcumin, Bisdemethoxycurcumin and Bisdemethylcurcumin by a Validated HPLC-UV Method. Int. J. Mol. Sci. 2023, 24, 6640. [Google Scholar] [CrossRef]
- Sudeep, V.H.; Gouthamchandra, K.; Chandrappa, S.; Naveen, P.; Reethi, B.; Venkatakrishna, K.; Shyamprasad, K. In vitro gastrointestinal digestion of a bisdemethoxycurcumin-rich Curcuma longa extract and its oral bioavailability in rats. Bull. Natl. Res. Cent. 2021, 45, 84. [Google Scholar] [CrossRef]
- Jain, R. Biological activities of bisdesmethoxycurcumin. J. Nat. Sci. Med. 2020, 3, 219. [Google Scholar] [CrossRef]
- Gouthamchandra, K.; Sudeep, H.V.; Chandrappa, S.; Raj, A.; Naveen, P.; Shyamaprasad, K. Efficacy of a Standardized Turmeric Extract Comprised of 70% Bisdemothoxy-Curcumin (REVERC3) Against LPS-Induced Inflammation in RAW264. 7 Cells and Carrageenan-Induced Paw Edema. J. Inflamm. Res. 2021, 14, 859. [Google Scholar] [CrossRef] [PubMed]
- Hv, S.; Raj, A.; Gouthamchandra, K.; Chandrappa, S.; Shyamprasad, K. Kinetics and computational analysis of cholinesterase inhibition by REVERC3, a bisdemethoxycurcumin-rich Curcuma longa extract: Relevance to the treatment of Alzheimer’s disease. SAGE Open Med. 2020, 8, 2050312120973499. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Slominski, R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer 2009, 124, 1470–1477. [Google Scholar] [CrossRef]
- Brożyna, A.A.; VanMiddlesworth, L.; Slominski, A.T. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int. J. Cancer 2008, 123, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Hengeltraub, S.F.; Gao, F.C.; Leivant, M.A.; Spandau, D.F. Aberrant NF-κB activity in HaCaT cells alters their response to UVB signaling. J. Investig. Dermatol. 2006, 126, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.; Piva, T.J. A comparative study of UV-induced cell signalling pathways in human keratinocyte-derived cell lines. Arch. Dermatol. Res. 2013, 305, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Boonkaew, B.; Kempf, M.; Kimble, R.; Cuttle, L. Cytotoxicity testing of silver-containing burn treatments using primary and immortal skin cells. Burns 2014, 40, 1562–1569. [Google Scholar] [CrossRef]
- Liu, L.; Xie, H.; Chen, X.; Shi, W.; Xiao, X.; Lei, D.; Li, J. Differential response of normal human epidermal keratinocytes and HaCaT cells to hydrogen peroxide-induced oxidative stress. Clin. Exp. Dermatol. 2012, 37, 772–780. [Google Scholar] [CrossRef]
- Sun, T.; Jackson, S.; Haycock, J.W.; MacNeil, S. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J. Biotechnol. 2006, 122, 372–381. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Deb, L.; Prasad, S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules 2014, 20, 185–205. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, Y.; Feng, J.; Wang, Y.; Lu, Y.; Chen, X. Tetrahydrocurcumin as a stable and highly active curcumin derivative: A review of synthesis, bioconversion, detection and application. Food Biosci. 2023, 53, 102591. [Google Scholar] [CrossRef]
- Tang, X.; Dong, Q.; Li, J.; Li, F.; Michniak-Kohn, B.B.; Zhao, D.; Ho, C.-T.; Huang, Q. Anti-melanogenic mechanism of tetrahydrocurcumin and enhancing its topical delivery efficacy using a lecithin-based nanoemulsion. Pharmaceutics 2021, 13, 1185. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.; Arora, C.; Saini, M.; Sharma, S.; Chitkara, D.; Kakkar, V. Preclinical safety of tetrahydrocurcumin loaded lipidic nanoparticles incorporated into tacrolimus ointment: In vitro and in vivo evaluation. Food Chem. Toxicol. 2022, 167, 113260. [Google Scholar] [CrossRef]
- Kakkar, V.; Kaur, I.P.; Kaur, A.P.; Saini, K.; Singh, K.K. Topical delivery of tetrahydrocurcumin lipid nanoparticles effectively inhibits skin inflammation: In vitro and in vivo study. Drug Dev. Ind. Pharm. 2018, 44, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Kanshide, A.; Peram, M.R.; Chandrasekhar, N.; Jamadar, A.; Kumbar, V.; Kugaji, M. Formulation, Optimization, and Antioxidant Evaluation of Tetrahydrocurcumin-Loaded Ultradeformable Nanovesicular Cream. J. Pharm. Innov. 2022, 18, 980–998. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, E.-S.; Bae, I.-H.; Hwang, J.-A.; Kim, S.-H.; Kim, D.-Y.; Park, N.-H.; Rho, H.S.; Kim, Y.J.; Oh, S.-G. Antimelanogenic efficacy of melasolv (3,4,5-trimethoxycinnamate thymol ester) in melanocytes and three-dimensional human skin equivalent. Ski. Pharmacol. Physiol. 2017, 30, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, L.; Mann, T.; Gerwat, W.; Batzer, J.; Ahlheit, S.; Scherner, C.; Wenck, H.; Stäb, F. 4-n-butylresorcinol, a highly effective tyrosinase inhibitor for the topical treatment of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Arct, J.; Ratz-Łyko, A.; Mieloch, M.; Witulska, M. Evaluation of skin colouring properties of curcuma longa extract. Indian J. Pharm. Sci. 2014, 76, 374. [Google Scholar] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goenka, S. Comparative Study of the Effects of Curcuminoids and Tetrahydrocurcuminoids on Melanogenesis: Role of the Methoxy Groups. Future Pharmacol. 2024, 4, 256-278. https://doi.org/10.3390/futurepharmacol4010016
Goenka S. Comparative Study of the Effects of Curcuminoids and Tetrahydrocurcuminoids on Melanogenesis: Role of the Methoxy Groups. Future Pharmacology. 2024; 4(1):256-278. https://doi.org/10.3390/futurepharmacol4010016
Chicago/Turabian StyleGoenka, Shilpi. 2024. "Comparative Study of the Effects of Curcuminoids and Tetrahydrocurcuminoids on Melanogenesis: Role of the Methoxy Groups" Future Pharmacology 4, no. 1: 256-278. https://doi.org/10.3390/futurepharmacol4010016
APA StyleGoenka, S. (2024). Comparative Study of the Effects of Curcuminoids and Tetrahydrocurcuminoids on Melanogenesis: Role of the Methoxy Groups. Future Pharmacology, 4(1), 256-278. https://doi.org/10.3390/futurepharmacol4010016