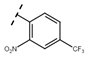
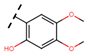
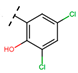
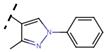
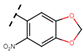
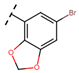
Abstract
Neglected tropical diseases (NTDs) are a significant global health problem. Additionally, anti-protozoan treatments are toxic, and their therapeutic regimens require prolonged treatment times and high concentrations of the drugs. Additionally, multi-resistant protozoan strains represent an important global emergency that must be addressed. For these reasons, global efforts are being made to identify new drug candidates that are capable of combating these kinds of diseases. This systematic review shows that 5-nitroimidazole derivatives have been successfully used against neglected tropical protozoan diseases (NTPDs), with a specific focus on three diseases: malaria, leishmaniasis, and human trypanosomiasis. Some nitroimidazole derivatives have been repurposed, and an important group of new drugs is available for the treatment of NTPDs. Finally, we address 5-nitroimidazoles using chemoinformatics and medicinal chemistry tools to describe the most recent and promising 5-nitroimidazole derivatives associated with anti-protozoal activity using their published in vitro and in vivo data. We show that 5-nitroimidazoles offer a broader spectrum of activity against a variety of protozoal pathogens. More importantly, these compounds demonstrate a significantly reduced systemic toxicity compared to other nitroimidazoles. This makes them a more favorable option in the treatment of protozoal infections, particularly in scenarios where the patient’s tolerance to drug side effects is a critical concern.
1. Introduction
5-Nitroimidazole (5-NI) derivatives are prodrugs which contain an imidazole ring with a nitro group that is linked to the carbon in the fifth position in their chemical structure. The mechanism of action of 5-NI derivatives can be explained in two steps: Firstly, they undergo reduction and activation through low-redox-potential reactions, mediated by proteins that are present in the target protozoans (e.g., ferredoxin, nitroreductases, or thioredoxin reductases). Secondly, several reactive species derived from 5-NI, including reactive nitrogen species and hydroxylamine intermediates, interact with cellular proteins and result in different endpoints that promote parasite death. Namely, the oxidated reactive species generate DNA damage and covalent adducts with structural proteins [1]. During 5-NI derivatives’ anaerobic nitroreduction, reactive metabolites are generated that bind to DNA, proteins, and lipids, constituting the key step in the chemotherapeutic action (Figure 1).
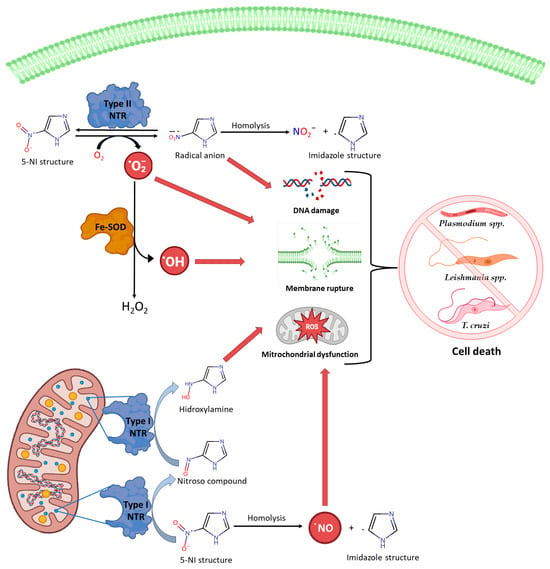
Figure 1.
Mechanisms of action of 5-NI and cytotoxic effects generated by the nitro group mediated by reactive species [Created with BioRender.com accessed on 18 December 2023].
Different anti-protozoan compounds contain nitro scaffolds that cause side effects in patients, (e.g., inducing the development of cancer). In contrast, 5-nitroimidazole scaffold derivatives exhibit antiparasitic activity without these side effects and offer new clinical opportunities to develop safe anti-protozoan agents [2].
These pharmacological molecules have been widely used as anti-protozoal agents, particularly against the Trypanosomatidae and Plasmodiidae families among many others, which use vectors for transmission and occur in tropical regions, particularly in economically disadvantaged regions. They belong to the group of 20 neglected tropical diseases (NTDs) which, according to WHO, affect more than one billion people in 149 nations located in tropical and subtropical regions [3].
In this systematic review, we focus on the main NTDs caused by protozoa that have been recorded with the highest number of deaths: malaria, caused by several species of Plasmodium; leishmaniasis, which is caused by various species of Leishmania; and American trypanosomiasis, which is caused by Trypanosoma cruzi.
2. Materials and Methods
A systematic electronic search was carried out following the guidelines of the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) using various databases [4]. A pre-specified study protocol was registered on the PROSPERO platform (CRD42024497822). The last query on all databases was performed on 30 September 2023. The search strategy is set out below and can be found in Figure 2 and Table S1 (see Supplementary Materials).

Figure 2.
Studies included in the results of this study according to the PRISMA guidelines [4].
1. Identification of Keywords and Related Terms: Keywords and terms that were relevant to the research focus were systematically identified. Specifically, a deliberate inquiry was conducted targeting the three primary neglected tropical protozoan diseases that exhibit the most substantial global mortality rates, along with pharmaceutical compounds derived from 5-NI. The following search terms were utilized: “5-Nitroimidazoles Derivatives”, “nitroimidazole”, “Host-Target Therapies”, “treatments”, “Chagas Disease”, “Trypanosoma cruzi”, “American trypanosomiasis”, “Malaria”, “Plasmodium”, “Leishmaniasis”, and “Leishmania”.
2. Employment of Boolean Operators (AND, OR, NOT) in Keyword Combinations: Boolean operators, including AND, OR, and NOT, were used to combine keywords and related terms to generate more specific and focused search inquiries.
3. Exploration of Scientific and Bibliographic Databases: Exhaustive explorations were undertaken in relevant scientific and bibliographic databases, such as PubMed, Google Scholar, EBSCO, Redalyc, and SciELO. Advanced search filters were applied, including date ranges (covering the period from 2018 to 2023), document types (clinical and experimental studies, as well as review articles, research, book chapters, and meta-analyses), specific domains of research (covering medicine, biology, and biochemistry), and accessibility options (open access), to fine-tune search outcomes and procure studies of greater relevance.
4. Assessment and Selection of Research Studies: A methodical evaluation was executed, involving scrutiny of the titles and abstracts of the identified studies to determine their conformity with the predetermined inclusion and exclusion criteria. The inclusion criteria included studies delineating the development and use of pharmaceutical agents derived from 5-NI, posited as prospective interventions within the sphere of neglected tropical diseases of paramount public health importance due to their high morbidity and mortality profiles. The scope of the incorporated research ranged from pre-clinical investigations to clinical trials. Conversely, the exclusion criteria included studies that diverged from the scope of the research, those characterized by methodological deficiencies, or cases of study duplication. The studies initially appearing relevant based on their title and abstract were subsequently subjected to extensive review to confirm their pertinence.
5. Documentation of the Search Process: An exhaustive and meticulous record of the search process was maintained. This comprehensive record encompassed particulars regarding the databases employed, the intricacies of the search queries, the dates of the search execution, the number of outcomes, and the ultimately chosen studies. This archival documentation serves a dual purpose, elucidating the search methodology employed within narrative reviews and facilitating the replication and refinement of the search efforts.
3. Results
3.1. 5-Nitroimidazole Derivatives with Antimalarial Activity
Malaria is a serious disease that results from infections by protozoa of the genus Plasmodium, which are transmitted through the bite of female mosquitoes belonging to the genus Anopheles. In the case of humans, six species of Plasmodium can cause the disease: P. falciparum, P. vivax, P. malariae, P. ovale wallikeri, P. ovale curtisi, and P. knowlesi [5]. With P. falciparum and P. vivax causing the majority of cases and deaths, they affect more than 200 million people globally and cause half a million deaths annually. Recent estimates indicate that approximately 50% of the world’s population faces a risk of contracting malaria [6].
In addition to the above, maternal malaria is responsible for the death of 200,000 children each year, which represented a loss of 56 million years by Disability-Adjusted Life Years (DALYs) in 2015 alone [7,8]. On the other hand, the clinical signs and symptoms of malaria could range from mild to chronic depending on the progression of the disease, including organ failure, blood abnormalities, cerebral malaria, and death [9].
Due to the high population-level risk that malaria represents, strategies have been developed that range from the control of mosquito vectors to vaccines for its prevention. However, due to the complexity of the parasitic life cycle and its species diversity, these strategies have had ineffective results. Therefore, drug therapies remain the best alternative to deal with this disease [10].
Currently, drugs used as malaria treatment are divided into four categories (Figure 3): quinolines or aminoquinolines (e.g., chloroquine and primaquine); antifolates (e.g., sulfadoxine and pyrimethamine); artemisinin derivatives; and macrocycles, such as tetracycline and doxycycline [11]. However, although there are different types of drugs and novel poly-drug therapies, a growing concern is the emergence of drug-resistant Plasmodium strains [10,12].
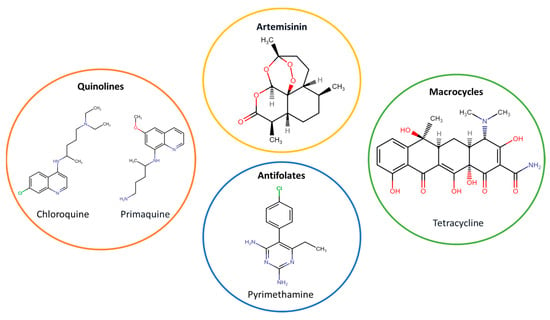
Figure 3.
Categories of drugs used as antimalarial treatments.
A representative example of this is the mutation in the Pfcrt gene, associated with the chloroquine (CQ) resistance in P. falciparum, which caused a two-to-three-fold increase in malaria-related deaths during the 1980s. To combat CQ resistance, sulfadoxine/pyrimethamine (SP) became the first choice for an antimalarial drug. However, in the early 2000s, the parasite developed resistance to SP. In recent years, history has been repeated, as artemisinin combination therapy regimens were adopted, and now, we have observed poly-drug resistance in Southeast Asia (associated with the Kelch 13 protein mutation) [13].
Currently, the emergence of resistant malaria strains creates an urgent need to develop new antimalarial drugs, especially against P. falciparum and P. vivax. Interestingly, a viable alternative is to develop novel 5-NI derivatives, which have demonstrated great potential in in vivo and in vitro models [14].
3.1.1. Quinoline-Based 5-Nitroimidazole Derivatives
One of the current strategies to combat resistant malaria strains is the generation of quinoline-based nitroimidazoles targeted explicitly at pyrimethamine-resistant P. falciparum (strain K1). Tukulula et al. were pioneers in this field, initially generating 10 compounds from 4-nitroimidazole hybridized with aminoquinolines (like primaquine and chloroquine) [15,16].
Subsequently, a second generation of nitroimidazoles was developed (Figure 4), with the nitro group at position 5 (5-NI). Their IC50 values ranged from 0.094 μM to 11.063 μM, which were lower than those of the controls (CQ = 0.213 μM; PQ = 0.643 μM) [6,16].
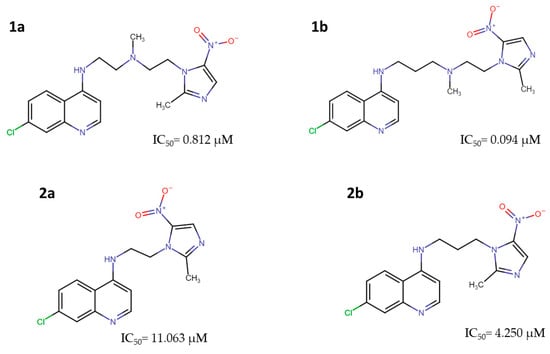
Figure 4.
Methylnitroimidazole-chloroquinoline: 2[{2-[(7-Chloroquinolin-4-yl)amino]ethyl}-(methyl)amino]ethyl [2-(2-methyl-5-nitroimidazole-1-yl (1a), 2[{3-[(7-Chloroquinolin-4-yl)amino]propyl}-(methyl)amino]ethyl[2-(2-methyl-5-nitroimidazole-1-yl (1b); and methylnitroimidazole-primaquine: (7-Chloroquinolon-4-yl)-[2-(2-methyl-5-nitroimidazol-1-yl)-ethyl]-amine (2a), (7-Chloroquinolon-4-yl)-[3-(2-methyl-5-nitroimidazol-1-yl)-propyl]-amine (2b) with anti-Plasmodium activity [16].
3.1.2. 5-Nitroimidazole-2-carbaldehyde Derivatives
In 2021, Navidpour et al. synthesized 16 derivatives from 3-(2H)-benzofuranone, 1-indadone, and 1,3-indanedione, which were hybridized with a 1-methyl-5-nitroimidazole-2-carbaldehyde scaffold (3a–n, 4, and 5) (Figure 5 and Table 1) [11].
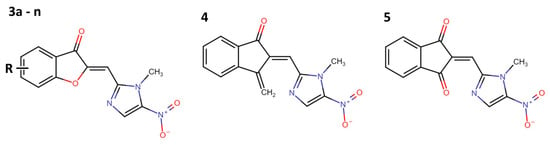
Figure 5.
Hybridized compounds with 5-nitroimidazole-2-carbaldehyde scaffold (3a–n, 4, and 5) with anti-pyrimethamine-resistant Plasmodium falciparum activity [11].

Table 1.
IC50 values of derivatives of 5-nitroimidazole-2-carbaldehyde derivatives against chloroquine- and pyrimethamine-resistant strains of P. falciparum [11].
Their work determined the IC50 values for each compound against CQ-resistant (3D7) and pyrimethamine-resistant (K1) P. falciparum strains (Table 1). These results demonstrate the high potential of 5-NI derivatives against pyrimethamine-resistant strains (IC50 values from <0.654 to 6.31 nM) in contrast with CQ treatment (IC50 = 206.3 nM). However, with respect to strain 3D7, all compounds were less active than CQ [11].
3.1.3. Bicyclic Nitroimidazole Drugs: Nitroimidazopyridazines
Bicyclic compounds such as nitroimidazopyridazines, particularly those within a 5-nitro group in the imidazole ring, have attracted interest for the treatment of a wide variety of anaerobic and semi-aerobic parasites like Plasmodium. For example, Zheng et al. synthesized and tested 19 nitroimidazopyridazine derivatives (6 to 24) (Figure 6) against P. falciparum. However, none of the compounds presented better activity than CQ (Table 2) [17].
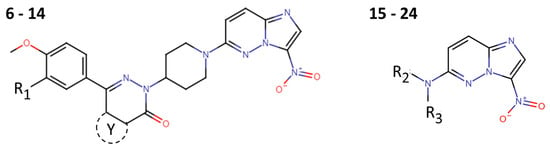
Figure 6.
Nitroimidazopyridazine derivatives (6–24) with anti-Plasmodium falciparum activity.

Table 2.
Nitroimidazopyridazine derivatives with activity against P. falciparum.
Additionally, these compounds exhibited anti-Leishmania infantum and anti-Trypanosoma cruzi activities, vide infra.
3.1.4. 2-Pyrazoline Spacer Derivatives
Lohidakshan et al., using a computer-aided drug design campaign, identified four 5-NI derivatives based on pyrazoline (25–28; Table 3). These compounds were synthesized and evaluated using in vitro tests, and their antimalarial activity (using around 50 mg/mL) was expressed as a percentage of the P. falciparum RKL-2 strain that was killed [18].

Table 3.
Anti-Plasmodium falciparum activity (% dead parasites) of 2-pyrazoline-based 5-nitroimidazole derivatives.
3.1.5. Metronidazole-Hybridized Derivatives
In parallel, metronidazole (an antimicrobial agent with a nitroimidazole scaffold) has been used as a starting point for developing novel anti-malarial agents. In 2023, Beteck et al. generated molecular hybrids with different Schiff bases, obtaining 21 new compounds (29 to 50) (Figure 7) [19].

Figure 7.
Metronidazole-hybridized derivatives with anti-Plasmodium potential.
These compounds show IC50 values ranging from >20 μM to 0.7 μM (Table 4). The most potent compounds exhibited a similar activity to that of the control (CQ IC50 = 0.05 μM) [19].

Table 4.
Activity of metronidazole-hybridized derivatives against chloroquine-susceptible P. falciparum strain (3D7) [19].
3.2. 5-Nitroimidazole Derivatives with Anti-Leishmanial Activity
Leishmaniasis is the second most deadly parasitic infection following malaria and is induced by several species from the Leishmania genus [20]. Transmission of this disease occurs through bites from infected female sandflies of the Lutzomyia genus (in America) and the Phlebotomus genus (in Asia and Europe) [20].
Clinically, leishmaniasis has three forms: cutaneous leishmaniasis (CL), the most common variant, appears as skin lesions that may become chronic, resulting in tissue destruction; mucocutaneous leishmaniasis (MCL), which causes partial or total destruction of the mucous membranes of the nose, mouth, and throat; and visceral leishmaniasis (VL), characterized by the spread of the parasites to organs such as the liver, spleen, and bone marrow, presenting a higher mortality rate [21].
It is important to highlight that each clinical manifestation is caused by specific species depending on their location. For instance, CL is mainly caused by L. major and L. tropica in the Americas and by L. infantum and L. mexicana in Europe. Meanwhile, VL is caused by L. donovani and L. infantum in Euro-Asia and Africa and in the Americas, respectively [22].
This disease is endemic in 98 countries, affecting 12 million people. Each year, around one million new cases are reported. Of these, approximately 50,000 to 90,000 are VL, and 600,000 to 1 million are various forms of CL and MCL. The annual death toll from leishmaniasis disease is between 26,000 and 65,000 [23,24]. Additionally, the economic impact per year has been estimated at 1.4 million Disability-Adjusted Life Years (DALYs), primarily associated with VL [8].
Currently, drug therapy is the main strategy for treating and preventing this disease. Four drugs (Figure 8) are commonly utilized either as monotherapy or in combination: Pentostam (a pentavalent antimony compound with a large list of side effects), amphotericin B (with poor oral bioavailability, limited chemical stability under extreme climatic conditions, and high cost), miltefosine (considered a second-line drug, has potential teratogenic effects), and paromomycin (has limited oral bioavailability) [24].

Figure 8.
Representative drugs used as treatments against leishmaniasis.
In addition to the above, these drugs are ineffective due to the emergence of resistant strains and the occurrence of unwanted side effects, which may include intestinal complications, vomiting, diarrhea, arthralgia, myalgia, hepatotoxicity, pancreatitis, nephrotoxicity, and ototoxicity [25]. All these factors emphasize the need to explore newer compounds with anti-leishmanial properties.
3.2.1. Fexinidazole Derivatives
Phase I clinical trials for fexinidazole (FXZ) began in 2009. It received FDA approval in 2021 for the treatment of human African trypanosomiasis, and it has generated significant interest due to its potential [26]. In the context of leishmaniasis, fexinidazole, along with its sulfoxide and sulfone metabolites (51–53; Figure 9), has been tested in both in vitro and in vivo models [27].
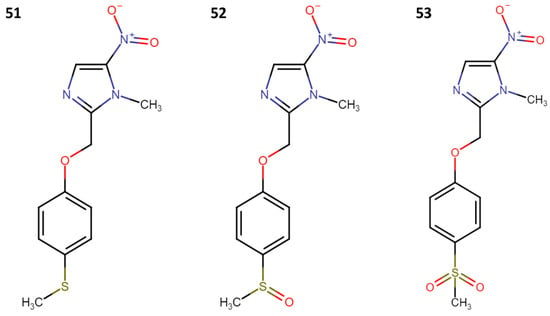
Figure 9.
Structure of fexinidazole (51), fexinidazole sulfoxide (52), and fexinidazole sulfone (53).
Using in vitro models, both axenic and intracellular promastigotes and amastigote strains of L. donovani strain (LdBOB) were evaluated, and their mean effective concentrations (EC50 values) were determined. Additionally, it was demonstrated that all drugs were inactive in human MRC5 fibroblast cells that were isolated from healthy lung tissue, even at concentrations higher than 50 μM. As for its in vivo efficacy, it was assessed in a murine model of VL induced by the L. donovani strain LV9 (Table 5). The drug was administered orally every 24 h for five days. It was concluded that the drug was well tolerated by the mice and proved to be an extremely effective inhibitor at a dose of 200 mg/kg, suppressing infection by 98.4% (mean effective dose). All the findings and their comparisons with the second-line drug, miltefosine, are summarized below [28].

Table 5.
In vitro and in vivo evaluation of fexinidazole, fexinidazole sulfoxide, and fexinidazole sulfone against L. donovani.
Phase II clinical trials were conducted to assess its effectiveness in patients aged 15 to 60 with visceral leishmaniasis in Sudan. However, while the study has been completed, the published results are not yet available [27,29].
3.2.2. 2-(1-Methyl-5-nitro-1H-imidazol-2-yl)-1,3,4-thiadiazole Derivatives
Since 2005, the Foroumadi research group has diligently focused on the development of 5-NI with anti-leishmanial activity. Initially, they synthesized seven compounds (54a–54g) based on 2-(1-methyl-5-nitro-1H-imidazole-2-yl)-1,3,4-thiadiazoles (Figure 10). These compounds exhibited inhibitory activity against L. major promastigotes, and their IC50 values were measured (Table 6) [30].

Figure 10.
2-(1-methyl-5-nitro-1H-imidazol-2-yl)-1,3,4-thiadiazole scaffold with anti-leishmanial activity.

Table 6.
IC50 of compounds based on 2-(1-methyl-5-nitro-1H-imidazol-2-yl)-1,3,4-thiadiazoles with anti-leishmanial activity.
Subsequently, the compounds were evaluated in axenic and intracellular amastigotes of L. donovani (Table 6), where compounds 54a, 54b, and 54g were identified as potential active agents against both amastigotes and promastigotes of Leishmania, with IC50 values of <1 µM [30].
3.2.3. Metronidazole Derivatives
Other research groups have started from metronidazole (55a). For example, in 2020, Rodriguez and collaborators carried out the synthesis of three series of molecules, starting with the replacement of the primary alcohol with a chlorine atom or a mesyl group (Figure 11) [31]. Subsequently, it was converted into a hydrochloride salt through a reaction with thionyl chloride. This salt was then treated with water and Et3N at a pH of 11, resulting in the synthesis of 2-[2-(2-methyl-5-nitro-1H-imidazole-1-yl)ethylthio]ethanol (56). From this precursor, 23 new molecules (57a–l, 58a–b, 59a–i) were synthesized (Figure 12) through coupling reactions with benzoic acids in the presence of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDCI) and 4-(dimethylamino)pyridine (DMAP) [31].
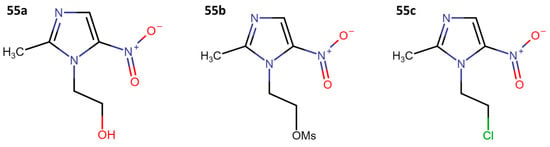
Figure 11.
Metronidazole (55a), mesylated metronidazole (55b), and 1-(2-chloroethyl)-2-methyl-5-nitroimidazole (55c).

Figure 12.
Metronidazole derivatives with anti-promastigote activity against L. braziliensis and L. mexicana.
Finally, they evaluated its anti-leishmanial activity by assessing the growth of L. braziliensis and L. mexicana promastigotes, and they determined the mean lethal concentration (LC50) (Table 7).

Table 7.
LC50 of metronidazole derivatives with anti-promastigote activity against L. braziliensis and L. mexicana [31].
Blanco et al. are pursuing a similar research direction, and in 2021, they synthesized molecules derived from metronidazole (55a). First, they replaced the primary alcohol with a chlorine atom (55c). Subsequently, they carried out nucleophilic substitution reactions with 2-mercaptoethanol, 1-methyl-2-mercaptoethanol, and a derivative of 2-thioacetic acid, resulting in the formation of compounds 60 and 61. Compound 62 was obtained through a coupling reaction with (S)-methyl 2-amino-4-methylpentanoate (Figure 13) [32].

Figure 13.
Second optimization cycle of metronidazole-based derivatives with anti-leishmanial activity.
Subsequently, they found that compounds 55c, 60, 61, and 62 reduced the growth of promastigotes of L. mexicana and L. braziliensis, and they measured the mean inhibitory concentrations (IC50) against intracellular amastigotes of both species (Table 8). Among these molecules, 61 and 62 proved to be the most effective. As a result, an in vivo evaluation was conducted using mouse models of cutaneous leishmaniasis (CL). This evaluation showed that both compounds reduced 96% of the lesions and cured 63% of the mice [32].

Table 8.
IC50 of metronidazole-based derivatives with activity against the amastigotes of L. braziliensis and L. mexicana.
3.2.4. Quinoline–Metronidazole Derivatives
Recent studies have been directed toward quinoline and metronidazole hybrids as a potential solution for VL. This approach stems from metronidazole’s promising results in the treatment of experimental leishmaniasis and the activity demonstrated by quinolones in both LV and LC experiments [33]. For instance, in 2019, Upadhyay and collaborators synthesized thirteen compounds (Figure 14) (63a–m) and assessed their inhibitory activity in vitro at concentrations of 50 µM and 25 µM on promastigotes and intracellular amastigotes of L. donovani [34].
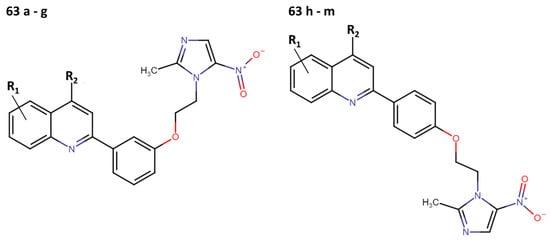
Figure 14.
Quinoline–metronidazole derivatives with anti-leishmanial activity.
While most of the compounds exhibited anti-leishmanial activity, compounds 63b and 63i demonstrated the highest efficacy against promastigote forms, with IC50 values of 9.54 μM and 5.42 μM, respectively, and IC50 values against intracellular amastigotes of 9.81 μM and 3.75 μM, respectively (Table 9) [34].

Table 9.
In vitro inhibitory activity of quinoline–metronidazole derivatives again L. donovani.
Subsequently, both compounds underwent in vivo evaluation in a murine model of VL caused by L. donovani. They were administered for 5 days, and compound 63i showed superior efficacy in the liver and spleen at doses of 25 mg/kg and 50 mg/kg/day (Table 10). However, in both trials, they were unable to surpass the effects achieved by miltefosine [34].

Table 10.
In vivo inhibitory activity of quinoline–metronidazole derivatives in a murine model of visceral leishmaniasis generated by L. donovani infection.
Finally, according to their main results, Upadhyay et al. suggest that compound 63i promotes oxidative stress, leading to a bioenergetic collapse and parasite apoptosis. They observed a reduction in the ATP production level, followed by a potential disruption of the mitochondrial membrane. Namely, the enhanced generation of ROS and NO from 5-NI contributes to the augmentation of metronidazole’s effectiveness [34].
3.2.5. Nitroimidazolyl–Benzofuranone Hybrids
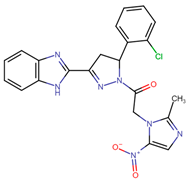
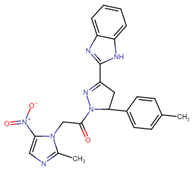
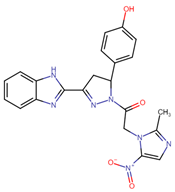
Another field of interest involves hybrids of nitroimidazolyl and benzofuranone, which are antimicrobial agents. These agents function by altering DNA through the formation of active radical anions and inhibiting chorismate synthase, which is essential for the synthesis of aromatic amino acids [35]. These properties make these compounds promising candidates for the development of new hybrids aimed at treating leishmaniasis. In this context, Navidpour et al. synthesized 16 new molecules (64a–o, 65) (Figure 15) [35].
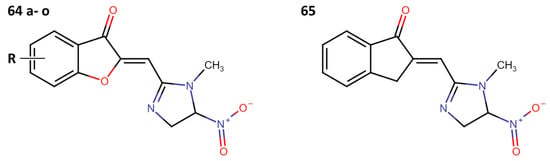
Figure 15.
Nitroimidazolyl–benzofuranone hybrids with anti-leishmanial activity.
Initially, they tested the compounds against L. major promastigotes and measured their IC50 values. Subsequently, the most effective ones (with IC50 values of <20 μM) were evaluated against L. donovani amastigotes (Table 11).

Table 11.
Nitroimidazolyl–benzofuranone hybrids with anti-leishmanial activity [35].
Bicyclic Nitroimidazole Drugs: 3-Nitroimidazo[1,2-a]Pyridine Derivatives
Among the first bicyclic compounds that were synthesized for the treatment of leishmaniasis, derivatives of 3-nitroimidazopyridines have emerged. The first use of this 5-NI derivative was reported in 2013 by the Castera-Ducros group, who synthesized 25 (66a–y) compounds and tested their anti-promastigote activity against L. donovani (Figure 16). The IC50 values obtained for this series of compounds, and the drugs miltefosine and amphotericin B, are presented in Table 12 [36].

Figure 16.
3-Nitroimidazopyridine scaffold with anti-leishmanial activity.

Table 12.
3-Nitroimidazopyridine derivatives with anti-leishmanial activity [36].
It was found that compound 66y had an IC50 of 1.8 µM (±0.8), which is lower than the IC50 of miltefosine (3.1 ± 0.06), the second-line treatment. Encouraged by these results, they tested the compound’s activity in the amastigote phase, where it achieved an IC50 of 5.5 (±0.2), surpassing the value obtained by the reference drug miltefosine (IC50 of 6.8 (±0.3)). Subsequently, they decided to assess its activity in promastigotes of another species, L. infantum, where it obtained an IC50 of 3.3 (±0.7). In contrast, miltefosine had an IC50 of 11.6 (±0.4) [36].
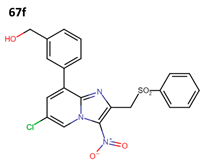
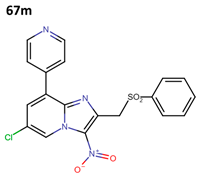
Subsequently, Fersing et al. optimized compound 66y and obtained 21 new compounds. When evaluating their anti-leishmanial activity, only 13 compounds exhibited activity (67a–m) (Figure 17) [37].
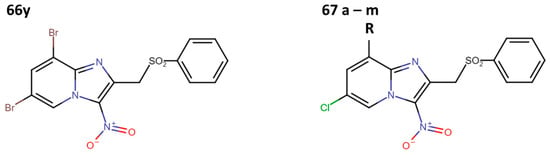
Figure 17.
First optimization cycle of 3-nitroimidazopyridine derivatives with anti-leishmanial activity [37].
They determined the IC50 values of compounds 67a–m against axenic amastigotes of L. infantum, surpassing the results obtained by Castera-Durcos, even for compound 66y, but not for compound 67g (Table 13) [37].

Table 13.
First optimization cycle of 3-nitroimidazopyridine derivatives with anti-leishmanial activity [37].
Finally, they evaluated compounds 67f and 67m against forms of L. donovani and L. infantum; their anti-leishmanial activity is shown in Table 14 [37].

Table 14.
Extended in vitro evaluation of best candidates selected from the first optimization cycle of 3-nitroimidazopyridine derivatives with anti-leishmanial activity.
To investigate the antiparasitic mechanism of action of these new nitrated molecules, they evaluated whether they could be substrates for kinetoplastid nitroreductases. This was tested by comparing the IC50 values measured against wild-type parasite strains with those measured against recombinant parasite strains overexpressing a nitroreductase (NTR1 or NTR2). They concluded that the aryl moiety addition in 66y improved the activity of all derivatives (67a–67m), which can be metabolized by type 1 nitroreductases (NTR1) [37].
In 2019, Fersing et al. synthesized new compounds containing a phenylthio or benzylthio residue in position 8 of the scaffold. Initially, they created a series of 26 compounds, but they could only evaluate the anti-leishmanial activity in 22 (68a–v) molecules (Figure 18) [38].
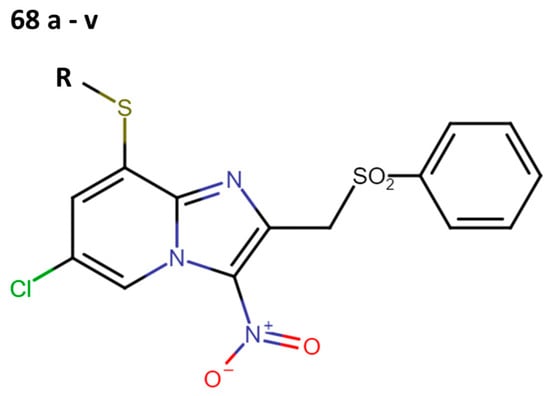
Figure 18.
Second optimization cycle of 3-nitroimidazopyridine scaffold with anti-leishmanial activity.
First, they determined the IC50 values (µM) against promastigotes of L. donovani (Table 15). Among these compounds, compounds 68b and 68e stood out not only for their inhibitory capacity but also for their low cytotoxicity.

Table 15.
Second optimization cycle of 3-nitroimidazopyridine derivatives with anti-leishmanial activity [38].
The best candidates were tested against the intracellular form of L. donovani, promastigotes and amastigote of L. major, and axenic amastigotes of L. infantum (Table 16).

Table 16.
Extended in vitro evaluation of the best candidates selected from the second optimization cycle of 3-nitroimidazopyridine derivatives with anti-leishmanial activity.
These results showed that the second optimization cycle increased the activity of the most active compound (68e) (Figure 19).
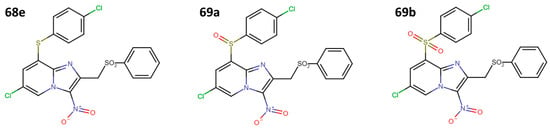
Figure 19.
Third optimization cycle of 3-nitroimidazopyridine derivatives with anti-leishmanial activity.
The optimized molecules (69a and 69b) showed an IC50 of 1.3 ± 0.3 μM against promastigotes of L. donovani, while for axenic amastigotes of L. infantum, it was 1.6 ± 0.4 μM for 69a and 3.7 ± 0.5 μM for 69b. This result suggests that the SO and SO2 groups improve the species selectivity, guided by the type 1 nitroreductase enzyme (NTR1) inhibition of L. donovani. Finally, these structures showed negative Ame’s test results (i.e., low mutagenic effects in human cells) and non-genetic damage (tested by comet assay), making them promising candidates for future research in more complex biological models [38].
Finally, in 2020, Fersing et al. reported a new series of 11 compounds (70a–k) in which they introduced a hydroxyalkynyl group at position 8 of the 3-nitroimidazo[1,2-a]pyridine scaffold with the aim of ensuring anti-leishmanial activity and microsomal stability (Figure 20) [39].

Figure 20.
Fourth optimization cycle of 3-nitroimidazopyridine scaffold with anti-leishmanial activity.
The in vitro IC50 values reported for promastigotes of L. donovani are shown in Table 17.

Table 17.
Fourth optimization cycle of 3-nitroimidazopyridine derivatives with anti-leishmanial activity [39].
Bicyclic Nitroimidazole Drugs: Nitroimidazopyridazines
Zheng et al. evaluated a series of bicyclic nitroimidazopyridazine compounds (6–24, see in Figure 5) against L. infantum; the results are shown in Table 18 [17].

Table 18.
Nitroimidazopyridazine derivatives with activity against L. infantum [17].
3.3. 5-Nitroimidazole Derivatives against American Trypanosomiasis
American trypanosomiasis, also known as Chagas disease (CD), is caused by the protozoan parasite Trypanosoma cruzi, which is mainly transmitted through the feces or urine of triatomine bugs (vector transmission) [40].
However, the parasite can also be transmitted congenitally, through blood transfusions, organ transplants, or by consuming contaminated foods. Although it is endemic in 21 Latin American countries, its reach has surpassed the borders of this continent due to globalization and migration, extending to non-endemic areas in America, Europe, Asia, and Oceania [41].
CD affects around 6 to 8 million people worldwide, causing 14,000 to 50,000 deaths annually. Additionally, recent estimates suggest that almost 70 to 100 million people are at risk of infection [42].
It is essential to understand the natural course of Chagas disease (CD), which is divided into two phases: acute and chronic. The acute phase occurs shortly after infection by the parasite and is characterized mainly by elevated parasitemia and an absence of symptoms. However, in some cases, nonspecific entry-point symptoms and signs may present, including fever, weakness, lymphadenopathy, hepatosplenomegaly, and inflammation at the inoculation site. Depending on the type of lesion, this is referred to as a chagoma (skin lesion) or Romaña’s sign (palpebral edema) [43].
Approximately 2 to 3 months after infection, patients transition to the chronic phase, which is characterized by a decrease in circulating parasites in the blood due to the immune system’s control of the infection. Within this framework, about 60–70% of cases remain in an indeterminate stage, meaning that they are infected with the T. cruzi parasite but do not develop the disease’s specific pathophysiology. In contrast, the remaining 30–40% develop Chagasic cardiomyopathy (cardiomegaly), heart failure, megacolon, megaesophagus, and even sudden death. It is worth noting that this phase of the disease can manifest between 10 and 30 years after the T. cruzi infection [44].
Chagas disease is the parasitic disease with the greatest socioeconomic impact in Latin America, as it results in more than 800,000 Disability-Adjusted Life Years (DALYs) [45]. Because Chagasic cardiomyopathy leads to a progressive inability to continue working, it results in an economic loss exceeding USD 1.2 billion annually. In non-endemic countries, a loss of over USD 7 billion is estimated due to treatment costs and productivity loss [46].
Among the strategies designed to curb the impact of Chagas disease on the global economy and public health, prevention stands paramount. However, a disheartening aspect is the lack of effective vaccines to prevent Chagas disease and, as of today, chemotherapy remains the only form of treatment [47].
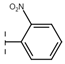
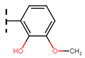
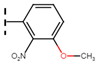
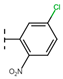
The current treatment is limited to two nitroheterocyclic drugs, each containing a nitro group linked to imidazole and furan rings: benznidazole (BNZ) and nifurtimox (NFX), respectively (Figure 21). These drugs were approved for use in 1971 and 1965, respectively [48].

Figure 21.
Drugs used as treatment against Chagas disease.
Furthermore, they are insufficient since, in the case of BNZ, it exhibits an efficacy of 70–80% during the acute phase, decreasing to around 10% in the chronic phase. On the other hand, NFX demonstrates an efficacy ranging from 70 to 100% in the acute phase, but this declines to 7–8% in the chronic phase, which is when most patients are diagnosed [49].
Patients treated with BNZ or NFX present adverse effects such as fever, nausea, headaches, muscle and joint pains, neurological reactions (restlessness, disorientation, amnesia, insomnia, spasms, paresthesias, polyneuritis, and seizures), dermatological manifestations (dermatitis with skin rashes, generalized or peritoneal edema), and bone marrow depression (lymphadenopathy, agranulocytosis, neutropenia, and thrombocytopenic purpura), and BNZ and NFX are also considered genotoxic, carcinogenic, and teratogenic [49,50]. Additionally, the pharmacological administration of BNZ or NFX occurs over an extended period of 60 to 90 days [51].
As a result, significant resources are currently dedicated to exploring new chemotherapeutic alternatives. These efforts span from utilizing in silico tools for assisted design and the discovery of alternative therapeutic targets to conducting high-throughput trials. These trials are focused not only on treatment but also on minimizing side effects and preventing drug resistance [52]. In this new direction, nitroimidazole-derived drugs are emerging, as described below.
3.3.1. Fexinidazole
Fexinidazole (FXZ) (51) has demonstrated activity against several strains of T. cruzi (CL Brener, Y, Colombian, and VL-10). Experiments have also been conducted on infected mice to compare its efficacy to that of BNZ. In this regard, it was more effective, with a cure rate exceeding 70% in animals who were infected by different strains and treated during the acute or chronic phases. Furthermore, mice infected with BNZ-resistant strains have been treated with fexinidazole at a dose of 300 mg/kg/day for 20 days, successfully reducing the parasitemia to undetectable levels (Figure 8) [53].
Recently, in the research conducted by Zuma and de Souza in 2022, they determined the IC50 that was required to halt the proliferation of amastigotes, which was found to be 1 µM. Furthermore, they described how FXZ intervenes in the structural organization of the parasite, massively disorganizing the reservosomes, causing detachment of the plasma membrane, and damaging the nuclear heterochromatin. They also noted alterations in the mitochondria, the Golgi apparatus, and the kinetoplast–mitochondria complex [54].
These results have encouraged the undertaking of a greater number of studies with FXZ. To cite an example, a phase II clinical trial was initiated in Spain, involving short-duration treatments (3, 7, and 10 days) with low doses of FXZ (600 and 1200 mg) in adults suffering from Chagas disease in its indeterminate chronic phase. The objective was to determine the minimum effective and safe dose for treatment. Although the results of this study were obtained in 2020, they have not yet been published [55].
Some research groups have used FXZ as a scaffold for the generation of new compounds or derived compounds. For example, fexinidazole sulfoxide (52) and fexinidazole sulfone (53) have shown in vitro and in vivo activities that were superior to those of BNZ and FXZ, with an IC50 in infected Macrophages of 5.8 μM for fexinidazole sulfone, 5.4 μM for fexinidazole sulfoxide, and 6.9 μM for BNZ. Regarding experimental models, mice were infected with the Y strain and treated in the acute phase with 100 mg/kg/day for 20 days, and both compounds had a 100% cure rate [56,57].
3.3.2. Megazol
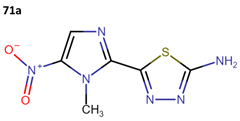
Megazol (71a) was designed as an antimicrobial drug; however, it has demonstrated activity against T. cruzi epimastigotes, with an IC50 of 9.9 µM for the Y strain and other strains (Table 19). Consequently, it has been used as a scaffold for the synthesis of new drugs, although to date, none of these derivatives have surpassed the efficacy of megazol. The IC50 ± SD of each new compound is detailed for each tested strain in Table 19 [58].

Table 19.
Megazol derivatives activity against Trypanosoma strains.
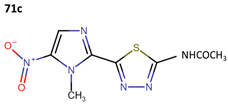
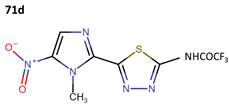
Some research groups used megazol to synthesize derivatives with the aim of enhancing its anti-leishmanial effectiveness. In this context, Maya et al. obtained three new compounds (71b–71d) as prodrugs of megazol (71a) through the action of cytosolic peptides in the acetyl region. The IC50 values against the Tulahuen, LQ, and Brener strains are shown in Table 19 [59].
3.3.3. Metronidazole Derivatives
Since the 1950s, metronidazole (55a) has been used in clinical practice to treat various infections caused by anaerobic bacteria and parasites, including its ongoing use in the treatment of trichomoniasis, amebiasis, giardiasis, and vaginosis [33].
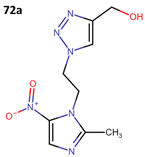
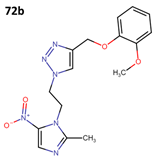
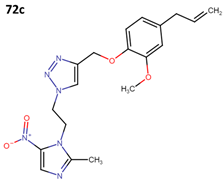
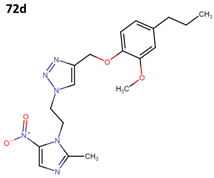
The activity of 55a has been assessed against T. cruzi epimastigote and trypomastigote forms in the Y strain; however, it did not surpass the efficacy of benznidazole (BNZ), which is the approved and currently used drug against Chagas disease. The results obtained from four derivatives of metronidazole (72a–d) that exhibit anti-T. cruzi activity are presented in Table 20 [60].

Table 20.
Metronidazole derivatives with activity against Trypanosoma cruzi strains.
3.3.4. MK-436
The drug MK-436 (73) (Figure 22) demonstrated efficacy in treating infections by T. cruzi in the acute and chronic stages in a murine model. In this model, the researchers administered two daily doses of 250 mg per kg of body weight and observed a total elimination of the parasitemia. Furthermore, a cure rate of 72% to 100% was achieved against different kinds of parasitic strains [53].
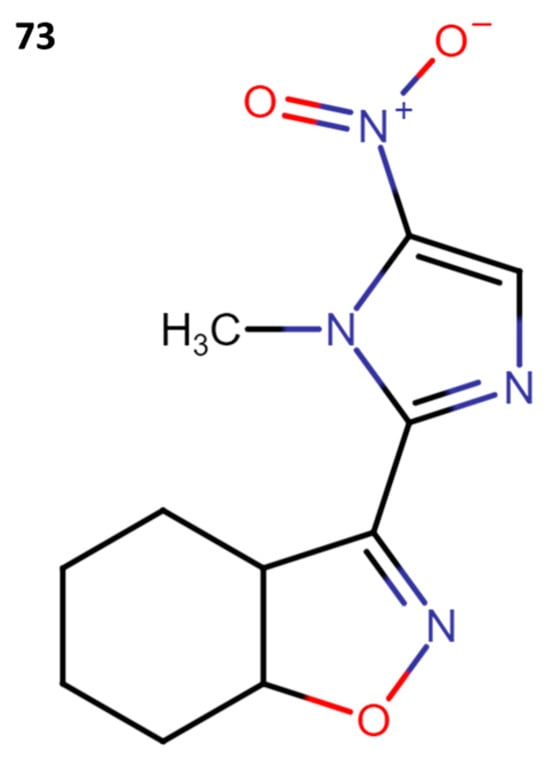
Figure 22.
Structure of MK-436.
3.3.5. Bicyclic Nitroimidazole Drugs: Nitroimidazopyridazines
Finally, Zheng et al. evaluated a series of bicyclic nitroimidazopyridazine compounds (6–24) (Figure 5) against T. cruzi; the results are shown in Table 21 [17].

Table 21.
Nitroimidazopyridazine derivatives with activity against T. cruzi.
4. Discussion
Starting from the premise that anti-protozoal treatments are toxic, and their therapeutic regimens require prolonged treatment times and high concentrations of drugs, in this systematic review, we demonstrate that 5-nitroimidazole derivatives offer a broader spectrum of activity against a variety of protozoal pathogens. Therefore, 5-nitroimidazole derivatives are proposed as pharmacophores against neglected tropical protozoan diseases. There are a plethora of chemical structures containing nitroimidazole rings that have been associated with multiple biological activities, such as anti-protozoan, anti-bacterial, anti-fungal, and anti-cancer effects [61]. One of the main advantages is that nitroindazoles exhibit fewer toxic effects and better clinical tolerance than reference drugs. Furthermore, it is evident that the nitro group can induce improvements in the activity of compounds, enabling them to interfere with critical enzymes of these parasites, such as iron superoxide dismutase (Fe-SOD) and trypanothione reductase (TR), leading to the generation of reactive oxygen and nitrogen species in protozoans, resulting in their death [58].
The anti-protozoal effect of all 5-NI derivatives is attributed to the nitro group reduction of 5-Nis, which results in intermediate compounds and free radicals that can interact with membranes, proteins, and nucleic acids through oxidation or covalent binding modifications. Due to this mechanism of action, the structures are considered “prodrugs” [62,63].
It is worth mentioning that 5-NI can be metabolized by both humans and protozoa. However, recent achievements have been documented in the selectivity of 5-NI derivatives to combat protozoa endpoints, significantly reducing their side effects in humans [64]. These new results have opened new perspectives for developing novel and safe anti-protozoan agents.
In addition to their anti-protozoan activity, 5-NI derivatives have also been associated with multi-inhibitory activity against different molecular targets involved in homeostasis, metabolism, and cancer [65,66,67,68,69,70].
In general, the main metabolic pathway involves the oxidation of the aliphatic side chains of these compounds, resulting in the generation of oxidized metabolites (hydroxylated and acetylated metabolites). Some of these metabolites, along with the parent molecule, are conjugated and excreted via urine and feces along with free metabolites. The literature findings suggest that, although the initial metabolic step is the formation of a nitro anion radical, in the presence of oxygen, this radical is reoxidized, leading to the generation of a superoxide anion. This cycle is believed to be the source of reactive oxygen species (ROS), without the loss of the parent molecule. Subsequently, after the formation of the superoxide anion, in the presence of superoxide dismutase (SOD) and traces of metal ions such as Fe2+ and Cu2+, reactions would occur, leading to the formation of a hydroxyl radical [71]. It is due to the action of ROS that nitroimidazole derivatives have been considered mutagenic, primarily in prokaryotic systems and to a lesser extent in eukaryotes, as mentioned earlier. However, the International Agency for Research on Cancer (IARC) does classify nitroimidazoles as carcinogens [72]. For this reason, it is important to continue the study of their possible “off-target” effects.
In the present work, using a systematic review protocol, we identified 213 5-NI derivatives with inhibitory activity against different protozoans: Plasmodium (62/29.1%), Leishmania (142/66.7%), and T. cruzi (9/4.2%). The activity ranged from 0.0005 to 74 µM, 0.001 to 84 µM, and 1 to 602 µM, respectively. Notably, a greater number of 5-NI derivatives were tested against Leishmania and demonstrated higher activity. The best scaffolds for each protozoan genus are 5-nitroimidazole-2-carbaldehyde derivatives for Plasmodium falciparum, metronidazole derivatives for Leishmania mexicana, and fexinidazole for Trypanosoma cruzi. Interestingly, three 5-NI scaffolds have been associated with multi-protozoan activity, which are distributed in 23 compounds (nitroimidazopyridazine derivates, compounds 6–24; metronidazole, compound 54a; and fexinidazole derivatives, compounds 51–53).
There are additional compounds within 5-NI scaffolds that were identified in the early stages of this systematic review, but their content was either not directly related or did not meet the inclusion criteria. An example of this is secnidazole (1-(2-methyl-5-nitro-1-imidazole-1-yl) propan-2-o), which was tested in 2017 by Eke et al. against Trypanosoma brucei, both in vitro and in vivo. They determined the minimum inhibitory concentration (MIC) that is required to inhibit motility in blood infected with 225,000 trypomastigotes, which was found to be 2.8–1.4 mg/mL after 10 min post-treatment. Regarding murine models, significantly lower parasitemia levels were observed (p < 0.05) compared to the untreated infected group after 6 days post-infection (post-treatment) [73]. While Trypanosoma cruzi or Leishmania spp. were not specifically evaluated, given that they are trypanosomatids, these results encourage further exploration of the application and development of new compounds based on 5-NI. For instance, compounds like tinidazole, ornidazole, ronidazole, and their derivatives could be potential candidates for future investigations [74].
The data collected from this work allow for a systematic chemoinformatic study using DataWarrior software version 5.5.0 [69]. For each compound, their physicochemical properties were calculated. For example, the total molecular weight, cLogP (octanol/water coefficient, solubility estimation), and the number of sp3 atoms (stereochemical complexity estimation) were calculated and statistically analyzed (Table S2). The results suggest that 3-nitroimidazopyridines derivatives (6–24), metronidazole hybridized derivatives (29–50), and quinoline–metronidazole derivatives (63a–63m) have high molecular weights compared with other 5-NI derivatives. These kinds of compounds are in the upper recommended limit to be considered “drug-like” compounds, according to the empirical rules proposed by Lipinsky [70].
Interestingly, these results also suggest that there are compounds with a hydrophobic profile, like the quinoline–metronidazole derivatives (63a–63m), and others with a hydrophilic profile like fexinidazole derivatives. Finally, the authors remark on the stereochemical complexity of 5-NI derivatives, particularly for cases like metronidazole-hybridized and metronidazole-based compounds (29–50, 55a–59i). All data are available in the Supplementary Materials section and can be interactively analyzed using DataWarrior software version 5.5.0 [75].
Although the 5-NI derivatives offer an interesting starting point for developing novel and safe anti-protozoan agents, there are still other important issues to resolve. For example, different research groups are leading the development of novel drug formulations and new chemical vehicles to reduce the 5-NI doses to significantly reduce their associated side effects. In parallel, the main purpose of studying 5-NI derivatives is to develop new chemical derivates with better protozoan selectivity and better clinical administration regimens [76,77]. Additionally, non-neglected tropical protozoan (e.g., Giardia lambia) strains have developed 5-NI derivative resistance through multiple mechanisms, which highlight the possibility that other types of protozoans (like Trypanosoma brucei or Trichomonas vaginalis) will develop similar resistance mechanisms in the near future [78,79].
Finally, the authors highlight that this systematic review has limitations that must be noted. For example, the use of a systematic method does not guarantee that all 5-NI derivatives with anti-protozoan activity have been presented in the final list of this work (see Supplementary Materials section). Also, this systematic review did not consider “non-open access journals” and “lower-impact journals” that could contain important data to discuss. However, this work shows representative successful cases that illustrate the potential of the 5-NI scaffold against protozoan endpoints.
Nevertheless, this systematic review shows us that the exploration of 5-NI derivatives and other new chemical entities in the treatment of NTPDs, including malaria, visceral leishmaniasis, and Chagas disease, underscores the importance of continued innovation and collaboration in drug discovery. These efforts are not only crucial in addressing the immediate challenges posed by these diseases but also in shaping future strategies for effective disease management in resource-limited settings [80].
Nitro-imidazoles, in general, have provided relief for various human and livestock infections, resulting in significant economic benefits for humanity. However, in addition to the resistance, multidrug resistance, and pan-resistance that the excessive and uncontrolled use of antimicrobials has triggered, there is also environmental harm. Both soils and continental and marine waters are increasingly being invaded by waste containing metabolites and antibiotics that promote resistance in microorganisms, reactivating cycles that are difficult to halt. That is why collaborative efforts are underway to develop new, more easily degradable antibiotics, such as metal nanoparticles and biopolymer-based ones [81]. Work is also being carried out on the design of devices that can identify contamination by nitroimidazoles or other antibiotics in water or soil, as well as on decontamination processes [82,83]. However, as mentioned earlier, infections by protozoa-causing diseases that are classified as neglected primarily affect a larger number of victims in resource-poor countries that are unable to access these benefits.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/futurepharmacol4010015/s1, Table S1: Search strategies and keywords according to PRISMA guidelines; Table S2: Comparison of physicochemical properties of different kinds of 5-nitroimidazole derivatives; File S1: Chemoinformatic analysis of 5-NTs with anti-protozoal activity. Generated with DataWarrior software version 5.5.0; File S2: PRISMA 2020 checklist.
Author Contributions
M.M.V.-R. and E.L.-L. generated the figures and tables and interpreted the data. C.B.-S. and E.L.-L. supervised the work of M.M.V.-R., participated in the conceptualization of the study, and helped draft the first version of the draft. C.B.-S., E.L.-L. and C.S.-C. contributed to the discussion of the concepts. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The Supplementary Materials is available at https://zenodo.org/doi/10.5281/zenodo.10251630 (accessed on 1 January 2024).
Acknowledgments
We acknowledge the support from CONAHCYT. M.M.V.-R. and E.L.-L. thank CONAHCYT, Mexico, for Ph.D. scholarships (numbers 859013 and 894234). The authors acknowledge the financial support for the DGAPA-PAPIIT: IN210023 project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lauwaet, T.; Miyamoto, Y.; Ihara, S.; Le, C.; Kalisiak, J.; Korthals, K.A.; Ghassemian, M.; Smith, D.K.; Sharpless, K.B.; Fokin, V.V.; et al. Click chemistry-facilitated comprehensive identification of proteins adducted by antimicrobial 5-nitroimidazoles for discovery of alternative drug targets against giardiasis. PLoS Negl. Trop. Dis. 2020, 14, e0008224. [Google Scholar] [CrossRef]
- Martín-Escolano, R.; Pérez-Cordón, G.; Arán, V.J.; Marín, C.; Sánchez-Moreno, M.; Rosales, M.J. 5-Nitroindazole derivatives as potential therapeutic alternatives against Acanthamoeba castellanii. Acta Trop. 2022, 232, 106538. [Google Scholar] [CrossRef]
- Borba, J.V.V.B.; Silva, A.C.; Lima, M.N.N.; Mendonca, S.S.; Furnham, N.; Costa, F.T.M.; Andrade, C.H. Chemogenomics and bioinformatics approaches for prioritizing kinases as drug targets for neglected tropical diseases. In Advances in Protein Chemistry and Structural Biology, 1st ed.; Rossen, D., Ed.; Elsevier: San Diego, CA, USA, 2021; Volume 124, pp. 187–223. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, 372. [Google Scholar] [CrossRef]
- Pinheiro, L.C.S.; Feitosa, L.M.; Silveira, F.F.D.; Boechat, N. Current Antimalarial Therapies and Advances in the Development of Semi-Synthetic Artemisinin Derivatives. Acad. Bras. Cienc. 2018, 90, 1251–1271. [Google Scholar] [CrossRef]
- Feng, L.S.; Xu, Z.; Chang, L.; Li, C.; Yan, X.F.; Gao, C.; Ding, C.; Zhao, F.; Shi, F.; Wu, X. Hybrid molecules with potential in vitro antiplasmodial and in vivo antimalarial activity against drug-resistant Plasmodium falciparum. Med. Res. Rev. 2020, 40, 931–971. [Google Scholar] [CrossRef]
- Boitel, E.; Desoubeaux, G. Antiparasitic treatments in pregnant women: Update and recommendations. Med. Mal. Infect. 2020, 50, 3–15. [Google Scholar] [CrossRef]
- Álvarez-Bardón, M.; Pérez-Pertejo, Y.; Ordóñez, C.; Sepúlveda-Crespo, D.; Carballeira, N.M.; Tekwani, B.L.; Murugesan, S.; Martinez-Valladares, M.; García-Estrada, C.; Reguera, R.M.; et al. Screening Marine Natural Products for New Drug Leads against Trypanosomatids and Malaria. Mar. Drugs 2020, 18, 187. [Google Scholar] [CrossRef]
- Pedra-Rezende, Y.; Macedo, I.S.; Midlej, V.; Mariante, R.M.; Menna-Barreto, R.F.S. Different drugs, same end: Ultrastructural hallmarks of autophagy in pathogenic protozoa. Front. Microbiol. 2022, 29, 856686. [Google Scholar] [CrossRef]
- Glennon, E.K.K.; Dankwa, S.; Smith, J.D.; Kaushansky, A. Opportunities for Host-targeted Therapies for Malaria. Trends Parasitol. 2018, 34, 843–860. [Google Scholar] [CrossRef]
- Navidpour, L.; Chibale, K.; Esmaeili, S.; Ghiaee, A.; Hadj-Esfandiari, N.; Irani, M.; Ahmadi Koulaei, S.; Yassa, N. Antimalarial Activities of (Z)-2-(Nitroheteroarylmethylene)-3(2H)-Benzofuranone Derivatives: In Vitro and In Vivo Assessment and β-Hematin Formation Inhibition Activity. Antimicrob. Agents Chemother. 2021, 65, e0268320. [Google Scholar] [CrossRef]
- Figueroa-Romero, A.; Pons-Duran, C.; Gonzalez, R. Drugs for intermittent preventive treatment of malaria in pregnancy: Current knowledge and way forward. Trop. Med. Infect. Dis. 2022, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Ochora, D.O.; Mogire, R.M.; Masai, R.J.; Yeda, R.A.; Mwakio, E.W.; Amwoma, J.G.; Wakoli, D.M.; Yenesew, A.; Akala, H.M. Ex vivo and In vitro antiplasmodial activities of approved drugs predicted to have antimalarial activities using chemogenomics and drug repositioning approach. Heliyon 2023, 9, e18863. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Anand, U.; Siddiqui, W.A.; Tripathi, R. Drug Development Strategies for Malaria: With the Hope for New Antimalarial Drug Discovery—An Update. Adv. Med. 2023, 2023, 5060665. [Google Scholar] [CrossRef] [PubMed]
- Chugh, A.; Kumar, A.; Verma, A.; Kumar, S.; Kumar, P. A review of antimalarial activity of two or three nitrogen atoms containing heterocyclic compounds. Med. Chem. Res. 2020, 29, 1723–1750. [Google Scholar] [CrossRef]
- Tukulula, M.; Sharma, R.K.; Meurillon, M.; Mahajan, A.; Naran, K.; Warner, D.; Huang, J.; Mekonnen, B.; Chibale, K. Synthesis and antiplasmodial and antimycobacterial evaluation of new nitroimidazole and nitroimidazooxazine derivatives. ACS Med. Chem. Lett. 2013, 4, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Müller, J.; Kunz, S.; Siderius, M.; Maes, L.; Caljon, G.; Müller, N.; Hemphill, A.; Sterk, G.J.; Leurs, R. 3-nitroimidazo[1,2-b]pyridazine as a novel scaffold for antiparasitics with sub-nanomolar anti-Giardia lamblia activity. Int. J. Parasitol. Drugs Drug Resist. 2022, 19, 47–55. [Google Scholar] [CrossRef]
- Lohidakshan, K.; Rajan, M.; Ganesh, A.; Paul, M.; Jerin, J. Pass and Swiss ADME collaborated in silico docking approach to the synthesis of certain pyrazoline spacer compounds for dihydrofolate reductase inhibition and antimalarial activity. Bangladesh J. Pharmacol. 2018, 13, 23. [Google Scholar] [CrossRef]
- Beteck, R.M.; Isaacs, M.; Legoabe, L.J.; Hoppe, H.C.; Tam, C.C.; Kim, J.H.; Petzer, J.P.; Cheng, L.W.; Quiambao, Q.; Land, K.M.; et al. Synthesis and in vitro antiprotozoal evaluation of novel metronidazole-Schiff base hybrids. Arch. Pharm. 2023, 356, e2200409. [Google Scholar] [CrossRef]
- Briones Nieva, C.A.; Cid, A.G.; Romero, A.I.; García-Bustos, M.F.; Villegas, M.; Bermúdez, J.M. An appraisal of the scientific current situation and new perspectives in the treatment of cutaneous leishmaniasis. Acta Trop. 2021, 221, 105988. [Google Scholar] [CrossRef]
- Varikuti, S.; Jha, B.K.; Volpedo, G.; Ryan, N.M.; Halsey, G.; Hamza, O.M.; McGwire, B.S.; Satoskar, A.R. Host-Directed Drug Therapies for Neglected Tropical Diseases Caused by Protozoan Parasites. Front. Microbiol. 2018, 9, 2655. [Google Scholar] [CrossRef]
- Pacheco-Fernandez, T.; Markle, H.; Verma, C.; Huston, R.; Gannavaram, S.; Nakhasi, H.L.; Satoskar, A.R. Field-Deployable Treatments For Leishmaniasis: Intrinsic Challenges, Recent Developments and Next Steps. Res. Rep. Trop. Med. 2023, 14, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Capela, R.; Moreira, R.; Lopes, F. An overview of drug resistance in protozoal diseases. Int. J. Mol. Sci. 2019, 20, 5748. [Google Scholar] [CrossRef]
- García-Estrada, C.; Pérez-Pertejo, Y.; Domínguez-Asenjo, B.; Holanda, V.N.; Murugesan, S.; Martínez-Valladares, M.; Balaña-Fouce, R.; Reguera, R.M. Further investigations of nitroheterocyclic compounds as potential antikinetoplastid drug candidates. Biomolecules 2023, 13, 637. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J.; Meena, L.P. Leishmaniasis: Treatment, drug resistance and emerging therapies. Expert Opin. Orphan Drugs 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Bernhard, S.; Kaiser, M.; Burri, C.; Mäser, P. Fexinidazole for Human African Trypanosomiasis, the Fruit of a Successful Public-Private Partnership. Diseases 2022, 10, 90. [Google Scholar] [CrossRef]
- Deeks, E.D. Fexinidazole: First global approval. Drugs 2019, 79, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, S.; Patterson, S.; Stojanovski, L.; Simeons, F.R.; Norval, S.; Kime, R.; Read, K.D.; Fairlamb, A.H. The anti-trypanosome drug fexinidazole shows potential for treating visceral leishmaniasis. Sci. Transl. Med. 2012, 4, 119re1. [Google Scholar] [CrossRef]
- Imran, M.; Khan, S.A.; Alshammari, M.K.; Alqahtani, A.M.; Alanazi, T.A.; Kamal, M.; Jawaid, T.; Ghoneim, M.M.; Alshehri, S.; Shakeel, F. Discovery, Development, Inventions and Patent Review of Fexinidazole: The First All-Oral Therapy for Human African Trypanosomiasis. Pharmaceuticals 2022, 15, 128. [Google Scholar] [CrossRef]
- Foroumadi, A.; Emami, S.; Pournourmohammadi, S.; Kharazmi, A.; Shafiee, A. Synthesis and in vitro leishmanicidal activity of 2-(1-methyl-5-nitro-1H-imidazol-2-yl)-5-substituted-1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2005, 40, 1346–1350. [Google Scholar] [CrossRef]
- Rodríguez, M.; Gutiérrez, J.; Domínguez, J.; Peixoto, P.A.; Fernández, A.; Rodríguez, N.; Deffieux, D.; Rojas, L.; Quideau, S.; Pouységu, L.; et al. Synthesis and leishmanicidal evaluation of sulfanyl- and sulfonyl-tethered functionalized benzoate derivatives featuring a nitroimidazole moiety. Arch. Pharm. 2020, 353, e2000002. [Google Scholar] [CrossRef]
- Blanco, Z.; Mijares, M.R.; Ramírez, H.; Fernandez-Moreira, E.; Oviedo, H.J.; Rodríguez, N.M.; Charris, J.E. In vitro evaluation and in vivo efficacy of nitroimidazole-sulfanyl ethyl derivatives against Leishmania (V.) braziliensis and Leishmania (L.) mexicana. Parasitol. Res. 2021, 120, 3307–3317. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.M.; Long, Y.; King, S.B. Nitroaromatic antibiotics as nitrogen oxide sources. Biomolecules 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Chandrakar, P.; Gupta, S.; Parmar, N.; Singh, S.K.; Rashid, M.; Kushwaha, P.; Wahajuddin, M.; Sashidhara, K.V.; Kar, S. Synthesis, Biological Evaluation, Structure-Activity Relationship, and Mechanism of Action Studies of Quinoline-Metronidazole Derivatives Against Experimental Visceral Leishmaniasis. J. Med. Chem. 2019, 62, 5655–5671. [Google Scholar] [CrossRef]
- Navidpour, L.; Lima, M.L.; Milne, R.; Wyllie, S.; Hadj-Esfandiari, N.; Choudhary, M.I.; Khan, S.; Yousuf, S. Antileishmanial Activities of (Z)-2-(Nitroimidazolylmethylene)-3(2H)-Benzofuranones: Synthesis, In Vitro Assessment, and Bioactivation by NTR 1 and 2. Antimicrob. Agents Chemother. 2022, 66, e0058322. [Google Scholar] [CrossRef]
- Castera-Ducros, C.; Paloque, L.; Verhaeghe, P.; Casanova, M.; Cantelli, C.; Hutter, S.; Tanguy, F.; Laget, M.; Remusat, V.; Cohen, A.; et al. Targeting the human parasite Leishmania donovani: Discovery of a new promising anti-infectious pharmacophore in 3-nitroimidazo[1,2-a]pyridine series. Bioorg. Med. Chem. 2013, 21, 7155–7164. [Google Scholar] [CrossRef]
- Fersing, C.; Boudot, C.; Pedron, J.; Hutter, S.; Primas, N.; Castera-Ducros, C.; Bourgeade-Delmas, S.; Sournia-Saquet, A.; Moreau, A.; Cohen, A.; et al. 8-Aryl-6-chloro-3-nitro-2-(phenylsulfonylmethyl)imidazo[1,2-a]pyridines as potent antitrypanosomatid molecules bioactivated by type 1 nitroreductases. Eur. J. Med. Chem. 2018, 157, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Fersing, C.; Basmaciyan, L.; Boudot, C.; Pedron, J.; Hutter, S.; Cohen, A.; Castera-Ducros, C.; Primas, N.; Laget, M.; Casanova, M.; et al. Nongenotoxic 3-Nitroimidazo[1,2-a]pyridines Are NTR1 Substrates That Display Potent In Vitro Antileishmanial Activity. ACS Med. Chem. Lett. 2019, 10, 34–39. [Google Scholar] [CrossRef]
- Fersing, C.; Boudot, C.; Castera-Ducros, C.; Pinault, E.; Hutter, S.; Paoli-Lombardo, R.; Primas, N.; Pedron, J.; Seguy, L.; Bourgeade-Delmas, S.; et al. 8-Alkynyl-3-nitroimidazopyridines display potent antitrypanosomal activity against both T. b. brucei and cruzi. Eur. J. Med. Chem. 2020, 202, 112558. [Google Scholar] [CrossRef] [PubMed]
- Reséndiz-Mora, A.; Barrera-Aveleida, G.; Sotelo-Rodríguez, A.; Galarce-Sosa, I.; Nevárez-Lechuga, I.; Santiago-Hernández, J.C.; Nogueda-Torres, B.; Meza-Toledo, S.; Gómez-Manzo, S.; Wong-Baeza, I.; et al. Effect of B-NIPOx in Experimental Trypanosoma cruzi Infection in Mice. Int. J. Mol. Sci. 2022, 24, 333. [Google Scholar] [CrossRef] [PubMed]
- García-Huertas, P.; Cardona-Castro, N. Advances in the treatment of Chagas disease: Promising new drugs, plants and targets. Biomed. Pharmacother. 2021, 142, 112020. [Google Scholar] [CrossRef] [PubMed]
- Martín-Escolano, R.; Rosales, M.J.; Marín, C. Biological characteristics of the Trypanosoma cruzi Arequipa strain make it a good model for Chagas disease drug discovery. Acta Trop. 2022, 236, 106679. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Molina, J.A.; Crespillo-Andújar, C.; Bosch-Nicolau, P.; Molina, I. Trypanocidal treatment of Chagas disease. Enferm. Infecc. Microbiol. Clin. 2021, 39, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.M.C.; Pedra-Rezende, Y.; Pereira, L.D.; de Melo, T.G.; Barbosa, H.S.; Lannes-Vieira, J.; de Castro, S.L.; Daliry, A.; Salomão, K. Benznidazole and amiodarone combined treatment attenuates cytoskeletal damage in Trypanosoma cruzi-infected cardiac cells. Front. Cell Infect. Microbiol. 2022, 12, 975931. [Google Scholar] [CrossRef] [PubMed]
- Kratz, J.M. Drug discovery for chagas disease: A viewpoint. Acta Trop. 2019, 198, 105107. [Google Scholar] [CrossRef]
- Vannier-Santos, M.A.; Suarez-Fontes, A.M.; Almeida-Silva, J.; Lifsitch Viçosa, A.; Chavez Perez, S.A.; Hasslocher-Moreno, A.M.; Parreiras Estolano da Silveira, G.; Fernandes Portela, L.; Magalhães Saraiva, R. Translational research on chagas disease: Focusing on drug combination and repositioning. In Chagas Disease—From Cellular and Molecular Aspects of Trypanosoma cruzi-Host Interactions to the Clinical Intervention, 1st ed.; Menna-Barreto, R., Ed.; IntechOpen: London, UK, 2022; pp. 1–35. [Google Scholar] [CrossRef]
- Pereira, P.M.L.; Fernandes, B.T.; Dos Santos, V.R.; Cabral, W.R.C.; Lovo-Martins, M.I.; Alonso, L.; Lancheros, C.A.C.; de Paula, J.C.; Camargo, P.G.; Suzukawa, H.T.; et al. Antiprotozoal Activity of Benzoylthiourea Derivatives against Trypanosoma cruzi: Insights into Mechanism of Action. Pathogens 2023, 12, 1012. [Google Scholar] [CrossRef]
- Francisco, A.F.; Jayawardhana, S.; Olmo, F.; Lewis, M.D.; Wilkinson, S.R.; Taylor, M.C.; Kelly, J.M. Challenges in Chagas disease drug development. Molecules 2020, 25, 2799. [Google Scholar] [CrossRef]
- Kawaguchi, W.H.; Cerqueira, L.B.; Fachi, M.M.; Campos, M.L.; Reason, I.J.M.; Pontarolo, R. Efficacy and safety of chagas disease drug therapy and treatment perspectives. In Chagas Disease—Basic Investigations and Challenges, 1st ed.; Nissapatorn, V., Oz, H.S., Eds.; InTech: London, UK, 2018; pp. 121–151. [Google Scholar] [CrossRef]
- Kratz, J.M.; Garcia Bournissen, F.; Forsyth, C.J.; Sosa-Estani, S. Clinical and pharmacological profile of benznidazole for treatment of Chagas disease. Expert. Rev. Clin. Pharmacol. 2018, 11, 943–957. [Google Scholar] [CrossRef]
- Barnadas-Carceller, B.; Martinez-Peinado, N.; Gómez, L.C.; Ros-Lucas, A.; Gabaldón-Figueira, J.C.; Diaz-Mochon, J.J.; Gascon, J.; Molina, I.J.; Pineda de Las Infantas y Villatoro, M.J.; Alonso-Padilla, J. Identification of compounds with activity against Trypanosoma cruzi within a collection of synthetic nucleoside analogs. Front. Cell Infect. Microbiol. 2022, 12, 1067461. [Google Scholar] [CrossRef]
- López-López, E.; Barrientos-Salcedo, C.; Prieto-Martínez, F.D.; Medina-Franco, J.L. In silico tools to study molecular targets of neglected diseases: Inhibition of TcSir2rp3, an epigenetic enzyme of Trypanosoma cruzi. In Advances in Protein Chemistry and Structural Biology, 1st ed.; Karabencheva-Christova, T., Christov, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 122, pp. 203–229. [Google Scholar] [CrossRef]
- Mazzeti, A.L.; Capelari-Oliveira, P.; Bahia, M.T.; Mosqueira, V.C.F. Review on Experimental Treatment Strategies Against Trypanosoma cruzi. J. Exp. Pharmacol. 2021, 13, 409–432. [Google Scholar] [CrossRef]
- Zuma, A.A.; de Souza, W. Fexinidazole interferes with the growth and structural organization of Trypanosoma cruzi. Sci. Rep. 2022, 12, 20388. [Google Scholar] [CrossRef]
- Oral Fexinidazole Dosing Regimens for the Treatment of Adults with Chronic Indeterminate Chagas Disease (FEXI12), CTG Labs—NCBI. Available online: https://clinicaltrials.gov/study/NCT03587766 (accessed on 30 September 2023).
- Ribeiro, V.; Dias, N.; Paiva, T.; Hagström-Bex, L.; Nitz, N.; Pratesi, R.; Hecht, M. Current trends in the pharmacological management of Chagas disease. Int. J. Parasitol. Drugs Drug Resist. 2020, 12, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Bahia, M.T.; Nascimento, A.F.; Mazzeti, A.L.; Marques, L.F.; Gonçalves, K.R.; Mota, L.W.; Diniz, L.D.E.F.; Caldas, I.S.; Talvani, A.; Shackleford, D.M.; et al. Antitrypanosomal activity of fexinidazole metabolites, potential new drug candidates for Chagas disease. Antimicrob. Agents Chemother. 2014, 58, 4362–4370. [Google Scholar] [CrossRef]
- da Silva Santos-Júnior, P.F.; Rocha Silva, L.; Quintans-Júnior, L.J.; Ferreira da Silva-Júnior, E. Nitro compounds against trypanosomatidae parasites: Heroes or villains? Bioorg. Med. Chem. Lett. 2022, 75, 128930. [Google Scholar] [CrossRef]
- Maya, J.D.; Bollo, S.; Nuñez-Vergara, L.J.; Squella, J.A.; Repetto, Y.; Morello, A.; Périé, J.; Chauvière, G. Trypanosoma cruzi: Effect and mode of action of nitroimidazole and nitrofuran derivatives. Biochem. Pharmacol. 2003, 65, 999–1006. [Google Scholar] [CrossRef]
- Pelozo, M.F.; Lima, G.F.S.; Cordeiro, C.F.; Silva, L.S.; Caldas, I.S.; Carvalho, D.T.; Lavorato, S.N.; Hawkes, J.A.; Franco, L.L. Synthesis of New Hybrid Derivatives from Metronidazole and Eugenol Analogues as Trypanocidal Agents. J. Pharm. Pharm. Sci. 2021, 24, 421–434. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, S.; Singh, R.; Vishwakarma, R.A.; Mignani, S.; Singh, P.P. Functionalized Nitroimidazole Scaffold Construction and Their Pharmaceutical Applications: A 1950–2021 Comprehensive Overview. Pharmaceuticals 2022, 15, 561. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Schmid, H. Pharmaco-toxicological mode of action of antimicrobial 5-nitroimidazole derivatives. J. Vet. Med. A Physiol. Pathol. Clin. Med. 1999, 46, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Graves, K.J.; Novak, J.; Secor, W.E.; Kissinger, P.J.; Schwebke, J.R.; Muzny, C.A. A systematic review of the literature on mechanisms of 5-nitroimidazole resistance in Trichomonas vaginalis. Parasitology 2020, 147, 1383–1391. [Google Scholar] [CrossRef]
- Pasupuleti, V.; Escobedo, A.A.; Deshpande, A.; Thota, P.; Roman, Y.; Hernandez, A.V. Efficacy of 5-nitroimidazoles for the treatment of giardiasis: A systematic review of randomized controlled trials. PLoS Negl. Trop. Dis. 2014, 8, e2733. [Google Scholar] [CrossRef]
- Aspatwar, A.; Parvathaneni, N.K.; Barker, H.; Anduran, E.; Supuran, C.T.; Dubois, L.; Lambin, P.; Parkkila, S.; Winum, J.Y. Design, synthesis, in vitro inhibition and toxicological evaluation of human carbonic anhydrases I, II and IX inhibitors in 5-nitroimidazole series. J. Enzym. Inhib. Med. Chem. 2020, 35, 109–117. [Google Scholar] [CrossRef]
- Peerzada, M.N.; Khan, P.; Khan, N.S.; Avecilla, F.; Siddiqui, S.M.; Hassan, M.I.; Azam, A. Design and Development of Small-Molecule Arylaldoxime/5-Nitroimidazole Hybrids as Potent Inhibitors of MARK4: A Promising Approach for Target-Based Cancer Therapy. ACS Omega 2020, 5, 22759–22771. [Google Scholar] [CrossRef]
- López-López, E.; Cerda-García-Rojas, C.M.; Medina-Franco, J.L. Tubulin Inhibitors: A Chemoinformatic Analysis Using Cell-Based Data. Molecules 2021, 26, 2483. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Duan, Y.; Wang, P.; Gao, R.; Chen, X.; Ou, Y.; Liang, M.; Wang, Z.; Yuan, Y.; Wang, L.; et al. DYT-40, a novel synthetic 2-styryl-5-nitroimidazole derivative, blocks malignant glioblastoma growth and invasion by inhibiting AEG-1 and NF-κB signaling pathways. Sci. Rep. 2016, 6, 27331. [Google Scholar] [CrossRef] [PubMed]
- López-López, E.; Naveja, J.J.; Medina-Franco, J.L. DataWarrior: An evaluation of the open-source drug discovery tool. Expert. Opin. Drug Discov. 2019, 14, 335–341. [Google Scholar] [CrossRef]
- Bajorath, J.; Chávez-Hernández, A.L.; Duran-Frigola, M.; Fernández-de Gortari, E.; Gasteiger, J.; López-López, E.; Maggiora, G.M.; Medina-Franco, J.L.; Méndez-Lucio, O.; Mestres, J.; et al. Chemoinformatics and artificial intelligence colloquium: Progress and challenges in developing bioactive compounds. J. Cheminform. 2022, 14, 82. [Google Scholar] [CrossRef]
- Campos-Fernández, L.; Ortiz-Muñiz, R.; Cortés-Barberena, E.; Mares-Sámano, S.; Garduño-Juárez, R.; Soriano-Correa, C. Imidazole and nitroimidazole derivatives as NADH-fumarate reductase inhibitors: Density functional theory studies, homology modeling, and molecular docking. J. Comput. Chem. 2022, 43, 1573–1595. [Google Scholar] [CrossRef]
- The Carcinogenic Potency Project (CPDB). Available online: https://files.toxplanet.com/cpdb/index.html (accessed on 18 December 2023).
- Eke, I.G.; Eze, I.O.; Ezeudu, T.A.; Eze, U.U.; Anaga, A.O.; Onyeyili, P.A. Anti-trypanosomal activity of secnidazole in vitro and in vivo. Trop. J. Pharm. Res. 2017, 16, 535. [Google Scholar] [CrossRef]
- Oliveira, A.A.; Oliveira, A.P.A.; Franco, L.L.; Ferencs, M.O.; Ferreira, J.F.G.; Bachi, S.M.P.S.; Speziali, N.L.; Farias, L.M.; Magalhães, P.P.; Beraldo, H. 5-Nitroimidazole-derived Schiff bases and their copper(II) complexes exhibit potent antimicrobial activity against pathogenic anaerobic bacteria. Biometals 2018, 31, 571–584. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Enanga, B.; Ndong, J.M.; Boudra, H.; Debrauwer, L.; Dubreuil, G.; Bouteille, B.; Chauvière, G.; Labat, C.; Dumas, M.; Périé, J.; et al. Pharmacokinetics, metabolism and excretion of megazol in a Trypanosoma brucei gambiense primate model of human African trypanosomiasis. Preliminary study. Arzneimittelforschung 2000, 50, 158–162. [Google Scholar]
- Galasse Rando, D.G.; de Oliveira Costa, H.G.; Fernanda Heitor, T.; de Moraes, J.; Amorim Pavani, T.F. Employing “red flags” to fight the most neglected diseases: Nitroaromatic as still suitable tools to treat human and veterinary parasitosis. Curr. Top. Med. Chem. 2023, 23, 816–832. [Google Scholar] [CrossRef] [PubMed]
- Leitsch, D.; Burgess, A.G.; Dunn, L.A.; Krauer, K.G.; Tan, K.; Duchêne, M.; Upcroft, P.; Eckmann, L.; Upcroft, J.A. Pyruvate:ferredoxin oxidoreductase and thioredoxin reductase are involved in 5-nitroimidazole activation while flavin metabolism is linked to 5-nitroimidazole resistance in Giardia lamblia. J. Antimicrob. Chemother. 2011, 66, 1756–1765. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.Y.; Wyllie, S.; Patterson, S.; Oza, S.L.; Read, K.D.; Fairlamb, A.H. Cross-resistance to nitro drugs and implications for treatment of human African trypanosomiasis. Antimicrob. Agents Chemother. 2010, 54, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- López-López, E.; Bajorath, J.; Medina-Franco, J.L. Informatics for chemistry, biology, and biomedical sciences. J. Chem. Inf. Model. 2021, 61, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Chen, A.; Chen, W.; Lai, J.; Pei, Y.; Wen, J.; Yang, C.; Luo, J.; Zhang, J.; Lei, C.; et al. Fabrication of Biodegradable and Biocompatible Functional Polymers for Anti-Infection and Augmenting Wound Repair. Pharmaceuticals 2022, 15, 120. [Google Scholar] [CrossRef]
- Jose, A.; Vijayan, V.; Sahadevan, R.; Porel, M.; Sadhukhan, S. Selective luminescent detection of 5-nitroimidazole antibiotics through self-aggregates of a single non-aromatic amino acid, L-lysine. Microchem. J. 2023, 197, 109802. [Google Scholar] [CrossRef]
- de Ilurdoz, M.S.; Sadhwani, J.J.; Reboso, J.V. Antibiotic removal processes from water & wastewater for the protection of the aquatic environment—A review. J. Water Proc. Eng. 2022, 45, 102474. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).