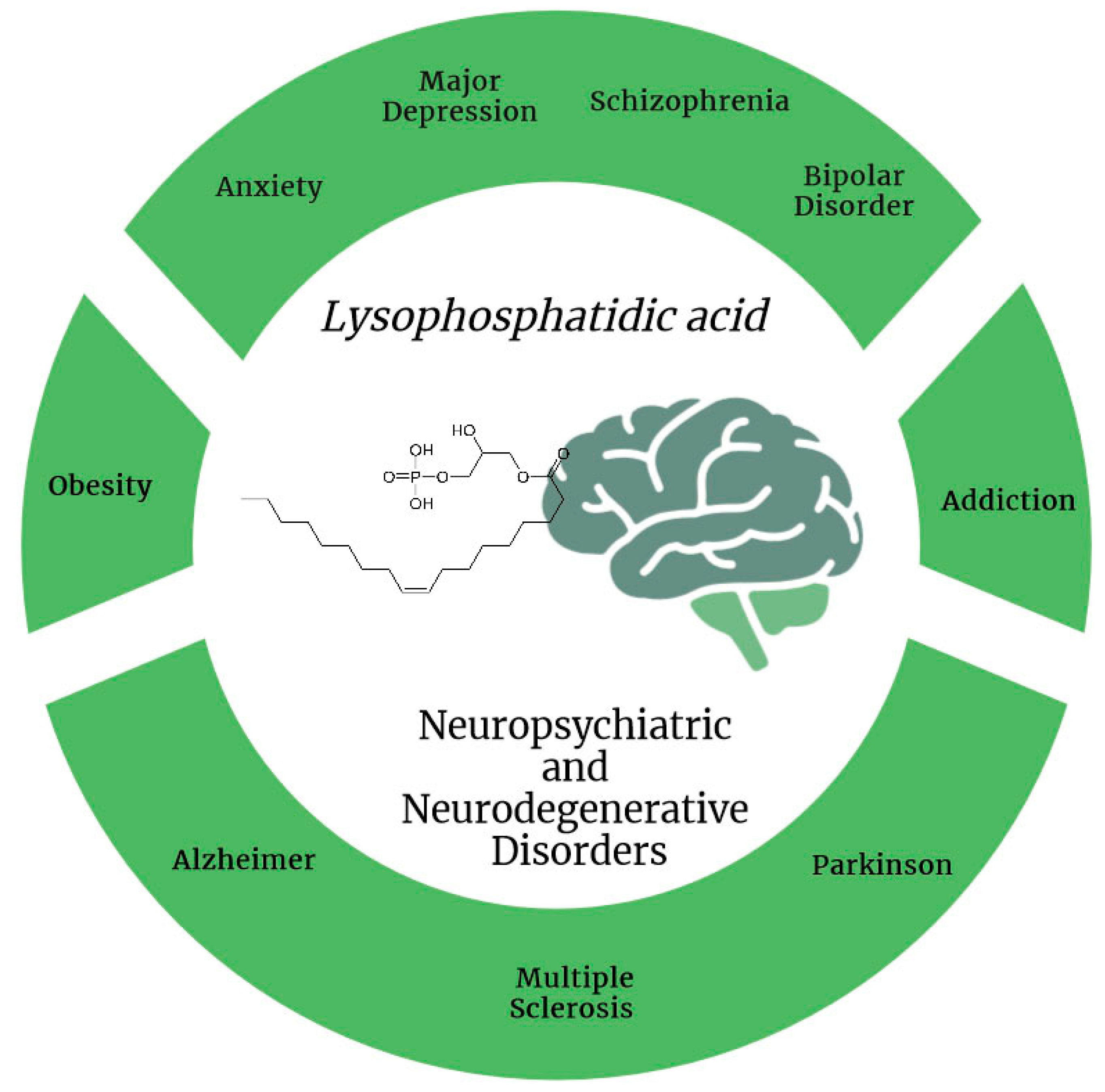
The Role of Lysophosphatidic Acid in Neuropsychiatric and Neurodegenerative Disorders
Abstract
1. Introduction
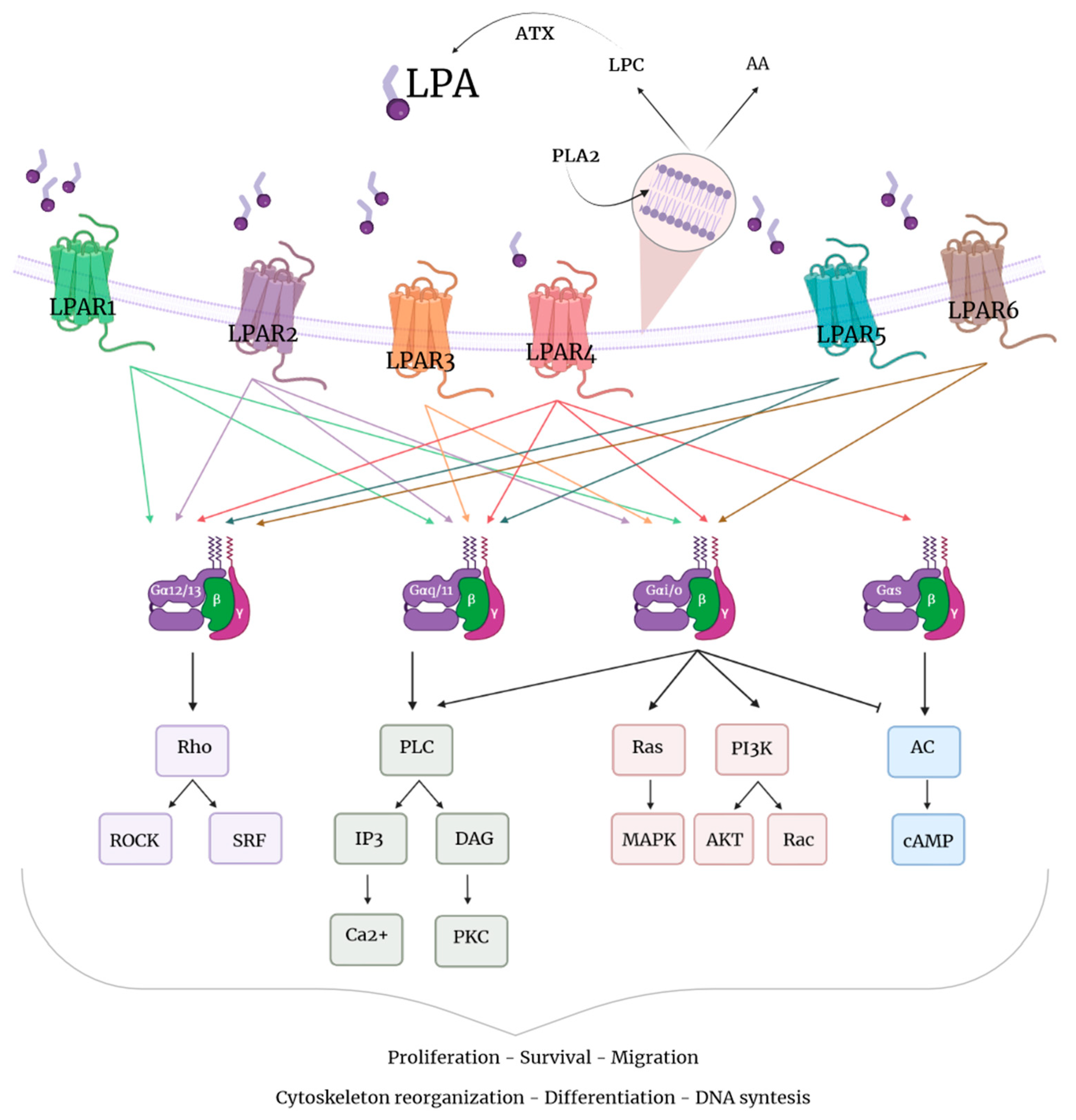
2. LPA and LPA Receptors: Overview
Roles of LPA in the Body
3. Involvement of LPA in Neuropsychiatric Disorders
3.1. Major Depressive Disorder: Clinical Studies
Major Depressive Disorder: Preclinical Studies
3.2. Schizophrenia: Clinical Studies Involving LPA and Its Receptors
Schizophrenia: Preclinical Studies
3.3. Anxiety and Bipolar Disorders: Clinical Evidence
Anxiety and Bipolar Disorders: Preclinical Studies
3.4. Dysregulated Gene Sets in Neuropsychiatric Diseases in Humans
4. LPA Involvement in Minor Neuropsychiatric Disorders
4.1. Obesity: Preclinical Studies
4.2. Addiction: Preclinical Studies
5. LPA in Neurodegenerative Disorders
5.1. Parkinson’s Disease and LPA: Preclinical Evidence
5.2. Multiple Sclerosis and LPA
5.2.1. LPA Levels in MS Patients
5.2.2. Preclinical Studies Conducted in Animal Models of MS
5.2.3. Comparative Study of LPA Levels between Patients and an Animal Model of MS
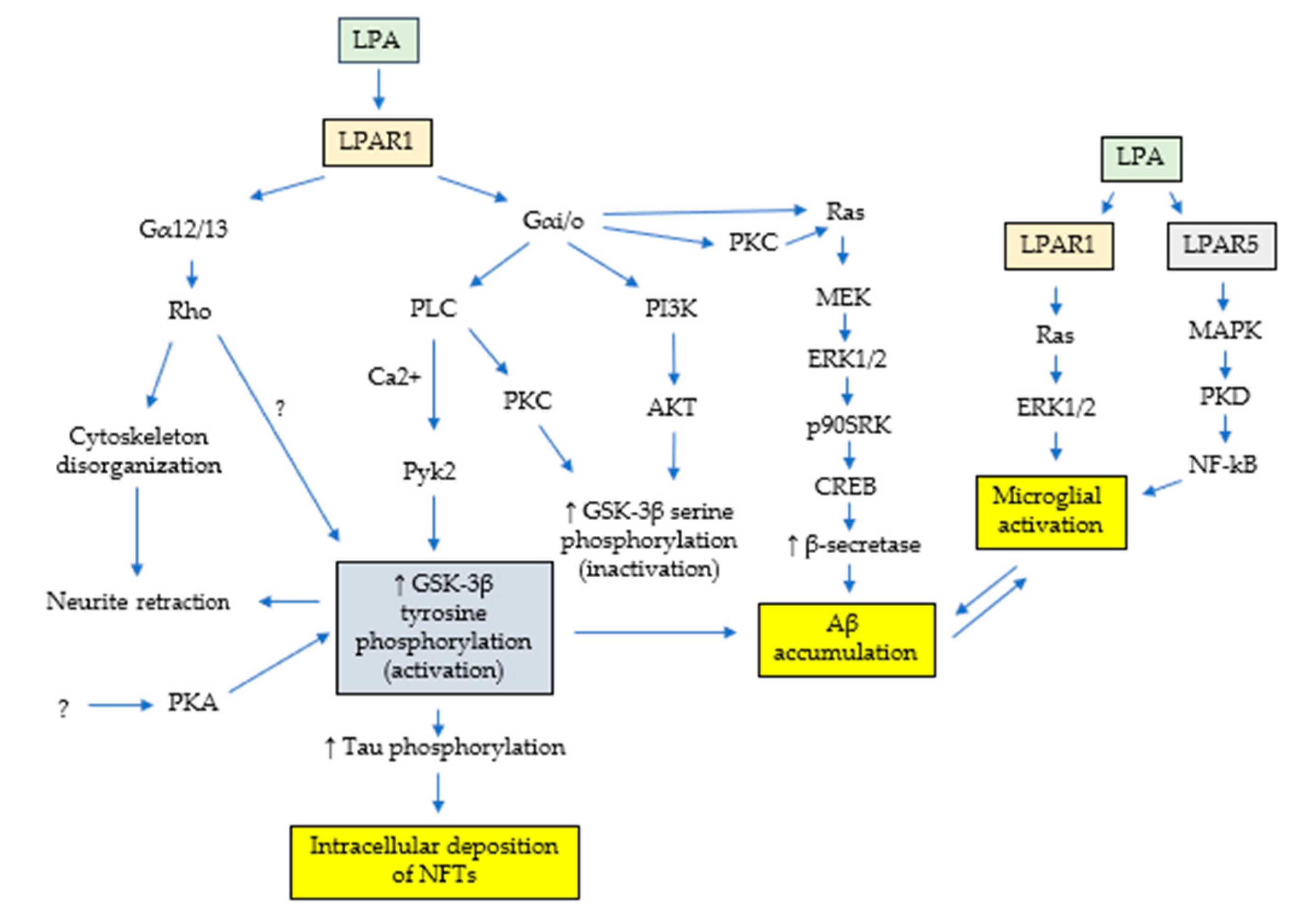
5.3. LPA and Alzheimer’s: In Vitro Main Evidence
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2016 (GBD 2016) Results. 2017. Available online: https://www.healthdata.org/research-analysis/gbd (accessed on 10 January 2024).
- Ding, C.; Wu, Y.; Chen, X.; Chen, Y.; Wu, Z.; Lin, Z.; Kang, D.; Fang, W.; Chen, F. Global, regional, and national burden and attributable risk factors of neurological disorders: The Global Burden of Disease study 1990–2019. Front. Public Health 2022, 10, 952161. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Martínez-González, M.B.; Benitez-Agudelo, J.C.; Navarro-Jiménez, E.; Beltran-Velasco, A.I.; Ruisoto, P.; Diaz Arroyo, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Impact of the COVID-19 Pandemic on Mental Disorders. A Critical Review. Int. J. Environ. Res. Public Health 2021, 18, 10041. [Google Scholar] [CrossRef] [PubMed]
- Cénat, J.M.; Farahi, S.M.M.M.; Dalexis, R.D.; Darius, W.P.; Bekarkhanechi, F.M.; Poisson, H.; Broussard, C.; Ukwu, G.; Auguste, E.; Nguyen, D.D.; et al. The global evolution of mental health problems during the COVID-19 pandemic: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2022, 315, 70–95. [Google Scholar] [CrossRef]
- Hjorthøj, C.; Stürup, A.E.; McGrath, J.J.; Nordentoft, M. Years of potential life lost and life expectancy in schizophrenia: A systematic review and meta-analysis. Lancet Psychiatry 2017, 4, 295–301. [Google Scholar] [CrossRef]
- Walker, E.R.; McGee, R.E.; Druss, B.G. Mortality in mental disorders and global disease burden implications a systematic review and meta-analysis. JAMA Psychiatry 2015, 72, 334–341. [Google Scholar] [CrossRef]
- van der Linde, R.M.; Dening, T.; Stephan, B.C.; Prina, A.M.; Evans, E.; Brayne, C. Longitudinal course of behavioural and psychological symptoms of dementia: A systematic review. Br. J. Psychiatry 2016, 209, 366–377. [Google Scholar] [CrossRef]
- Perrakis, A.; Moolenaar, W.H. Autotaxin: Structure-function and signaling. J. Lipid Res. 2014, 55, 1010–1018. [Google Scholar] [CrossRef]
- Aoki, J.; Inoue, A.; Okudaira, S. Two pathways for lysophosphatidic acid production. Biochim. Biophys. Acta 2008, 1781, 513–518. [Google Scholar] [CrossRef]
- Kranenburg, O.; Moolenaar, W.H. Ras-MAP kinase signaling by lysophosphatidic acid and other G protein-coupled receptor agonists. Oncogene 2001, 20, 1540–1546. [Google Scholar] [CrossRef]
- Kim, J.H.; Adelstein, R.S. LPA(1)-induced migration requires nonmuscle myosin II light chain phosphorylation in breast cancer cells. J. Cell Physiol. 2011, 226, 2881–2893. [Google Scholar] [CrossRef]
- Olianas, M.C.; Dedoni, S.; Onali, P. Antidepressants activate the lysophosphatidic acid receptor LPA(1) to induce insulin-like growth factor-I receptor transactivation, stimulation of ERK1/2 signaling and cell proliferation in CHO-K1 fibroblasts. Biochem. Pharmacol. 2015, 95, 311–323. [Google Scholar] [CrossRef]
- Matas-Rico, E.; García-Diaz, B.; Llebrez-Zayas, P.; López-Barroso, D.; Santín, L.; Pedraza, C.; Smith-Fernández, A.; Fernández-Llebrez, P.; Tellez, T.; Redondo, M.; et al. Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol. Cell Neurosci. 2008, 39, 342–355. [Google Scholar] [CrossRef]
- Olianas, M.C.; Dedoni, S.; Onali, P. LPA1 is a key mediator of intracellular signalling and neuroprotection triggered by tetracyclic antidepressants in hippocampal neurons. J. Neurochem. 2017, 143, 183–197. [Google Scholar] [CrossRef]
- Olianas, M.C.; Dedoni, S.; Onali, P. LPA1 Mediates Antidepressant-Induced ERK1/2 Signaling and Protection from Oxidative Stress in Glial Cells. J. Pharmacol. Exp. Ther. 2016, 359, 340–353. [Google Scholar] [CrossRef]
- Lu, W.Y.; Xiong, Z.G.; Lei, S.; Orser, B.A.; Dudek, E.; Browning, M.D.; MacDonald, J.F. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat. Neurosci. 1999, 2, 331–338. [Google Scholar] [CrossRef]
- Cuenca-Bermejo, L.; Prinetti, A.; Kublickiene, K.; Raparelli, V.; Kautzky-Willer, A.; Norris, C.M.; Pilote, L.; GOING-FWD Consortium; Herrero, M.T. Fundamental Neurochemistry Review: Old brain stories- Influence of age and sex on the neurodegeneration-associated lipid changes. J. Neurochem. 2023, 166, 427–452. [Google Scholar] [CrossRef]
- Moolenaar, W.H.; van Meeteren, L.A.; Giepmans, B.N. The ins and outs of lysophosphatidic acid signaling. Bioessays 2004, 26, 870–881. [Google Scholar] [CrossRef]
- Riya, S.; Sultana, S.; Daria, S.; Proma, M.A.; Bhuiyan, M.A.; Haque, M.A.; Islam, M.R. Evaluation of Serum Lysophosphatidic Acid and Lysophosphatidylcholine Levels in Major Depressive Disorder Patients. Cureus 2020, 12, e12388. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, L.; Yamada, M.; Hattori, K.; Sasayama, D.; Noda, T.; Yoshida, S.; Kunugi, H.; Yamada, M. Lysophosphatidic acid levels in cerebrospinal fluid and plasma samples in patients with major depressive disorder. Heliyon 2019, 5, e01699. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.; Winter, P.; Shilliam, C.S.; Hughes, Z.A.; Langmead, C.; Maycox, P.R.; Dawson, L.A. Neurochemical changes in LPA1 receptor deficient mice--a putative model of schizophrenia. Neurochem. Res. 2005, 30, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Orellana, A.; Kohler, I.; Frölich, L.; de Rojas, I.; Gil, S.; Boada, M.; Hernández, I.; Hausner, L.; Bakker, M.H.M.; et al. Association of lysophosphatidic acids with cerebrospinal fluid biomarkers and progression to Alzheimer’s disease. Alzheimers Res. Ther. 2020, 12, 124. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhao, E.Y.; Zhuang, W.X.; Sun, F.X.; Han, H.L.; Han, H.R.; Lin, Z.J.; Pan, Z.F.; Qu, M.H.; Zeng, X.W.; et al. LPA signaling is required for dopaminergic neuron development and is reduced through low expression of the LPA1 receptor in a 6-OHDA lesion model of Parkinson’s disease. Neurol. Sci. 2015, 36, 2027–2033. [Google Scholar] [CrossRef]
- Vogt, W. The chemical nature of Darmstoff. J. Physiol. 1957, 137, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Subramanian, P.; Sevilmis, G.; Globke, B.; Soehnlein, O.; Karshovska, E.; Megens, R.; Heyll, K.; Chun, J.; Saulnier-Blache, J.S.; et al. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing CXCL1 from the endothelium. Cell Metab. 2011, 13, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.L.; Umstot, E.S.; Desiderio, D.M.; Tigyi, G.J. Quantitative analysis of lysophosphatidic acid in human blood fractions. Ann. N. Y Acad. Sci. 2000, 905, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Chun, J. Lysophospholipids and their receptors in the central nervous system. Biochim. Biophys. Acta 2013, 1831, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J. Mechanisms of lysophosphatidic acid production. Semin. Cell Dev. Biol. 2004, 15, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Okudaira, S.; Yukiura, H.; Aoki, J. Biological roles of lysophosphatidic acid signaling through its production by autotaxin. Biochimie 2010, 92, 698–706. [Google Scholar] [CrossRef]
- Pagès, C.; Simon, M.F.; Valet, P.; Saulnier-Blache, J.S. Lysophosphatidic acid synthesis and release. Prostaglandins Other Lipid Mediat. 2001, 64, 1–10. [Google Scholar] [CrossRef]
- Hecht, J.H.; Weiner, J.A.; Post, S.R.; Chun, J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J. Cell Biol. 1996, 135, 1071–1083. [Google Scholar] [CrossRef]
- Chun, J.; Hla, T.; Lynch, K.R.; Spiegel, S.; Moolenaar, W.H. International union of basic and clinical pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2010, 62, 579–587. [Google Scholar] [CrossRef]
- Ueda, H. LPA receptor signaling as a therapeutic target for radical treatment of neuropathic pain and fibromyalgia. Pain Manag. 2020, 10, 43–53. [Google Scholar] [CrossRef]
- Ueda, H.; Neyama, H.; Matsushita, Y. Lysophosphatidic Acid Receptor 1- and 3-Mediated Hyperalgesia and Hypoalgesia in Diabetic Neuropathic Pain Models in Mice. Cells 2020, 9, 1906. [Google Scholar] [CrossRef]
- Funke, M.; Knudsen, L.; Lagares, D.; Ebener, S.; Probst, C.K.; Fontaine, B.A.; Franklin, A.; Kellner, M.; Kühnel, M.; Matthieu, S.; et al. Lysophosphatidic Acid Signaling through the Lysophosphatidic Acid-1 Receptor Is Required for Alveolarization. Am. J. Respir. Cell Mol. Biol. 2016, 55, 105–116. [Google Scholar] [CrossRef]
- Chen, X.; Walther, F.J.; van Boxtel, R.; Laghmani, E.H.; Sengers, R.M.; Folkerts, G.; DeRuiter, M.C.; Cuppen, E.; Wagenaar, G.T. Deficiency or inhibition of lysophosphatidic acid receptor 1 protects against hyperoxia-induced lung injury in neonatal rats. Acta Physiol. 2016, 216, 358–375. [Google Scholar] [CrossRef]
- Contos, J.J.; Fukushima, N.; Weiner, J.A.; Kaushal, D.; Chun, J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc. Natl. Acad. Sci. USA 2000, 97, 13384–13389. [Google Scholar] [CrossRef]
- Gennero, I.; Laurencin-Dalicieux, S.; Conte-Auriol, F.; Briand-Mésange, F.; Laurencin, D.; Rue, J.; Beton, N.; Malet, N.; Mus, M.; Tokumura, A.; et al. Absence of the lysophosphatidic acid receptor LPA1 results in abnormal bone development and decreased bone mass. Bone 2011, 49, 395–403. [Google Scholar] [CrossRef]
- Alioli, C.A.; Demesmay, L.; Laurencin-Dalacieux, S.; Beton, N.; Farlay, D.; Follet, H.; Saber, A.; Duboeuf, F.; Chun, J.; Rivera, R.; et al. Expression of the type 1 lysophosphatidic acid receptor in osteoblastic cell lineage controls both bone mineralization and osteocyte specification. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020, 1865, 158715. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Zhang, F.; Wu, D.D.; An, D.; Shi, J.; Li, G.; Xu, X.; Cui, M.Z. Lysophosphatidic acid-induced vascular neointimal formation in mouse carotid arteries is mediated by the matricellular protein CCN1/Cyr61. Am. J. Physiol. Cell Physiol. 2016, 311, C975–C984. [Google Scholar] [CrossRef] [PubMed]
- Panchatcharam, M.; Miriyala, S.; Salous, A.; Wheeler, J.; Dong, A.; Mueller, P.; Sunkara, M.; Escalante-Alcalde, D.; Morris, A.J.; Smyth, S.S. Lipid phosphate phosphatase 3 negatively regulates smooth muscle cell phenotypic modulation to limit intimal hyperplasia. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Kobayashi, D.; Aoi, K.; Sasaki, N.; Sugiura, Y.; Igarashi, H.; Tohya, K.; Inoue, A.; Hata, E.; Akahoshi, N.; et al. Fibroblastic reticular cell-derived lysophosphatidic acid regulates confined intranodal T-cell motility. Elife 2016, 5, e10561. [Google Scholar] [CrossRef]
- Endle, H.; Horta, G.; Stutz, B.; Muthuraman, M.; Tegeder, I.; Schreiber, Y.; Snodgrass, I.F.; Gurke, R.; Liu, Z.W.; Sestan-Pesa, M.; et al. AgRP neurons control feeding behaviour at cortical synapses via peripherally derived lysophospholipids. Nat. Metab. 2022, 4, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Bitar, L.; Uphaus, T.; Thalman, C.; Muthuraman, M.; Gyr, L.; Ji, H.; Domingues, M.; Endle, H.; Groppa, S.; Steffen, F.; et al. Inhibition of the enzyme autotaxin reduces cortical excitability and ameliorates the outcome in stroke. Sci. Transl. Med. 2022, 14, eabk0135. [Google Scholar] [CrossRef] [PubMed]
- Park, G.Y.; Lee, Y.G.; Berdyshev, E.; Nyenhuis, S.; Du, J.; Fu, P.; Gorshkova, I.A.; Li, Y.; Chung, S.; Karpurapu, M.; et al. Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am. J. Respir. Crit. Care Med. 2013, 188, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Cai, L.; Wang, F.; Xu, C.; Pei, S.; Guo, H.; Sun, X.; Chun, J.; Cong, X.; Zhu, W.; et al. LPA2 Contributes to Vascular Endothelium Homeostasis and Cardiac Remodeling After Myocardial Infarction. Circ. Res. 2022, 131, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Hama, K.; Contos, J.J.; Anliker, B.; Inoue, A.; Skinner, M.K.; Suzuki, H.; Amano, T.; Kennedy, G.; Arai, H.; et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 2005, 435, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Uchida, H.; Nagai, J.; Inoue, M.; Chun, J.; Aoki, J.; Ueda, H. Lysophosphatidic acid-3 receptor-mediated feed-forward production of lysophosphatidic acid: An initiator of nerve injury-induced neuropathic pain. Mol. Pain 2009, 5, 64. [Google Scholar] [CrossRef]
- Wang, F.; Liu, S.; Pei, J.; Cai, L.; Liu, N.; Liang, T.; Dong, X.; Cong, X.; Chun, J.; Chen, J.; et al. LPA3-mediated lysophosphatidic acid signaling promotes postnatal heart regeneration in mice. Theranostics 2020, 10, 10892–10907. [Google Scholar] [CrossRef]
- Sumida, H.; Noguchi, K.; Kihara, Y.; Abe, M.; Yanagida, K.; Hamano, F.; Sato, S.; Tamaki, K.; Morishita, Y.; Kano, M.R.; et al. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood 2010, 116, 5060–5070. [Google Scholar] [CrossRef]
- Siess, W.; Zangl, K.J.; Essler, M.; Bauer, M.; Brandl, R.; Corrinth, C.; Bittman, R.; Tigyi, G.; Aepfelbacher, M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc. Natl. Acad. Sci. USA 1999, 96, 6931–6936. [Google Scholar] [CrossRef]
- Yasuda, D.; Kobayashi, D.; Akahoshi, N.; Ohto-Nakanishi, T.; Yoshioka, K.; Takuwa, Y.; Mizuno, S.; Takahashi, S.; Ishii, S. Lysophosphatidic acid-induced YAP/TAZ activation promotes developmental angiogenesis by repressing Notch ligand Dll4. J. Clin. Investig. 2019, 129, 4332–4349. [Google Scholar] [CrossRef]
- Yanagida, K.; Igarashi, H.; Yasuda, D.; Kobayashi, D.; Ohto-Nakanishi, T.; Akahoshi, N.; Sekiba, A.; Toyoda, T.; Ishijima, T.; Nakai, Y.; et al. The Gα12/13-coupled receptor LPA4 limits proper adipose tissue expansion and remodeling in diet-induced obesity. JCI Insight 2018, 3, e97293. [Google Scholar] [CrossRef]
- Liu, Y.B.; Kharode, Y.; Bodine, P.V.; Yaworsky, P.J.; Robinson, J.A.; Billiard, J. LPA induces osteoblast differentiation through interplay of two receptors: LPA1 and LPA4. J. Cell Biochem. 2010, 109, 794–800. [Google Scholar] [CrossRef]
- Igarashi, H.; Akahoshi, N.; Ohto-Nakanishi, T.; Yasuda, D.; Ishii, S. The lysophosphatidic acid receptor LPA4 regulates hematopoiesis-supporting activity of bone marrow stromal cells. Sci. Rep. 2015, 5, 11410. [Google Scholar] [CrossRef]
- Mathew, D.; Kremer, K.N.; Strauch, P.; Tigyi, G.; Pelanda, R.; Torres, R.M. LPA5 Is an inhibitory receptor that suppresses CD8 T-cell cytotoxic function via disruption of early TCR signaling. Front. Immunol. 2019, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Jenkin, K.A.; He, P.; Yun, C.C. Expression of lysophosphatidic acid receptor 5 is necessary for the regulation of intestinal Na+/H+ exchanger 3 by lysophosphatidic acid in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G433–G442. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; He, P.; Han, Y.; Dong, L.; Yun, C.C. Control of Intestinal Epithelial Permeability by Lysophosphatidic Acid Receptor 5. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 1073–1092. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.E.; Rivera, R.R.; Chun, J. Targeted deletion of LPA5 identifies novel roles for lysophosphatidic acid signaling in development of neuropathic pain. J. Biol. Chem. 2012, 287, 17608–17617. [Google Scholar] [CrossRef]
- Shimomura, Y.; Wajid, M.; Ishii, Y.; Shapiro, L.; Petukhova, L.; Gordon, D.; Christiano, A.M. Disruption of P2RY5, an orphan G protein-coupled receptor, underlies autosomal recessive woolly hair. Nat. Genet. 2008, 40, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.A.; Suárez-Pozos, E.; Soto-Verdugo, J.; Wang, H.; Afshari, F.S.; Li, G.; Manam, S.; Yasuda, D.; Ortega, A.; Lister, J.A.; et al. Lysophosphatidic acid signaling via LPA6: A negative modulator of developmental oligodendrocyte maturation. J. Neurochem. 2022, 163, 478–499. [Google Scholar] [CrossRef]
- Inaba, A.; Harada, H.; Ikezaki, S.; Kumakami-Sakano, M.; Arai, H.; Azumane, M.; Ohshima, H.; Morikawa, K.; Kano, K.; Aoki, J.; et al. LPA6-RhoA signals regulate junctional complexes for polarity and morphology establishment of maturation stage ameloblasts. J. Oral Biosci. 2022, 64, 85–92. [Google Scholar] [CrossRef]
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Patten, S.B.; Freedman, G.; Murray, C.J.; Vos, T.; Whiteford, H.A. Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study. PLoS Med. 2013, 10, e1001547. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, K.; Takebayashi, M.; Abe, H.; Shibasaki, C.; Kajitani, N.; Okada-Tsuchioka, M.; Hattori, K.; Yoshida, S.; Kunugi, H.; Yamawaki, S. Reduced Serum and Cerebrospinal Fluid Levels of Autotaxin in Major Depressive Disorder. Int. J. Neuropsychopharmacol. 2019, 22, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Omori, W.; Kano, K.; Hattori, K.; Kajitani, N.; Okada-Tsuchioka, M.; Boku, S.; Kunugi, H.; Aoki, J.; Takebayashi, M. Reduced Cerebrospinal Fluid Levels of Lysophosphatidic Acid Docosahexaenoic Acid in Patients with Major Depressive Disorder and Schizophrenia. Int. J. Neuropsychopharmacol. 2021, 24, 948–955. [Google Scholar] [CrossRef]
- Moreno-Fernández, R.D.; Pérez-Martín, M.; Castilla-Ortega, E.; Rosell Del Valle, C.; García-Fernández, M.I.; Chun, J.; Estivill-Torrús, G.; Rodríguez de Fonseca, F.; Santín, L.J.; Pedraza, C. maLPA1-null mice as an endophenotype of anxious depression. Transl. Psychiatry 2017, 7, e1077. [Google Scholar] [CrossRef] [PubMed]
- Tabbai, S.; Moreno-Fernández, R.D.; Zambrana-Infantes, E.; Nieto-Quero, A.; Chun, J.; García-Fernández, M.; Estivill-Torrús, G.; Rodríguez de Fonseca, F.; Santín, L.J.; Oliveira, T.G.; et al. Effects of the LPA1 Receptor Deficiency and Stress on the Hippocampal LPA Species in Mice. Front. Mol. Neurosci. 2019, 12, 146. [Google Scholar] [CrossRef]
- Tafet, G.E.; Nemeroff, C.B. The Links Between Stress and Depression: Psychoneuroendocrinological, Genetic, and Environmental Interactions. J. Neuropsychiatry Clin. Neurosci. 2016, 28, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Nagata, W.; Koizumi, A.; Nakagawa, K.; Takahashi, S.; Gotoh, M.; Satoh, Y.; Ishizuka, T. Treatment with lysophosphatidic acid prevents microglial activation and depression-like behaviours in a murine model of neuropsychiatric systemic lupus erythematosus. Clin. Exp. Immunol. 2023, 212, 81–92. [Google Scholar] [CrossRef]
- Olianas, M.C.; Dedoni, S.; Onali, P. Inhibition of TNF-α-induced neuronal apoptosis by antidepressants acting through the lysophosphatidic acid receptor LPA1. Apoptosis 2019, 24, 478–498. [Google Scholar] [CrossRef]
- Olianas, M.C.; Dedoni, S.; Onali, P. Differential targeting of lysophosphatidic acid LPA1, LPA2, and LPA3 receptor signalling by tricyclic and tetracyclic antidepressants. Eur. J. Pharmacol. 2023, 959, 176064. [Google Scholar] [CrossRef]
- Harrison, S.M.; Reavill, C.; Brown, G.; Brown, J.T.; Cluderay, J.E.; Crook, B.; Davies, C.H.; Dawson, L.A.; Grau, E.; Heidbreder, C.; et al. LPA1 receptor-deficient mice have phenotypic changes observed in psychiatric disease. Mol. Cell Neurosci. 2003, 24, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, R.J.; Kinsella, A.; Baldwin, P.; Waddington, J.L. 3D laser surface imaging and geometric morphometrics resolve frontonasal dysmorphology in schizophrenia. Biol. Psychiatry 2007, 61, 1187–1194. [Google Scholar] [CrossRef]
- Mirendil, H.; Thomas, E.A.; De Loera, C.; Okada, K.; Inomata, Y.; Chun, J. LPA signaling initiates schizophrenia-like brain and behavioral changes in a mouse model of prenatal brain hemorrhage. Transl. Psychiatry 2015, 5, e541. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, L.; Zhang, T.; Li, R.; Cui, Z.Q.; Du, J.; Yang, J.B.; Xue, F.; Chen, Y.H.; Tan, Q.R.; Peng, Z.W. Alterations in the Plasma Lipidome of Adult Women with Bipolar Disorder: A Mass Spectrometry-Based Lipidomics Research. Front. Psychiatry 2022, 13, 802710. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Ortega, E.; Escuredo, L.; Bilbao, A.; Pedraza, C.; Orio, L.; Estivill-Torrus, G.; Santin, L.J.; de Fonseca, F.R.; Pavon, F.J. 1-Oleoyl lysophosphatidic acid: A new mediator of emotional behavior in rats. PLoS ONE 2014, 9, e85348. [Google Scholar] [CrossRef]
- Yamada, M.; Tsukagoshi, M.; Hashimoto, T.; Oka, J.; Saitoh, A.; Yamada, M. Lysophosphatidic acid induces anxiety-like behavior via its receptors in mice. J. Neural Transm. 2015, 122, 487–494. [Google Scholar] [CrossRef]
- Rosell-Valle, C.; Martínez-Losa, M.; Matas-Rico, E.; Castilla-Ortega, E.; Zambrana-Infantes, E.; Gómez-Conde, A.I.; Sánchez-Salido, L.; Ladrón de Guevara-Miranda, D.; Pedraza, C.; Serrano-Castro, P.J.; et al. GABAergic deficits in absence of LPA1 receptor, associated anxiety-like and coping behaviors, and amelioration by interneuron precursor transplants into the dorsal hippocampus. Brain Struct. Funct. 2021, 226, 1479–1495. [Google Scholar] [CrossRef]
- Guan, J.; Cai, J.J.; Ji, G.; Sham, P.C. Commonality in dysregulated expression of gene sets in cortical brains of individuals with autism, schizophrenia, and bipolar disorder. Transl. Psychiatry 2019, 9, 152. [Google Scholar] [CrossRef]
- D’Souza, K.; Paramel, G.V.; Kienesberger, P.C. Lysophosphatidic Acid Signaling in Obesity and Insulin Resistance. Nutrients 2018, 10, 399. [Google Scholar] [CrossRef]
- D’Souza, K.; Nzirorera, C.; Cowie, A.M.; Varghese, G.P.; Trivedi, P.; Eichmann, T.O.; Biswas, D.; Touaibia, M.; Morris, A.J.; Aidinis, V.; et al. Autotaxin-LPA signaling contributes to obesity-induced insulin resistance in muscle and impairs mitochondrial metabolism. J. Lipid Res. 2018, 59, 1805–1817. [Google Scholar] [CrossRef]
- Rancoule, C.; Dusaulcy, R.; Tréguer, K.; Grès, S.; Attané, C.; Saulnier-Blache, J.S. Involvement of autotaxin/lysophosphatidic acid signaling in obesity and impaired glucose homeostasis. Biochimie 2014, 96, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Rancoule, C.; Viaud, M.; Gres, S.; Viguerie, N.; Decaunes, P.; Bouloumié, A.; Langin, D.; Bascands, J.L.; Valet, P.; Saulnier-Blache, J.S. Pro-fibrotic activity of lysophosphatidic acid in adipose tissue: In vivo and in vitro evidence. Biochim. Biophys. Acta 2014, 1841, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Yea, K.; Kim, J.; Lim, S.; Park, H.S.; Park, K.S.; Suh, P.G.; Ryu, S.H. Lysophosphatidic acid regulates blood glucose by stimulating myotube and adipocyte glucose uptake. J. Mol. Med. 2008, 86, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Ladrón de Guevara-Miranda, D.; Moreno-Fernández, R.D.; Gil-Rodríguez, S.; Rosell-Valle, C.; Estivill-Torrús, G.; Serrano, A.; Pavón, F.J.; Rodríguez de Fonseca, F.; Santín, L.J.; Castilla-Ortega, E. Lysophosphatidic acid-induced increase in adult hippocampal neurogenesis facilitates the forgetting of cocaine-contextual memory. Addict. Biol. 2019, 24, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Lee, B.H.; Choi, S.H.; Kim, H.J.; Jung, S.W.; Kim, H.S.; Shin, H.C.; Park, H.J.; Park, K.H.; Lee, M.K.; et al. Gintonin, a novel ginseng-derived lysophosphatidic acid receptor ligand, stimulates neurotransmitter release. Neurosci. Lett. 2015, 584, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jang, M.; Oh, S.; Nah, S.Y.; Cho, I.H. Multi-Target Protective Effects of Gintonin in 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Mediated Model of Parkinson’s Disease via Lysophosphatidic Acid Receptors. Front. Pharmacol. 2018, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Gruntz, K.; Bloechliger, M.; Becker, C.; Jick, S.S.; Fuhr, P.; Meier, C.R.; Rüegg, S. Parkinson disease and the risk of epileptic seizures. Ann. Neurol. 2018, 83, 363–374. [Google Scholar] [CrossRef]
- Choi, J.H.; Kwon, T.W.; Jo, H.S.; Ha, Y.; Cho, I.H. Gintonin, a Panax ginseng-derived LPA receptor ligand, attenuates kainic acid-induced seizures and neuronal cell death in the hippocampus via anti-inflammatory and anti-oxidant activities. J. Ginseng Res. 2023, 47, 390–399. [Google Scholar] [CrossRef]
- Hwang, S.H.; Shin, E.J.; Shin, T.J.; Lee, B.H.; Choi, S.H.; Kang, J.; Kim, H.J.; Kwon, S.H.; Jang, C.G.; Lee, J.H.; et al. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates Alzheimer’s disease-related neuropathies: Involvement of non-amyloidogenic processing. J. Alzheimers Dis. 2012, 31, 207–223. [Google Scholar] [CrossRef]
- Louapre, C.; Papeix, C.; Lubetzki, C.; Maillart, E. Multiple sclerosis and aging. Geriatr. Psychol. Neuropsychiatr. Vieil. 2017, 15, 402–408. (In English) [Google Scholar] [CrossRef]
- Balood, M.; Zahednasab, H.; Siroos, B.; Mesbah-Namin, S.A.; Torbati, S.; Harirchian, M.H. Elevated serum levels of lysophosphatidic acid in patients with multiple sclerosis. Hum. Immunol. 2014, 75, 411–413. [Google Scholar] [CrossRef]
- Jiang, D.; Ju, W.; Wu, X.; Zhan, X. Elevated lysophosphatidic acid levels in the serum and cerebrospinal fluid in patients with multiple sclerosis: Therapeutic response and clinical implication. Neurol. Res. 2018, 40, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Zuroff, L.; Rezk, A.; Shinoda, K.; Espinoza, D.A.; Elyahu, Y.; Zhang, B.; Chen, A.A.; Shinohara, R.T.; Jacobs, D.; Alcalay, R.N.; et al. Immune aging in multiple sclerosis is characterized by abnormal CD4 T cell activation and increased frequencies of cytotoxic CD4 T cells with advancing age. EBioMedicine 2022, 82, 104179. [Google Scholar] [CrossRef]
- Peng, H.Y.; Lucavs, J.; Ballard, D.; Das, J.K.; Kumar, A.; Wang, L.; Ren, Y.; Xiong, X.; Song, J. Metabolic Reprogramming and Reactive Oxygen Species in T Cell Immunity. Front. Immunol. 2021, 12, 652687. [Google Scholar] [CrossRef]
- Lu, Y.S.; Pu, L.Y.; Li, X.C.; Wang, X.H. Methylprednisolone inhibits activated CD4+ T cell survival promoted by toll-like receptor ligands. Hepatobiliary Pancreat. Dis. Int. 2010, 9, 376–383. [Google Scholar] [PubMed]
- Inoue, M.; Rashid, M.H.; Fujita, R.; Contos, J.J.; Chun, J.; Ueda, H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat. Med. 2004, 10, 712–718. [Google Scholar] [CrossRef]
- Nagai, J.; Uchida, H.; Matsushita, Y.; Yano, R.; Ueda, M.; Niwa, M.; Aoki, J.; Chun, J.; Ueda, H. Autotaxin and lysophosphatidic acid1 receptor-mediated demyelination of dorsal root fibers by sciatic nerve injury and intrathecal lysophosphatidylcholine. Mol. Pain 2010, 6, 78. [Google Scholar] [CrossRef]
- Thirunavukkarasu, K.; Tan, B.; Swearingen, C.A.; Rocha, G.; Bui, H.H.; McCann, D.J.; Jones, S.B.; Norman, B.H.; Pfeifer, L.A.; Saha, J.K. Pharmacological Characterization of a Potent Inhibitor of Autotaxin in Animal Models of Inflammatory Bowel Disease and Multiple Sclerosis. J. Pharmacol. Exp. Ther. 2016, 359, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, R.; Yamamoto, S.; Yoshikawa, K.; Gotoh, M.; Tsukahara, T.; Neyama, H.; Ishii, S.; Akahoshi, N.; Yanagida, K.; Sumida, H.; et al. LPA5 signaling is involved in multiple sclerosis-mediated neuropathic pain in the cuprizone mouse model. J. Pharmacol. Sci. 2018, 136, 93–96. [Google Scholar] [CrossRef]
- Fransson, J.; Gómez-Conde, A.I.; Romero-Imbroda, J.; Fernández, O.; Leyva, L.; de Fonseca, F.R.; Chun, J.; Louapre, C.; Van-Evercooren, A.B.; Zujovic, V.; et al. Activation of Macrophages by Lysophosphatidic Acid through the Lysophosphatidic Acid Receptor 1 as a Novel Mechanism in Multiple Sclerosis Pathogenesis. Mol. Neurobiol. 2021, 58, 470–482. [Google Scholar] [CrossRef]
- Ninou, I.; Sevastou, I.; Magkrioti, C.; Kaffe, E.; Stamatakis, G.; Thivaios, S.; Panayotou, G.; Aoki, J.; Kollias, G.; Aidinis, V. Genetic deletion of Autotaxin from CD11b+ cells decreases the severity of experimental autoimmune encephalomyelitis. PLoS ONE 2020, 15, e0226050. [Google Scholar] [CrossRef]
- Choi, J.H.; Oh, J.; Lee, M.J.; Bae, H.; Ko, S.G.; Nah, S.Y.; Cho, I.H. Inhibition of lysophosphatidic acid receptor 1-3 deteriorates experimental autoimmune encephalomyelitis by inducing oxidative stress. J. Neuroinflammation 2021, 18, 240. [Google Scholar] [CrossRef]
- Schmitz, K.; Brunkhorst, R.; de Bruin, N.; Mayer, C.A.; Häussler, A.; Ferreiros, N.; Schiffmann, S.; Parnham, M.J.; Tunaru, S.; Chun, J.; et al. Dysregulation of lysophosphatidic acids in multiple sclerosis and autoimmune encephalomyelitis. Acta Neuropathol. Commun. 2017, 5, 42. [Google Scholar] [CrossRef]
- Salgado-Polo, F.; Borza, R.; Matsoukas, M.T.; Marsais, F.; Jagerschmidt, C.; Waeckel, L.; Moolenaar, W.H.; Ford, P.; Heckmann, B.; Perrakis, A. Autotaxin facilitates selective LPA receptor signaling. Cell Chem. Biol. 2023, 30, 69–84.e14. [Google Scholar] [CrossRef]
- Geraldo, L.H.M.; Spohr, T.C.L.S.; Amaral, R.F.D.; Fonseca, A.C.C.D.; Garcia, C.; Mendes, F.A.; Freitas, C.; dosSantos, M.F.; Lima, F.R.S. Role of lysophosphatidic acid and its receptors in health and disease: Novel therapeutic strategies. Signal Transduct. Target. Ther. 2021, 6, 45. [Google Scholar] [CrossRef]
- Gao, Y.; Tan, L.; Yu, J.T.; Tan, L. Tau in Alzheimer’s Disease: Mechanisms and Therapeutic Strategies. Curr. Alzheimer Res. 2018, 15, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Chuprun, J.K.; Raymond, J.R.; Blackshear, P.J. The heterotrimeric G protein G alpha i2 mediates lysophosphatidic acid-stimulated induction of the c-fos gene in mouse fibroblasts. J. Biol. Chem. 1997, 272, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Jalink, K.; van Corven, E.J.; Hengeveld, T.; Morii, N.; Narumiya, S.; Moolenaar, W.H. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J. Cell Biol. 1994, 126, 801–810. [Google Scholar] [CrossRef]
- Tigyi, G.; Fischer, D.J.; Sebök, A.; Yang, C.; Dyer, D.L.; Miledi, R. Lysophosphatidic acid-induced neurite retraction in PC12 cells: Control by phosphoinositide-Ca2+ signaling and Rho. J. Neurochem. 1996, 66, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Sayas, C.L.; Moreno-Flores, M.T.; Avila, J.; Wandosell, F. The neurite retraction induced by lysophosphatidic acid increases Alzheimer’s disease-like Tau phosphorylation. J. Biol. Chem. 1999, 274, 37046–37052. [Google Scholar] [CrossRef] [PubMed]
- Sayas, C.L.; Avila, J.; Wandosell, F. Regulation of neuronal cytoskeleton by lysophosphatidic acid: Role of GSK-3. Biochim. Biophys. Acta 2002, 1582, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Sayas, C.L.; Ariaens, A.; Ponsioen, B.; Moolenaar, W.H. GSK-3 is activated by the tyrosine kinase Pyk2 during LPA1-mediated neurite retraction. Mol. Biol. Cell 2006, 17, 1834–1844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hughes, K.; Nikolakaki, E.; Plyte, S.E.; Totty, N.F.; Woodgett, J.R. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphor-ylation. EMBO J. 1993, 12, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Qureshi, H.Y.; Cafferty, P.W.; Sobue, K.; Agarwal-Mawal, A.; Neufield, K.D.; Paudel, H.K. Glyco-gen synthase kinase-3b is complexed with Tau protein in brain microtubules. J. Biol. Chem. 2002, 277, 11933–11940. [Google Scholar] [CrossRef] [PubMed]
- Agarwal-Mawal, A.; Qureshi, H.Y.; Cafferty, P.W.; Yuan, Z.; Han, D.; Loin, R.; Paudel, H.K. 14-3-3 Connects glycogen synthase kinase-3b to Tau within a brain microtubule-associated Tau phosphorylation com- plex. J. Biol. Chem. 2003, 278, 12722–12728. [Google Scholar] [CrossRef] [PubMed]
- Spittaels, K.; Van den Haute, C.; Van Dorpe, J.; Geerts, H.; Mercken, M.; Bruynseels, K.; Lasrado, R.; Vandezande, K.; Laenen, I.; Boon, T.; et al. Glycogen synthase kinase-3beta phosphorylates protein tau and rescues the axonopathy in the central nervous system of human four-repeat tau transgenic mice. J. Biol. Chem. 2000, 275, 41340–41349. [Google Scholar] [CrossRef]
- Liu, S.J.; Wang, J.Z. Alzheimer-like tau phosphorylation induced in vivo by wortmannin and itsattenuation by melatonin. Acta Pharmacol. Sinica. 2002, 23, 183–187. [Google Scholar]
- Liu, S.J.; Zhang, A.H.; Li, H.L.; Wang, Q.; Deng, H.M.; Netzer, W.J.; Xu, H.; Wang, J.Z. Overactivation of glycogen synthase kinase-3 by inhibition of phosphoinositol-3 kinase and protein kinase C leads to hyperphosphorylation of tau and impairment of spatial memory. J. Neurochem. 2003, 87, 1333–1344. [Google Scholar] [CrossRef]
- Jalink, K.; van Corven, E.J.; Moolenaar, W.H. Lysophosphatidic acid, but not phosphatidic acid, is a potent Ca2+-mobilizing stimulus for fibroblasts. Evidence for an extracellular site of action. J. Biol. Chem. 1990, 265, 12232–12239. [Google Scholar] [CrossRef]
- Li, X.; Lu, F.; Tian, Q.; Yang, Y.; Wang, Q.; Wang, J.Z. Activation of glycogen synthase kinase-3 induces Alzheimer-like tau hyperphosphorylation in rat hippocampus slices in culture. J. Neural Transm. 2006, 113, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kim, N.H.; Yang, H.; Kim, S.H.; Huh, S.O. Lysophosphatidic acid induces neurite retraction in differentiated neuroblastoma cells via GSK-3β activation. Mol. Cells 2011, 31, 483–489. [Google Scholar] [CrossRef]
- Takashima, A. GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J. Alzheimer’s Dis. 2006, 9, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.; Gómez de Barreda, E.; Fuster-Matanzo, A.; Lucas, J.J.; Avila, J. GSK3: A possible link between beta amyloid peptide and tau protein. Exp. Neurol. 2010, 223, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Leroy, K.; Yilmaz, Z.; Brion, J.-P. Increased level of active GSK-3beta in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol. Appl. Neurobiol. 2007, 33, 43–55. [Google Scholar] [CrossRef]
- Ramesh, S.; Govindarajulu, M.; Suppiramaniam, V.; Moore, T.; Dhanasekaran, M. Autotaxin−Lysophosphatidic Acid Signaling in Alzheimer’s Disease. Int. J. Mol. Sci. 2018, 19, 1827. [Google Scholar] [CrossRef]
- Umemura, K.; Yamashita, N.; Yu, X.; Arima, K.; Asada, T.; Makifuchi, T.; Murayama, S.; Saito, Y.; Kanamaru, K.; Goto, Y.; et al. Autotaxin expression is enhanced in frontal cortex of Alzheimer-type dementia patients. Neurosci. Lett. 2006, 400, 97–100. [Google Scholar] [CrossRef]
- Sayas, C.L.; Ávila, J. GSK-3 and Tau: A Key Duet in Alzheimer’s Disease. Cells 2021, 10, 721. [Google Scholar] [CrossRef]
- de Leeuw, F.A.; Peeters, C.F.W.; Kester, M.I.; Harms, A.C.; Struys, E.A.; Hankemeier, T.; van Vlijmen, H.W.T.; van der Lee, S.J.; van Duijn, C.M.; Scheltens, P.; et al. Blood-based metabolic signatures in Alzheimer’s disease. Alzheimers Dement. 2017, 8, 196–207. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomedicine. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Draczynska-Lusiak, B.; Doung, A.; Sun, A.Y. Oxidized lipoproteins may play a role in neuronal cell death in Alzheimer disease. Mol. Chem. Neuropathol. 1998, 33, 139–148. [Google Scholar] [CrossRef]
- Giasson, B.I.; Ischiropoulos, H.; Lee, V.M.; Trojanowski, J.Q. The relationship between oxidative/nitrative stress and pathological inclusions in Alzheimer’s and Parkinson’s diseases. Free Radic. Biol. Med. 2002, 32, 1264–1275. [Google Scholar] [CrossRef]
- Sun, Y.X.; Minthon, L.; Wallmark, A.; Warkentin, S.; Blennow, K.; Janciauskiene, S. Inflammatory markers in matched plasma and cerebrospinal fluid from patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2003, 16, 136–144. [Google Scholar] [CrossRef]
- Shi, J.; Dong, Y.; Cui, M.Z.; Xu, X. Lysophosphatidic acid induces increased BACE1 expression and Aβ formation. Biochim. Biophys. Acta 2013, 1832, 29–38. [Google Scholar] [CrossRef]
- McLimans, K.E.; Willette, A.A. Alzheimer’s Disease Neuroimaging Initiative. Autotaxin is Related to Metabolic Dysfunction and Predicts Alzheimer’s Disease Outcomes. J. Alzheimers Dis. 2017, 56, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Herr, D.R.; Chew, W.S.; Satish, R.L.; Ong, W.Y. Pleotropic Roles of Autotaxin in the Nervous System Present Opportunities for the Development of Novel Therapeutics for Neurological Diseases. Mol. Neurobiol. 2020, 57, 372–392. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Awada, R.; Rondeau, P.; Grès, S.; Saulnier-Blache, J.S.; Lefebvre d’Hellencourt, C.; Bourdon, E. Autotaxin protects microglial cells against oxidative stress. Free Radic. Biol. Med. 2012, 52, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Awada, R.; Saulnier-Blache, J.S.; Grès, S.; Bourdon, E.; Rondeau, P.; Parimisetty, A.; Orihuela, R.; Harry, G.J.; d’Hellencourt, C.L. Autotaxin downregulates LPS-induced microglia activation and pro-inflammatory cytokines production. J. Cells Biochem. 2014, 115, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Gaire, B.P.; Park, S.J.; Shin, D.Y.; Choi, J.W. Identifying lysophosphatidic acid receptor subtype 1 (LPA1) as a novel factor to modulate microglial activation and their TNF-α production by activating ERK1/2. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2018, 1863, 1237–1245. [Google Scholar] [CrossRef]
- Plastira, I.; Bernhart, E.; Goeritzer, M.; DeVaney, T.; Reicher, H.; Hammer, A.; Lohberger, B.; Wintersperger, A.; Zucol, B.; Graier, W.F.; et al. Lysophosphatidic acid via LPA-receptor 5/protein kinase D-dependent pathways induces a motile and pro-inflammatory microglial phenotype. J. Neuroinflammation 2017, 14, 253. [Google Scholar] [CrossRef]
- Plastira, I.; Bernhart, E.; Joshi, L.; Koyani, C.N.; Strohmaier, H.; Reicher, H.; Malle, E.; Sattler, W. MAPK signaling determines lysophosphatidic acid (LPA)-induced inflammation in microglia. J. Neuroinflammation 2020, 17, 127. [Google Scholar] [CrossRef]
- Liu, W.; Hopkins, A.M.; Hou, J. The development of modulators for lysophosphatidic acid receptors: A comprehensive review. Bioorg Chem. 2021, 117, 105386. [Google Scholar] [CrossRef]
- Meduri, B.; Pujar, G.V.; Durai Ananda Kumar, T.; Akshatha, H.S.; Sethu, A.K.; Singh, M.; Kanagarla, A.; Mathew, B. Lysophosphatidic acid (LPA) receptor modulators: Structural features and recent development. Eur. J. Med. Chem. 2021, 222, 113574. [Google Scholar] [CrossRef] [PubMed]
- González-Gil, I.; Zian, D.; Vázquez-Villa, H.; Hernández-Torres, G.; Martínez, R.F.; Khiar-Fernández, N.; Rivera, R.; Kihara, Y.; Devesa, I.; Mathivanan, S.; et al. A Novel Agonist of the Type 1 Lysophosphatidic Acid Receptor (LPA1), UCM-05194, Shows Efficacy in Neuropathic Pain Amelioration. J. Med. Chem. 2020, 63, 2372–2390. [Google Scholar] [CrossRef]
- Ueno, A.; Nagao, R.; Watanabe, T.; Ohta, H.; Yagi, M.; Inventors; Kirin Brewery Co Ltd., Assignee. Isoxazole and Thiazole Compounds and Use Thereof as Medicine. U.S. Patent 6,964,975, 15 November 2005. [Google Scholar]
- An, S.; Li, C.; Zhou, G.; Huang, C. Compound as Antagonist of Lysophosphatidic Acid Receptor, Composition, and Use Thereof, CUREGENIX Inc., USA. U.S. Patent 8,785,442, 22 July 2014. [Google Scholar]
- Hoshino, Y.; Okuno, T.; Saigusa, D.; Kano, K.; Yamamoto, S.; Shindou, H.; Aoki, J.; Uchida, K.; Yokomizo, T.; Ito, N. Lysophosphatidic acid receptor1/3 antagonist inhibits the activation of satellite glial cells and reduces acute nociceptive responses. FASEB J. 2022, 36, e22236. [Google Scholar] [CrossRef]
- Gaire, B.P.; Sapkota, A.; Song, M.R.; Choi, J.W. Lysophosphatidic acid receptor 1 (LPA1) plays critical roles in microglial activation and brain damage after transient focal cerebral ischemia. J. Neuroinflammation 2019, 16, 170. [Google Scholar] [CrossRef]
- Sepehrinezhad, A.; Shahbazi, A.; Joghataei, M.T.; Larsen, F.S.; Sahab Negah, S. Inhibition of autotaxin alleviates pathological features of hepatic encephalopathy at the level of gut-liver-brain axis: An experimental and bioinformatic study. Cell Death Dis. 2023, 14, 490. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Lee, S.; Norman, D.D.; Tigyi, G.J. Designing Dual Inhibitors of Autotaxin-LPAR GPCR Axis. Molecules 2022, 27, 5487. [Google Scholar] [CrossRef] [PubMed]
- Plastira, I.; Joshi, L.; Bernhart, E.; Schoene, J.; Specker, E.; Nazare, M.; Sattler, W. Small-molecule lysophosphatidic acid receptor 5 (LPAR5) antagonists: Versatile pharmacological tools to regulate inflammatory signaling in BV-2 microglia cells. Front. Cells Neurosci. 2019, 13, 531. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dedoni, S.; Camoglio, C.; Siddi, C.; Scherma, M.; Fratta, W.; Fadda, P. The Role of Lysophosphatidic Acid in Neuropsychiatric and Neurodegenerative Disorders. Future Pharmacol. 2024, 4, 199-221. https://doi.org/10.3390/futurepharmacol4010014
Dedoni S, Camoglio C, Siddi C, Scherma M, Fratta W, Fadda P. The Role of Lysophosphatidic Acid in Neuropsychiatric and Neurodegenerative Disorders. Future Pharmacology. 2024; 4(1):199-221. https://doi.org/10.3390/futurepharmacol4010014
Chicago/Turabian StyleDedoni, Simona, Chiara Camoglio, Carlotta Siddi, Maria Scherma, Walter Fratta, and Paola Fadda. 2024. "The Role of Lysophosphatidic Acid in Neuropsychiatric and Neurodegenerative Disorders" Future Pharmacology 4, no. 1: 199-221. https://doi.org/10.3390/futurepharmacol4010014
APA StyleDedoni, S., Camoglio, C., Siddi, C., Scherma, M., Fratta, W., & Fadda, P. (2024). The Role of Lysophosphatidic Acid in Neuropsychiatric and Neurodegenerative Disorders. Future Pharmacology, 4(1), 199-221. https://doi.org/10.3390/futurepharmacol4010014





