Fatty Acid-Binding Protein 5 Gene Deletion Enhances Nicotine-Conditioned Place Preference: Illuminating the Putative Gateway Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Statistical Analysis
2.4. Nicotine-Conditioned Place Preference (CPP)
3. Results
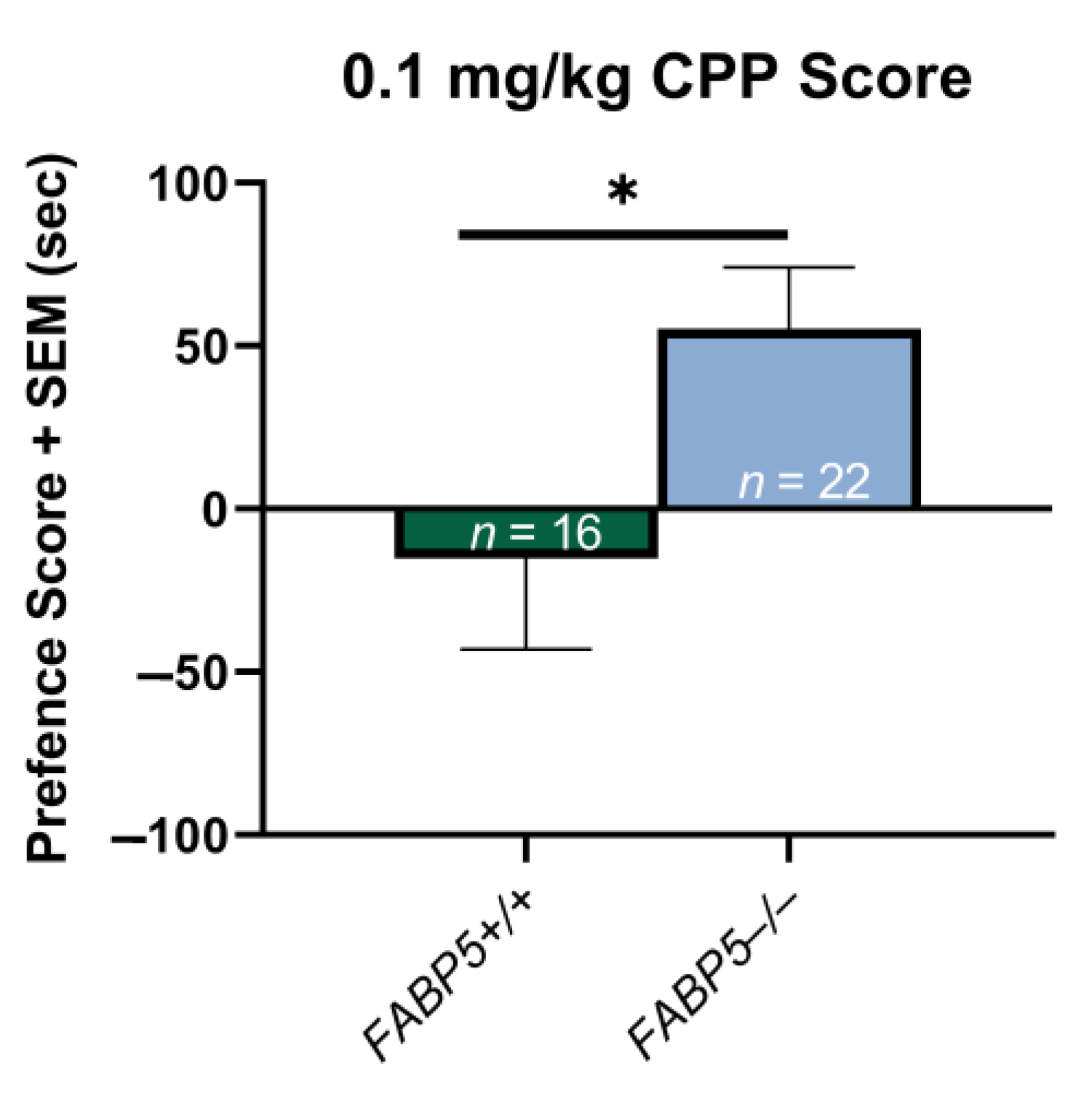
3.1. Nicotine CPP
3.2. Nicotine CPP Locomotor Activity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lariscy, J.T.; Hummer, R.; Rogers, R. Cigarette Smoking and All-Cause and Cause-Specific Adult Mortality in the United States. Demography 2018, 55, 1855–1885. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L. Pharmacology of Nicotine: Addiction, Smoking-Induced Disease, and Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, L.; Liang, Y.; Siapas, A.G.; Zhou, F.-M.; Dani, J.A. Dopamine Signaling Differences in the Nucleus Accumbens and Dorsal Striatum Exploited by Nicotine. J. Neurosci. 2009, 29, 4035. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W. Dopamine signals for reward value and risk: Basic and recent data. Behav. Brain Funct. 2010, 6, 24. [Google Scholar] [CrossRef]
- Febo, M.; Blum, K.; Badgaiyan, R.D.; Baron, D.; Thanos, P.K.; Colon-Perez, L.M.; Demotrovics, Z.; Gold, M.S. Dopamine homeostasis: Brain functional connectivity in reward deficiency syndrome. Front. Biosci. 2017, 22, 669–691. [Google Scholar]
- Melis, M.; Muntoni, A.; Pistis, M. Endocannabinoids and the Processing of Value-Related Signals. Front. Pharmacol. 2012, 3, 7. [Google Scholar] [CrossRef]
- Parsons, L.H.; Hurd, Y. Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 2015, 16, 579–594. [Google Scholar] [CrossRef]
- Murillo-Rodríguez, E.; Vázquez, E.; Millán-Aldaco, D.; Palomero-Rivero, M.; Drucker-Colin, R. Effects of the fatty acid amide hydrolase inhibitor URB597 on the sleep-wake cycle, c-Fos expression and dopamine levels of the rat. Eur. J. Pharmacol. 2007, 562, 82–91. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Zhou, Y.-Q.; Yu, Z.-P.; Zhang, X.-Q.; Shi, J.; Shen, H.-W. Restoring glutamate homeostasis in the nucleus accumbens via endocannabinoid-mimetic drug prevents relapse to cocaine seeking behavior in rats. Neuropsychopharmacology 2021, 46, 970–981. [Google Scholar] [CrossRef]
- Gunduz-Cinar, O.; MacPherson, K.P.; Cinar, R.; Gamble-George, J.; Sugden, K.; Williams, B.; Godlewski, G.; Ramikie, T.S.; Gorka, A.X.; Alapafuja, S.O.; et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol. Psychiatry 2013, 18, 813–823. [Google Scholar] [CrossRef]
- Garani, R.; Watts, J.; Mizrahi, R. Endocannabinoid system in psychotic and mood disorders, a review of human studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 110096. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Khalsa, J.; Cadet, J.L.; Baron, D.; Bowirrat, A.; Boyett, B.; Lott, L.; Brewer, R.; Gondré-Lewis, M.; Bunt, G.; et al. Cannabis-Induced Hypodopaminergic Anhedonia and Cognitive Decline in Humans: Embracing Putative Induction of Dopamine Homeostasis. Front. Psychiatry 2021, 12, 623403. [Google Scholar] [CrossRef] [PubMed]
- Le Foll, B.; Goldberg, S. Rimonabant, a CB1 antagonist, blocks nicotine-conditioned place preferences. Neuroreport 2004, 15, 2139–2143. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.; Perrault, G.; Griebel, G.; Soubrié, P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: Reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716). Neuropsychopharmacology 2005, 30, 145–155. [Google Scholar] [CrossRef]
- Le Foll, B.; Perrault, G.; Griebel, G.; Soubrié, P. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: Insights from pre-clinical and clinical studies. Addict. Biol. 2008, 13, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Cheer, J.F.; Wassum, K.M.; Sombers, L.A.; Heien, M.L.A.V.; Ariansen, J.L.; Aragona, B.J.; Phillips, P.E.M.; Wightman, R.M. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J. Neurosci. 2007, 27, 791–795. [Google Scholar] [CrossRef]
- Cohen, C.; Perrault, G.; Voltz, C.; Steinberg, R.; Soubrié, P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav. Pharmacol. 2002, 13, 451–463. [Google Scholar] [CrossRef]
- Grieder, T.E.; George, O.; Tan, H.; George, S.R.; Le Foll, B.; Laviolette, S.R.; van der Kooy, D. Phasic D1 and tonic D2 dopamine receptor signaling double dissociate the motivational effects of acute nicotine and chronic nicotine withdrawal. Proc. Natl. Acad. Sci. USA 2012, 109, 3101–3106. [Google Scholar] [CrossRef]
- Forget, B.; Coen, K.; Le Foll, B. Inhibition of fatty acid amide hydrolase reduces reinstatement of nicotine seeking but not break point for nicotine self-administration—Comparison with CB(1) receptor blockade. Psychopharmacology 2009, 205, 613–624. [Google Scholar] [CrossRef]
- Kandel, E.R.; Kandel, D.B. A molecular basis for nicotine as a gateway drug. N. Engl. J. Med. 2014, 371, 932–943. [Google Scholar] [CrossRef]
- Rodríguez de Fonseca, F.; del Arco, I.; Bermudez-Silva, F.J.; Bilbao, A.; Cippitelli, A.; Navarro, M. The endocannabinoid system: Physiology and pharmacology. Alcohol Alcohol. 2005, 40, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanuš, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef]
- Merritt, L.L.; Martin, B.R.; Walters, C.; Lichtman, A.H.; Damaj, M.I. The endogenous cannabinoid system modulates nicotine reward and dependence. J. Pharmacol. Exp. Ther. 2008, 326, 483–492. [Google Scholar] [CrossRef]
- Pavon, F.J.; Serrano, A.; Sidhpura, N.; Polis, I.; Stouffer, D.; de Fonseca, F.R.; Cravatt, B.F.; Martin-Fardon, R.; Parsons, L.H. Fatty acid amide hydrolase (FAAH) inactivation confers enhanced sensitivity to nicotine-induced dopamine release in the mouse nucleus accumbens. Addict. Biol. 2018, 23, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Kaczocha, M.; Glaser, S.; Deutsch, D. Identification of intracellular carriers for the endocannabinoid anandamide. Proc. Natl. Acad. Sci. USA 2009, 106, 6375–6380. [Google Scholar] [CrossRef] [PubMed]
- Kaczocha, M.; Glaser, S.T.; Maher, T.; Clavin, B.; Hamilton, J.; Joseph, O.; Rebecchi, M.; Puopolo, M.; Owada, Y.; Thanos, P.K. Fatty acid binding protein deletion suppresses inflammatory pain through endocannabinoid/N-acylethanolamine-dependent mechanisms. Mol. Pain 2015, 11, 52. [Google Scholar] [CrossRef]
- Fauzan, M.; Oubraim, S.; Yu, M.; Glaser, S.T.; Kaczocha, M.; Haj-Dahmane, S. Fatty Acid-Binding Protein 5 Modulates Brain Endocannabinoid Tone and Retrograde Signaling in the Striatum. Front. Cell. Neurosci. 2022, 16, 936939. [Google Scholar] [CrossRef]
- Yu, S.; Levi, L.; Casadesus, G.; Kunos, G.; Noy, N. Fatty acid-binding protein 5 (FABP5) regulates cognitive function both by decreasing anandamide levels and by activating the nuclear receptor peroxisome proliferator-activated receptor β/δ (PPARβ/δ) in the brain. J. Biol. Chem. 2014, 289, 12748–12758. [Google Scholar] [CrossRef]
- Buczynski, M.W.; Polis, I.; Parsons, L. The volitional nature of nicotine exposure alters anandamide and oleoylethanolamide levels in the ventral tegmental area. Neuropsychopharmacology 2013, 38, 574–584. [Google Scholar] [CrossRef]
- Maeda, K.; Uysal, K.T.; Makowski, L.; Görgün, C.Z.; Atsumi, G.; Parker, R.A.; Brüning, J.; Hertzel, A.V.; Bernlohr, D.A.; Hotamisligil, G.S. Role of the fatty acid binding protein mal1 in obesity and insulin resistance. Diabetes 2003, 52, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Thanos, K.; Bermeo, C.; Wang, G.-J.; Volkowa, N.D. D-cycloserine accelerates the extinction of cocaine-induced conditioned place preference in C57bL/c mice. Behav. Brain Res. 2009, 199, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Thanos, K.; Malave, L.; Delis, F.; Mangine, P.; Kane, K.; Grunseich, A.; Vitale, M.; Greengard, P.; Volkow, N.D. Knockout of p11 attenuates the acquisition and reinstatement of cocaine conditioned place preference in male but not in female mice. Synapse 2016, 70, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Thanos, P.K.; Bermeo, C.; Rubinstein, M.; Suchland, K.L.; Wang, G.J.; Grandy, D.K.; Volkow, N.D. Conditioned place preference and locomotor activity in response to methylphenidate, amphetamine and cocaine in mice lacking dopamine D4 receptors. J. Psychopharmacol. 2010, 24, 897–904. [Google Scholar] [CrossRef]
- Hamilton, J.; Marion, M.; Figueiredo, A.; Clavin, B.H.; Deutsch, D.; Kaczocha, M.; Haj-Dahmane, S.; Thanos, P.K. Fatty acid binding protein deletion prevents stress-induced preference for cocaine and dampens stress-induced corticosterone levels. Synapse 2018, 72, e22031. [Google Scholar] [CrossRef]
- Ananth, M.; Hetelekides, E.M.; Hamilton, J.; Thanos, P.K. Dopamine D4 receptor gene expression plays important role in extinction and reinstatement of cocaine-seeking behavior in mice. Behav. Brain Res. 2019, 365, 1–6. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Demarest, K.; Patricelli, M.P.; Bracey, M.H.; Giang, D.K.; Martin, B.R.; Aron, H. Lichtman Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. USA 2001, 98, 9371. [Google Scholar] [CrossRef]
- Haj-Dahmane, S.; Shen, R. Regulation of plasticity of glutamate synapses by endocannabinoids and the cyclic-AMP/protein kinase A pathway in midbrain dopamine neurons. J. Physiol. 2010, 588, 2589–2604. [Google Scholar] [CrossRef]
- Mansvelder, H.D.; Mertz, M.; Role, L. Nicotinic modulation of synaptic transmission and plasticity in cortico-limbic circuits. Semin. Cell. Dev. Biol. 2009, 20, 432–440. [Google Scholar] [CrossRef]
- Oleson, E.B.; Beckert, M.V.; Morra, J.T.; Lansink, C.S.; Cachope, R.; Abdullah, R.A.; Loriaux, A.L.; Schetters, D.; Pattij, T.; Roitman, M.F.; et al. Endocannabinoids shape accumbal encoding of cue-motivated behavior via CB1 receptor activation in the ventral tegmentum. Neuron 2012, 73, 360–373. [Google Scholar] [CrossRef]
- Lupica, C.R.; Riegel, A.C. Endocannabinoid release from midbrain dopamine neurons: A potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology 2005, 48, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Szabo, B.; Siemes, S.; Wallmichrath, I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur. J. Neurosci. 2002, 15, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Pistis, M.; Perra, S.; Muntoni, A.L.; Pillolla, G.; Gessa, G.L. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J. Neurosci. 2004, 24, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Pillolla, G.; Luchicchi, A.; Muntoni, A.L.; Yasar, S.; Goldberg, S.R.; Pistis, M. Endogenous fatty acid ethanolamides suppress nicotine-induced activation of mesolimbic dopamine neurons through nuclear receptors. J. Neurosci. 2008, 28, 13985–13994. [Google Scholar] [CrossRef]
- Figueiredo, A.; Hamilton, J.; Marion, M.; Blum, K.; Kaczocha, M.; Haj-Dahmane, S.; Deutsch, D.; Thanos, P.K. Pharmacological Inhibition of Brain Fatty Acid Binding Protein Reduces Ethanol Consumption in Mice. J. Reward Defic. Syndr. Addict. Sci. 2017, 3, 21–27. [Google Scholar] [CrossRef]
- Cohen, C.; Kodas, E.; Griebel, G. CB1 receptor antagonists for the treatment of nicotine addiction. Pharmacol. Biochem. Behav. 2005, 81, 387–395. [Google Scholar] [CrossRef]
- Blum, K.; Oscar-Berman, M.; Braverman, E.R.; Febo, M.; Li, M.; Gold, M.S. Enhancing Brain Pregnenolone May Protect Cannabis Intoxication but Should Not Be Considered as an Anti-addiction Therapeutic: Hypothesizing Dopaminergic Blockade and Promoting Anti-Reward. J. Reward Defic. Syndr. 2015, 1, 20–23. [Google Scholar] [CrossRef]
- Tullis, L.M.; Dupont, R.; Frost-Pineda, K.; Gold, M.S. Marijuana and tobacco: A major connection? J. Addict. Dis. 2003, 22, 51–62. [Google Scholar] [CrossRef]
- Small, E.; Shah, H.P.; Davenport, J.J.; Geier, J.E.; Yavarovich, K.R.; Yamada, H.; Sabarinath, S.N.; Derendorf, H.; Pauly, J.R.; Gold, M.S.; et al. Tobacco smoke exposure induces nicotine dependence in rats. Psychopharmacology 2010, 208, 143–158. [Google Scholar] [CrossRef]
- Bruijnzeel, A.W.; Gold, M.S. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res. Rev. 2005, 49, 505–528. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roeder, N.; Richardson, B.; Mihalkovic, A.; Penman, S.; White, O.; Hamilton, J.; Gupta, A.; Blum, K.; Gold, M.S.; Thanos, P.K. Fatty Acid-Binding Protein 5 Gene Deletion Enhances Nicotine-Conditioned Place Preference: Illuminating the Putative Gateway Mechanisms. Future Pharmacol. 2023, 3, 108-116. https://doi.org/10.3390/futurepharmacol3010007
Roeder N, Richardson B, Mihalkovic A, Penman S, White O, Hamilton J, Gupta A, Blum K, Gold MS, Thanos PK. Fatty Acid-Binding Protein 5 Gene Deletion Enhances Nicotine-Conditioned Place Preference: Illuminating the Putative Gateway Mechanisms. Future Pharmacology. 2023; 3(1):108-116. https://doi.org/10.3390/futurepharmacol3010007
Chicago/Turabian StyleRoeder, Nicole, Brittany Richardson, Abrianna Mihalkovic, Samantha Penman, Olivia White, John Hamilton, Ashim Gupta, Kenneth Blum, Mark S. Gold, and Panayotis K. Thanos. 2023. "Fatty Acid-Binding Protein 5 Gene Deletion Enhances Nicotine-Conditioned Place Preference: Illuminating the Putative Gateway Mechanisms" Future Pharmacology 3, no. 1: 108-116. https://doi.org/10.3390/futurepharmacol3010007
APA StyleRoeder, N., Richardson, B., Mihalkovic, A., Penman, S., White, O., Hamilton, J., Gupta, A., Blum, K., Gold, M. S., & Thanos, P. K. (2023). Fatty Acid-Binding Protein 5 Gene Deletion Enhances Nicotine-Conditioned Place Preference: Illuminating the Putative Gateway Mechanisms. Future Pharmacology, 3(1), 108-116. https://doi.org/10.3390/futurepharmacol3010007








