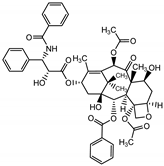
Abstract
The objectives of this study were to investigate whether elicitors induce the production of taxoids in Taxus globosa by testing the hypothesis that the cells induce a greater accumulation of taxoids depending on the type and concentration of the elicitor treatment tested. Cell cultures were initiated from Taxus globosa friable calli for producing taxoids using the Gamborg medium supplemented with different initial combinations of growth regulators as follows: naphthaleneacetic acid, benzylaminopurine, picloram, and polyvinylpyrrolidone. The cell suspension cultures were then used for evaluating taxoid production through different elicitor treatments, such as methyl jasmonate, ethanol, buthionine sulphoximine, and hydrogen peroxide. The cell suspension cultures showing the best growth characteristics were found in the medium supplemented with 10.74 µM of naphthaleneacetic acid and 3.33 µM of picloram. The highest biomass for the cell cultures was obtained in the EtOH-2 treatment (0.12 ± 0.005 mg·g−1 of dry weight) after 8 days, while the biomass in the control treatment at that time was 0.095 ± 0.2 mg·g−1 of dry weight. The exogenous application of a combination of elicitors buthionine and hydrogen peroxide in the cell suspension cultures significantly increased the concentration of the 10-deacetylbaccatin (1662 µg·g−1 of dry weight), cephalomannine (334.32 µg·g−1 of dry weight), and the production of taxol (157.0 µg·g−1 of dry weight).
1. Introduction
The yew (Taxus spp.) is a gymnosperm belonging to the Taxaceae family, which comprises nine species with a global distribution as follows: Taxus baccata, Taxus canadensis, Taxus brevifolia, Taxus floridana, Taxus mairei, Taxus globosa, Taxus chinensis, Taxus sumatrana, and Taxus wallichiana. Taxoids are a group of specific diterpene compounds with a pentamethyl tricyclopentadecane taxane skeleton, including taxol and other taxoids, which are found in the foliage and bark of yews. There are more than 350 unique compounds in the taxoid class, categorized by their structural differences [1]. Each group within the taxoid diterpenoid class differs in polarity and pharmaceutical properties, particularly in biological activity [2,3]. Taxol was the first-ever drug used against cancer. It is a highly effective drug with a unique action mechanism and chemical structure. Taxol is lipophilic (log P 3,5) and water-insoluble (0.3 ± 0.02 µg mL−1) [4]. This drug has not only been approved for the clinical treatment of ovarian and breast cancer by the Food and Drugs Administration in 1992 (FDA, EE.UU.), but also shows significant activity against malignant melanoma, lung cancer, and other solid tumors. In addition, taxol has shown promising results in the treatments for HIV, Kaposi’s sarcoma, and Alzheimer’s disease [5]. Taxol’s antitumoral properties are based on its capacity to join and stabilize microtubules, blocking cell replication in the late G2-M phase of the cellular cycle. Driven by the need for cancer treatment, the global demand for taxol is rising, the estimated amount of purified drug is approximately 250 kg annually [6]. The estimated amount of required purified taxol for treating 500 cancer patients is 1 kg, which amounts to nearly 10 tons of bark or the felling of 700 trees. On average, the production of 1 kg of taxol pure requires as much as 7000–10,000 kg of bark, equivalent to 750,000 trees to meet the current global demand for this drug. However, the natural supply of this drug in Taxus spp. is poor: only 0.01–0.06% on average, and only 0.01–0.1% in the needles and dry bark of Taxus brevifolia [6,7]. Additionally, the natural regeneration of Taxus spp. is very low, due to long seed dormancy and the seed pericarp preventing efficient germination. This low production capacity, coupled with the growing demand for taxol, means that a large source of plants must be secured for extraction [7]. Because of this, all Taxus species are exposed to the risk of extinction. Studying the taxol biosynthesis pathway is valuable, as it can provide insight into taxoid production and help advance research into transgenic plants or genetically modified microorganisms. Many studies have been carried out to identify the genes and the enzymes involved in taxol biosynthesis. In vitro cultures of plant cells are acknowledged as a possible alternative to the large-scale production of taxol [8]. Additionally, several techniques for tissue culturing are used to improve the yield of secondary metabolites, by eliciting a stress response against the use of elicitors or activators, precursors, biotransformation, variable environmental conditions, and modifications of medium constituents. Elicitors can be used as biosynthesis catalysts for plant secondary metabolism and play an important role in biosynthetic pathways for the large production of commercially important compounds such as ajmalicine (antihypertensive), vincristine and vinblastine (antileukemic), and ginsenoside (neuroprotective). Some plant metabolites assayed in in vitro cell suspension cultures can be induced by the exogenous supply of methyl jasmonate [9]. Jasmonic acid and methyl jasmonate act as signal transduction compounds, inducing proteinase inhibitors and defense genes and increasing the secondary metabolism of a large variety of plants [10,11]. Research has found that hydroxide peroxide (H2O2) can induce secondary metabolism in plant cells [12]. Glutathione (GSH), an antioxidant, plays a fundamental role in the defense systems of plants against environmental stress [13]. The influence of biotic and abiotic elicitors to improve the production and storage of taxol through tissue culture has also been studied [14,15,16,17,18,19,20]. In vitro cell and tissue cultures of Taxus spp. are also being extensively studied to increase taxoid yield. Taxoid analysis requires a quick identification and measurement of the main compounds present in in vitro culture systems of Taxus cells. High-Performance Liquid Chromatography (HPLC) is used mainly for the separation and determination of taxoids in plant materials and cell culture. Thus, the objective of this research was to study the effects of methyl jasmonate, ethanol, buthionine sulphoximine, and hydrogen peroxide as elicitors in the production of three taxoids produced and quantified in HPLC in cell suspension cultures of Taxus globosa due to its low production in in vitro cultures.
2. Materials and Methods
2.1. Experimental Conditions and Treatments
Friable calli of Taxus globosa supplied by Dr. Ramos-Valdivia in the Plant Cells Biotechnology Lab (Cinvestav, Zacatenco, Mexico City, Mexico) were used. The calli from Gamborg medium were induced from needle explants of T. globosa (Schlecht.) and immersed into a liquid B5 medium [21], supplemented with sucrose (20 g L−1), Na2EDTA (100 µM); B5 vitamins: myoinositol (550 µM), thiamine (30 µM), pyridoxine (0.45 µM), nicotinic acid (8.2 µM); and growth regulators such as 1-naphthaleneacetic acid (NAA), benzylaminopurine (BAP), and picloram (PIC) at different concentrations; at pH 6.1 ± 1 during 20–30 days. In order to reduce tissue oxidation, an antioxidant was used: polyvinylpyrrolidone (PVP, 0.4%). Cultures were incubated at 25 ± 1 °C in the dark, with an orbital shaker (120 rpm), and were sub-cultured every two weeks.
The cells were cultured in 1-L flasks containing 200 mL of medium with an inoculum size of 20 g. The medium was sub-cultured every two weeks and the cells were sub-cultured every third week. The flasks were put inside a rotary shaker at 100 rpm under continuous lighting at 25 ± 1 °C.
2.2. Inoculation and Elicitation
The experiments were carried out in 125-mL Erlenmeyer flasks containing 30 mL of fresh medium. Each flask was inoculated with 2 g of cell fresh weight. After 14 days of growth, sterile biotic and abiotic elicitors were added. Methyl jasmonate (MeJA) was diluted in ethanol and a final concentration of 60 µM (50 µL), 100 and 300 µL of ethanol, and a combination of 0.8 µM of buthionine sulphoximine (BSO) and 0.2 µM of hydrogen peroxide (H2O2) was used. Sterile water was added to the control flasks. The cells were kept in an orbital shaker at 25 °C for 6, 8, and 10 days. Samples in the liquid phase were recovered after 10 days of incubation and analyzed to quantify taxol, cephalomannine, and 10-deacetylbaccatin. All the experiments were made in triplicate. All the elicitors were filtered with a 0.45 µm membrane filter.
The cells were recovered by vacuum filtering, and filtrates were recovered to measure the volume of the spent medium and its pH with a potentiometer. Cell growth was evaluated by measuring the increase in cell dry weight. A cell viability assay was performed with the fluorescein diacetate method (FDA) using fluorescence microscopy.
2.3. HPLC Analysis of Taxoids
The HPLC reverse phase method was developed to quantify the extracted levels of taxol, cephalomannine, and 10-deacetylbaccatin III. An Agilent Technologies HPLC equipment, model 1100, with a photodiode array detector (Model 1200) was used. Chromatographic separation was performed using a Fluophase C18 reverse phase HPLC column (4.6 mm × 150 mm). The mobile phase consisted of acetonitrile-trifluoroacetic acid (87:13). The mobile phase was eluted at an isocratic velocity of 1.8 mL min−1 at 65 °C, pH 3.03, and the effluent was monitored at 205 nm. All samples and extractions were filtered using 47 mm Acrodisk filters with a nylon membrane (0.45 μM, Millipore Co., Burlington, MA, USA) prior to the HPLC analysis. In total, 50 µL of each extract was used, which was injected into the HPLC column. The quantification was obtained by measuring the peak area of the compound to the inner pattern. The identification of taxol, cephalomannine, and 10-deacetylbaccatin was carried out by comparing retention times with authentic standards (Sigma, St. Louis, MO, USA).
All the chemical products used were of analytical grade. Metabolite content was expressed as micrograms of metabolite per gram of dry weight of the analyzed sample. The experimental results indicate the mean of the triplicate, deviations were defined as standard errors, data were subjected to variance analysis (ANOVA), and the difference between means was compared by the Tukey test (p < 0.05).
2.4. Statistical Analysis
Data were analyzed with ANOVA (two ways) in the statistical package SPSS Statistics version 17 for Windows.
3. Results
3.1. Cell Culture
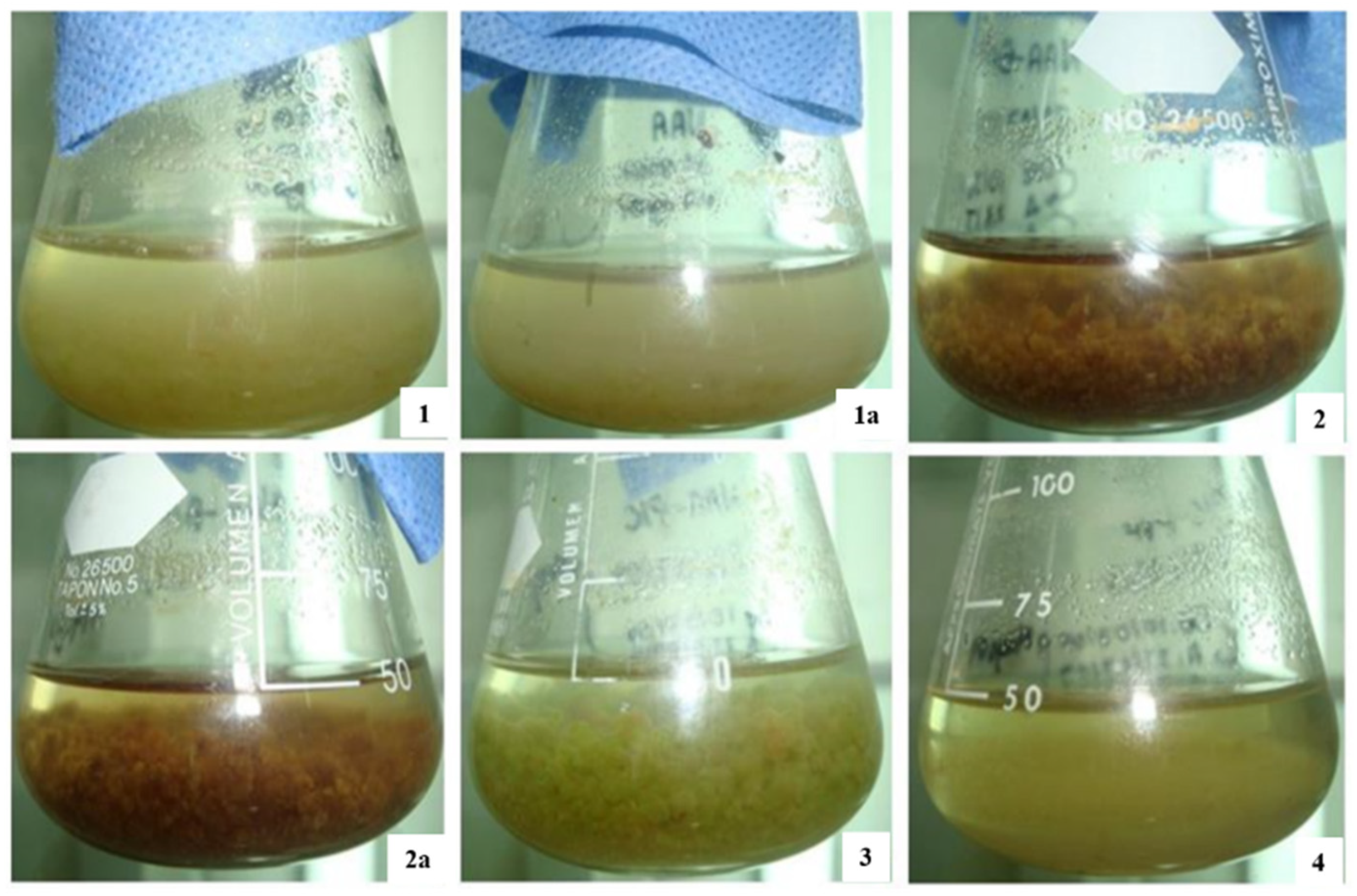
The culture of Taxus spp. cells are regarded as a possible stable source of taxol and related taxoid compounds for cancer drug synthesis. The low production of taxoids is an obstacle in the solid in vitro culture of callus. A suspension culture develops through the immersion of a relatively friable portion of calli into a liquid medium, kept in adequate conditions of aeration, shaking, lighting, temperature, and other environmental parameters [22]. This method has the advantage to provide a continuous and reliable source of the natural product. The color and texture results of the cell suspension culture of Taxus globosa during our experiment are shown in Figure 1. The best in vitro cell suspension cultures of Taxus globosa originated in the B5 medium, with added naphthaleneacetic acid (10.74 µM) and picloram (3.33 µM): the cells had a friable aspect, were green in color, and demonstrated high cell viability. These treatments were used for both control treatment and elicitation experiments.
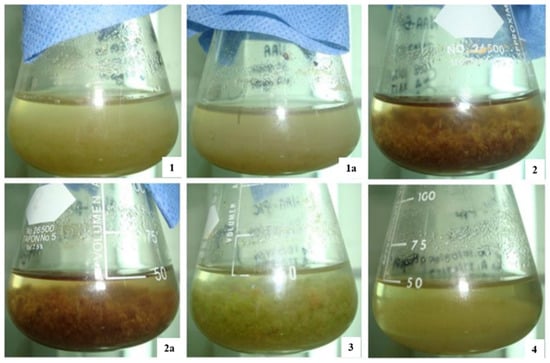
Figure 1.
Maintenance of cell suspension cultures of Taxus globosa. (1,1a) Suspension culture growth in Gamborg medium with addition of naphthaleneacetic acid hormone (10 µM); (2,2a) Suspension culture growth in Gamborg medium with addition of naphthaleneacetic acid hormone (5 µM) + Benzylaminopurine hormone (5 µM); (3) Suspension culture growth in Gamborg medium with addition of naphthaleneacetic acid hormone (10 µM) + Picloram auxin (3 µM); (4) Suspension culture growth in Gamborg medium with addition of picloram auxin (4 µM) + Polyvinylpyrrolidone antioxidant (0.2%) Picture: Corresponding Author.
3.2. Cell Viability
The cell viability of Taxus globosa cell cultures during the growth phase was 75–98% after 10 days, according to the determination by fluorescein diacetate (FDA) staining under fluorescent microscopy.
3.3. Biomass Accumulation
The effect of elicitors on biomass accumulation in cell suspension cultures of Taxus globosa was studied. Maximum biomass of the cell suspension cultures was measured in the EtOH-2 treatment, yielding 0.12 ± 0.005 mg·g−1 of dry weight after 8 days, while the biomass in the control treatment was 0.095 ± 0.2 mg·g−1 of dry weight after the same amount of time.
3.4. pH Profiles
In the control cell cultures and cultures elicited of T. globosa, the pH of the culture medium was measured at six, eight, and ten days (Table 1).

Table 1.
pH measurement in cell cultures of T. globosa.
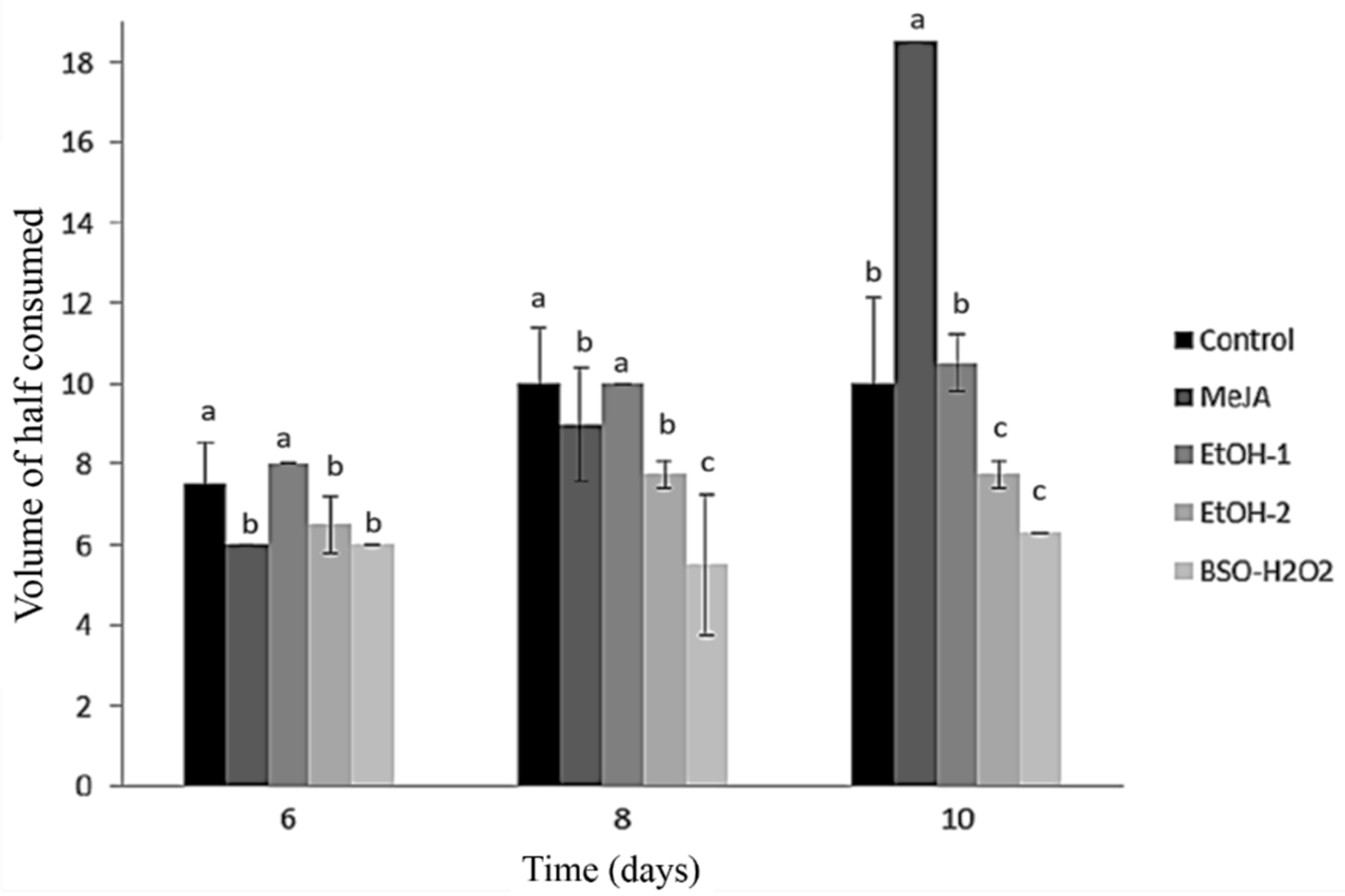
Fett-Neto et al. (1994) suggested that the transitory increase in intracellular pH was due to NH4+ absorption [23]. Wickremesinhe and Arteca (1994), when studying suspension cultures of Taxus media, found no substantive differences between pH values of the medium after 21 days of growth: with an initial medium pH value adjusted to between 3 and 7, the final pH at day 21 was always 5.4–5.6 [24], similarly to our results. This suggests that the ammonium ion flux stimulates the initial liberation of taxol in the culture medium, as it is also thought to explain alkaloid release in Cinchona spp. [25]. In Figure 2, we present the effect of the elicitors ethanol (EtOH), methyl jasmonate (MeJA), and a combination of buthionine sulphoximine and hydrogen peroxide (BSO + H2O2) on the spent medium volume of cell suspension cultures of Taxus globosa. The highest values were that consumed by the control treatment and the cultures elicited with MeJA and EtOH-1 (Figure 2).
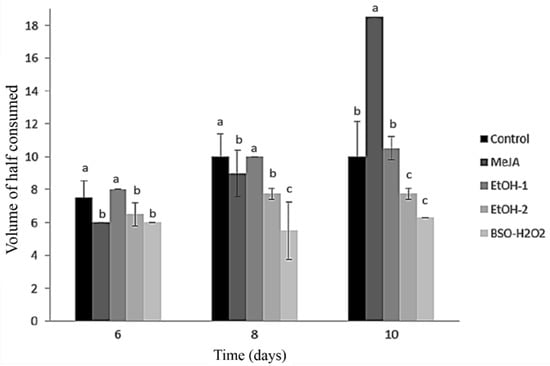
Figure 2.
Effect of the elicitors ethanol (EtOH), methyl jasmonate (MeJA), and a combination of buthionine sulphoximine and hydrogen peroxide (BSO + H2O2) on the spent medium volume of cell suspension cultures of Taxus globosa. a,b,c: means with the same letter are not statistically different (Tukey, p < 0.05).
Different basal media such as Gamborg (B5), Murashige and Skoog (MS), Schenk and Hildebrandt (SH), and Woody Plant Medium (WPM) have all been traditionally employed to initiate and maintain Taxus cultures [26]. However, we found no study on the relationship between basal media and the synthesis and secretion of taxoid compounds in Taxus spp. cells. It appears that nutrient concentration and composition in the medium does not only affect cell growth and viability, but also metabolite biosynthesis [27]. The medium of a cell culture works as a nutrient source for growing cells, however, bacterial, fungal, and animal cells cultured in vitro actively secrete a series of metabolites and proteins in the culture medium. This suggests that, to some extent, culture medium also works as an external storage compartment [28]. In normal systems of plant cell culture, most compounds are formed in the stationary phase.
3.5. Effect of Elicitors on the Production of 10-Diacetylbaccatin
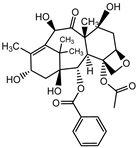
The maximum concentration of 10-deacetylbaccatin was 1662.15 µg·g−1 of dry weight, after elicitation using a combination of buthionine sulphoximine (0.8 µM) and hydrogen peroxide (0.2 µM). Compared to the control, the combination of BSO + H2O2 increased the contents of 10-deacetylbaccatin by a factor of 80.7 in 6 days (at pH 6) and by a factor of 21.66 in 10 days (pH 6.3). There was no 10-DAB synthesis accumulation on day 8. Our results showed that the combination of the BSO-H2O2 elicitors induced the synthesis of 10-DAB in the cell suspension cultures of Taxus globosa. Compared to the control, the 60 µM MeJA treatment induced the highest contents of 10-deacetylbaccatin, increasing them by a factor of 2.85 times 8 days after elicitation (at pH 5.73). The treatment with EtOH-1 and EtOH-2 at a concentration of 100 µL and 300 µL induced the highest contents of 10-deacetylbaccatin, increasing them 13.19 times and 1.04 times 10 days after elicitation, respectively (at pH of 5.54 and 5.40, respectively).
3.6. Effect of Elicitors on the Production of Cephalomannine
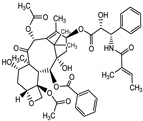
The maximum concentration of cephalomannine was 334.32 µg·g−1 of dry weight, with a combination of buthionine sulphoximine (0.8 µM) and hydrogen peroxide (0.2 µM). Compared to the control, the BSO + H2O2 combination increased the contents of cephalomannine by a factor of 1.82 in 8 days (pH 5.5) and by a factor of 78.74 in 10 days (pH 6.3). Our results showed that the BSO-H2O2 elicitors induced cephalomannine synthesis in cell suspension cultures of Taxus globosa. The amount of cephalomannine produced in the presence of ethanol decreased compared to the MeJA treatment (120 μg g−1 of dry weight in 8 days) and to the BSO + H2O2 treatment.
3.7. Effect of Elicitors on Taxol Production
The maximum amount of taxol production in the presence of the combination of buthionine sulphoximine (0.8 µM) and hydrogen peroxide (0.2 µM) was 86.78, 84.2, and 157.0 µg·g−1 of dry weight consecutively at six, eight and ten days after elicitation. In contrast, the maximum total accumulation of 10-DAB, cephalomannine, and taxol was 309.12, 217.69, and 157.00 µg·g−1 of dry weight, respectively, for the BSO-H2O2 treatment after 10 days. The total maximum accumulation of 10-DAB was 193.50 µg·g−1 of dry weight, with the EtOH-1 treatment after 10 days, while no accumulation of cephalomannine or taxol was observed with the concentrations of 100 and 300 μL of EtOH-1 and EtOH-2. Similarly, the 60-μM MeJA treatment resulted in no accumulation of taxol; and no accumulation of 10-DAB with the 300 μL EtOH-2 treatment. These results emphasize the importance of the elicitor concentration in the culture medium, which changes according to the plant species used. This could be due to the cell or enzymatic activity related to the metabolism of plant cells through the addition of biotic or abiotic elicitors [29]. Table 2 summarizes the highest production of taxoids obtained from the suspended cells of T. globosa.

Table 2.
Production of taxoids obtained from suspended cells of T. globosa.
4. Discussion
The response of the elicitor also depends on the growth stage in most culture systems. With some exceptions, most in vitro cultures show a response to elicitation during the growth stage. The growth stage of a culture can impact not only the quantitative response to the elicitor treatment but also the production pattern. Plants respond to biotic and abiotic stress by activating several defense mechanisms, such as hypersensitive cell death and the accumulation of secondary metabolites [30].
Many plant species kept in in vitro cell cultures in suspension accumulate secondary metabolites induced by the exogenous provision of MeJA [8]. These molecules (jasmonates) are involved in the regulation of many biosynthetic pathways, which lead to the production of secondary metabolites [31]. For example, jasmonic acid and MeJA act as signaling compounds in the biosynthesis pathways. For example, MeJA induces the biosynthesis of rosmarinic acid in cultures in the suspension of Lithospermum erythrorhizon cells [32]. Taxol stimulation in Taxus cuspidate and Taxus canadensis with ethylene and MeJA has been reported [33].
Taxol production in cell suspension cultures of Taxus canadensis increased when the cultures were elicitated with a combination of acetyl ketohexose and MeJA. MeJA, a lipid-derived elicitor, was also applied as an elicitor in combination with chitopentose in cell suspension cultures of Taxus chinensis to increase the production of podophyllotoxin. The production of the taxoid baccatin III increased after the addition of the concentration of treatment of MeJA, which increased the activity of 10-DAB III acetyltransferase. Thus, induction by MeJA is essential to produce 10-deacetylbaccatin III and baccatin III [34]. Luo et al. (1999) reported a taxol production of up to 1.17% within 5 days of elicitation with MeJA, together with other taxoids, such as 13-acetyl-9-dihydrobaccatin, 9-dihydrobaccatin III, and baccatin VI [35]. The jasmonate signaling pathway connects to other signaling pathways, forming a complex regulation network. Genes regulated by the treatment with MeJA include those implicated in jasmonate biosynthesis, secondary metabolism, cell wall formation, and the ones coding for protective-stress and defense proteins [36].
The effect of elicitors depends on many factors, such as elicitor concentration, elicitation time, and the stage at which the elicitor is applied. Additionally, elicitors can have a synergistic effect: glutathione (GSH) plays a fundamental role as an antioxidant in plant defense systems against environmental stress and is regarded as a protective agent against oxidative stress. L-γ-glutamyl-L-cysteinyl-glycine and cysteine protect against oxidative stress. GSH is the most abundant intracellular antioxidant, playing an important role in the protection against reactive oxygen species, in nutrient and xenobiotic metabolism, and in intracellular redox status regulation [37]. The molecule of L-buthionine sulphoximine (BSO) is a specific inhibitor of γ-glutamylcysteine synthetase, which blocks the limiting step of the velocity of glutathione biosynthesis and, in doing this, depletes the intracellular reserve of GSH in culture cells (Lewis-Wambi et al., 2008), causing oxidative stress that leads to secondary metabolite generation and production as a defense mechanism [38]. In respect to cell cultures of Taxus cuspidata (yield of 0.02% of dry weight), cell suspension cultures of Taxus baccata had a taxol yield of 1.5 mg/L. Kim et al. (1995) established a similar taxol level from cell suspension cultures of Taxus brevifolia after 10 days of culture with an optimized medium containing 6% fructose [39]. The addition of carbohydrates during the growth cycle increased the taxol yield rate accumulating in the culture medium (14.78 mg/L). The influence of biotic and abiotic elicitors has also been studied to improve the production and accumulation of taxol through cultures [40]. Conversely, the interaction of ethanol with the cell membrane inhibits the interaction with membrane proteins or cell receptors. Ethanol and other small alcohols are natural surfactants of organic/aqueous interphases, and also affect the cell membrane by mechanical stress and by the inhibition of the natural absorption of terminal groups in the interphases.
The increase in the concentration of some elicitors such as methanol, ethanol, hydrogen peroxide, buthionine sulphoximine, and methyl jasmonate could improve biosynthesis and production of taxoids. Yamamoto et al. (2014) found that an increase in alcohols and hydrocarbons with greater p values [41], as well as triglycerides, were effective and reported that lauric acid significantly increased taxol productivity in a two-phase culture system. In their study, the effect of lauryl alcohol on taxol and baccatin III productivity was 0.023 and 0.014 mg/L/day, respectively. Taxol concentration in the culture medium with lauric acid was 0.018 mg/L, compared to 0.032 mg/L in the control [42]. Taxol concentration inhibited the growth of T. globosa cells in rates exceeding 0.02 mg/L [41]. Therefore, the high growth of calli in the culture with lauric acid was a result of the decrease in taxol concentration in the medium with a concentration of 0.02 mg/L per in situ taxol extraction with lauric acid. Taxol and cephalomannine concentrations in the medium with added lauric acid decreased in comparison with the control. In contrast, baccatin III and 10-deacetylbaccatin III concentrations in the medium with added lauric acid increased compared to the control [42]. As an elicitor, methanol (MeOH) is a biochemical waste product, with no known biological role in plants [36]. The main source of ethanol in plants comes from pectin demethylation by pectin methylesterase. The exogenous addition of 1% methanol in rice accelerates tryptophane and serotonin biosynthesis, with the corresponding gene induction [43]. No other solvent, including ethanol, produced a tryptophan induction effect [44]. The transduction of signals and the expression of genes coding for taxoid biosynthesis enzymes in in vitro cell cultures with exogenous elicitation of ethanol and buthionine sulphoximine are unknown. The results show that taxoids in Taxus globosa are present in different concentrations, which may be due to related cell or enzymatic activity, as a function of plant metabolism, through the addition of the elicitor type, elicitation time, and elicitor concentration, as well as genotype, which are all important parameters for taxoid accumulation. Applications of the quantification method used in this study for the determination of taxol, cephalomannine, 10-deacetylbaccatin, and other related taxoids could serve as a parameter for different pharmaceutical extracts of diverse Taxus species in the presence of this type of anticarcinogens compounds.
5. Conclusions
The best cell suspension cultures for Taxus globosa originated in the Gamborg medium, with added naphthaleneacetic acid and picloram. Adding a combination of elicitors buthionine sulphoximine and hydrogen peroxide increased the content of taxol, cephalomannine, and 10-deacetylbaccatin. Cell cultures were initiated from Taxus globosa friable calli for producing taxoids using the Gamborg medium supplemented with different initial combinations of growth regulators as follows: naphthaleneacetic acid, benzylaminopurine, picloram, and polyvinylpyrrolidone.
The cell suspension cultures were then used for evaluating taxoid production through different elicitor treatments, such as methyl jasmonate, ethanol, buthionine sulphoximine, and hydrogen peroxide.
The cell suspension cultures showing the best growth characteristics were found in the medium supplemented with 10.74 µM of naphthaleneacetic acid and 3.33 µM of picloram. The highest biomass for the cell cultures was obtained in the EtOH-2 treatment (0.12 ± 0.005 mg·g−1 of dry weight) after 8 days, while the biomass in the control treatment at that time was 0.095 ± 0.2 mg·g−1 of dry weight.
The exogenous application of a combination of elicitors buthionine and hydrogen peroxide in the cell suspension cultures significantly increased the concentration of the taxoid 10-deacetylbaccatin (1662 µg·g−1 of dry weight), cephalomannine (334.32 µg·g−1 of dry weight), and the production of taxol (157.0 µg·g−1 of dry weight).
Author Contributions
Conceptualization, A.C.R.V. and M.S.H.; methodology, H.J.B.-C. and A.C.R.V.; software, H.J.B.-C. and M.S.H.; validation, H.J.B.-C. and M.S.H.; formal analysis, H.J.B.-C. and M.S.H.; resources, H.J.B.-C. and M.S.H.; original—draft preparation, H.J.B.-C. and M.S.H.; writing—review and editing, H.J.B.-C. and M.S.H.; visualization, M.S.H.; supervision, M.S.H.; project administration, H.J.B.-C.; funding acquisition, H.J.B.-C. and M.S.H. All authors have read and agreed to the published version of the manuscript.
Funding
his research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The authors are grateful to Consejo Nacional de Ciencia y Tecnología (CONACyT, Mexico) and Colegio de Postgraduados, Campus Montecillo.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chun, F.; Long, J.; Li, Q. Simultaneous identification and determination of major taxoids from extracts of Taxus chinensis cell cultures. J. Chem. Sci. 2007, 62, 1–10. [Google Scholar]
- Ojima, I.; Kumar, K.; Awasthi, D.; Vineberg, J.G. Drug discovery targeting cell division proteins, microtubules and FtsZ. Bioorganic Med. Chem. 2014, 22, 5060–5077. [Google Scholar] [CrossRef]
- Wordeman, L.; Vicente, J.J. Microtubule Targeting Agents in Disease: Classic Drugs, Novel Roles. Cancers 2021, 13, 5650. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Kohli, K.; Mir, S. Development and validation of RP-HPLC method for analysis of novel self-emulsifying paclitaxel formulation. J. Pharm. Biomed. Anal. 2013, 2, 17–27. [Google Scholar]
- Su, J.; Zhang, Z.; Deng, J. Study on the taxol content in Taxus yunnanensis of different age and different provenance. Eur. J. Res. 2005, 18, 369–374. [Google Scholar]
- Hussain, A.; Ahmed, Q.; Nazir, H. In vitro callogenesis and organogenesis in Taxus wallichiana Zucc. The himalayan yew. Pak. J. Bot. 2013, 45, 1755–1759. [Google Scholar]
- Escrich, A.; Almagro, L.; Moyano, E.; Cusido, R.M.; Bonfill, M.; Hosseini, B.; Palazon, J. Improved biotechnological production of paclitaxel in Taxus media cell cultures by the combined of corona tine and calixarenes. Plant Phys. Biochem. 2021, 163, 68–75. [Google Scholar] [CrossRef]
- Liao, Z.; Chen, M.; Sun, X. Micropropagation of endangered plant species. Methods Mol. Biol. 2006, 318, 179–185. [Google Scholar]
- Khosroushahi, A.; Valizadeh, M.; Ghasempour, A. Improved taxol production by combination of inducing factors in suspension cell culture of Taxus baccata. Cell Biol. Int. 2006, 30, 262–269. [Google Scholar] [CrossRef]
- Linden, J.; Phisalaphong, M. Oligosaccharides potentiate methyl jasmonate-induced production of paclitaxel in Taxus canadensis. Plant Sci. 2000, 158, 41–51. [Google Scholar] [CrossRef]
- Ruan, J.; Zhou, Y.; Zhou, M. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Abe, H.; Gen-Ichuro, A. Jasmonates induce both defense responses and communication in monocotyledonous and dicotyledonous plantas. Plant Cell Physiol. 2015, 56, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Guo, H.; Zhang, J. Hydrogen peroxide is involved in salicylic acid-elicited rosmarinic acid production in Salvia miltiorrhiza cell cultures. Sci. World J. 2014, 2014, 843764. [Google Scholar] [CrossRef] [PubMed]
- Ransy, C.; Vaz, C.; Lombés, A.; Bouillaud, F. Use of H2O2 to cause oxidative stress, the catalase issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef]
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Sarmadi, M.; Karimi, N.; Palazón, J.; Ghassempour, A.; Mirjalili, M. The effects of salicylic acid and glucose on biochemical traits and taxane production in a Taxus baccata callus culture. Plant Physiol. Biochem. 2018, 132, 271–280. [Google Scholar] [CrossRef]
- Nakagawara, S.; Nakamura, N.; Guo, Z. Enhanced formation of constitutive sesquiterpenoid in cultured cells of Liverwort. Plant Cell Physiol. 1992, 34, 421–429. [Google Scholar]
- Ghafoori, R.; Bernard, F.; Abolmaali, S. Improved effect of glutathione on the induction and growth of Taxus baccata L. callus. Ann. Biol. Res. 2012, 4, 1726–1730. [Google Scholar]
- Halliwell, B. Reflections of an aging free radical. Free. Rad. Biol. Med. 2020, 161, 234–245. [Google Scholar] [CrossRef]
- Yukimune, Y.; Tabata, H.; Higashi, Y. Methyl jasmonate induced overproduction of paclitaxel and baccatin III in Taxus cell suspension cultures. Nat. Biotechnol. 1996, 14, 1129–1132. [Google Scholar] [CrossRef]
- Gamborg, O.; Millar, R.; Djma, A. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Farkya, S.; Srivastava, A. Bioprocess considerations for production of secondary metabolites by plant cell suspension cultures. Biotechnol. Bioprocess Eng. 2002, 7, 138–149. [Google Scholar] [CrossRef]
- Fett-Neto, A.; Melanson, S.; Nicholson, S. Improved taxol yield by aromatic carboxylic acid and amino acid feeding to cell cultures of Taxus cuspidate. Biotechnol. Bioeng. 1994, 44, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Wickremesinhe, E.; Arteca, R. Taxus callus cultures: Optimizing growth and production of taxol. J. Plant Physiol. 1994, 144, 183–188. [Google Scholar] [CrossRef]
- Ateacha, D.N.; Koch, U.; Engelhard, C. Direct analysis of alkaloids in natural Cinchona bark and commercial extracts using time-of-flight secondary ion mass spectrometry. Anal. Methods 2018, 10, 950–958. [Google Scholar] [CrossRef]
- Maheshwari, P.; Garg, S.; Kumar, A. Taxoids: Biosynthesis and in vitro production. Biotechnol. Mol. Biol. Rev. 2008, 3, 71–87. [Google Scholar]
- Abbasi, K.; Moghim, S.; Reza, M. Optimization of the basal medium for improving production and secretion of taxanes from suspension cell culture of Taxus baccata L. J. Pharm. Sci. 2012, 20, 54–59. [Google Scholar]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 25. [Google Scholar] [CrossRef]
- Gull, A.; Lone, A.; Ul, N.; Wani, I. Biotic and abiotic stresses in plants. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2019; pp. 1–6. [Google Scholar]
- Marsik, P.; Langhansova, L.; Dvorakova, M. Increased ginsenosides production by elicitation of In vitro cultivated Panax Ginseng adventitious roots. J. Med. Aromat. Plants 2014, 3, 39. [Google Scholar]
- Mirjalili, N.; Linden, J. Methyl jasmonate induced production of taxol in suspension cultures of Taxus cuspidata: Ethylene interaction and induction models. Biotechnol. Prog. 1996, 12, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, Z. Effect of methyl jasmonic acid on baccatin III biosynthesis. Tsinghua Sci. Technol. 2006, 11, 363–367. [Google Scholar] [CrossRef]
- Luo, J.; Mu, Q.; Gu, Y. Protoplast culture and paclitaxel production by Taxus yunnanensis. Plant Cell Tiss. Org. 1999, 59, 25–29. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.; Yang, S. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Lewis-Wambi, J.; Kim, H.; Wambi, C. Buthionine sulphoximine sensitizes antihormone-resistant human breast cancer cells to estrogen-induced apoptosis. Breast Cancer Res. 2008, 10, R104. [Google Scholar] [CrossRef]
- Kim, J.; Yun, J.; Hwang, Y. Production of taxol and related taxanes in Taxus brevifolia cell cultures: Effect of sugar. Biotechnol. Lett. 1995, 17, 101–106. [Google Scholar] [CrossRef]
- Parc, G.; Canaguier, A.; Landre, P. Production of taxane with biological activity by plants and callus culture from selected Taxus genotypes. Phytochemistry 2002, 59, 725–730. [Google Scholar] [CrossRef]
- Yamamoto, S.; Taura, K.; Hayashi, S. Effect of lauryl alcohol on production of taxanes in a suspension callus culture. Solvent Extr. Res. Dev. 2014, 21, 95–101. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, S.; Ogawa, K.; Hayashi, S. Effect of increased volume fraction of organic solvents on callus growth and taxol production in simultaneous suspension callus culture of Taxus baccata and in situ extraction. Solvent Extr. Res. Dev. 2007, 14, 71–77. [Google Scholar]
- Dornenburg, H.; Knorr, D. Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzym. Microb. Technol. 1995, 17, 674–684. [Google Scholar] [CrossRef]
- Kang, K.; Park, S.; Natsagdorj, U. Methanol is an endogenous elicitor molecule for the synthesis of tryptophan and tryptophan-derived secondary metabolites upon senescence of detached rice leaves. Plant J. 2011, 66, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Fall, R.; Benson, A. Leaf methanol-the simplest natural product from plants. Trends Plant Sci. 1996, 1, 296–301. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).