Abstract
With humans living longer and the median age of the population increasing, there is an ever-increasing demand for better biomedical implants. Titanium implants have a long history of successful use, but their naturally forming amorphous oxide surfaces are not ideal to promote bone growth. Therefore, titanium surfaces are often modified to improve bioactivity through electrochemical processes such as anodization which can crystallize the oxide into more bioactive titanium oxide phases, form hierarchical micro- and nano-scale roughness profiles, and incorporate beneficial bone chemistry into the oxide layer to improve interactions with bone cells. We have recently developed three innovative anodization electrolytes based on combinations of citrus fruit juices and commercially available calcium compounds. Anodization in these electrolytes produced citrus-based oxides exhibiting surface Ca/P ratios within the range of human bone, unique cauliflower-like hierarchical micro- and nano-scale surface roughness profiles, and the formation of titanate compounds which have been shown to be precursors for subsequent apatite formation. Thus, our titanate-containing citrus-based oxides show much promise for improving future osseointegration.
1. Introduction
With humans living longer, the need for implants is constantly increasing. The global dental implant market reached USD 4.6 billion in 2019 [1], and the orthopedic implant market is projected to reach nearly USD 9 billion by 2025 [2,3]. Titanium and its alloys are commonly used for dental and orthopedic implant materials due to their high strength, beneficial strength-to-weight ratio, and excellent corrosion resistance. The corrosion resistance is primarily due to a thin amorphous oxide layer that is naturally formed when titanium is exposed to oxygenated environments. However, the bio-inertness of this amorphous oxide leads to less-than-ideal osseointegration when compared to more bioactive materials [2,4]. Because of this, modifications of titanium implant surfaces have been meticulously explored to achieve improved bone tissue interactions in vivo [5]. Common commercially used titanium surface modification techniques include sandblasting, acid etching, plasma spraying, and anodization [6]. Anodization is an electrochemical process that can be used to modify titanium oxide surface roughness, porosity, crystallinity, and chemistry [7].
Micro- and nano-scale surface roughness levels can be achieved on titanium surfaces through anodization processes. Anodization processes that use a forming voltage exceeding the dielectric limit of the oxide are known to experience generate sparking and the form micro- or nano-scale surface porosity and thus micro- and nano-scale roughness profiles. Previous studies on micro-scale roughness oxide surfaces have shown improved mechanical bone interlocking, while studies on oxide surfaces with nano-scale roughness have shown increased protein adsorption, osteoblast cell attachment, and differentiation [8,9,10]. More recent studies have shown surfaces exhibiting hierarchical micro- and nano-scale surface roughness profiles promote cell proliferation, differentiation, and extracellular matrix formation [5,11,12].
In addition to modifying surface topography, anodization can incorporate beneficial dopant elements present within the electrolyte solution directly into the oxides. Since human bones are largely made of calcium phosphates, developing anodization processes to incorporate calcium (Ca) and phosphorus (P) into these oxide layers has become an area of substantial research interest [2]. Ca- or P-doped oxide surfaces have individually been shown to increase the mineralization of osteoblasts, bone-to-implant contact (BIC), and implant screw removal torques compared to non-anodized titanium control surfaces [13,14]. A recent study in our laboratory created P-doped oxides through anodization followed by subsequent addition of Ca onto the oxide surfaces using hydrothermal soak treatments [15]. This two-step process resulted in oxides exhibiting similar surface Ca/P ratios to those found in hard tissues like bone and showed significantly improved osteoblast mineralization compared to non-anodized control surfaces [15]. Several other anodization studies have simultaneously incorporated Ca and P oxide dopants into CaP titanium oxides and achieved promising surface Ca/P ratios using optimized anodization electrolytes and waveforms [16,17,18,19]. Surfaces containing Ca and P have been shown to improve the bone-implant interface by increasing the local mineral content and are thus considered osteoinductive [20,21]. Early anodization studies forming CaP oxides with a single-step anodization process used electrolytes combining phosphoric acid and various calcium compounds and produced oxides exhibiting less than desirable Ca/P ratios below 1.0 [17,22,23]. Later CaP oxide studies [24]. Switched to glycerophosphate and calcium acetate containing electrolytes and which generated oxide Ca/P ratios within the range of hydroxyapatite (1.67) and human bone (1.50–1.70) [17,22,23]. Nonetheless, these early CaP oxides with beneficial Ca/P ratios still showed difficulty forming apatite during simulated body fluid bioactivity testing [2,18,25]. More recent CaP oxide studies have continued using the glycerophosphate and calcium acetate electrolyte and shown improvement in osseointegration in both in vitro cell culture studies [19,26,27,28], and in vivo tests with anodized screw implants in rabbits [26].
Anodization processes are also able to thicken and crystallize the oxide layer into anatase and rutile phases. Crystalline anatase and rutile oxides have been shown to be generally be more bioactive compared to amorphous counterparts and the anatase phase to generally be preferred over rutile [29,30,31]. Additionally, the atomic lattice of the anatase phase is a better match to the lattice for apatite, so anatase phase oxide surfaces should promote apatite formation [32,33,34]. Calcium titanate (CaTiO3), also known as perovskite, is a bio-ceramic material having good biocompatibility and is suggested for use as a coating material over implants [35,36]. Previously, ion implantation and radiofrequency sputtering have been used for the formation of calcium titanate coatings on titanium substrates and have been shown to improve bioactivity when soaked in SBF (simulated body fluids) or Hank’s solution [37,38,39]. Furthermore, Ca- and CaP-doped oxides that also formed crystalline titanates during the anodization process exhibited an improved ability to form apatite during bioactivity testing [2,18,25,40]. Previously, nano-structured CaTiO3 coatings on porous Ti6Al4V scaffolds, created using hydrothermal treatment, have been shown to promote macrophage polarization toward the anti-inflammatory M2 phenotype by suppressing TNF-α signalling and enhancing oxidative phosphorylation [41]. The titanate coatings also exhibited enhanced osteogenic differentiation of pre-osteoblasts, as evidenced by increased ALP activity and mineralization in vitro. Additionally, the titanate coatings showed significant improvement in bone ingrowth, osseointegration, and neurovascular integration, compared to uncoated counterparts in vivo [41].
The present study introduces innovative anodization processes to form CaP oxides in electrolytes based on citrus fruit juice and commercially available calcium compounds. The resulting citrus-based oxides exhibit a strategic combination of beneficial surface characteristics including hierarchical micro- and nano-scale surface textures, surface Ca/P ratios within the range of human bone, and calcium titanate formation. Thus, the innovative citrus-based CaP oxide anodization processes show much promise to improve healing and osseointegration on future titanium implants.
2. Materials and Methods
2.1. Specimen Preparation
Commercially pure titanium grade 4 (CPTi4) disc specimens were produced by cutting a 12.7 mm bar stock into 3 mm discs. The cut disc specimens were cleaned ultrasonically using laboratory detergent (Alconox®, White Plains, NY, USA), followed by rinsing with distilled water. Disc specimens were then dipped in a 10:1 ratio solution of nitric acid-hydrofluoric acid (TURCO NITRADD, Henkel Corporation, Madison Heights, MI, USA) for a period of 30 s to activate the surface for anodization.
2.2. Anodization
The compositions of the three citrus fruit-based electrolytes developed for this study are listed in Table 1. The first electrolyte (CFOJ) consisted of a mixture of commercially available calcium-fortified orange juice (Kroger, Cincinnati, OH, USA) and 0.15 M calcium acetate (99% Spectrum chemical, New Brunswick, NJ, USA). The other two electrolytes required juicing either navel oranges (Kroger, Cincinnati, OH, USA) or mandarins (Kroger, Cincinnati, OH, USA) using a commercially available fruit juicer (Eurolux ELCJ-1800S, 300 W, Chesnut Ridge, NY, USA). The juiced-orange electrolyte (OJ) consisted of a mixture of juice from juicing 5 navel oranges, monobasic calcium phosphate (90%, Thermo Fisher Scientific, Waltham, MA, USA), and calcium acetate. The juiced-mandarin electrolyte (MJ) consisted of a mixture of juice from juicing 7 mandarins, monobasic calcium phosphate, and calcium acetate. The molarities listed in Table 1 for calcium phosphate and calcium acetate represent the final molarities in the electrolyte solution once the respective powders were dissolved into the fruit juices. The pH of all three electrolytes was recorded prior to anodization. The anodization reaction cell consisted of a 500 mL beaker of each electrolyte, the CPTi4 disc anode, and two CPTi4 strip cathodes. Anodization of activated disc specimens was performed using a DC rectifier (350 V, 10 A, Dynatronix, Amery, WI, USA) using a galvanostatic direct current density waveform. Current densities of 700 mA/cm2 were applied for 20 s in the CFOJ electrolyte, and current densities of 1000 mA/cm2 were applied for 20 s in the OJ and MJ electrolytes. Multiple specimens (n = 4) were anodized in each electrolyte, and the final forming voltage generated during the anodization of each oxide specimen was recorded.

Table 1.
Composition of anodization electrolytes for forming CaP oxides.
2.3. Oxide Surface Characterization
Optical microscopy imaging (Keyence, Osaka, Japan, VHX-1000) and Scanning electron microscopy (SEM, Zeiss, Jena, Germany, Supra 40) were used to document the surface topographies of the anodized surfaces on micro- and nano-scale dimensions. SEM imaging was conducted using a 12 kV accelerating voltage. Atomic force microscopy (AFM, Bruker, Santa Barbara, CA, USA Bioscope Catalyst) was also used in ScanAssyst mode (0.25 Hz, and 512 samples/line) to capture the micro- and nano-scale surface roughness topographies of the anodized surfaces. Scan areas with dimensions of 50 × 50 µm2 and 1 × 1 µm2 were collected on multiple specimens (n = 4) from each anodized group. Gwyddion software was used to calculate average roughness (Sa) and peak-to-valley roughness (Sz) values for each specimen. Energy dispersive X-ray spectroscopy (EDS, EDAX, Mahwah, NJ, USA, TEAM EDS Software Suite) was used to collect bulk chemistry spectra from the anodized oxide surfaces using an SEM accelerating voltage of 12 kV, magnification of 5000×, and a 30 µm SEM aperture to identify the bulk oxide chemistry. Spectra were collected from triplicate areas from each specimen on each oxide group (n = 4). Average atomic percentage values for each element present in each oxide group were calculated from the EDS spectra datasets. This methodology allowed the calculation of the Ca and P dopant uptake levels into the anodized oxide layers and, thus, the calculation of the surface oxide Ca/P ratio for each oxide group. Since the below-surface interaction volumes generated during EDS penetrated to depths that extended through the anodized oxide layer thickness and into the CPTi substrate material surface, it was of interest to examine the oxide-only surface chemistry using a different analytical technique. The oxide-only surface chemistry was measured using X-ray photoelectron spectroscopy (XPS, Thermo Scientific, Waltman, MA, K-Alpha XPS System Advantage v5.9911 Software Suite) for comparison to the EDS datasets. The XPS system consisted of a monochromatic X-ray source of 1486.6 eV, corresponding to the Al Kα line. A 75 W X-ray power set at 12 kV was used with a spot size set at 400 µm2. The K-Alpha instrument was adjusted to a base pressure of 9.0 × 10−10 mbar and was calibrated to give a binding energy of 84.0 eV for Au 4f7/2 and 284.8 eV for the C1s line of adventitious (aliphatic) carbon present on the non-sputtered oxide specimens. Photoelectrons were collected from a take-off angle of 90° relative to the sample surface. A series of XPS spectra were performed in the Constant Analyzer Energy mode. Triplicate survey spectra were collected from selected areas of a representative specimen (n = 1) from each oxide group using a pass energy of 200 eV and an energy step size of 1.0 eV. Triplicate high resolution (HR) core level spectra of C1s, O1s, P2p, Ca2p, and Ti2p were also collected from selected areas of a representative specimen (n = 1) from each oxide group using a 40 eV pass energy, an energy step size of 0.1eV, and an average of 50 scans. Thin-film X-ray diffraction (XRD, XDS 2000, Scintag, Franklin, MA, USA) was utilized to check for the presence of crystalline anatase or rutile titanium oxide phases and titanate compound formation within the anodized oxide layers. Triplicate representative specimens from each group were rotated 1° away from the copper X-ray source (0.154 nm Cu-Kα) to enhance the X-ray interaction volume within the thin anodized layers. XRD scans were conducted at two-theta angles ranging from 20° to 80° since this range contains the highest intensity diffraction peaks for anatase, rutile, and titanate compounds. Attenuated total reflectance Fourier transform infrared spectroscopy (ATR FTIR) was used on representative specimens from each oxide group to determine molecular differences. A Spectrum 100 FTIR (Perkin-Elmer, Waltham, MA, USA) was used over the range of 650–4000 cm−1 at 100 cm−1 spectral resolution.
2.4. Oxide Cross-Sectional Characterization
Representative specimens from each oxide group were sectioned, mounted in conductive epoxy, and polished to view the cross-sectional thicknesses of the citrus-based oxide layers in the SEM. Five oxide cross-sectional images were collected per specimen, and five thickness measurements were performed on each oxide image, resulting in a total of 25 thickness measurements per oxide group.
2.5. Oxide Adhesion Quality
The VDI 3198 standard Rockwell C indentation test was performed to evaluate the adhesion quality of the anodized CFOJ oxide group [42,43,44]. The CFOJ group was selected for further characterization since the surface characteristics of the oxides were all similar and the commercially available calcium-fortified orange juice electrolyte component did not depend on seasonal availability of the citrus fruits for juicing. Multiple indentations (n = 3) were performed on representative CFOJ oxide specimens using a Rockwell C indenter at a load value of 150 kg, followed by capturing an imaging using an optical microscope. The resulting images were compared to the VDI 3198 standard maps for adhesion quality assessment [2,42,43,44,45,46,47].
2.6. Ion Release Rates
Inductively coupled plasma optical emission spectrometry (ICP-OES, SPECTRO AMETEK, SPECTROGREEN, Kleve, Germany, SPECTRO ICP Analyzer Pro Software) was used for measuring the release profiles of Ca2+ ions from the CFOJ group. Representative anodized disc specimens (n = 3) were immersed in 10 mL of calcium-free phosphate-buffered saline (PBS) solution (MP Biomedicals, Santa Ana, CA, USA). The specimens were transferred to fresh solution at specific time points of 2, 4, 6, 8, 10, 12, 14, 18, 22, and 30 days. Any remaining particulate matter in each collected solution was dissolved by adding 300 μL of nitric acid and 50 μL of hydrochloric acid, followed by filtration using 0.4 μm syringe filters. The filtered solutions were then analyzed for Ca2+ ion release concentrations in parts per million (ppm).
2.7. Pre-Osteoblast Cell Viability Testing
Mouse pre-osteoblastic cells (MC3T3-E1, Subclone 4; American Type Culture Collection, Manassas, VA, USA) were cultured and maintained in alpha-modified Eagle’s minimum essential medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin, at 37 °C in 5% CO2. Representative specimens (n = 3) from the CFOJ oxide group and non-anodized CPTi controls were sonicated in distilled water to clean the surface, followed by autoclaving, and seeding with 50,000 cells/cm2. Media changes were performed every 48 h up to a period of 7 days.
To assess the cell viability, a Live/Dead™ viability/cytotoxicity assay kit (Invitrogen, Carlsbad, CA, USA) was used on day 7. Imaging was performed using an epifluorescence microscope (IX81, Olympus, Hachioji, Tokyo, Japan) in combination with image analysis software (OLYMPUS cellSens Dimension 3.2 and Slidebook 4.2.0.10, Olympus, Center Valley, PA, USA).
Cell viability was quantified using an MTT assay on day 7, as previously described [48]. The media were removed and replaced with MTT solution (Invitrogen, Carlsbad, CA, USA), followed by a 4 h incubation at 37 °C in 5% CO2. Wells containing only culture medium and MTT solution without any pre-osteoblast cells served as controls. Following incubation, the MTT solution was removed, dimethyl sulfoxide (DMSO; Sigma Aldrich, St. Louis, MO, USA) was added, and the oxide specimens were incubated for 10 min. The resulting solutions from each well were collected, and three replicates per group were plated in a 96-well plate. Absorbance was then measured using an ELx800 plate reader (Biotek, Winooski, VT, USA) at a wavelength of 540 nm.
2.8. Statistical Analysis
A one-way ANOVA (α = 0.05) with post hoc Tukey analyses was performed to determine significant differences in the oxide surface roughness values and the oxide cross-sectional thickness values. An unpaired t-test (α = 0.05) was performed to determine significant differences in the MTT assay cell viability results.
3. Results
3.1. Anodization Process Results
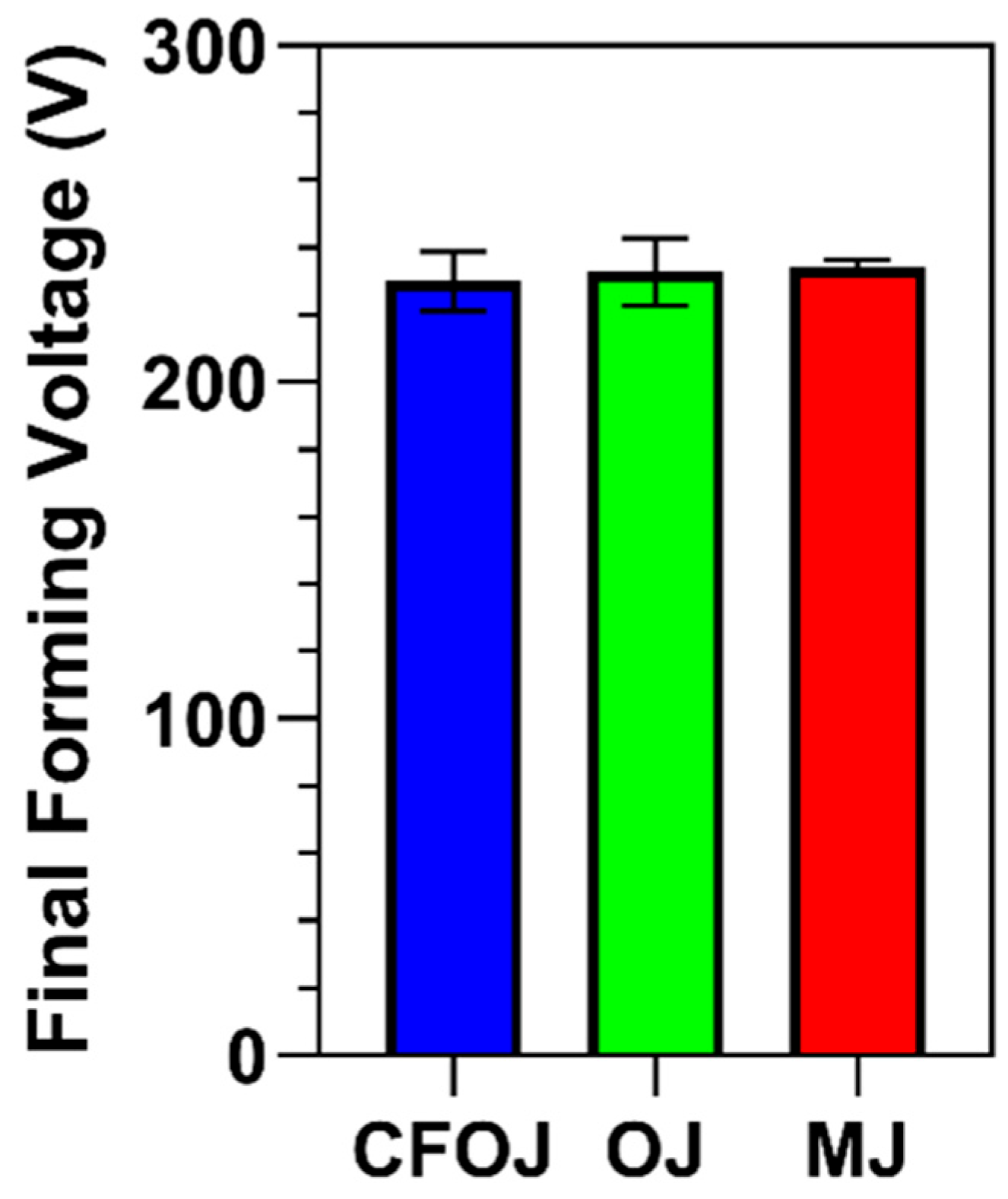
The CFOJ, OJ, and MJ citrus fruit-based electrolyte mixtures shown in Table 1 revealed pH values of 4.96, 5.14, and 5.10, respectively. The final forming voltage ranges recorded for all the specimens anodized using galvanostatic waveforms in the CFOJ, OJ, and MJ electrolytes are shown in Figure 1. Interestingly, the citrus fruit-based oxides formed in each electrolyte revealed similar final forming voltage ranges of 230 ± 9 V, 233 ± 10 V, and 234 ± 2 V, respectively.
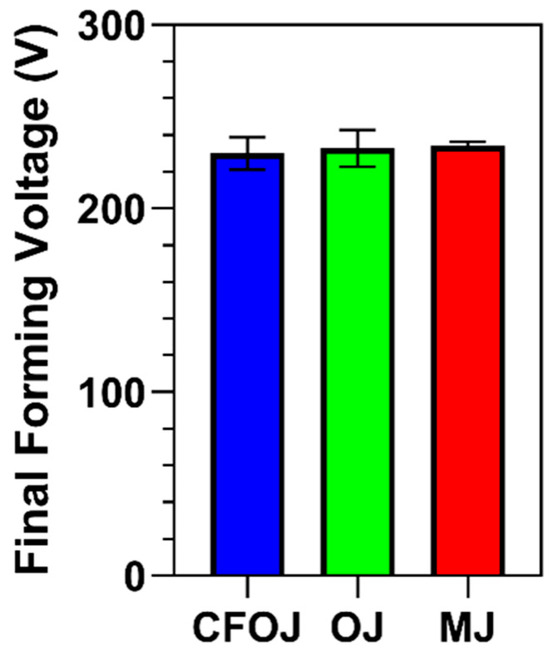
Figure 1.
Final forming voltages recorded during galvanostatic anodization in each citrus fruit-based electrolyte. The citrus fruit-based oxides formed in each electrolyte revealed similar final forming voltage ranges of 230 ± 9 V (CFOJ), 233 ± 10 V (OJ), and 234 ± 2 V (MJ).
3.2. Oxide Surface Topography and Roughness
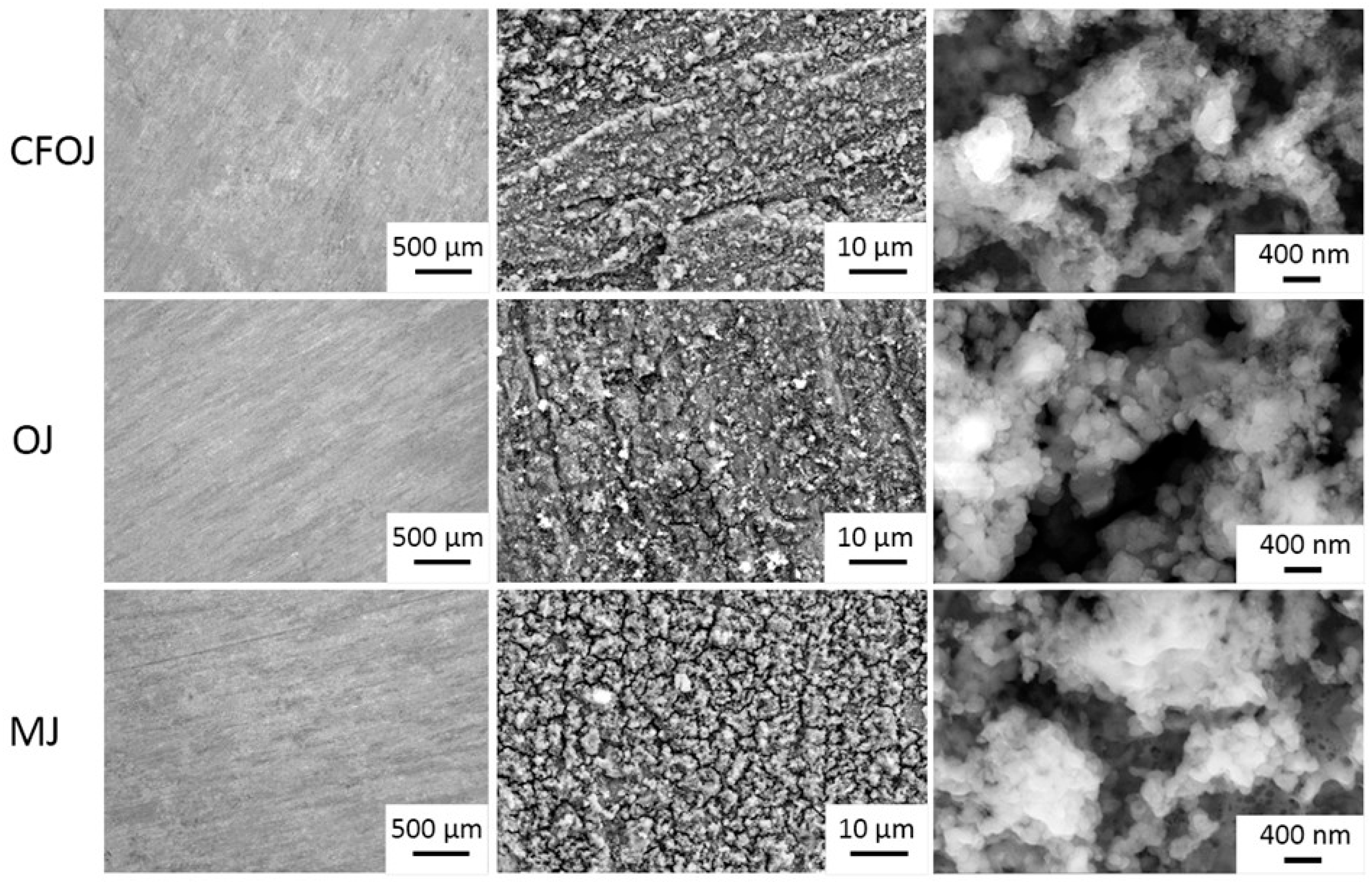
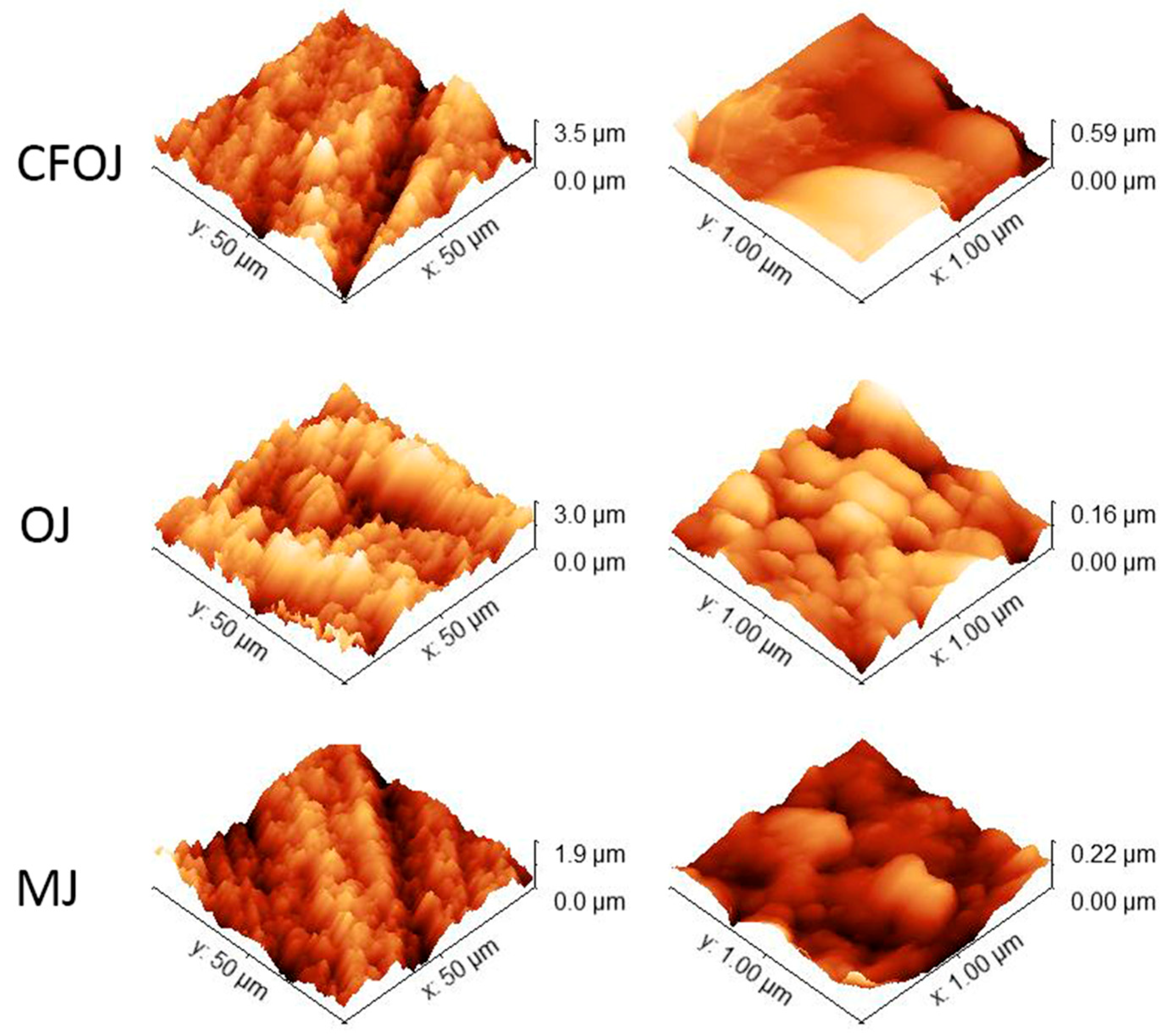
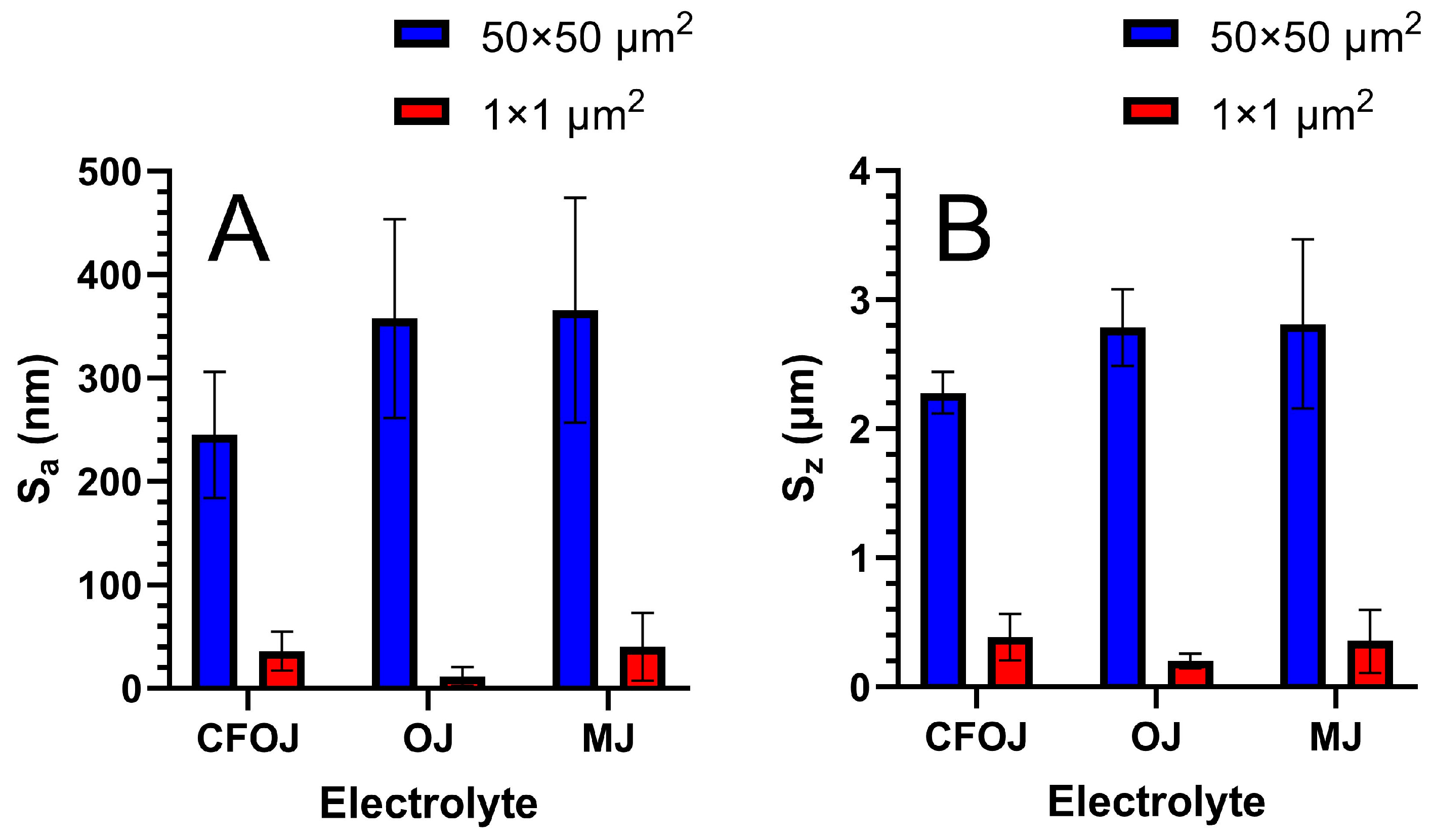
The optical microscopy and SEM images for each citrus fruit-based oxide group are shown in Figure 2. Low-magnification optical microscopy images in the left column of Figure 2 reveal a greyish-white surface appearance for each oxide group. SEM images at higher magnifications revealed a complex micro- and nano-scale surface topography for each oxide, as shown in the middle and right columns of Figure 2. The nano-scale surface topography for each oxide group exhibited a unique cauliflower-like surface topography. Representative AFM 50 × 50 µm2 and 1 × 1 µm2 surface scan areas from each citrus-based oxide group are shown in Figure 3. The complex surfaces depicted confirmed the presence of multiscale micro- and nano-scale surface roughness profiles for each oxide. The average surface roughness, Sa, and peak-to-valley roughness, Sz, values for each oxide group are compiled in Figure 4. All the oxide groups revealed statistically similar 50 × 50 µm2 scan Sa (p = 0.922) and Sz (p = 0.482) values and statistically similar 1 × 1 µm2 scan Sa (p = 0.766) and Sz (p = 0.702) values. The 50 × 50 µm2 scan areas revealed micro-roughness Sa values with magnitudes less than 500 nm. The 1 × 1 µm2 scan areas showed true nano-roughness Sa values below 90 nm. Additionally, the unique cauliflower-like nano-scale oxide surface topographies shown in the right column SEM images of Figure 2 were also apparent in the 1 × 1 µm2 AFM roughness profiles in Figure 3.
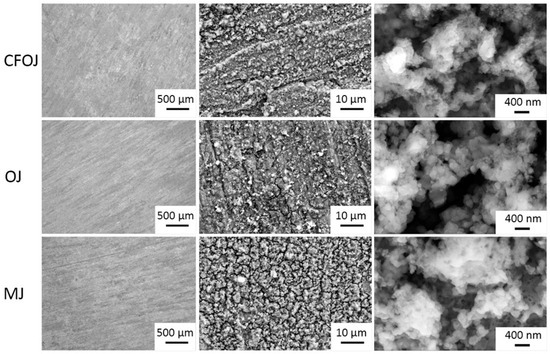
Figure 2.
Optical Microscopy and SEM images of citrus fruit-based oxide groups showing complex micro- and nano-scale surface topographies. Left column—optical micro-topography at 100×; middle column—SEM micro-topography at 5000×; right column—SEM nano-topography. Optical microscopy images reveal a greyish-white surface appearance for each oxide group. The nano-scale SEM surface topography for each oxide group exhibited a cauliflower-like surface topography.
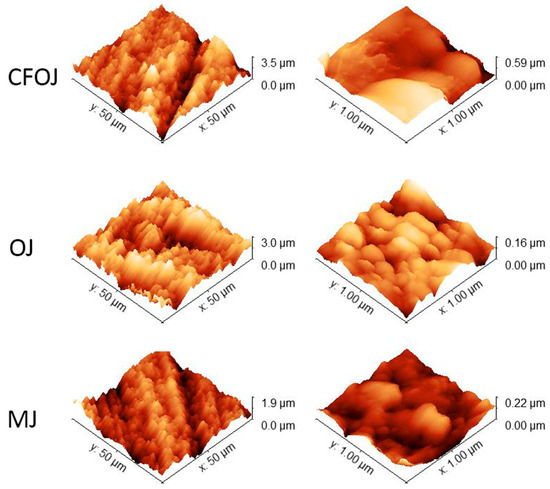
Figure 3.
AFM images of citrus fruit-based anodized oxide groups showing micro- and nano-scale roughness profiles. Left column 50 × 50 µm2 scan areas; right column—1 × 1 µm2 scan areas. Multiscale micro- and nano-scale surface roughness profiles were observed for each oxide.
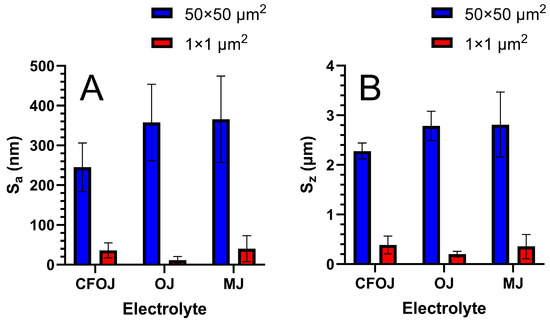
Figure 4.
Surface roughness values for each citrus-based oxide group. (A) Average surface roughness (Sa) values, (B) peak-to-valley roughness (Sz) surface roughness values. 50 × 50 µm2 scan areas showed Sa values less than 500 nm, while 1 × 1 µm2 scan areas showed true nano-roughness Sa values below 90 nm. All the oxide groups revealed statistically similar 50 × 50 µm2 scan and 1 × 1 µm2 scan values. Additionally, the unique cauliflower-like nano-scale surface topographies were confirmed within the 1 × 1 µm2 AFM surface roughness profiles for each group.
3.3. Oxide Surface Chemistry
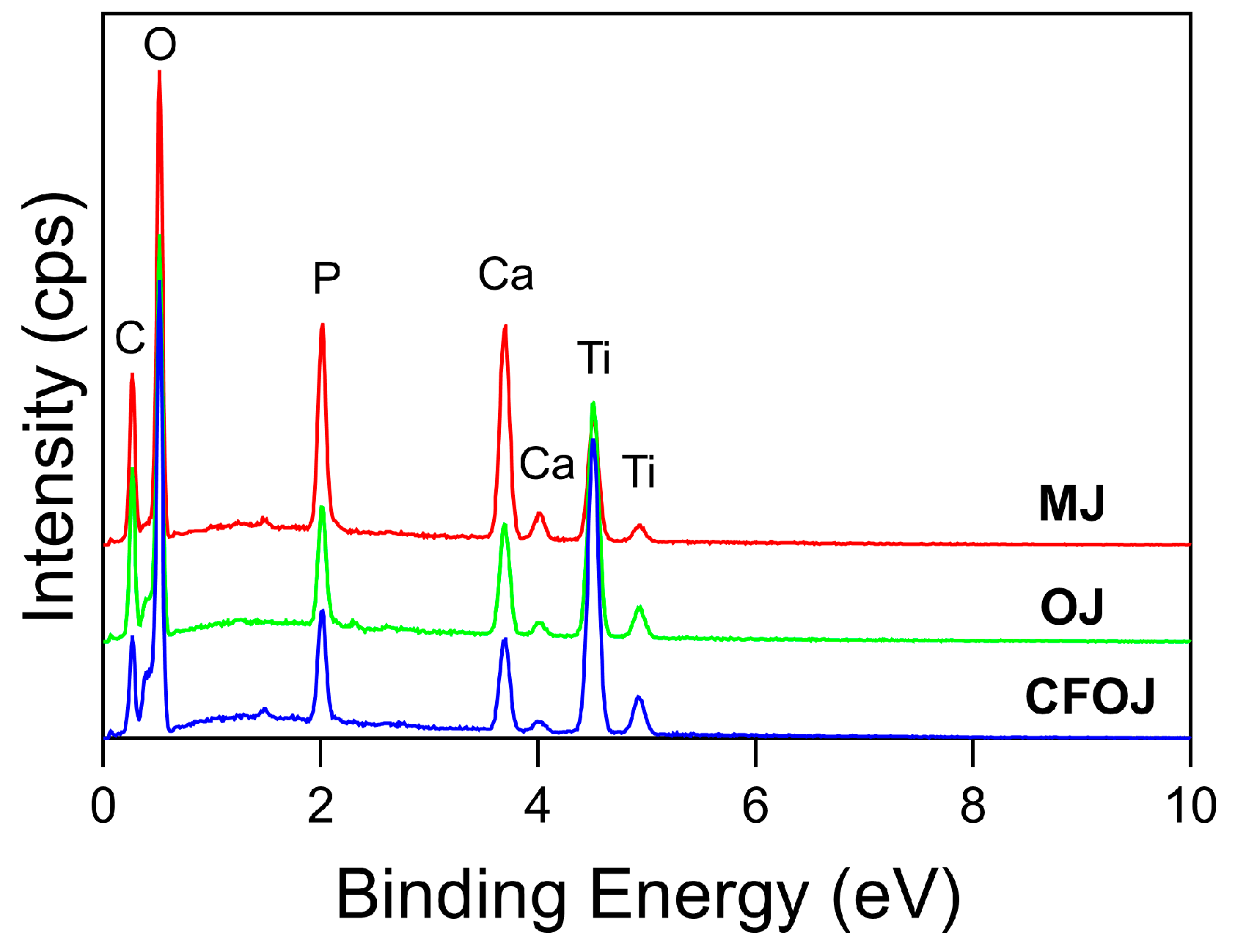
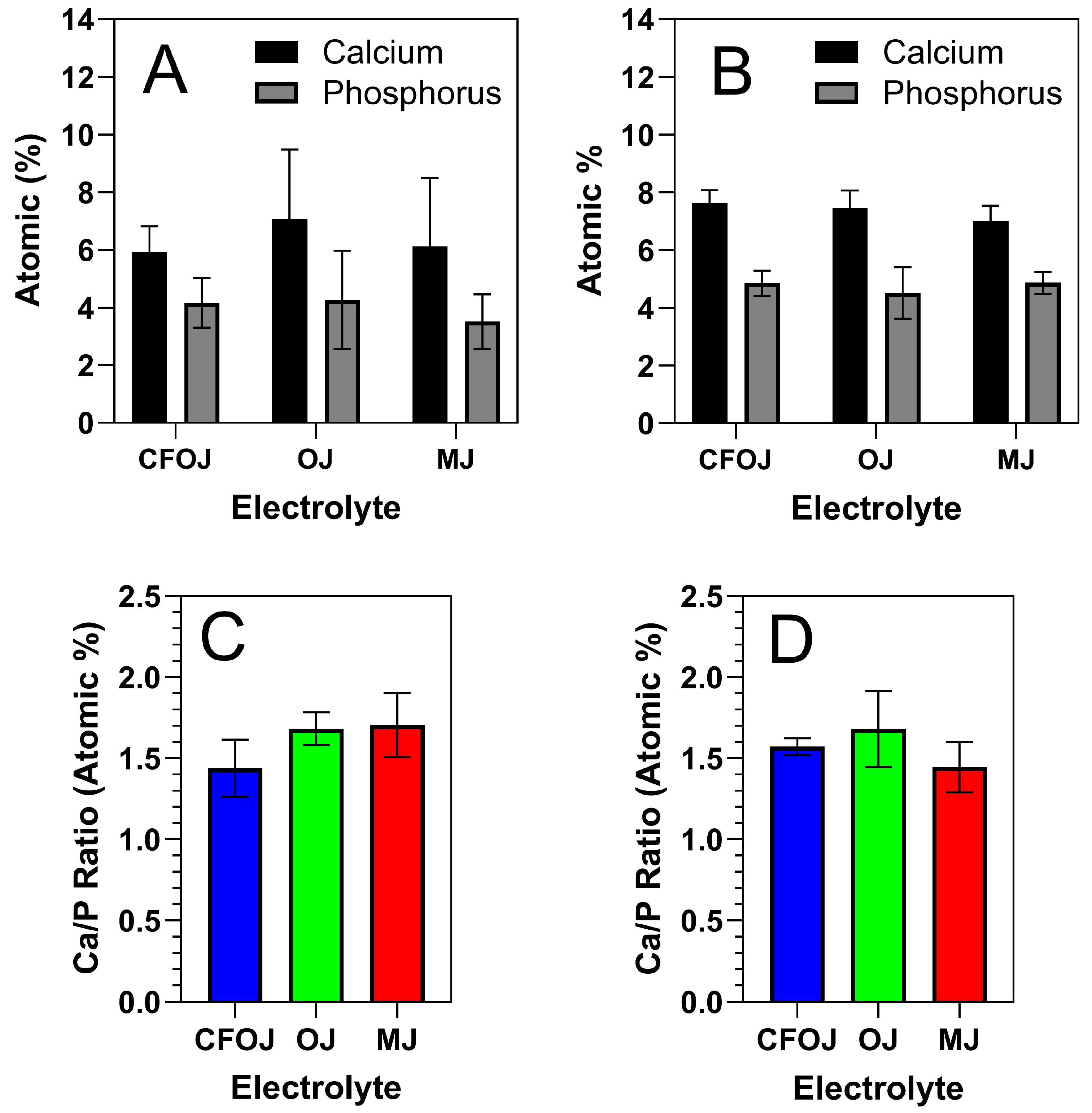
Representative EDS and XPS spectra for each oxide group are shown in Figure 5 and Figure 6. EDS and XPS dopant uptake levels for Ca and P within each oxide group are compiled in Figure 7. EDS spectra revealed significant dopant uptake of Ca and P in each oxide group, as shown in Figure 5 and Figure 7. Approximately 6 at % of Ca and 4 at % of P were incorporated into each oxide group according to the EDS results in Figure 7A. The resulting average EDS Ca/P ratios for CFOJ, OJ, and MJ, as shown in Figure 7C, were 1.44 ± 0.18, 1.69 ± 0.1, and 1.7 ± 0.2, respectively. Thus, the EDS oxide surface Ca/P ratios were all shown to fall within the 1.5 to 1.7 Ca/P ratio range commonly reported for human bone [17,22,23].
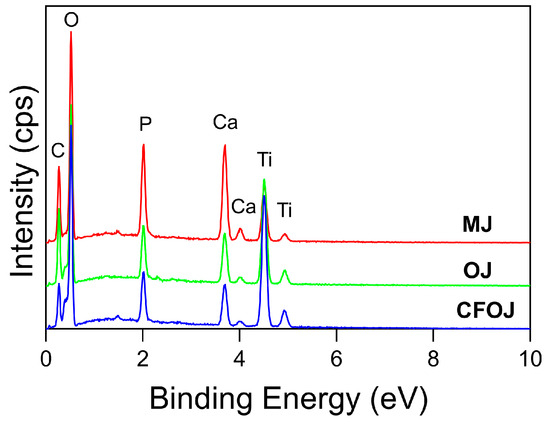
Figure 5.
Representative EDS spectra from each citrus fruit-based oxide group. All three oxide spectra revealed prominent dopant uptake peaks for Ca, and P in addition to the Ti and O peaks.
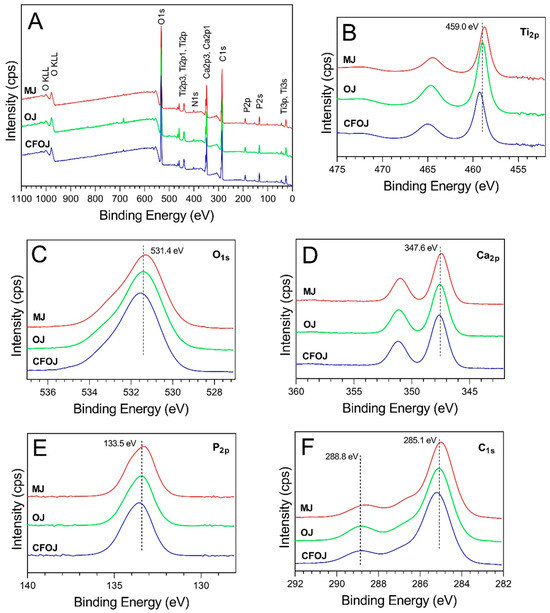
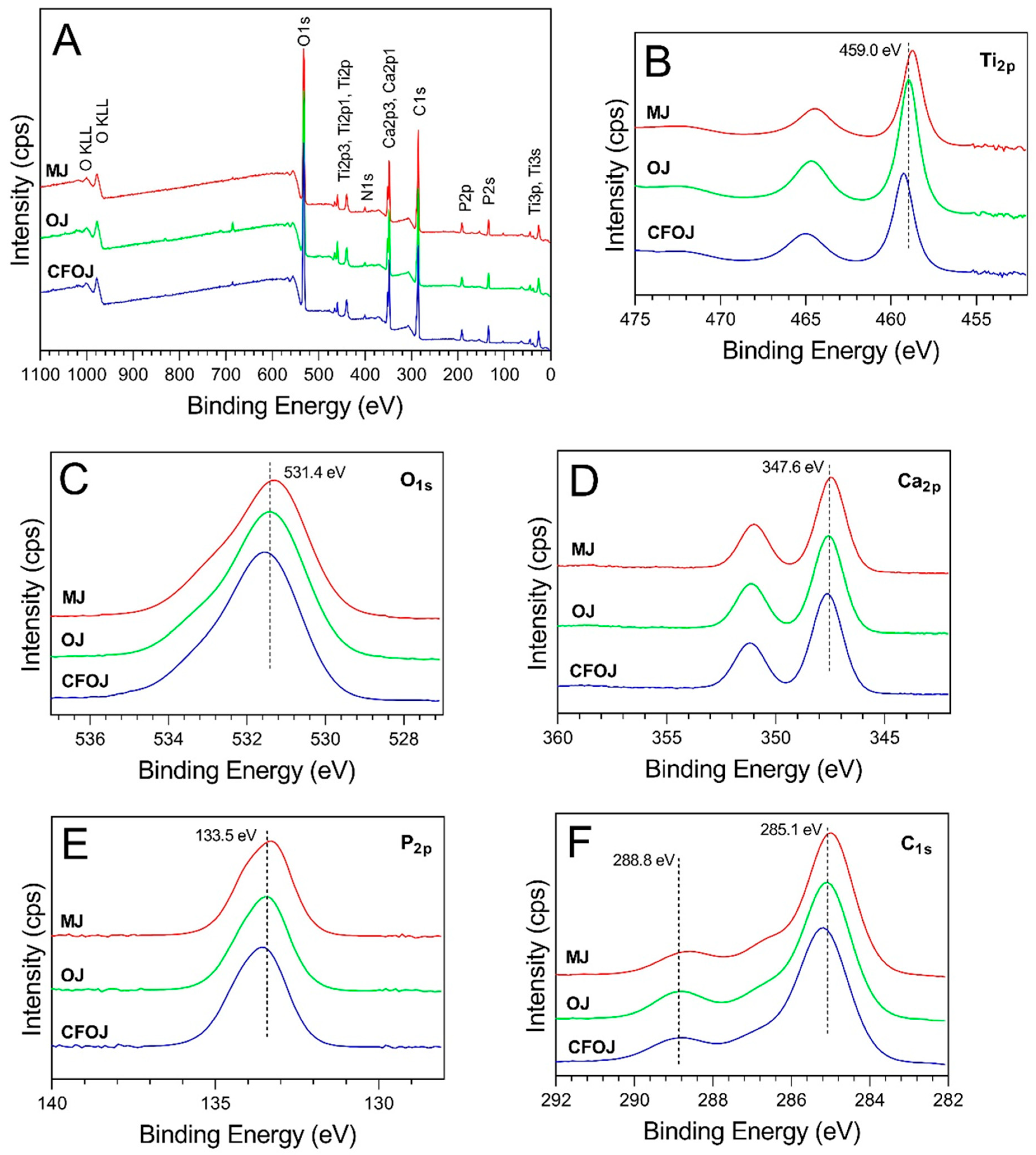
Figure 6.
Representative XPS spectra for each citrus fruit-based oxide group. (A) Survey full spectra, high resolution spectra for (B) Ti2p, (C) O1s, (D) Ca2p, (E) P2p, and (F) C1s.
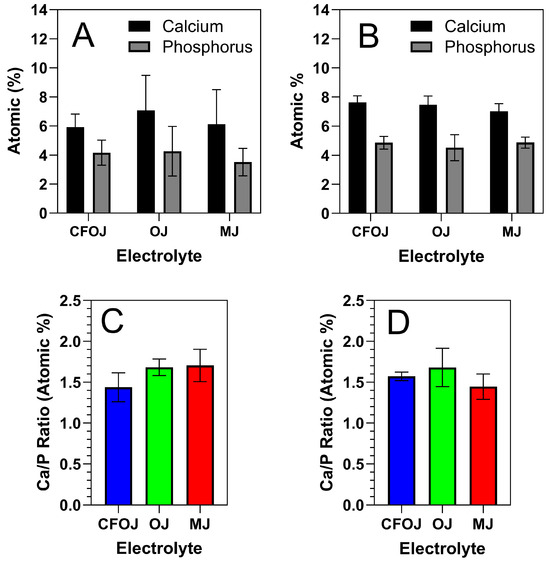
Figure 7.
EDS and XPS Ca and P dopant uptake and Ca/P uptake ratios for each citrus fruit-based oxide group. (A) EDS Ca and P dopant uptake levels, (B) XPS Ca and P dopant uptake levels, (C) EDS Ca/P uptake ratios, and (D) XPS Ca/P uptake ratios. The EDS and XPS oxide surface Ca/P ratios for all oxide groups were shown to fall within the 1.5 to 1.7 range commonly reported for human bone.
XPS survey spectra collected from the outermost surface of the citrus fruit-based oxides exhibited Ti, O, Ca, P, N, and C peaks, as shown in Figure 6A. The high-resolution spectra for the Ti2p peaks are shown in Figure 6B. The formation of TiO2 is represented by the Ti2p3/2 peaks having a binding energy of 459 eV [27,49]. Figure 6C shows the O1s peaks to exhibit binding energies of approximately 531.5 and indicate the formation of calcium phosphate-containing oxides [49,50]. The Ca2p3/2 peaks shown in Figure 6D revealed binding energies of approximately 347.6 eV and indicate the formation of CaTiO3 and calcium phosphates [27,30,49,50,51,52]. The P2p peaks shown in Figure 6E exhibited binding energies of approximately 133.6 eV and represent the formation of phosphorus-containing titanium oxides and phosphates [27,50,51]. Finally, the C1s peaks shown in Figure 6F exhibited binding energies of approximately 285 eV and 289 eV and indicate the presence of C-C bonds and carbonates [27,50]. The 285 eV binding energy peaks for C1s are generally attributed to carbon surface contamination [53,54].
The Ca and P dopant uptake shown in the XPS spectra for the outermost oxide surfaces of each citrus fruit-based oxide group is compiled in Figure 7B. Comparing Figure 7A,B, the XPS Ca and P dopant uptake levels were similar to those shown by the EDS spectra. However, the XPS spectra exhibited slightly higher Ca and P uptake levels which most likely stemmed from the lower interaction volumes produced in the XPS analyses. Nonetheless, the resulting XPS spectra Ca/P ratios from the outermost oxide surfaces were still all shown to fall within the ranges commonly reported for human bone [17,22,23].
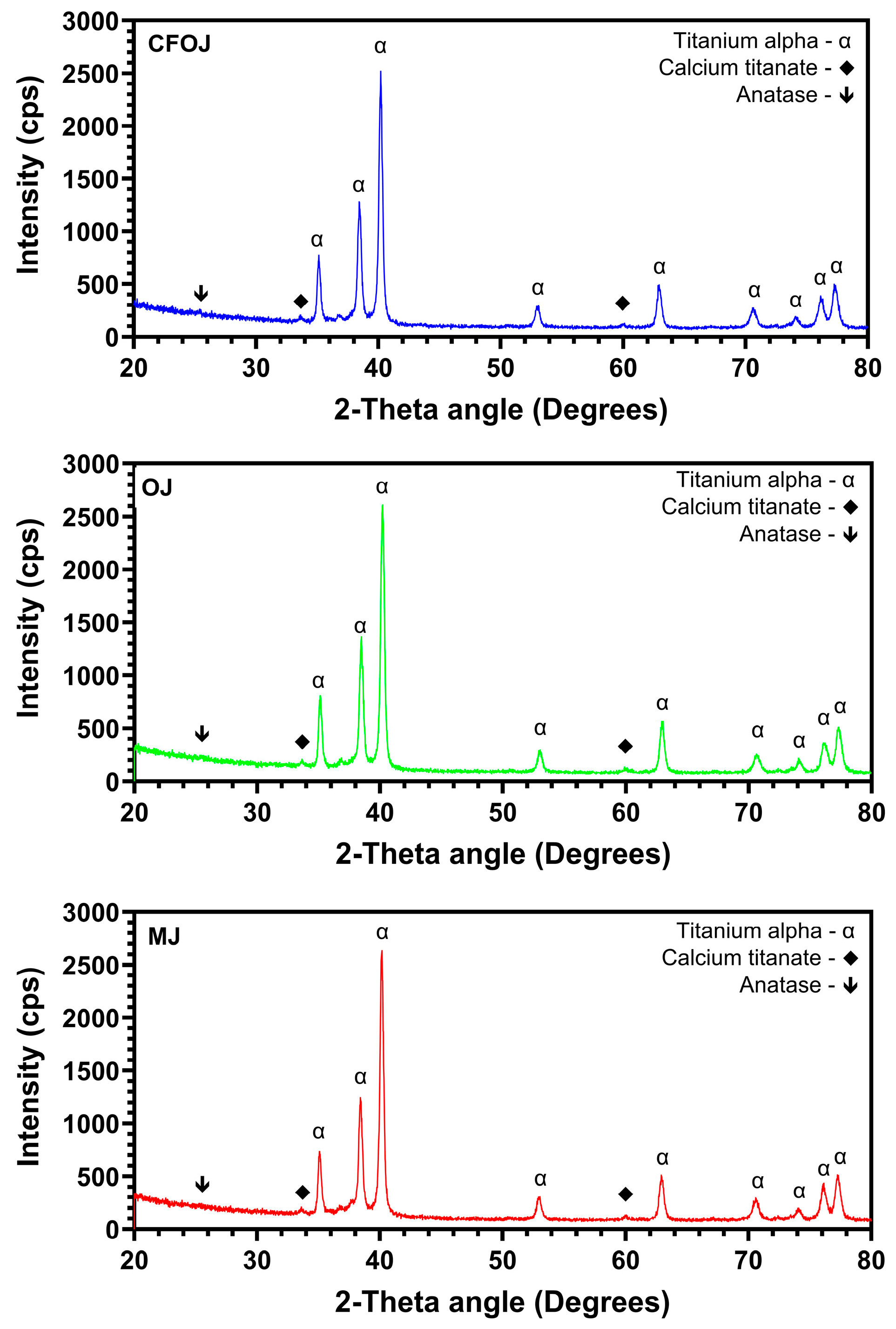
3.4. Oxide Crystallinity
Representative XRD 20° to 80° two-theta scans for each of the citric fruit-based oxides are shown in Figure 8. CaTiO3 was formed in each of the citrus-based oxides, which agrees well with the XPS high-resolution Ca2P spectra results shown in Figure 6D. Additionally, each showed diffraction peaks for anatase phase titanium dioxide the α-phase CPTi substrate material.
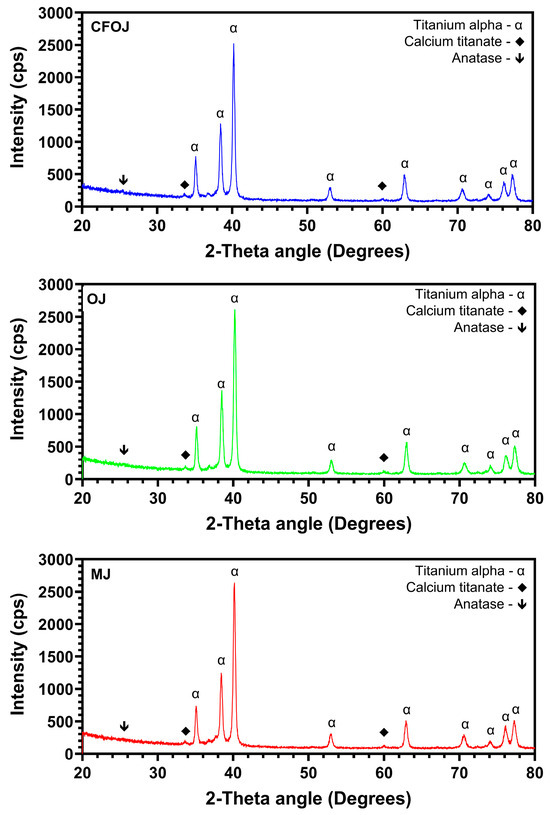
Figure 8.
Representative X-ray diffraction scans of the citric fruit-based oxides. The two-theta scans for each oxide indicated the formation of anatase phase titanium dioxide and calcium titanate.
3.5. Oxide Molecular Structure Analyses
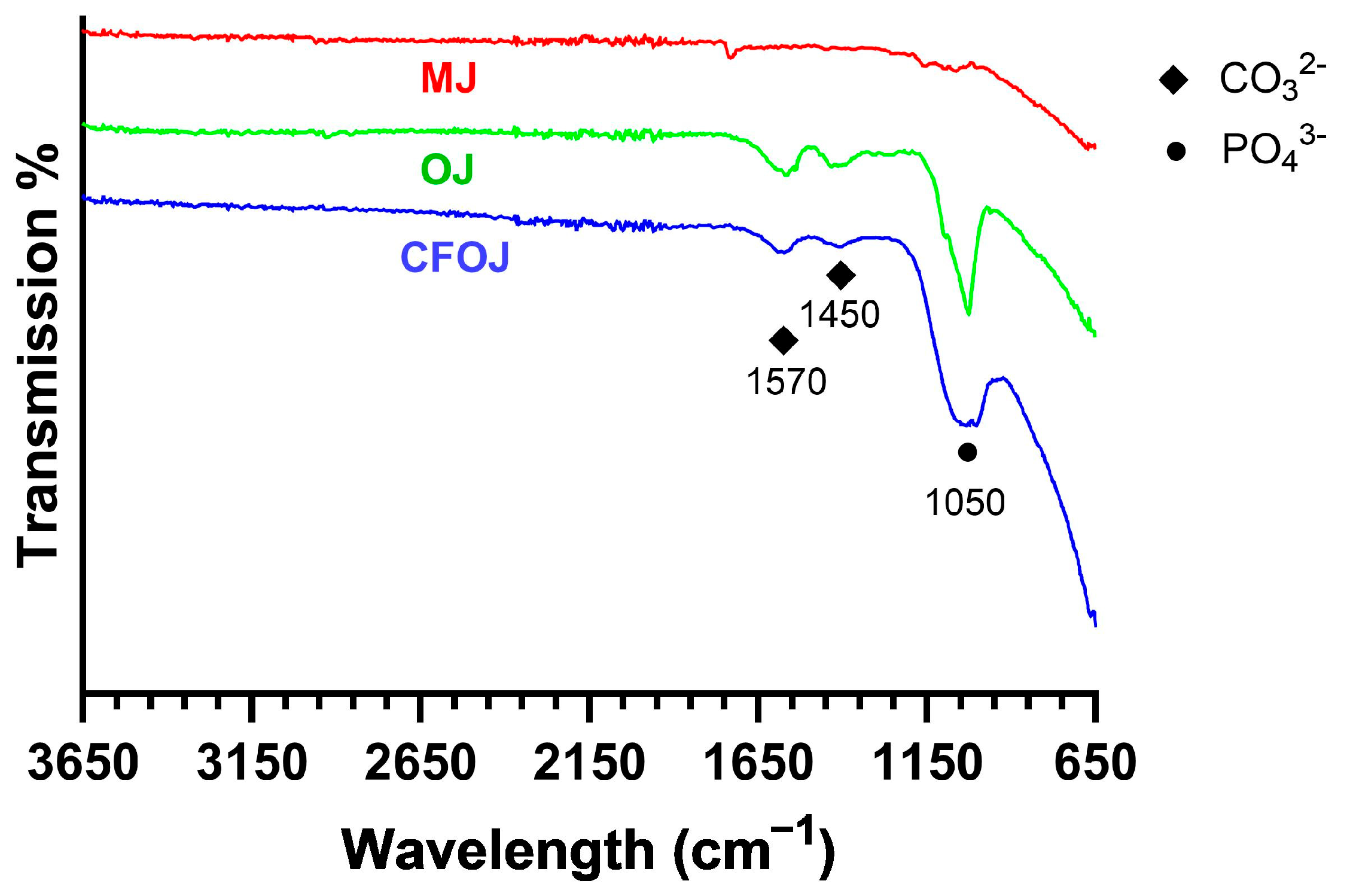
Representative FTIR scans of the citric fruit-based oxides are shown in Figure 9. Oxide MJ showed a poorly defined phosphate (PO43−) peak around 1050 cm−1. Whereas the CFOJ and OJ oxides showed comparatively higher intensity (PO43−) absorption peaks at 1050 cm−1, indicating the presence of calcium phosphate compounds in the respective oxide [24,45]. This result agreed well with the XPS analyses shown in Figure 6D. Additionally, the CFOJ and OJ oxides revealed weak carbonate (CO32−) absorption peaks at 1450 and 1570 cm−1 [24,45,55]. This combination of high-intensity PO43− peaks and weak CO32− absorption peaks at 1450 and 1570 cm−1 and the lack of crystalline phosphate peaks in the XRD spectra in Figure 8, likely indicates the presence of amorphous carbonated phosphates in these oxides [54,55]. The amorphous carbonated phosphates are likely precursors of carbonated tricalcium phosphate and hydroxyapatite formation based on other studies [54,55].
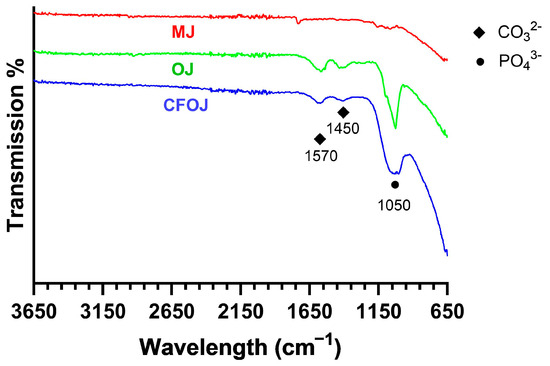
Figure 9.
Representative FTIR spectra for each citric fruit-based oxide. MJ showed a poorly defined PO43− peak around 1050 cm−1. Oxides CFOJ and OJ revealed comparatively high-intensity PO43− peaks and weak CO32− absorption peaks at 1450 and 1570 cm−1.
3.6. Oxide Thickness
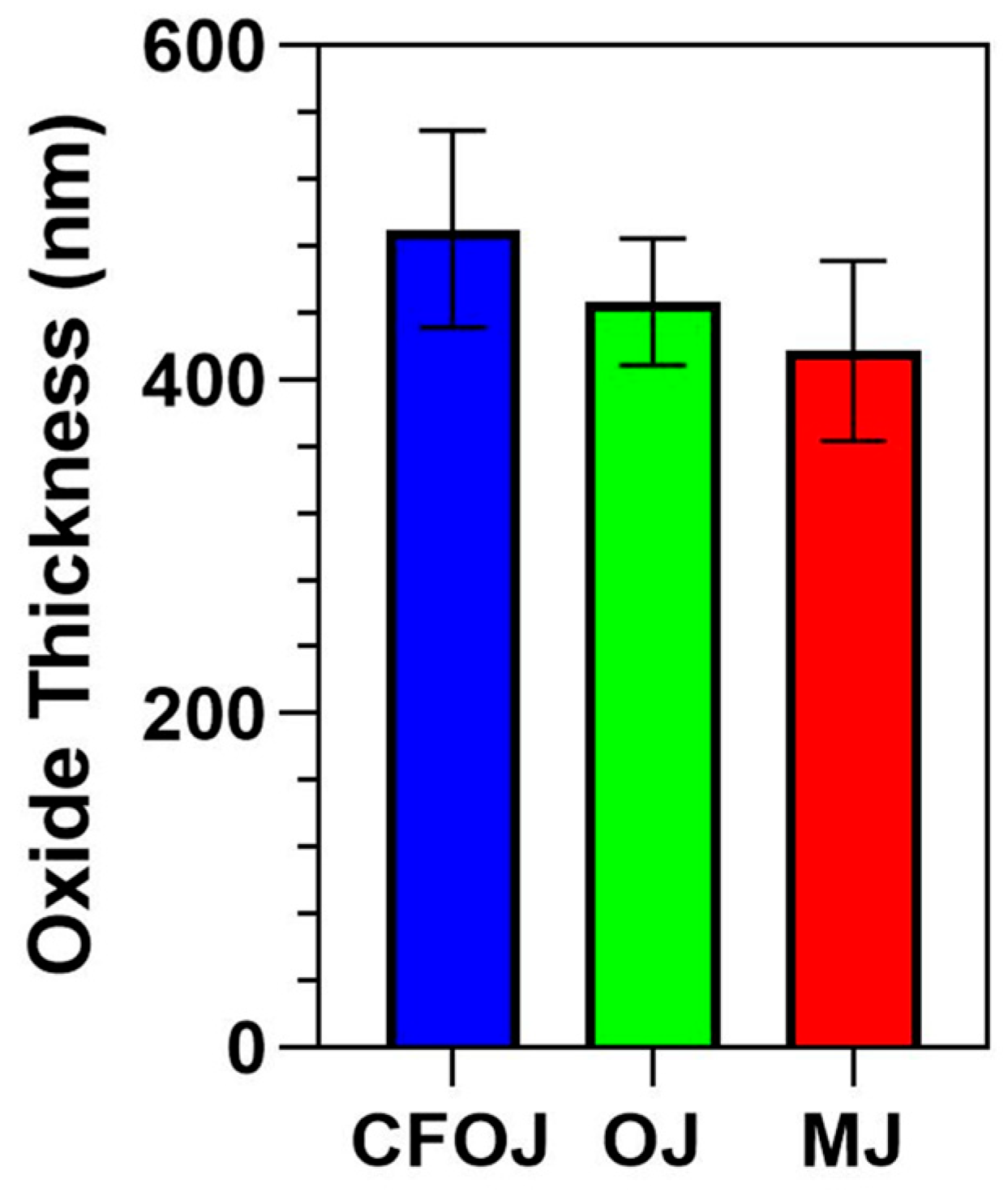
The oxide thickness values from the citrus fruit-based oxide are shown in Figure 10. The average oxide thickness value for each group was less than 500 nm. The CFOJ electrolyte oxide was shown to be significantly thicker than the OJ and MJ electrolyte oxides (p < 0.0001). Specifically, the CFOJ electrolyte oxide revealed thickness values of 490 ± 59 nm, the OJ electrolyte oxide showed thickness values of 447± 38 nm, and the MJ electrolyte oxide revealed oxide thickness values of 417 ± 54 nm.
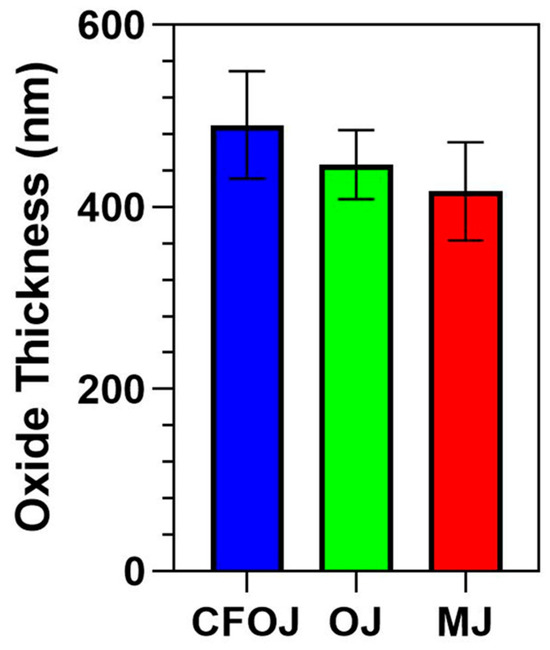
Figure 10.
Cross-sectional thickness values for each citrus fruit-based oxide group. The average oxide thickness value for each of the oxide groups was determined to be less than 500 nm.
The commercially available calcium fortified orange juice CFOJ electrolyte component was determined to be more readily available compared to the juiced orange and mandarin components of their respective electrolytes, since citrus produce availability varies based on location and growing seasons within the calendar year. Because of the increased availability of the CFOJ electrolyte components and the similar SEM, EDS, XPS, and AFM-derived surface characteristics shown for all three citrus-based oxide groups produced in this study, the CFOJ oxides were selected for additional coating and performance testing protocols. The additional testing for the CFOJ oxides included oxide adhesion quality testing, Ca ion release studies, and cell viability assessments.
3.7. Oxide Adhesion Results
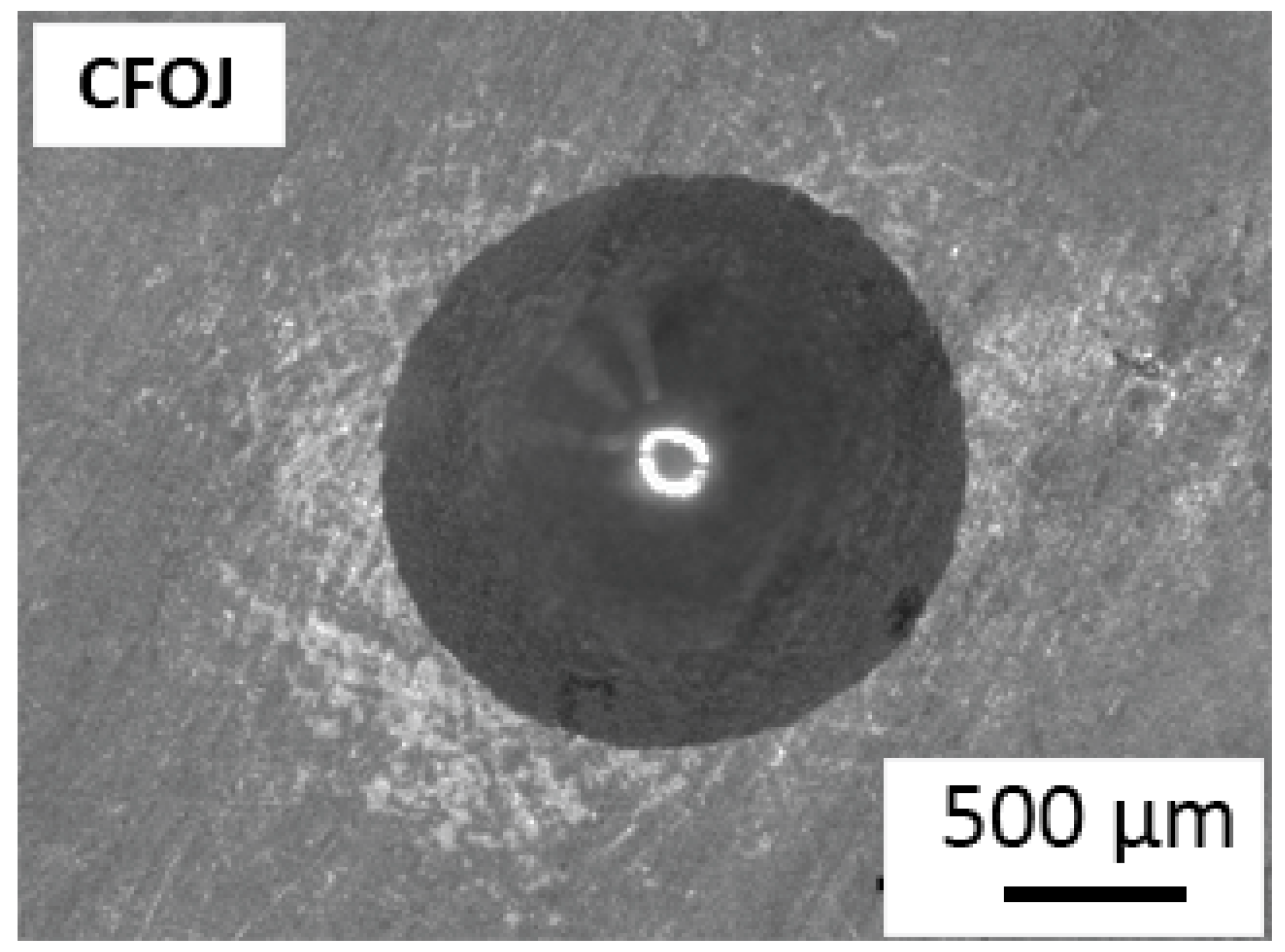
Figure 11 shows the representative VDI 3198 standard oxide layer adhesion results for the CFOJ group. The oxide showed good adhesion to the substrate titanium with some microcracking, but no visible delamination. According to the VDI 3198 qualitative adhesion quality reference charts, this performance represents acceptable oxide adhesion for the CFOJ oxides.
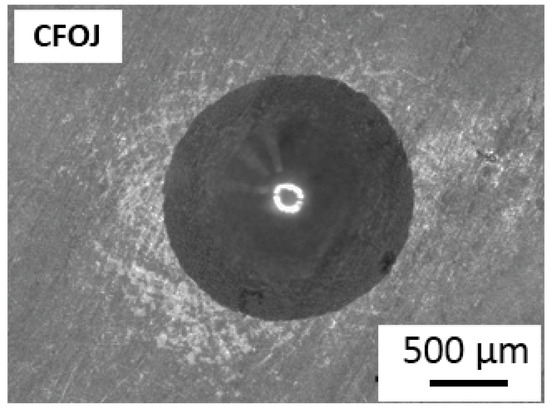
Figure 11.
Oxide layer adhesion quality results for CFOJ oxide showing acceptable adhesion to titanium substrate without some microcracking but no visible delamination.
3.8. Calcium Ion Release Profiles
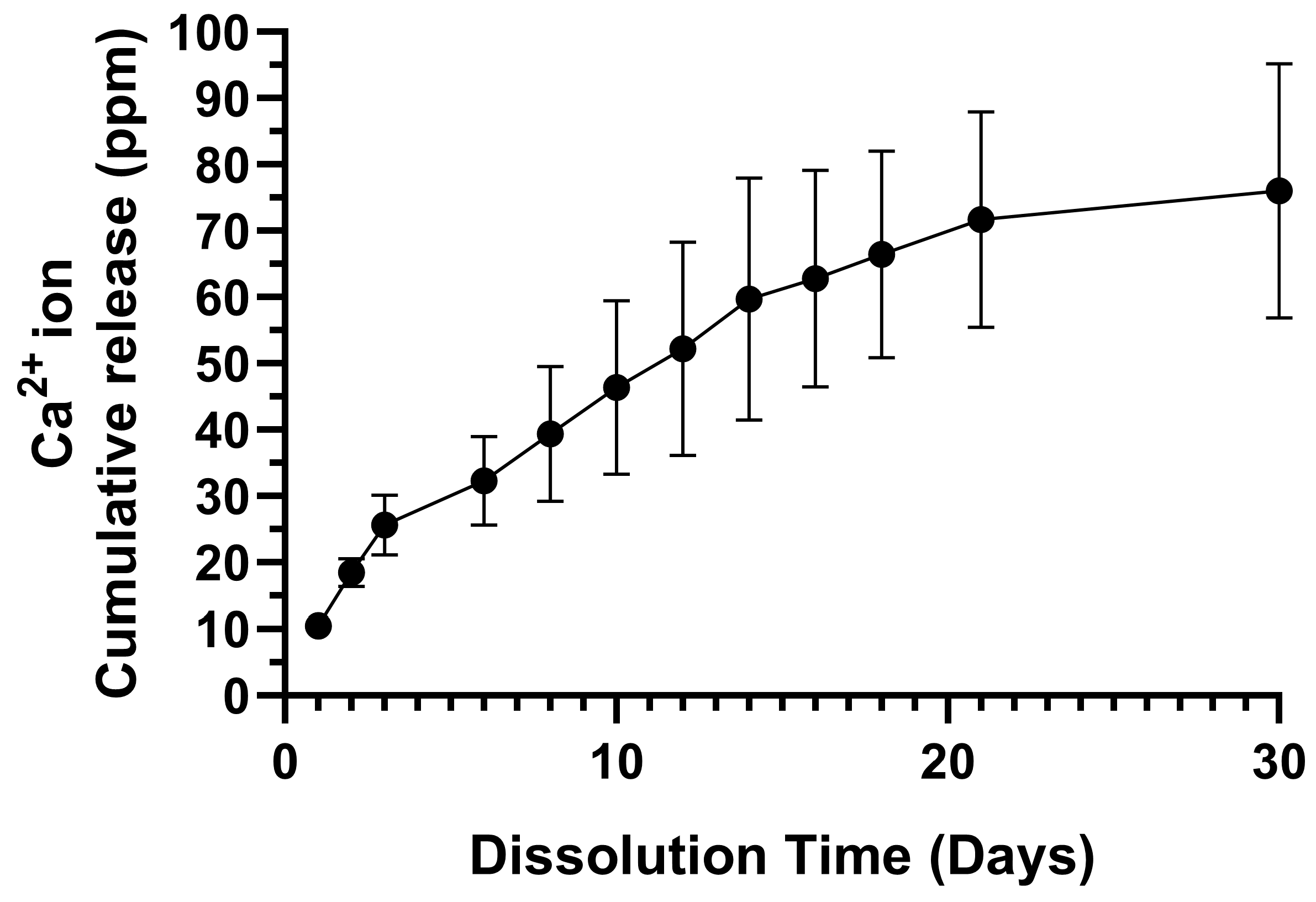
The ICP-OES-derived 30-day Ca2+ ion cumulative release profile from the CFOJ oxide group is provided in Figure 12. The profile revealed an initial burst release followed by a sustained release over a period of 30 days, with the average cumulative amount released from each CFOJ oxide specimen reaching 76 ppm by day 30.
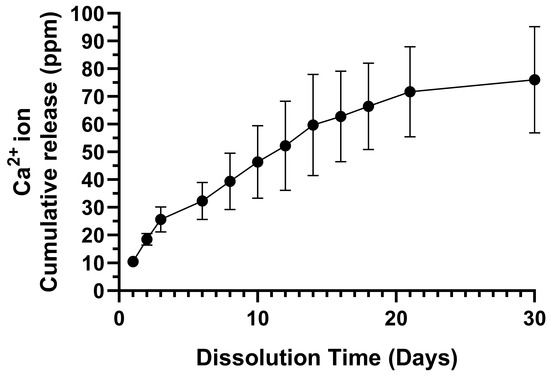
Figure 12.
Ca2+ ion cumulative release profile for the CFOJ oxide group over a 30-day period. An initial burst release was observed, followed by a sustained release reaching an average cumulative amount of 76 ppm by day 30.
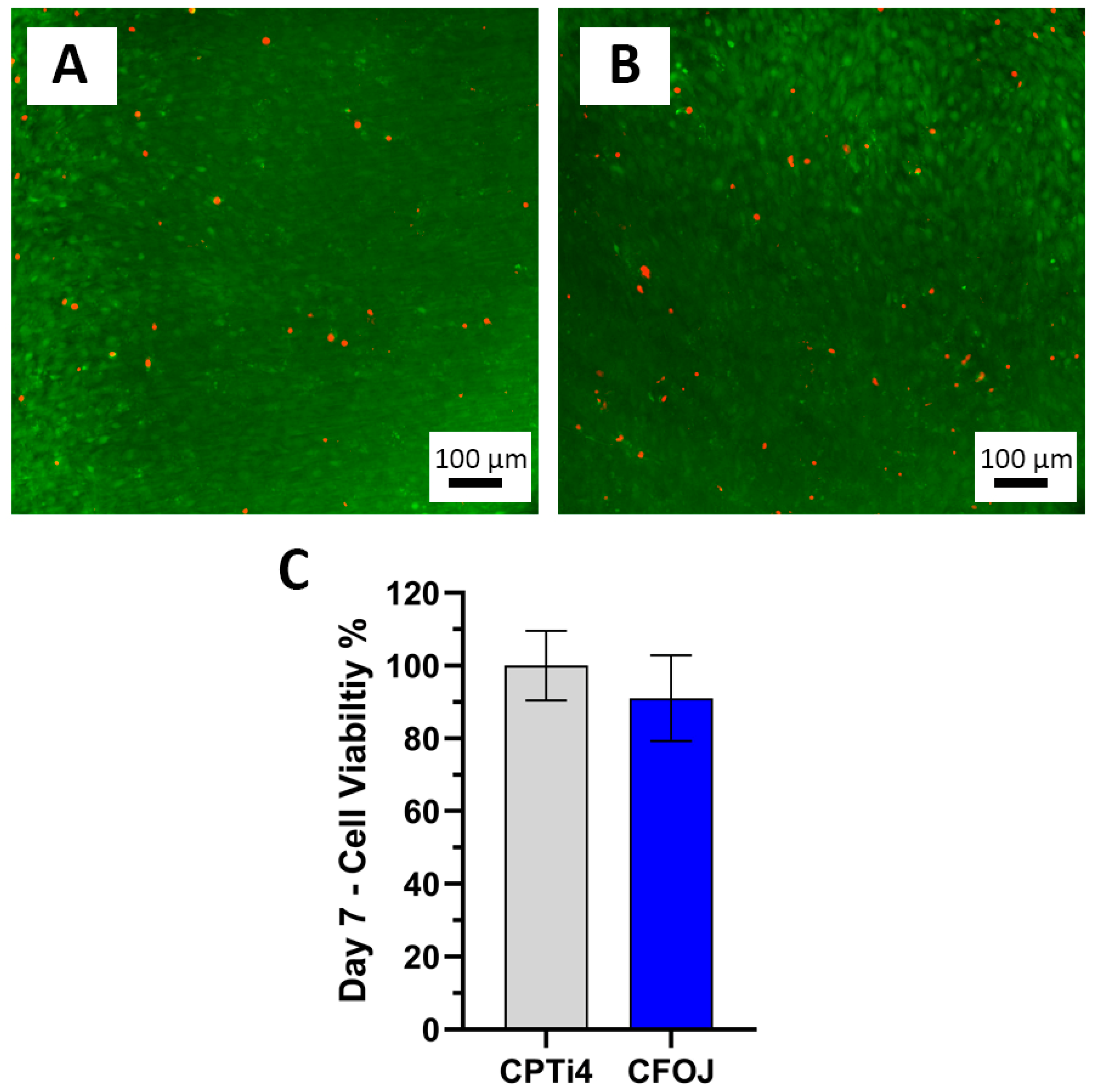
3.9. Cell Viability Testing Results
Figure 13 shows the pre-osteoblast cell viability assessment results for the CFOJ oxide group. Figure 13A,B show the 7-day live/dead assay results which reveal a high quantity of live cells (green) with very few dead cells (red) on the surfaces of the CPTi4 non-anodized materials and the anodized CFOJ oxide group after. Figure 13C reveals the MTT assay results showing that the CFOJ oxide group exhibited statistically similar (p = 0.566) cell viability compared to the non-anodized CPTi controls. A lower (but not statistically lower) cell viability mean of approximately 91% was noted for the CFOJ oxides. However, we have shown a similar phenomenon in some of our previous anodization studies on phosphorus-doped oxides [13,15]. In those previous cell culture studies, the phosphorus containing oxides showed a lower 7-day performance, but caught up and passed the non-anodized control surfaces at later timepoints [13,15].
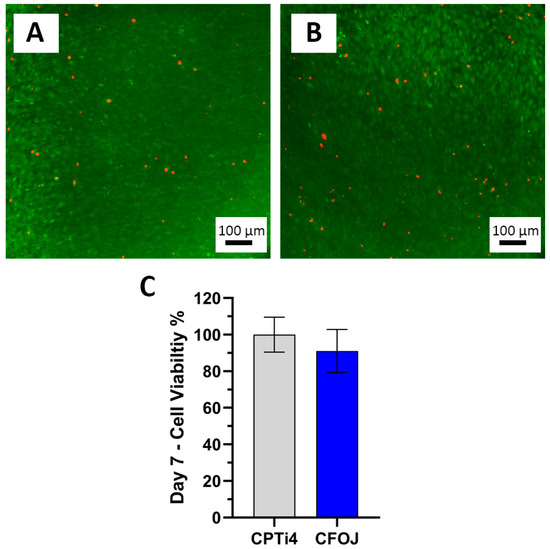
Figure 13.
Cell viability results. Representative day 7 live/dead images showing mostly live (green) cells with very few dead (red) cells on the (A) CPTi4 non-anodized material surface and the (B) CFOJ oxide surface. (C) MTT assay results for non-anodized CPTi4 surfaces and anodized CFOJ surfaces indicating statistically similar (p = 0.566) day 7 cell viability values.
4. Discussion
Titanium anodization processes incorporating Ca and P dopants into CaP oxide layers have been extensively explored previously with the goal of improving osseointegration [16,22,28,40,56]. Most of these CaP oxide studies have continued to use anodization electrolytes similar to the original β-glycerophosphate and calcium acetate-based electrolyte formula that was shown to obtain oxide surface Ca/P ratios within the commonly reported range of human bone [2,22,25]. In the present study, we developed novel anodization electrolytes based on combinations of juiced citrus fruit and commercially available calcium additives, which readily formed oxides that exhibited hierarchical micro- and nano-scale surface topographies and surface Ca/P ratios within the range of human bone. Anodization in citric acid-based electrolytes has been previously explored for other purposes, such as the hard anodization of aluminum alloys and improving the corrosion resistance and oxide crystallinity of titanium alloys [57,58,59,60]. However, to the best of our knowledge, the innovative use of citrus fruit-based electrolytes, containing both citric and ascorbic acid components along with commercially available calcium additives, has never been explored for the purposes of forming Ca- and P-doped titanium oxides on implant surfaces. The electrolyte calcium additives used in this study included calcium acetate and monobasic calcium phosphate additives, which are interestingly also commonly used commercially as food stabilizers and leavening agents in baking products [19,61].
While a number of previous CaP oxide studies have successfully achieved surface Ca/P ratios above 1.0 and even within the range of human bone, the Ca/P ratio alone does not provide sufficient information as to the actual amounts of Ca and P incorporated into the oxide layers. Earlier anodization studies incorporating either Ca or P dopants revealed oxide groups showing higher dopant uptake to exhibit increased alkaline phosphatase activity in cell culture studies and increased removal torques from anodized screws implanted into rabbits [13,30,53]. Nonetheless, relatively few CaP oxide studies have reported the Ca and P dopant uptake percentages along with the surface Ca/P ratios. One study combining EDTA with a pure calcium phosphate powder and calcium acetate in the electrolyte showed that the Ca dopant uptake and the surface Ca/P ratio increased with electrolyte pH [49]. Specifically, increasing the electrolyte pH from 6 to 14 by adding EDTA increased the Ca dopant uptake from 10 to 16% [49]. Another study anodizing in a β-glycerophosphate disodium and calcium acetate electrolyte showed approximately 5% Ca dopant uptake [27]. Our EDS and XPS analyses for oxides formed in the citrus fruit-based electrolytes revealed a similar range of Ca dopant uptake of 6% to 8%. Additionally, a sustained release of substantial amounts of Ca2+ ions was shown for the CFOJ oxide group over a 30-day period in PBS solution. Interestingly, the CFOJ and OJ oxides exhibited PO43− absorption peaks and weak CO32− absorption peaks in FTIR analysis, indicating the presence of carbonates and phosphates. However, the lack of calcium phosphates and or carbonate peaks in the XRD spectra from these oxides (Figure 8) indicates beneficial compounds were likely in an amorphous state. Nonetheless, these amorphous phosphates and carbonates shown in the CFOJ and OJ oxides in the present study are likely precursors of carbonated tricalcium phosphate and hydroxyapatite formation shown in our previous citrus-based anodization studies [62,63].
It is well known that the adhesion strength of anodized titanium oxides typically decreases with increasing oxide cross-sectional thickness [15,30,64]. Many previous Ca- and CaP-doped oxide studies have used galvanostatic waveforms with low current densities of up to 50 mA/cm2, resulting in oxide thickness values ranging from 5 µm to 200 µm [13,22,40]. One recent CaP-oxide study used galvanostatic waveforms with higher current densities ranging from 400 to 1200 mA/cm2 and formed oxides with thickness values between 3 µm and 8 µm [17]. In contrast, our citrus-based CaP oxides formed using galvanostatic waveforms with similar applied current densities of 700 or 1000 mA/cm2 revealed much thinner oxides of approximately 0.5 µm. These comparatively thin citrus-based CaP oxides were still shown to exhibit multiscale micro and nano surface topographies, high levels of Ca and P dopant uptake, surface Ca/P ratios within the range of human bone, and the formation of titanate compounds that have been shown to be important precursors for apatite formation.
Since most CaP oxide studies have used electrolytes similar to the β-glycerophosphate and calcium acetate combinations, the resulting oxides have, almost exclusively, shown micro- and nano-scale porous surface topographies and the formation of a combination of anatase and rutile phase oxide crystallinity [16,22]. However, these oxides have notably shown difficulty forming apatite during SBF-soaking bioactivity testing methodologies such as the ISO 23317 standard methodology [56]. In fact, some of the early CaP oxides required subsequent hydrothermal autoclaving treatments at extreme temperatures of 300 °C for a two-hour duration for apatite formation on the oxide surfaces [16,22].
The surface topography and the chemical composition of an implant surface have both been shown to play a critical role in the resulting biological response [2]. Several research groups have explored alterations in the anodization electrolyte chemistries with the goal of improving the bioactivity of CaP oxides [18,25,65]. Previous Ca- and CaP-doped oxide studies have shown titanate compound formation to be an important precursor for future apatite formation [18,35,40]. In light of this finding, some more recent CaP oxide studies have used post-anodization microwave or UV irradiation treatments in order to facilitate titanate formation [27,66]. Another study used high forming voltage of 350 V and a current density of 70 mA/cm2 in aβ-glycerophosphate, calcium acetate, and sodium hydroxide electrolyte to form oxides with nanoflower surface topographies containing anatase, rutile, and sodium titanate oxide crystallinity [18]. The nanoflower titanate-containing oxide layers were shown to precipitate apatite on the surfaces after only a seven-day soak in SBF bioactivity testing [18]. Calcium titanate containing coatings have also been shown to enhance the osteogenic differentiation of pre-osteoblasts in vitro and significantly improve bone ingrowth, osseointegration, and neurovascular integration, compared to uncoated counterparts in vivo in a rat femoral model study [41]. Interestingly, our novel CaP oxides, produced from anodization in novel fruit-based electrolytes, exhibited unique cauliflower-like nano-scale surface topographies with anatase and calcium titanate crystallinity, as evidenced by SEM, AFM, XPS, and XRD analyses. Subsequent MTT assay and live/dead analyses of the CFOJ citrus-based oxides revealed good cell viability after 7 days of culture. Thus, our citrus fruit-based CaP oxides exhibiting calcium titanate formation show much promise to improve clinical outcomes on future titanium implants.
5. Conclusions
Surface modification using anodization has been extensively explored for the purpose of improving the performance of titanium implants. Anodization processes allow modifications of the oxide surface topography and the incorporation of beneficial dopant elements, such as Ca and P, into the oxide layers with the goal of improving osseointegration. In the present study, we developed novel anodization electrolytes based on juiced citrus fruits and commercially available calcium additions to form CaP oxides. Our novel citrus-based CaP oxides exhibited a hierarchical micro- and nano-scale surface topography with a unique cauliflower-like surface topography on the nano-scale. In addition, the citrus-based CaP oxides revealed significant Ca and P dopant uptake into the oxide layers, leading to a favourable Ca/P ratio in the range of human bone, and showed a sustained release of Ca2+ ions. Structurally, the citrus-based oxides were shown to contain both anatase and calcium titanate phase formation, which are both favourable precursors for crystalline apatite phase formation. Finally, the oxides exhibited sub-micron oxide thickness values and showed good adhesion strengths to titanium substrate materials during VDI indentation testing. Thus, our citrus-based CaP oxides show a strong potential to improve healing and osseointegration around future titanium implants.
6. Patents
This work is a part of patent application PCT/US25/18963.
Author Contributions
Conceptualization, M.D.R., A.P. and A.V.J.; methodology, M.D.R. and A.P.; investigation, A.P. and P.K.; writing—original draft preparation, A.P.; writing—review and editing, A.V.J. and M.D.R.; visualization, A.P. and M.D.R.; supervision, M.D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by UMMC IRSP Grant no. DN00554.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data for this study are also a portion of a patent application.
Acknowledgments
The authors would like to acknowledge Fort Wayne Metals for donating the CPTi used in this study. The authors would also like to acknowledge Felio Perez at the Integrated Microscope Center at the University of Memphis for performing the XPS analyses for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface modification techniques of titanium and titanium alloys for biomedical dental applications: A review. Mater. Today-Proc. 2021, 39, 84–90. [Google Scholar] [CrossRef]
- Alipal, J.; Lee, T.C.; Koshy, P.; Abdullah, H.Z.; Idris, M.I. Evolution of anodised titanium for implant applications. Heliyon 2021, 7, e07408. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.; McFadden, R.; Chan, C.-W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef] [PubMed]
- El Awadly, T.A.; Wu, G.; Ayad, M.; Radi, I.A.W.; Wismeijer, D.; Abo El Fetouh, H.; Osman, R.B. A histomorphometric study on treated and untreated ceramic filled PEEK implants versus titanium implants: Preclinical in vivo study. Clin. Oral Implant. Res. 2020, 31, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.; Zhong, Y.; Wang, L. Multi-Scale Surface Treatments of Titanium Implants for Rapid Osseointegration: A Review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kumar, M.A.; Chowdhary, R. Anodized dental implant surface. Indian J. Dent. Res. 2017, 28, 76–99. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.A.-O.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.A.-O.; Li, C.A.-O. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 6. [Google Scholar] [CrossRef]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef]
- Ding, D.; Xie, Y.; Li, K.; Huang, L.; Zheng, X. Micro/Nano Structural Tantalum Coating for Enhanced Osteogenic Differentiation of Human Bone Marrow Stem Cells. Materials 2018, 11, 546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, R.; Yang, Y.; Liang, C.; Yu, X.; Liu, Y.; Wang, T.; Yu, Y.; Deng, F. Micro/nano-textured hierarchical titanium topography promotes exosome biogenesis and secretion to improve osseointegration. J. Nanobiotechnol. 2021, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Williamson, R.S.; Janorkar, A.V.; Griggs, J.A.; Roach, M.D. Osteoblast response to nanostructured and phosphorus-enhanced titanium anodization surfaces. J. Biomater. Appl. 2019, 34, 419–430. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Y.; Hu, R.; Shi, B.; Zhang, L.; Huang, Q.; Yang, Y.; Tang, P.; Lin, C. Advanced surface engineering of titanium materials for biomedical applications: From static modification to dynamic responsive regulation. Bioact. Mater. 2023, 27, 15–57. [Google Scholar] [CrossRef]
- Nelson, J.; Jain, S.; Pal, P.; Johnson, H.A.; Nobles, K.P.; Janorkar, A.V.; Williamson, R.S.; Roach, M.D. Anodized titanium with calcium and phosphorus surface enhancements for dental and orthopedic implant applications. Thin Solid Film. 2022, 745, 139117. [Google Scholar] [CrossRef]
- Ishizawa, H.; Ogino, M. Characterization of thin hydroxyapatite layers formed on anodic titanium oxide films containing Ca and P by hydrothermal treatment. J. Biomed. Mater. Res. 1995, 29, 1071–1079. [Google Scholar] [CrossRef]
- Laurindo, C.A.; Torres, R.D.; Mali, S.A.; Gilbert, J.L.; Soares, P. Incorporation of Ca and P on anodized titanium surface: Effect of high current density. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 37, 223–231. [Google Scholar] [CrossRef]
- Lee, T.C.; Abdullah, H.Z.; Koshy, P.; Idris, M.I. Deposition of novel bioactive nanoflower-like sodium titanate on TiO coating via anodic oxidation for biomedical applications. Mater. Lett. 2018, 216, 256–260. [Google Scholar] [CrossRef]
- Zhu, X.; Kim, K.H.; Jeong, Y. Anodic oxide films containing Ca and P of titanium biomaterial. Biomaterials 2001, 22, 2199–2206. [Google Scholar] [CrossRef]
- Chang, Y.L.; Stanford, C.M.; Keller, J.C. Calcium and phosphate supplementation promotes bone cell mineralization: Implications for hydroxyapatite (HA)-enhanced bone formation. J. Biomed. Mater. Res. 2000, 52, 270–278. [Google Scholar] [CrossRef]
- Furko, M.; Balázsi, C. Calcium Phosphate Based Bioactive Ceramic Layers on Implant Materials Preparation, Properties, and Biological Performance. Coatings 2020, 10, 823. [Google Scholar] [CrossRef]
- Ishizawa, H.; Ogino, M. Formation and characterization of anodic titanium oxide films containing Ca and P. J. Biomed. Mater. Res. 1995, 29, 65–72. [Google Scholar] [CrossRef]
- Wu, S.L.; Weng, Z.Y.; Liu, X.M.; Yeung, K.W.K.; Chu, P.K. Functionalized TiO Based Nanomaterials for Biomedical Applications. Adv. Funct. Mater. 2014, 24, 5464–5481. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy-Materials Science, Engineering and Technology; Theophile, T., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 6. [Google Scholar] [CrossRef]
- Alipal, J.; Lee, T.C.; Koshy, P.; Abdullah, H.Z.; Idris, M.I. Influence of altered Ca-P based electrolytes on the anodised titanium bioactivity. Surf. Coat. Technol. 2021, 412, 127041. [Google Scholar] [CrossRef]
- Li, L.H.; Kong, Y.M.; Kim, H.W.; Kim, Y.W.; Kim, H.E.; Heo, S.J.; Koak, J.Y. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials 2004, 25, 2867–2875. [Google Scholar] [CrossRef]
- Lin, D.J.; Fuh, L.J.; Chen, W.C. Nano-morphology, crystallinity and surface potential of anatase on micro-arc oxidized titanium affect its protein adsorption, cell proliferation and cell differentiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110204. [Google Scholar] [CrossRef]
- Kim, K.; Lee, B.A.; Piao, X.H.; Chung, H.J.; Kim, Y.J. Surface characteristics and bioactivity of an anodized titanium surface. J. Periodontal Implant. Sci. 2013, 43, 198–205. [Google Scholar] [CrossRef]
- Park, T.E.; Choe, H.C.; Brantley, W.A. Bioactivity evaluation of porous TiO2 surface formed on titanium in mixed electrolyte by spark anodization. Surf. Coat. Technol. 2013, 235, 706–713. [Google Scholar] [CrossRef]
- Sul, Y.T. The significance of the surface properties of oxidized titanium to the bone response: Special emphasis on potential biochemical bonding of oxidized titanium implant. Biomaterials 2003, 24, 3893–3907. [Google Scholar] [CrossRef]
- Yang, B.; Uchida, M.; Kim, H.M.; Zhang, X.; Kokubo, T. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 2004, 25, 1003–1010. [Google Scholar] [CrossRef]
- Svetina, M.; Ciacchi, L.C.; Sbaizero, O.; Meriani, S.; De Vita, A. Deposition of calcium ions on rutile (110): A first-principles investigation. Acta Mater. 2001, 49, 2169–2177. [Google Scholar] [CrossRef]
- Xia, W.; Lindahl, C.; Lausmaa, J.; Engqvist, H. Biomimetic Hydroxyapatite Deposition on Titanium Oxide Surfaces for Biomedical Application. In Advances in Biomimetics; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Roach, M.; Williamson, R.S.; Blakely, I.P.; Didier, L.M. Tuning anatase and rutile phase ratios and nanoscale surface features by anodization processing onto titanium substrate surfaces. Mater. Sci. Eng. C 2015, 58, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xu, K.W.; Han, Y. Preparation and apatite layer formation of plasma electrolytic oxidation film on titanium for biomedical application. Mater. Lett. 2005, 59, 185–189. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Li, Y.Y.; Ren, Z.H.; Ahmad, Z.S.; Li, X.; Han, G.R. Synthesis of porous CaTiO nanotubes with tunable hollow structures via single-nozzle electrospinning. Mater. Lett. 2015, 152, 82–85. [Google Scholar] [CrossRef]
- Krupa, D.; Baszkiewicz, J.; Kozubowski, J.A.; Barcz, A.; Sobczak, J.W.; Biliniski, A.; Lewandowska-Szumiel, M.D.; Rajchel, B. Effect of calcium-ion implantation on the corrosion resistance and biocompatibility of titanium. Biomaterials 2001, 22, 2139–2151. [Google Scholar] [CrossRef]
- Ohtsu, N.; Sato, K.; Saito, K.; Asami, K.; Hanawa, T. Calcium phosphates formation on CaTiO3 coated titanium. J. Mater. Sci. Mater. Med. 2007, 18, 1009–1016. [Google Scholar] [CrossRef]
- Wadge, M.D.; McGuire, J.; Thomas, K.G.; Stuart, B.W.; Felfel, R.M.; Ahmed, I.; Grant, D.M. Developing alkaline titanate surfaces for medical applications. Int. Mater. Rev. 2023, 68, 677–724. [Google Scholar] [CrossRef]
- Song, W.H.; Ryu, H.S.; Hong, S.H. Apatite induction on Ca-containing titania formed by micro-arc oxidation. J. Am. Ceram. Soc. 2005, 88, 2642–2644. [Google Scholar] [CrossRef]
- Yu, D.; Tang, Z.; Bao, S.; Guo, S.; Chen, C.; Wu, Q.; Wang, M.; Zheng, Z.; Cao, P.; Xu, B.; et al. Immunoregulatory Neuro-Vascularized Osseointegration Driven by Different Nano-Morphological CaTiO3 Bioactive Coatings on Porous Titanium Alloy Scaffolds. Adv. Healthc. Mater. 2025, 14, e2404647. [Google Scholar] [CrossRef]
- Oss Giacomelli, R.; Mattos, J.; Soprano, P.; Salvaro, D.; Binder, C.; Klein, A.; Biasoli de Mello, J.D. Assessment of multifunctional coating adhesion: Comparison between indentation and scratch tests. In Proceedings of the 2nd International Brazilian Conference on Tribology, Foz do Iguaçu, Brazil, 3–5 November 2014; pp. 4059–4068. [Google Scholar] [CrossRef]
- Aziz, I.; Mulyani, E.; Yusuf, Y. Morphological, mechanical and antibacterial properties of Ti-Cu-N thin films deposited by sputtering DC. Heliyon 2023, 9, e17170. [Google Scholar] [CrossRef]
- Gorman, D.; Green, C.; Booth-Downs, F.; Gee, M.; Fry, T. Rockwell Indentation Test for Evaluation of Adhesion of Coatings-Aka Daimler-Benz Adhesion Test; NPL Report. MAT 107; NPL Management Limited: Teddington, UK, 2022. [Google Scholar]
- Alipal, J.; Saidin, S.; Lo, A.Z.K.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T. surface efficacy of CaP-based anodised titanium for bone implants. Surf. Interfaces 2023, 39, 102872. [Google Scholar] [CrossRef]
- Drobný, P.; Mercier, D.; Koula, V.; Škrobáková, S.I.; Čaplovič, Ľ.; Sahul, M. Evaluation of Adhesion Properties of Hard Coatings by Means of Indentation and Acoustic Emission. Coatings 2021, 11, 919. [Google Scholar] [CrossRef]
- Ali, A.; Chowdhury, S.; Janorkar, A.; Marquart, M.; Griggs, J.A.; Bumgardner, J.; Roach, M.D. A novel single-step anodization approach for PANI-doping oxide surfaces to improve the photocatalytic activity of titanium implants. Biomed. Mater. 2022, 18, 015010. [Google Scholar] [CrossRef] [PubMed]
- Bruni, C.L.; Johnson, H.A.; Ali, A.; Parekh, A.; Marquart, M.E.; Janorkar, A.V.; Roach, M.D. Phosphorus-and-Silver-Doped Crystalline Oxide Coatings for Titanium Implant Surfaces. Oxygen 2024, 4, 402–420. [Google Scholar] [CrossRef]
- Frauchiger, V.M.; Schlottig, F.; Gasser, B.; Textor, M. Anodic plasma-chemical treatment of CP titanium surfaces for biomedical applications. Biomaterials 2004, 25, 593–606. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, J.; Scheideler, L.; Reichl, R.; Geis-Gerstorfer, J. Effects of topography and composition of titanium surface oxides on osteoblast responses. Biomaterials 2004, 25, 4087–4103. [Google Scholar] [CrossRef]
- Durdu, S.; Deniz, Ö.F.; Kutbay, I.; Usta, M. Characterization and formation of hydroxyapatite on Ti6Al4V coated by plasma electrolytic oxidation. J. Alloys Compd. 2013, 551, 422–429. [Google Scholar] [CrossRef]
- Ohtsu, N.; Ito, A.; Saito, K.; Hanawa, T. Characterization of calcium titanate thin films deposited on titanium with reactive sputtering and pulsed laser depositions. Surf. Coat. Technol. 2007, 201, 7686–7691. [Google Scholar] [CrossRef]
- Sul, Y.T.; Johansson, C.B.; Petronis, S.; Krozer, A.; Jeong, Y.; Wennerberg, A.; Albrektsson, T. Characteristics of the surface oxides on turned and electrochemically oxidized pure titanium implants up to dielectric breakdown: The oxide thickness, micropore configurations, surface roughness, crystal structure and chemical composition. Biomaterials 2002, 23, 491–501. [Google Scholar] [CrossRef]
- Arce, J.E.; Arce, A.E.; Aguilar, Y.; Yate, L.; Moya, S.; Rincón, C.; Gutiérrez, O. Calcium phosphate-calcium titanate composite coatings for orthopedic applications. Ceram. Int. 2016, 42, 10322–10331. [Google Scholar] [CrossRef]
- Liu, S.M.; Yang, X.J.; Cui, Z.D.; Zhu, S.L.; Wei, Q.A. One-step synthesis of petal-like apatite/titania composite coating on a titanium by micro-arc oxidation. Mater. Lett. 2011, 65, 1041–1044. [Google Scholar] [CrossRef]
- ISO 23317:2014; Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials. International Standard Organization: Geneva, Switzerland, 2014.
- Cabral-Miramontes, J.; Almeraya-Calderón, F.; López, F.E.; Banda, M.L.; Olguin-Coca, J.; López-León, L.D.; Castañeda-Robles, I.; Alcalá, M.A.E.; Zambrano-Robledo, P.; Gaona-Tiburcio, C. Citric Acid as an Alternative to Sulfuric Acid for the Hard Anodizing of AA6061. Metals 2021, 11, 1838. [Google Scholar] [CrossRef]
- Munirathinam, B.; Neelakantan, L. Titania nanotubes from weak organic acid electrolyte: Fabrication, characterization and oxide film properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Tao, B.Q.; Fang, C.Q.; Shao, H.; Xie, L.; Feng, J.Q.; Yan, G. Anodization of titanium in reduced graphene oxide citric acid electrolyte. Results Phys. 2021, 24, 104060. [Google Scholar] [CrossRef]
- Kieser, T.A.; Kunst, S.R.; Morisso, F.D.P.; Machado, T.C.; Oliveira, C.T. Anodização de titânio em ácido cítrico. Res. Soc. Dev. 2022, 11, e25311830872. [Google Scholar] [CrossRef]
- Miller, R. Leavening Agents. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 523–528. [Google Scholar] [CrossRef]
- Parekh, A.; Tahincioglu, A.; Walters, C.; Chisolm, C.; Williamson, S.; Janorkar, A.V.; Roach, M.D. Citrus-Fruit-Based Hydroxyapatite Anodization Coatings on Titanium Implants. Materials 2025, 18, 1163. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.; Moore, M.; Janorkar, A.V.; Roach, M.D. Mg-Doped Carbonated Hydroxyapatite and Tricalcium Phosphate Anodized Coatings on Titanium Implant Alloys. Appl. Sci. 2024, 14, 11831. [Google Scholar] [CrossRef]
- Jain, S.; Williamson, R.S.; Roach, M.D. Surface characterization, shear strength, and bioactivity of anodized titanium prepared in mixed-acid electrolytes. Surf. Coat. Technol. 2017, 325, 594–603. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, B.E.; Zhou, L.X.; Ma, J.W.; Zhang, X.L.; Li, H.P.; Liang, C.Y.; Liu, S.M.; Wang, H.S. Influence of surface structures on biocompatibility of TiO2/HA coatings prepared by MAO. Mater. Chem. Phys. 2018, 215, 339–345. [Google Scholar] [CrossRef]
- Lee, T.C.; Abdullah, H.Z.; Koshy, P.; Idris, M.I. Ultraviolet-assisted biomimetic coating of bone-like apatite on anodised titanium for biomedical applications. Thin Solid Film. 2018, 660, 191–198. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).