Soybean Reproductive Traits Evaluated in Response to Temperature Stress and Elevated Oxygen; Three Peroxidase Transgenes Reduce Seed Abortion
Abstract
1. Introduction
2. Materials and Methods
2.1. Soybean Plant Materials
2.2. Transgenic Constructs
2.3. Statistical Analyses
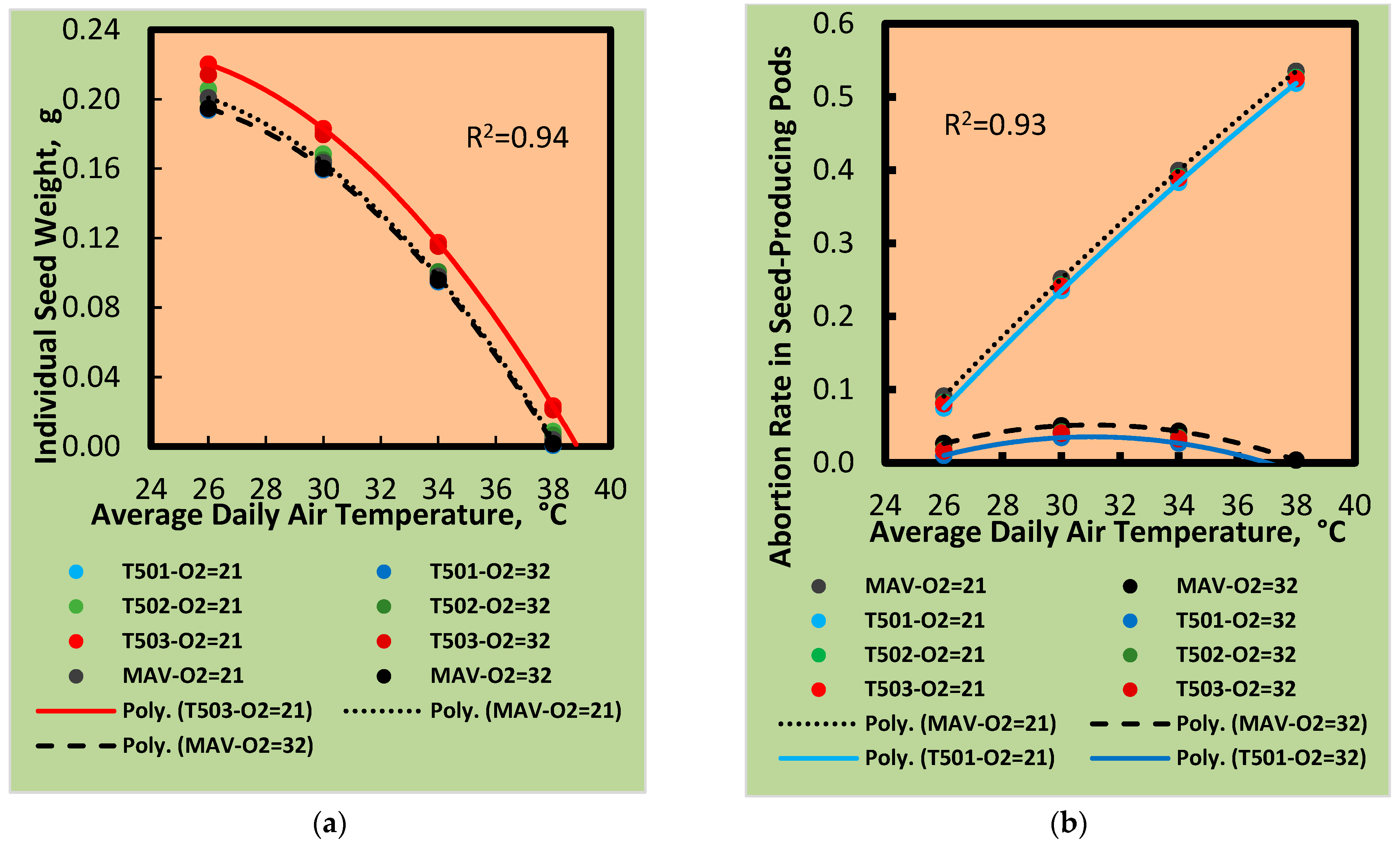
3. Results and Discussion
3.1. Soybean Growth and Seed Oxygen Concentration
3.2. Seed Abortion
3.3. Plant Fertility in Response to Temperature and Oxygen

| Dependent Variable | PODNUM | SEEDNUM | SEEDABORT | PODABORT |
|---|---|---|---|---|
| Mean | 124. | 245. | 39.1 | 3.68 |
| R2 | 0.63 | 0.69 | 0.80 | 0.58 |
| Probability column | Pr > |t| | Pr > |t| | Pr > |t| | Pr > |t| |
| Intercept | <0.0001 | <0.0001 | <0.0001 | 00.328 |
| O2TR 21 | 0.459 | 0.044 | 0.001 | <0.0001 |
| O2TR 32 | ||||
| TTRT | <0.0001 | <0.0001 | <0.0001 | 0.312 |
| TTR × TTR | <0.0001 | <0.0001 | <0.0001 | 0.351 |
| TTR × TTR × O2TR 21 | 0.238 | 0.865 | <0.0001 | <0.0001 |
| TTR × TTR × O2TR 32 | ||||
| PER17-OE(T501) | 0.580 | 0.802 | 0.027 | 0.264 |
| PER28-OE(T502) | 0.012 | 0.045 | 0.035 | 0.148 |
| PER29-OE(T503) | 0.002 | 0.007 | 0.003 | 0.007 |
| MAVERICK | ||||
| Tukey Mean for O2TR 21 | 150. | 297 | 68.4 | 4.73 |
| Tukey Mean for O2TR 32 | 98.6 | 195 | 10.7 | 2.65 |
| O2TRT32/O2TRT21 | 0.66 | 0.66 | 0.16 | 0.56 |

3.4. Seed Responses to Temperature and Oxygen



3.5. Heterologous Expression of PER17 in Soybean (Blue Lines)
3.6. Heterologous Expression of PER28 in Soybean (Green Lines in the Figures)
3.7. Heterologous Expression of PER29 in Soybean (Red Lines)
3.8. Growth and Yield Responses to Temperature and O2
3.9. PER Function During Seed Formation
3.10. Exposure of Seed Pods Only Versus Whole Plants
3.11. Supplemental O2 Decreased Yields
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, L.H.; Pan, D.; Boote, K.J.; Pickering, N.B.; Jones, J.W. Carbon Dioxide and Temperature Effects on Evapotranspiration and Water Use Efficiency of Soybean. Agron. J. 2003, 95, 1071–1081. [Google Scholar] [CrossRef]
- Allen, L.H.; Boote, K.J.; Jones, J.W.; Jones, P.H.; Pickering, N.B.; Baker, J.T.; Vu, J.C.V.; Gesch, R.W.; Thomas, J.M.G.; Prasad, P.V.V. Sunlit, Controlled-environment Chambers Are Essential for Comparing Plant Responses to Various Climates. Agron. J. 2020, 112, 4531–4549. [Google Scholar] [CrossRef]
- Boote, K.J.; Allen, L.H.; Prasad, P.V.V.; Baker, J.T.; Gesch, R.W.; Snyder, A.M.; Pan, D.; Thomas, J.M.G. Elevated Temperature and CO2 Impacts on Pollination, Reproductive Growth, and Yield of Several Globally Important Crops. J. Agric. Meteorol. 2005, 60, 469–474. [Google Scholar] [CrossRef]
- Thomas, J.M.G.; Boote, K.J.; Allen, L.H.; Gallo-Meagher, M.; Davis, J.C. Elevated Temperature and Carbon Dioxide Effects on Soybean Seed Composition and Transcript Abundance. Crop Sci. 2003, 43, 1548–1557. [Google Scholar] [CrossRef]
- Fleisher, D.H.; Timlin, D.; Reddy, K.R.; Reddy, V.R.; Yang, Y.; Kim, S.-H. Effects of CO2 and Temperature on Crops: Lessons from SPAR Growth Chambers. In ICP Series on Climate Change Impacts, Adaptation, and Mitigation; Imperial College Press: London, UK, 2010. [Google Scholar]
- Tacarindua, R.P.; Shiraiwa, T.; Homma, K.; Kumagai, E.; Sameshima, R. The effects of increased temperature on crop growth and yield of soybean grown in a temperature gradient chamber. Field Crops Res. 2013, 154, 74–81. [Google Scholar] [CrossRef]
- Egli, D.B.; TeKrony, D.M.; Heitholt, J.J.; Rupe, J.C. Air Temperature during Seed Filling and Soybean Seed Germination and Vigor. Crop Sci. 2005, 45, 1329–1335. [Google Scholar] [CrossRef]
- Chamarthi, S.K.; Kaler, A.S.; Abdel-Haleem, H.; Fritschi, F.B.; Gillman, J.D.; Ray, J.D.; Smith, J.A.; Dhanapal, A.P.; King, C.H.; Purcell, L.C. Identification and Confirmation of LOCI Associated with Canopy Wilting in Soybean Using Genome-Wide Association Mapping. Front. Plant Sci. 2021, 12, 698116. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.M.G.; Boote, K.J.; Pan, D.; Allen, L.H. Elevated temperature delays onset of reproductive growth and reduces seed growth rate of soybean. J. AgroCrop Sci. 2010, 1, 19–32. [Google Scholar]
- Borisjuk, L.; Rolletschek, H. The Oxygen Status of the Developing Seed. New Phytol. 2009, 182, 17–30. [Google Scholar] [CrossRef]
- Porterfield, D.M.; Kuang, A.; Smith, P.; Crispi, M.L.; Musgrave, M.E. Oxygen-Depleted Zones inside Reproductive Structures of Brassicaceae: Implications for Oxygen Control of Seed Development. Can. J. Bot. 2000, 77, 1439–1446. [Google Scholar] [CrossRef]
- Rolletschek, H.; Borisjuk, L.; Koschorreck, M.; Wobus, U.; Weber, H. Legume Embryos Develop in a Hypoxic Environment. J. Exp. Bot. 2002, 53, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Thorne, J.C. Temperature and Oxygen Effects on 14C-Photosynthate Unloading and Accumulation in Developing Soybean Seeds. Plant Physiol. 1982, 69, 48–53. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Ward, J.; Randall, C.W. Soybean Seed Growth in Response to Long-Term Exposures to Differing Oxygen Partial Pressures. Plant Physiol. 1987, 83, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, T.R. Oxygen and Temperature Effects on Soybean Seed Coat Respiration Rates. Plant Physiol. 1988, 86, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Richard, T. Calculating the Oxygen Diffusion Coefficient in Water. Available online: https://compost.css.cornell.edu/oxygen/oxygen.diff.water.html (accessed on 15 April 2025).
- Vigeolas, H.; Van Dongen, J.T.; Waldeck, P.; Hühn, D.; Geigenberger, P. Lipid Storage Metabolism Is Limited by the Prevailing Low Oxygen Concentrations Within Developing Seeds of Oilseed Rape. Plant Physiol. 2003, 133, 2048–2060. [Google Scholar] [CrossRef]
- Rolletschek, H.; Radchuk, R.; Klukas, C.; Schreiber, F.; Wobus, U.; Borisjuk, L. Evidence of a Key Role for Photosynthetic Oxygen Release in Oil Storage in Developing Soybean Seeds. New Phytol. 2005, 167, 777–786. [Google Scholar] [CrossRef]
- Borisjuk, L.; Nguyen, T.D.; Neuberger, T.; Rutten, T.; Tschiersch, H.; Claus, B.E.H.; Feussner, I.; Webb, A.G.; Jakob, P.M.; Weber, H.; et al. Gradients of Lipid Storage, Photosynthesis and Plastid Differentiation in Developing Soybean Seeds. New Phytol. 2005, 167, 761–776. [Google Scholar] [CrossRef]
- Dat, J.F.; Vandenabeele, S.; Vranová, E.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Dual Action of the Active Oxygen Species during Plant Stress Responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef]
- Overmyer, K.; Brosché, M.; Kangasjärvi, J. Reactive Oxygen Species and Hormonal Control of Cell Death. Trends Plant Sci. 2003, 8, 335–342. [Google Scholar] [CrossRef]
- Bailly, C. Active Oxygen Species and Antioxidants in Seed Biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Wagner, D.; Przybyla, D.; Camp, R.O.D.; Kim, C.; Landgraf, F.; Lee, K.; Würsch, M.; Laloi, C.; Nater, M.; Hideg, É.; et al. The Genetic Basis of Singlet Oxygen–Induced Stress Responses of Arabidopsis thaliana. Science 2004, 306, 1183–1185. [Google Scholar] [CrossRef]
- Almagro, L.; Ros, L.V.G.; Belchí-Navarro, S.; Bru, R.; Barceló, A.R.; Pedreño, M.A. Class III Peroxidases in Plant Defence Reactions. J. Exp. Bot. 2008, 60, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Bestwick, C.S.; Brown, I.H.; Mansfield, J.W. Localized Changes in Peroxidase Activity Accompany Hydrogen Peroxide Generation During the Development of a Nonhost Hypersensitive Reaction in Lettuce. Plant Physiol. 1998, 118, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Tschiersch, H.; Borisjuk, L.; Rutten, T.; Rolletschek, H. Gradients of Seed Photosynthesis and Its Role for Oxygen Balancing. Bio-Systems 2011, 103, 302–308. [Google Scholar] [CrossRef]
- Brazel, A.J.; Ó’Maoiléidigh, D.S. Photosynthetic Activity of Reproductive Organs. J. Exp. Bot. 2019, 70, 1737–1754. [Google Scholar] [CrossRef] [PubMed]
- Benamar, A.; Rolletschek, H.; Borisjuk, L.; Avelange-Macherel, M.-H.; Curien, G.; Mostefai, H.A.; Andriantsitohaina, R.; Macherel, D. Nitrite–Nitric Oxide Control of Mitochondrial Respiration at the Frontier of Anoxia. Biochim. Biophys. Acta-Bioenerg. 2008, 1777, 1268–1275. [Google Scholar] [CrossRef]
- Kumar, S.; Chintagunta, A.D.; Reddy, Y.C.J.; Rajjou, L.; Garlapati, V.K.; Agarwal, D.C.; Prasad, S.; Simal-Gandara, J. Implications of Reactive Oxygen and Nitrogen Species in Seed Physiology for Sustainable Crop Productivity Under Changing Climate Conditions. Curr. Plant Biol. 2021, 26, 100197. [Google Scholar] [CrossRef]
- Borisjuk, L.; Macherel, D.; Benamar, A.; Wobus, U.; Rolletschek, H. Low Oxygen Sensing and Balancing in Plant Seeds: A Role for Nitric Oxide. New Phytol. 2007, 176, 813–823. [Google Scholar] [CrossRef]
- Sun, K.; Hunt, K.; Hauser, B.A. Ovule Abortion in Arabidopsis Triggered by Stress. Plant Physiol. 2004, 135, 2358–2367. [Google Scholar] [CrossRef]
- Allen, L.H.; Zhang, L.; Boote, K.J.; Hauser, B.A. Elevated Temperature Intensity, Timing, and Duration of Exposure Affect Soybean Internode Elongation, Mainstem Node Number, and Pod Number per Plant. Crop J. 2018, 6, 148–161. [Google Scholar] [CrossRef]
- Zhang, L.; Allen, L.H.; Vaughan, M.; Hauser, B.A.; Boote, K.J. Solar Ultraviolet Radiation Exclusion Increases Soybean Internode Lengths and Plant Height. Agric. For. Meteorol. 2014, 184, 170–178. [Google Scholar] [CrossRef]
- Sleper, D.A.; Nickell, C.D.; Noel, G.R.; Cary, T.R.; Thomas, D.J.; Clark, K.M.; Rao-Arelli, A.P. Registration of ‘Maverick’ soybean. Crop Sci. 1998, 38, 549–550. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Hauser, B.A.; Allen, L.H.; Boote, K.J.; Karplus, E.; McLamore, E.S. A Multiplexing Fiber Optic Microsensor System for Monitoring Oxygen Concentration in Plants. Proc. SPIE—Int. Soc. Opt. Eng. 2014, 8719, 87190O. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Taguchi, M.; Burrs, S.L.; Hauser, B.A.; Salim, W.W.A.W.; Claussen, J.C.; McLamore, E.S. Emerging Technologies for Non-Invasive Quantification of Physiological Oxygen Transport in Plants. Planta 2013, 238, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Hauser, B.A.; Foster, J.M.; Karplus, E.; Levine, L.H.; Coutts, J.L.; Richards, J.T.; Vanegas, D.C.; McLamore, E.S. A Multiplexing Fiber Optic Microsensor System for Monitoring Spatially Resolved Oxygen Patterns. Sens. Actuators B-Chem. 2014, 196, 71–79. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Head, D.J.; Hauser, B.A. During Water Stress, Fertility Modulated by ROS Scavengers Abundant in Arabidopsis Pistils. Plants 2023, 12, 2182. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Vadnais, D.A.; Zhang, Z.P.; Polacco, J.C. Refined Glufosinate Selection in Agrobacterium-Mediated Transformation of Soybean [Glycine max (L.) Merrill]. Plant Cell Rep. 2004, 22, 478–482. [Google Scholar] [CrossRef]
- Yang, R.-C.; Juskiw, P. Analysis of Covariance in Agronomy and Crop Research. Can. J. Plant Sci. 2011, 91, 621–641. [Google Scholar] [CrossRef]
- Leppink, J. Analysis of Covariance (ANCOVA) vs. Moderated Regression (MODREG): Why the Interaction Matters. Health Prof. Educ. 2018, 4, 225–232. [Google Scholar] [CrossRef]
- Kim, H.J. Statistical Notes for Clinical Researchers: Analysis of Covariance (ANCOVA). Restor. Dent. Endod. 2018, 43, e43. [Google Scholar] [CrossRef]
- Cosio, C.; Ranocha, P.; Francoz, E.; Burlat, V.; Zheng, Y.; Perry, S.E.; Ripoll, J.G.; Yanofsky, M.F.; Dunand, C. The Class III Peroxidase PRX 17 Is a Direct Target of the MADS-box Transcription Factor AGAMOUS-LIKE15 (AGL 15) and Participates in Lignified Tissue Formation. New Phytol. 2016, 213, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Shin, R.; Schachtman, D.P. Hydrogen Peroxide Mediates Plant Root Cell Response to Nutrient Deprivation. Proc. Natl. Acad. Sci. USA 2004, 101, 8827–8832. [Google Scholar] [CrossRef]
- Boote, K.J.; Prasad, P.V.V.; Allen, L.H.; Singh, P.K.; Jones, J.W. Modeling Sensitivity of Grain Yield to Elevated Temperature in the DSSAT Crop Models for Peanut, Soybean, Dry Bean, Chickpea, Sorghum, and Millet. Eur. J. Agron. 2018, 100, 99–109. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H.; Thomas, J.M.G. Effects of Elevated Temperature and Carbon Dioxide on Seed-Set and Yield of Kidney Bean (Phaseolus vulgaris L.). Glob. Change Biol. 2002, 8, 710–721. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H.; Thomas, J.M.G. Super-Optimal Temperatures Are Detrimental to Peanut (Arachis hypogaea L.) Reproductive Processes and Yield at Both Ambient and Elevated Carbon Dioxide. Glob. Change Biol. 2003, 9, 1775–1787. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H. Adverse High Temperature Effects on Pollen Viability, Seed-Set, Seed Yield and Harvest Index of Grain-Sorghum [Sorghum bicolor (L.) Moench] Are More Severe at Elevated Carbon Dioxide Due to Higher Tissue Temperatures. Agric. For. Meteorol. 2006, 139, 237–251. [Google Scholar] [CrossRef]
- Salem, M.A.; Kakani, V.G.; Koti, S.; Reddy, K.R. Pollen-Based Screening of Soybean Genotypes for High Temperatures. Crop Sci. 2007, 47, 219–231. [Google Scholar] [CrossRef]
- Simon, D.C.; Keith, B. The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell. Biol. 2008, 20, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.L.; Povilus, R.A.; Williams, B.P.; Gehrig, M. Transcriptional and imprinting complexity in Arabidopsis seeds at single-nucleus resolution. Nat. Plants 2021, 7, 730–738. [Google Scholar] [CrossRef]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases Have More Functions than a Swiss Army Knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, G.; Peng, X. Photorespiration in plant adaptation to environmental changes. Crop Environ. 2024, 4, 203–212. [Google Scholar] [CrossRef]

| Room Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| [CO2] Mean | 701 | 698 | 694 | 703 | 696 | 696 | 698 | 697 |
| Std. Dev | 82 | 81 | 73 | 85 | 79 | 79 | 74 | 73 |
| [O2] Mean Std. Dev | 32.0 | Amb | 32.0 | Amb | 31.9 | Amb | 31.6 | Amb |
| 0.8 | ≈zero | 0.8 | ≈zero | 0.8 | ≈zero | 1.3 | ≈zero | |
| Mean Daytime Temp °C Std. Dev | 30.0 | 30.0 | 34.0 | 34.0 | 38.0 | 38.0 | 42.0 | 42.0 |
| 0.9 | 0.8 | 0.7 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |
| Mean RH % | 57.2 | 55.9 | 55.5 | 55.7 | 57.0 | 58.9 | 63.8 | 60.2 |
| Std. Dev | 2.9 | 2.0 | 2.7 | 1.3 | 3.2 | 4.8 | 4.5 | 4.4 |
| Mean PPFD | 314.0 | 334.4 | 338.0 | 330.5 | 342.2 | 339.2 | 359.0 | 342.8 |
| Mean DLI | 13.56 | 14.45 | 14.60 | 14.29 | 14.78 | 14.65 | 15.51 | 14.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hauser, B.A.; Wang, Y.-Y.; Boote, K.J.; Chaturvedi, P.; McLamore, E.S.; Allen, L.H., Jr. Soybean Reproductive Traits Evaluated in Response to Temperature Stress and Elevated Oxygen; Three Peroxidase Transgenes Reduce Seed Abortion. Oxygen 2025, 5, 5. https://doi.org/10.3390/oxygen5020005
Hauser BA, Wang Y-Y, Boote KJ, Chaturvedi P, McLamore ES, Allen LH Jr. Soybean Reproductive Traits Evaluated in Response to Temperature Stress and Elevated Oxygen; Three Peroxidase Transgenes Reduce Seed Abortion. Oxygen. 2025; 5(2):5. https://doi.org/10.3390/oxygen5020005
Chicago/Turabian StyleHauser, Bernard A., Ya-Ying Wang, Kenneth J. Boote, Prachee Chaturvedi, Eric S. McLamore, and Leon H. Allen, Jr. 2025. "Soybean Reproductive Traits Evaluated in Response to Temperature Stress and Elevated Oxygen; Three Peroxidase Transgenes Reduce Seed Abortion" Oxygen 5, no. 2: 5. https://doi.org/10.3390/oxygen5020005
APA StyleHauser, B. A., Wang, Y.-Y., Boote, K. J., Chaturvedi, P., McLamore, E. S., & Allen, L. H., Jr. (2025). Soybean Reproductive Traits Evaluated in Response to Temperature Stress and Elevated Oxygen; Three Peroxidase Transgenes Reduce Seed Abortion. Oxygen, 5(2), 5. https://doi.org/10.3390/oxygen5020005








