Hyperbaric Oxygen in Otorhinolaryngology: Current Concepts in Management and Therapy
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. HBOT in Obstructive Sleep Apnea Syndrome (OSAS)
3.2. HBOT in Bell’s Palsy
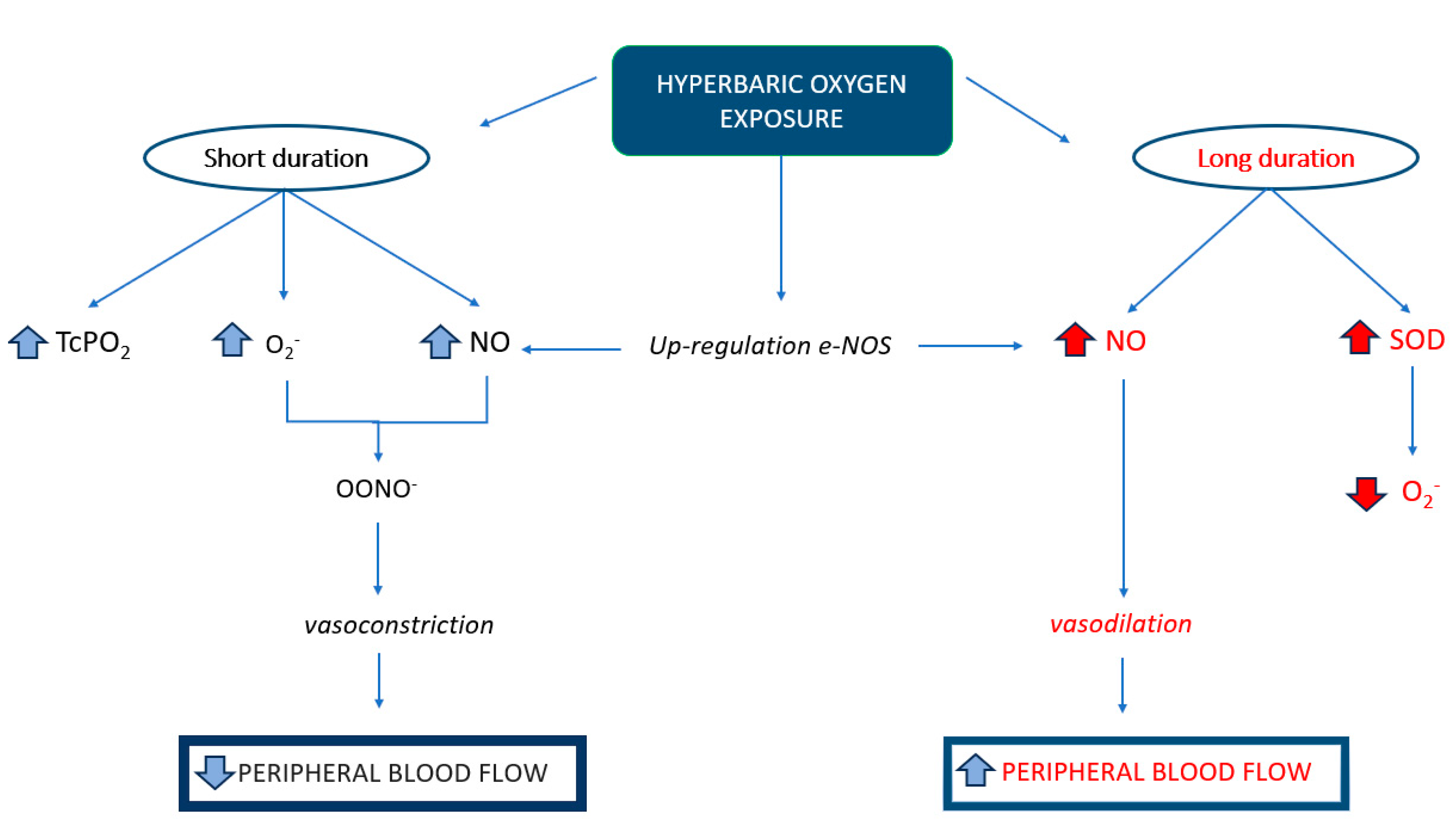
3.3. HBOT in the Treatment of Sudden Sensorineural Hearing Loss
3.4. HBOT in Radiation Necrosis
3.5. Could the HBOT Be an Effective Treatment for Malignant Otitis Externa?
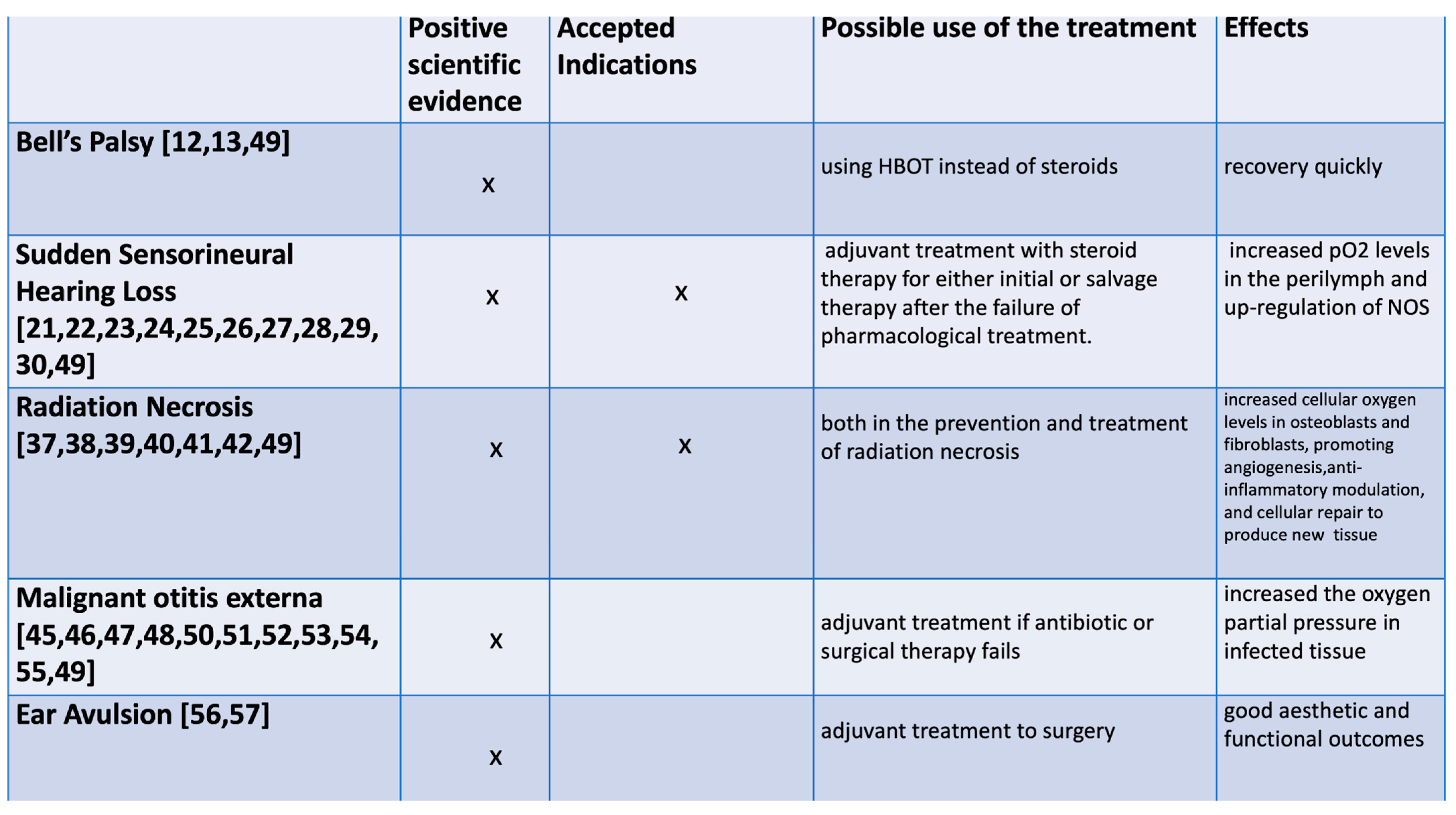
3.6. Primary Repair of Ear Avulsion with Adjuvant Hyperbaric Therapy
3.7. HBOT and Infections
3.8. Side Effects of Hyperbaric Oxygen Treatment
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lavie, L.; Lavie, P. Molecular mechanisms of cardiovascular disease in OSAHS: The oxidative stress link. Eur. Respir. J. 2009, 33, 1467–1484. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Takada, R.; Maeda, T.; Yoshii, T.; Okawa, A.; Yagishita, K. Microcirculation and tissue oxygenation in the head and limbs during hyperbaric oxygen treatment. Diving Hyperb. Med. 2021, 51, 338–344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gul, F.; Muderris, T.; Yalciner, G.; Sevil, E.; Bercin, S.; Ergin, M.; Babademez, M.A.; Kiris, M. A comprehensive study of oxidative stress in sudden hearing loss. Eur. Arch. Otorhinolaryngol. 2017, 274, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Meliante, P.G.; Zoccali, F.; Cascone, F.; Di Stefano, V.; Greco, A.; de Vincentiis, M.; Petrella, C.; Fiore, M.; Minni, A.; Barbato, C. Molecular Pathology, Oxidative Stress, and Biomarkers in Obstructive Sleep Apnea. Int. J. Mol. Sci. 2023, 24, 5478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lv, R.; Liu, X.; Zhang, Y.; Dong, N.; Wang, X.; He, Y.; Yue, H.; Yin, Q. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduct. Target. Ther. 2023, 8, 218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faber, J.; Faber, C.; Faber, A.P. Obstructive sleep apnea in adults. Dent. Press. J. Orthod. 2019, 24, 99–109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, J.G.; Ramar, K.; Olson, E.J. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin. Proc. 2011, 86, 549–554, quiz 554–555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Truschel, B.; Polkey, M.I. Optimizing Tracheal Oxygen Tension and Diffusion Ratio When Choosing High-Flow Oxygen Therapy or CPAP for the Treatment of Hypoxemic Respiratory Failure: Insights from Ex Vivo Physiologic Modelling. J. Clin. Med. 2023, 12, 2878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2006, 174, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Sivri, B.; Sezen, O.S.; Akbulut, S.; Coskuner, T. The effect of continuous positive airway pressure on middle ear pressure. Laryngoscope 2013, 123, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Racic, G.; Denoble, P.J.; Sprem, N.; Bojic, L.; Bota, B. Hyperbaric oxygen as a therapy of Bell’s palsy. Undersea Hyperb. Med. 1997, 24, 35–38. [Google Scholar] [PubMed]
- Holland, N.J.; Bernstein, J.M.; Hamilton, J.W. Hyperbaric oxygen therapy for Bell’s palsy. Cochrane Database Syst. Rev. 2012, 2012, CD007288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rinaldi, M.; Cavallaro, G.; Cariello, M.; Scialpi, N.; Quaranta, N. Metabolic syndrome and idiopathic sudden sensori-neural hearing loss. PLoS ONE 2020, 15, e0238351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuhn, M.; Heman-Ackah, S.E.; Shaikh, J.A.; Roehm, P.C. Sudden sensorineural hearing loss: A review of diagnosis, treatment, and prognosis. Trends Amplif. 2011, 15, 91–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prince, A.D.P.; Stucken, E.Z. Sudden Sensorineural Hearing Loss: A Diagnostic and Therapeutic Emergency. J. Am. Board. Fam. Med. 2021, 34, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Plontke, S.K.; Meisner, C.; Agrawal, S.; Cayé-Thomasen, P.; Galbraith, K.; Mikulec, A.A.; Parnes, L.; Premakumar, Y.; Reiber, J.; Schilder, A.G.; et al. Intratympanic corticosteroids for sudden sensorineural hearing loss. Cochrane Database Syst. Rev. 2022, 7, CD008080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ciorba, A.; Gasparini, P.; Chicca, M.; Pinamonti, S.; Martini, A. Reactive oxygen species in human inner ear perilymph. Acta Otolaryngol. 2010, 130, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Mastino, P.; Rosati, D.; de Soccio, G.; Romeo, M.; Pentangelo, D.; Venarubea, S.; Fiore, M.; Meliante, P.G.; Petrella, C.; Barbato, C.; et al. Oxidative Stress in Obstructive Sleep Apnea Syndrome: Putative Pathways to Hearing System Impairment. Antioxidants 2023, 12, 1430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamm, K.; Arnold, W. Successful treatment of noise-induced cochlear ischemia, hypoxia, and hearing loss. Ann. N. Y. Acad. Sci. 1999, 884, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.D.; Wei, I.H.; Lai, C.H.; Hsia, T.C.; Kao, M.C.; Tsai, M.H.; Wu, C.H.; Tsai, M.H. Hyperbaric oxygen upregulates cochlear constitutive nitric oxide synthase. BMC Neurosci. 2011, 12, 21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aldè, M.; Cantarella, G.; Piatti, G.; Ambrosetti, U. Sudden hearing loss and early hyperbaric oxygen therapy: A preliminary study. Undersea Hyperb. Med. 2023, 50, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.S.; Lee, T.Y.; Chen, Y.W.; Wu, M.F. Idiopathic Sudden Sensorineural Hearing Loss: Is Hyperbaric Oxygen Treatment the Sooner and Longer, the Better? J. Pers. Med. 2022, 12, 1652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cavaliere, M.; De Luca, P.; Scarpa, A.; Strzalkowski, A.M.; Ralli, M.; Calvanese, M.; Savignano, L.; Viola, P.; Cassandro, C.; Chiarella, G.; et al. Combination of Hyperbaric Oxygen Therapy and Oral Steroids for the Treatment of Sudden Sensorineural Hearing Loss: Early or Late? Medicina 2022, 58, 1421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Capuano, L.; Cavaliere, M.; Parente, G.; Damiano, A.; Pezzuti, G.; Lopardo, D.; Iemma, M. Hyperbaric oxygen for idiopathic sudden hearing loss: Is the routine application helpful? Acta Otolaryngol. 2015, 135, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Holy, R.; Navara, M.; Dosel, P.; Fundova, P.; Prazenica, P.; Hahn, A. Hyperbaric oxygen therapy in idiopathic sudden sensorineural hearing loss (ISSNHL) in association with combined treatment. Undersea Hyperb. Med. 2011, 38, 137–142. [Google Scholar] [PubMed]
- Chandrasekhar, S.S.; Tsai Do, B.S.; Schwartz, S.R.; Bontempo, L.J.; Faucett, E.A.; Finestone, S.A.; Hollingsworth, D.B.; Kelley, D.M.; Kmucha, S.T.; Moonis, G.; et al. Clinical Practice Guideline: Sudden Hearing Loss (Update). Otolaryngol. Head Neck Surg. 2019, 161 (Suppl. 1), S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Ajduk, J.; Ries, M.; Trotic, R.; Marinac, I.; Vlatka, K.; Bedekovic, V. Hyperbaric Oxygen Therapy as Salvage Therapy for Sudden Sensorineural Hearing Loss. J. Int. Adv. Otol. 2017, 13, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Pezzoli, M.; Magnano, M.; Maffi, L.; Pezzoli, L.; Marcato, P.; Orione, M.; Cupi, D.; Bongioannini, G. Hyperbaric oxygen therapy as salvage treatment for sudden sensorineural hearing loss: A prospective controlled study. Eur. Arch. Otorhinolaryngol. 2015, 272, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.; Cheng, X.; Gu, J.; Hong, Y.; Wang, Z.; Zhang, Z. Prognostic factors for hearing outcomes in patients that undergo adjuvant hyperbaric oxygen therapy for sudden sensorineural hearing loss. Laryngoscope Investig. Otolaryngol. 2022, 7, 592–598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dova, S.; Psillas, G.; Tsaligopoulos, M.; Nikolaidis, V.; Stefanidou, S.; Karagiannis, G.; Kotsiou, M.; Kaltzidis, T.; Markou, K. The effectiveness of hyperbaric oxygen therapy on the final outcome of patients with sudden sensorineural hearing loss. Am. J. Otolaryngol. 2022, 43, 103564. [Google Scholar] [CrossRef] [PubMed]
- Çekin, E.; Cincik, H.; Ulubil, S.A.; Gungor, A. Effectiveness of hyperbaric oxygen therapy in management of sudden hearing loss. J. Laryngol. Otol. 2009, 123, 609–612. [Google Scholar] [CrossRef]
- Yücel, A.; Özbuğday, Y. Comparison of Steroid Treatment with and without Hyperbaric Oxygen Therapy for Idiopathic Sudden Sensorineural Hearing Loss. J. Audiol. Otol. 2020, 24, 127–132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hajikarimloo, B.; Kavousi, S.; Jahromi, G.G.; Mehmandoost, M.; Oraee-Yazdani, S.; Fahim, F. Hyperbaric oxygen therapy as an alternative therapeutic option for radiation-induced necrosis following radiotherapy for intracranial pathologies. World Neurosurg. 2024, 186, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.D.; Hanley, M.E.; Cooper, J.S. Osteoradionecrosis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Myers, R.A.; Marx, R.E. Use of hyperbaric oxygen in postradiation head and neck surgery. NCI Monogr. 1990, 9, 151–157. [Google Scholar] [PubMed]
- Jenwitheesuk, K.; Mahakkanukrauh, A.; Punjaruk, W.; Jenwitheesuk, K.; Chowchuen, B.; Jinaporntham, S.; Uraiwan, K.; Limrattanapimpa, P. Efficacy of Adjunctive Hyperbaric Oxygen Therapy in Osteoradionecrosis. Biores. Open Access 2018, 7, 145–149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ashamalla, H.L.; Thom, S.R.; Goldwein, J.W. Hyperbaric oxygen therapy for the treatment of radiation-induced sequelae in children. The University of Pennsylvania experience. Cancer 1996, 77, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.H.; Feldmeier, J.; Smee, R.; Milross, C. Hyperbaric oxygenation for tumour sensitisation to radiotherapy. Cochrane Database Syst. Rev. 2012, 2012, CD005007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leach, R.M.; Rees, P.J.; Wilmshurst, P. Hyperbaric oxygen therapy. BMJ 1998, 317, 1140–1143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forner, L.E.; Dieleman, F.J.; Shaw, R.J.; Kanatas, A.; Butterworth, C.J.; Kjeller, G.; Alsner, J.; Overgaard, J.; Hillerup, S.; Hyldegaard, O.; et al. Hyperbaric oxygen treatment of mandibular osteoradionecrosis: Combined data from the two randomized clinical trials DAHANCA-21 and NWHHT2009-1. Radiother. Oncol. 2022, 166, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Korambayil, P.M.; Ambookan, P.V.; Pillai, S.; Karangath, R.R.; George, D. Role of Hyperbaric Medicine for Osteoradionecrosis and Post Irradiation Wounds: An Institutional Experience. Indian. J. Surg. Oncol. 2020, 11, 469–474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Treviño González, J.L.; Reyes Suárez, L.L.; Hernández de León, J.E. Malignant otitis externa: An updated review. Am. J. Otolaryngol. 2021, 42, 102894. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Friedman, P. The diagnostic criteria of malignant external otitis. J. Laryngol. Otol. 1987, 101, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Arsovic, N.; Radivojevic, N.; Jesic, S.; Babac, S.; Cvorovic, L.; Dudvarski, Z. Malignant Otitis Externa: Causes for Various Treatment Responses. J. Int. Adv. Otol. 2020, 16, 98–103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Byun, Y.J.; Patel, J.; Nguyen, S.A.; Lambert, P.R. Hyperbaric oxygen therapy in malignant otitis externa: A systematic review of the literature. World J. Otorhinolaryngol. Head Neck Surg. 2020, 7, 296–302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mardassi, A.; Turki, S.; Lahiani, R.; Mbarek, H.; Benzarti, S.; Gharsallah, H. Is there a real benefit of hyperbaric oxygenotherapy in the treatment of necrotizing otitis externa? Tunis. Med. 2016, 94, 863. [Google Scholar] [PubMed]
- Amaro, C.E.; Espiney, R.; Radu, L.; Guerreiro, F. Malignant (necrotizing) externa otitis: The experience of a single hyperbaric centre. Eur. Arch. Otorhinolaryngol. 2019, 276, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, D.; Marroni, A.; Kot, J. Tenth European Consensus Conference on Hyperbaric Medicine: Recommendations for accepted and non-accepted clinical indications and practice of hyperbaric oxygen treatment. Diving Hyperb. Med. 2017, 47, 24–32, Erratum in Diving Hyperb. Med. 2017, 47, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Al Siyabi, A.; Al Farsi, B.; Al-Shidhani, A.; Al Hinai, Z.; Al Balushi, Y.; Al Qartoobi, H. Management of Malignant Otitis Externa with Hyperbaric Oxygen Therapy: A Case Series of 20 Patients. Oman Med. J. 2023, 38, e512. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davis, J.C.; Gates, G.A.; Lerner, C.; Davis MGJr Mader, J.T.; Dinesman, A. Adjuvant hyperbaric oxygen in malignant external otitis. Arch. Otolaryngol. Head Neck Surg. 1992, 118, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Narozny, W.; Kuczkowski, J.; Stankiewicz, C.; Kot, J.; Mikaszewski, B.; Przewozny, T. Value of hyperbaric oxygen in bacterial and fungal malignant external otitis treatment. Eur. Arch. Otorhinolaryngol. 2006, 263, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Narozny, W.; Kuczkowski, J.; Mikaszewski, B. Hyperbaric oxygen to treat malignant external otitis. Am. Fam. Physician 2004, 70, 1860. [Google Scholar] [PubMed]
- Mader, J.T.; Love, J.T. Malignant external otitis. Cure with adjunctive hyperbaric oxygen therapy. Arch. Otolaryngol. 1982, 108, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Leahy, T.W.; Sader, C. A rare case of bilateral malignant otitis externa and osteomyelitis with lower cranial nerve sequelae. BMJ Case Rep. 2011, 2011, bcr0320113957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Favede, C.; Bradshaw, C.; Sethia, R.; Kramer, S.; Jatana, K.; Elmaraghy, C.; Grischkan, J. Near-Total Ear Avulsion Repaired with Primary Closure and Hyperbaric Oxygen: A Case Series and Review of the Literature. Ann. Otol. Rhinol. Laryngol. 2023, 132, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Archibald, H.; Tibesar, M.; Thacker, J.; Masters, T.; Chinnadurai, S.; Tibesar, R. Primary Repair of Ear Avulsion with Adjuvant Hyperbaric Therapy and Nitroglycerin Ointment. Facial Plast. Surg. Aesthet. Med. 2024. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Nedrebø, T.; Bruun, T.; Skjåstad, R.; Holmaas, G.; Skrede, S. Hyperbaric oxygen treatment in three cases of necrotizing infection of the neck. Infect. Dis. Rep. 2012, 4, e21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cimşit, M.; Uzun, G.; Yildiz, S. Hyperbaric oxygen therapy as an anti-infective agent. Expert. Rev. Anti Infect. Ther. 2009, 7, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Maroon, J.C. The effect of hyperbaric oxygen therapy on cognition, performance, proteomics, and telomere length-The difference between zero and one: A case report. Front. Neurol. 2022, 13, 949536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yip, W.L. Influence of oxygen on wound healing. Int. Wound J. 2015, 12, 620–624. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hunt, T.K.; Hopf, H.W. Wound healing and wound infection. What surgeons and anesthesiologists can do. Surg. Clin. N. Am. 1997, 77, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Capó, X.; Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Batle, J.M.; Tur, J.A.; Pons, A.; Sureda, A.; Tejada, S. Hyperbaric Oxygen Therapy Reduces Oxidative Stress and Inflammation, and Increases Growth Factors Favouring the Healing Process of Diabetic Wounds. Int. J. Mol. Sci. 2023, 24, 7040. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Wolde, S.D.; Hulskes, R.H.; Weenink, R.P.; Hollmann, M.W.; Van Hulst, R.A. The Effects of Hyperbaric Oxygenation on Oxidative Stress, Inflammation and Angiogenesis. Biomolecules 2021, 11, 1210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Camporesi, E.M. Side effects of hyperbaric oxygen therapy. Undersea Hyperb. Med. 2014, 41, 253–257. [Google Scholar] [PubMed]
- Nasole, E.; Zanon, V.; Marcolin, P.; Bosco, G. Middle ear barotrauma during hyperbaric oxygen therapy; a review of occurrences in 5962 patients. Undersea Hyperb Med. 2019, 46, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Edinguele, W.F.O.P.; Barberon, B.; Poussard, J.; Thomas, E.; Reynier, J.C.; Coulange, M. Middle-ear barotrauma after hyperbaric oxygen therapy: A five-year retrospective analysis on 2610 patients. Undersea Hyperb Med. 2020, 47, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.A.; Farage, L.; Cury, M.C.; Bahamad, F., Jr. Update on middle ear barotrauma after hyperbaric oxygen therapy-insights on pathophysiology. Int. Arch. Otorhinolaryngol. 2014, 18, 204–209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Micun, Z.; Dobrzyńska, W.; Sieśkiewicz, M.; Zawadzka, I.; Dmuchowska, D.A.; Wojewodzka-Zelezniakowicz, M.; Konopińska, J. Hyperbaric Oxygen Therapy in Ophthalmology: A Narrative Review. J. Clin. Med. 2023, 13, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collettini, A.; Zoccali, F.; Barbato, C.; Minni, A. Hyperbaric Oxygen in Otorhinolaryngology: Current Concepts in Management and Therapy. Oxygen 2024, 4, 150-162. https://doi.org/10.3390/oxygen4020010
Collettini A, Zoccali F, Barbato C, Minni A. Hyperbaric Oxygen in Otorhinolaryngology: Current Concepts in Management and Therapy. Oxygen. 2024; 4(2):150-162. https://doi.org/10.3390/oxygen4020010
Chicago/Turabian StyleCollettini, Andrea, Federica Zoccali, Christian Barbato, and Antonio Minni. 2024. "Hyperbaric Oxygen in Otorhinolaryngology: Current Concepts in Management and Therapy" Oxygen 4, no. 2: 150-162. https://doi.org/10.3390/oxygen4020010
APA StyleCollettini, A., Zoccali, F., Barbato, C., & Minni, A. (2024). Hyperbaric Oxygen in Otorhinolaryngology: Current Concepts in Management and Therapy. Oxygen, 4(2), 150-162. https://doi.org/10.3390/oxygen4020010






