Abstract
Wheat-flour crackers represent a staple snack option, although they lack nutritional value. Agricultural by-products such as olive and grape seeds, cereals such as barley and legumes such as lupine and chickpea are rich in bioactive compounds; therefore, flours obtained from those could represent a better option for bakery products fortification. The purpose of the present study was the investigation of total phenolic content and antioxidant activity before and after the baking of wheat crackers enriched with 10–30% olive seed, 10–30% grape seed, 10–40% lupine, 10–30% barley and 20–60% and 80% chickpea flours and the evaluation of the predicted bioavailability after in vitro digestion of crackers demonstrating the highest values. Crackers and doughs were processed and analyzed using Folin–Ciocâlteu and ferric reducing antioxidant power (FRAP) assays, respectively. Crackers with the highest properties were subjected to in vitro gastrointestinal digestion. Baking resulted in an increase in total phenolics and antioxidant activity in the majority of crackers. Olive and grape seed flour crackers demonstrated the highest antioxidant properties. Following in vitro digestion, 30% olive seed flour crackers retained the majority of polyphenols and antioxidant activity. Crackers enriched with 30% olive seed flour could represent a healthy functional bakery snack regarding their increased antioxidant properties.
1. Introduction
Crackers are low-moisture wheat-flour-formulated bakery products and represent one of the most popular easy-to-eat snack food options of consumers worldwide because of their long shelf-life, generally low cost and wide production range of flavors and tastes. Due to their formulation, they score high in calories but relatively low in nutritional value. This can be explained by their high content of rapidly digested carbohydrates and fats and low levels of fiber [1,2]. As the prevalence of celiac disease and metabolic conditions such as type II diabetes, metabolic syndrome and obesity has globally increased, individuals suffering from those conditions are unable to consume those snacks. Therefore, due to the increasing demand for functional food options, the modern food industry aims to develop gluten-free bakery snacks enriched with bioactive compounds that exhibit distinct antioxidant activity in order to provide both nutritional value and possible health benefits for the consumers [3]. Consumption of cereals, fruits and legumes, which are rich in antioxidants and fiber, is associated with a lower incidence of metabolic syndrome and chronic diseases [4]. In this context, alternative flours from agricultural by-products from fruit processing such as olive and grape seeds, along with cereal grains such as barley and legumes such as lupine and chickpea, are exploited to fortify bakery products because of their high protein, dietary fiber, fatty acids and polyphenol content.
Barley grain has a low glycemic index (GI) as it contains a vast array of antioxidant compounds such as tannins, proanthocyanins, phenolic acids, flavonols, flavones, flavanones and lignans [5]. Additionally, barley is considered superior to other grains, in terms of phenolic compound content, as it contains all eight vitamin E forms: tocopherols and the corresponding tocotrienols [6]. Furthermore, barley β-glucans possess the physiological benefits of insoluble dietary fiber regarding the increase in fecal bulk and the promotion of bowel health [7], plus the biological benefits of soluble dietary fiber linked to the reduction in serum cholesterol levels and postprandial glucose levels [8]. Compared to wheat flour, which lacks nutritional value because of the loss of the outer layers of the grain, which possess bioactive compounds, during the milling process, barley flour preserves most of the vital nutrients and beneficial compounds [9]. Therefore, there is a growing demand for incorporation of barley flour into various food products in order to improve their nutritional value.
Lupine contains a high amount of dietary fiber and a significant amount of oil, characterized by the presence of saturated fatty acids (10%) and unsaturated fatty acids (90%). Lupine also contains a number of polyphenols, mainly tannins and flavonoids [10,11], as well as phytosterols [12] and tocopherols [13]. Consumption of food products enriched with lupine has been linked to the prevention of cardiovascular disease, diabetes, obesity and digestive tract pathological conditions [14]. Lupine flour, due to its high protein (~40%) and low starch content because of gluten’s absence, is highly recommended for food product supplementation. Furthermore, due to its high dietary fiber content (~28%), it has been proposed for the enrichment of wheat-formulated bakery products [15].
Chickpea is a good source of polyunsaturated fatty acids, minerals and vitamins [16,17], whereas bioactive compounds such as tocopherols, phytosterols, carotenoids, phenolic compounds and phenolic acids are quite abundant in chickpea seeds [16,18,19]. Chickpea also contains both soluble and insoluble dietary fiber [20]. Chickpea consumption is associated with reduction in blood pressure, serum cholesterol levels, glucose absorption rate and insulin resistance. Thus, if incorporated in the diet, it could minimize the risks of cardiovascular or metabolic disease [16,19]. Chickpea flour has a low glycemic index, is rich in dietary fiber and is gluten free, compared to wheat flour [16]. Furthermore, the lipid content in chickpea flour is almost twice higher than in wheat flour [21].
Olive seed is a rich source of dietary fiber [22] and other bioactive compounds, such as polyphenols [23,24,25,26]. Olive polyphenols possess possible antioxidant, anti-inflammatory, hypolipidemic, hypoglycemic, cardio-protective, immune-modulatory and gastro-protective properties [27]. Olive seed contains a significant amount of oil, which possesses a high amount of monounsaturated fatty acids (~63%), moderate amounts of polyunsaturated amino acids (25%) and a low amount of saturated fatty acids (~12%) [26]. Furthermore, olive seed oil is rich in tocopherols and phytosterols [26,28]. Dietary fiber content is quite high, where insoluble and soluble fractions are present in equal amounts. Incorporation of olive stone flour into bakery products, apart from their enrichment with bioactive compounds, increases their protein, fat and dietary fiber content. Moreover, antioxidant capacity is increased in the final bakery products because of the enrichment with the high phenolic content present in olive seed flour [29,30].
Grape seed is reported to contain about 40% dietary fiber [31] and represents a rich source of polyphenols, such as the anthocyanins, proanthocyanidins, flavanols and catechins that are present at about 60–70% in grape seeds [32]. Grape polyphenols are reported to have a protective role against cardiovascular disease development because of their positive effects on plasma lipid and blood pressure levels, as well as their role in inhibition of LDL oxidation, platelet aggregation and inflammation [33]. Grape seeds contain 8–20% oil, which is rich in phenolic compounds, vitamin E, unsaturated fatty acids and phytosterols [34,35,36]. Grape seed flour is a good source of polyphenols, mainly proanthocyanidins and fatty acids, as the valuable fraction of those micronutrients is preserved. Due to its high fiber and protein content, it has been proposed as an alternative flour to be utilized in the food industry in order to increase the antioxidant properties of various food products [37,38].
Polyphenols represent a vast group of plant secondary metabolites that exert high antioxidant activity because of their ability to act as metal chelators and free-radical scavengers. Therefore, those compounds have been linked to a vast array of health benefits such as protective effects against serious pathological conditions (cardiovascular disease, diabetes, obesity and cancer) [39]. To exert those beneficial effects, their bioavailability in the respective tissues or organs is essential; in other words, these compounds need to be released from the food matrix during food processing, be accessible for metabolism in the gastrointestinal tract and finally reach the respective target tissues or organs. Bio-accessibility is defined as the fraction of a specific compound present in the gastrointestinal tract, which is available for absorption through the gut lumen, as a result of its release from the food matrix. Compounds mainly present in the small intestine constitute the soluble bio-accessible fraction that will be transported through the circulatory system, whereas the non-bioaccessible fraction will be secreted in the feces [40]. A number of factors may affect phenolic compound bioavailability, including gut microbiota, food matrix and food processing [41]. Industrial or domestic food processing methods can affect phenolic compound content, antioxidant activity, bio-accessibility and bioavailability in different ways. Some may lead to their degradation, while others can enhance their absorption and bioavailability [42]. Therefore, the final total phenolic content and bioavailability in the formulated processed food product highly depends on the nature and duration of the food processing, as well as on the food matrix exposed to the particular food processing.
The bioavailability of polyphenols following food processing is a major concern in the formulation of functional food products. Despite being carriers of bioactivity, conflicting evidence of polyphenol absorption and the underlying mechanisms of metabolism is present in the literature, especially when food composition and the complexity or dietary intake of specific food products are considered [43]. Generally, their absorption is low, which is attributed to some extent to the different structures of polyphenols that subsequently affect their gut absorption [44]. Other factors include their release from food matrices, especially from solid ones and their stability under gastrointestinal tract conditions that are affected by many physicochemical (temperature and pH) and biochemical parameters (presence and action of enzymes and bile salts) [45].
A certain number of studies have evaluated the nutritional profiles of bakery snacks other than crackers enriched with olive seed, grape seed and barley flours, whereas limited studies have focused on the potential of enrichment with chickpea and lupine flours. Additionally, no studies have been conducted to evaluate the effect of baking on total phenolic content and antioxidant activity in cracker dough enriched with these flours. Furthermore, there is limited information in the literature concerning simulated in vitro gastrointestinal digestion performed in crackers, whereas no studies have focused on the fate of polyphenols of those alternative flours following cracker digestion. This is the only study that focuses on assessing the potential of enrichment with these alternative flours on wheat-based crackers in parallel, in order to determine the most added-value cracker formulation.
In this framework, the aim of the present study was to assess the antioxidant activity and total phenolic content in wheat crackers fortified with barley, lupine, chickpea, olive and grape seed flours and compare the results to those of the respective dough samples in order to address changes induced in those properties throughout the cracker-making process. Furthermore, crackers that demonstrated the highest antioxidant profile were subjected to simulated in vitro gastrointestinal digestion in order to investigate the predicted bioavailability of phenolic compounds and the subsequent changes in antioxidant activity.
2. Materials and Methods
2.1. Cracker Dough Preparation and Baking Conditions
A standard cracker recipe was prepared. The ingredients of each cracker formulation are presented in Table 1. All ingredients were weighed and mixed in a dough by using a KMC570 (Kenwood, United Kingdom) mixer machine for 8 min. The produced dough was covered with a food wrapper to prevent excessive moisture loss and was allowed to rest at room temperature for 30 min. Then, the dough was divided, kneaded to release the air inside the dough, sheeted to 2 mm thickness using a manual dough-molding machine (Hendi, Rhenen, The Netherlands) and cut in dimensions 10 × 7.5. Nine punches were made in each cracker sample. Each cracker sample was placed in an electric heating air oven (North, FK-60W, Sotirios D.Prodanas & Co, National Road Kilkis-Thessaloniki, Greece) to provide uniform heat distribution over the dough during the baking process and baked at 170 °C for 15 min. Then, the cracker samples were allowed to cool at room temperature for 30 min and stored in polyethylene bags at 20 °C.

Table 1.
Recipes of the formulated crackers.
2.2. Extraction
Then, 2 g baked cracker powder or 2 g dough were extracted into 100 mL of 70% methanol at 70 °C for 1 h in an ultrasonic water bath [46]. The extracts were filtered and then used to determine total phenolic content and antioxidant activity by Folin–Ciocalteu and ferric reducing antioxidant power (FRAP) assays, respectively [47].
2.3. Simulated In Vitro Gastrointestinal Digestion
The protocol followed was according to the digestion process described by Kapsokefalou and Miller, 1991 [48], with some modifications. Ground cracker samples were prepared in order to provide 4 g of protein/100 mL sample and homogenized with ddH2O. The amount of 5 mL HCl 0.1 M was added to the samples, and the pH was fixed with concentrated HCl; 2 mL of each sample were transferred to wells in a six-well plate. In each well, 0.1 mL of pepsin was added, and the plates were covered with a plastic lid and placed on a shaking incubator at 37 °C for 2 h. At the end of the incubation period, a cylindrical insert with a piece of dialysis membrane fastened to one end with an elastic band was placed in each well. Each ring was filled with 2 mL 0.1 M PIPES buffer pH 6.5. The plates were incubated for another 30 min at 37 °C. After 30 min, the inserts were slightly lifted, and 0.5 mL of a pancreatin–bile salt mixture was added to the samples. The inserts were replaced, and the incubation continued for another 2 h. At the end of the incubation period, the inserts with the dialysis membranes were removed. Dialysates were centrifuged at 4000× g for 15 min at 4 °C, and the supernatants were used to determine total phenolic content and antioxidant activity through Folin–Ciocalteu and FRAP assays [49,50].
2.4. Determination of Total Phenolic Content (Folin–Ciocalteu Assay)
Total phenolic content was measured by the Folin–Ciocalteu method [51]. In a 96-well microplate, 50 mL of sample, 20 μL Na2CO3 solution and 20 μL Folin–Ciocalteu reagent were added, respectively. The samples were analyzed in triplicates. The 96-well microplate remained in the dark for 30 min, and then, the relative absorbance of the samples was measured at 765 nm in a spectrophotometer using Magellan™ data analysis software. Gallic acid was used as the reference standard, and the data were expressed as gallic acid equivalents (GAE), as mg GAE/10 mL [52].
2.5. Determination of Antioxidant Activity (FRAP Assay)
For the determination of antioxidant activity, the ferric reducing antioxidant power assay (FRAP) was applied. The FRAP assay is based on the reduction of yellow ferric-tripyridyltriazine complex (Fe(III)-TPTZ) to blue ferrous complex (Fe(II)-TPTZ) by the phenolics through the action of electron-donating antioxidants [53]. In a 96-well microplate, 20μL of sample and 80μL FRAP reagent were added. The samples were analyzed in triplicates. The 96-well microplate remained in the dark for 30 min and then the relative absorbance of the samples was measured at 595 nm in a spectrophotometer using Magellan™ data analysis software. The data were expressed as FeSO4 concentration (µM) [47,52,54].
2.6. Statistical Analysis
Normal distribution of the data was confirmed by Shapiro–Wilk’s test. One-way analysis of variance (ANOVA) and a post hoc Tukey test were used for comparison of the means between samples. Data are presented as mean ± standard deviation (SD) and analyzed using the Statistical Package for Social Sciences (SPSS version 17.0). The significance level for the differences between the sample means was set at p ≤ 0.05. Data were also evaluated using Pearson’s correlation coefficients for identification of any relationships between total phenolic content in crackers and their antioxidant activities as determined by Folin–Ciocalteu and FRAP assays.
3. Results
3.1. Total Phenolic Content of Novel Wheat Crackers
The results for total phenolics of novel wheat crackers and the respective doughs are presented in Diagram 1. In dough formulations, the highest total phenolic content was observed in the L4, G3, L3, G2 and L2 samples, respectively. On the other hand, the lowest content in total phenolics was demonstrated by the O1, C1, O2, C2 and C3 sample doughs. In crackers, the highest total phenolics were demonstrated by the G3, G2, O3, G1 and O2 samples, respectively. The lowest phenolic content was observed in the C1, L1, W, C2 and C3 samples. Total phenolic content increased in all dough formulations as the level of flour fortification increased; in the case of lupine and chickpea flour enrichment, the increase was significant (p ≤ 0.05). Doughs samples O3, G2, G3, B1–B3, L1–L4 and C4–C6 demonstrated higher total phenolic values compared to the W dough (control); in the case of the B1, L2–L4 and C6 doughs, the increase was significant. The remaining dough formulations demonstrated slightly lower total phenolic values compared to the W dough (control) (Figure 1).
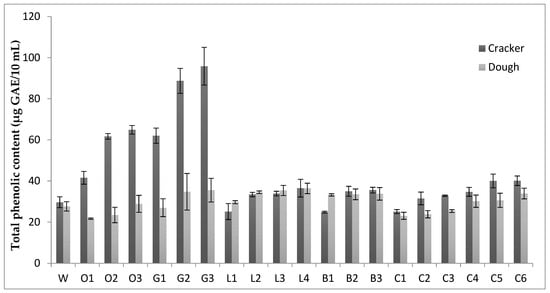
Figure 1.
Phenolic content of the novel wheat crackers. Phenolic content was expressed as gallic acid equivalents (GAE) in mg per 10 mL of crackers. Results are expressed as mean ± standard deviation from triplicate measurements. Mean values are significantly different (p ≤ 0.05) compared to 100% wheat flour crackers (controls).
The O1–O3 and G1–G3 cracker samples demonstrated a significantly (p ≤ 0.05) higher total phenolic content compared to the respective doughs. The L1–L3 cracker samples exhibited a slight decrease in total phenolics compared to their doughs, whereas a slight increase was observed in the L4 cracker samples. The B1 and B2–B3 crackers demonstrated a significant (p ≤ 0.05) decrease and a slight increase compared to the dough samples, respectively. The C1–C6 crackers demonstrated an increase in total phenolic content compared to their doughs, which in the case of the C2, C3, C5 and C6 crackers was significant (p ≤ 0.05)
The O1–O3, G1–G3, B3, C5 and C6 crackers demonstrated a significant increase in total phenolic content compared to the W crackers (controls). The B2, L2–L4 and C2–C4 crackers displayed higher total phenolic values compared to the W crackers but not at the significance level. On the other hand, the B1, L1 and C1 crackers demonstrated a decrease in total phenolics compared to the W crackers; in the case of the B1 crackers, the decrease was significant (p ≤ 0.05). One-way ANOVA showed a statistically significant increase (p ≤ 0.05) in total phenolic content of each cracker formulation as the level of flour fortification increased.
3.2. Antioxidant Activity of Novel Wheat Crackers
The results for antioxidant activity of the novel wheat crackers and the respective doughs are presented in Table 2. In cracker doughs, the highest antioxidant activity was observed in the O3, G2, O2, G3 and G1 samples, respectively. The lowest values were observed in the L1–L4 and C1 doughs. As the level of fortification increased, the antioxidant activity of all enriched doughs increased significantly (p ≤ 0.05). In crackers, the highest antioxidant activity was demonstrated by the G3, G2, O3, O2 and G1 samples, whereas the lowest values were observed in the B1, B2, C1, L1 and C2 samples, respectively. All enriched dough formulations demonstrated significantly (p ≤ 0.05) higher antioxidant activity values compared to the W dough (control).

Table 2.
Total antioxidant activity of dough and cracker formulations.
The O1–O3, G1–G3 and L1–L4 crackers demonstrated a significant (p ≤ 0.05) increase in antioxidant activity compared to the respective doughs. The B1–B3 crackers demonstrated a decrease in antioxidant activity, which in the B1 and B2 samples was significant (p ≤ 0.05). The C1 crackers exhibited lower antioxidant activity values compared to their doughs, whereas the C2–C6 crackers had significantly higher (p ≤ 0.05) antioxidant activity compared to the respective doughs (Table 2).
The O1–O3, G1–G3, B3, L2–L4 and C2–C6 crackers demonstrated significantly (p ≤ 0.05) higher antioxidant activity values than the W crackers (controls). The L1 crackers showed slightly higher values, whereas the B1–B2 and C1 crackers demonstrated lower values than the W crackers. One-way ANOVA showed a statistically significant (p ≤ 0.05) increase in antioxidant activity of all enriched crackers as the level of flour fortification increased (Table 2).
Pearson’s correlation coefficients between total phenolic content and antioxidant activity of the novel wheat crackers are presented in Table 3. A positive correlation between total phenolic content and antioxidant activity of the alternative-flour-enriched crackers was observed; in the case of olive seed, grape seed, lupine and chickpea flour crackers, the relationship was significant (p ≤ 0.05). There was a moderate positive association between phenolic content and antioxidant activity in barley and lupine flour crackers (r2 = 0.558 and r2 = 0.689, respectively) and a strong positive association in chickpea, olive and grape seed flour crackers (r2 = 0.830, r2 = 0.891 and r2 = 0.914, respectively). The strongest positive association between phenolic content and antioxidant activity was observed in olive- and grape-seed-flour-enriched crackers (Table 3).

Table 3.
Correlation of phenolic content and antioxidant activity in novel wheat crackers.
3.3. Simulated In Vitro Gastrointestinal Digestion
Following in vitro gastrointestinal digestion, the total phenolic content of all cracker formulations demonstrated a decrease, which in the case of the G1–G3 and W formulations was significant (p ≤ 0.05). More than half of the total polyphenols (53.89%) were preserved after digestion in the G1 crackers, whereas the greatest decrease was observed in the G2 and G3 samples, as only 47.71% and 46.23% of the total phenolics were preserved, respectively. The O3 crackers demonstrated the highest bio-accessibility values, as 93.01% of the total polyphenols were preserved after the digestion process. One-way ANOVA indicated significantly (p ≤ 0.05) higher total phenolic content of all digested enriched crackers compared to the digested 100% wheat flour crackers (controls) (Table 4).

Table 4.
Total phenolic content of undigested and digested cracker samples.
Antioxidant activity of all enriched crackers demonstrated significantly (p ≤ 0.05) lower values after digestion with the greatest decrease observed in the G3 crackers, as only 44.17% of the antioxidant activity was preserved. The W crackers demonstrated a significant (p ≤ 0.05) increase in antioxidant activity following digestion, whereas the O3 crackers preserved the majority of their antioxidants among the enriched crackers (AA% = 86.90%). All digested enriched crackers demonstrated significantly (p ≤ 0.05) higher antioxidant activity values compared to the 100% wheat flour crackers (controls) (Table 5).

Table 5.
Antioxidant activity of undigested and digested cracker samples.
4. Discussion
This study indicates that the adding of alternative flours in wheat-formulated bakery products, such as crackers, can increase their phenolic content and the antioxidant activity, especially after the baking process, therefore rendering a possible health effect to the consumers selecting those crackers as a snack option. Moreover, through the in vitro simulation of gastrointestinal digestion in the cracker samples demonstrating the highest phenolic and antioxidant activity profiles, the adding of alternative flours seems to lead to important bioaccessibility, thereby predicting bioavailability.
The B2 and B3 crackers, although they exhibited relatively low values in phenolics, demonstrated higher phenolic content than the W crackers, whereas total phenolic content of barley crackers significantly increased as the level of barley flour increased. In terms of antioxidant activity, the B3 crackers resulted in a significantly higher antioxidant activity than the W crackers. A significant increase was also observed in the antioxidant activity of crackers as the level of barley flour increased, whereas a moderate positive association was determined between phenolic content and antioxidant activity of the barley-flour-enriched crackers. Our findings indicate the added values of 20% and 30% barley flour enrichments in improving the phenolic and antioxidant profile of wheat-formulated products. The results of our study are supported by a certain number of studies. Punia et al. reported a gradual increase in the total phenolic content values of wheat rusks substituted with 10%, 20% and 30% barley flour, respectively. In parallel, increasing barley flour substitution in wheat–barley flour rusks, their antioxidant activity progressively increased [55]. Furthermore, Aly et al. also demonstrated an increase in antioxidant activity of cookies substituted with 20% and 40% barley flour compared to the control wheat cookies [56], which is in accordance with the antioxidant profile of the B2 crackers in our study. Several other researchers have reported the increase in total phenolics and antioxidant activity in cookies and chapattis as the fortification of barley flour increased [57,58,59,60].
Regarding the effect of baking, the present study resulted in the B2 and B3 cracker samples demonstrating a slightly higher phenolic content than that of the respective doughs, whereas in the B1 samples, a significant decrease was observed. In terms of antioxidant activity, all crackers demonstrated lower values compared to the respective doughs; in the case of the B1 and B2 samples, the decrease was significant. Our results concerning antioxidant activity are in accordance with the study of Holtekjølen et al., 2008, in which the authors reported that antioxidant activities measured by FRAP assay were decreased during the baking process in wheat-formulated breads fortified with 40% barley flour [5,61]. Nevertheless, in another study, the baking of cookie dough enriched with 0%, 25%, 50% and 100% barley flour led to a significant decrease of up to 19.2% in total phenolic content in the formulated cookies [57]. The decrease in total phenolic content is addressed, possibly, as a result of a particular change in the molecular structure of phenolic compounds, probably polymerization that leads to oxidation and a decrease in their extractability [62]. Furthermore, during dough making, certain oxidative enzymes such as polyphenol oxidase are activated by flour hydration, and the oxidation may result in phenolic compound degradation [63]. The increase in antioxidant activity has been attributed to the formation of dark brown pigments during baking caused by the Maillard reactions that are reported to have antioxidant activity [64]. Therefore, our findings concerning the decrease in antioxidant activity could be explained by the thermal degradation of antioxidants present in barley flour, although the slight increase in phenolic content in the B2 and B3 samples could be due to the greater number of phenolic compounds supplied by the increase in barley flour substitution and their subsequent thermal release.
The L2–L4 samples demonstrated higher total phenolic content values than the W crackers, whereas L1 had slightly lower values. Regarding antioxidant activity, lupine flour enrichment resulted in an increase in the formulated crackers compared to the 100% wheat crackers; in the case of the L2–L4 samples, the increase was significant. A significant increase in total phenolic content and antioxidant activity of lupine-flour-enriched crackers was observed as the proportion of lupine flour increased, whereas a significantly moderate positive association was determined between phenolic content and antioxidant activity of the barley-flour-enriched crackers. Therefore, our results indicate the improvement of the antioxidant profile of the formulated crackers by the addition of 20–40% lupine flour. Our results are in accordance with the limited number of studies that investigated the potential of lupine flour addition in bakery products. The inclusion of 4, 8 and 12% of sweet lupine flour in wheat flour cookies resulted in a significant increase in total polyphenols compared to the control biscuits and a significant increase in antioxidant activity [63]. The incorporation of germinated Australian sweet lupine flour in muffins increased the total phenolic content and antioxidant activity of muffins [65]. The replacement of wheat flour with lupine flour at levels of 10, 20 and 30% in bread resulted in a significant increase in total phenolic content, compared to the controlled wheat-formulated bread, with the highest values observed in the bread fortified with 30% lupine flour. The same trends were observed in the measurement of antioxidant activity, as the level of lupine flour fortification increased, compared to the control wheat bread [66]. Many studies have highlighted the positive correlation of total phenolic content and antioxidant activity [11], although some others have concluded that in lupine there is no correlation, as certain compounds such as carotenoids and tocopherols that are present in lupine seeds can also contribute to the antioxidant activity [67].
After baking, the L1–L3 crackers demonstrated a slightly lower total phenolic content compared to their dough formulations, whereas the L4 crackers showed a slight increase. Nevertheless, the antioxidant activity of all lupine-flour-enriched crackers significantly increased compared to their respective doughs. In a single study retrieved, increasing lupine flour enrichment did not result in an increase in phenolic content of the baked products, as concluded from our findings, although the increase in antioxidant activity is in accordance with our results. Muffins enriched with 4% and 6% germinated Australian sweet lupine flour demonstrated significantly higher total phenolic content than their batters. The authors linked that increase to the release of phenolics from the matrix wall and the formation of phenolic products because of thermal degradation induced by baking. Additionally, baking increased the antioxidant activity of all muffin formulations. The higher antioxidant activity was attributed to the high phenolic content, as well as the formation of products with high radical-scavenging capacity during the baking process [65]. Therefore, we can conclude that 40% lupine flour fortification can improve the phenolic content of the crackers, resisting heat degradation, and heat treatment results in the production of a greater number of antioxidant compounds.
Regarding chickpea-flour-enriched crackers, there is limited literature available concerning the investigation of the phenolic and antioxidant profile of bakery products fortified with chickpea flour. In the present study, fortification with the C2–C6 crackers demonstrated higher phenolic content compared to control crackers, which in the case of the C5 and C6 samples was significant. Additionally, the antioxidant activity of the C2–C6 chickpea-flour-enriched crackers was significantly higher than that of the control crackers. As the level of chickpea flour increased, the total phenolic content and antioxidant activity of the formulated crackers increased significantly. Furthermore, there was a significantly strong positive association between phenolic content and antioxidant activity in chickpea-flour-enriched crackers. Two studies retrieved are in accordance with our results. Incorporation of 40% chickpea flour in wheat remilled-semolina-based bread, focaccia and pizza crust resulted in a significant increase in phenolic compound content and antioxidant activity compared to the control durum-wheat-formulated products [68]. Moreover, supplementation of wheat bread with 2% chickpea husk extract resulted in an increase in total phenolic content and antioxidant activity [69].
Furthermore, baking resulted in an increase in total phenolic content of all chickpea-flour-enriched crackers compared to their doughs; in the C2, C3, C5 and C6 samples, the increase was significant. The antioxidant activity of the C2–C6 crackers increased compared to the respective doughs; in the case of the C2 and C4–C6 samples, the increase was significant. Although we report the significant increase in phenolics and antioxidant activity of the 30% to 80% enrichments, in a single study retrieved, baking did not affect the levels of total phenolic content or antioxidant activity measured by FRAP assay relative to bread dough enriched with 400 g desi-type black seed coat chickpea flour in the final products [70]. However, longer baking times have been reported to trigger an increase in total phenolic content and antioxidant activity in pulse-flour-enriched wheat crackers, which may be attributed to the depolymerization of fibers during mechanical mixing and baking, release of fiber-associated phenolic compounds and the presence of Maillard reaction antioxidant products [71,72,73]. Therefore, our findings can be attributed to those procedures taking place during cracker production and baking, as well as the added value of bioactive compounds present in that flour as the level of fortification increased.
Olive-seed- and grape-seed-flour-enriched crackers demonstrated the highest total phenolic content and antioxidant activity among the tested novel crackers. Olive-seed-flour-enriched crackers exhibited significantly higher phenolic content and antioxidant activity compared to the control 100% wheat crackers. There was a significant increase in phenolic content and antioxidant activity as the proportion of olive seed flour increased, whereas there was a significantly strong positive association between phenolic content and antioxidant activity in olive-seed-flour-enriched crackers. In a study by Bolek et al., 2020, wheat flour replacement by 5%, 10% and 15% olive stone powder in cookies significantly increased phenolic content and antioxidant activity as the proportion of olive stone powder increased [29]. Similarly, substitution of wheat flour with 15%, 25%, and 35% olive stone powder in sponge cakes increased the total phenolic content of the samples as the percentage of olive stone powder substitution increased [30]. Our study in crackers adds to those reports, as higher antioxidant and phenolic properties were achieved through olive seed flour fortification and highlight the potential of this alternative flour as a nutritional carrier in functional bakery products. Olive-seed-flour-enriched crackers also demonstrated significantly higher total phenolics and antioxidant activity values compared to their dough formulations. In a single study, the baking of olive pomace cookie dough resulted in a slight increase in total phenolic content and a slightly lower antioxidant activity after baking. The authors attributed the increase to the release of phenolics, respectively because of the heat treatment [74].
Grape-seed-flour-enriched crackers demonstrated significantly higher phenolic content and antioxidant activity compared to the control crackers. Phenolic content and antioxidant activity of the crackers significantly increased as the proportion of grape seed flour increased, whereas there was a significantly strong association between those properties. A number of studies in a range of bakery products have also highlighted the potential of grape seed flour in improving, to a large extent, the nutritional and antioxidant profile of wheat-formulated bakery products. In a study by Acun and Gül, 2014, incorporation of 5%, 7.5% and 10% grape seed flour in cookies demonstrated a gradual increase in total phenolic content. As the level of grape seed flour substitution increased, the antioxidant activity of the cookies also increased [75]. Furthermore, inclusion of 2.5 and 5% grape seed flour in wheat cookies resulted in a linear increase in total phenolics compared to control wheat cookies [76]. In a study by Antonic et al., grape seed flour was used for the fortification of waffles in concentrations of 1, 3, 5 and 10%, where the highest phenolic and antioxidant profile was demonstrated by 10% inclusion of grape seed flour [77]. Enrichment of whole-wheat-, whole-siyez-wheat- and whole-oat-flour-formulated muffins with grape seed flour at 7.5% and 15% ratios resulted in a significant increase in antioxidant activity and total phenolics [78,79].
Grape-seed-flour-enriched crackers demonstrated significantly higher total phenolic content and antioxidant activity values compared to the corresponding dough formulations. In other studies, total phenolic content of breads enriched with 2.5, 5, 7.5 or 10 g grape seed flour/100 g wheat flour increased compared to the corresponding doughs. The authors argued that the increase in bread was essentially due to the combination of the thermal stability of grape seed flour phenolic compounds, as they are not degraded in temperatures lower than 180 °C, and the conversion of insoluble phenolic compounds bound to the dough gluten matrix to soluble forms upon baking [80,81]. Baked breads enriched with 2.5, 5, 7.5 or 10 g grape seed flour/100 g wheat flour exhibited significantly higher antioxidant activity values compared to the corresponding doughs [80], whereas the same trend was observed in grape-seed-containing breads compared to the corresponding doughs [82].
In the literature, the increase in total phenolic content in baked products is often addressed as a side effect of the baking process. Baking increased total phenolic content by affecting the solubility of bound forms of phenolic acids. In addition, it is important to underline that both the Folin–Ciocalteu and FRAP methods being unspecific methods as part of the reported measurements might be due to interfering compounds. Specifically, a Folin–Ciocalteu reagent not only measures the amount of phenolic compounds, but it also may react with any reducing substance. It therefore measures the total reducing capacity of a sample. Heat treatment induces the formation of compounds from the Maillard reactions that possess reductone structures, which are Folin–Ciocalteu reactive substances and are reported to contribute to the increase in the total phenolics. Total phenolic content could also be affected by polyphenolic oxidation and caramelization products. Some of these compounds are reported to have antioxidant activity, whereas others simply act as false positives in the test [83,84]. Additionally, certain interfering agents can act as false positives, such as ascorbic acid, which is an additive in commercial wheat baking flours (3 g/kg) [85], but also saccharides, phytic acids and amino acids [86]. Therefore, those events contribute to the increase in total phenolic compounds measured by Folin–Ciocalteu assay [83,84].
During baking, hazardous chemical products known as Maillard reaction products, including α-dicarbonyl compounds (DCs), furan, 5-hydroxymethylfurfural (HMF), furosine and acrylamide, can be generated. Those can react with other compounds in the formulated products, resulting in the formation of compounds critical for flavor, aroma and color. Baked products are abundant in DCs because of their high concentration of sugars, low moisture content and their processing. DCs do represent critical precursors of brown and volatile aromatic compounds, which are highly associated with color and aroma development. Furan compounds can derive from ascorbic acid, carbohydrates, amino acids, fatty acids and carotenoids. The development of browning in bakery products is a process mainly influenced by temperature and water activity and results from the production and accumulation of mainly HMF and melanoidins during baking. Bakery product formulation, baking conditions and use of sugars are responsible for the high variability of furan and α-dicarbonyl compounds (DCs) generated through processing. Use of sucrose instead of fructose or glucose in cookie recipes results in lower levels of both HMF and DCs without being influenced by baking temperature and time. Lower baking temperatures ranging from 150–170 °C are generally preferable for the containment of those compounds [87,88,89]. According to the recipes used, per batch, grape seed flour crackers contained 12.22–12.68 g sugars, olive seed flour crackers contained 12.11–12.35 g sugars, barley flour crackers contained 10.39–11.39 g sugars, lupine flour crackers contained 11.59–11.89 g sugars and chickpea flour crackers contained 14.51–22.07 g sugars. As raw material, grape seed flour contained 5.15 g sugars/100 g, wheat flour contained 4 g sugars/100 g, barley flour contained 1 g sugars/100 g, olive seed flour contained 4.6 g sugars/100 g, chickpea flour contained 10.3 g/100 g and lupine flour contained 3.5 g sugars/100 g. The sugar type added to the recipe was sucrose in a rate of 4 g per batch, and the baking conditions were 15 min in 170 °C. Color during baking intensified compared to the raw material and ranged in the formulated crackers from golden yellow (at a greater extent observed in lupine- and chickpea-flour-enriched crackers) to dark brown (mainly observed in olive-seed- and grape-seed-flour-enriched crackers). Although monitoring and calculation of Maillard compounds were not carried out, with the progressive browning and intensification of the aroma observed in the majority of the formulated enriched crackers during baking and taking into account the literature findings, we can conclude that the conditions and the recipe followed resulted in the generation of a substantial amount of those compounds that resulted in the higher phenolic and antioxidant activity values observed.
Since the O3, G1–G3 crackers yielded the highest total phenolic and antioxidant activity values, a simulated in vitro gastrointestinal model was employed in order to fully elucidate the events in the fate of those bioactive compounds during cracker digestion and help to determine which cracker formulation could act as the most potent nutritional carrier. Although the in vitro models cannot fully depict the digestion process taking place in the human gastrointestinal tract, as the anatomy and morphology, as well as the peristaltic movements, cannot be mimicked, it does represent an easy and rapid method to study the possible action of digestive enzymes on specific food products and the subsequent release of nutrients and antioxidants [90].
Very few studies have addressed the in vitro digestion of polyphenols present in non-naturally enriched food matrices such as bakery products. Interestingly, according to our knowledge, no studies have investigated the fate of polyphenols in complex food matrices of bakery products enriched with grape and olive seed flours. Therefore, the bio-accessible total polyphenol content, as well as the antioxidant activity of grape- and olive-seed-flour-enriched crackers were evaluated in this study. Generally, following gastrointestinal digestion, grape-seed-flour-enriched crackers demonstrated a significant decrease in phenolic content and antioxidant activity ranging from 46.11–53.77% and 31.07–55.83%, respectively. These properties were also significantly higher compared to those of 100% wheat-flour crackers even after digestion. The control crackers demonstrated a significant decrease in total phenolic content but a significant increase in antioxidant activity. The results indicated that increasing inclusion of grape seed flour in cracker enrichment resulted in an increase in the bio-accessible polyphenols, therefore suggesting the added value of grape flour in bakery products formulation. A limited number of studies has highlighted the value of grape polyphenol enrichment in bakery products by increasing the bio-accessible phytochemicals and, thus, affecting human health beneficially. In digested spaghetti supplemented with 15% red grape marc flour, the bio-accessible fraction demonstrated an increase in total phenolic content and a decrease in total antioxidant activity compared to the undigested samples. In the same study, the enriched spaghetti displayed higher total phenolics and antioxidant activity, even after the digestion process, compared to the control durum wheat semolina spaghetti. In the control spaghetti, similarly to our findings, an increase in antioxidant activity was observed after digestion. The authors attributed the increase to the release of amino acids from wheat durum semolina proteins and phenolic acids such as ferulic acid, which exhibit antioxidant activity because of the action of enzymes during the digestion process [91]. Our results are generally in accordance with the present study, although we reported a decrease in total phenolics, which can be attributed to the loss of grape seed anthocyanins that are unstable and can be destroyed from the transition of the acidic gastric conditions to the mild alkaline (pH = 7.2–7.6) intestinal environment. Anthocyanins exist in a colorless chalcone pseudobase molecular form in neutral pH values. Prolonged exposure to this form can trigger the degradation of the B and C rings, resulting in the destruction of the anthocyanin chromophore, thereby leading to anthocyanin degradation [92]. In another study, following in vitro gastric and small intestine digestive phases, a decrease in antioxidant capacity of breads enriched with 5 g and 10 g/100 g grape pomace powder was reported [93]. Similarly, our results are fully in accordance with these findings and further highlight the nutritional improvement of a wheat-formulated product with the addition of grape seed flour.
Our major finding of the in vitro gastrointestinal digestion was the high predicted bio-accessibility of olive seed flour polyphenols and antioxidant activity retained during the process. The O3 crackers exhibited a minor decrease in total polyphenols (−6.99%) and antioxidant activity (−13.1%) and demonstrated a significantly higher total phenolic content and antioxidant activity compared to the W crackers following digestion. A number of studies further support our findings. During the gastric and small-intestinal digestive conditions of the in vitro digestion process, total phenolic content of 10% dry olive-paste-flour-enriched breads remained relatively stable compared to the white flour control breads (59.3% recovery for the control and 72.1% recovery for the enriched bread). The authors attributed the polyphenol stability in a simulated gastro-intestinal environment to the high bio-accessibility of olive oil by-product compounds present through the bread enrichment [94]. In another study, polyphenol content and antioxidant activity increased after in vitro digestion in tarallis with and without olive leaf extract. The enriched tarallis showed a significantly higher concentration of bio-accessible total polyphenols than the controls and a higher antioxidant activity [95]. The results were explained by the action of digestive enzymes promoting the release of bound phenolic acids, as well as to the amino acids present in wheat proteins contained in the flour used for the product formulation. The amino acids can interact with the Folin–Ciocalteu reagent, thus contributing to a positive reaction [96,97]. Our study is in accordance with these reports, as the majority of polyphenols and antioxidants present in the O3 crackers were retained after digestion, therefore suggesting this cracker formulation as a potent nutritional carrier.
This study has some limitations. The first limitation is that the phenolic compounds were quantified through Folin–Ciocalteu assay but not individually characterized by other analytical techniques such as LC-MS and HPLC, especially for comparison in the phenolic profiles of undigested and digested samples. Another limitation is that a second antioxidant activity assay was not performed to validate the results, as the identification of phenolic compounds was not performed. In addition, no evaluation was performed about the composition of the unbaked enriched crackers in sugars and asparagine to fully elucidate the changes observed in the different formulations responsible for generating Maillard compounds. Furthermore, there is a very limited number of prior research studies on the fortification of wheat products and particularly no study on crackers enriched with chickpea, barley, lupine, olive and grape seed flours and the subsequent measurements of their phenolic content and antioxidant activity before and after baking, since these alternative flours have limited applications, so far, as raw materials. Finally, there were no literature findings concerning the digestion of olive- and grape-seed-flour-enriched bakery products, so it was difficult to compare our results; therefore, our findings were correlated to those acquired in bakery products fortified with flours resulting from other olive and grape by-products.
5. Conclusions
The present study indicated that crackers could potentially represent a suitable bioactive compound vehicle, since total phenolic content and antioxidant activity demonstrated higher values after baking to the majority of the cracker formulations because of the relatively low heat treatment at short time, which is typical of cracker baking. Among the tested alternative flours, olive and grape seed flours substantially improved the phenolic content and the antioxidant activity of wheat crackers. The findings of in vitro digestion suggested that enrichment of crackers with 30% olive seed flour can increase their beneficial nutritional properties by increasing the predicted bio-accessibility of compounds with antioxidant activity. Therefore, 30% olive-seed-flour-enriched crackers could represent a functional bakery snack that could act as a carrier of a vast array of health benefits. Future studies, especially nutritional interventional and prospective epidemiological studies, are needed in order to further investigate the possible effect of the novel crackers on disease biomarkers and human health promotion.
Author Contributions
Conceptualization, A.E.K.; data curation, D.C., C.K., P.P. and O.P.; formal analysis, C.K.; funding acquisition, K.G. (Konstantinos Gkatzionis); investigation, D.C., C.K. and P.P.; methodology, D.C., C.K., P.P., O.P., K.G. (Konstantinos Giannoutsos), D.I.K., D.S., K.G. (Konstantinos Gkatzionis) and A.E.K.; project administration, A.E.K.; resources, D.C., K.G. (Konstantinos Giannoutsos), D.I.K., D.S. and K.G. (Konstantinos Gkatzionis); software, D.C., P.P. and D.S.; supervision, A.E.K.; validation, D.C., O.P. and K.G. (Konstantinos Gkatzionis); visualization, O.P. and A.E.K.; writing—original draft, D.C.; writing—review and editing, A.E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-funded by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH–CREATE–INNOVATE (project code: T2EDK-02137).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We would like to thank all the contributors in all aspects of the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmed, Z.S.; Abozed, S.S. Functional and antioxidant properties of novel snack crackers incorporated with Hibiscus sabdariffa by-product. J. Adv. Res. 2015, 6, 79–87. [Google Scholar] [CrossRef]
- Giarnetti, M.; Paradiso, V.M.; Caponio, F.; Summo, C.; Pasqualone, A. Fat replacement in shortbread cookies using an emulsion filled gel based on inulin and extra virgin olive oil. LWT 2015, 63, 339–345. [Google Scholar] [CrossRef]
- Mir, S.A.; Bosco, S.J.D.; Shah, M.A.; Santhalakshmy, S.; Mir, M.M. Effect of apple pomace on quality characteristics of brown rice based cracker. J. Saudi Soc. Agric. Sci. 2017, 16, 25–32. [Google Scholar] [CrossRef]
- Satija, A.; Hu, F.B. Cardiovascular Benefits of Dietary Fiber. Curr. Atheroscler. Rep. 2012, 14, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Holtekjølen, A.K.; Bævre, A.B.; Rødbotten, M.; Berg, H.; Knutsen, S.H. Antioxidant properties and sensory profiles of breads containing barley flour. Food Chem. 2008, 110, 414–421. [Google Scholar] [CrossRef]
- Panfili, G.; Fratianni, A.; Irano, M. Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J. Agric. Food Chem. 2003, 51, 3940–3944. [Google Scholar] [CrossRef]
- El Khoury, D.; Cuda, C.; Luhovyy, B.L.; Anderson, G.H. Beta glucan: Health benefits in obesity and metabolic syndrome. J. Nutr. Metab. 2012, 2012, 851362. [Google Scholar] [CrossRef]
- Brennan, C.S.; Cleary, L.J. The potential use of cereal (1→3,1→4)-β-d-glucans as functional food ingredients. J. Cereal Sci. 2005, 42, 1–13. [Google Scholar] [CrossRef]
- Otles, S. Cereal based functional foods and nutraceuticals. Acta Sci. Pol. Technol. Aliment. 2006, 5, 107–112. [Google Scholar]
- Lampart-Szczapa, E.; Siger, A.; Trojanowska, K.; Nogala-Kalucka, M.; Malecka, M.; Pacholek, B. Chemical composition and antibacterial activities of lupin seeds extracts. Nahr. Food 2003, 47, 286–290. [Google Scholar] [CrossRef]
- Siger, A.; Czubinski, J.; Kachlicki, P.; Dwiecki, K.; Lampart-Szczapa, E.; Nogala-Kalucka, M. Antioxidant activity and phenolic content in three lupin species. J. Food Compos. Anal. 2012, 25, 190–197. [Google Scholar] [CrossRef]
- Hamama, A.A.; Bhardwaj, H.L. Phytosterols, triterpene alcohols, and phospholipids in seed oil from white lupin. JAOCS J. Am. Oil Chem. Soc. 2004, 81, 1039–1044. [Google Scholar] [CrossRef]
- Boschin, G.; Arnoldi, A. Legumes are valuable sources of tocopherols. Food Chem. 2011, 127, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Duranti, M. Grain legume proteins and nutraceutical properties. Fitoterapia 2006, 77, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, V. Effect of lupin flour incorporation on the physical characteristics of dough and biscuits. Qual. Assur. Saf. Crop. Foods 2011, 3, 140–147. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef]
- Szefer, P.; Lebiedzin, A. Food Chemistry Vitamins B in grain and cereal—Grain food, soy-products and seeds. Food Chem. 2006, 95, 116–122. [Google Scholar] [CrossRef]
- Dixon, R.A. Phytoestrogens. Annu. Rev. Plant Biol. 2004, 55, 225–261. [Google Scholar] [CrossRef]
- Rachwa-Rosiak, D.; Nebesny, E.; Budryn, G. Chickpeas—Composition, Nutritional Value, Health Benefits, Application to Bread and Snacks: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1137–1145. [Google Scholar] [CrossRef]
- Rodríguez, R.; Jiménez, A.; Fernández-Bolaños, J.; Guillén, R.; Heredia, A. Dietary fibre from vegetable products as source of functional ingredients. Trends Food Sci. Technol. 2006, 17, 3–15. [Google Scholar] [CrossRef]
- Nikolić, N.Č.; Todorović, Z.B.; Stojanović, J.S.; Veličković, D.T.; Lazić, M.L. The fatty acids and acylglycerols in chickpea and lentil flour. Agro Food Ind. Hi Tech 2013, 24, 66–68. [Google Scholar]
- Cuevas, M.; García, J.F.; Hodaifa, G.; Sánchez, S. Oligosaccharides and sugars production from olive stones by autohydrolysis and enzymatic hydrolysis. Ind. Crops Prod. 2015, 70, 100–106. [Google Scholar] [CrossRef]
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J. Olive stone an attractive source of bioactive and valuable compounds. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef]
- Chanioti, S.; Siamandoura, P.; Tzia, C. Evaluation of Extracts Prepared from Olive Oil By-Products Using Microwave-Assisted Enzymatic Extraction: Effect of Encapsulation on the Stability of Final Products. Waste Biomass Valorization 2016, 7, 831–842. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.; Rodríguez, G.; Rodríguez, R.; Guillén, R.; Jiménez, A. Extraction of interesting organic compounds from olive oil waste. Grasas Aceites 2006, 57, 95–106. [Google Scholar] [CrossRef]
- Maestri, D.; Barrionuevo, D.; Bodoira, R.; Zafra, A.; Jiménez-López, J.; Alché, J.d.D. Nutritional profile and nutraceutical components of olive (Olea europaea L.) seeds. J. Food Sci. Technol. 2019, 56, 4359–4370. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Prenzler, P.D.; Omar, S.H.; Ismael, R.; Servili, M.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Urbani, S. Pharmacology of Olive Biophenols; Elsevier: Amsterdam, The Netherlands, 2012; Volume 6, ISBN 9780444593894. [Google Scholar]
- Ranalli, A.; Pollastri, L.; Contento, S.; Di Loreto, G.; Iannucci, E.; Lucera, L.; Russi, F. Acylglycerol and fatty acid components of pulp, seed, and whole olive fruit oils. Their use to characterize fruit variety by chemometrics. J. Agric. Food Chem. 2002, 50, 3775–3779. [Google Scholar] [CrossRef]
- Bolek, S. Olive stone powder: A potential source of fiber and antioxidant and its effect on the rheological characteristics of biscuit dough and quality. Innov. Food Sci. Emerg. Technol. 2020, 64, 102423. [Google Scholar] [CrossRef]
- Jahanbakhshi, R.; Ansari, S. Physicochemical Properties of Sponge Cake Fortified by Olive Stone Powder. J. Food Qual. 2020, 2020, 1493638. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C. Varietal differences among the phenolic profiles and antioxidant activities of seven table grape cultivars grown in the south of Italy based on chemometrics. J. Agric. Food Chem. 2011, 59, 9815–9826. [Google Scholar] [CrossRef]
- Xia, E.; Deng, G.; Guo, Y.; Li, H. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Bada, J.C.; León-Camacho, M.; Copovi, P.; Alonso, L. Characterization of grape seed oil from wines with protected denomination of origin (PDO) from Spain. Grasas Aceites 2015, 66, e085. [Google Scholar] [CrossRef]
- Horvath, G.; Wessjohann, L.; Bigirimana, J.; Monica, H.; Jansen, M.; Guisez, Y.; Caubergs, R.; Horemans, N. Accumulation of tocopherols and tocotrienols during seed development of grape (Vitis vinifera L. cv. Albert Lavallée). Plant Physiol. Biochem. 2006, 44, 724–731. [Google Scholar] [CrossRef]
- Pardo, J.E.; Fernández, E.; Rubio, M.; Alvarruiz, A.; Alonso, G.L. Characterization of grape seed oil from different grape varieties (Vitis vinifera). Eur. J. Lipid Sci. Technol. 2009, 111, 188–193. [Google Scholar] [CrossRef]
- Özvural, E.B.; Vural, H. Grape seed flour is a viable ingredient to improve the nutritional profile and reduce lipid oxidation of frankfurters. MESC 2011, 88, 179–183. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Saura-calixto, F.; Gon, I. Food Chemistry Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef]
- Koli, R.; Erlund, I.; Jula, A.; Marniemi, J.; Mattila, P.; Alfthan, G. Bioavailability of various polyphenols from a diet containing moderate amounts of berries. J. Agric. Food Chem. 2010, 58, 3927–3932. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef] [PubMed]
- Cilla, A.; Bosch, L.; Barberá, R.; Alegría, A. Effect of processing on the bioaccessibility of bioactive compounds—A review focusing on carotenoids, minerals, ascorbic acid, tocopherols and polyphenols. J. Food Compos. Anal. 2018, 68, 3–15. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C. Polyphenols: Food source and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.Y.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Isik, F.; Yapar, A. Effect of tomato seed supplementation on chemical and nutritional properties of tarhana. J. Food Meas. Charact. 2017, 11, 667–674. [Google Scholar] [CrossRef]
- Drakou, M.; Birmpa, A.; Koutelidakis, A.E.; Komaitis, M.; Panagou, E.Z.; Kapsokefalou, M. Total antioxidant capacity, total phenolic content and iron and zinc dialyzability in selected Greek varieties of table olives, tomatoes and legumes from conventional and organic farming. Int. J. Food Sci. Nutr. 2015, 66, 197–202. [Google Scholar] [CrossRef]
- Kapsokefalou, M.; Miller, D.D. Effects of Meat and Selected Food Components on the Valence of Nonheme Iron during In Vitro Digestion. J. Food Sci. 1991, 56, 352–355. [Google Scholar] [CrossRef]
- Argyri, K.; Birba, A.; Miller, D.D.; Komaitis, M.; Kapsokefalou, M. Predicting relative concentrations of bioavailable iron in foods using in vitro digestion: New developments. Food Chem. 2009, 113, 602–607. [Google Scholar] [CrossRef]
- Argyri, K.; Athanasatou, A.; Bouga, M.; Kapsokefalou, M. The potential of an in vitro digestion method for predicting glycemic response of foods and meals. Nutrients 2016, 8, 209. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Koutelidakis, A.E.; Andritsos, N.D.; Kabolis, D.; Kapsokefalou, M.; Drosinos, E.H.; Komaitis, M. Antioxidant and antimicrobial properties of tea and aromatic plant extracts against bacterial foodborne pathogens: A comparative evaluation. Curr. Top. Nutraceutical Res. 2016, 14, 133–142. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kandyliari, A.; Elmaliklis, I.N.; Kontopoulou, O.; Tsafkopoulou, M.; Komninos, G.; Ntzatha, C.; Petsas, A.; Karantonis, H.C.; Koutelidakis, A.E. An epidemiological study report on the antioxidant and phenolic content of selected mediterranean functional foods, their consumption association with the body mass index, and consumers purchasing behavior in a sample of healthy greek adults. Appl. Sci. 2021, 11, 7818. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Kaur, M. Quantification of phenolic acids and antioxidant potential of wheat rusks as influenced by partial replacement with barley flour. J. Food Sci. Technol. 2020, 57, 3782–3791. [Google Scholar] [CrossRef]
- Aly, A.A.; El-Deeb, F.E.; Abdelazeem, A.A.; Hameed, A.M.; Abdulaziz Alfi, A.; Alessa, H.; Alrefaei, A.F. Addition of Whole Barley Flour as a Partial Substitute of Wheat Flour to Enhance the Nutritional Value of Biscuits. Arab. J. Chem. 2021, 14, 103112. [Google Scholar] [CrossRef]
- Sharma, P.; Gujral, H.S. Cookie making behavior of wheat-barley flour blends and effects on antioxidant properties. LWT 2014, 55, 301–307. [Google Scholar] [CrossRef]
- Narwal, S.; Kumar, D.; Sheoran, S.; Verma, R.P.S.; Gupta, R.K. Hulless barley as a promising source to improve the nutritional quality of wheat products. J. Food Sci. Technol. 2017, 54, 2638–2644. [Google Scholar] [CrossRef]
- Gupta, M.; Bawa, A.S.; Abu-Ghannam, N. Effect of barley flour and freeze-thaw cycles on textural nutritional and functional properties of cookies. Food Bioprod. Process. 2011, 89, 520–527. [Google Scholar] [CrossRef]
- Sharma, P.; Gujral, H.S. Antioxidant potential of wheat flour chapattis as affected by incorporating barley flour. LWT 2014, 56, 118–123. [Google Scholar] [CrossRef]
- Holtekjølen, A.K.; Knutsen, S.H. Antioxidant Activity and Phenolics in Breads with Added Barley Flour; Elsevier Inc.: Amsterdam, The Netherlands, 2011; ISBN 9780123808868. [Google Scholar]
- Altan, A.; McCarthy, K.L.; Maskan, M. Effect of extrusion process on antioxidant activity, total phenolics and β-glucan content of extrudates developed from barley-fruit and vegetable by-products. Int. J. Food Sci. Technol. 2009, 44, 1263–1271. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, C.R. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Technol. 2000, 11, 340–346. [Google Scholar] [CrossRef]
- Abd El-Maasoud, S.; Ghaly, M. Influence of Addition Sweet Lupine Flour on Quality and Antioxidant Characteristics of Biscuits. J. Food Dairy Sci. 2018, 9, 163–170. [Google Scholar] [CrossRef]
- Rumiyati, R.; James, A.P.; Jayasena, V. Effects of lupin incorporation on the physical properties and stability of bioactive constituents in muffins. Int. J. Food Sci. Technol. 2015, 50, 103–110. [Google Scholar] [CrossRef]
- Plustea, L.; Negrea, M.; Cocan, I.; Radulov, I.; Tulcan, C.; Berbecea, A.; Popescu, I.; Obistioiu, D.; Hotea, I.; Suster, G.; et al. Lupin (Lupinus spp.)-Fortified Bread: A Sustainable, Nutritionally, Functionally, and Technologically Valuable Solution for Bakery. Foods 2022, 11, 2067. [Google Scholar] [CrossRef] [PubMed]
- Rumiyati; Jayasena, V.; James, A.P. Total Phenolic and Phytosterol Compounds and the Radical Scavenging Activity of Germinated Australian Sweet Lupin Flour. Plant Foods Hum. Nutr. 2013, 68, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; De Angelis, D.; Squeo, G.; Difonzo, G.; Caponio, F.; Summo, C. The effect of the addition of apulian black chickpea flour on the nutritional and qualitative properties of durum wheat-based bakery products. Foods 2019, 8, 504. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Muy-Rangel, D.; De La Garza, A.L.; Rubio-Carrasco, W.; Pérez-Meza, B.; Araujo-Chapa, A.P.; Gutiérrez-Álvarez, K.A.; Urías-Orona, V. Dietary fiber from chickpea (Cicer arietinum) and soybean (Glycine max) husk byproducts as baking additives: Functional and nutritional properties. Molecules 2019, 24, 991. [Google Scholar] [CrossRef]
- Segev, A.; Badani, H.; Galili, L.; Hovav, R.; Kapulnik, Y.; Shomer, I.; Galili, S. Effects of Baking, Roasting and Frying on Total Polyphenols and Antioxidant Activity in Colored Chickpea Seeds. Food Nutr. Sci. 2012, 3, 369–376. [Google Scholar] [CrossRef]
- Collar, C.; Jiménez, T.; Conte, P.; Fadda, C. Impact of ancient cereals, pseudocereals and legumes on starch hydrolysis and antiradical activity of technologically viable blended breads. Carbohydr. Polym. 2014, 113, 149–158. [Google Scholar] [CrossRef]
- Millar, K.A.; Barry-Ryan, C.; Burke, R.; Hussey, K.; McCarthy, S.; Gallagher, E. Effect of pulse flours on the physiochemical characteristics and sensory acceptance of baked crackers. Int. J. Food Sci. Technol. 2017, 52, 1155–1163. [Google Scholar] [CrossRef]
- Vogrinčič, M.; Timoracka, M.; Melichacova, S.; Vollmannova, A.; Kreft, I. Degradation of rutin and polyphenols during the preparation of tartary buckwheat bread. J. Agric. Food Chem. 2010, 58, 4883–4887. [Google Scholar] [CrossRef]
- Argyri, E.A.; Piromalis, S.P.; Koutelidakis, A.; Kafetzopoulos, D.; Petsas, A.S.; Skalkos, D.; Nasopoulou, C.; Dimou, C.; Karantonis, H.C. Olive paste-enriched cookies exert increased antioxidant activities. Appl. Sci. 2021, 11, 5515. [Google Scholar] [CrossRef]
- Acun, S.; Gül, H. Effects of grape pomace and grape seed flours on cookie quality. Qual. Assur. Saf. Crop. Foods 2014, 6, 81–88. [Google Scholar] [CrossRef]
- Maman, R.; Yu, J. Chemical Composition and Particle Size of Grape Seed Flour and Their Effects on the Characteristics of Cookies. J. Food Res. 2019, 8, 111. [Google Scholar] [CrossRef]
- Antonic, B.; Dordevic, D.; Jancikova, S.; Holeckova, D.; Tremlova, B.; Kulawik, P. Effect of grape seed flour on the antioxidant profile, textural and sensory properties ofwaffles. Processes 2021, 9, 131. [Google Scholar] [CrossRef]
- Yalcin, E.; Ozdal, T.; Gok, I. Investigation of textural, functional, and sensory properties of muffins prepared by adding grape seeds to various flours. J. Food Process. Preserv. 2022, 46, e15316. [Google Scholar] [CrossRef]
- Yalcin, E.; Gok, I.; Ozdal, T. Effect of Grape Seed Flour on the Phenolic Profile, Antioxidant Capacity and Sensory Properties of Muffins. Lat. Am. Appl. Res. 2022, 52, 213–220. [Google Scholar] [CrossRef]
- Hoye, C. Value-Added Product Development Utilizing Washington State Grape Seed Flour; Washington State University: Pullman, WA, USA, 2009. [Google Scholar]
- Lee, S.C.; Kim, J.H.; Jeong, S.M.; Kim, D.R.; Ha, J.U.; Nam, K.C.; Ahn, D.U. Effect of far-infrared radiation on the antioxidant activity of rice hulls. J. Agric. Food Chem. 2003, 51, 4400–4403. [Google Scholar] [CrossRef] [PubMed]
- Meral, R.; Erim Köse, Y. The effect of bread-making process on the antioxidant activity and phenolic profile of enriched breads. Qual. Assur. Saf. Crop. Foods 2019, 11, 171–181. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Mennella, C.; Barba, F.; Russo, M.; Russo, G.L.; Krome, K.; Erbersdobler, H.F.; Faist, V.; Fogliano, V. Characterization of coloured compounds obtained by enzymatic extraction of bakery products. Food Chem. Toxicol. 2003, 41, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Žilić, S.; Kocadağli, T.; Vančetović, J.; Gökmen, V. Effects of baking conditions and dough formulations on phenolic compound stability, antioxidant capacity and color of cookies made from anthocyanin-rich corn flour. LWT 2016, 65, 597–603. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of phenolic compounds and their antioxidant activity in fruits and cereals. Talanta 2007, 71, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Saura-Calixto, F. Literature data may underestimate the actual antioxidant capacity of cereals. J. Agric. Food Chem. 2005, 53, 5036–5040. [Google Scholar] [CrossRef]
- Fallico, B.; Grasso, A.; Arena, E. Hazardous Chemical Compounds in Cookies: The Role of Sugars and the Kinetics of Their Formation during Baking. Foods 2022, 11, 4066. [Google Scholar] [CrossRef]
- Cincotta, F.; Brighina, S.; Condurso, C.; Arena, E.; Verzera, A.; Fallico, B. Sugars Replacement as a Strategy to Control the Formation of α-Dicarbonyl and Furanic Compounds during Cookie Processing. Foods 2021, 10, 2101. [Google Scholar] [CrossRef]
- Purlis, E. Browning development in bakery products—A review. J. Food Eng. 2010, 99, 239–249. [Google Scholar] [CrossRef]
- Tu, J.; Brennan, M.A.; Wu, G.; Bai, W.; Cheng, P.; Tian, B.; Brennan, C.S. Delivery of phenolic compounds, peptides and β-glucan to the gastrointestinal tract by incorporating dietary fibre-rich mushrooms into sorghum biscuits. Foods 2021, 10, 1812. [Google Scholar] [CrossRef]
- Marinelli, V.; Padalino, L.; Conte, A.; Del Nobile, M.A.; Briviba, K. Red grape marc flour as food ingredient in durum wheat spaghetti: Nutritional evaluation and bioaccessibility of bioactive compounds. Food Sci. Technol. Res. 2018, 24, 1093–1100. [Google Scholar] [CrossRef]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red wine—Their stability under simulated gastrointestinal digestion. Phytochemistry 2005, 66, 2540–2548. [Google Scholar] [CrossRef]
- Rocchetti, G.; Rizzi, C.; Cervini, M.; Rainero, G.; Bianchi, F.; Giuberti, G.; Lucini, L.; Simonato, B. Impact of grape pomace powder on the phenolic bioaccessibility and on in vitro starch digestibility of wheat based bread. Foods 2021, 10, 507. [Google Scholar] [CrossRef]
- Cedola, A.; Cardinali, A.; Del Nobile, M.A.; Conte, A. Amalia Conte Enrichment of Bread with Olive Oil Industrial By-Product. J. Agric. Sci. Technol. B 2019, 9, 119–127. [Google Scholar] [CrossRef]
- Cedola, A.; Cardinali, A.; D’Antuono, I.; Conte, A.; Del Nobile, M.A. Cereal foods fortified with by-products from the olive oil industry. Food Biosci. 2020, 33, 100490. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, D.; Świeca, M.; Sȩczyk, Ł.; Rózyło, R.; Szymanowska, U. Bread enriched with Chenopodium quinoa leaves powder—The procedures for assessing the fortification efficiency. LWT 2015, 62, 1226–1234. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
