Abstract
Globally, among different types of cancers, breast cancer is identified as the chief cause of mortality among females, and it is a challenge to find new effective treatment strategies with minimal side effects and increased efficacy. Plants are an integral part of the traditional indigenous healthcare system and are becoming the concrete source of new drug discovery. Thus, there is a need to obtain a scientific basis for applying traditionally used plants in cancer treatments that may harbour novel phytochemicals. Therefore, this study aims to investigate the antioxidant and anticancer potential of selected plants of ethnobotanical importance. Five plants of ethnobotanical importance were selected and screened to determine their antioxidant potential through various in vitro free radical scavenging assays (such as DPPH, ABTS, hydroxyl, and superoxide radical scavenging), ferric chelation, and total antioxidant potential, and the total phenolic and flavonoid content was estimated for the selected plants. In contrast, the anticancer potential of crude plant extracts was assessed using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) against different breast cancer (MCF-7, MDA-MB-231, and MDA-MB-435S) and hepatic cancer cell lines (HepG2), and human PBMCs (peripheral blood mononuclear cells) were used for toxicity studies. The MTT results showed that among all of the crude plant extracts (CAN = Etlingera linguiformis, SES = Sesbania grandiflora, LEX = Smilax ovalifolia, DES = Desmodium triflorum, and CA = Chenopodium album), it was CAN and LEX that showed the best cytotoxic potential on exposed breast cancer cell lines in contrast to SES, DES, and CA. In addition, at the selected dosages that were exposed to breast cancer cells, none of the extracts from any of the five plants showed any cytotoxicity against human PBMCs. Thus, the crude extracts can be explored further for chemopreventive and anticancer activity on murine models to understand their underlying mechanism for effective cancer management.
1. Introduction
Among the various noncommunicable diseases (NCDs), cancer stands second in mortality [1]. Globally, in 2020, about 19.3 million new cases of cancers were diagnosed and 10 million cancer deaths were reported [2], with the expected rise of new cases to 32.2 million by 2040 [3,4], which is a matter of concern affecting humanity worldwide. Among various types of cancer, breast cancer is the most common malignancy spotted globally in females. The apparent factors responsible for the development of breast cancer are hormonal and environmental, strongly related to oxidative stress induced by reactive oxygen species [5]. Conventional therapies, such as chemotherapy, surgery, and radiation therapy, where radiotherapy and chemotherapeutic agents kill cancer cells along with normal cells by inducing ROS, which leads to apoptosis or necrosis of cells, and results in several side effects that make life miserable and thus deteriorate the quality of life of both patients and caregivers [6]. Therefore, alternative medicines offering optimal therapy with minimal adverse effects become the top priority to improve patient quality of life [7]. For thousands of years, plants have been known to cure various diseases due to the presence of phytochemicals [8]. Recently, plant-based products have gained global interest from researchers looking to discover drugs of plant-based origins for use against cancer; a literature survey further summarised the anticancer activity of these phytochemical-rich plants, demonstrating their cytotoxic effect against different cancer cell lines [9,10,11,12,13,14,15,16,17]. Apart from the anticancer activity displayed by plants of medicinal importance, by modulating the signalling cascades correlated with cell cycle regulation and apoptosis, it has also been found that these plants display potent anti-inflammatory, antioxidant, and chemotherapeutic effects [12,13,14,15,16,17,18]. Interestingly these phytochemicals exhibit more cytotoxic effects on cancer cells than on normal cells [6,19] due to their pro-oxidant or antioxidant role depending on their exposure dose and vicinity [6]. Therefore, high performance is essential for plant-based products to act as therapeutic agents against cancer.
In this study, selected five plants of ethnobotanical importance (Etlingera linguiformis, Sesbania grandiflora, Smilax Ovalifolia, Desmodium triflorum, and Chenopodium album), which have been found to grow commonly in the north-eastern part of India, collected specifically from Assam and Meghalaya. North eastern region of India which is also hot spot of biodiversity in flora and fauna and inhabited by different ethnic tribes, plants are integral part of their day today life style and are being used for various medicinal purpose and in culinary activity, such as Etlingera linguiformis (Zingiberaceae) used to cure sore throat, jaundice, leafy vegetables etc. [20], whereas the anticancer activity of the dried flower extract of Etlingera elatior plant belongs to the same genus of family Zingiberaceae and exhibits anticancer activity against breast cancer cell lines [21]. Sesbania grandiflora (Fabaceae), used by locals for the treatment of nasal catarrh and stomatalgia, and used as an antipyretic, etc. [22], also showed anticancer efficacy against different cancer cell lines, such as HepG2, A549, HCT-15, and others [23]. Smilax zylenica, a plant of the genus Smilax (Smilacaceae), has been documented to show antioxidant and anticancer potential, whereas the potent anticancer efficacy of Smilax ovalifolia (Smilacaceae), although used by locals for the treatment of skin problems, urinary complaints, wound healing, tuberculosis, rheumatic arthritis, muscular sprain, jaundice, dysentery, etc. [24,25], is still yet to be explored. Desmodium triflorum (Fabaceae), used in malaria, toothache, kidney problems, gastric ailments, bronchitis, fever, liver complaints, etc., also displays strong antioxidant activity but induces insignificant toxicity against liver cancer cells (HepG2) and human prostate carcinoma cell lines (PC3) [26,27]. Chenopodium album (Chenopodiaceae) is used as an anti-helminthic and an aphrodisiac and is used for constipation, intestinal worms, and skin ailments, among others [28]. Nevertheless, their antioxidant and anticancer efficacies are not well explored against breast cancer. Free radicals, such as superoxide anions (O2•–) and hydroxyl radicals (•OH), and reactive oxygen species, e.g., H2O2 generated during oxidative stress, are found to be a common cause of various diseases, including cancer, which are neutralized by antioxidants [29]. Therefore, free radical scavenging activity assays such as ABTS, DPPH, SO, and OH are widely known methods conducted to evaluate the antioxidant potential of plant extracts, whereas their reducing potential is estimated by using the reduction of ferricyanide complexes into ferrous forms, and antioxidant-rich plants tend to show anticancer activity [27,29]. Therefore, this study aimed to investigate the antioxidant and anticancer potential of hydro-alcoholic leaf extracts of collected plants in following manner:
- By using cell-free chemical-based methods using various free radical scavenging assays, such as DPPH, superoxide reduction, hydroxyl reduction, ABTS total reducing, and Fe2+ chelation
- whereas, Anticancer properties of plant extracts (PE) of selected five (CAN = Etlingera linguiformis, SES = Sesbania grandiflora, LEX = Smilax ovalifolia, DES = Desmodium triflorum, and CA = Chenopodium album) plants was investigated by using an MTT assay against breast cancer cell lines (MCF-7, MDA-MB-231, and MDA-MB-435S), and hepatic cancer cell lines (HepG2) in contrast to normal PBMCs.
2. Materials and Methods
- (a)
- General chemical and cell lines
2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), potassium persulfate (K2S2O8), 2-deoxy-2-ribose, sodium nitroprusside (SNP), potassium ferricyanide, triton X-100, ethylenediaminetetraacetic acid (EDTA), bovine serum albumin (BSA), sodium pyruvate, thiobarbituric acid (TBA), sodium pyruvate, and L-glutamine were obtained from Sigma-Aldrich, USA. Folin–Ciocalteu reagent, xylenol orange, and N,N-dimethyl-4-nitrosoaniline were obtained from Merck, Mumbai, India. Butylated hydroxyanisole (BHA), nitroblue tetrazolium chloride (NBT), ferrozine trichloroacetic acid (TCA), ascorbic acid, gallic acid, and 1,1-Diphenyl-2-Picrylhydrazyl (DPPH), were purchased from HiMedia Laboratories, Mumbai, India. Cell culture-wares were purchased from Thermo Scientific, USA. Fetal bovine serum (FBS) and antibiotic antimycotic solution were obtained from Thermo Fisher Scientific, Waltham, MA, USA.
- (b)
- Plant material collection
Plant samples were collected from the two states, namely Assam (approximately 50–80 km north of Guwahati) and Meghalaya (from Phulbari town), India. Selected plants for the study were identified at the Department of Botany, Guwahati University, Guwahati, Assam (India) and further authenticated at the Botanical Survey of India (BSI), Eastern Regional Centre, Shillong, Meghalaya (India), vide identification No.: BSI/ERC/Tech./2019/03, dated 2 April 2019.
- (c)
- Extract preparation
Healthy fresh leaves were collected from the plants and washed, followed by shade-drying. They were then chopped and ground into a powdered form using a grinder, macerated thrice with hydro alcohol (80% Ethanol), and left for seven days at room temperature in the dark. The extracts obtained were filtered through Whatman No.1 filter paper, dried to remove the solvents, and, finally, semi-solid forms of the extracts were stored at −20 °C. The stock solution of dry extract from herbal raw materials obtained from individual plant species at a concentration of 100 mg/mL was used in all of the subsequent studies.
- (d)
- Total phenolic and flavonoid content
The total phenolic content (TPC) was measured using the method from Singleton and Rossi (1965) [30] with little modification. In brief, to a mixture of plant extract and FC reagent (diluted 10 times), 0.4 mL of Na2CO3 solution was added, and the mixture was kept for 2 h at 22 °C, with absorbance being recorded at 725 nm. In contrast, total flavonoid content was estimated using the protocol of Zhishen et al. (1999) [31]. Briefly, different concentrations were mixed with NaNO2 (1.5%) for 5 min at 25 °C. Later, NaOH (200 mM) was added, and absorbance was measured at 510 nm.
- (e)
- ABTS radical scavenging activity
The ABTS radical scavenging assay was estimated, as described by Arnao et al. (2001) [32]. As per the protocol, ABTS+ radical was pre-generated by mixing (7.4 mM) ABTS stock solution with (2.6 mM) potassium persulfate (final concentration) and incubated in the dark for 12–16 h at room temperature. After stabilization, this working solution was mixed with various concentrations of plant extracts and kept for 2 h. Later, the OD was recorded at 734 nm, and total antioxidant activity was calculated in terms of % scavenging of ABTS+ radicals.
% Of scavenging Activity (SA) = (AC − ATS)/AC × 100
AC = absorbance of the control, ATS = absorbance of the test sample
- (f)
- DPPH radical
DPPH free radical scavenging activity was assessed as described by Kitts et al. (2000) and Shahidi et al. (2007) with slight modification [33,34]. Briefly, various concentrations of extract or BHA were added to a 0.135 mM DPPH solution in methanol, maintaining a total volume of 1 mL, which was vortexed, and after 30 min of incubation in dark the absorbance was recorded at 517 nm using a gas spectrophotometer against a blank consisting of methanol, and its DPPH scavenging activity was calculated.
- (g)
- Superoxide radical scavenging activity
The superoxide radical scavenging activity was estimated as described by Srinivasan et al. (2007) and Kumara et al. (2012) [35,36]. Briefly, 0.1 mL of NBT solution was added to the alkaline DMSO to which 0.3 mL of plant extract of various concentrations was added, and the OD was recorded at 560 nm against a blank containing DMSO.
- (h)
- Hydroxyl radical scavenging activity
Hydroxyl radical scavenging activity was performed as described by Halliwell B and Gutteridge [37,38]. Briefly, various concentrations of plant extract were added to a reaction mixture (containing 20 mM FeCl3, 0.1 mM EDTA, 0.28 mM 2-deoxyribose, 0.2 mM H2O2, and 0.3 mM ascorbic acid). After incubation for 1 h at 37 °C, 1 mL of TCA and 1 mL of TBA were added, and the mixtures were heated for 15 min in a boiling water bath at 80 °C. After cooling down, the OD was recorded at 532 nm against a blank reagent.
- (i)
- Ferric chelation inhibitory activity
Ferric chelation inhibitory activity was performed as described by Dinis et al., 1994 [39]. In brief, a mixture containing plant extract or EDTA and 1 mM FeCl2 was prepared, to which 1 mM ferrozine was added, the mixture volume was adjusted to 1 mL with methanol, shaken vigorously, left for 10 min, and the absorbance was then recorded at 562 nm.
- (j)
- Total reducing power
Reducing power was assessed as described by Zhu et al. (2011) [40] with slight modification. A volume of 1% potassium ferricyanide was added to an equal volume of 0.2 M potassium buffer (pH 6.6) along with various concentrations of plant extract/ascorbic acid and the mixture was incubated for 20 min at 50 °C. Later 10% TCA was added and the mixture was centrifuged at 3000 rpm for 10 min. A total of 0.5 mL of each MQ water and 0.1% ferric chloride was added to the supernatant and mixed thoroughly, and the absorbance was recorded at 700 nm.
- (k)
- PBMCs and Cell linesCulture condition
Pre-informed consent was obtained from the volunteers who donated blood for obtaining the peripheral blood mononuclear cells (PBMCs) needed for this experiment. Ethical committee permission was also obtained from the Tezpur University Ethical Committee (TUEC), Tezpur University, Tezpur (Protocol no. DoRD/TUEC/10-14/4361 dated 28.03.2014) for the use of human blood. PBMCs were isolated by density gradient centrifugation and seeded in RPMI-1640 media supplemented with 10% FBS. Whereas cancer cell lines MCF-7 cells were cultured in MEM medium containing 10% foetal bovine serum, and MDA-MB-231 and MDA-MB-435S cells were cultured in Leibovitz-15 (L-15), HepG-2 cells were cultured in DMEM medium containing 10% FBS, 100 U/mL penicillin, and 100 mg/L streptomycin. All cell lines were grown in a stable environment with 5% CO2 at 37 °C.
- (l)
- Cell viability using an MTT assay
Cell viability/cell proliferation ability by plant extract was determined using a thiazolyl blue tetrazolium bromide (MTT) assay [41]. All of the cell lines MCF-7, MDA-MB-231, MDA-MB-435S, and HepG2 cells 2 × 103 cells were seeded per well in 96-well plates and allowed to adhere overnight in a CO2 incubator at 37 °C. Cells were then treated with three different concentrations of plant extract (25, 50, and 100 µg/mL), (0.1%) DMSO was taken as a vehicle control and all were incubated for 48 h at 37 °C. To study the effect of plant extract on human peripheral blood mononuclear cells (PBMCs), 2 × 103 cells were seeded per well in a 96-well plate. A similar method was followed for HepG2 cells with a slight modification in the treatment conditions of the plant extracts in response to H2O2 treatment (pre-, post-and co-treatment) to analyze the protective role of plant extracts in response to H2O2-mediated toxicity to HepG2 cells at various time intervals. Before experiment termination, 10 µL of MTT reagent was added to each well and incubated for a further 2 h. Later, the supernatant was aspirated, 200 µL of DMSO was added, and the OD was recorded at 570 nm.
- (m)
- Intracellular reactive oxygen species (ROS) measurement
The generation of intracellular ROS was monitored using DCFH-DA [42]. The DCFH-DA passively enters the cell, reacting with ROS to form the highly fluorescent compound dichlorofluorescein (DCF). To estimate intracellular ROS, cells were grown in a 6-well plate at 1 × 105 cells/well and allowed to adhere overnight in a CO2 incubator at 37 °C. Then, cells were treated with three different concentrations of plant extract (25, 50, and 100 µg/mL) and incubated for 48 h at 37 °C. At the end of the respective treatment period, cells were washed twice with PBS, followed by incubation with an equal volume of the assay media (20 mM TrisHCl, 130 mM KCl, 5 mM MgCl2, 20 mM NaH2PO4, 30 mM glucose, and 5 µM DCFDA) at 37 °C for 15 min. The formation of DCF was measured at the excitation wavelength of 488 nm and emission wavelength of 610 nm for 10 min using a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
- (n)
- Statistical analysis
All of the data were expressed as mean ± SEM, n = 3, from three independent experiments. Statistical analysis was performed using one-way and two-way ANOVA along with Tukey’s multiple comparison tests. Three levels of significant differences were considered, a p ≤ 0.001, b p ≤ 0.01, and c p ≤ 0.05 (PRISM software, Boston, MA, USA). The IC50 values were calculated using CompuSyn software [43].
3. Results
- (a)
- Total phenolic and flavonoid content
Phenolic and flavonoids are two major compounds present in plants that possess potent antioxidative and free radical scavenging activities. Phytochemical analysis revealed the presence of total phenolic content as 4.45 ± 0.09, 3.64 ± 0.04, 3.64 ± 0.04, 1.87 ± 0.04, 4.49 ± 0.07, and 19.75 ± 2.79 µg/mL gallic acid equivalent (GAE) per mg for CAN, LEX, SES, DES, and CA, respectively. Further total flavonoid contents in CAN, LEX, SES, DES, and CA were determined and found to be 29.29 ± 0.48, 23.51 ± 1.75, 15.49 ± 0.48, 34.50 ± 2.74, and 23.26 ± 2.31 µg/mL epicatechin equivalent (ECE) per mg of the extract, respectively (Table 1).

Table 1.
Total phenolic and flavonoid contents.
- (b)
- ABTS radical
The total antioxidant activity of the CAN, LEX, SES, DES, and CA was evaluated using ABTS+ radical. In this assay, ABTS+ radical was generated by mixing ABTS with potassium persulfate overnight. The antioxidant reduces the blue-coloured ABTS+ radical into ABTS, lessening the blue colouring [32]. As shown in (Figure 1a), the extracts had significant antioxidant activities at all doses. The ABTS+ radical scavenging activity of the five crude extracts was relatively significant compared to the standard with the IC50 value for CA, as shown in (Table 2). The relative IC50 values of all five plants were SES > CAN > LEX > DES > CA.
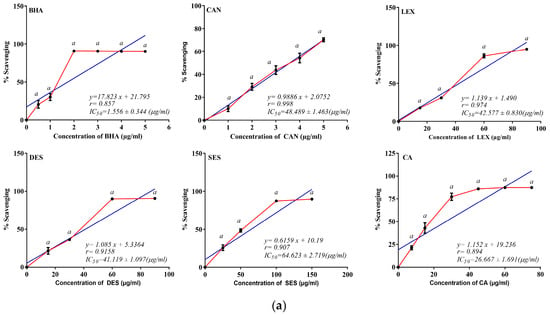

Figure 1.
Antioxidative and free radical scavenging activity of CAN, LEX, SES, DES, and CA for (a) ABTS, and (b) DPPH were evaluated using in vitro cell-free chemical-based reactions. Values are expressed as mean ± SEM; n = 3; values were significant (a p ≤ 0.001, b p ≤ 0.01, and c p ≤ 0.05) when compared to the control. BHA was taken as a positive control for both ABTS and DPPH radical activity.

Table 2.
Antioxidative and free radical scavenging activity of CAN, LEX, DES, SES, and CA: DPPH, superoxide, hydroxyl radical scavenging activity, Fe3+ reducing activities, Fe2+ chelation inhibitory activity, and ABTS were evaluated using in vitro cell-free chemical-based reactions. Abbreviations: BHA: butylated hydroxy anisole; AA: ascorbic acid; GA: gallic acid; and EDTA: Ethylene diamine tetra acetic acid is represented in the parentheses for the corresponding standard (STD).
- (c)
- DPPH
The DPPH assay is a widely used method for determining antioxidant and free radical scavenging properties. The violet-coloured DPPH is a synthetic, stable radical that accepts an electron or hydrogen from an antioxidant and becomes a stable DPPH-H. The discolouration of the DPPH can be spectrophotometrically measured at 517 nm [33]. The DPPH radical scavenging activity of all five plants has been shown (Figure 1b), indicating that CAN, LEX, SES, DES, and CA scavenge the DPPH radical in a dose-dependent manner. DPPH free radical scavenging activity is excellent in the case of CAN (IC50 ≈ 50.80 µg/mL) (Table 2). Further, relative IC50 values for the five plants were observed as SES > CA > DES > LEX > CAN.
- (d)
- Superoxide radical scavenging activity (SO)
In the present study, the alkaline DMSO method evaluates superoxide radical scavenging activity [35,36]. In this method, superoxide radicals were generated by adding sodium hydroxide to air-saturated DMSO, and the generated superoxide reduces nitro-blue tetrazolium (NBT) into formazan dye at room temperature, which can be measured at 560 nm. As shown in (Figure 2a), all five plant extracts showed a significant dose-dependent increase in superoxide radical scavenging activity (a p ≤ 0.001), with relative IC50 values observed in the order of CA > SES > DES > LEX > CAN when compared to the standard compound, ascorbic acid. Relatively higher values of IC50 against superoxide radical scavenging activity were observed for all of the samples except for CAN, which displayed relatively better scavenging activity (IC50 ≈ 69.32 µg/mL) (Table 2).

Figure 2.
The antioxidative and free radical scavenging activity of CAN, LEX, SES, DES, and CA against (a) superoxide and (b) hydroxyl radicals were evaluated using in vitro cell-free chemical-based reactions. Values were expressed as mean ± SEM; n = 3; values are significant a p ≤ 0.001, b p ≤ 0.01, and c p ≤ 0.05 when compared to the control. Ascorbic acid and gallic acid were used as a positive control for both superoxide and hydroxyl radical activity.
- (e)
- Hydroxyl radical
The hydroxyl radical (•OH) is a major active oxygen species that cause lipid peroxidation and exerts various biological damages. (•OH) radicals were generated by incubating Fe3+-EDTA with ascorbic acid and H2O2 and reacting this mixture with 2-deoxyribose to yield a malondialdehyde (MDA)-like product, which, upon heating, formed a pink-coloured chromogen. Antioxidant substances remove the hydroxyl radicals from the sugar and prevent their action [37,38]. The results of this study indicated that all concentrations of all studied plant extracts showed a significant (a p ≤ 0.001) dose-dependent increase in their free radical scavenging activity. The IC50 values for the hydroxyl radical scavenging activities of all five plant extracts were determined and compared against gallic acid (GA) as a standard (IC50 ≈ 285.3 µg/mL), as shown in (Table 2) and (Figure 2b), and the relative IC50 values were in the order CA > CAN > SES > LEX > DES.
- (f)
- Ferric chelation (FC)
Transition metals such as iron induce lipid peroxidation through the Fenton reaction and accelerate lipid peroxidation by converting lipid hydroperoxides into peroxyl and alkoxyl radicals, which later initiate a chain reaction. Therefore, metal chelating activity is significant in maintaining healthy cellular status [39]. The results of this study present data which demonstrate the ion chelation activity of CAN, LEX, SES, DES, and CA, with the relative IC50 values CAN > DES > SES > LEX > CA. EDTA is used as a standard with an IC50 ≈ 3.586 µg/mL. All of the extracts showed relatively sound chelating effects and CA exhibited the minimum IC50 ≈ 105.55 µg/mL (Table 2 and Figure 3).

Figure 3.
The Fe2+ chelation inhibitory activities of CAN, LEX, SES, DES, and CA were evaluated using in vitro cell-free chemical-based reactions. Values are expressed as mean ± SEM; n = 3; values were significant a p ≤ 0.001, b p ≤ 0.01, and c p ≤ 0.05 when compared to the control. EDTA was used as a positive control.
- (g)
- Total reducing power
The reducing potential of substances from Fe3+ to Fe2+ (total reducing power) is a significant indicator of antioxidant activity, exerting antioxidant activity by preventing free radical chain initiation by donating hydrogen atoms, decomposing peroxides, and scavenging free radicals [40]. Here, we found that the reducing potential for CAN, LEX, SES, DES, and CA was dose-dependent, with ascorbic acid taken as standard, as shown in (Figure 4). The corresponding IC50 values were found to be 467.83 ± 0.8.75, 887.66 ± 10.26, 951.00 ± 1.00, 889.01 ± 6.55, 15.68 ± 0.971 and 28.118 ± 0.281 µg/mL for CAN, LEX, SES, DES, and CA, respectively, as shown in (Table 2).

Figure 4.
Fe3+ reducing ability of CAN, LEX, SES, DES, and CA: The reductive ability of CAN, LEX, SES, DES, CA, and ascorbic acid was evaluated using in vitro cell-free chemical-based reactions. Values are expressed as mean ± SEM; n = 3; values are significant when a p ≤ 0.001, b p ≤ 0.01, and c p ≤ 0.05 compared to the control. Ascorbic acid was taken as the positive control.
- (h)
- Cell viability by MTT
All of the crude extracts (CAN, LEX, SES, DES, and CA) showed anticancer activity with IC50 values below 300 µg/mL against all of the studied breast cancer cell types (MCF-7, MDA-MB-231, and MDA-MB-435S). However, all CAN (MCF-7 ≈ 117.62 ± 2.54, MDA-MB 231 ≈ 109.21 ± 3.51, and MDA-MB-435S ≈ 126.82 ± 2.35 µg/mL) and LEX (MCF-7 ≈ 104.81 ± 7.54, MDA-MB 231 ≈ 132 ± 2.46, and MDA-MB-435S ≈ 218.20 ± 3.45 µg/mL) showed low IC50 against the exposed breast cancer cell line in contrast to SES, DES, and CA. There were some exceptions observed for SES, which exhibited a relatively high IC50 value against MDA-MB-435S (≈869.59 ± 4.52 µg/mL) (Table 3). Further, a time-point study (Figure 5) of the crude extract (CAN, LEX, SES, DES, and CA) revealed a significant (a p ≤ 0.001, b p ≤ 0.01, and c p ≤ 0.05) dose-dependent decrease in % cell viability at 48 h compared to the control.

Table 3.
IC50 values for the hydro-alcoholic crude extracts against three breast cancer cell lines.

Figure 5.
The effect of cell viability of CAN, LEX, SES, DES, and CA in HepG2 cells and breast cancer cell lines (MCF-7, MDA-MB-231, and MDA-MB-435S) at 48 h, measured using an MTT-based method. Values are expressed as mean ± SEM; n = 3; values are significant when a p ≤ 0.0001, b p ≤ 0.001, c p ≤ 0.01 and d p ≤ 0.05 compared to the control.
- (i)
- Effect of plant extract on human Peripheral blood mononuclear cells (PBMCs)
Cells were exposed to different concentrations of each extract (25, 50, and 100 µg/mL), as shown in (Figure 6). The exposure of human lymphocytes to CAN extract exhibited excellent cell viability, even for higher concentrations (250 µg/mL) at all time points (12, 18, and 36 h). It is worth mentioning that the CAN extract was found to enhance the PBMC proliferation significantly (a p ≤ 0.001), and the cell viability was found to be greater (128.63 ± 3.56, 138.88 ± 3.9, and 184.21 ± 14.64%) than that of the control with increasing concentrations at all time points of 12, 18 and 36 h of exposure, respectively (Table 4). Similarly, LEX also exhibited no cellular toxicity against PBMCs, even at higher doses (a p ≤ 0.001) at all time points of 12, 18, and 36 h (130.88 ± 3.27%, 131.14 ± 4.31%, and 128.27 ± 1.31%, respectively) when compared to control. Similar results were also found in the case of DES, SES, and CA, as stated in (Table 4). Thus, the results indicated that plant extracts did not confer any toxicity to normal PBMCs (a p ≤ 0.001) at all time points of 12, 18, and 36 h when compared to the control, indicating that plant extracts do not confer any toxicity to normal healthy PBMCs.

Figure 6.
The effect on cell viability of CAN, LEX, SES, DES, and CA in PBMC cells at 12, 18, and 36 h, measured using an MTT-based method. Values are expressed as mean ± SEM; n = 3; values were significant when a p ≤ 0.001, b p ≤ 0.01, and c p ≤ 0.05 compared to the control.

Table 4.
The effect on cell viability of CAN, LEX, DES, SES, and CA in PBMC cells was measured using an MTT-based method. The viability was calculated as % of the control (100%) and expressed as mean ± SD, n = 3.
- (j)
- Protective role of plants extracts against H2O2-induced cellular toxicity
In order to analyze the protective role of the plant extracts at various time points of exposure to H2O2, H2O2-mediated toxicity in HepG2 cell lines was measured using an MTT assay at three time points (pre-, post-, and co-treatment) (Table 5, Figure 7). The time-point study revealed that among the various plant extracts, CAN showed a protective role with significantly (x p ≤ 0.001, y p ≤ 0.01, and z p ≤ 0.05) increased cell viability at all concentrations (25, 50, and 100 µg/mL) in pretreated (84.91 ± 3.71, 83.52 ± 4.84, and 97.26 ± 1.84, respectively) and co-treated (80.60 ± 2.54, 78.81 ± 5.04, and 93.37 ± 5.10) groups in a dose-dependent manner compared to H2O2-alone treatment (positive control) (values are pre- 67.07 ± 2.78 and co- 71.08 ± 3.79). However, for post-treated, there was no significant increased cell viability observed in contrast to a positive control (61.37 ± 6.00), thereby indicating the protective function of the plant extracts against H2O2-mediated cytotoxicity in only pre- and co-treated groups. In contrast to the negative control, however, it continued to show a significant (a p ≤ 0.001 and b p ≤ 0.01) decrease in cell viability. Further, other plant extracts (LEX, SES, DES, and CA) also followed a similar pattern, where, in general, cell viability was found to increase significantly (x p ≤ 0.001, y p ≤ 0.01, and z p ≤ 0.05) in the pre- and co-treated groups when compared to the positive control. In contrast, the post-treated group at the various concentrations did not show significant increases in cell viability compared to the positive control. Further % cell viability tends to decrease significantly (a p ≤ 0.001, b p ≤ 0.01, and c p ≤ 0.05) when compared to the negative control (Table 5, Figure 7).

Table 5.
The effect on cell viability of CAN, LEX, DES, SES, and CA on H2O2-exposed HepG2 cells, measured using an MTT-based method.

Figure 7.
The effect on cell viability of CAN, LEX, DES, SES, and CA in HepG2 cells, measured at 48 h using an MTT-based method. Values are expressed as the mean ± SEM; n = 3; All of the values were compared with both negative (0.1% DMSO) and positive (0.2% H2O2) controls. Values are significant (a p ≤ 0.001, b p ≤ 0.01, and c p ≤ 0.05) for the negative control.
- (k)
- Protective role of plants extracts against H2O2-induced Intracellular ROS level
Further, the protective mechanisms of the plant extracts were analysed using the scavenging of ROS estimated by DCFDA, where intracellular ROS were generated by treating the cells with H2O2 in HepG2 cells (positive control). The level was evaluated using a DCFH-DA fluorescent probe, which was further compared with HepG2 cells treated with H2O2 and various doses of plant extract (25, 50, and 100 µg/mL). The results showed that the DCF fluorescence intensity in H2O2-treated HepG2 cells significantly (a p ≤ 0.001) increased 5.65 ± 1.00 fold compared to the control for the one exposed to CAN. In contrast, the fluorescence intensity was significantly (x p ≤ 0.001) reduced in HepG2 cells along with H2O2 in a dose-dependent manner (4.30 ± 1.46, 3.91 ± 1.16, and 3.21 ± 1.68) in contrast to those that received H2O2 only (5.65 ± 1.00), a similar trend was observed in HepG2 cells treated with H2O2 along with LEX (5.05 ± 0.58, 4.54 ± 1.58, and 3.77 ± 0.23), SES (5.28 ± 0.31, 4.95 ± 0.44, and 4.11 ± 0.96), DES (4.85 ± 0.05, 4.75 ± 0.11, and 2.16 ± 1.21), and CA (5.42 ± 0.18, 4.62 ± 0.77, and 4.41 ± 0.53) in a dose-dependent manner compared to those exposed to H2O2 (Table 6, Figure 8).

Table 6.
The relative fluorescence unit of reactive oxygen species (ROS) for CAN, LEX, DES, SES, and CA in HepG2 cells at 48 h, measured using the fluorometric-based method.

Figure 8.
The effect of reactive oxygen species (ROS) on CAN, LEX, DES, SES, and CA in HepG2 cells at 48 h, measured using a fluorometric-based method. The relative fluorescence unit is calculated as a fold change relative to the negative control and is expressed as the mean ± SD, n = 3. All of the values were compared to both negative (0.1% DMSO) and positive (0.2% H2O2) controls. Values were significant (a p ≤ 0.001) for the negative control and (x p ≤ 0.001) for the positive control.
4. Discussion
Plants have been known since the beginning of human civilization, serving as a rich source of medicinal assets [44]. Around 35,000–70,000 plant species have reportedly been used so far as medicines [45], around 14–28% of the 250,000 plant species that are thought to exist globally [46]. Such medicinal plant species are enriched with various bioactive compounds offering anticancer, antibacterial, antioxidant, and anti-inflammatory activities [47]. Further exhaustive screening of plant extracts is required to confirm the presence of bioactive compounds for their biological activities and therapeutic potential against various diseases, including cancers [36,48]. Breast cancer has been the leading cause of death, where chemotherapy is the most appropriate therapy after surgery. However, chemotherapy offers a significant drawback in the non-specific killing of normal cells and cancer cells [36,49,50]. An increase in the use of herbal formulation for the treatment of cancer over synthetic drugs has been witnessed due to the harbor of various noble functions that originated from the natural source., These properties are found to correlate with the presence of specific phytochemicals in plants, such as their antioxidant potential, anti-inflammatory properties, and antiproliferative activities. Most importantly, these are known to have cytotoxic potential against cancer cells butremain nontoxic to a normal cell. Isolation of such active compounds may not only fulfil the need to serve as a drug, but has led to the development of anticancer compounds, such as vincristine, vinblastine, and paclitaxel, which can be used for the treatment of dreadful diseases [36]. Abnormally high concentrations of free radical generation are found to be responsible for various diseases, such as parasitic infections, inflammation, lung damage, reperfusion injury, cardiovascular disorders, ageing, atherosclerosis, and neoplastic diseases, including cancer [51]. Furthermore, antioxidants are compounds that neutralize interactions with free radicals, thus preventing the damage induced by reactive oxygen species [51]. This study aimed to screen the potential of the selected plants based on their antioxidative and radical scavenging properties, which have played a key role in minimizing diseases, such as cancer, as estimated through various assays, such as total antioxidant activity, DPPH radical scavenging activity, superoxide scavenging activity, hydroxyl radical scavenging, Fe2+ chelating assay, and total reducing potential. All of the crude plant extracts of CAN, LEX, SES, DES, and CA, display significantly high free radical scavenging activities, superoxide scavenging activities, hydroxyl radical scavenging activities, reducing power, antioxidant, and Fe2+ chelating effects in a dose-dependent manner. Among these, in general, CAN and LEX display comparatively low IC50 and high free radical scavenging activities (Table 2. Figure 2, Figure 3, Figure 4 and Figure 5). Thus, plant extracts exhibiting lesser IC50 values showed higher antioxidant potential [47,52], whereas high IC50 values reflect low antioxidant potential [53]. Various free radical scavenging methods are used to measure the potential antioxidant source in treating oxidative stress-mediated diseases [54]. Additionally, free radical scavenging indirectly measures phytochemicals (flavonols, catechin, and anthocyanins), whose function strengthens the redox potential, thereby neutralizing the free radical, decomposing hydrogen peroxides, or quenching singlet oxygen [55]. High phenolic content is often correlated with strong antioxidant capacity, as reported by Ghasemzadeh and Jaafar in 2013, where Pandanus amaryllifolius Roxb. extracts exhibited high total phenolics and flavonoid content and showed high free radical scavenging and anticancer activity [56]. In our study, plant extracts (CAN and LEX) showed a lower phenolic content (Table 1) with more potent free radical scavenging activity (Figure 2, Figure 3, Figure 4 and Figure 5). This additional antioxidant activity might be due to other bioactive compounds screened during the preliminary investigation (Supplementary Materials, Table S1). Similar results were obtained in a study suggesting phenolic content is not the only factor for their antioxidant activity [53]. Further MTT assays were conducted on HepG2 and breast cancer cell lines, including MCF-7, MDA-MB-435S, and MDA-MB-231, to document the anticancer efficacy of selected plant extracts (CAN, LEX, SES, DES, and CA). The results showed a dose-dependent decrease in cell viability in studied breast cancer cell lines compared to the control, among which CAN and LEX exhibited profoundly decreased cell viability compared to the rest of the extract (Figure 5). Similar results were observed in the leaf extract of Simarouba glauca, with a high antioxidant capacity found to inhibit cancer activity against the T-24 bladder cancer cell line. A methanolic extract of Euphorbia tirucalli was found to show anticancer activity against the MiaPaCa-2 pancreatic cancer cell line, and Tinospora cordifolia and Withania sominifera showed anticancer activity against breast cancer cell lines MCF-7 and MDA-MB231 [57,58,59].
On the other hand, the cytotoxic effects of studied plant extracts (CAN, LEX, SES, DES, and CA) on normal human cells (PBMCs) exhibited cell proliferation. Among these, CAN and LEX showed a marked increase in cell viability as the dose increased with different time frames, except DES, which showed increased cell proliferation only at 12 h, and a decrease in cell viability at 18 and 36 h compared to the control was observed (Figure 6). Similar results were observed in a study where crude plant extracts showed a protective effect on PBMCs [60]. Further, to understand toxic or cytoprotective effects against the studied compounds, HepG2 was considered a good tool because a steady-state antioxidant resistance level is higher in HepG2 cells than in other hepatic cells [55]. Therefore, HepG2 cells exposed to crude plant extract (CAN, LEX, SES, DES, and CA) showed decreased cell viability compared to the negative and vehicle control, whereas positive control, pre-, and co-treated HepG2 cells continued to proliferate against H2O2 in all of the studied plant extracts in a dose-dependent manner compared to post-treated cells exposed to H2O2 (Figure 7), thus showing a protective effect against assaulted HepG2 cells. Similar results have been observed in a study where Ocimum gratissimum L. extract showed a protective effect on HepG2 cells when exposed to H2O2 [61]. Hydrogen peroxide (H2O2) is considered the leading intermediary for oxidative stress-mediated cytotoxicity. This reactive oxygen species (ROS) scavenging activity of plant extract to positive control resulted in a dose-dependent decrease in ROS levels (Figure 8). Similarly, the in vitro antioxidant potential of Theobroma cacao, where a methanolic extract of various parts, such as leaf, husk, bark, unfermented and fermented shell, root, pith, and cherelle exhibited anticancer activity on breast cancer MCF-7 cell lines. However, normal cells remain unaffected [62], and Euphorbia tirucalli in [54]. The exhibited anticancer activity of a plant extract having anticancer potential may be due to the presence of various bioactive compounds, such as epicatechin, rutin, gallic acid, kaempferol [51], myricetin, and catechin [58]. Such studies decipher the hidden anticancer potential of plants of ethnobotanical importance to screen the bioactive compounds that can be used for therapeutic implications in the field of cancer biology. Such observed antioxidant potential of plants can be later used for the treatment of various diseases, including chemotherapeutic implications.
5. Conclusions
From the results, among the selected plants, Etlingera linguiformis and Smilax ovalifolia showed comparatively potent antioxidant and free radical scavenging potential along with iron chelation and reducing power. They also showed high anticancer potential with nominal toxicity to the normal cells (PBMCs) compared to the other selected plants. The in vitro assays indicated that both plant extracts have significant sources of natural antioxidants, which might be helpful in the protection from oxidative stress generation. Nevertheless, the components accountable for the antioxidative activity are still blurred. Therefore, further study is required to establish the underlying mechanism of the above activity and determine the anticancer potential of plants of ethnobotanical importance, and their safety, before clinical use.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/oxygen3020015/s1, Supplementary Materials Table S1. References [63,64] are cited in the supplementary materials.
Author Contributions
M.K.D. participated in the design of the study, carried out the sample collection, performed experimental work and data analysis, and prepared the manuscript. N.S. participated in data analysis and manuscript writing and revision. P.R. provided technical advice and assisted in the revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Tezpur University Ethical Committee (TUEC), Tezpur University, Tezpur (Protocol no. DoRD/TUEC/10-14/4361 dated 28.03.2014).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The author acknowledges the Department of Molecular Biology and Biotechnology, Tezpur University, which provided the laboratory facilities in which the experimental work was conducted. The author acknowledges the University Grant Commission, New Delhi, India for Providing RGNF fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ABTS | 2,2′-azino-di 3-ethylbenthiazolinesulfonate |
| DPPH | 2,2′-diphenyl-1-picrylhydrazy |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| DMSO | dimethyl sulfoxide |
| OD | optical density |
| ANOVA | analysis of variance |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | foetal bovine serum |
| IC50 | The half-maximal inhibitory concentration |
| ND | Not determined |
| PBS | phosphate buffer saline |
| SEM | standard error of the mean |
| BHA | beta Hydroxy Acids |
| TCA | trichloroacetic acid |
| FC | Folin–Ciocalteu Phenol reagent |
References
- Bodeker, G.; Ong, C.-K. WHO Global Atlas of Traditional, Complementary and Alternative Medicine; World Health Organization: Geneva, Switzerland, 2005; Volume 1. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bouali, N.; Hamadou, W.S.; Badraoui, R.; Lajimi, R.H.; Hamdi, A.; Alreshidi, M.; Adnan, M.; Soua, Z.; Siddiqui, A.J.; Noumi, E.; et al. Phytochemical Composition, Antioxidant, and Anticancer Activities of Sidr Honey: In Vitro and In Silico Computational Investigation. Life 2022, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Dyba, T.; Randi, G.; Martos, C.; Giusti, F.; Calvalho, R.; Neamtiu, L.; Nicholson, N.; Flego, M.; Dimitrova, N.; Bettio, M. 1501O Long-term estimates of cancer incidence and mortality for the EU and EFTA countries according to different demographic scenarios. Ann. Oncol. 2021, 32, S1102. [Google Scholar] [CrossRef]
- Calaf, G.M.; Urzua, U.; Termini, L.; Aguayo, F. Oxidative stress in female cancers. Oncotarget 2018, 9, 23824. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, A.; Guler, E.M.; Dikilitas, M. Role of Antioxidant Phytochemicals in Prevention, Formation and Treatment of Cancer; IntechOpen Limited: London, UK, 2018; pp. 21–45. [Google Scholar]
- Matowa, P.R.; Gundidza, M.; Gwanzura, L.; Nhachi, C.F. A survey of ethnomedicinal plants used to treat cancer by traditional medicine practitioners in Zimbabwe. BMC Complement. Med. Ther. 2020, 20, 278. [Google Scholar] [CrossRef]
- Wang, H.; Oo Khor, T.; Shu, L.; Su, Z.-Y.; Fuentes, F.; Lee, J.-H.; Tony Kong, A.-N. Plants vs. cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anti-Cancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Gu, I.; Brownmiller, C.; Howard, L.; Lee, S.-O. Chemical Composition of Volatile Extracts from Black Raspberries, Blueberries, and Blackberries and Their Antiproliferative Effect on A549 Non-Small-Cell Lung Cancer Cells. Life 2022, 12, 2056. [Google Scholar] [CrossRef]
- Noumi, E.; Ahmad, I.; Bouali, N.; Patel, H.; Ghannay, S.; ALrashidi, A.A.; Abdulhakeem, M.A.; Patel, M.; Ceylan, O.; Badraoui, R.; et al. Thymus musilii Velen. Methanolic Extract: In Vitro and In Silico Screening of Its Antimicrobial, Antioxidant, Anti-Quorum Sensing, Antibiofilm, and Anticancer Activities. Life 2023, 13, 62. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.; Al-Hamoud, K.; Adil, S.F.; Shaik, M.R.; Alkhathlan, H.Z. Comprehensive Phytochemical Analysis of Various Solvent Extracts of Artemisia judaica and Their Potential Anticancer and Antimicrobial Activities. Life 2022, 12, 1885. [Google Scholar] [CrossRef]
- Ly, H.T.; Truong, T.M.; Nguyen, T.T.H.; Nguyen, H.D.; Zhao, Y.; Le, V.M. Phytochemical screening and anticancer activity of the aerial parts extract of Xanthium strumarium L. on HepG2 cancer cell line. Clin. Phytosci. 2021, 7, 14. [Google Scholar] [CrossRef]
- Wagh, A.S.; Butle, S.R. Phytochemical analysis and in-vitro anticancer activity of Duranta erecta L. (Verbenaceae). Int. J. Pharm. Sci. Res. 2019, 10, 2941–2946. [Google Scholar]
- Bhandari, J.; Muhammad, B.; Thapa, P.; Shrestha, B.G. Study of phytochemical, anti-microbial, anti-oxidant, and anti-cancer properties of Allium wallichii. BMC Complement. Altern. Med. 2017, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Al-Shehri, M.; Moustafa, M. Anticancer, Antibacterial, and Phytochemicals Derived From Extract of Aerva javanica (Burm.f.) Juss. ex Schult. Grown Naturally in Saudi Arabia. Trop. Conserv. Sci. 2019, 12, 194008291986426. [Google Scholar] [CrossRef]
- Jacobs, E.C. Potential Therapeutic Effects of Phytochemicals and Medicinal Herbs for Cancer Prevention and Treatment. Arch. Gen. Intern. Med. 2018, 2, 44–48. [Google Scholar] [CrossRef]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Chandrashekar, K.S.; Pai, K.S.R.; Setty, M.M.; Devkar, R.A.; Reddy, N.D.; Shoja, M.H. Evaluation of antioxidant and anticancer activity of extract and fractions of Nardostachys jatamansi DC in breast carcinoma. BMC Complement. Altern. Med. 2015, 15, 50. [Google Scholar] [CrossRef]
- Ahmad, N.; Gupta, S.; Husain, M.M.; Heiskanen, K.M.; Mukhtar, H. Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin. Cancer Res. 2000, 6, 1524–1528. [Google Scholar]
- Hossan, M.A.; Ibrahim, M.; Ahsan, M.Q.; Aktar, F.; Kuddus, M.R.; Chowdhury, M.M.U.; Rashid, M.A. Pharmacological and phytochemical screenings of ethanol extract of Etlingera linguiformis (Roxb.) RM Sm. growing in Bangladesh. Bangladesh Pharm. J. 2013, 16, 33–37. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A.; Ashkani, S. Secondary metabolites constituents and antioxidant, anticancer and antibacterial activities of Etlingera elatior (Jack) RM Sm grown in different locations of Malaysia. BMC Complement. Altern. Med. 2015, 15, 335. [Google Scholar] [CrossRef]
- Roy, A.; Bhoumik, D.; Sahu, R.K.; Dwivedi, J. Phytochemical screening and antioxidant activity of Sesbania grandiflora leaves extracts. Asian J. Res. Pharm. Sci. 2014, 4, 16–21. [Google Scholar]
- Pajaniradje, S.; Mohankumar, K.; Pamidimukkala, R.; Subramanian, S.; Rajagopalan, R. Antiproliferative and apoptotic effects of Sesbania grandiflora leaves in human cancer cells. BioMed Res. Int. 2014, 2014, 474953. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.K.; Bioscience, A. Antioxidant activity and estimation of total phenols and flavonoids in extracts of Smilax ovalifolia leaves. Int. J. Pure Appl. Biosci. 2015, 3, 174–177. [Google Scholar]
- Malge, N.R.; Bandara, A.M.; Keerthirathna, W.L.; Dissanayake, D.M.; Perera, P.K.; Witharana, C.; Peiris, L.D. Antioxidant and Anti-proliferative Activities of Smilax Zeylanica Root and Rhizome Extract against Liver Carcinoma Cells. J. Herbs Spices Med. Plants 2021, 27, 188–199. [Google Scholar] [CrossRef]
- Singh, S.; Parmar, N.; Patel, B. A review on Shalparni (Desmodium gangeticum DC.) and Desmodium species (Desmodium triflorum DC. & Desmodium laxiflorum DC.)—Ethnomedicinal perspectives. J. Med. Plants 2015, 3, 38–43. [Google Scholar]
- Lai, S.-C.; Ho, Y.-L.; Huang, S.-C.; Huang, T.-H.; Lai, Z.-R.; Wu, C.-R.; Lian, K.-Y.; Chang, Y.-S. Antioxidant and antiproliferative activities of Desmodium triflorum (L.) DC. Am. J. Chin. Med. 2010, 38, 329–342. [Google Scholar] [CrossRef]
- Chamkhi, I.; Charfi, S.; Hachlafi, N.E.; Mechchate, H.; Guaouguaou, F.-E.; El Omari, N.; Bakrim, S.; Balahbib, A.; Zengin, G.; Bouyahya, A. Genetic diversity, antimicrobial, nutritional, and phytochemical properties of Chenopodium album: A comprehensive review. Food Res. Int. 2022, 154, 110979. [Google Scholar] [CrossRef]
- Al-Dabbagh, B.; Elhaty, I.A.; Al Hrout, A.; Al Sakkaf, R.; El-Awady, R.; Ashraf, S.S.; Amin, A. Antioxidant and anticancer activities of Trigonella foenum-graecum, Cassia acutifolia and Rhazya stricta. BMC Complement. Altern. Med. 2018, 18, 240. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Kitts, D.D.; Wijewickreme, A.N.; Hu, C. Antioxidant properties of a North American ginseng extract. Mol. Cell. Biochem. 2000, 203, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Alasalvar, C.; Liyana-Pathirana, C.M. Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L.) and hazelnut byproducts. J. Agric. Food Chem. 2007, 55, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Chandrasekar, M.J.; Nanjan, M.J.; Suresh, B. Antioxidant activity of Caesalpinia digyna root. J. Ethnopharmacol. 2007, 113, 284–291. [Google Scholar] [CrossRef]
- Thakkar, K.; Prasad, A.; Nayak, J.; Iyer, S.V.; Kumar, S. Antioxidant and in vitro cytotoxic activity of extracts of aerial parts of Cocculus hirsutus (L) using cell line cultures (breast cell line). J. Phytopharm. 2014, 3, 395–399. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: The role of superoxide and hydroxyl radicals. FEBS Lett. 1981, 128, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Hazra, B.; Sarkar, R.; Biswas, S.; Mandal, N. Comparative study of the antioxidant and reactive oxygen species scavenging properties in the extracts of the fruits of Terminalia chebula, Terminalia belerica and Emblica officinalis. BMC Complement. Altern. Med. 2010, 10, 20. [Google Scholar] [CrossRef]
- Dinis, T.C.; Maderia, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Zhu, K.-X.; Lian, C.-X.; Guo, X.-N.; Peng, W.; Zhou, H.-M. Antioxidant activities and total phenolic contents of various extracts from defatted wheat germ. Food Chem. 2011, 126, 1122–1126. [Google Scholar] [CrossRef]
- Das, A.J.; Das, M.K.; Singh, S.P.; Saikia, P.P.; Singh, N.; Islam, J.; Ansari, A.; Chattopadhyay, P.; Rajamani, P.; Miyaji, T.; et al. Synthesis of salicylic acid phenylethyl ester (SAPE) and its implication in immunomodulatory and anticancer roles. Sci. Rep. 2022, 12, 8735. [Google Scholar] [CrossRef]
- Manna, P.; Bhattacharyya, S.; Das, J.; Ghosh, J.; Sil, P.C. Phytomedicinal role of Pithecellobium dulce against CCl4-mediated hepatic oxidative impairments and necrotic cell death. Evid.-Based Complement. Altern. Med. 2011, 2011, 832805. [Google Scholar] [CrossRef]
- Chou, T.C.; Martin, N. CompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values; ComboSyn: Paramus, NJ, USA, 2005. [Google Scholar]
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines on Good Agricultural and Collection Practices (GACP) for Medicinal Plants; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Volenzo, T.; Odiyo, J. Integrating endemic medicinal plants into the global value chains: The ecological degradation challenges and opportunities. Heliyon 2020, 6, e04970. [Google Scholar] [CrossRef] [PubMed]
- Ralte, L.; Bhardwaj, U.; Singh, Y.T. Traditionally used edible Solanaceae plants of Mizoram, India have high antioxidant and antimicrobial potential for effective phytopharmaceutical and nutraceutical formulations. Heliyon 2021, 7, e07907. [Google Scholar] [CrossRef] [PubMed]
- Hostettmann, K.; Wolfender, J.-L.; Terreaux, C. Modern screening techniques for plant extracts. Pharm. Biol. 2001, 39, 18–32. [Google Scholar]
- Balamurugan, V.; Balakrishnan, V.; Robinson, J.P.; Ramakrishnan, M. Anti-cancer and apoptosis-inducing effects of Moringa concanensis using hepG2 cell lines. Bangladesh J. Pharmacol. 2014, 9, 604–609. [Google Scholar] [CrossRef]
- Robinson, J.P.; Suriya, K.; Subbaiya, R.; Ponmurugan, P. Antioxidant and cytotoxic activity of Tecoma stans against lung cancer cell line (A549). Braz. J. Pharm. Sci. 2017, 53. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Huang, L. Correlation between antioxidant activities and phenolic contents of radix Angelicae sinensis (Danggui). Molecules 2009, 14, 5349–5361. [Google Scholar] [CrossRef]
- Matuszewska, A.; Jaszek, M.; Stefaniuk, D.; Ciszewski, T.; Matuszewski, Ł. Anticancer, antioxidant, and antibacterial activities of low molecular weight bioactive subfractions isolated from cultures of wood degrading fungus Cerrena unicolor. PLoS ONE 2018, 13, e0197044. [Google Scholar] [CrossRef]
- Tonisi, S.; Okaiyeto, K.; Mabinya, L.V.; Okoh, A.I. Evaluation of bioactive compounds, free radical scavenging and anticancer activities of bulb extracts of Boophone disticha from Eastern Cape Province, South Africa. Saudi J. Biol. Sci. 2020, 27, 3559–3569. [Google Scholar] [CrossRef]
- Aliyu, A.B.; Ibrahim, M.A.; Musa, A.M.; Musa, A.O.; Kiplimo, J.J.; Oyewale, A.O. Free radical scavenging and total antioxidant capacity of root extracts of Anchomanes difformis Engl. (Araceae). Acta Pol. Pharm. 2013, 70, 115–121. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z. Profiling of phenolic compounds and their antioxidant and anticancer activities in pandan (Pandanus amaryllifolius Roxb.) extracts from different locations of Malaysia. BMC Complement. Altern. Med. 2013, 13, 341. [Google Scholar] [CrossRef] [PubMed]
- Puranik, S.I.; Ghagane, S.C.; Nerli, R.B.; Jalalpure, S.S.; Hiremath, M.B. Evaluation of in vitro antioxidant and anticancer activity of Simarouba glauca leaf extracts on T-24 bladder cancer cell line. Pharmacogn. J. 2017, 9, 906–912. [Google Scholar] [CrossRef]
- Maliyakkal, N.; Udupa, N.; Pai, K.; Rangarajan, A. Cytotoxic and apoptotic activities of extracts of Withania somnifera and Tinospora cordifolia in human breast cancer cells. Int. J. Appl. Res. Nat. Prod. 2013, 6, 1–10. [Google Scholar]
- Munro, B.; Vuong, Q.V.; Chalmers, A.C.; Goldsmith, C.D.; Bowyer, M.C.; Scarlett, C.J. Phytochemical, antioxidant and anti-cancer properties of Euphorbia tirucalli methanolic and aqueous extracts. Antioxidants 2015, 4, 647–661. [Google Scholar] [CrossRef]
- Nasser, M.; Damaj, R.; Merah, O.; Hijazi, A.; Trabolsi, C.; Wehbe, N.; Nasser, M.; Al-Khatib, B.; Damaj, Z. Potency of Combining Eucalyptus camaldulensis subsp. camaldulensis with Low-Dose Cisplatin in A549 Human Lung Adenocarcinomas and MCF-7 Breast Adenocarcinoma. Medicines 2020, 7, 40. [Google Scholar] [CrossRef]
- Chiu, Y.-W.; Lo, H.-J.; Huang, H.-Y.; Chao, P.-Y.; Hwang, J.-M.; Huang, P.-Y.; Huang, S.-J.; Liu, J.-Y.; Lai, T.-J. The antioxidant and cytoprotective activity of Ocimum gratissimum extracts against hydrogen peroxide-induced toxicity in human HepG2 cells. J. Food Drug Anal. 2013, 21, 253–260. [Google Scholar] [CrossRef]
- Baharum, Z.; Akim, A.M.; Taufiq-Yap, Y.H.; Hamid, R.A.; Kasran, R. In vitro antioxidant and antiproliferative activities of methanolic plant part extracts of Theobroma cacao. Molecules 2014, 19, 18317–18331. [Google Scholar] [CrossRef]
- Kokate, C.K.; Purohit, A.P.; Gokhale, S.B. Pharmacognosy; Nirali Prakashan: Pune, India, 2007. [Google Scholar]
- Jeet, K.; Thakur, R.; Sharma, A.K.; Shukla, A. Pharmacognostic and phytochemical investigation of whole aerial part of Argyreia nervosa. Int. J. Biol. Pharm. Res. 2012, 3, 713–717. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).