Cell Localization of DPI-Dependent Production of Superoxide in Reproductive Tissues of the Olive Tree (Olea europaea L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. In Vitro Pollen Germination
2.3. CLSM Detection of ROS Production
2.4. Light Microscopy (LM) Chromogenic Detection of O2●− Production
2.5. Transmission Electron Microscopy (TEM) Localization of O2●− Production in Mature Pollen Grains and Pollen Tubes
2.6. Statistical Methods
3. Results
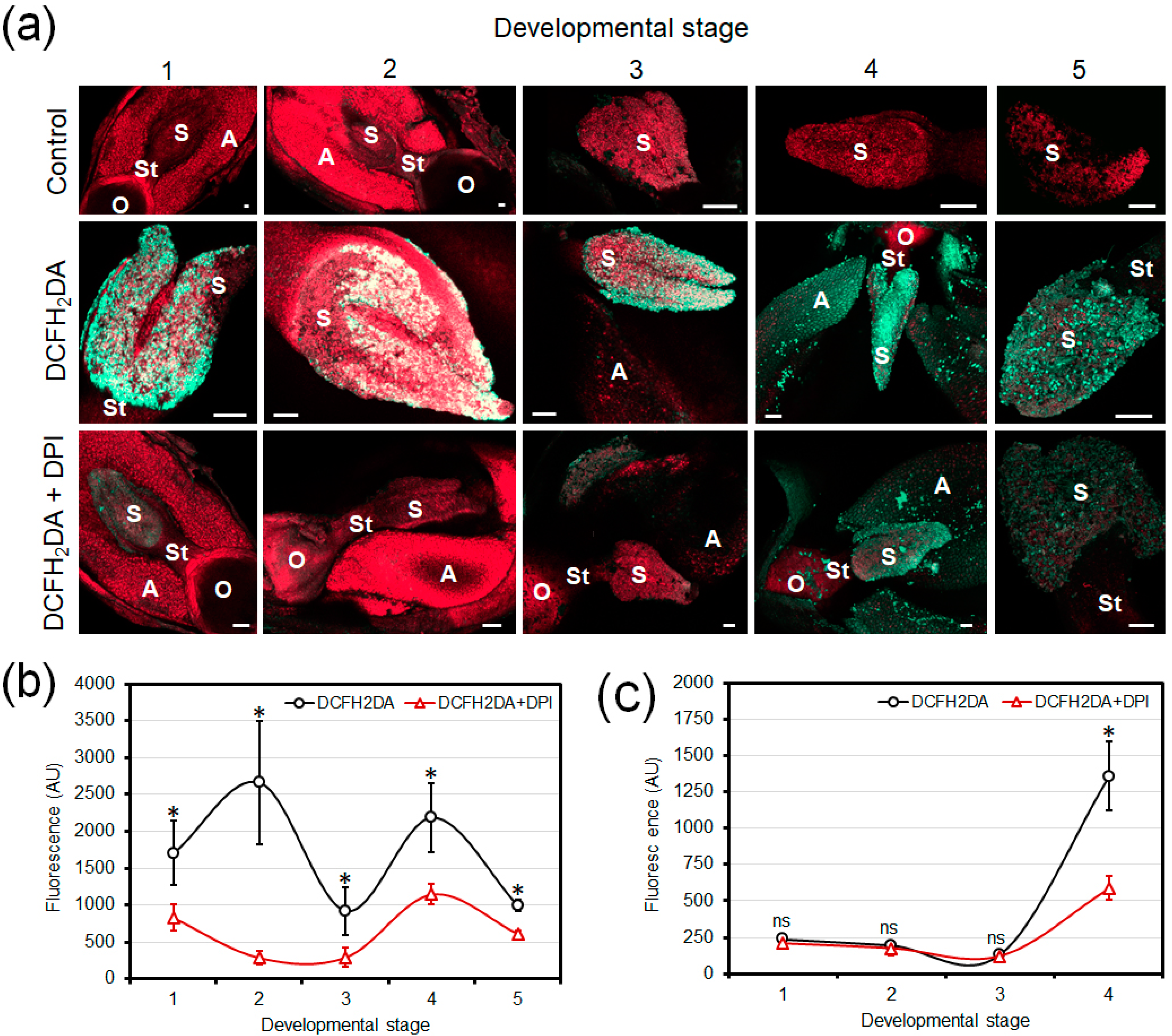
3.1. Fluorescence Detection of Developmental Production of ROS in Olive Floral Organs
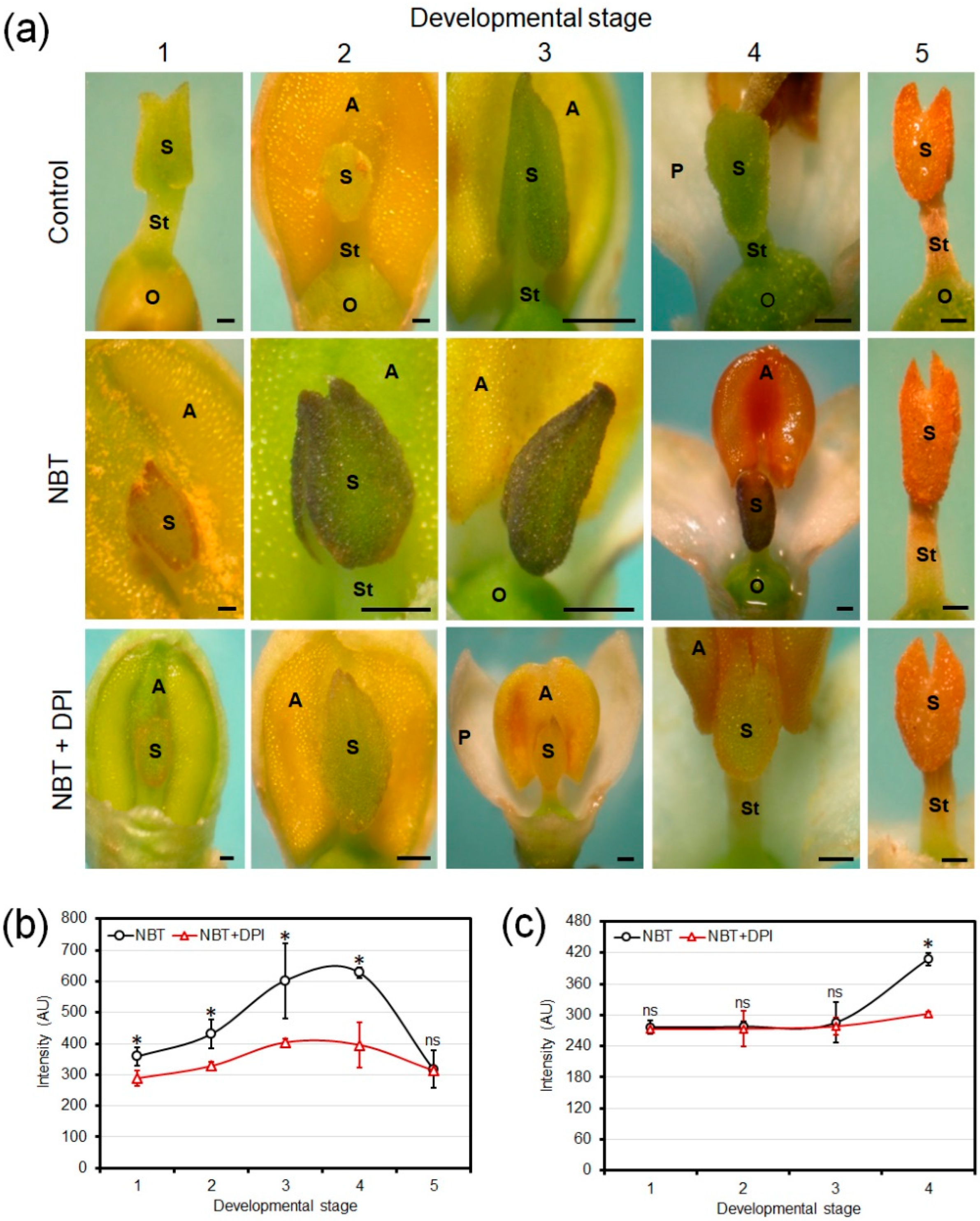
3.2. Chromogenic Detection of Developmental Production of Superoxide in Olive Floral Organs
3.3. Ultrastructural Localization of Superoxide in Olive Pollen Grains
3.4. Ultrastructural Localization of Superoxide in Olive Pollen Tubes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Traverso, J.A.; Pulido, A.; Rodriguez-Garcia, M.I.; Alche, J.D. Thiol-based redox regulation in sexual plant reproduction: New insights and perspectives. Front. Plant Sci. 2013, 4, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingos, P.; Prado, A.M.; Wong, A.; Gehring, C.; Feijo, J.A. Nitric oxide: A multitasked signaling gas in plants. Mol. Plant 2015, 8, 506–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, I.; Romero-Puertas, M.C.; Sandalio, L.M.; Olmedilla, A. The role of reactive oxygen species and nitric oxide in programmed cell death associated with self-incompatibility. J. Exp. Bot. 2015, 66, 2869–2876. [Google Scholar] [CrossRef] [Green Version]
- Breygina, M.; Klimenko, E. ROS and Ions in Cell Signaling during Sexual Plant Reproduction. Int. J. Mol. Sci. 2020, 21, 9476. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Quesada, M.J.; Traverso, J.A.; Alché, J.D. NADPH oxidase dependent superoxide production in plant reproductive tissues. Front. Plant Sci. 2016, 7, 00359. [Google Scholar] [CrossRef] [Green Version]
- Hao, H.; Fan, L.; Chen, T.; Li, R.; Li, X.; He, Q.; Botella, M.A.; Lin, J. Clathrin and membrane microdomains cooperatively regulate RbohD dynamics and activity in Arabidopsis. Plant Cell 2014, 26, 1729–1745. [Google Scholar] [CrossRef] [Green Version]
- Takeda, S.; Gapper, C.; Kaya, H.; Bell, E.; Kuchitsu, K.; Dolan, L. Local positive feedback regulation determines cell shape in root hair cells. Science 2008, 319, 1241–1244. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Kaya, H.; Hiraoka, G.; Yumoto, F.; Kimura, S.; Kadota, Y.; Hishinuma, H.; Senzaki, E.; Yamagoe, S.; Nagata, K.; et al. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J. Biol. Chem. 2008, 283, 8885–8892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drerup, M.M.; Schlücking, K.; Hashimoto, K.; Manishankar, P.; Steinhorst, L.; Kuchitsu, K.; Kudla, J. The calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol. Plant 2013, 6, 559–569. [Google Scholar] [CrossRef] [Green Version]
- Ono, E.; Wong, H.L.; Kawasaki, T.; Hasegawa, M.; Kodama, O.; Shimamoto, K. Essential role of the small GTPase Rac in disease resistance of rice. Proc. Natl. Acad. Sci. USA 2001, 98, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, H.; Zhang, Q.; Li, M.; Yan, M.; Wang, R.; Wang, L.; Welti, R.; Zhang, W.; Wang, X. Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 2009, 21, 2357–2377. [Google Scholar] [CrossRef] [Green Version]
- Yun, B.W.; Feechan, A.; Yin, M.; Saidi, N.B.; Le Bihan, T.; Yu, M.; Moore, J.W.; Kang, J.G.; Kwon, E.; Spoel, S.H.; et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef]
- Potocký, M.; Jones, M.A.; Bezvoda, R.; Smirnoff, N.; Žárský, V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007, 174, 742–751. [Google Scholar] [CrossRef]
- Potocký, M.; Pejchar, P.; Gutkowska, M.; Jiménez Quesada, M.J.; Potocká, A.; Alché, J.D.; Kost, B.; Zárský, V. NADPH oxidase activity in pollen tubes is affected by calcium ions, signaling phospholipids and Rac/Rop GTPases. J. Plant Physiol. 2012, 169, 1654–1663. [Google Scholar] [CrossRef]
- Boisson-Dernier, A.; Lituiev, D.S.; Nestorova, A.; Franck, C.M.; Thirugnanarajah, S.; Grossniklaus, U. ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol. 2013, 11, e1001719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, H.; Nakajima, R.; Iwano, M.; Kanaoka, M.M.; Kimura, S.; Takeda, S.; Kawarazaki, T.; Senzaki, E.; Hamamura, Y.; Higashiyama, T.; et al. Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 2014, 26, 1069–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassig, R.; Gutermuth, T.; Bey, T.D.; Konrad, K.R.; Romeis, T. Pollen tube NAD (P) H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J. 2014, 78, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Speranza, A.; Crinelli, R.; Scoccianti, V.; Geitmann, A. Reactive oxygen species are involved in pollen tube initiation in kiwifruit. Plant Biol. 2012, 14, 64–76. [Google Scholar] [CrossRef]
- Kaya, H.; Iwano, M.; Takeda, S.; Kanaoka, M.M.; Kimura, S.; Abe, M.; Kuchitsu, K. Apoplastic ROS production upon pollination by RbohH and RbohJ in Arabidopsis. Plant Signal. Behav. 2015, 10, e989050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Quesada, M.J.; Traverso, J.A.; Potocký, M.; Zarsky, V.; Alché, J.D. Generation of superoxide by OeRbohH, a NADPH oxidase activity during olive (Olea europaea L.) pollen development and germination. Front. Plant Sci. 2019, 10, 1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafra, A.; Rejón, J.D.; Hiscock, S.J.; Alché, J.D. Patterns of ROS Accumulation in the Stigmas of Angiosperms and Visions into Their Multi-Functionality in Plant Reproduction. Front. Plant Sci. 2016, 7, 1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafra, A.; Rodríguez-García, M.I.; Alché, J.D. Cellular localization of ROS and NO in olive reproductive tissues during flower development. BMC Plant Biol. 2010, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- M’rani-Alaoui, M.; Castro, A.; Alché, J.D.; Wang, W.; Fernández, M.C.; Rodríguez-García, M.I. Expression of ole E 1, the major olive pollen allergen, during in-vitro pollen germination. Acta Hortic. 2002, 586, 465–468. [Google Scholar] [CrossRef] [Green Version]
- McInnis, S.M.; Desikan, R.; Hancock, J.T.; Hiscock, S.J. Production of ROS and RNS by angiosperm stigmas and pollen: Potential signalling crosstalk? New Phytol. 2006, 172, 221–228. [Google Scholar] [CrossRef]
- Carter, C.; Healy, R.; O’Tool, N.M.; Naqvi, S.S.; Ren, G.; Park, S.; Beattie, G.A.; Horner, H.T.; Thornburg, R.W. Tobacco nectaries express a novel NADPH oxidase implicated in the defense of floral reproductive tissues against microorganisms. Plant Physiol. 2007, 143, 389–399. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, M.I.; Fernandez, M.C.; Alché, J.D.; Olmedilla, A. Endoplasmic reticulum as a storage site for allergenic proteins in pollen grains of several Oleaceae. Protoplasma 1995, 197, 111–116. [Google Scholar] [CrossRef]
- Doussiere, J.; Gaillard, J.; Vignais, P.V. The heme component of the neutrophil NADPH oxidase complex is a target for aryliodonium compounds. Biochemistry 1999, 38, 3694–3703. [Google Scholar] [CrossRef]
- Bernas, T.; Dobrucki, J.W. The role of plasma membrane in bioreduction of two tetrazolium salts, MTT and CTC. Arch. Biochem. Biophys. 2000, 380, 108–116. [Google Scholar] [CrossRef]
- Overmyer, K.; Brosché, M.; Kangasjärvi, J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003, 8, 335–342. [Google Scholar] [CrossRef]
- Sagi, M.; Fluhr, R. Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol. 2001, 126, 1281–1290. [Google Scholar] [CrossRef] [Green Version]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Allan, A.C.; Fluhr, R. Two distinct sources of elicitated reactive oxygen species in tobacco epidermal cells. Plant Cell 1997, 9, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Jagnandan, D.; Church, J.E.; Bánfi, B.; Stuehr, D.J.; Marrero, M.B.; Fulton, D.J. Novel mechanism of activation of NADPH oxidase 5. Calcium sensitization via phosphorylation. J. Biol. Chem. 2007, 282, 6494–6507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambasta, R.K.; Kumar, P.; Griendling, K.K.; Schmidt, H.H.; Busse, R.; Brandes, R.P. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J. Biol. Chem. 2004, 279, 45935–45941. [Google Scholar] [CrossRef] [Green Version]
- Li, J.M.; Shah, A.M. Intracellular localization and pre-assembly of the NADPH oxidase complex in cultured endothelial cells. JBC 2002, 277, 19952–19960. [Google Scholar] [CrossRef] [Green Version]
- Leshem, Y.; Melamed-Book, N.; Cagnac, O.; Ronen, G.; Nishri, Y.; Solomon, M.; Cohen, G.; Levine, A. Suppression of Arabidopsis vesicle-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proc. Natl. Acad. Sci. USA 2006, 103, 18008–18013. [Google Scholar] [CrossRef] [Green Version]
- Leshem, Y.; Seri, L.; Levine, A. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J. 2007, 51, 185–197. [Google Scholar] [CrossRef]
- Fluhr, R. Reactive Oxygen-Generating NADPH oxidases in plants. In Reactive Oxygen Species in Plant Signaling; Del Río, L.A., Puppo, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–25. [Google Scholar]
- Rodríguez-García, M.I.; Fernández, M.C. Ultrastructural evidence of endoplasmic reticulum changes during the maturation of the olive pollen grain (Olea europaea L., Oleaceae). Plant Syst. Evol. 1990, 171, 221–231. [Google Scholar] [CrossRef]
- Butowt, R.; Rodríguez-García, M.I.; Alché, J.D.; Gorska-Brylass, A. Calcium in electron-dense globoids during pollen grain maturation in Chlorophytum elatum R. Br. Planta 1997, 203, 413–421. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Quesada, M.J.; Castro, A.J.; Lima-Cabello, E.; Alché, J.d.D. Cell Localization of DPI-Dependent Production of Superoxide in Reproductive Tissues of the Olive Tree (Olea europaea L.). Oxygen 2022, 2, 79-90. https://doi.org/10.3390/oxygen2020007
Jiménez-Quesada MJ, Castro AJ, Lima-Cabello E, Alché JdD. Cell Localization of DPI-Dependent Production of Superoxide in Reproductive Tissues of the Olive Tree (Olea europaea L.). Oxygen. 2022; 2(2):79-90. https://doi.org/10.3390/oxygen2020007
Chicago/Turabian StyleJiménez-Quesada, María José, Antonio Jesús Castro, Elena Lima-Cabello, and Juan de Dios Alché. 2022. "Cell Localization of DPI-Dependent Production of Superoxide in Reproductive Tissues of the Olive Tree (Olea europaea L.)" Oxygen 2, no. 2: 79-90. https://doi.org/10.3390/oxygen2020007
APA StyleJiménez-Quesada, M. J., Castro, A. J., Lima-Cabello, E., & Alché, J. d. D. (2022). Cell Localization of DPI-Dependent Production of Superoxide in Reproductive Tissues of the Olive Tree (Olea europaea L.). Oxygen, 2(2), 79-90. https://doi.org/10.3390/oxygen2020007






