Free Radical Properties, Source and Targets, Antioxidant Consumption and Health
Abstract
1. Introduction
2. Free Radicals
2.1. Properties of Free Radicals
2.1.1. Superoxide Anion Radical (O2•−)
2.1.2. Hydroxyl Radical (HO•)
2.1.3. Peroxyl Radical (ROO•)
2.1.4. Hydroperoxyl Radical (HO2•)
2.1.5. Hydrogen Peroxide (H2O2)
2.1.6. Molecular Oxygen (O2••) and Singlet Oxygen (1O2)
2.1.7. Ozone (O3)
2.1.8. Hypochlorous Acid (HOCl)
2.1.9. Carbonate Radical Anion (CO3•−)
2.1.10. Nitric Oxide (NO•)
2.1.11. Nitrogen Dioxide (NO2•)
2.1.12. Peroxynitrite (ONOO−)
2.1.13. Reactive Sulfur Species
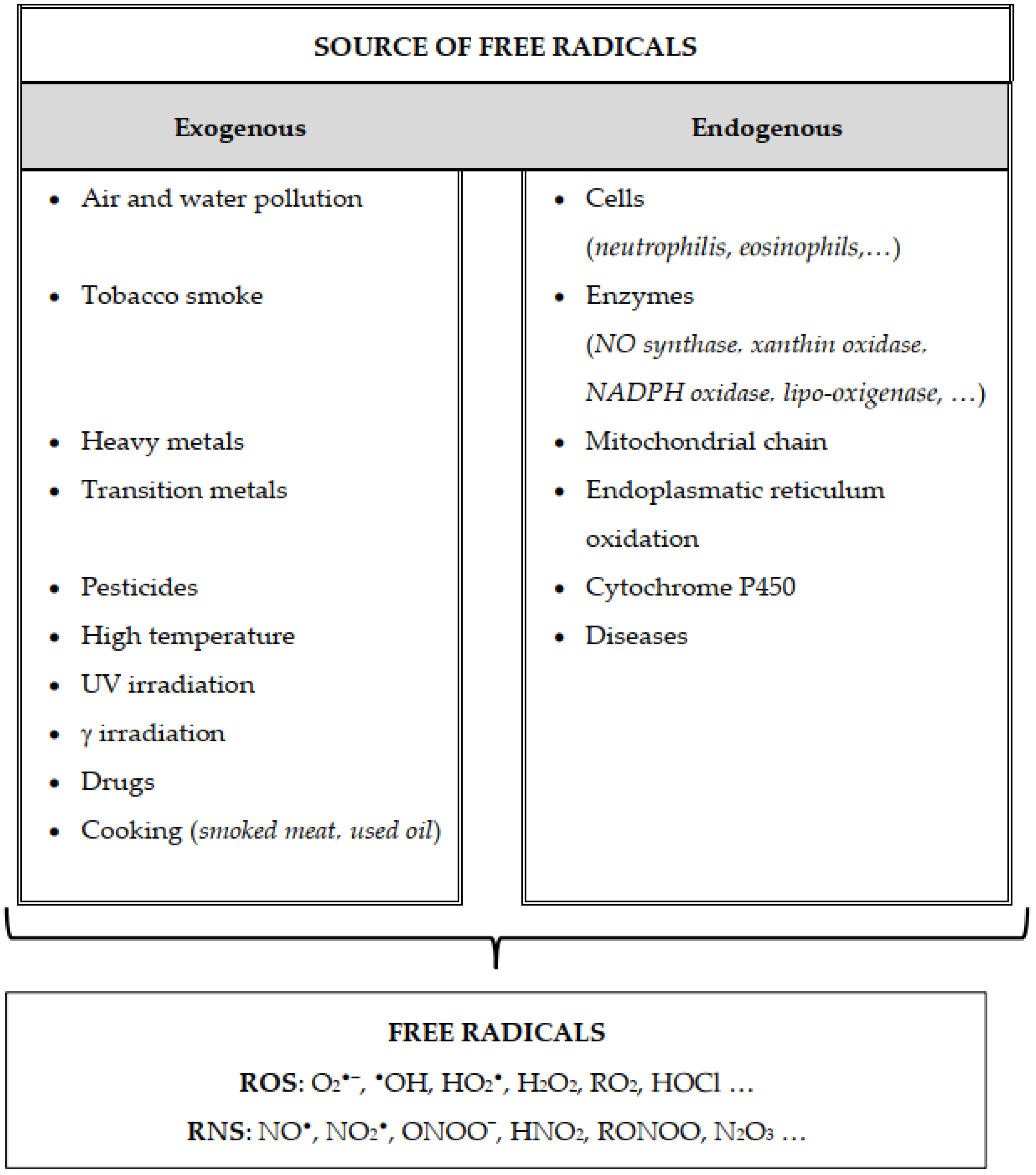
2.2. Generation of Free Radicals
2.2.1. Mitochondria
2.2.2. Peroxisomes
2.2.3. Endoplasmic Reticulum
2.2.4. Role of the Enzyme System
2.2.5. Role of Metals
2.3. Detection of ROS and RNS
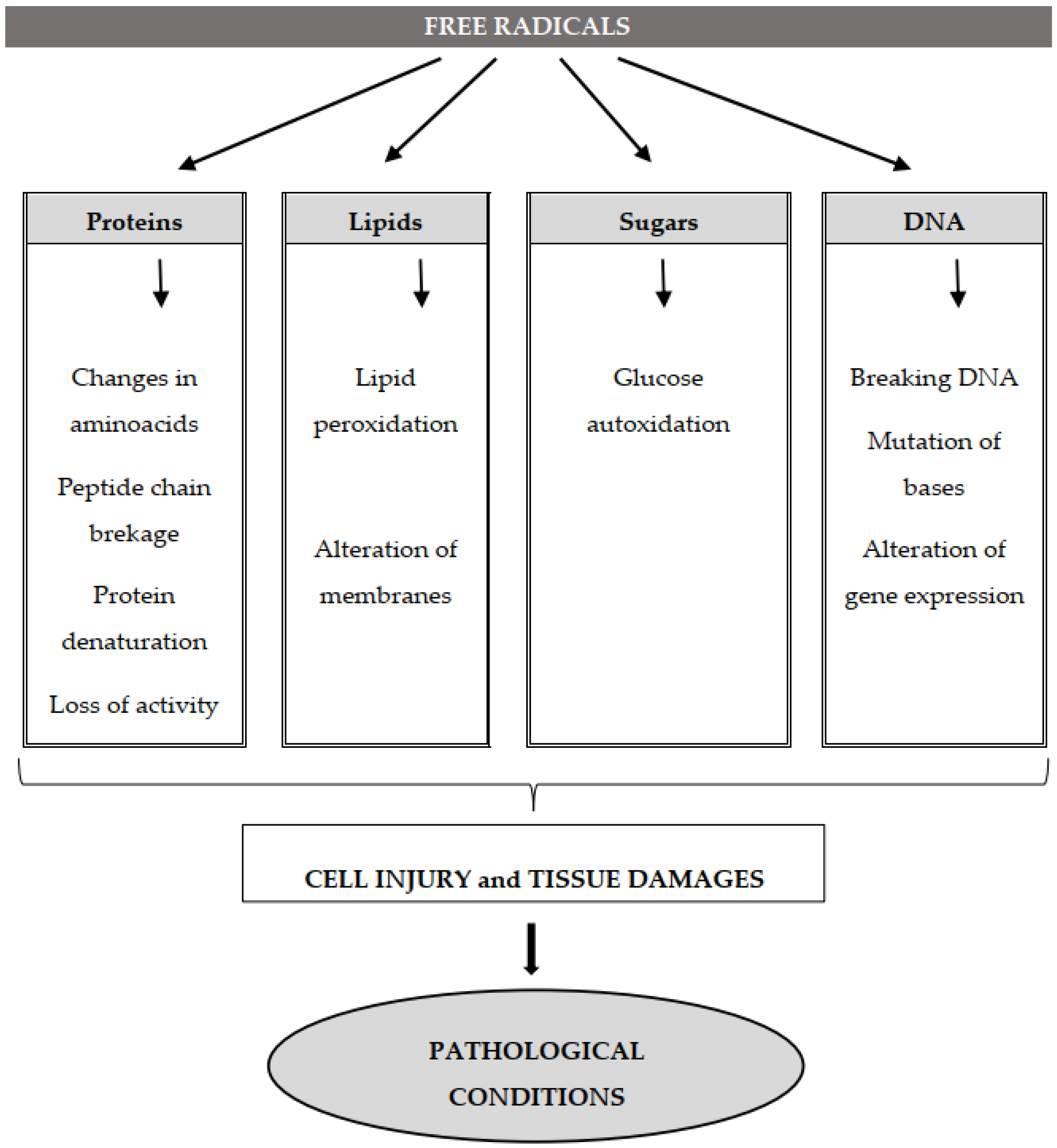
2.4. Molecular Targets of Free Radicals
2.4.1. Deoxyribonucleic (DNA) and Ribonucleic (RNA) Acids
2.4.2. Lipid Oxidation
Cholesterol Oxidation
Lipid Peroxides in Disease and Death
2.4.3. Protein Oxidation
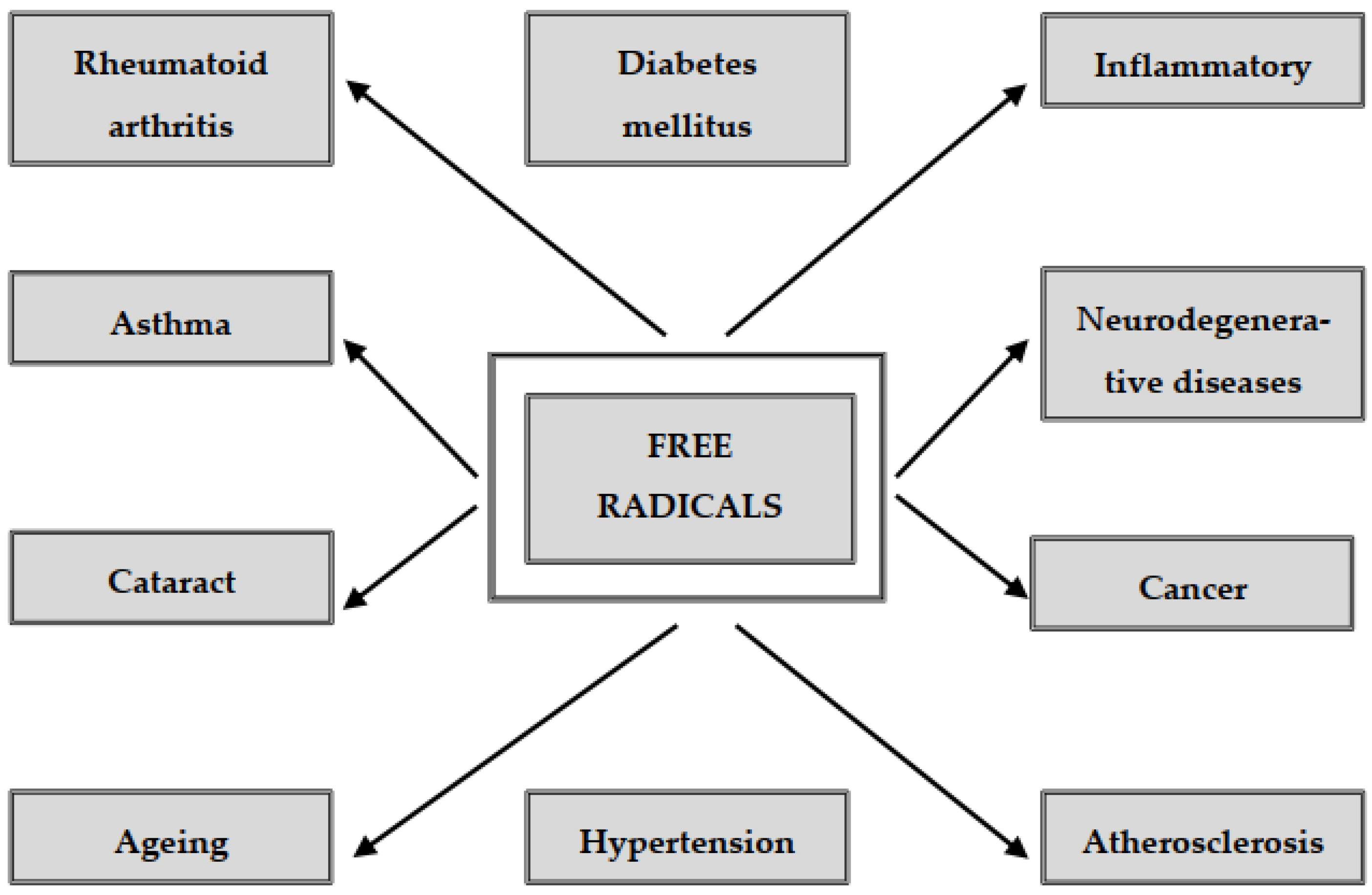
2.5. Free Radicals and Diseases
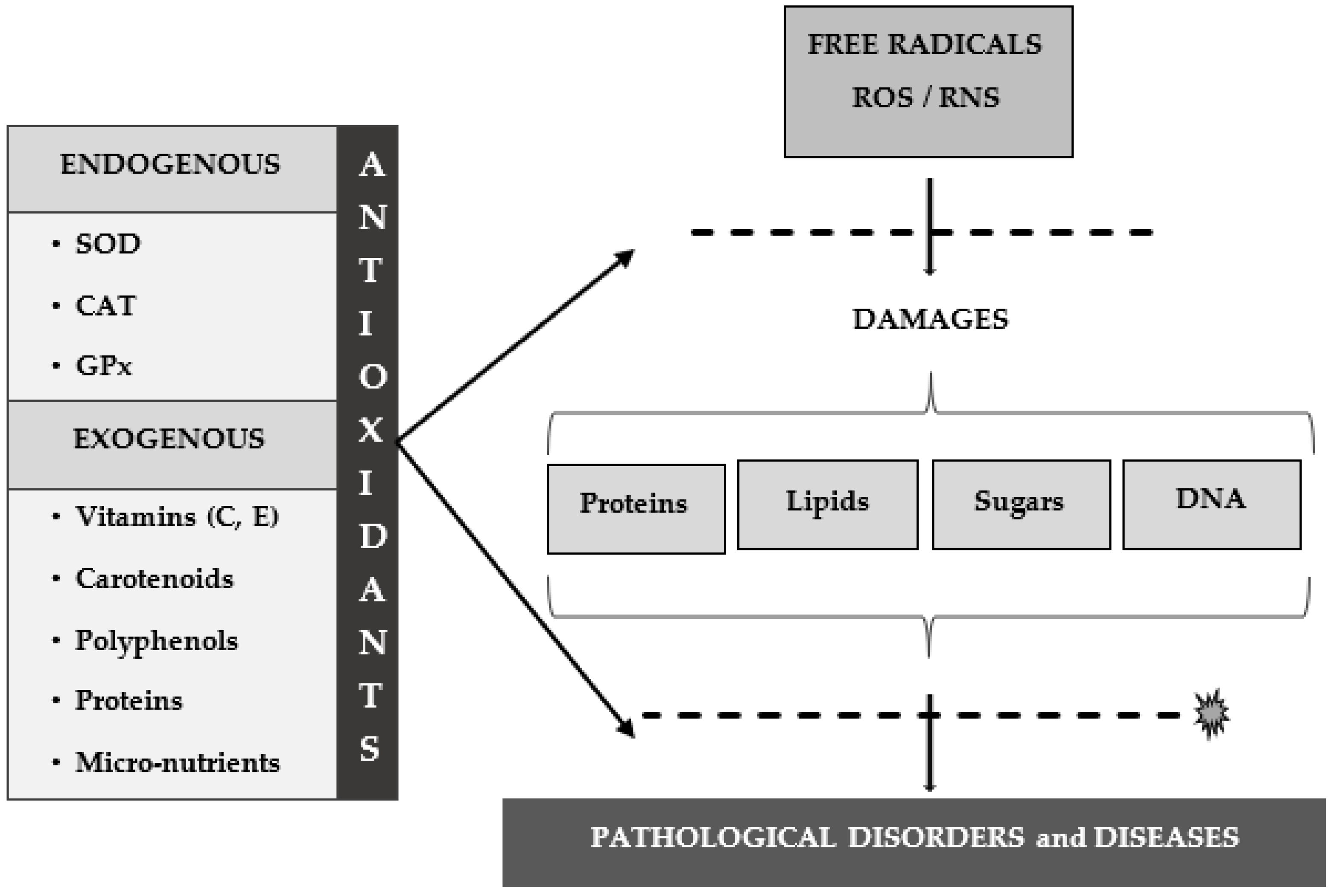
3. Antioxidants
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Michaelis, L. Free radicals as intermediate steps of oxidation- reduction. Cold Spring Harb. Symp. Quant. Biol. 1939, 7, 33–49. [Google Scholar] [CrossRef]
- Gerschman, R.; Gilbert, D.L.; Nye, S.W.; Dwyer, P.; Fenn, W.O. Oxygen poisoning and X-irradiation: A mechanism in common. Science 1954, 119, 62362–62366. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide dismutase, an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Loschen, G.; Flohe, L.; Chance, B. Respiratory chain linked H2O2 production in pigeon heart mitochondria. FEBS Lett. 1971, 18, 261–264. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [CrossRef]
- Palmer, R.M.; Rees, D.D.; Ashton, D.S.; Moncada, S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem. Biophys. Res. Commun. 1988, 153, 1251–1256. [Google Scholar] [CrossRef]
- Ferrari, C.K.; Souto, P.C.; França, E.L.; Honorio-França, A.C. Oxidative and nitrosative stress on phagocytes’ function: From effective defense to immunity evasion mechanisms. Arch. Immunol. Ther. Exp. 2011, 59, 441–448. [Google Scholar] [CrossRef]
- Stone, J.R.; Yang, S. Hydrogen peroxide: A signaling messenger. Antioxid. Redox Signal. 2006, 243–270. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Halliwell, B., Gutteridge, J.M.C., Eds.; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress and antioxidants. Toxicol. Pathol. 2002, 30, 620–630. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, A.; Chandel, N.S. ROS. Curr. Biol. 2013, 23, R100–R102. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.; Higgs, E. Nitric oxide: Physiology, patophysiology and pharmacology. Pharmacol. Rev. 1991, 43, 109–141. [Google Scholar] [PubMed]
- Genestra, M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell. Signal. 2007, 19, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.I.; Jacob, C. Reactive sulfur species: An emerging concept in oxidative stress. J. Biol. Chem. 2002, 383, 375–388. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and SODs. Ann. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef]
- Babcock, G.T. How oxygen is activated and reduced in respiration. Proc. Natl. Acad. Sci. USA 1999, 96, 13114–13117. [Google Scholar] [CrossRef]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Inoue, M.; Sato, E.F.; Nishikawa, M.; Parke, A.; Kira, Y.; Imada, I.; Utsumi, K. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr. Med. Chem. 2003, 10, 2495–2505. [Google Scholar] [CrossRef]
- Min, D.B.; Boff, J.B. Chemistry and reaction of singlet oxygen in foods. Compr. Rev. Food Sci. Food Saf. 2002, 1, 58–72. [Google Scholar] [CrossRef]
- Stief, T.W. The physiology and pharmacology of singlet oxygen. Med. Hypotheses 2003, 60, 567–572. [Google Scholar] [CrossRef]
- Sayre, L.M.; Moreira, P.I.; Smith, M.A.; Perry, G. Metal ions and oxidative protein modification in neurological disease. Ann. Ist. Super. Sanità 2005, 41, 143–164. [Google Scholar] [PubMed]
- Knight, J.A. Biochemistry of free radicals and oxidative stress. In Free radicals, Antioxidants, Ageing and Disease; Knight, J.A., Ed.; AACC Press: Washington, DC, USA, 1999; pp. 21–43. [Google Scholar]
- Lane, N. Oxygen: The Molecule That Made the World, revised ed.; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Korycka-Dahl, M.B.; Richardson, T. Activated oxygen species and oxidation of food constituents. Crit. Rev. Food Sci. Nutr. 1978, 10, 209–241. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products—A review. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Davies, M.J.A. The oxidative environment and protein damage. Biochim. Biophys. Acta 2005, 1703, 93–109. [Google Scholar] [CrossRef]
- Vijayalaxmi, R.J.; Reiter, D.X.; Tan, T.S.; Herman, C.R., Jr. Thomas, Melatonin as a radioprotective agent: A review. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 639–653. [Google Scholar] [CrossRef]
- Lipinski, B.; Pretorius, E. Hydroxyl radical-modified fibrinogen as a marker of thrombosis: The role of iron. Hematology 2012, 17, 241–247. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P. Mechanisms of free radical induced damage to DNA. Free Radic. Res. 2012, 46, 382–419. [Google Scholar] [CrossRef]
- Gutowski, M.; Kowalczyk, S. A study of free radical chemistry: Their role and pathophysiological significance. Acta Biochim. Pol. 2013, 60, 1–13. [Google Scholar] [CrossRef]
- Augusto, O.; Miyamoto, S. Oxygen radicals and related species. In Principles of Free Radical Biomedicine; Pantopoulos, K., Schipeer, H.M., Eds.; Nova Science Publishers: New York, NY, USA, 2011; pp. 19–42. [Google Scholar]
- León-Carmona, J.R.; Galano, A. Is caffeine a good scavenger of oxygenated free radicals? J. Phys. Chem. B 2011, 115, 4538–4546. [Google Scholar] [CrossRef]
- Galano, A. On the direct scavenging activity of melatonin towards hydroxyl and a series of peroxyl radicals. Phys. Chem. Chem. Phys. 2011, 13, 7178–7188. [Google Scholar] [CrossRef] [PubMed]
- De Grey, A. HO2•: The forgotten radical. DNA Cell Biol. 2002, 21, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Bielski, B.H.J.; Cabelli, B.H.; Arudi, R.L.; Ross, A.B. Reactivity of RO2/O2 radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Winterbourn, C.C. The biological chemistry of hydrogen peroxide. Methods Enzymol. 2013, 528, 3–25. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Kesheri, M.; Kanchan, S.; Richa, R.P. Sinha. Oxidative stress: Challenges and its mitigation mechanisms in cyanobacteria. In Biological Sciences: Innovations and Dynamics; Rajeshwar, P., Sinha, Richa, Rastogi, R.P., Eds.; New India Publishing Agency: New Delhi, India, 2015; pp. 311–324. [Google Scholar]
- Malanga, G.; Puntarulo, S. The use of electron para-magnetic resonance in studies of oxidative damage to lipids in aquatic systems. In Oxidative Stress in Aquatic Ecosystems; Abele, D., Vazquez-Medina, J., Zenteno-Savin, T., Eds.; Wiley & Sons: London, UK, 2011; pp. 448–457. [Google Scholar]
- Ryter, S.W.; Tyrrell, R.M. Singlet molecular oxygen (1O2): A possible effector of eukaryotic gene expression. Free Radic. Biol. Med. 1998, 24, 1520–1534. [Google Scholar] [CrossRef]
- Agnez-Lima, L.F.; Melo, J.T.; Silva, A.E.; Oliveira, A.H.S.; Timoteo, A.R.S.; Lima-Bessa, K.M.; Martinez, G.R.; Medeiros, M.H.G.; Di Mascio, P.; Galhardo, R.S.; et al. DNA damage by singlet oxygen and cellular protective mechanisms. Mutat. Res. Rev. Mutat. Res. 2012, 751, 15–28. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Petrou, A.L.; Terzidaki, A.A. Meta-analysis and review examining a possible role for oxidative stress and singlet oxygen in diverse diseases. Biochem. J. 2017, 474, 2713–2727. [Google Scholar] [CrossRef]
- Altenhofer, S.; Radermacher, K.A.; Kleikers, P.W.; Wingler, K.; Schmidt, H.H. Evolution of NADPH oxidase inhibitors: Selectivity and mechanisms for target engagement. Antioxid. Redox Signal. 2015, 23, 406–427. [Google Scholar] [CrossRef]
- Goldstein, B.D.; Lodi, C.; Collinson, C.; Balchum, O.J. Ozone and lipid peroxidation. Arch. Environm. Heath 1969, 18, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Graham, N.J.D. Oxidation of amino acids, peptides and proteins by ozone: A review. Ozone Sci. 2010, 32, 81–90. [Google Scholar] [CrossRef]
- Lerner, R.A.; Eschenmoser, A. Ozone in biology. Proc. Natl. Acad. Sci. USA 2003, 100, 3013–3015. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Kettle, A.J. Biomarkers of myeloperoxidase derived hypochlorous acid. Free Rad. Biol. Med. 2000, 29, 403–409. [Google Scholar] [CrossRef]
- Prütz, W.A. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch. Biochem. Biophys. 1996, 332, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Cope, V.W.; Hoffman, M. Behaviour of CO3- radicals generated in the flash photolysis of arbonatoamine complexes of cobalt (III) in aqueous solution. J. Phys. Chem. 1973, 77, 1111–1116. [Google Scholar] [CrossRef]
- Meli, R.; Nauser, T.; Latal, P.; Koppenol, W.H. Reaction of peroxynitrite with carbon dioxide: Intermediates and determination of the yield of CO3• and NO2•. J. Biol. Inorg. Chem. 2002, 7, 31–36. [Google Scholar] [CrossRef]
- Hoffman, A.; Goldstein, S.; Samuni, A.; Borman, J.B.; Schwalb, H. Effect of nitric oxide and nitroxide SOD-mimic on the recovery of isolated rat heart following ischemia and reperfusion. Biochem. Pharmacol. 2003, 66, 1279–1286. [Google Scholar] [CrossRef]
- Radi, R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. USA 2004, 101, 4003–4008. [Google Scholar] [CrossRef]
- Liochev, S.I.; Fridovich, I. CO2, not HCO3−, facilitates oxidations by Cu, Zn superoxide dismutase plus H2O2. Proc. Natl. Acad. Sci. USA 2004, 101, 743–744. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein oxidation in ageing and age-related diseases. Ann. N. Y. Acad. Sci. 2000, 928, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Surmeli, N.B.; Litterman, N.K.; Miller, A.F.; Groves, J.T. Peroxynitrite mediates active site tyrosine nitration in manganese superoxide dismutase. Evidence of a role for the carbonate radical anion. J. Am. Chem. Soc. 2010, 132, 17174–17185. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, J.; Liu, K.; Li, B.; Wang, H.; Jia, J. Peroxynitrite-induced nitration of cyclooxygenase- 2 and inducible nitric oxide synthase promotes their binding in diabetic angiopathy. Mol. Med. 2010, 16, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Douki, T.; Cadet, J. Peroxynitrite mediated oxidation of purine bases of nucleosides and isolated DNA. Free Radic. Res. 1996, 24, 369–380. [Google Scholar] [CrossRef]
- Ghafourifar, P.; Cadenas, E. Mitochondrial nitric oxide synthase. Trends Pharmacol. Sci. 2005, 26, 190–195. [Google Scholar] [CrossRef]
- Nagase, S.; Takemura, K.; Ueda, A.; Hirayama, A.; Aoyagi, K.; Kondoh, M.; Koyama, A. A novel nonenzymatic pathway for the generation of nitric oxide by the reaction of hydrogen peroxide and D- or L-arginine. Biochem. Biophys. Res. Commun. 1997, 233, 150–153. [Google Scholar] [CrossRef]
- Singh, R.J.; Hogg, N.; Joseph, J.; Kalyanaraman, B. Mechanism of nitric oxide release from S- nitrosothiols. J. Biol. Chem. 1996, 27, 18596–18603. [Google Scholar] [CrossRef]
- Repetto, M.; Semprine, J.; Boveris, A. Lipid peroxidation: Chemical mechanism, biological implications and analytical determination. In Lipid Peroxidation; Catala, D.A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 3–30. [Google Scholar]
- Noguchi, N.; Niki, E. Chemistry of active oxygen species and antioxidants. In Antioxidant Status, Diet, Nutrition, and Health; Papas, A.M., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 3–20. [Google Scholar]
- Papas, A.M. Diet and antioxidant status. Food. Chem. Toxicol. 1999, 37, 999–1007. [Google Scholar] [CrossRef]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. Cell Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef]
- Olson, K.R.; Straub, K.D. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. Physiology 2016, 31, 60–72. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. H2S: A novel gasotransmitter that signals by sulfhydration. Trends Biochem. Sci. 2015, 40, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Banerjee, R. Enzymology of H2S biogenesis, decay and signaling. Antioxid. Redox Signal. 2014, 20, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Goubern, M.; Andriamihaja, M.; Nübel, T.; Blachier, F.; Bouillaud, F. Sulfide, the first inorganic substrate for human cells. FASEB J. 2007, 21, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.I.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from Dcysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef]
- Nicholls, P. Inhibition of cytochrome c oxidase by sulphide. Biochem. Soc. Trans. 1975, 3, 316–319. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Sikka, G.; Gazi, S.K.; Steppan, J.; Jung, S.M.; Bhunia, A.K.; Barodka, V.M.; Gazi, F.K.; Barrow, R.K.; Wang, R.; et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ. Res. 2011, 109, 1259–1268. [Google Scholar] [CrossRef]
- Xie, Z.Z.; Shi, M.M.; Xie, L.; Wu, Z.Y.; Li, G.; Hua, F.; Bian, J.S. Sulfhydration of p66Shc at cysteine59 mediates the antioxidant effect of hydrogen sulfide. Antioxid. Redox Signal. 2014, 21, 2531–2542. [Google Scholar] [CrossRef]
- Zhou, H.; Ding, L.; Wu, Z.; Cao, X.; Zhang, Q.; Lin, L.; Bian, J.S. Hydrogen sulfide reduces RAGE toxicity through inhibition of its dimer formation. Free Radic. Biol. Med. 2017, 104, 262–271. [Google Scholar] [CrossRef]
- Li, L.; Bhatia, M.; Zhu, Y.Z.; Ramnath, R.D.; Wang, Z.J.; Anuar, F.B.M.; Moore, P.K.; Zhu, Y.C.; Whiteman, M.; Salto-Tellez, M. Hydrogen sulfide is a novel mediator of lipopolysaccharide- induced inflammation in the mouse. FASEB J. 2005, 19, 1196–1198. [Google Scholar] [CrossRef]
- Zanardo, R.C.O.; Brancaleone, V.; Distrutti, E.; Fiorucci, S.; Cirino, G.; Wallace, J.L. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006, 20, 2118–2120. [Google Scholar] [CrossRef]
- Hellmich, M.R.; Coletta, C.; Chao, C.; Szabo, C. The therapeutic potential of cystathionine b- synthetase/hydrogen sulfide inhibition in cancer. Antioxid. Redox Signal. 2015, 22, 424–448. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.Y.; Ogasawara, N.; Shibuya, H.; Kimura, K. Ishii. Polysulfide exerts a protective effect against cytotoxicity caused by t-buthylhydroperoxide through Nrf2 signaling in neuroblastoma cells. FEBS Lett. 2013, 587, 3548–3555. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Untereiner, A.; Wu, L.; Wang, R. Hydrogen sulfide and the pathogenesis of atherosclerosis. Antioxid. Redox Signal. 2014, 20, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Elrod, J.W.; Calvert, J.W.; Morrison, J.; Doeller, J.E.; Kraus, D.W.; Tao, L.; Jiao, X.; Scalia, R.; Kiss, L.; Szabo, C.; et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA 2007, 104, 15560–15565. [Google Scholar] [CrossRef]
- Bianco, C.L.; Akaike, T.; Ida, T.; Nagy, P.; Bogdandi, V.; Toscano, J.P.; Kumagai, Y.; Henderson, C.F.; Goddu, R.N.; Lin, J.; et al. The reaction of hydrogen sulfide with disulfides: Formation of a stable trisulfide and implications for Biological systems. J. Pharmacol. 2019, 176, 671–683. [Google Scholar] [CrossRef]
- Cuevasanta, E.; Lange, M.; Bonanata, J.; Coitiño, E.L.; Ferrer-Sueta, G.; Filipovic, M.R.; Alvarez, B. Reaction of hydrogen sulfide with disulfide and sulfenic acid to form the strongly nucleophilic persulfide. J. Biol. Chem. 2015, 290, 26866–26880. [Google Scholar] [CrossRef]
- Symons, M.C.R. Radicals generated by bone cutting and fracture. Free Radic. Biol. Med. 1996, 20, 831–835. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Srinivasan, S.; Avadhani, N. Cytochrome c oxidase dysfunction in oxidative stress. Free Rad. Biol. Med. 2012, 53, 1252–1263. [Google Scholar] [CrossRef]
- Rich, P.R.; Marechal, A. The mitochondrial respiratory chain. Essays Biochem. 2010, 47, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Deas, E.; Cremades, N.; Angelova, P.R.; Ludtmann, M.H.R.; Yao, Z.; Chen, S.; Horrocks, M.H.; Banushi, B.; Little, D.; Devine, M.J.; et al. Alpha-synuclein oligomers interact with metal ions to induce oxidative stress and neuronal death in Parkinson’s disease. Antioxid. Redox Sign. 2016, 24, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and ageing. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Lüthje, S.; Möller, B.; Perrineau, F.C.; Wöltje, K. Plasma membrane electron pathways and oxidative stress. Antioxid. Redox Signal. 2013, 18, 2163–2183. [Google Scholar] [CrossRef]
- Vartanian, L.S.; Gurevich, S.M. NADH- and NADPH-dependent formation of superoxide radicals in liver nuclei. Biokhimiia 1989, 54, 1020–10255. [Google Scholar]
- Brignac-Huber, L.; Reed, J.R.; Backes, W.L. Organization of NADPH-cytochrome P450 reductase and CYP1A2 in the endoplasmic reticulum microdomain localization affects monooxygenase function. Mol. Pharmacol. 2011, 79, 549–557. [Google Scholar] [CrossRef]
- Wang, W.; Gong, G.; Wang, X.; Wei-LaPierre, L.; Cheng, H.; Dirksen, R.; Sheu, S.S. Mitochondrial flash: Integrative reactive oxygen species and pH signals in cell and organelle biology. Antioxid. Redox Signal. 2016, 25, 534–549. [Google Scholar] [CrossRef]
- Wong, H.S.; Dighe, P.A.; Mezera, V.; Monternier, P.A.; Brand, M.D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 2017, 292, 16804–16809. [Google Scholar] [CrossRef]
- Zou, X.; Ratti, B.A.; O’Brien, J.G.; Lautenschlager, S.O.; Gius, D.R.; Bonini, M.G.; Zhu, Y. Manganese superoxide dismutase (SOD2): Is there a center in the universe of mitochondrial redox signaling? J. Bioenerg. Biomembr. 2017, 49, 325–333. [Google Scholar] [CrossRef]
- Leitão, E.F.V.; de Ventura, E.; Souza, M.A.F.; Riveros, J.M.; do Monte, S.A. Spin-forbidden branching in the mechanism of the intrinsic haber-weiss reaction. Chemistry Open 2017, 6, 360–363. [Google Scholar] [CrossRef]
- Mahaseth, T.; Kuzminov, A. Potentiation of hydrogen peroxide toxicity: From catalase inhibition to stable DNA-iron complexes. Mutat. Res. 2017, 773, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Pollack, M.; Leeuwenburgh, C. Molecular mechanisms of oxidative stress in ageing: Free radicals, ageing, antioxidants and disease. In Handbook of Oxidants and Antioxidants in Exercise; Sen, C.K., Paker, O., Hannine, L., Eds.; Elsevier: Amesterdam, The Nederland, 1999; pp. 881–923. [Google Scholar]
- Hauptmann, N.; Grimsby, J.; Shih, J.C.; Cadenas, E. The metabolism of tyramine by monoamine oxidase A/B causes oxidative damage to mitochondrial DNA. Arch. Biochem. Biophys. 1996, 335, 295–304. [Google Scholar] [CrossRef]
- Fhan, S.; Cohen, G. The oxidant stress hypothesis in Parkinson’s disease: Evidence supporting it. Ann. Neurol. 1992, 32, 804–812. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Perevoshchikova, I.V.; Brand, M.D. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013, 1, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, A.Y.; Kushnareva, Y.E.; Murphy, A.N.; Starkov, A.A. Mitochondrial ROS metabolism: 10 years later. Biochemistry 2015, 80, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Grivennikova, V.G.; Vinogradov, A.D. Partitioning of superoxide and hydrogen peroxide production by mitochondrial respiratory complex I. Biochim. Biophys. Acta 2013, 1827, 446–454. [Google Scholar] [CrossRef][Green Version]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar] [CrossRef]
- Ishii, T.; Yasuda, K.; Akatsuka, A.; Hino, O.; Hartman, P.S.; Ishii, N. A mutation in the SDHC gene of complex II increases oxidative stress, resulting in apoptosis and tumorigenesis. Cancer Res. 2005, 65, 203–209. [Google Scholar]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Heather, L.C.; Carr, C.A.; Stuckey, D.J.; Pope, S.; Morten, K.J.; Carter, E.E.; Edwards, L.M.; Clarke, K. Critical role of complex III in the early metabolic changes following myocardial infarction. Cardiovasc. Res. 2010, 85, 127–136. [Google Scholar] [CrossRef]
- Dröse, S. Differential effects of complex II on mitochondrial ROS production and their relation to ardioprotective pre- and postconditioning. Biochim. Biophys. Acta 2013, 1827, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Talamanca, A.A.; Castello, G.; Cordero, M.D.; D’Ischia, M.; Gadaleta, M.N.; Pallardó, F.V.; Petrović, S.; Tiano, L.; Zatterale, A. Oxidative stress and mitochondrial dysfunction across broad-ranging pathologies: Toward mitochondria-targeted clinical strategies. Oxid. Med. Cell. Longev. 2014, 2014, 541230. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010, 35, 505–513. [Google Scholar] [CrossRef]
- Ristow, M.; Schmeisser, K. Mitohormesis: Promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response 2014, 12, 288–341. [Google Scholar] [CrossRef] [PubMed]
- Scheibye-Knudsen, M.; Fang, E.F.; Croteau, D.L.; Wilson, D.M.; Bohr, V.A. Protecting the mitochondrial powerhouse. Trends Cell Biol. 2015, 25, 158–170. [Google Scholar] [CrossRef] [PubMed]
- De Duve, C.; Baudhuin, P. Peroxisomes (microbodies and related particles). Physiol. Rev. 1966, 46, 323–357. [Google Scholar] [CrossRef]
- Iuliano, L. Pathways of cholesterol oxidation via non-enzymatic mechanisms. Chem. Phys. Lipids 2011, 164, 457–468. [Google Scholar] [CrossRef]
- Schrader, M.; Fahimi, H.D. Peroxisomes and oxidative stress. Biochim. Biophys. Acta 2006, 1763, 1755–1766. [Google Scholar] [CrossRef]
- Islinger, M.; Voelkl, A.; Fahimi, H.D.; Schrader, M. The peroxisome: An update on mysteries 2.0. Histochem. Cell Biol. 2018, 150, 443–471. [Google Scholar] [CrossRef]
- Cheeseman, K.H.; Slater, T.F. An introduction to free radicals chemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef]
- Gross, E.; Sevier, C.S.; Heldman, N.; Vitu, E.; Bentzur, M.; Kaiser, C.A.; Thorpe, C.; Fass, D. Generating disulfides enzymatically: Reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. Proc. Natl. Acad. Sci. USA 2006, 103, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D. Glucose and reactive oxygen species. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Spiteller, G. Lipid oxidation in ageing and age-dependent disease. Exp. Gerontol. 2001, 36, 1425–1457. [Google Scholar] [CrossRef]
- Rosen, G.M.; Pou, S.; Ramos, C.L.; Cohen, M.S.; Britigan, B.E. Free radicals and phagocytic cells. FASEB J. 1995, 9, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Kohchi, C.; Inagawa, H.; Nishizawa, T.; Soma, G. ROS and innate immunity. Anticancer Res. 2009, 29, 817–821. [Google Scholar]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Heinecke, J.W.; Li, W.; Francis, G.A.; Goldstein, J.A. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J. Clin. Investig. 1993, 91, 2866–2872. [Google Scholar] [CrossRef]
- Lieber, C.S. Cytochrome P450 2E1: Its physiological and pathological role. Physiol. Rev. 1997, 77, 517–544. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control: A review. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Lu, S.C. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001, 15, 1335–1349. [Google Scholar] [CrossRef] [PubMed]
- Liochev, S.I.; Fridovich, I. Lucigenin as mediator of superoxide production: Revisited. Free Radic. Biol. Med. 1998, 25, 926–928. [Google Scholar] [CrossRef]
- Khramtsov, V.V. In vivo electron paramagnetic resonance: Radical concepts for translation to the clinical cetting. Antioxid. Redox Signal. 2018, 28, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; von Minckwitz, G.; Weber, S.; Peter, H.S.; Schini-Kerth, V.B.; Lobysheva, I.; Nepveu, F.; Wolf, G.; Strebhardt, K.; Kaufmann, M. Expression of endothelial and inducible nitric oxide synthase in benign and malignant lesions of the breast and measurement of nitric oxide using electron paramagnetic resonance spectroscopy. Cancer 2002, 95, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat. Res. 1997, 387, 147–163. [Google Scholar] [CrossRef]
- Hiraku, Y. Formation of 8-nitroguanine, a nitrative DNA lesion, in inflammation-related carcinogenesis and its significance, Environ. Health Prev. Med. 2010, 15, 63–72. [Google Scholar] [CrossRef]
- Hofer, T.; Badouard, C.; Bajak, E.; Ravanat, J.L.; Mattsson, A.; Cotgreave, I.A. Hydrogen peroxide causes greater oxidation in cellular RNA than in DNA. Biol. Chem. 2005, 386, 33–337. [Google Scholar] [CrossRef]
- Abe, T.; Tohgi, H.; Isobe, C.; Murata, T.; Sato, C. Remarkable increase in the concentration of 8- hydroxyguanosine in cerebrospinal fluid from patients with Alzheimer’s disease. J. Neurosci. Res. 2002, 70, 447–450. [Google Scholar] [CrossRef]
- Kikuchi, A.; Takeda, A.; Onodera, H.; Kimpara, T.; Hisanaga, K.; Sato, N.; Nunomura, A.; Castellani, R.J.; Perry, G.; Smith, M.A.; et al. Systemic increase of oxidative nucleic acid damage in Parkinson’s disease and multiple system atrophy. Neurobiol. Dis. 2002, 9, 244–248. [Google Scholar] [CrossRef]
- Martinet, W.; de Meyer, G.R.; Herman, A.G.; Kockx, M.M. Reactive oxygen species induce RNA damage in human atherosclerosis. Eur. J. Clin. Investig. 2004, 34, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Broedbaek, K.; Poulsen, H.E.; Weimann, A.; Kom, G.D.; Schwedhelm, E.; Nielsen, P.; Böger, R.H. Urinary excretion of biomarkers of oxidatively damaged DNA and RNA in hereditary hemochromatosis. Free Radic. Biol. Med. 2009, 47, 1230–1233. [Google Scholar] [CrossRef] [PubMed]
- Tateyama, M.; Takeda, A.; Onodera, Y.; Matsuzaki, M.; Hasegawa, T.; Nunomura, A.; Hirai, K.; Perry, G.; Smith, M.A.; Itoyama, Y. Oxidative stress and predominant Aβ42(43) deposition in myopathies with rimmed vacuoles. Acta Neuropathol. 2003, 105, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malondialdehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Nawar, W.F. Lipids. In Food Chemistry; Fennema, O.R., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 225–320. [Google Scholar]
- Elmore, S.; Mottram, D.S.; Enser, M.; Wood, J.D. Effect of the polyunsaturated fatty acid composition of beef muscle on the profile of aroma volatiles. J. Agric. Food Chem. 1999, 47, 1619–1625. [Google Scholar] [CrossRef]
- Marnett, L.J. Lipid peroxidation—DNA damage by malondialdehyde. Mutat. Res. 1999, 424, 83–95. [Google Scholar] [CrossRef]
- Schneider, C.; Pratt, D.A.; Porter, N.A.; Brash, A.R. Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem. Biol. 2007, 4, 473–488. [Google Scholar] [CrossRef]
- Ingólfsson, H.I.; Melo, M.N.; van Eerden, F.J.; Arnarez, C.; Lopez, C.A.; Wassenaar, T.A.; Periole, X.; de Vries, A.H.; Tieleman, D.P.; Marrink, S.J. Lipid organization of the plasma membrane. J. Am. Chem. Soc. 2014, 136, 14554–14559. [Google Scholar] [CrossRef]
- Brown, A.J.; Jessup, W. Oxysterols and atherosclerosis. Atherosclerosis 1999, 142, 1–28. [Google Scholar] [CrossRef]
- Diczfalusy, U. Analysis of cholesterol oxidation products in biological samples. J. AOAC Int. 2004, 87, 467–473. [Google Scholar] [CrossRef]
- Schroepfer, G.J., Jr. Oxysterols: Modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000, 80, 361–554. [Google Scholar] [CrossRef] [PubMed]
- Vicente, S.J.V.; Sampaio, G.R.; Ferrari, C.K.B.; Torres, E.A.F.S. Oxidation of cholesterol in foods and its importance for human health. Food Rev. Int. 2012, 28, 47–70. [Google Scholar] [CrossRef]
- Kulig, W.; Cwiklik, L.; Jurkiewicz, P.; Rog, T.; Vattulainen, I. Cholesterol oxidation products and their biological importance. Chem. Phys. Lipids 2016, 199, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Kloudová, A.; Guengerich, F.P.; Soucek, P. The role of oxysterols in human cancer. Trends Endocrinol. Metab. 2017, 28, 485–496. [Google Scholar] [CrossRef]
- Bast, A. Oxidative stress and calcium homeostasis. In DNA and Free Radicals; Halliwell, B., Aruoma, O.I., Eds.; Ellis Horwood: London, UK, 1993; pp. 95–108. [Google Scholar]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 360438. [Google Scholar] [CrossRef]
- Camandola, S.; Poli, G.; Mattson, M. The lipid peroxidation product 4-hydroxy-2,3-nonenal inhibits constitutive and inducible activity of nuclear factor-bin neurons. Brain Res. Mol. Brain Res. 2000, 85, 53–60. [Google Scholar] [CrossRef]
- Ackermann, J.A.; Hofheinz, K.; Zaiss, M.M.; Kronke, G. The double-edged role of 12/15-lipoxygenase during inflammation and immunity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1862, 371–381. [Google Scholar] [CrossRef]
- Domínguez, R.O.; Marschoff, E.R.; Guareschi, E.M.; Repetto, M.G.; Famulari, A.L.; Pagano, M.A.; Serra, J.A. Insulin, glucose and glycated haemoglobin in Alzheimer’s and vascular dementia with and without superimposed Tpe II diabetes mellitus condition. J. Neural Transm. 2008, 115, 77–84. [Google Scholar] [CrossRef]
- Lovell, M.A.; Ehmann, W.D.; Buffer, B.M.; Markesberry, W.R. Elevated thiobarbituric acid reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology 1995, 45, 1594–1601. [Google Scholar] [CrossRef]
- West, J.D.; Marnett, L.J. Endogenous reactive intermediates as modulators of cell signaling and cell death. Chem. Res. Toxicol. 2006, 19, 173–194. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Gueraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by lipid peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I. Reactive oxygen species, lipid peroxidation and antioxidative defense mechanism. Not. Bot. Horti Agrobo. 2013, 41, 44–57. [Google Scholar] [CrossRef]
- Fedotcheva, N.I.; Litvinova, E.G.; Amerkhanov, Z.G.; Kamzolova, S.V.; Morgunov, I.G.; Kondrashova, M.N. Increase in the contribution of transamination to the respiration of mitochondria during arousal. Cryo Lett. 2008, 29, 35–42. [Google Scholar] [PubMed]
- Hermes-Lima, M.; Willmore, W.G.; Storey, K.B. Quantification of lipid peroxidation in tissue extracts based on Fe(III) xylenol orange complex formation. Free Radic. Biol. Med. 1995, 19, 271–280. [Google Scholar] [CrossRef]
- Brannian, J.D.; Zhao, Y.; Burbach, J.A. Localization of lipid peroxidation-derived protein epitopes in the porcine corpus luteum. Biol. Reprod. 1997, 57, 1461–1466. [Google Scholar] [CrossRef]
- Dean, R.T.; Fu, S.; Stocker, R.; Davies, M.J. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 1997, 324, 1–18. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Koppal, T.; Howard, B.; Subramaniam, R.; Hall, N.; Hensley, K.; Yatin, S.; Allen, K.; Aksenov, M.; Aksenova, M.; et al. Structural and functional changes in proteins induced by free radical-mediated oxidative stress and protective action of the antioxidants N-tert-butyl-alpha-phenylnitrone and vitamin E. Ann. N. Y. Acad. Sci. 1998, 854, 448–462. [Google Scholar] [CrossRef]
- Chevion, M.; Berenshtein, E.; Stadtman, E.R. Human studies related to protein oxidation: Protein carbonyl content as a marker of damage. Free Radic. Res. 2000, 33, S99–S108. [Google Scholar]
- Floor, E.; Wetzel, M.G. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J. Neurochem. 1998, 70, 268–275. [Google Scholar] [CrossRef]
- Barreiro, E.; Hussain, S. Protein carbonylation in skeletal muscles: Impact on function. Antioxid. Redox Signal. 2010, 12, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.L.; Rubin, B.R.; Gracy, R.W. Increased carbonyl content of proteins in synovial fluid from patients with rhematoid arthritis. J. Rheumatol. 1989, 16, 15–18. [Google Scholar]
- Krisko, A.; Radman, M. Protein damage, ageing and age-related diseases. Open Biol. 2019, 9, 180–249. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Stadtman, E.R. Oxidative modification of proteins during ageing. Exp. Gerontol. 2001, 36, 1495–1502. [Google Scholar] [CrossRef]
- Baraibar, M.A.; Liu, L.; Ahmed, E.K.; Friguet, B. Protein oxidative damage at the crossroads of cellular senescence, ageing, and age-related diseases. Oxid. Med. Cell Longev. 2012, 2012, 919832. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, J.P.; Klotz, L.O. Free radicals and related reactive species as mediators of tissue injury and disease: Implications for health. Crit. Rev. Toxicol. 2015, 45, 765–798. [Google Scholar] [CrossRef]
- Bashan, N.; Kovsan, J.; Kachko, I.; Ovadia, H.; Rudich, A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol. Rev. 2009, 89, 27–71. [Google Scholar] [CrossRef]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 1–19. [Google Scholar] [CrossRef]
- Pan, X.D.; Zhu, Y.G.; Lin, N.; Zhang, J.; Ye, Q.Y.; Huang, H.P.; Chen, X.C. Microglial phagocytosis induced by fibrillar β-amyloid is attenuated by oligomeric β-amyloid: Implications for Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 1–17. [Google Scholar] [CrossRef]
- Sevcsik, E.; Trexler, A.J.; Dunn, J.M.; Rhoades, E. Allostery in a disordered protein: Oxidative modifications to a-synuclein act distally to regulate membrane binding. J. Am. Chem. Soc. 2011, 133, 7152–7158. [Google Scholar] [CrossRef]
- Ha, A.D.; Fung, V.S. Huntington’s disease. Curr. Opin. Neurol. 2012, 25, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Varghese, M.; Yemul, S.; Pan, Y.; Cheng, A.; Marano, P.; Hassan, S.; Vempati, P.; Chen, F.; Qian, X.; et al. Peroxisome proliferator activator receptor gamma coactivator- 1alpha (PGC-1α) improves motor performance and survival in a mouse model of amyotrophic lateral sclerosis. Mol. Neurodegener. 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Witherick, J.; Wilkins, A.; Scolding, N.; Kemp, K. Mechanisms of oxidative damage in multiple sclerosis and a cell therapy approach to treatment. Autoimmune Dis. 2011, 1–11. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.S.; Jones, A.; Fujiyama, C.; Harris, A.L.; Bicknell, R. Thymidine phosphorylase induces carcinoma cell oxidative stress and promotes secretion of angiogenic factors. Cancer Res. 2000, 60, 6298–6302. [Google Scholar] [PubMed]
- Lim, S.D.; Sun, C.; Lambeth, J.D.; Marshall, F.; Amin, M.; Chung, L.; Petros, J.A.; Arnold, R.S. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate 2005, 62, 200–207. [Google Scholar] [CrossRef]
- Azad, N.; Rojanasakul, Y.; Vallyathan, V. Inflammation and lung cancer: Roles of reactive oxygen/ nitrogen species. J. Toxicol. Environ. Health 2008, 11, 1–15. [Google Scholar] [CrossRef]
- Zhang, N.; Bradley, T.A.; Zhang, C. Inflammation and reactive oxygen species in cardiovascular disease. World J. Cardiol. 2010, 2, 408–410. [Google Scholar] [CrossRef]
- Yin, Y.; Pastrana, J.L.; Li, X.; Huang, X.; Mallilankaraman, K.; Choi, E.T.; Madesh, M.; Wang, H.; Yang, X.F. Inflammasomes: Sensors of metabolic stresses for vascular inflammation. Front. Biosci. 2013, 18, 638–649. [Google Scholar]
- WHO. A global brief on hypertension. In World Health Day 2013; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Costagliola, C.; Iuliano, G.; Menzione, M.; Nesti, A.; Simonelli, F.; Rinaldi, E. Systemic human diseases as oxidative risk factors in cataractogenesis I. Diabetes. Ophthalmic Res. 1988, 20, 308–316. [Google Scholar] [CrossRef]
- Costagliola, C.; Iuliano, G.; Menzione, M.; Simonelli, F.; Tortori, A.; Masturzi, B.; di Benedetto, A.; Rinaldi, E. Systemic human diseases as oxidative risk factors in cataractogenesis. II. Chronic renal failure. Exp. Eye Res. 1990, 51, 631–635. [Google Scholar] [CrossRef]
- Vasanthi, P.; Nalini, G.; Rajasekhar, G. Status of oxidative stress in rheumatoid arthritis. Int. J. Rheum. Dis. 2009, 12, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T. Role of oxygen radicals on bronchial asthma. Curr. Drug Targets Inflamm. Allergy 2005, 4, 505–509. [Google Scholar] [CrossRef]
- Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; Dhaka, N. Potential applications of antioxidants—A review. J. Pharm. Res. 2013, 7, 828–835. [Google Scholar] [CrossRef]
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef]
- Costagliola, C.; Libondi, T.; Menzione, M.; Rinaldi, E.; Auricchio, G. Vitamin E and red blood cell glutathione. Metabolism 1985, 34, 712–714. [Google Scholar] [CrossRef]
- Mandal, S.; Yadav, S.; Yadav, S.; Nema, R.K. Antioxidants: A review. J. Chem. Pharm. Res. 2009, 1, 102–104. [Google Scholar]
- Akbarirad, H.; Ardabili, G.A.; Kazemeini, S.M.; Khaneghah, M.A. An overview on some of important sources of natural antioxidants. Int. Food Res. J. 2016, 23, 928–933. [Google Scholar]
- Seifried, H.E.; Anderson, D.E.; Fisher, E.I.; Milner, J.A. A review of the interaction among the dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007, 18, 567–579. [Google Scholar] [CrossRef]
- Bitiren, M.; Karakilcik, A.Z.; Zerin, M.; Ozardali, I.; Selek, S.; Nazligül, Y.; Ozgonul, A.; Musa, D.; Uzunkoy, A. Protective effects of selenium and vitamin E combination on experimental colitis in blood plasma and colon of rats. Biol. Trace Elem. Res. 2010, 136, 87–95. [Google Scholar] [CrossRef]
- Poljsak, B.; Suput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 956792, 1–11. [Google Scholar] [CrossRef] [PubMed]
- King, D.A.; Cordova, F.; Scharf, S.M. Nutritional aspects of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2008, 5, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Alzoghaibi, M.A. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 2013, 19, 6540–6547. [Google Scholar] [CrossRef]
- Schols, A.M.; Ferreira, I.M.; Franssen, F.M.; Gosker, H.R.; Janssens, W.; Muscaritoli, M.; Rutten-van Mölken, M.; Slinde, F.; Steiner, M.C.; Tkacova, R.; et al. Nutritional assessment and therapy in COPD: A European respiratory society statement. Eur. Respir. J. 2014, 44, 1504–1520. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Stahl, W.; Sevanian, A. Nutritional, dietary and postprandial oxidative stress. J. Nutr. 2005, 135, 969–972. [Google Scholar] [CrossRef]
- Varraso, R.; Chiuve, S.E.; Fung, T.T.; Barr, R.G.; Hu, F.B.; Willett, W.C.; Camargo, C.A. Alternate healthy eating index 2010 and risk of chronic obstructive pulmonary disease among US women and men: Prospective study. BMJ 2015, 350, h286. [Google Scholar] [CrossRef]
- Sideri, O.; Tsaousis, K.T.; Li, H.J.; Viskadouraki, M.; Tsinopoulos, I.T. The potential role of nutrition on lens pathology: A systematic review and meta-analysis. Surv. Ophthalmol. 2019, 64, 668–678. [Google Scholar] [CrossRef]
- Zino, S.; Skeaff, M.; Williams, S.; Mann, J. Randomised controlled trial of effect of fruit and vegetable consumption on plasma concentrations of lipids and antioxidants. BMJ 1997, 314, 1787–1791. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef]
- Vita, J.A. Polyphenols and cardiovascular disease: Effects on endothelial and platelet function. Am. J. Clin. Nutr. 2005, 81, 292s–297s. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Quinones, M.; Miguel, M.; Aleixandre, A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.P.; Anthony, J.C.; Khachaturian, A.S.; Stone, S.V.; Gustafson, D.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A.; Breitner, J.C.S. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: The Cache County Study. Arch Neurol. 2004, 61, 82–88. [Google Scholar] [CrossRef]
- Wang, J.; Yang, D.; Yu, Y.; Shao, G.; Wang, Q. Vitamin D and sunlight exposure in newly- diagnosed Parkinson’s disease. Nutrients 2016, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Sechi, G.; Sechi, E.; Fois, C.; Kumar, N. Advances in clinical determinants and neurological manifestations of B vitamin deficiency in adults. Nutr. Rev. 2016, 74, 281–300. [Google Scholar] [CrossRef]
- Mazo, N.A.; Echeverria, V.; Cabezas, R.; Avila-Rodriguez, M.; Tarasov, V.V.; Yarla, N.S.; Aliev, G.; Barreto, G.E. Medicinal plants as protective strategies against Parkinson’s disease. Curr. Pharm. Des. 2017, 23, 4180–4188. [Google Scholar] [CrossRef]
- Kang, H.; Ascherio, A.; Grodstein, F. Fruit and vegetable consumption and cognitive decline in ageing women. Ann. Neurol. 2005, 57, 713–720. [Google Scholar] [CrossRef]
- Kandola, K.; Bowman, A.; Birch-Machin, M.A. Oxidative stress–a key emerging impact factor in health, ageing, lifestyle and aesthetics. Int. J. Cosmet. Sci. 2015, 37 (Suppl. 2), 1–8. [Google Scholar] [CrossRef]
- Nagata, C.; Nakamura, K.; Wada, K.; Oba, S.; Hayashi, M.; Takeda, N.; Yasuda, K. Association of dietary fat, vegetables and antioxidant micronutrients with skin ageing in Japanese women. Br. J. Nutr. 2010, 103, 1493–1498. [Google Scholar] [CrossRef]
- Inserra, P.F.; Jiang, S.; Solkoff, D. Immune function in elderly smokers and nonsmokers improves during supplementation with fruit and vegetable extracts. Integr. Med. 1999, 2, 3–10. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in inflammatory bowel disease: Clinical aspects and animal models. Saudi. J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Rossi, M.; Pelucchi, C.; Levi, F.; La Vecchia, C. Fruit and vegetables and cancer risk: A review of southern European studies. Br. J. Nutr. 2015, 113 (Suppl. 2), S102–S110. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef]
- Manikandan, R.; Beulaja, M.; Arulvasu, C.; Sellamuthu, S.; Dinesh, D.; Prabhu, D.; Babu, G.; Vaseeharan, B. Synergistic anticancer activity of curcumin and catechin: An in vitro study using human cancer cell lines. Microsc. Res. Technol. 2012, 75, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr. Cancer 2014, 66, 177–193. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
- Curti, V.; Di Lorenzo, A.; Da Crema, M.; Xiao, J.B.; Nabavi, S.M.; Daglia, M. In vitro polyphenol effects on apoptosis: An update of literature data. Semin. Canc. Biol. 2017, 46, 119–131. [Google Scholar] [CrossRef]
- Pan, P.; Skaer, C.; Yu, J.; Zhao, H.; Ren, H.; Oshima, K.; Wang, L.S. Berries and other natural products in the pancreatic cancer chemoprevention in human clinical trials. J. Berry Res. 2017, 7, 147–161. [Google Scholar] [CrossRef]
- Aqil, F.; Jeyabalan, J.; Kausar, H.; Munagala, R.; Singh, I.P.; Gupta, R. Lung cancer inhibitory activity of dietary berries and berry polyphenolics. J. Berry Res. 2016, 6, 105–114. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Cordero, M.D.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Santos-Buelga, C.; González-Paramás, A.M.; Astolfi, P.; Rubini, C.; et al. Strawberry consumption improves ageing-associated impairments, mitochondrial biogenesis and functionality through the AMP-activated protein kinase signaling cascade. Food Chem. 2017, 234, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo- Rodriguez, P.; Amici, A.; Quiles, J.L.; Battino, M. Inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cells growth. Part 1: Suppression of proliferation, promotion of apoptosis and arrest of cell cycle. Food Funct. 2018, 9, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.T.; Chuang, C.H.; Yeh, C.L.; Liao, J.W.; Liu, K.L.; Tseng, M.J.; Yeh, S.L. Quercetin supplementation suppresses the secretion of pro-inflammatory cytokines in the lungs of Mongolian gerbils and in A549 cells exposed to benzo[a]pyrene alone or in combination with β-carotene: In vivo and ex vivo studies. J. Nutr. Biochem. 2012, 23, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.B.; Rajendiran, V.; Kasinathan, N.K.; Venkatabalasubramanian, P.A.S.; Murali, M.R.; Devaraj, H.; Devaraj, S.N. Differential cytotoxic activity of quercetin on colonic cancer cells depends on ROS generation through COX-2 expression. Food Chem. Toxicol. 2017, 106, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Ye, T.; Xiang, Y.; Shi, Z.; Zhang, J.; Lou, B.; Zhang, F.; Chen, B.; Zhou, M. Quercetin inhibits epithelial-mesenchymal transition, decreases invasiveness and metastasis, and reverses IL-6 induced epithelial-mesenchymal transition, expression of MMP by inhibiting STAT3 signaling in pancreatic cancer cells. OncoTargets Ther. 2017, 10, 4719–4729. [Google Scholar] [CrossRef]
- Xiao, J.B.; Högger, P. Stability of dietary polyphenols under the cell culture condition: Avoiding erroneous conclusions. J. Agri. Food Chem. 2015, 63, 1547–1557. [Google Scholar] [CrossRef]
- Iacopetta, D.; Grande, F.; Caruso, A.; Mordocco, R.A.; Plutino, M.R.; Scrivano, L.; Ceramella, J.; Muià, N.; Saturnino, C.; Puoci, F.; et al. New insights for the use of quercetin analogs in cancer treatment. Future Med. Chem. 2017, 9, 2011–2028. [Google Scholar] [CrossRef]
- Yang, F.; Song, L.; Wang, H.; Wang, J.; Xu, Z.; Xing, N. Quercetin in prostate cancer: Chemotherapeutic and chemopreventive effects, mechanisms and clinical application potential (Review). Oncol. Rep. 2015, 33, 2659–2668. [Google Scholar] [CrossRef]
- Norat, T.; Scoccianti, C.; Boutron-Ruault, M.C.; Anderson, A.; Berrino, F.; Cecchini, M.; Espina, C.; Key, T.; Leitzmann, M.; Powers, H.; et al. European code against cancer 4th edition: Diet and cancer. Cancer Epidemiol. 2015, 39 (Suppl. 1), S56–S66. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Margaritelis, N.V. Antioxidants as therapeutics in the intensive care unit: Have we ticked the redox boxes? Pharmacol. Res. 2016, 111, 126–132. [Google Scholar] [CrossRef]
- Abourashed, E.A. Bioavailability of plant-derived antioxidants. Antioxidants 2013, 2, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Kumar, S. The importance of antioxidants and their role in pharmaceutical science—A review. Asian J. Res. Chem. Pharm. Sci. 2014, 1, 27–44. [Google Scholar]
- Ferdinandusse, S.; Finckh, B.; de Hingh, Y.C.; Stroomer, L.E.; Denis, S.; Kohlschütter, A.; Wanders, R.J. Evidence for increased oxidative stress in peroxisomal D-bifunctional protein deficiency. Mol. Genet Metab. 2003, 79, 281–287. [Google Scholar] [CrossRef]
| Reactive Oxygen Species (ROS) | Reactive Nitrogen Species (RNS) | ||||
|---|---|---|---|---|---|
| Name | Symbol | Half-Life (s) | Name | Symbol | Half-Life 1 |
| Radicals | |||||
| Superoxide | O•− | 10−6 s | Nitric oxide | NO• | s |
| Hydroxyl | •OH | 10−10 s | Nitrogen dioxide | NO2• | s |
| Hydroperoxyl | HO2• | s | Nitrate radical | NO3• | s |
| Peroxyl | ROO• | 17 s | |||
| Alkoxyl Organic hydroperoxide | RO• ROOH | 10−6 s Stable | |||
| Non-radicals | |||||
| Hydrogen peroxide | H2O2 | Stable | Nitrous acid | HNO2 | s |
| Ozone | O3 | s | Nitrosonium cation | NO+ | s |
| Singlet oxygen | (1O2Dg) | 10−6 s | Nitroxyl anion | NO− | s |
| Hypochlorous acid | HOCl | Stable (min) | Peroxynitrite | ONOO− | 10−3 s |
| Peroxynitrite | ONOO− | 10−3 s | Dinitrogen trioxide | N2O3 | s |
| Dinitrogen tetroxide | N2O4 | s | |||
| Peroxynitrous acid | ONOOH | Fair stable | |||
| Nitryl chloride | NO2Cl | s | |||
| Reactive Oxygen Species (ROS) | Reactive Nitrogen Species (RNS) | Reactive Sulfur Species (RSS) | |||
|---|---|---|---|---|---|
| Name | Symbol | Name | Symbol | Name | Symbol |
| Radicals | |||||
| Oxygen (bi-radical) | O2·· | Nitric oxide | NO• | Thiyl radical S• | RS• |
| Hydroxyl | •OH | Nitrogen dioxide | NO2• | ||
| Hydroperoxyl | HO2• | Nitrate radical | NO3• | ||
| Carbonate | CO3•− | ||||
| Peroxyl | ROO• | ||||
| Alkoxyl | RO• | ||||
| Carbon dioxide radical | CO2•− | ||||
| Non-radicals | |||||
| Hydrogen peroxide | H2O2 | Nitrosyl cation | NO+ | Hydrogen sulfide | H2S |
| Ozone | O3 | Nitrous acid | HNO2 | Disulfide | RSSR |
| Singlet oxygen | 1O2 | Nitroxyl anion | NO− | Disulfide-S-monoxide | RS(O)SR |
| Hypobromous acid | HOBr | Dinitrogen trioxide | N2O3 | Disulfide-S-dioxide | RS(O)2SR |
| Hypochlorous acid | HOCl | Dinitrogen tetroxide | N2O4 | Sulfenic acid | RSOH |
| Hypoiodous acid | HOI | Dinitrogen pentoxide | N2O5 | Thiol/sulfide | RSR |
| Organic peroxides | ROOH | Alkyl peroxynitrites | ROONO | ||
| Peroxynitrite | ONOO− | Alkyl peroxynitrates | RO2ONO | ||
| Peroxynitrate | O2NOO− | Nitryl chloride | NO2Cl | ||
| Peroxynitrous acid | ONOOH | Peroxyacetyl nitrate | CH3C(O)OONO2 | ||
| Peroxomono-carbonate | HOOCO2− | ||||
| Carbon monoxide | CO | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48-78. https://doi.org/10.3390/oxygen2020006
Martemucci G, Costagliola C, Mariano M, D’andrea L, Napolitano P, D’Alessandro AG. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen. 2022; 2(2):48-78. https://doi.org/10.3390/oxygen2020006
Chicago/Turabian StyleMartemucci, Giovanni, Ciro Costagliola, Michele Mariano, Luca D’andrea, Pasquale Napolitano, and Angela Gabriella D’Alessandro. 2022. "Free Radical Properties, Source and Targets, Antioxidant Consumption and Health" Oxygen 2, no. 2: 48-78. https://doi.org/10.3390/oxygen2020006
APA StyleMartemucci, G., Costagliola, C., Mariano, M., D’andrea, L., Napolitano, P., & D’Alessandro, A. G. (2022). Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen, 2(2), 48-78. https://doi.org/10.3390/oxygen2020006






