The Reactive Oxygen Species Singlet Oxygen, Hydroxy Radicals, and the Superoxide Radical Anion—Examples of Their Roles in Biology and Medicine
Abstract
1. Introduction
2. Effect of Environment on ROS
2.1. Excited States
2.2. Radicals
3. Experimental Techniques
3.1. LFP and PR
3.2. Singlet Oxygen (1O2)
3.3. Electron Paramagnetic Resonance (EPR) and Spin Trapping
4. Applications
4.1. Carotenoids in the Eye
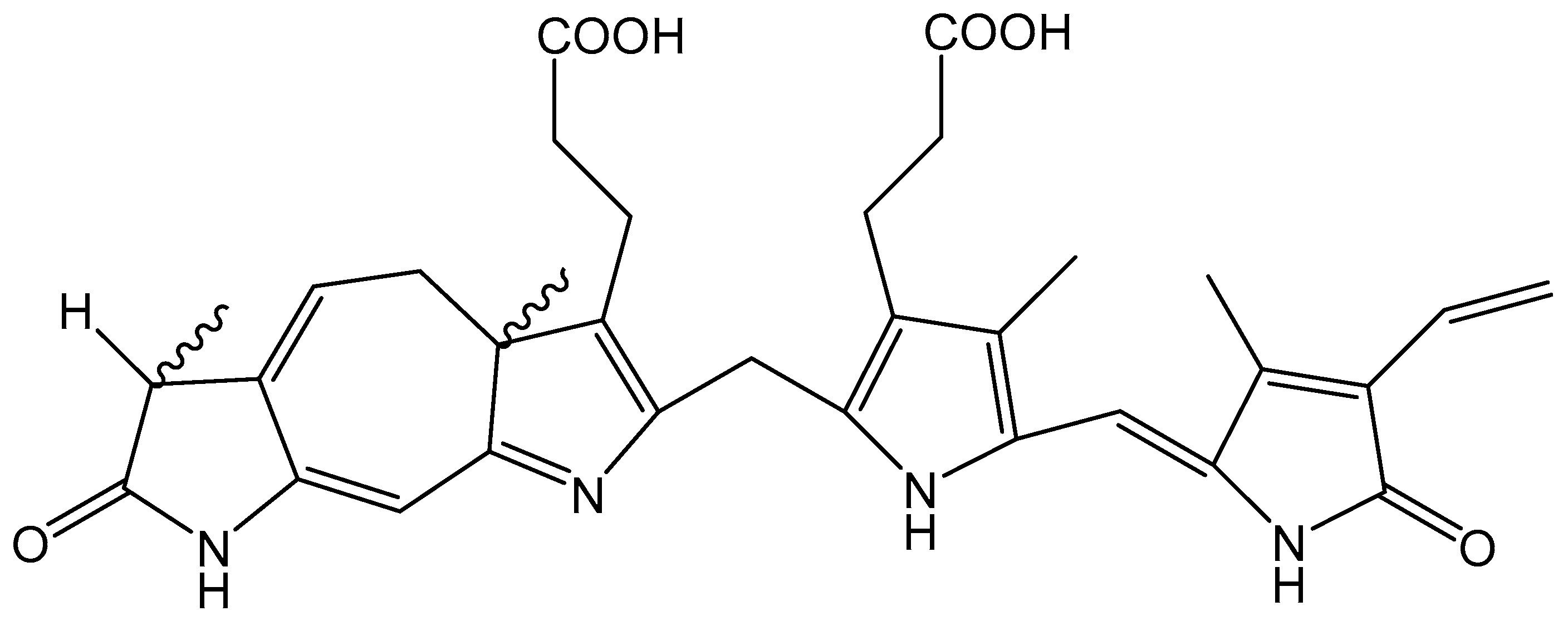
4.2. Melanins and COVID-19
4.3. Psoriasis and UVA
4.4. Neonatal Jaundice
4.5. Commercial Sunscreens
4.5.1. Inorganic Sunscreens
4.5.2. Organic Sunscreens
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edge, R. Radiolytic and photolytic production of free radicals and reactive oxygen species: Interactions with antioxidants and biomolecules. In Applied Photochemistry; Evans, R.C., Douglas, P., Burrows, H.D., Eds.; Springer Science: Dordrecht, The Netherlands, 2013; pp. 305–330. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Halliwell, B.; Adhikary, A.; Dingfelder, M.; Dizdaroglu, M. Hydroxyl radical is a significant player in oxidative DNA damage in vivo. Chem. Soc. Rev. 2021, 50, 8355–8360. [Google Scholar] [CrossRef]
- Prado, F.M.; Scalfo, A.C.; Miyamoto, S.; Medeiros, M.H.G.; Di Mascio, P. Generation of Singlet Molecular Oxygen by Lipid Hydroperoxides and Nitronium Ion. Photochem. Photobiol. 2020, 96, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.F.; Medeiros, M.H.G.; Di Mascio, P. Singlet oxygen-induced protein aggregation: Lysozyme crosslink formation and nLC-MS/MS characterization. J. Mass. Spec. 2019, 54, 894–905. [Google Scholar] [CrossRef]
- Pospíšil, P.; Ankush Prasad, A. Formation of singlet oxygen and protection against its oxidative damage in Photosystem II under abiotic stress. J. Photochem. Photobiol. B Biol. 2014, 137, 39–48. [Google Scholar] [CrossRef]
- Pospíšil, P. Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 218–231. [Google Scholar] [CrossRef]
- Dikalov, S.; Itani, H.; Richmond, B.; Vergeade, A.; Rahman, S.M.J.; Boutaud, O.; Blackwell, T.; Massion, P.P.; Harrison, D.G.; Dikalova, A. Tobacco smoking induces cardiovascular mitochondrial oxidative stress, promotes endothelial dysfunction, and enhances hypertension. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H639–H646. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Allen, A.O. Mechanism of the disproportionation of superoxide radicals. J. Phys. Chem. 1977, 81, 1048–1050. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/O−2 radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Bensasson, R.V.; Land, E.J.; Truscott, T.G. Excited States in Biology and Medicine; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Rodgers, M.A.J. Time resolved studies of 1.27 μm luminescence from singlet oxygen generated in homogeneous and microheteregeneous fluids. Photochem. Photobiol. 1983, 37, 99–103. [Google Scholar] [CrossRef]
- Salokhiddinov, K.I.; Dzhagarov, B.M.; Byteva, I.M.; Gurinovich, G.P. Photosensitized luminescence of singlet oxygen in solutions at 1588 nm. Chem. Phys. Lett. 1980, 76, 85–87. [Google Scholar] [CrossRef]
- Bosio, G.N.; Breitenbach, T.; Parisi, J.; Reigosa, M.; Blaikie, F.H.; Pedersen, B.W.; Silva, E.F.F.; Martire, D.O.; Ogilby, P.R. Antioxidant β-carotene does not quench singlet oxygen in mammalian cells. J. Am. Chem. Soc. 2013, 135, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Telfer, A. Singlet oxygen production by PSII under light stress: Mechanism, detection and the protective role of β-carotene. Plant Cell Physiol. 2014, 55, 1216–1223. [Google Scholar] [CrossRef]
- Edge, R.; Truscott, T.G. Singlet oxygen and free radical reactions of retinoids and carotenoids—A review. Antioxidants 2018, 7, 5. [Google Scholar] [CrossRef]
- Dillon, J.; Gaillard, E.R.; Bilski, P.; Chignell, C.F.; Reszka, K.J. The photochemistry of the retinoids as studied by steady-state and pulsed methods. Photochem. Photobiol. 1996, 63, 680–685. [Google Scholar] [CrossRef]
- El-Agamey, A.; Fukuzumi, S. Laser flash photolysis study on the retinol radical cation in polar solvents. Org. Biomol. Chem. 2011, 9, 6437–6446. [Google Scholar] [CrossRef]
- Teraoka, J.; Hashimoto, H.; Matsudaira, S.; Koyama, Y. Resonance Raman spectra of excited triplet-states of β-carotene isomers. Chem Lett. 1985, 14, 311–314. [Google Scholar] [CrossRef]
- Edge, R.; Land, E.J.; McGarvey, D.J.; Burke, M.; Truscott, T.G. The reduction potential of the β-Carotene•+/β-Carotene couple in an aqueous micro-heterogeneous environment. FEBS Lett. 2000, 471, 125–127. [Google Scholar] [CrossRef]
- Burke, M.; Edge, R.; Land, E.J.; McGarvey, D.J.; Truscott, T.G. One-electron reduction potentials of dietary carotenoid radical cations in aqueous micellar environments. FEBS Lett. 2001, 500, 132–136. [Google Scholar] [CrossRef]
- Mackor, A.; Wajer, T.A.J.W.; de Boer, T.J.; van Voorst, J.D.W. C-nitroso compounds. Part I. The formation of nitroxides by photolysis of nitroso compounds as studied by electron spin resonance. Tetrahedron Lett. 1966, 7, 2115–2123. [Google Scholar] [CrossRef]
- Mackor, A.; Wajer, T.A.J.W.; de Boer, T.J.; van Voorst, J.D.W. C-nitroso compounds. Part III. Alkoxy-alkyl-nitroxides as intermediates in the reaction of alkoxy-radicals with nitroso compounds. Tetrahedron Lett. 1967, 8, 385–390. [Google Scholar] [CrossRef]
- Mackor, A.; Wajer, T.A.J.W.; de Boer, T.J. C-nitroso compounds—VI: Acyl-alkyl-nitroxides from acyl radicals and nitroso compounds as studied by ESR. Tetrahedron Lett. 1968, 9, 1623–1631. [Google Scholar] [CrossRef]
- Janzen, E.G.; Blackburn, B.J. Detection and identification of short-lived free radicals by an electron spin resonance trapping technique. J. Am. Chem. Soc. 1968, 90, 5909–5910. [Google Scholar] [CrossRef]
- Iwamura, M.; Inamoto, N. Reactions of Nitrones with Free Radicals. II. Formation of Nitroxides. Bull. Chem. Soc. Jpn. 1970, 43, 860–863. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Kruk, I.; Lichszteld, K.; Michalska, T.; Kladna, A.; Marczynski, S.; Ölgen, S. Scavenging of reactive oxygen species by N-substituted indole-2 and 3-carboxamides. Luminescence 2004, 19, 1–7. [Google Scholar] [CrossRef]
- Polivka, T. Effects of self-assembled aggregation on excited states. In Carotenoids: Physical, Chemical, and Biological Functions and Properties; Landrum, J.T., Ed.; CRC Press: Boca Raton, FL, USA, 2010; Chapter 8; pp. 137–157. [Google Scholar]
- Sliwka, H.-R.; Partali, V.; Lockwood, S.F. Hydrophilic carotenoids: Carotenoid aggregates. In Carotenoids: Physical, Chemical, and Biological Functions and Properties; Landrum, J.T., Ed.; CRC Press: Boca Raton, FL, USA, 2010; Chapter 3; pp. 31–58. [Google Scholar]
- Edge, R.; Truscott, T.G. Properties of carotenoid radicals and excited states and their potential role in biological systems. In Carotenoids: Physical, Chemical, and Biological Functions and Properties; Landrum, J.T., Ed.; CRC Press: Boca Raton, FL, USA, 2010; Chapter 14; pp. 283–307. [Google Scholar]
- Boehm, F.; Edge, R.; Truscott, T.G. Anti- and pro-oxidative mechanisms comparing the macular carotenoids zeaxanthin and lutein with other dietary carotenoids—A singlet oxygen, free-radical in vitro and ex vivo study. Photochem. Photobiol. Sci. 2020, 19, 1001–1008. [Google Scholar] [CrossRef]
- Mares-Perlman, J.A.; Brady, W.E.; Klein, R.; Klein, B.E.; Bowen, P.; Stacewicz-Sapuntzakis, M.; Palta, M. Serum antioxidants and age-related macular degeneration in a population-based case-control study. Arch. Ophthalmol. 1995, 113, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.Z.; Li, W.; Xin, P.; Mitchell, D.L. Melanin: A two edged sword? Pigment Cell Res. 1997, 10, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Bacchiocchi, A.; Wakamatsu, K.; Bechara, E.J.H.; Halaban, R.; Douki, T.; Brash, D.E. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science 2015, 347, 842–847. [Google Scholar] [CrossRef]
- Thomson, A.; Land, E.J.; Chedekel, M.R.; Subbaro, K.V.; Truscott, T.G. A pulse radiolysis investigation of the oxidation of the melanin precursors 3,4-dihydroxyphenylalanine (dopa) and the cysteinyldopas. Biochim. Et Biophys. Acta 1985, 843, 49–57. [Google Scholar] [CrossRef]
- Mustafa, F.H.; Jaafar, M.S. Comparison of wavelength-dependent penetration depths of lasers in different types of skin in photodynamic therapy. Indian J. Phys. 2013, 87, 203–209. [Google Scholar] [CrossRef]
- Khunti, K.; Platt, L.; Routen, A.; Abbasi, K. COVID-19 and ethnic minorities: An urgent agenda for overdue action. BMJ 2020, 369, m2503. [Google Scholar] [CrossRef] [PubMed]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- Israel, A.; Cicurel, A.A.; Feldhamer, I.; Dror, Y.; Giveon, S.M.; Gillis, D.; Strich, D.; Lavie, G. The link between vitamin D deficiency and COVID-19 in a large population. medRxiv bioRxiv 2020. [Google Scholar] [CrossRef]
- Riley, P.A.; Truscott, T.G. Does iron chelation by melanin explain the ethnic link with COVID-19 fatality? Biomed. J. Sci. Tech. Res. 2020, 31, 24527–24528. [Google Scholar]
- Khunti, K.; Platt, L.; Routen, A.; Abbasi, K. Ethnicity and COVID-19. BMJ 2020, 369, m2282. [Google Scholar]
- Pareek, M.; Bangash, M.N.; Pareek, N.; Pan, D.S.; Sze, S.; Minhas, J.S.; Hanif, W.; Khunti, K. Ethnicity and COVID-19: An urgent public health research priority. Lancet 2020, 395, 1421–1422. [Google Scholar] [CrossRef]
- Edge, R.; Truscott, T.G. COVID-19 and the ethnicity link—Is there a photochemical link? Photochem. Photobiol. Sci. 2021, 20, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, X.; Huang, Y.; Liao, B.; Cheng, L.; Ren, B. Reactive Oxygen Species in Pathogen Clearance: The Killing Mechanisms, the Adaption Response, and the Side Effects. Front. Microbiol. 2021, 11, 622534. [Google Scholar] [CrossRef]
- Clarke, K.; Edge, R.; Johnson, V.; Land, E.J.; Navaratnam, S.; Truscott, T.G. The carbonate Radical: Its reactivity with oxygen, ammonia, amino acids and melanins. J. Phys. Chem. A 2008, 112, 10147–10151. [Google Scholar] [CrossRef] [PubMed]
- Chedekel, M.R.; Smith, S.K.; Post, P.W.; Pokora, A.; Vessell, D.L. Photodestruction of pheomelanin: Role of oxygen. Proc. Natl. Acad. Sci. USA 1978, 75, 5395–5399. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Sinclair, R.S.; Truscott, T.G.; Land, E.J.; Chedekel, M.R.; Liu, C.-T. Photochemistry of benzothiazole models of pheomelanin. Photochem. Photobiol. 1984, 39, 5–10. [Google Scholar] [CrossRef]
- Land, E.J.; Thompson, A.; Truscott, T.G.; Subbarao, K.V.; Chedekel, M.R. Photochemistry of melanin precursors: Dopa, 5-S-cysteinyldopa and 2,5-S S’-dicysteinyldopa. Photochem. Photobiol. 1986, 44, 697–702. [Google Scholar] [CrossRef]
- Nofsinger, J.B.; Lui, Y.; Simon, J.D. Aggregation of eumelanin mitigates photogeneration of reactive oxygen species. Free Radic. Biol. Med. 2002, 32, 720–730. [Google Scholar] [CrossRef]
- Kassouf, N.; Kay, C.W.M.; Volkov, A.; Chiang, S.-H.; Birch-Machin, M.A.; El-Khamisy, S.F.; Haywood, R.M. UVA-induced carbon-centred radicals in lightly pigmented cells detected using ESR spectroscopy. Free Radic. Biol. Med. 2018, 126, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, S.; Jung, S.; Müller, R.; Lademann, J.; Zuberbier, T.; Zastrow, L.; Reble, C.; Beckers, I.; Meinke, M.C. Skin type differences in solar-simulated radiation-induced oxidative stress. Br. J. Dermatol. 2019, 180, 597–603. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Kladna, A.; Kruk, I.; Lichszteld, K.; Michalska, T. Effect of psoralens on Fenton-like reaction generating reactive oxygen species. Biopolym. Biospectroscopy 2003, 72, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Collet, M.; Hoebeke, M.; Piette, J.; Jakobs, A.; Lindqvist, L.; Van de Vorst, A. Photosensitized generation of hydroxyl radical by eight new sulfur and selenium analogs of psoralen. J. Photochem. Photobiol. B Biol. 1996, 35, 221–231. [Google Scholar] [CrossRef]
- Knox, C.N.; Land, E.J.; Truscott, T.G. Singlet oxygen generation by furocoumarins triplet states—1. Linear furocoumarins (Psoralens). Photochem. Photobiol. 1986, 43, 359–363. [Google Scholar] [CrossRef]
- Craw, M.; Chedekal, M.R.; Truscott, T.G.; Land, E.J. The photochemical interaction between the triplet state of 8-methoxypsoralen and the melanin precursor L-3,4 dihydroxyphenylalanine. Photochem. Photobiol. 1984, 39, 155–159. [Google Scholar] [CrossRef]
- Bacci, M.; Linari, R. UV excitable fluorescence of lumirubin. J. Photochem. Photobiol. B Biol. 1989, 3, 419–427. [Google Scholar] [CrossRef]
- Jasprova, J.; Dal Ben, M.; Vianello, E.; Goncharova, I.; Urbanova, M.; Vyroubalova, K.; Gazzin, S.; Tiribelli, C.; Sticha, M.; Cerna, M.; et al. The biological effects of bilirubin photoisomers. PLoS ONE 2016, 11, e0148126. [Google Scholar] [CrossRef]
- Böhm, F.; Drygalla, F.; Charlesworth, P.; Böhm, K.; Truscott, T.G.; Jokiel, K. Bilirubin phototoxicity to human cells by green light phototherapy in vitro. Photochem. Photobiol. 1995, 62, 980–983. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, A.; Pospíšilová, K.; Žížalová, K.; Capková, N.; Muchová, L.; Vecka, M.; Vrzáčková, N.; Křížová, J.; Zelenka, J.; Vítek, L. The effects of bilirubin and lumirubin on metabolic and oxidative stress markers. Front. Pharmacol. 2021, 12, 567001. [Google Scholar] [CrossRef]
- Lowe, N.J.; Shaath, N.A. Sunscreens: Development, Evaluation and Regulatory Aspects; Marcel Dekker: New York, NY, USA, 1990. [Google Scholar]
- Trivedi, M.; Murase, J. Titanium dioxide in sunscreen. In Application of Titanium Dioxide; Janus, M., Ed.; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/chapters/55103 (accessed on 10 August 2021).
- Wu, M.J.; Bak, T.; O’Doherty, P.J.; Moffitt, M.C.; Nowotny, J.; Bailey, T.D.; Kersaitis, C. Photocatalysis of titanium dioxide for water disinfection: Challenges and future perspectives. Int. J. Photochem. 2014, 2014, 973484. [Google Scholar] [CrossRef]
- Newman, M.D.; Stotland, M.; Ellis, J.I. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J. Am. Acad. Dermatol. 2009, 61, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N.; Salinaro, A.; Emeline, A. Deleterious effects of sunscreen titanium dioxide nanoparticles on DNA: Efforts to limit DNA damage by particle surface modification. In Proceedings of the BiOS 2001 the International Symposium on Biomedical Optics, San Jose, CA, USA, 20 January 2001; Volume 4258, pp. 86–98. [Google Scholar]
- Hidaka, H.; Kobayashi, H.; Koike, T.; Serpone, N. DNA damage photoinduced by cosmetic pigments and sunscreen agents under solar exposure and artificial UV illumination. J. Oleo Sci. 2006, 55, 249–261. [Google Scholar] [CrossRef][Green Version]
- Knowland, J.; McKenzie, E.A.; McHugh, P.J.; Cridland, N.A. Sunlight-induced mutagenicity of a common sunscreen ingredient. FEBS Lett. 1993, 324, 309–313. [Google Scholar] [CrossRef]
- Mturi, G.J.; Martincigh, B. Photostability of the sunscreening agent 4- tert-butyl-4′-methoxydibenzoylmethane (avobenzone) in solvents of different polarity and proticity. J. Photochem. Photobiol. A Chem. 2008, 200, 410–420. [Google Scholar] [CrossRef]
- Andrae, I.; Bringhen, A.; Böhm, F.; Gonzenbach, H.; Hill, T.; Mulroy, L.; Truscott, T.G. A UVA filter (4-tert-butyl-4′-methoxydibenzoylmethane): Photoprotection reflects photophysical properties. J. Photochem. Photobiol. B Biol. 1997, 37, 147–150. [Google Scholar] [CrossRef]
- Cantrell, A.; McGarvey, D.J. Photochemical studies of 4-tert-butyl-4′-methoxydibenzoylmethane (BM-DBM). J. Photochem. Photobiol. B Biol. 2001, 64, 117–122. [Google Scholar] [CrossRef]
- Hill, T.J. Molecular Mechanisms of Photoprotection. Ph.D. Thesis, Keele University, Keele, UK, 1994. [Google Scholar]
- Paris, C.; Lhiaubet-Vallet, V.; Jimenez, O.; Trullas, C.; Miranda, M.A. A blocked diketo form of avobenzone: Photostability, photosensitizing properties and triplet quenching by a triazine-derived UVB-filter. Photochem. Photobiol. 2009, 85, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Lhiaubet-Vallet, V.; Marin, M.; Jimenez, O.; Gorchs, O.; Trullas, C.; Miranda, M.A. Filter–filter interactions. Photostabilization, triplet quenching and reactivity with singlet oxygen. Photochem. Photobiol. Sci. 2010, 9, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Berenbeim, J.A.; Wong, N.G.K.; Cockett, M.C.R.; Berden, G.; Oomens, J.; Rijs, A.M.; Dessent, C.E.H. Unravelling the keto–enol tautomer dependent photochemistry and degradation pathways of the protonated UVA filter avobenzone. J. Phys. Chem. A 2020, 124, 2919–2930. [Google Scholar] [CrossRef]
- Damiani, E.; Rosati, L.; Castagna, R.; Carloni, P.; Greci, L. Changes in ultraviolet absorbance and hence in protective efficacy against lipid peroxidation of organic sunscreens after UVA irradiation. J. Photochem. Photobiol. B Biol. 2006, 82, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Oguchi, N.; Yagi, M. Optical and electron paramagnetic resonance studies of the excited states of 4-tert-butyl-4′-methoxydibenzoylmethane and 4-tert-butyl-4′-methoxydibenzoylpropane. J. Phys. Chem. A 2009, 113, 13492–13497. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, T.; Pan, J.; Scheurich, R.; Pfluecker, F.; Graf, R.; Epstein, H. Superior two step approach to completely photoprotect avobenzone with a designed organic redox pair. SOFW J. 2009, 135, 14–18. [Google Scholar]
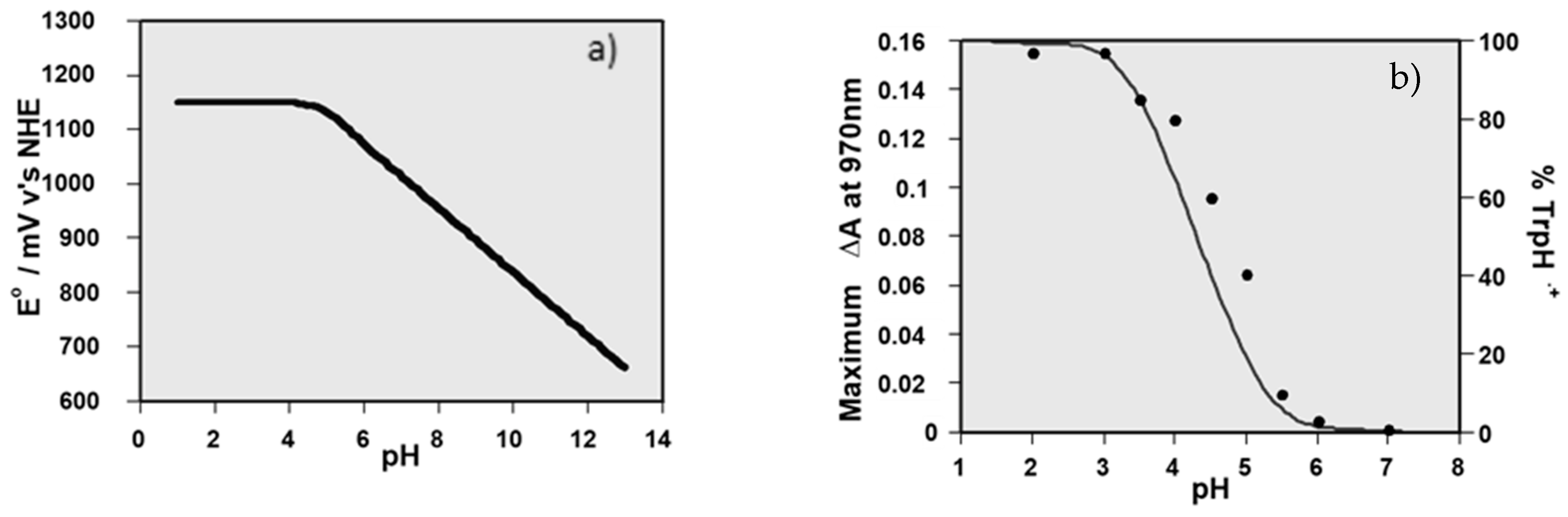
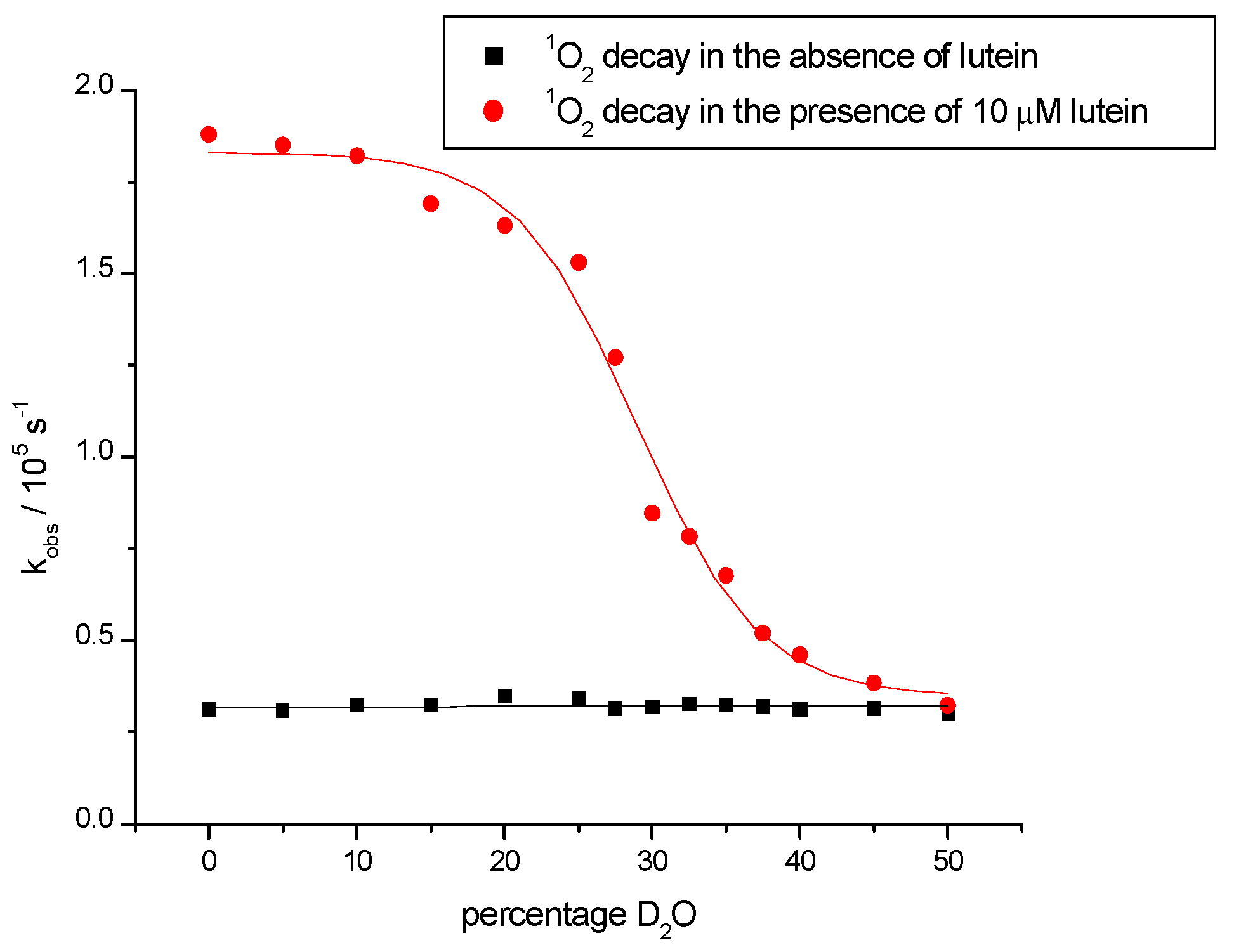

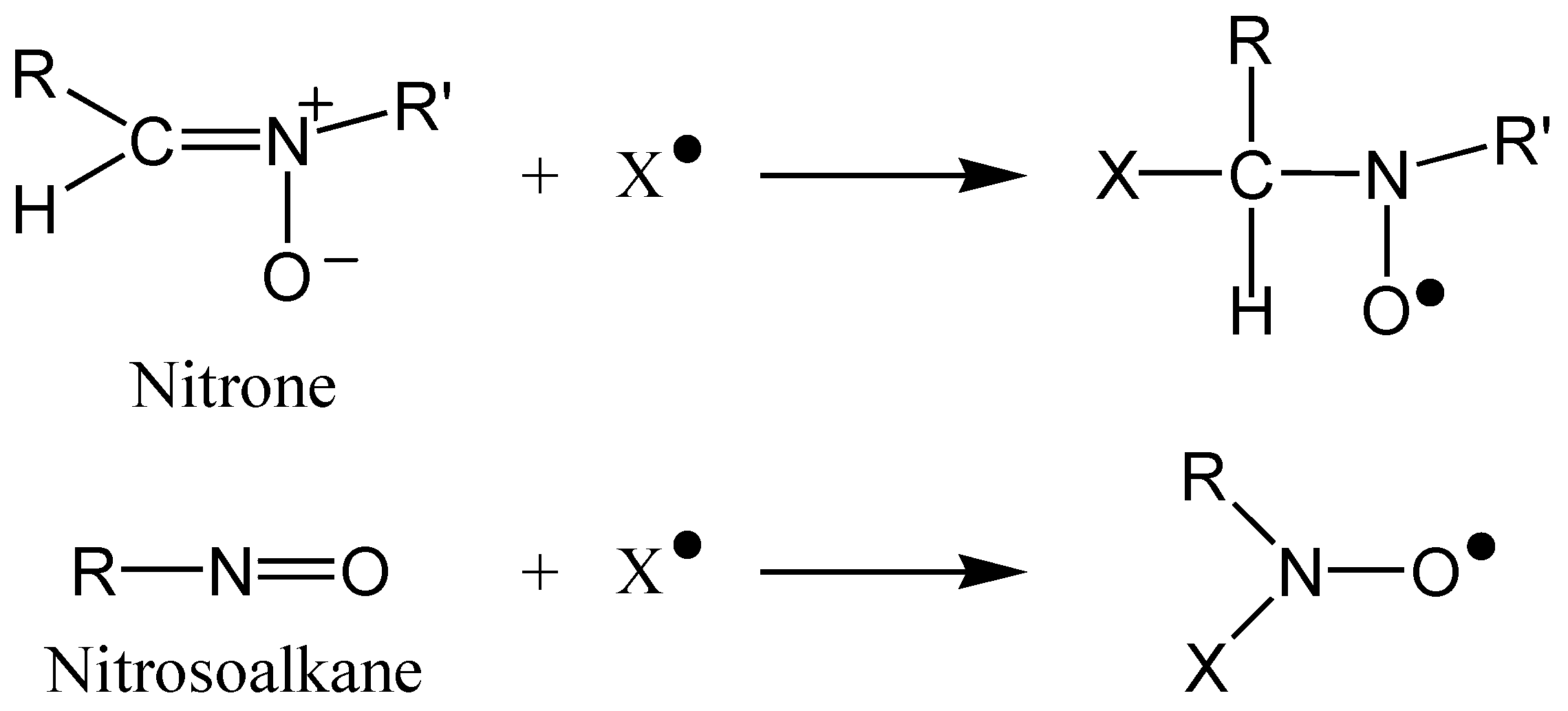

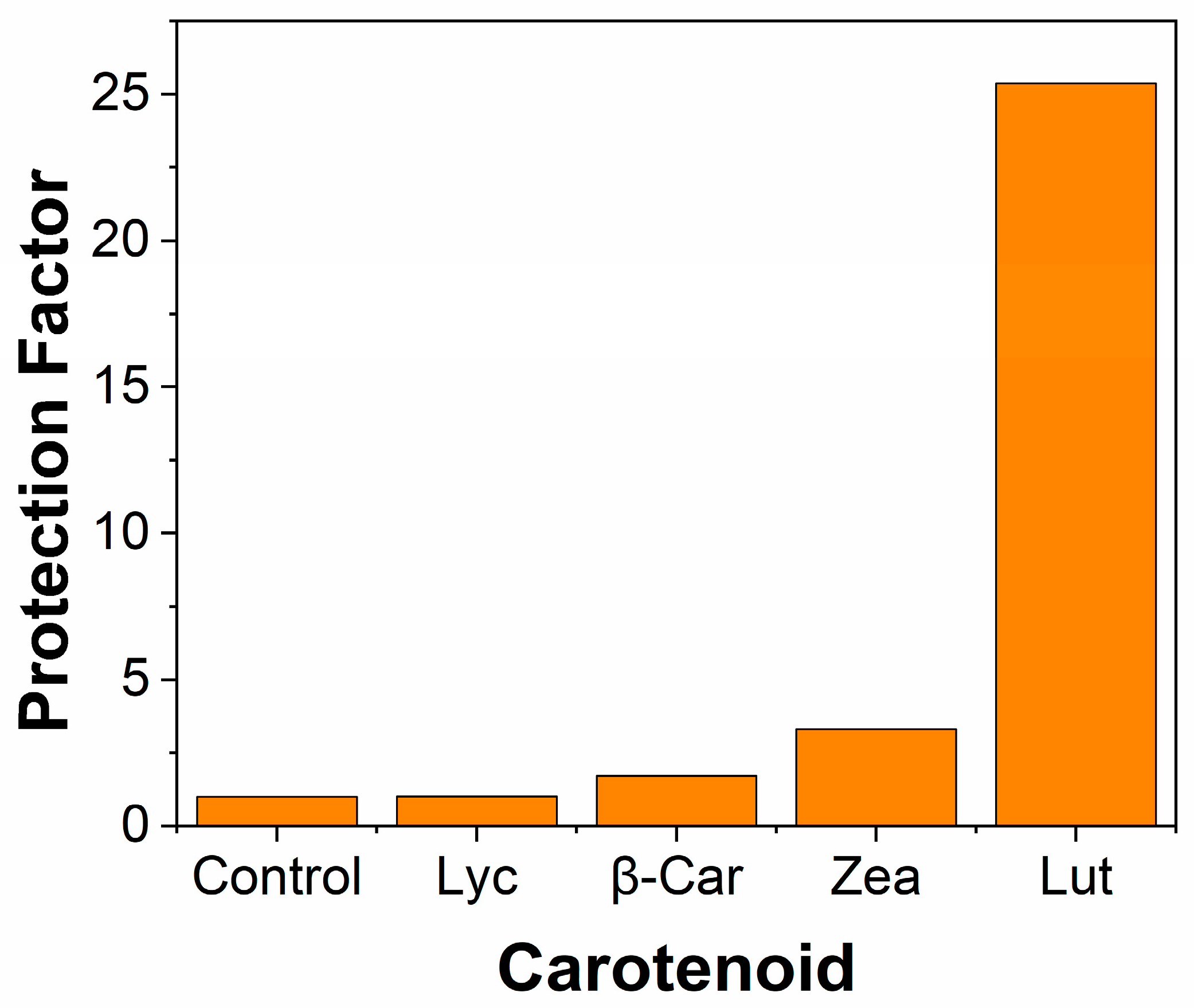

| Carotenoid | Protection Factor |
|---|---|
| Lyc | 4.9 |
| β-Car | 5.0 |
| Zea | 8.7 |
| Lut | 26 |
| SOD | 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edge, R.; Truscott, T.G. The Reactive Oxygen Species Singlet Oxygen, Hydroxy Radicals, and the Superoxide Radical Anion—Examples of Their Roles in Biology and Medicine. Oxygen 2021, 1, 77-95. https://doi.org/10.3390/oxygen1020009
Edge R, Truscott TG. The Reactive Oxygen Species Singlet Oxygen, Hydroxy Radicals, and the Superoxide Radical Anion—Examples of Their Roles in Biology and Medicine. Oxygen. 2021; 1(2):77-95. https://doi.org/10.3390/oxygen1020009
Chicago/Turabian StyleEdge, Ruth, and T. George Truscott. 2021. "The Reactive Oxygen Species Singlet Oxygen, Hydroxy Radicals, and the Superoxide Radical Anion—Examples of Their Roles in Biology and Medicine" Oxygen 1, no. 2: 77-95. https://doi.org/10.3390/oxygen1020009
APA StyleEdge, R., & Truscott, T. G. (2021). The Reactive Oxygen Species Singlet Oxygen, Hydroxy Radicals, and the Superoxide Radical Anion—Examples of Their Roles in Biology and Medicine. Oxygen, 1(2), 77-95. https://doi.org/10.3390/oxygen1020009







