Acute Impacts of Hurricane Ian on Benthic Habitats, Water Quality, and Microbial Community Composition on the Southwest Florida Shelf
Abstract
1. Introduction
2. Materials and Methods
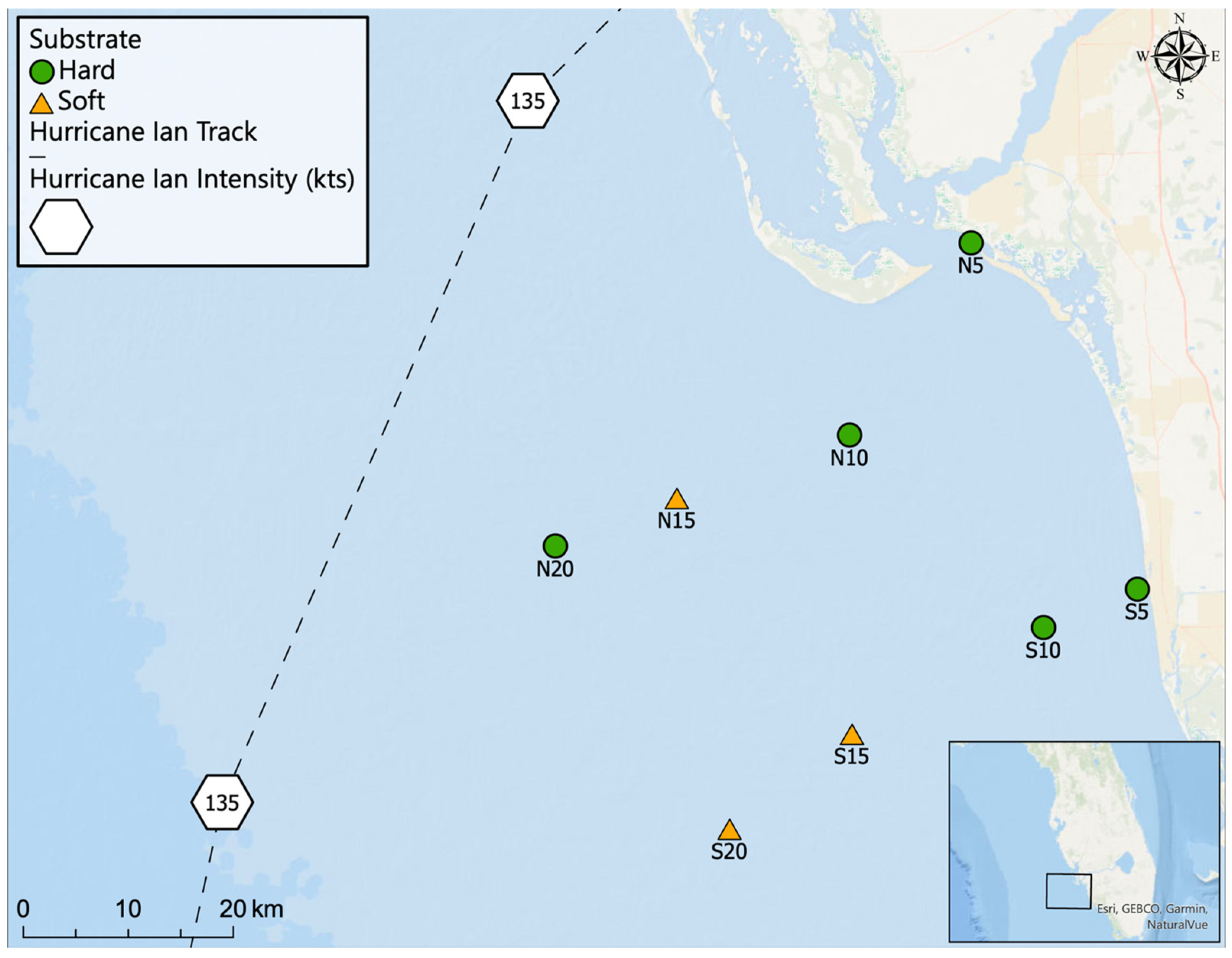
2.1. Sampling Locations
2.2. Water Quality Parameters
2.3. Benthic Video Surveys
2.4. Sediment Characteristics
2.5. Microbial Community Analysis
2.6. Statistical Analysis
3. Results
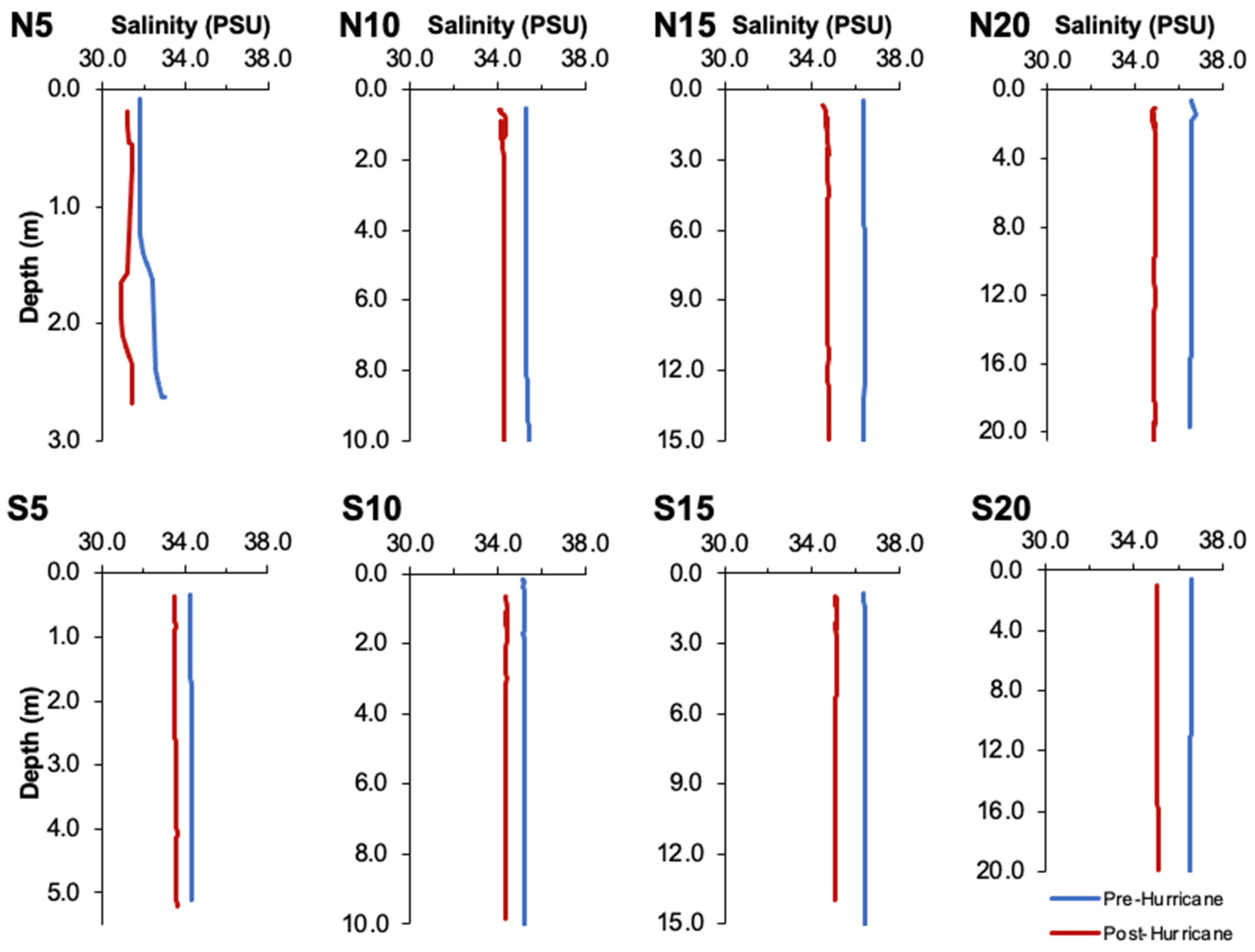
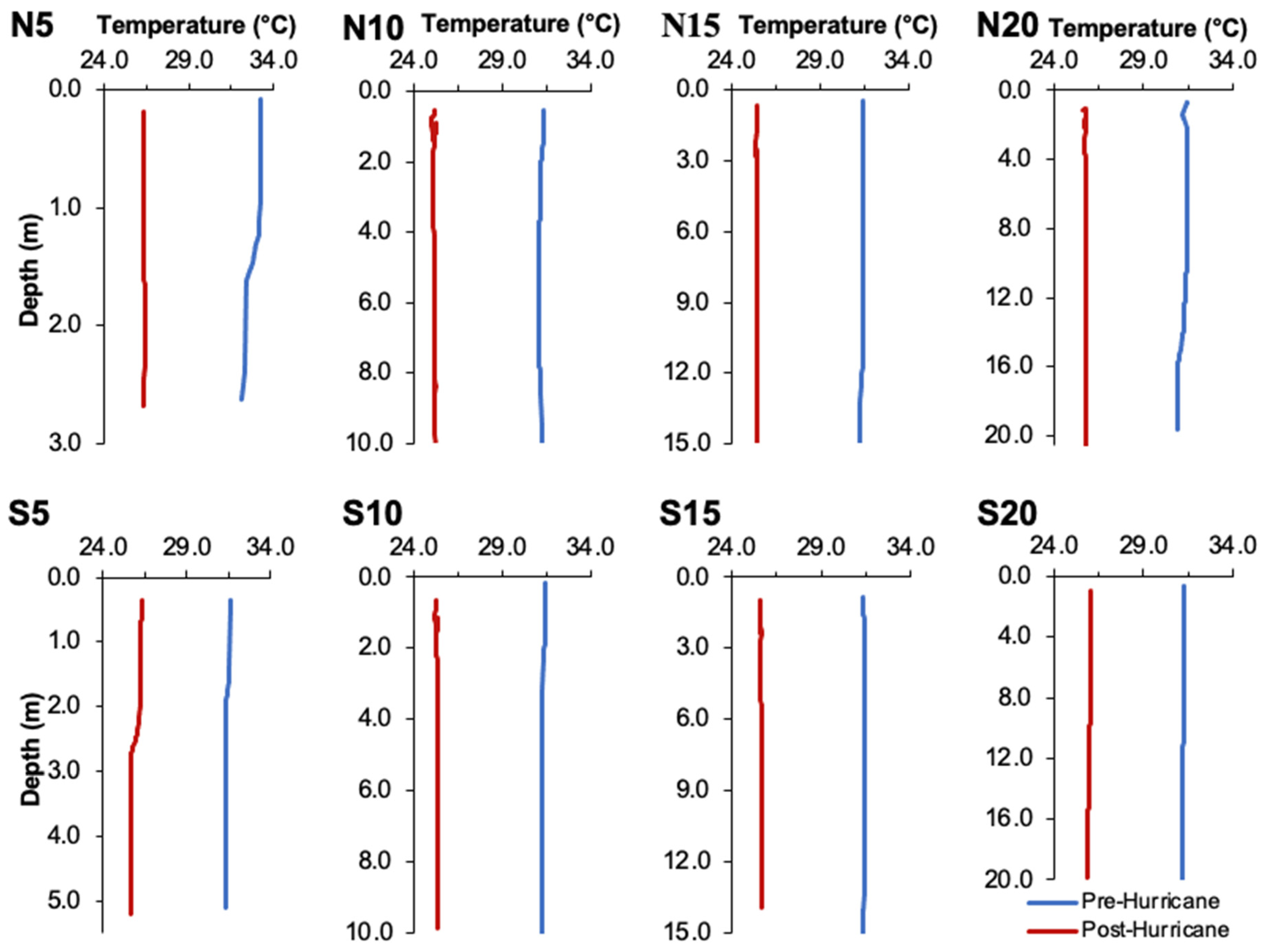
3.1. Physical Effects of Hurricane Ian
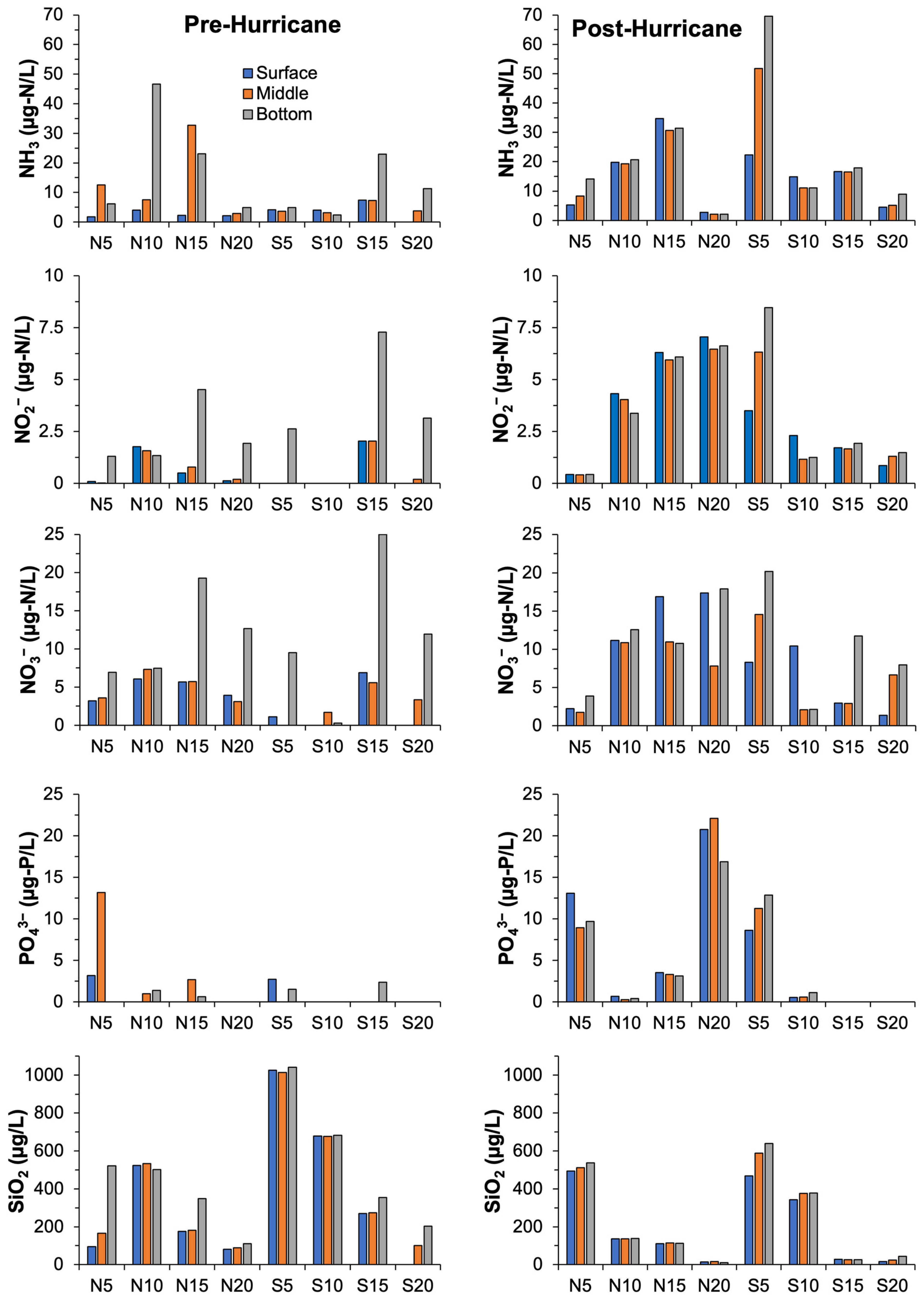
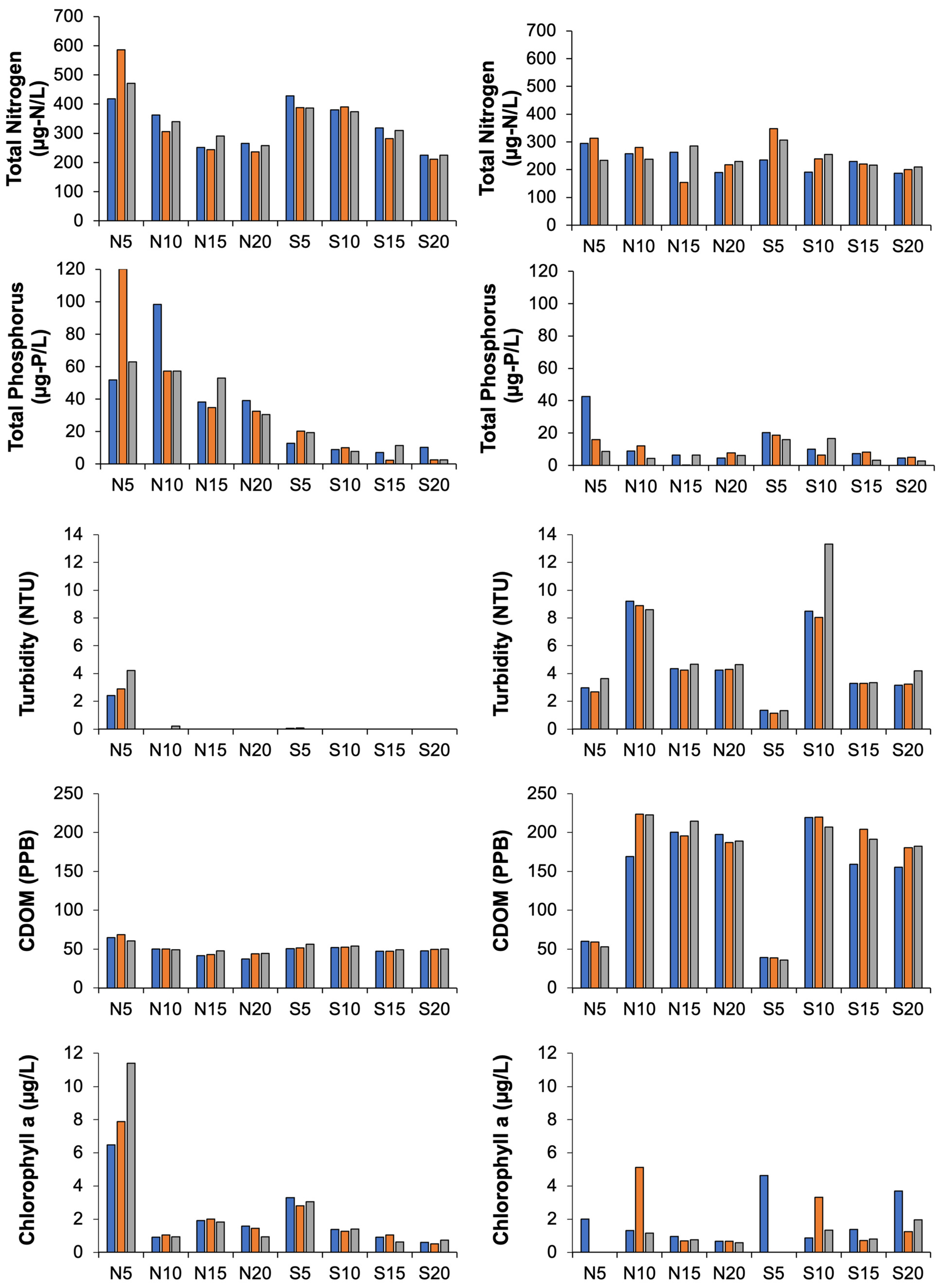
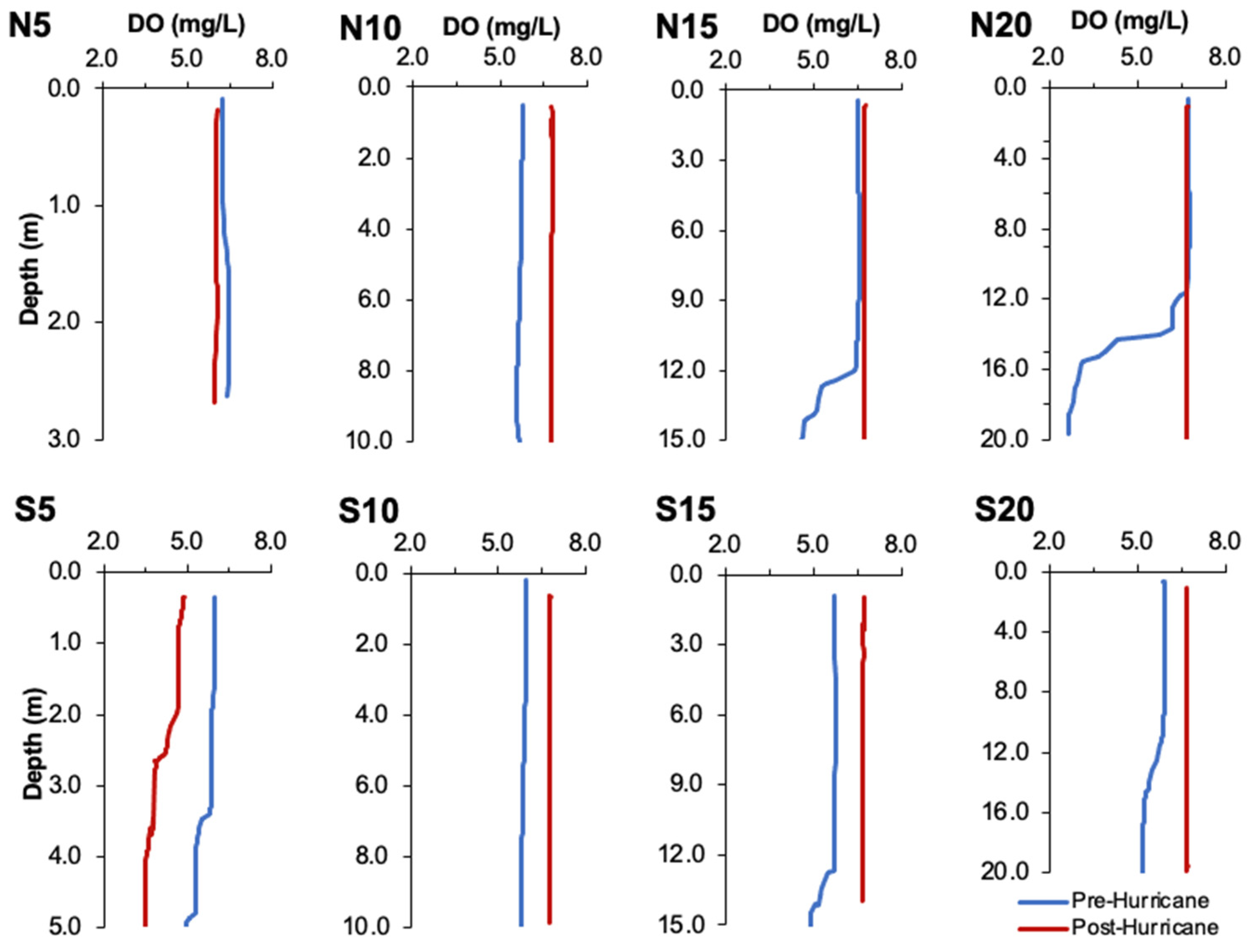
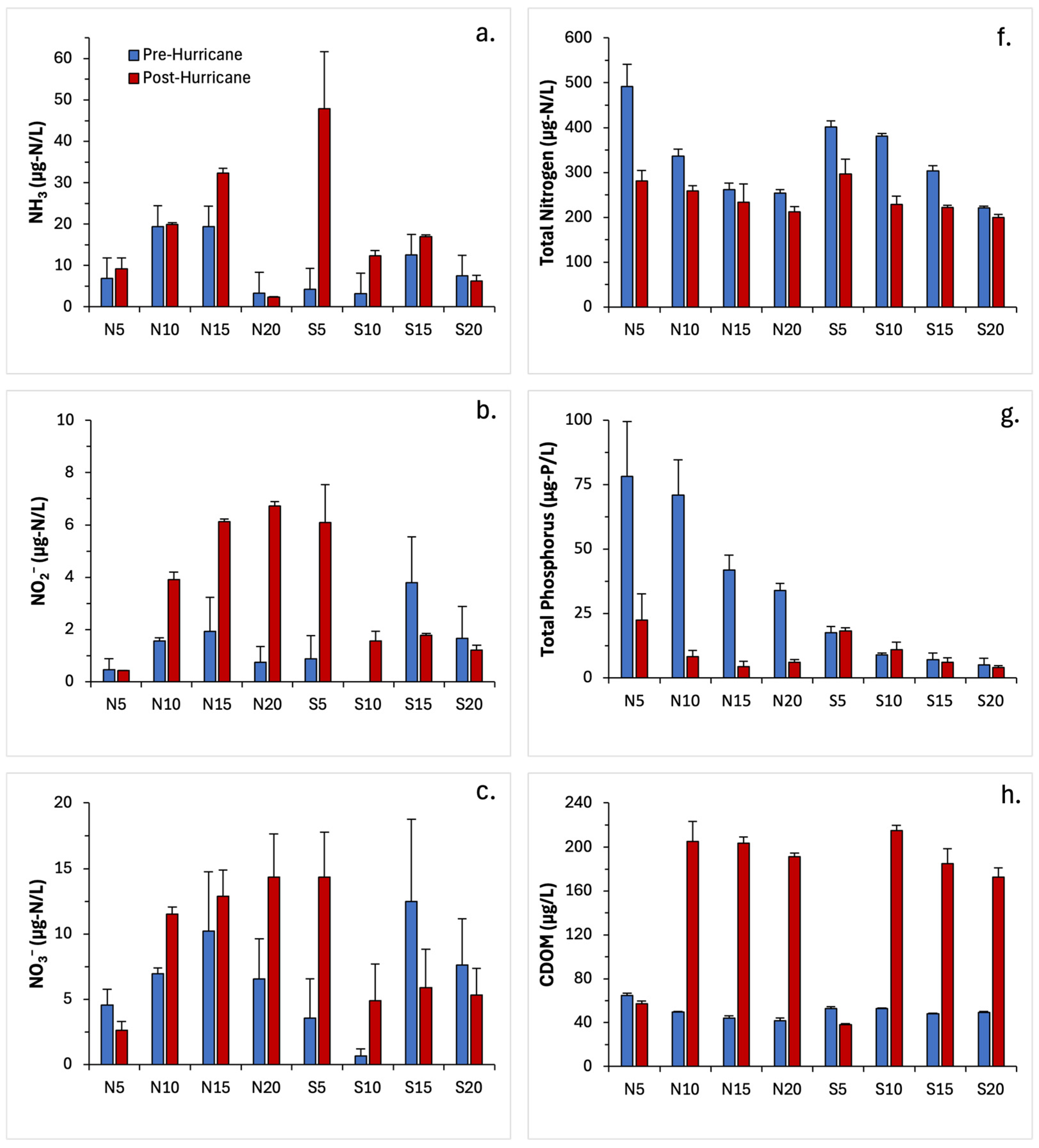
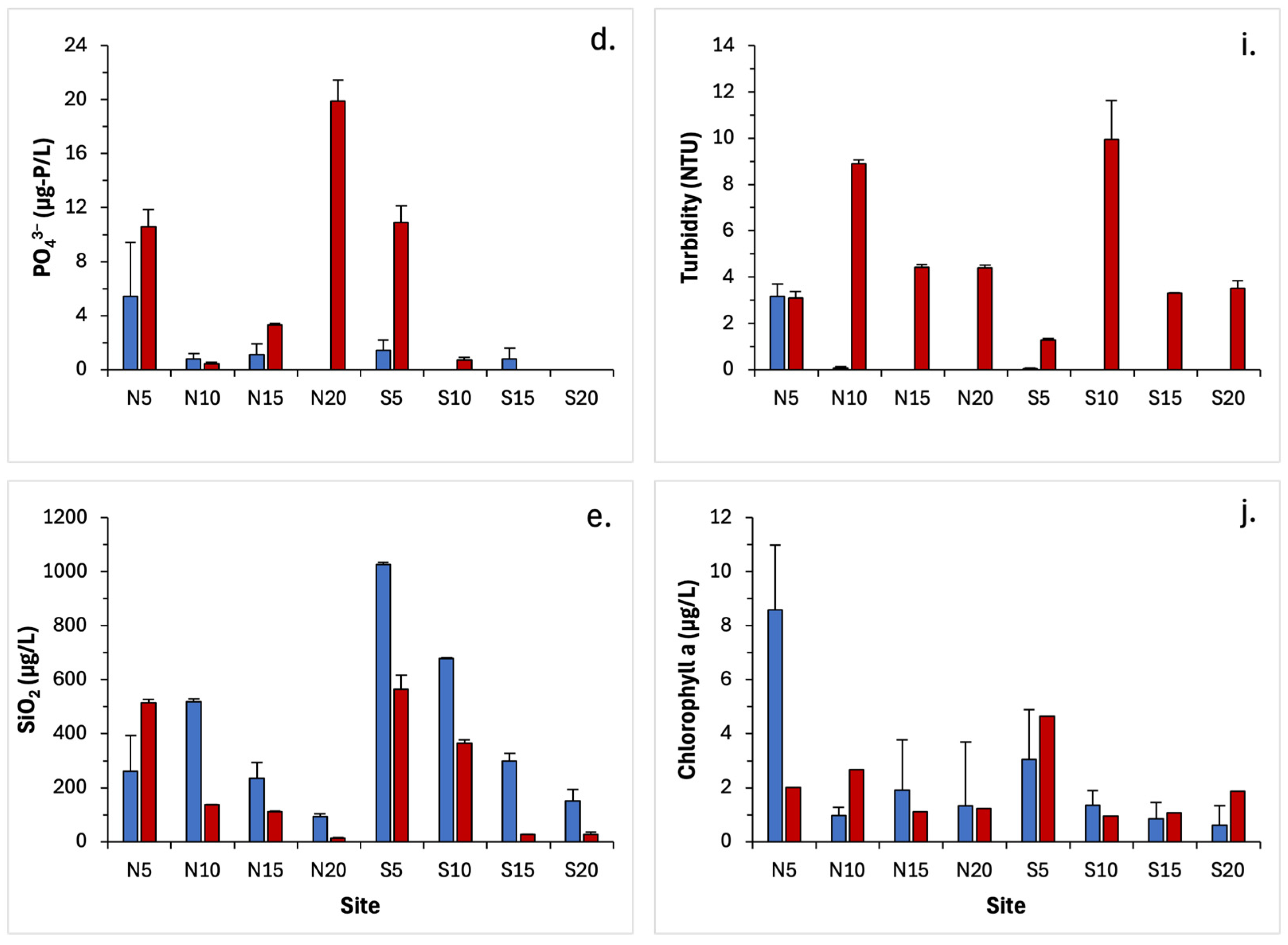
3.2. Water Quality Parameters
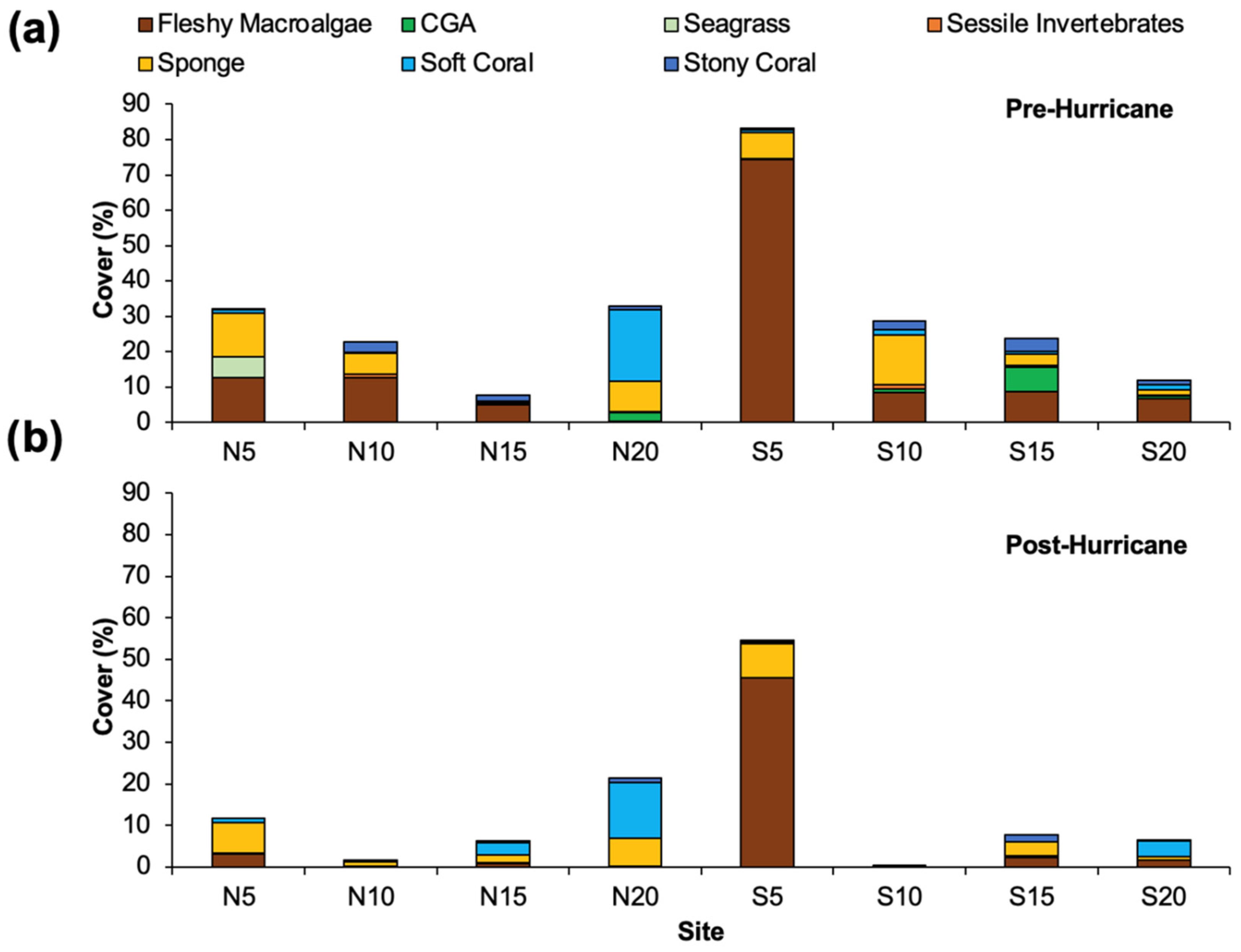
3.3. Epibenthos and Substrate
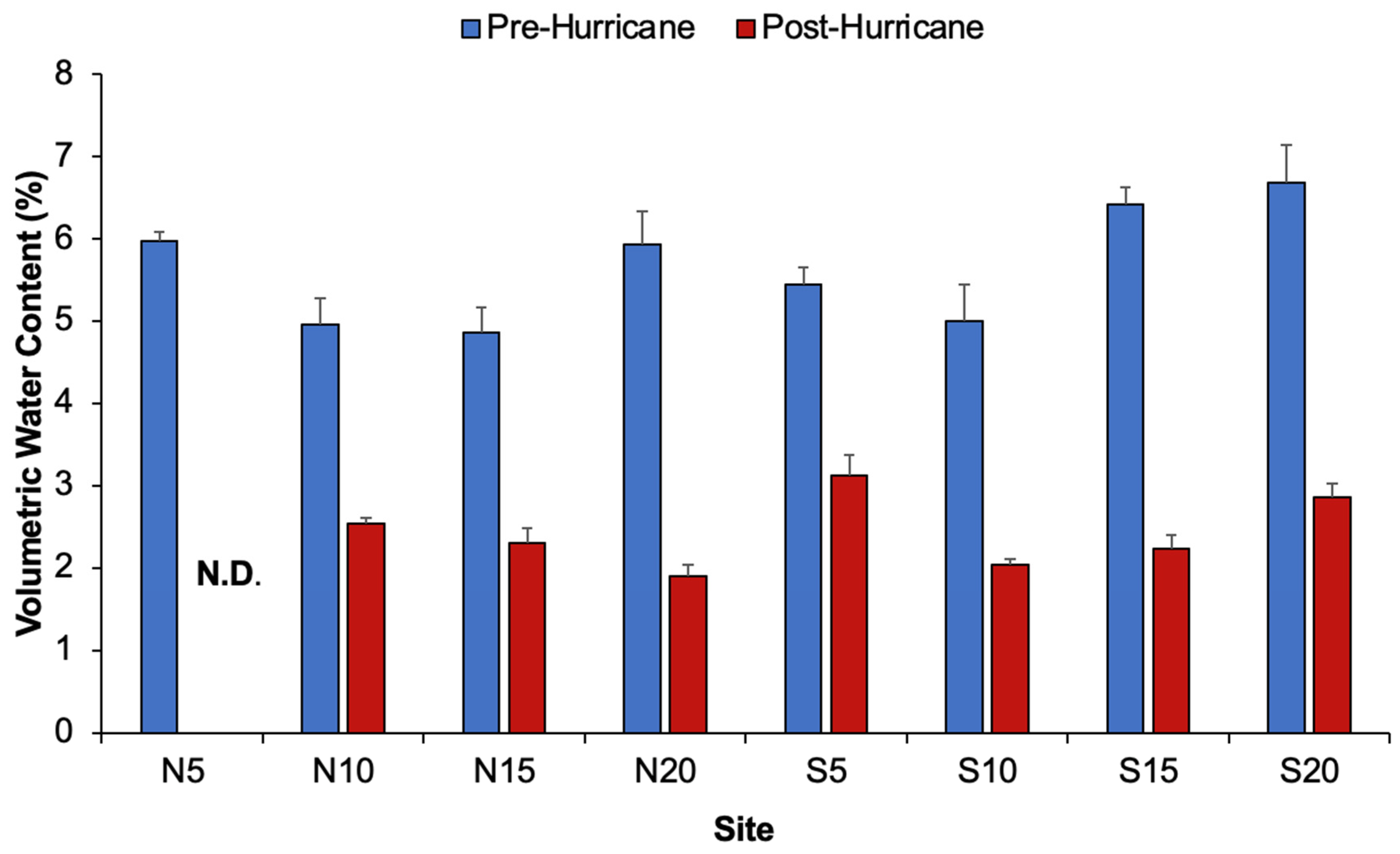
3.4. Sediment Characteristics
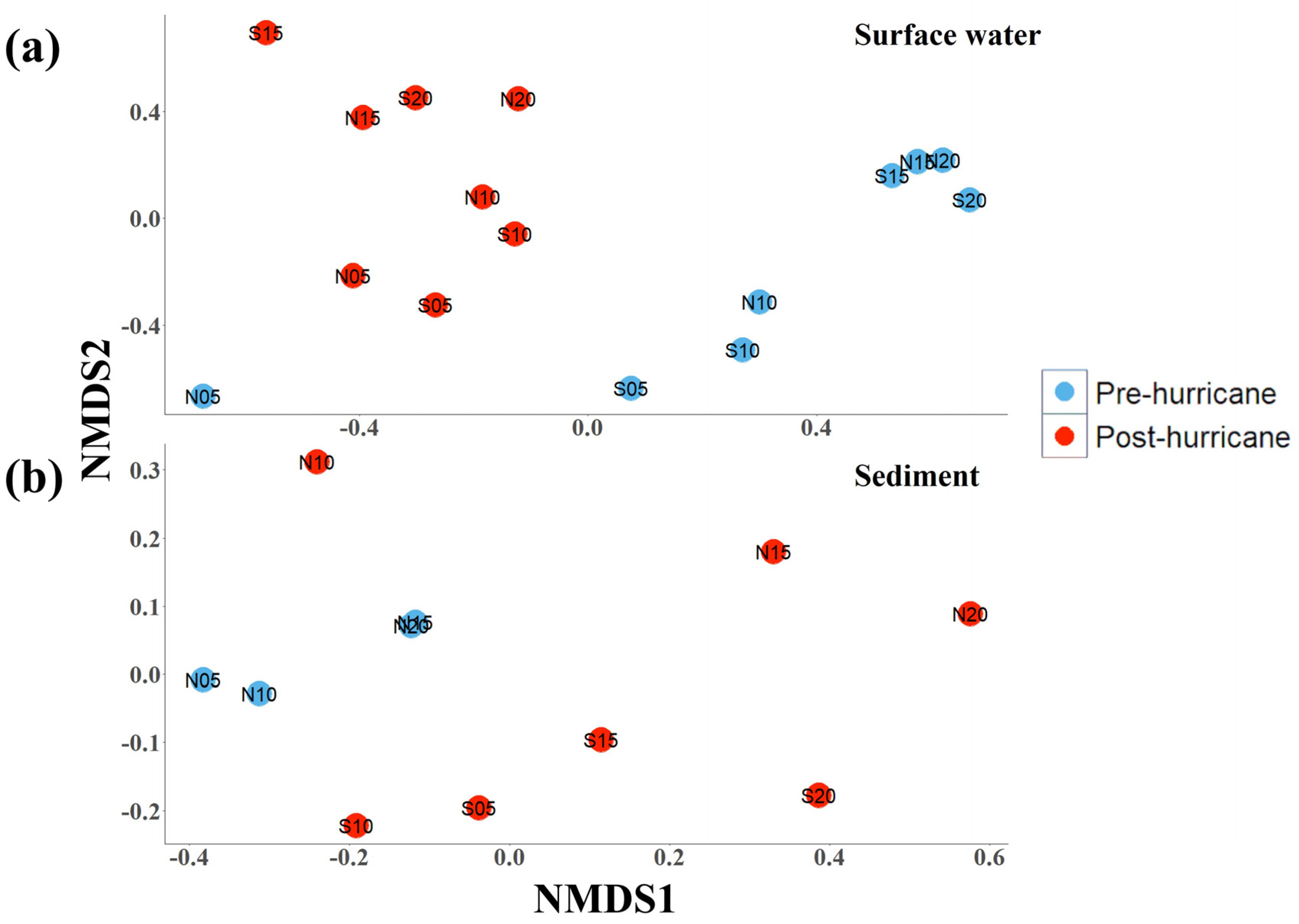
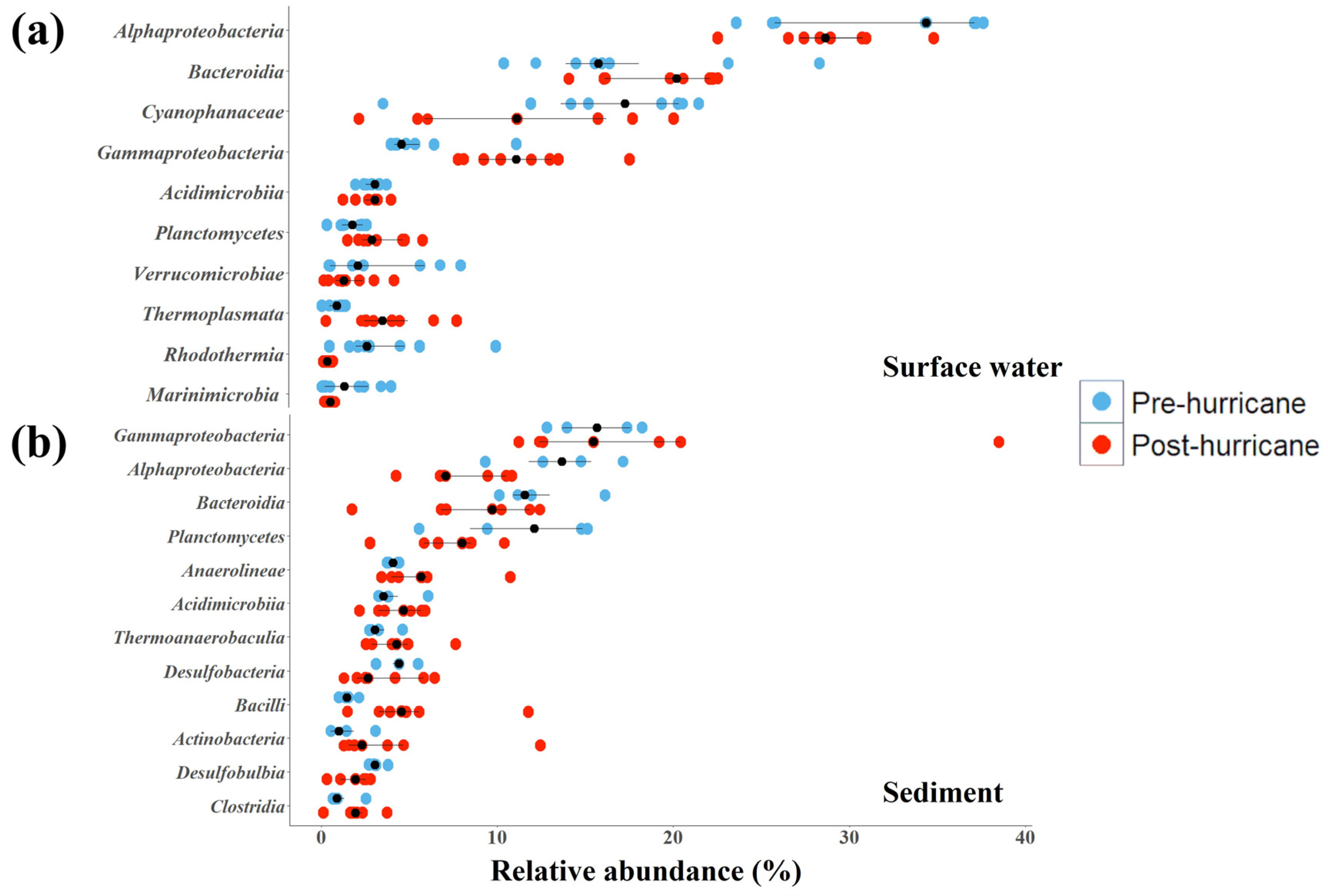
3.5. Microbial Community Structure
4. Discussion
4.1. Hurricane Ian in Historic Context
4.2. Changes in Water Quality
4.3. Changes in Substrates and Benthos
4.4. Microbial Community Changes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DO | Dissolved Oxygen |
| DOC | Dissolved Organic Carbon |
| CRE | Caloosahatchee River Estuary |
| SWFS | Southwest Florida Shelf |
| FGCU | Florida Gulf Coast University |
| GOM | Gulf of Mexico |
| DOM | Dissolved Organic Matter |
| CTSB | Cross-Shelf Transport Barrier |
| FIO | Florida Institute of Oceanography |
| US EPA | United States Environmental Protection Agency |
| PSU | Practical Salinity Unit |
| NTU | Nephelometric Turbidity Unit |
| CDOM | Chromophoric Dissolved Organic Matter |
Appendix A
| Site | Depth (m) | Latitude and Longitude | Video | Water Quality | Sediment Grain Size | Sediment Microbial | Water Microbial | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre- | Post- | Pre- | Post- | Pre- | Post- | Pre- | Post- | Pre- | Post- | |||
| N5 | 3 | 26.4722° N, 081.9771° W | 9/2 | 11/2 | 8/23 | 11/2 | 9/2 | 9/2 | 8/23 | 11/2 | ||
| N10 | 10 | 26.3086° N, 082.0963° W | 9/2 | 10/21 | 8/24 | 10/21 | 9/2 | 10/21 | 9/2 | 10/21 | 8/24 | 10/21 |
| N15 | 15 | 26.25751° N, 082.2623° W | 9/6 | 10/22 | 8/25 | 10/22 | 9/6 | 10/22 | 9/6 | 10/22 | 8/25 | 10/22 |
| N20 | 20 | 26.21683° N, 082.3791° W | 9/6 | 10/22 | 8/26 | 10/22 | 9/6 | 10/22 | 9/6 | 10/22 | 8/26 | 10/22 |
| S5 | 5 | 26.17203° N, 081.8234° W | 9/1 | 11/2 | 8/25 | 11/2 | 9/1 | 11/2 | 11/2 | 8/25 | 11/2 | |
| S10 | 10 | 26.14049° N, 081.9140° W | 9/1 | 10/23 | 8/26 | 10/23 | 9/1 | 10/23 | 10/23 | 8/26 | 10/23 | |
| S15 | 15 | 26.05257° N, 082.0984° W | 9/8 | 10/23 | 8/27 | 10/23 | 9/8 | 10/23 | 10/23 | 8/27 | 10/23 | |
| S20 | 20 | 25.97247° N, 082.2164° W | 9/8 | 10/23 | 8/28 | 10/23 | 9/8 | 10/23 | 10/23 | 8/28 | 10/23 | |
| Parameter | In Situ Method | Laboratory Analysis |
|---|---|---|
| Salinity (PSU) | YSI EXO II conductivity sensor | N/A |
| Temperature (°C) | YSI EXO II thermistor sensor | N/A |
| Dissolved oxygen (mg/L) | YSI EXO II optical sensor | N/A |
| Turbidity (NTU) | YSI EXO II turbidity sensor | N/A |
| Chromophoric Dissolved Organic Matter (CDOM) | Collected with Van Dorn, or Niskin bottle, filtered seawater with 0.45 µm nylon filter | Trilogy Fluorometer, CDOM/fDOM module |
| Chlorophyll a | Collected with Van Dorn, or Niskin bottle, filtered onto 0.7 µm GF/F filter, wrapped in aluminum foil and frozen at −20 °C | Filters extracted with 90% acetone and quantified with Trilogy Fluorometer with module CHL-A-ACID (Model 7200–040 |
| Ammonia (NH3) | Collected with Van Dorn, or Niskin bottle, filtered seawater with 0.45 µm nylon filter, frozen until analysis | SEAL AA500 autoanalyzer, method A-043-19 |
| Nitrite (NO2) | Collected with Van Dorn, or Niskin bottle, filtered seawater with 0.45 µm nylon filter, frozen until analysis | SEAL AA500 autoanalyzer, A-044-19 method |
| Nitrate (NO3) | Collected with Van Dorn, or Niskin bottle, filtered seawater with 0.45 µm nylon filter, frozen until analysis | SEAL AA500 autoanalyzer, A-044-19 method |
| Orthophosphate (oP) | Collected with Van Dorn, or Niskin bottle, filtered seawater with 0.45 µm nylon filter, frozen until analysis | SEAL AA500 autoanalyzer, A-005-19 method |
| Total nitrogen (TN) | Van Dorn/Niskin bottle, frozen until analysis | Shimadzu TOC with TNM-L accessory |
| Total phosphorus (TP) | Van Dorn, frozen until analysis | Manual persulfate digestion, SEAL AA500 Autoanalyzer, A005-19-3 method |
| Response Variable | Factors & Interactions | Sample Size (n) | Test | p-Values | Samples Excluded |
|---|---|---|---|---|---|
| Benthos and Substrate | |||||
| Hard Bottom Cover | Date Transect Date × Transect | 300 | Permutation Two-way ANOVA | <2 × 10−16 *** <2 × 10−16 *** 0.5811 | N/A |
| Total Biotic Cover | Date Transect Date × Transect | 300 | Permutation Two-way ANOVA | <2 × 10−16 *** <2 × 10−16 *** 0.6545 | N/A |
| Stony Coral % Cover | Date Transect Date × Transect | 300 | Permutation Two-way ANOVA | <2 × 10−16 *** 0.3194 0.1061 | N/A |
| Soft Coral % Cover | Date Transect Date × Transect | 300 | Permutation Two-way ANOVA | 0.8235 <2 × 10−16 *** 0.6545 | N/A |
| Sponge % Cover | Date Transect Date × Transect | 300 | Permutation Two-way ANOVA | 0.00620 ** 0.3402 0.1714 | N/A |
| Sessile Invertebrate % Cover | Date Transect Date × Transect | 300 | Permutation Two-way ANOVA | <2 × 10−16 *** 0.1401 0.1510 | N/A |
| Seagrass % Cover | Date Transect Date × Transect | 300 | Permutation Two-way ANOVA | 0.0008 *** 0.0040 ** 0.0112 * | N/A |
| Calcareous Green Algae % Cover | Date Transect Date × Transect | 300 | Permutation Two-way ANOVA | <2 × 10−16 *** 0.0024 ** 0.0102 * | N/A |
| Fleshy Macroalgae % Cover | Date Transect Date × Transect | 300 | Permutation Two-way ANOVA | <2 × 10−16 *** <2 × 10−16 *** 0.2377 | N/A |
| Water Quality and Nutrients | |||||
| Chlorophyll a | Date Transect Date × Transect | 48 | Permutation Two-way ANOVA | 0.9804 0.8627 0.6429 | N/A |
| Ammonia (NH3) | Date Transect Date × Transect | 48 | Permutation Two-way ANOVA | 0.0044 * 0.4622 0.4444 | N/A |
| Nitrate (NO3) | Date Transect Date × Transect | 48 | Permutation Two-way ANOVA | 0.2922 0.2182 0.7647 | N/A |
| Nitrite (NO2) | Date Transect Date × Transect | 48 | Permutation Two-way ANOVA | <0.001 ** 0.3924 0.1523 | N/A |
| Orthophosphate (oP) | Date Transect Date × Transect | 48 | Permutation Two-way ANOVA | 0.0146 * 0.2153 0.7451 | N/A |
| Silica (SiO2) | Date Transect Date × Transect | 48 | Permutation Two-way ANOVA | 0.0158 * 0.0548 0.0633 | N/A |
| Total Nitrogen (TN) | Date Transect Date × Transect | 48 | Permutation Two-way ANOVA | <0.0001 *** 0.6667 0.9804 | N/A |
| Total Phosphorus (TP) | Date Transect Date × Transect | 48 | Permutation Two-way ANOVA | <0.001 ** <0.001 ** <0.001 ** | N/A |
| CDOM (PPB) | Date Transect Date × Transect | 48 | Permutation Two-way ANOVA | <0.001 ** 0.8824 0.5542 | N/A |
| Turbidity (NTU) | Date Transect Date × Transect | 48 | Permutation Two-way ANOVA | <0.001 ** 0.1924 0.8039 | N/A |
| Sediment Characteristics | |||||
| Volumetric Water Content (%) | Date Transect Date × Transect | 7 | Permutation Two-way ANOVA | <0.001 * 0.3552 0.9608 | N5 |
| Organic Matter Content (%) | Date Transect Date × Transect | 7 | Two-way ANOVA | 0.0124 * 0.1515 0.9623 | N5 |
| Shell fraction (%) | Date Transect Date × Transect | 7 | Permutation Two-way ANOVA | 0.8039 1.0000 0.2170 | N5 |
| Fine shell fraction (%) | Date Transect Date × Transect | 7 | Permutation Two-way ANOVA | 0.6429 0.5412 0.8431 | N5 |
| Coarse sand fraction (%) | Date Transect Date × Transect | 7 | Permutation Two-way ANOVA | 0.3043 0.5412 0.7255 | N5 |
| Fine sand fraction (%) | Date Transect Date × Transect | 7 | Permutation Two-way ANOVA | 0.9216 0.0188 * 0.4906 | N5 |
| Silt fraction (%) | Date Transect Date × Transect | 7 | Permutation Two-way ANOVA | 0.0570 0.0140 * 0.1230 | N5 |
| Microbial Alpha Diversity and Abundances | |||||
| Surface water -Richness | Date | 8 | Paired T-test | <0.001 ** | N/A |
| Surface water -Hill-Shannon | Date | 8 | Paired T-test | <0.001 ** | N/A |
| Surface water -Hill-InvSimpson | Date | 8 | Paired T-test | 0.011 * | N/A |
| Sediment-Richness | Date | 6 | Paired T-test | 0.095 | N5, ST |
| Sediment -Hill-Shannon | Date | 6 | Paired T-test | 0.187 | N5, ST |
| Sediment -Hill-InvSimp | Date | 6 | Paired T-test | 0.717 | N5, ST |
| Pre-SW + Sed Richness | Sample type | 8 | Paired T-test | <0.001 ** | ST |
| Pre-SW + Sed Hill-Shannon | Sample type | 8 | Paired T-test | <0.001 ** | ST |
| Pre-SW + Sed Hill- InvSimp | Sample type | 8 | Paired T-test | <0.001 ** | ST |
| Post-SW + Sed Richness | Sample type | 14 | Two-Sample Fisher-Pitman Permutation Test | 0.5664 | N5 |
| Post-SW + Sed Hill-Shannon | Sample type | 14 | Two-Sample Fisher-Pitman Permutation Test | 0.2805 | N5 |
| Post-SW + Sed Hill-InvSimp | Sample type | 14 | Two-Sample Fisher-Pitman Permutation Test | 0.1806 | N5 |
| Total cell count (surface water) | Date Transect Date × Transect | 16 | 2-way ANOVA | 0.231 0.295 0.848 | N/A |
| Picocyanobacteria counts (surface water) | Date Transect Date × Transect | 16 | 2-way ANOVA | 0.0034 * 0.5528 0.4950 | N/A |
| Species Richness | Hill-Shannon | Hill-InvSimpson | ||||
|---|---|---|---|---|---|---|
| Site | Pre | Post | Pre | Post | Pre | Post |
| N5 | 287 | 353 | 94.15 | 109.23 | 44.17 | 45.66 |
| N10 | 263 | 386 | 66.75 | 127.61 | 28.36 | 61.34 |
| N15 | 287 | 448 | 103.12 | 180.82 | 56.63 | 100.55 |
| N20 | 247 | 373 | 82.15 | 146.67 | 42.48 | 72.20 |
| S5 | 226 | 486 | 53.31 | 118.35 | 16.89 | 38.64 |
| S10 | 240 | 389 | 55.87 | 109.94 | 18.85 | 41.37 |
| S15 | 253 | 347 | 93.54 | 133.49 | 49.54 | 77.27 |
| S20 | 272 | 369 | 87.82 | 144.27 | 42.96 | 83.38 |
| Mean | 259 ± 22 | 394 ± 48 | 79.59 ± 18.72 | 133.80 ± 23.71 | 37.48 ± 14.47 | 65.05 ± 22.18 |
| Species Richness | Hill-Shannon | Hill-InvSimpson | ||||
|---|---|---|---|---|---|---|
| Site | Pre | Post | Pre | Post | Pre | Post |
| N5 | 1606 | n.d | 909.79 | n.d | 411.27 | n.d |
| N10 | 1098 | 1213 | 666.69 | 867.73 | 337.06 | 589.46 |
| N15 | 1339 | 371 | 914.63 | 261.69 | 585.9 | 174.09 |
| N20 | 1799 | 167 | 813.4 | 95.87 | 135.6 | 51.07 |
| S5 | n.d | 994 | n.d | 470.06 | n.d | 92.65 |
| S10 | n.d | 673 | n.d | 426.79 | n.d | 239.42 |
| S15 | n.d | 461 | n.d | 302.1 | n.d | 159.46 |
| S20 | n.d | 310 | n.d | 105.42 | n.d | 15.78 |
| Mean of N10, N15, N20 | 1412 ± 356 | 584 ± 554 | 798.24 ± 124.66 | 408.45 ± 406.31 | 352.85 ± 225.56 | 271.54 ± 282.11 |
| Taxon | Time | N5 | N10 | N15 | N20 | S5 | S10 | S15 | S20 | Mean |
|---|---|---|---|---|---|---|---|---|---|---|
| Alphaproteobacteria | Pre | 38.48 | 26.42 | 41.72 | 38.57 | 28.74 | 28.95 | 41.60 | 42.17 | 35.83 ± 6.64 |
| Post | 29.73 | 34.68 | 31.77 | 34.45 | 25.26 | 32.43 | 39.01 | 30.73 | 32.26 ± 4.04 | |
| Cyanophyceae | Pre | 3.93 | 23.01 | 13.36 | 21.66 | 24.05 | 22.73 | 17.02 | 15.89 | 17.70 ± 6.78 |
| Post | 17.63 | 12.44 | 6.78 | 12.50 | 22.43 | 19.81 | 2.41 | 6.14 | 12.52 ± 7.11 | |
| Bacteroidia | Pre | 31.73 | 25.92 | 16.22 | 13.66 | 17.43 | 17.86 | 18.36 | 11.62 | 19.10 ± 6.60 |
| Post | 18.02 | 23.04 | 22.21 | 15.76 | 24.74 | 18.10 | 24.92 | 25.24 | 21.50 ± 3.70 | |
| Gammaproteobacteria | Pre | 12.42 | 4.46 | 4.82 | 4.45 | 5.39 | 5.97 | 4.71 | 7.18 | 6.18 ± 2.69 |
| Post | 19.63 | 11.45 | 14.57 | 10.35 | 9.06 | 8.71 | 15.10 | 13.38 | 12.78 ± 3.66 | |
| Verrucomicrobiae | Pre | 2.00 | 0.48 | 6.30 | 8.88 | 0.59 | 0.53 | 2.69 | 7.56 | 3.63 ± 3.43 |
| Post | 0.45 | 1.55 | 2.43 | 1.35 | 0.18 | 1.11 | 3.36 | 4.65 | 1.88 ± 1.52 | |
| Acidimicrobiia | Pre | 2.90 | 3.72 | 3.23 | 2.72 | 3.61 | 3.70 | 2.18 | 4.16 | 3.28 ± 0.65 |
| Post | 3.47 | 4.44 | 3.01 | 3.42 | 2.17 | 3.59 | 1.37 | 3.42 | 3.11 ± 0.94 | |
| Planctomycetes | Pre | 1.52 | 2.83 | 1.24 | 1.37 | 2.59 | 2.89 | 2.44 | 0.35 | 1.90 ± 0.92 |
| Post | 1.65 | 3.51 | 5.32 | 6.44 | 5.17 | 2.98 | 2.72 | 2.35 | 3.77 ± 1.68 | |
| Thermoplasmata | Pre | 1.53 | 1.34 | 0.52 | 0.03 | 1.02 | 0.48 | 0.94 | 1.23 | 0.89 ± 0.51 |
| Post | 2.83 | 3.31 | 4.51 | 7.17 | 0.28 | 2.56 | 4.99 | 8.63 | 4.29 ± 2.67 | |
| Rhodothermia | Pre | 0.52 | 6.27 | 3.06 | 1.80 | 5.02 | 11.12 | 2.79 | 2.33 | 4.11 ± 3.36 |
| Post | 0.52 | 0.49 | 0.30 | 0.12 | 0.72 | 0.73 | 0.23 | 0.15 | 0.41 ± 0.24 | |
| Marinimicrobia (SAR406_clade) | Pre | 0.05 | 0.55 | 3.81 | 2.73 | 1.45 | 0.25 | 2.38 | 4.44 | 1.79 ± 1.78 |
| Post | 0.19 | 0.40 | 0.61 | 0.85 | 1.67 | 0.68 | 0.55 | 0.58 | 0.51 ± 0.22 |
| Taxon | Time | N5 | N10 | N15 | N20 | S5 | S10 | S15 | S20 | Mean N10, N15, N20 |
|---|---|---|---|---|---|---|---|---|---|---|
| Gammaproteobacteria | Pre | 18.23 | 17.36 | 13.95 | 12.82 | n.d. | n.d. | n.d. | n.d. | 14.71 ± 2.36 |
| Post | n.d. | 11.22 | 15.46 | 12.58 | 20.40 | 12.37 | 19.20 | 38.49 | 13.09 ± 2.16 | |
| Planctomycetes | Pre | 5.56 | 9.43 | 14.78 | 15.11 | n.d. | n.d. | n.d. | n.d. | 13.11 ± 3.19 |
| Post | n.d. | 5.85 | 8.40 | 2.75 | 10.40 | 7.99 | 8.50 | 6.64 | 5.67 ± 2.83 | |
| Alphaproteobacteria | Pre | 14.75 | 12.60 | 9.32 | 17.13 | n.d. | n.d. | n.d. | n.d. | 13.02 ± 3.92 |
| Post | n.d. | 6.97 | 9.45 | 6.74 | 10.81 | 7.07 | 10.52 | 4.23 | 7.72 ± 1.51 | |
| Bacteroidia | Pre | 16.11 | 10.10 | 11.94 | 11.16 | n.d. | n.d. | n.d. | n.d. | 11.07 ± 0.92 |
| Post | n.d. | 11.85 | 9.72 | 10.23 | 12.41 | 7.09 | 6.82 | 1.74 | 10.60 ± 1.11 | |
| Acidimicrobiia | Pre | 3.29 | 6.08 | 3.78 | 3.24 | n.d. | n.d. | n.d. | n.d. | 4.36 ± 1.51 |
| Post | n.d. | 2.16 | 5.08 | 3.24 | 5.70 | 4.67 | 5.91 | 3.59 | 3.49 ± 1.48 | |
| Anaerolineae | Pre | 4.35 | 4.42 | 3.82 | 3.73 | n.d. | n.d. | n.d. | n.d. | 3.99 ± 0.37 |
| Post | n.d. | 5.67 | 4.00 | 5.73 | 4.38 | 10.72 | 6.01 | 3.42 | 5.13 ± 0.98 | |
| Thermoanaerobaculia | Pre | 2.73 | 2.85 | 4.61 | 3.24 | n.d. | n.d. | n.d. | n.d. | 3.57 ± 0.92 |
| Post | n.d | 2.55 | 4.93 | 4.03 | 2.89 | 4.89 | 4.28 | 7.63 | 3.84 ± 1.20 | |
| Desulfobacteria | Pre | 5.49 | 3.11 | 4.40 | 4.42 | n.d. | n.d. | n.d. | n.d. | 3.98 ± 0.75 |
| Post | n.d. | 4.19 | 2.02 | 5.80 | 1.28 | 6.44 | 2.51 | 2.64 | 4.00 ± 1.90 | |
| Bacilli | Pre | 1.00 | 2.13 | 1.54 | 1.38 | n.d. | n.d. | n.d. | n.d. | 1.68 ± 0.40 |
| Post | n.d. | 3.27 | 5.57 | 11.75 | 1.49 | 3.91 | 4.56 | 4.81 | 6.86 ± 4.39 | |
| Actinobacteria | Pre | 1.44 | 3.07 | 0.53 | 0.53 | n.d. | n.d. | n.d. | n.d. | 1.38 ± 1.47 |
| Post | n.d. | 1.28 | 4.67 | 12.44 | 1.87 | 1.56 | 2.31 | 3.75 | 6.13 ± 5.72 | |
| Clostridia | Pre | 0.66 | 2.53 | 0.91 | 0.86 | n.d. | n.d. | n.d. | n.d. | 1.43 ± 0.95 |
| Post | n.d. | 3.74 | 1.95 | 0.12 | 2.33 | 2.02 | 1.80 | 1.66 | 1.93 ± 1.81 | |
| Desulfobulbia | Pre | 2.70 | 3.12 | 2.98 | 3.79 | n.d. | n.d. | n.d. | n.d. | 3.30 ± 0.43 |
| Post | n.d. | 2.78 | 2.43 | 1.07 | 1.09 | 2.53 | 1.95 | 0.31 | 2.09 ± 0.90 |




References
- Caddy, J.F.; Carocci, F.; Coppola, S. Have Peak Fishery Production Levels Been Passed in Continental Shelf Areas? Some Perspectives Arising from Historical Trends in Production per Shelf Area. J. Northwest Atl. Fish. Sci. 1998, 23, 191–220. [Google Scholar] [CrossRef]
- Nelson, J.R.; Eckman, J.E.; Robertson, C.Y.; Marinelli, R.L.; Jahnke, R.A. Benthic Microalgal Biomass and Irradiance at the Sea Floor on the Continental Shelf of the South Atlantic Bight: Spatial and Temporal Variability and Storm Effects. Cont. Shelf Res. 1999, 19, 477–505. [Google Scholar] [CrossRef]
- Ogston, A.S.; Cacchione, D.A.; Sternberg, R.W.; Kineke, G.C. Observations of Storm and River Flood-Driven Sediment Transport on the Northern California Continental Shelf. Cont. Shelf Res. 2000, 20, 2141–2162. [Google Scholar] [CrossRef]
- Harris, P.T.; Heap, A.D. Cyclone-Induced Net Sediment Transport Pathway on the Continental Shelf of Tropical Australia Inferred from Reef Talus Deposits. Cont. Shelf Res. 2009, 29, 2011–2019. [Google Scholar] [CrossRef]
- Feehan, C.J.; Filbee-Dexter, K.; Thomsen, M.S.; Wernberg, T.; Miles, T. Ecosystem Damage by Increasing Tropical Cyclones. Commun. Earth Environ. 2024, 5, 674. [Google Scholar] [CrossRef]
- Rees, E.I.S.; Nicholaidou, A.; Laskaridou, P. The Effects of Storms on the Dynamics of Shallow Water Benthic Associations. In Biology of Benthic Organisms; Elsevier: Amsterdam, The Netherlands, 1977; pp. 465–474. [Google Scholar] [CrossRef]
- Harris, P.T.; Coleman, R. Estimating Global Shelf Sediment Mobility Due to Swell Waves. Mar. Geol. 1998, 150, 171–177. [Google Scholar] [CrossRef]
- Harris, P.T. On Seabed Disturbance, Marine Ecological Succession and Applications for Environmental Management: A Physical Sedimentological Perspective. In Sediments, Morphology and Sedimentary Processes on Continental Shelves; Li, M.Z., Sherwood, C.R., Hill, P.R., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 387–404. [Google Scholar] [CrossRef]
- Fiorendino, J.M.; Gaonkar, C.C.; Henrichs, D.W.; Campbell, L. Drivers of Microplankton Community Assemblage Following Tropical Cyclones. J. Plankton Res. 2023, 45, 205–220. [Google Scholar] [CrossRef]
- Wada, A.; Midorikawa, T.; Ishii, M.; Motoi, T. Carbon System Changes in the East China Sea Induced by Typhoons Tina and Winnie in 1997. J. Geophys. Res. 2011, 116, 2010JC006701. [Google Scholar] [CrossRef]
- Zhang, H.; He, H.; Zhang, W.-Z.; Tian, D. Upper Ocean Response to Tropical Cyclones: A Review. Geosci. Lett. 2021, 8, 1. [Google Scholar] [CrossRef]
- Chen, F.; Lao, Q.; Lu, X.; Wang, C.; Chen, C.; Liu, S.; Zhou, X. A Review of the Marine Biogeochemical Response to Typhoons. Mar. Pollut. Bull. 2023, 194, 115408. [Google Scholar] [CrossRef]
- Chen, C.; Hsieh, C.; Cheng, Y.; Huang, W.; Chou, W.; Shiah, F.; Gong, G.; Chen, T. Effect of a Tropical Cyclone on the Pelagic Ecosystem of a Continental Shelf. Limnol. Oceanogr. 2024, 69, 2975–2990. [Google Scholar] [CrossRef]
- Pan, G.; Chai, F.; Tang, D.; Wang, D. Marine Phytoplankton Biomass Responses to Typhoon Events in the South China Sea Based on Physical-Biogeochemical Model. Ecol. Model. 2017, 356, 38–47. [Google Scholar] [CrossRef]
- Wang, T.; Liu, G.; Gao, L.; Zhu, L.; Li, D. Biological Responses to Nine Powerful Typhoons in the East China Sea. Reg. Environ. Change 2017, 17, 465–476. [Google Scholar] [CrossRef]
- Lao, Q.; Lu, X.; Chen, F.; Jin, G.; Chen, C.; Zhou, X.; Zhu, Q. Effects of Upwelling and Runoff on Water Mass Mixing and Nutrient Supply Induced by Typhoons: Insight from Dual Water Isotopes Tracing. Limnol. Oceanogr. 2023, 68, 284–295. [Google Scholar] [CrossRef]
- Chen, C.; Lao, Q.; Zhou, X.; Jin, G.; Zhu, Q.; Chen, F. Tracks of Typhoon Movement (Left and Right Sides) Control Marine Dynamics and Eco-Environment in the Coastal Bays after Typhoons: A Case Study in Zhanjiang Bay. Sci. Total Environ. 2024, 912, 168944. [Google Scholar] [CrossRef]
- Kurtay, G.; Prevost, H.J.; Stauffer, B.A. Pico- and Nanoplankton Communities on a near to Offshore Transect along the Continental Shelf of the Northwestern Gulf of Mexico in the Aftermath of Hurricane Harvey. Limnol. Oceanogr. 2021, 66, 2779–2796. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs: High Diversity of Trees and Corals Is Maintained Only in a Nonequilibrium State. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- VanBlaricom, G.R. Disturbance, Predation, and Resource Allocation in a High-Energy Sublittoral Sand-Bottom Ecosystem: Experimental Analyses of Critical Structuring Processes for the Infaunal Community. Ph.D. Thesis, University of California San Diego, La Jolla, CA, USA, 1978. [Google Scholar]
- Thistle, D. Natural Physical Disturbances and Communities of Marine Soft Bottoms. Mar. Ecol. Prog. Ser. 1981, 6, 223–228. [Google Scholar] [CrossRef]
- Harris, P.T. Shelf and Deep-Sea Sedimentary Environments and Physical Benthic Disturbance Regimes: A Review and Synthesis. Mar. Geol. 2014, 353, 169–184. [Google Scholar] [CrossRef]
- O’Leary, J.K.; Micheli, F.; Airoldi, L.; Boch, C.; De Leo, G.; Elahi, R.; Ferretti, F.; Graham, N.A.J.; Litvin, S.Y.; Low, N.H.; et al. The Resilience of Marine Ecosystems to Climatic Disturbances. BioScience 2017, 67, 208–220. [Google Scholar] [CrossRef]
- Hughes, T.P. Catastrophes, Phase Shifts, and Large-Scale Degradation of a Caribbean Coral Reef. Science 1994, 265, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.J.E.; McBride, J.L.; Klotzbach, P.J.; Balachandran, S.; Camargo, S.J.; Holland, G.; Knutson, T.R.; Kossin, J.P.; Lee, T.; Sobel, A.; et al. Tropical Cyclones and Climate Change. WIREs Clim. Change 2016, 7, 65–89. [Google Scholar] [CrossRef]
- Knutson, T.; Camargo, S.J.; Chan, J.C.L.; Emanuel, K.; Ho, C.-H.; Kossin, J.; Mohapatra, M.; Satoh, M.; Sugi, M.; Walsh, K.; et al. Tropical Cyclones and Climate Change Assessment: Part II: Projected Response to Anthropogenic Warming. Bull. Am. Meteorol. Soc. 2020, 101, E303–E322. [Google Scholar] [CrossRef]
- Ibarra-García, E.C.; Abarca-Arenas, L.G.; Ortiz, M.; Rodríguez-Zaragoza, F.A. Impact of Hurricane Dean on Chinchorro Bank Coral Reef (Western Caribbean): Temporal Variation in the Food Web Structure. Ecol. Indic. 2020, 118, 106712. [Google Scholar] [CrossRef]
- Keenan, S.F.; Switzer, T.S.; Knapp, A.; Weather, E.J.; Davis, J. Spatial Dynamics of the Quantity and Diversity of Natural and Artificial Hard Bottom Habitats in the Eastern Gulf of Mexico. Cont. Shelf Res. 2022, 233, 104633. [Google Scholar] [CrossRef]
- Walker, B.K.; Eagan, S.; Ames, C.; Brooke, S.; Keenan, S.; Baumstark, R. Shallow-Water Coral Communities Support the Separation of Marine Ecoregions on the West-Central Florida Gulf Coast. Front. Ecol. Evol. 2020, 8, 210. [Google Scholar] [CrossRef]
- Able, K.W. A Re-Examination of Fish Estuarine Dependence: Evidence for Connectivity between Estuarine and Ocean Habitats. Estuar. Coast. Shelf Sci. 2005, 64, 5–17. [Google Scholar] [CrossRef]
- Milbrandt, E.C.; Martignette, A.J.; Thompson, M.A.; Bartleson, R.D.; Phlips, E.J.; Badylak, S.; Nelson, N.G. Geospatial Distribution of Hypoxia Associated with a Karenia Brevis Bloom. Estuar. Coast. Shelf Sci. 2021, 259, 107446. [Google Scholar] [CrossRef]
- Medina, M.; Kaplan, D.; Milbrandt, E.C.; Tomasko, D.; Huffaker, R.; Angelini, C. Nitrogen-Enriched Discharges from a Highly Managed Watershed Intensify Red Tide (Karenia Brevis) Blooms in Southwest Florida. Sci. Total Environ. 2022, 827, 154149. [Google Scholar] [CrossRef]
- Urakawa, H.; Adhikari, P.L.; Urakawa, H.E.; Bartleson, R.D.; Rumbold, D.G. Microbial Assessment of the Ecological Linkage between a Red Tide of Karenia Brevis and Bottom Water Anoxia off the Coast of Fort Myers and Sanibel Island, Florida. Reg. Stud. Mar. Sci. 2024, 78, 103765. [Google Scholar] [CrossRef]
- Hu, C.; Muller-Karger, F.E.; Swarzenski, P.W. Hurricanes, Submarine Groundwater Discharge, and Florida’s Red Tides. Geophys. Res. Lett. 2006, 33, 2005GL025449. [Google Scholar] [CrossRef]
- Phlips, E.J.; Badylak, S.; Nelson, N.G.; Havens, K.E. Hurricanes, El Niño and Harmful Algal Blooms in Two Sub-Tropical Florida Estuaries: Direct and Indirect Impacts. Sci. Rep. 2020, 10, 1910. [Google Scholar] [CrossRef]
- Alarcon, J.; Ward, L.; Pan, K.; Gonsoroski, E.; Uejio, C.K.; Beitsch, L.; Lichtveld, M.Y.; Harville, E.W.; Sherchan, S. HABs Karenia Brevis and Pseudo-Nitzschia Pre- and Post-Hurricane Michael. J. Water Health 2023, 21, 491–500. [Google Scholar] [CrossRef]
- Yang, H.; Weisberg, R.H.; Niiler, P.P.; Sturges, W.; Johnson, W. Lagrangian Circulation and Forbidden Zone on the West Florida Shelf. Cont. Shelf Res. 1999, 19, 1221–1245. [Google Scholar] [CrossRef]
- Olascoaga, M.J.; Rypina, I.I.; Brown, M.G.; Beron-Vera, F.J.; Koçak, H.; Brand, L.E.; Halliwell, G.R.; Shay, L.K. Persistent Transport Barrier on the West Florida Shelf. Geophys. Res. Lett. 2006, 33, 2006GL027800. [Google Scholar] [CrossRef]
- Hampel, J.J.; McCarthy, M.J.; Reed, M.H.; Newell, S.E. Short Term Effects of Hurricane Irma and Cyanobacterial Blooms on Ammonium Cycling along a Freshwater–Estuarine Continuum in South Florida. Front. Mar. Sci. 2019, 6, 640. [Google Scholar] [CrossRef]
- Turley, B.D.; Karnauskas, M.; Campbell, M.D.; Hanisko, D.S.; Kelble, C.R. Relationships between Blooms of Karenia Brevis and Hypoxia across the West Florida Shelf. Harmful Algae 2022, 114, 102223. [Google Scholar] [CrossRef]
- Bucci, L.; Alaka, L.; Hagen, A.; Delgado, S.; Beven, J. Hurricane Ian (AL092022); National Hurricane Center Tropical Cyclone Report; National Hurricane Center: Miami, FL, USA, 2023; p. 72. Available online: https://www.nhc.noaa.gov/data/tcr/AL092022_Ian.pdf (accessed on 26 April 2025).
- Kirby-Smith, W.W.; Ustach, J. Resistance to Hurricane Disturbance of an Epifaunal Community on the Continental Shelf off North Carolina. Estuar. Coast. Shelf Sci. 1986, 23, 433–442. [Google Scholar] [CrossRef]
- Lugo-Fernández, A.; Gravois, M. Understanding Impacts of Tropical Storms and Hurricanes on Submerged Bank Reefs and Coral Communities in the Northwestern Gulf of Mexico. Cont. Shelf Res. 2010, 30, 1226–1240. [Google Scholar] [CrossRef]
- Landsea, C.W.; Franklin, J.L. Atlantic Hurricane Database Uncertainty and Presentation of a New Database Format. Mon. Weather Rev. 2013, 141, 3576–3592. [Google Scholar] [CrossRef]
- Martin, T.; Muller, J. The Geologic Record of Hurricane Irma in a Southwest Florida Back-Barrier Lagoon. Mar. Geol. 2021, 441, 106635. [Google Scholar] [CrossRef]
- Javaruski, J.; Adhikari, P.L.; Muller, J.; Parsons, M.L. Preservation of Brevetoxins in Southwest Florida Coastal Sediments. Harmful Algae 2022, 114, 102222. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.J. Loss on Ignition Method. In Soil Test Methods from the Southeastern United States; Southern Cooperative Series; Clemson University: Clemson, SC, USA; pp. 155–157.
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every Base Matters: Assessing Small Subunit rRNA Primers for Marine Microbiomes with Mock Communities, Time Series and Global Field Samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Robeson, M.S.; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible Sequence Taxonomy Reference Database Management. PLoS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wheeler, B.; Torchiano, M. lmPerm: Permutation Tests for Linear Models. 2025. Available online: https://cran.r-project.org/web/packages/lmPerm/lmPerm.pdf (accessed on 26 April 2025).
- Hothorn, T.; Hornik, K.; Wiel, M.A.V.D.; Zeileis, A. Implementing a Class of Permutation Tests: The Coin Package. J. Stat. Soft. 2008, 28, 1–23. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Li, D. hillR: Taxonomic, Functional, and Phylogenetic Diversity and Similarity through Hill Numbers. JOSS 2018, 3, 1041. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2001, 2, 321–326. [Google Scholar] [CrossRef]
- Wickham, H. Data Analysis. In Ggplot2: Elegant Graphics for Data Analysis; Wickham, H., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 189–201. [Google Scholar] [CrossRef]
- Weisberg, R.H.; Zheng, L. Hurricane Storm Surge Simulations for Tampa Bay. Estuaries Coasts 2006, 29, 899–913. [Google Scholar] [CrossRef]
- Pasch, R.J.; Brown, D.P.; Blake, E.S. Hurricane Charley (AL032004). In National Hurricane Center Tropical Cyclone Report; National Hurricane Center: Miami, FL, USA, 2004; p. 23. Available online: https://www.nhc.noaa.gov/data/tcr/AL032004_Charley.pdf (accessed on 9 May 2025).
- Pasch, R.J.; Blake, E.S.; Cobb III, H.D.; Roberts, D.P. Hurricane Wilma (AL252005); National Hurricane Center: Miami, FL, USA, 2006; p. 27. Available online: https://www.nhc.noaa.gov/data/tcr/AL252005_Wilma.pdf (accessed on 9 May 2025).
- Cangialosi, J.P.; Latto, A.S.; Berg, R. Hurricane Irma (AL112017). In National Hurricane Center Tropical Cyclone Report; National Hurricane Center: Miami, FL, USA, 2021; p. 111. Available online: https://www.nhc.noaa.gov/data/tcr/AL112017_Irma.pdf (accessed on 26 April 2025).
- Bregy, J.C.; Wallace, D.J.; Totten, R.L.; Cruz, V.J. 2500-Year Paleotempestological Record of Intense Storms for the Northern Gulf of Mexico, United States. Mar. Geol. 2018, 396, 26–42. [Google Scholar] [CrossRef]
- Hansen, K. Watercolor seas in the wake of Hurricane Ian. NASA Earth Observatory. Available online: https://earthobservatory.nasa.gov/images/150435/watercolor-seas-in-the-wake-of-hurricane-ian (accessed on 26 April 2025).
- Rumbold, D.G.; Doering, P.H. Water Quality and Source of Freshwater Discharge to the Caloosahatchee Estuary, Florida: 2009–2018. Fla. Sci. 2020, 83, 1–20. [Google Scholar]
- Reidenbach, L.; Bartleson, R.; Depaolis, M. Caloosahatchee and Estuary Conditions Report September 20–26, 2022; Memorandum; Sanibel Captiva Conservation Foundation: Sanibel, FL, USA, 2022; p. 3. Available online: https://sccf.org/wp-content/uploads/2023/06/Sept-27-2022.pdf (accessed on 26 April 2025).
- Reidenbach, L.; Bartleson, R.; Depaolis, M. Caloosahatchee and Estuary Conditions Report October 18-24, 2022; Caloosahatchee & Estuary Conditions Report; Memorandum; Sanibel Captiva Conservation Foundation: Sanibel, Florida, USA, 2022; p. 3. Available online: https://sccf.org/wp-content/uploads/2023/06/Oct-25-2022.pdf (accessed on 26 April 2025).
- Price, J.F. Upper Ocean Response to a Hurricane. J. Phys. Oceanogr. 1981, 11, 153–175. [Google Scholar] [CrossRef]
- D’Asaro, E.A.; Sanford, T.B.; Niiler, P.P.; Terrill, E.J. Cold Wake of Hurricane Frances. Geophys. Res. Lett. 2007, 34, 2007GL030160. [Google Scholar] [CrossRef]
- Monaldo, F.M.; Sikora, T.D.; Babin, S.M.; Sterner, R.E. Satellite Imagery of Sea Surface Temperature Cooling in the Wake of Hurricane Edouard (1996). Mon. Wea. Rev. 1997, 125, 2716–2721. [Google Scholar] [CrossRef]
- Avila-Alonso, D.; Baetens, J.M.; Cardenas, R.; De Baets, B. Oceanic Response to Hurricane Irma (2017) in the Exclusive Economic Zone of Cuba and the Eastern Gulf of Mexico. Ocean Dyn. 2020, 70, 603–619. [Google Scholar] [CrossRef]
- Le Hénaff, M.; Domingues, R.; Halliwell, G.; Zhang, J.A.; Kim, H.; Aristizabal, M.; Miles, T.; Glenn, S.; Goni, G. The Role of the Gulf of Mexico Ocean Conditions in the Intensification of Hurricane Michael (2018). JGR Ocean. 2021, 126, e2020JC016969. [Google Scholar] [CrossRef]
- Tomasko, D.A.; Anastasiou, C.; Kovach, C. Dissolved Oxygen Dynamics in Charlotte Harbor and Its Contributing Watershed, in Response to Hurricanes Charley, Frances, and Jeanne—Impacts and Recovery. Estuaries Coasts 2006, 29, 932–938. [Google Scholar] [CrossRef]
- Montagna, P. Benthic Infauna Are Resistant and Resilient to Hurricane Disturbance. Mar. Ecol. Prog. Ser. 2023, 707, 1–13. [Google Scholar] [CrossRef]
- Steichen, J.L.; Labonté, J.M.; Windham, R.; Hala, D.; Kaiser, K.; Setta, S.; Faulkner, P.C.; Bacosa, H.; Yan, G.; Kamalanathan, M.; et al. Microbial, Physical, and Chemical Changes in Galveston Bay Following an Extreme Flooding Event, Hurricane Harvey. Front. Mar. Sci. 2020, 7, 186. [Google Scholar] [CrossRef]
- Acker, J.; Lyon, P.; Hoge, F.; Shen, S.; Roffer, M.; Gawlikowski, G. Interaction of Hurricane Katrina with Optically Complex Water in the Gulf of Mexico: Interpretation Using Satellite-Derived Inherent Optical Properties and Chlorophyll Concentration. IEEE Geosci. Remote Sens. Lett. 2009, 6, 209–213. [Google Scholar] [CrossRef]
- Da Silva, C.E.; Castelao, R.M. Mississippi River Plume Variability in the Gulf of Mexico from SMAP and MODIS-Aqua Observations. JGR Ocean. 2018, 123, 6620–6638. [Google Scholar] [CrossRef]
- Yan, G.; Labonté, J.M.; Quigg, A.; Kaiser, K. Hurricanes Accelerate Dissolved Organic Carbon Cycling in Coastal Ecosystems. Front. Mar. Sci. 2020, 7, 248. [Google Scholar] [CrossRef]
- Gilbes, F.; Armstrong, R.A.; Webb, R.M.T.; Müller-Krager, F.E. SeaWiFS Helps Assess Hurricane Impact on Phytoplankton in Caribbean Sea. EoS Trans. 2001, 82, 529–533. [Google Scholar] [CrossRef]
- Anglès, S.; Jordi, A.; Campbell, L. Responses of the Coastal Phytoplankton Community to Tropical Cyclones Revealed by High-frequency Imaging Flow Cytometry. Limnol. Oceanogr. 2015, 60, 1562–1576. [Google Scholar] [CrossRef]
- Huang, S.-M.; Oey, L. Right-side Cooling and Phytoplankton Bloom in the Wake of a Tropical Cyclone. JGR Ocean. 2015, 120, 5735–5748. [Google Scholar] [CrossRef]
- McGee, L.; He, R. Mesoscale and Submesoscale Mechanisms behind Asymmetric Cooling and Phytoplankton Blooms Induced by Hurricanes: A Comparison between an Open Ocean Case and a Continental Shelf Sea Case. Ocean Dyn. 2018, 68, 1443–1456. [Google Scholar] [CrossRef]
- Chapman, P.; DiMarco, S.F.; Knap, A.H.; Quigg, A.; Walker, N.D. The Effects of Hurricane Harvey on Texas Coastal-Zone Chemistry. Ocean Sci. 2023, 19, 209–227. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Tranvik, L.; Kronberg, L.; Slätis, T.; Martinsen, W. Photochemical Production of Ammonium and Transformation of Dissolved Organic Matter in the Baltic Sea. Mar. Chem. 2007, 104, 227–240. [Google Scholar] [CrossRef]
- Burkhardt, B.G.; Watkins-Brandt, K.S.; Defforey, D.; Paytan, A.; White, A.E. Remineralization of Phytoplankton-Derived Organic Matter by Natural Populations of Heterotrophic Bacteria. Mar. Chem. 2014, 163, 1–9. [Google Scholar] [CrossRef]
- Moran, M.A.; Kujawinski, E.B.; Stubbins, A.; Fatland, R.; Aluwihare, L.I.; Buchan, A.; Crump, B.C.; Dorrestein, P.C.; Dyhrman, S.T.; Hess, N.J.; et al. Deciphering Ocean Carbon in a Changing World. Proc. Natl. Acad. Sci. USA 2016, 113, 3143–3151. [Google Scholar] [CrossRef] [PubMed]
- Hemsley, V.; Füssel, J.; Duret, M.T.; Rayne, R.R.; Iversen, M.H.; Henson, S.A.; Sanders, R.; Lam, P.; Trimmer, M. Suspended Particles Are Hotspots of Microbial Remineralization in the Ocean’s Twilight Zone. Deep Sea Res. Part II Top. Stud. Oceanogr. 2023, 212, 105339. [Google Scholar] [CrossRef]
- Smil, V. Global Material Cycles and Energy. In Encyclopedia of Energy; Elsevier: Amsterdam, The Netherlands, 2004; pp. 23–32. [Google Scholar] [CrossRef]
- Ward, B.B. Nitrification in Marine Systems. In Nitrogen in the Marine Environment; Elsevier: Amsterdam, The Netherlands, 2008; pp. 199–261. [Google Scholar] [CrossRef]
- Carlson, C.A.; Hansell, D.A. DOM Sources, Sinks, Reactivity, and Budgets. In Biogeochemistry of Marine Dissolved Organic Matter; Elsevier: Amsterdam, The Netherlands, 2015; pp. 65–126. [Google Scholar] [CrossRef]
- Lavrentyev, P.; Gardner, W.; Johnson, J. Cascading Trophic Effects on Aquatic Nitrification: Experimental Evidence and Potential Implications. Aquat. Microb. Ecol. 1997, 13, 161–175. [Google Scholar] [CrossRef]
- Heiss, E.M.; Fulweiler, R.W. Coastal Water Column Ammonium and Nitrite Oxidation Are Decoupled in Summer. Estuar. Coast. Shelf Sci. 2016, 178, 110–119. [Google Scholar] [CrossRef]
- Wang, P.; Hu, B.; Wang, C.; Lei, Y. Phosphorus Adsorption and Sedimentation by Suspended Sediments from Zhushan Bay, Taihu Lake. Env. Sci. Pollut. Res. 2015, 22, 6559–6569. [Google Scholar] [CrossRef]
- Jiang, C.; Cao, R.; Lao, Q.; Chen, F.; Zhang, S.; Bian, P. Typhoon Merbok Induced Upwelling Impact on Material Transport in the Coastal Northern South China Sea. PLoS ONE 2020, 15, e0228220. [Google Scholar] [CrossRef]
- Penna, A.; Magnani, M.; Fenoglio, I.; Fubini, B.; Cerrano, C.; Giovine, M.; Bavestrello, G. Marine Diatom Growth on Different Forms of Particulate Silica: Evidence of Cell/Particle Interaction. Aquat. Microb. Ecol. 2003, 32, 299–306. [Google Scholar] [CrossRef]
- Nodine, E.R.; Gaiser, E.E. Seasonal Differences and Response to a Tropical Storm Reflected in Diatom Assemblage Changes in a Southwest Florida Watershed. Ecol. Indic. 2015, 57, 139–148. [Google Scholar] [CrossRef]
- Babin, S.M.; Carton, J.A.; Dickey, T.D.; Wiggert, J.D. Satellite Evidence of Hurricane-induced Phytoplankton Blooms in an Oceanic Desert. J. Geophys. Res. 2004, 109, C03043. [Google Scholar] [CrossRef]
- Hanshaw, M.N.; Lozier, M.S.; Palter, J.B. Integrated Impact of Tropical Cyclones on Sea Surface Chlorophyll in the North Atlantic. Geophys. Res. Lett. 2008, 35, L01601. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, L.; Hou, Y.; Zhao, H.; Qi, Y.; Yi, X. Tropical Storm-Induced Turbulent Mixing and Chlorophyll-a Enhancement in the Continental Shelf Southeast of Hainan Island. J. Mar. Syst. 2014, 129, 405–414. [Google Scholar] [CrossRef]
- Avila-Alonso, D.; Baetens, J.M.; Cardenas, R.; De Baets, B. The Impact of Hurricanes on the Oceanographic Conditions in the Exclusive Economic Zone of Cuba. Remote Sens. Environ. 2019, 233, 111339. [Google Scholar] [CrossRef]
- Oey, L.-Y.; Ezer, T.; Wang, D.-P.; Fan, S.-J.; Yin, X.-Q. Loop Current Warming by Hurricane Wilma. Geophys. Res. Lett. 2006, 33, L08613. [Google Scholar] [CrossRef]
- Martínez-López, B.; Zavala-Hidalgo, J. Seasonal and Interannual Variability of Cross-Shelf Transports of Chlorophyll in the Gulf of Mexico. J. Mar. Syst. 2009, 77, 1–20. [Google Scholar] [CrossRef]
- Son, Y.B.; Gardner, W.D.; Richardson, M.J.; Ishizaka, J.; Ryu, J.-H.; Kim, S.-H.; Lee, S.H. Tracing Offshore Low-Salinity Plumes in the Northeastern Gulf of Mexico during the Summer Season by Use of Multispectral Remote-Sensing Data. J. Ocean. 2012, 68, 743–760. [Google Scholar] [CrossRef]
- Muller-Karger, F.E.; Smith, J.P.; Werner, S.; Chen, R.; Roffer, M.; Liu, Y.; Muhling, B.; Lindo-Atichati, D.; Lamkin, J.; Cerdeira-Estrada, S.; et al. Natural Variability of Surface Oceanographic Conditions in the Offshore Gulf of Mexico. Prog. Oceanogr. 2015, 134, 54–76. [Google Scholar] [CrossRef]
- Morey, S.L.; Martin, P.J.; O’Brien, J.J.; Wallcraft, A.A.; Zavala-Hidalgo, J. Export Pathways for River Discharged Fresh Water in the Northern Gulf of Mexico. J. Geophys. Res. 2003, 108, 3303. [Google Scholar] [CrossRef]
- Morey, S.L.; Schroeder, W.W.; O’Brien, J.J.; Zavala-Hidalgo, J. The Annual Cycle of Riverine Influence in the Eastern Gulf of Mexico Basin. Geophys. Res. Lett. 2003, 30, 1867. [Google Scholar] [CrossRef]
- Riley, S.J.; Bryant, T. The Relationship between Settling Velocity and Grainsize Values. J. Geol. Soc. Aust. 1979, 26, 313–315. [Google Scholar] [CrossRef]
- Bender, E.A.; Case, T.J.; Gilpin, M.E. Perturbation Experiments in Community Ecology: Theory and Practice. Ecology 1984, 65, 1–13. [Google Scholar] [CrossRef]
- Finkl, C.W.; Benedet, L.; Andrews, J.L.; Suthard, B.; Locker, S.D. Sediment Ridges on the West Florida Inner Continental Shelf: Sand Resources for Beach Nourishment. J. Coast. Res. 2007, 231, 143–159. [Google Scholar] [CrossRef]
- Harrison, S.E.; Locker, S.D.; Hine, A.C.; Edwards, J.H.; Naar, D.F.; Twichell, D.C.; Mallinson, D.J. Sediment-Starved Sand Ridges on a Mixed Carbonate/Siliciclastic Inner Shelf off West-Central Florida. Mar. Geol. 2003, 200, 171–194. [Google Scholar] [CrossRef]
- Blair, S.M.; McIntosh, T.L.; Mostkoff, B.J. Impacts of Hurricane Andrew on the Offshore Reef Systems of Central and Northern Dade County, Florida. Bull. Mar. Sci. 1994, 54, 961–973. [Google Scholar]
- Fourqurean, J.W.; Rutten, L.M. The Impact of Hurricane Georges on Soft-Bottom, Back Reef Communities: Site- and Species-Specific Effects in South Florida Seagrass Beds. Bull. Mar. Sci. 2004, 75, 239–257. [Google Scholar]
- Krause, J.R.; Lopes, C.C.; Wilson, S.S.; Boyer, J.N.; Briceño, H.O.; Fourqurean, J.W. Status and Trajectories of Soft-Bottom Benthic Communities of the South Florida Seascape Revealed by 25 Years of Seagrass and Water Quality Monitoring. Estuaries Coasts 2023, 46, 477–493. [Google Scholar] [CrossRef]
- Phillips, N.W.; Gettleson, D.A.; Spring, K.D. Benthic Biological Studies of the Southwest Florida Shelf. Am. Zool. 1990, 30, 65–75. [Google Scholar] [CrossRef]
- Bainbridge, Z.T.; Wolanski, E.; Álvarez-Romero, J.G.; Lewis, S.E.; Brodie, J.E. Fine Sediment and Nutrient Dynamics Related to Particle Size and Floc Formation in a Burdekin River Flood Plume, Australia. Mar. Pollut. Bull. 2012, 65, 236–248. [Google Scholar] [CrossRef]
- Avnimelech, Y.; Ritvo, G.; Meijer, L.E.; Kochba, M. Water Content, Organic Carbon and Dry Bulk Density in Flooded Sediments. Aquac. Eng. 2001, 25, 25–33. [Google Scholar] [CrossRef]
- Goni, M.A.; Alleau, Y.; Corbett, R.; Walsh, J.P.; Mallinson, D.; Allison, M.A.; Gordon, E.; Petsch, S.; Dellapenna, T.M. The Effects of Hurricanes Katrina and Rita on the Seabed of the Louisiana Shelf. TSR 2007, 5, 4–9. [Google Scholar] [CrossRef]
- Cong, S.; Wu, X.; Ge, J.; Bi, N.; Li, Y.; Lu, J.; Wang, H. Impact of Typhoon Chan-Hom on Sediment Dynamics and Morphological Changes on the East China Sea Inner Shelf. Mar. Geol. 2021, 440, 106578. [Google Scholar] [CrossRef]
- Meyneng, M.; Lemonnier, H.; Le Gendre, R.; Plougoulen, G.; Antypas, F.; Ansquer, D.; Serghine, J.; Schmitt, S.; Siano, R. Subtropical Coastal Microbiome Variations Due to Massive River Runoff after a Cyclonic Event. Environ. Microbiome 2024, 19, 10. [Google Scholar] [CrossRef]
- Edwards, J.L.; Smith, D.L.; Connolly, J.; McDonald, J.E.; Cox, M.J.; Joint, I.; Edwards, C.; McCarthy, A.J. Identification of Carbohydrate Metabolism Genes in the Metagenome of a Marine Biofilm Community Shown to Be Dominated by Gammaproteobacteria and Bacteroidetes. Genes 2010, 1, 371–384. [Google Scholar] [CrossRef]
- Bianucci, L.; Balaguru, K.; Smith, R.W.; Leung, L.R.; Moriarty, J.M. Contribution of Hurricane-Induced Sediment Resuspension to Coastal Oxygen Dynamics. Sci. Rep. 2018, 8, 15740. [Google Scholar] [CrossRef]
- Garrison, C.E.; Roozbehi, S.; Mitra, S.; Corbett, D.R.; Field, E.K. Coastal Microbial Communities Disrupted during the 2018 Hurricane Season in Outer Banks, North Carolina. Front. Microbiol. 2022, 13, 816573. [Google Scholar] [CrossRef]
- Morris, R.M.; Rappé, M.S.; Connon, S.A.; Vergin, K.L.; Siebold, W.A.; Carlson, C.A.; Giovannoni, S.J. SAR11 Clade Dominates Ocean Surface Bacterioplankton Communities. Nature 2002, 420, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Csűros, M.; Hughes, A.L.; Moran, M.A. Evolution of Divergent Life History Strategies in Marine Alphaproteobacteria. mBio 2013, 4, e00373-13. [Google Scholar] [CrossRef]
- Duran, R.; Bielen, A.; Paradžik, T.; Gassie, C.; Pustijanac, E.; Cagnon, C.; Hamer, B.; Vujaklija, D. Exploring Actinobacteria Assemblages in Coastal Marine Sediments under Contrasted Human Influences in the West Istria Sea, Croatia. Environ. Sci. Pollut. Res. 2015, 22, 15215–15229. [Google Scholar] [CrossRef]
- Wang, F.-Y.; Liu, M.-Y. Microbial Community Diversity of Coral Reef Sediments on Liuqiu Island, Southwestern Taiwan. JMSE 2023, 11, 85. [Google Scholar] [CrossRef]
- Bianchi, T.S. The Role of Terrestrially Derived Organic Carbon in the Coastal Ocean: A Changing Paradigm and the Priming Effect. Proc. Natl. Acad. Sci. USA 2011, 108, 19473–19481. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.A.R.; Silveira, W.B.D.; Passos, F.M.L.; Zucchi, T.D. Laccases from Actinobacteria—What We Have and What to Expect. AiM 2014, 4, 285–296. [Google Scholar] [CrossRef]
- Goecke, F.; Thiel, V.; Wiese, J.; Labes, A.; Imhoff, J.F. Algae as an Important Environment for Bacteria – Phylogenetic Relationships among New Bacterial Species Isolated from Algae. Phycologia 2013, 52, 14–24. [Google Scholar] [CrossRef]
- Lage, O.M.; Bondoso, J. Planctomycetes and Macroalgae, a Striking Association. Front. Microbiol. 2014, 5, 267. [Google Scholar] [CrossRef]
- McKew, B.A.; Dumbrell, A.J.; Taylor, J.D.; McGenity, T.J.; Underwood, G.J.C. Differences between Aerobic and Anaerobic Degradation of Microphytobenthic Biofilm-Derived Organic Matter within Intertidal Sediments. FEMS Microbiol. Ecol. 2013, 84, 495–509. [Google Scholar] [CrossRef]
- Dupont, J.; Hallock, P.; Jaap, W. Ecological Impacts of the 2005 Red Tide on Artificial Reef Epibenthic Macroinvertebrate and Fish Communities in the Eastern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2010, 415, 189–200. [Google Scholar] [CrossRef]
- Stys, B.; Foster, T.; Fuentes, M.; Glazer, B.; Karish, K.; Montero, N.; Reece, J. Climate Change Impacts on Florida’s Biodiversity and Ecology. In Florida’s Climate: Changes, Variations, & Impacts; Florida Climate Institute: Gainesville, FL, USA, 2017. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tillman, M.C.; Smith, R.M.; Tubbs, T.R.; Catasus, A.B.; Urakawa, H.; Adhikari, P.L.; Douglass, J.G. Acute Impacts of Hurricane Ian on Benthic Habitats, Water Quality, and Microbial Community Composition on the Southwest Florida Shelf. Coasts 2025, 5, 16. https://doi.org/10.3390/coasts5020016
Tillman MC, Smith RM, Tubbs TR, Catasus AB, Urakawa H, Adhikari PL, Douglass JG. Acute Impacts of Hurricane Ian on Benthic Habitats, Water Quality, and Microbial Community Composition on the Southwest Florida Shelf. Coasts. 2025; 5(2):16. https://doi.org/10.3390/coasts5020016
Chicago/Turabian StyleTillman, Matthew Cole, Robert Marlin Smith, Trevor R. Tubbs, Adam B. Catasus, Hidetoshi Urakawa, Puspa L. Adhikari, and James G. Douglass. 2025. "Acute Impacts of Hurricane Ian on Benthic Habitats, Water Quality, and Microbial Community Composition on the Southwest Florida Shelf" Coasts 5, no. 2: 16. https://doi.org/10.3390/coasts5020016
APA StyleTillman, M. C., Smith, R. M., Tubbs, T. R., Catasus, A. B., Urakawa, H., Adhikari, P. L., & Douglass, J. G. (2025). Acute Impacts of Hurricane Ian on Benthic Habitats, Water Quality, and Microbial Community Composition on the Southwest Florida Shelf. Coasts, 5(2), 16. https://doi.org/10.3390/coasts5020016







