Experimental Study on Foam Stability in Tailwater Discharge from Coastal Power Plants in Marine Environments
Abstract
1. Introduction
2. Materials and Methods
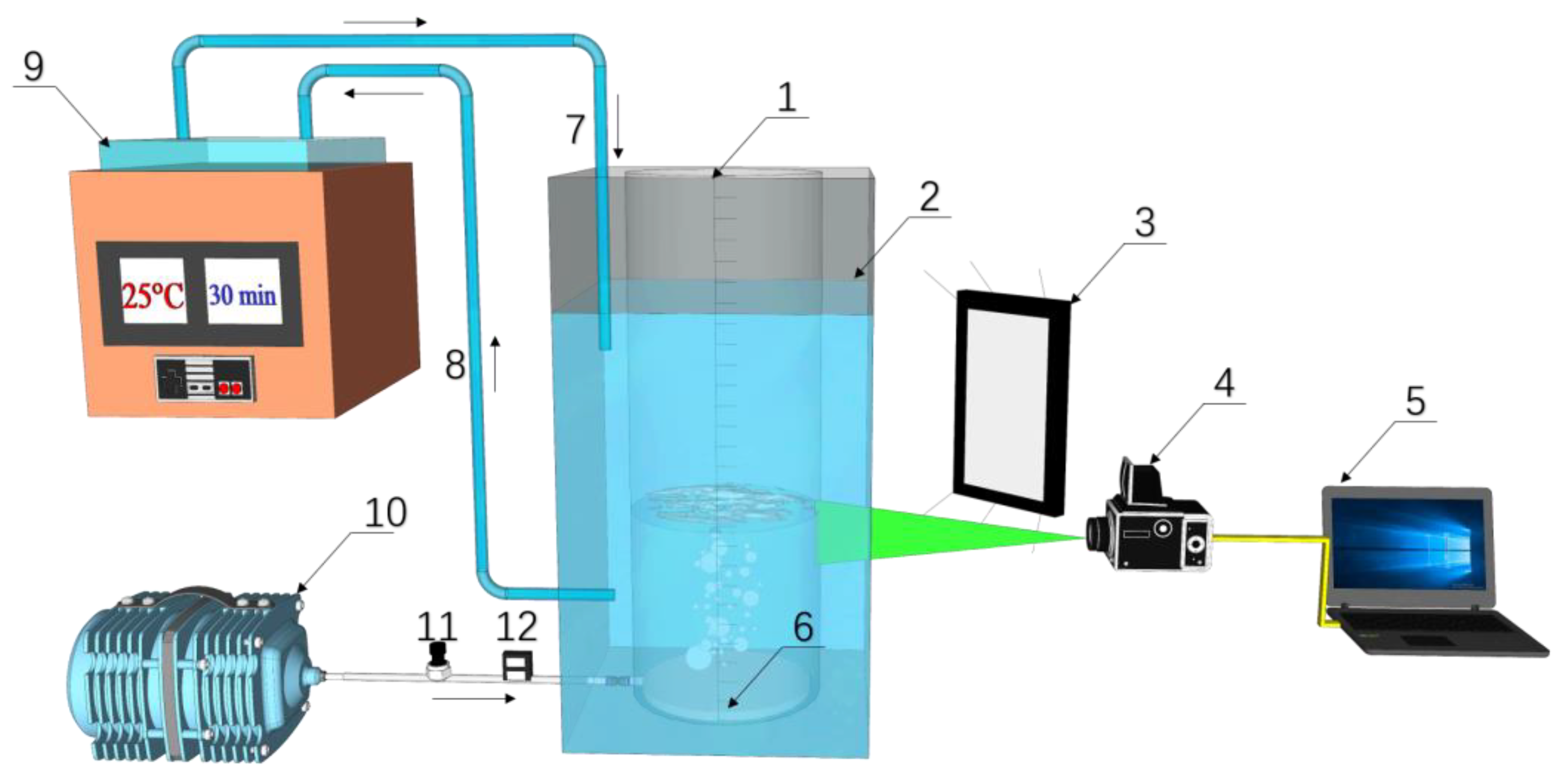
2.1. Experimental Setup
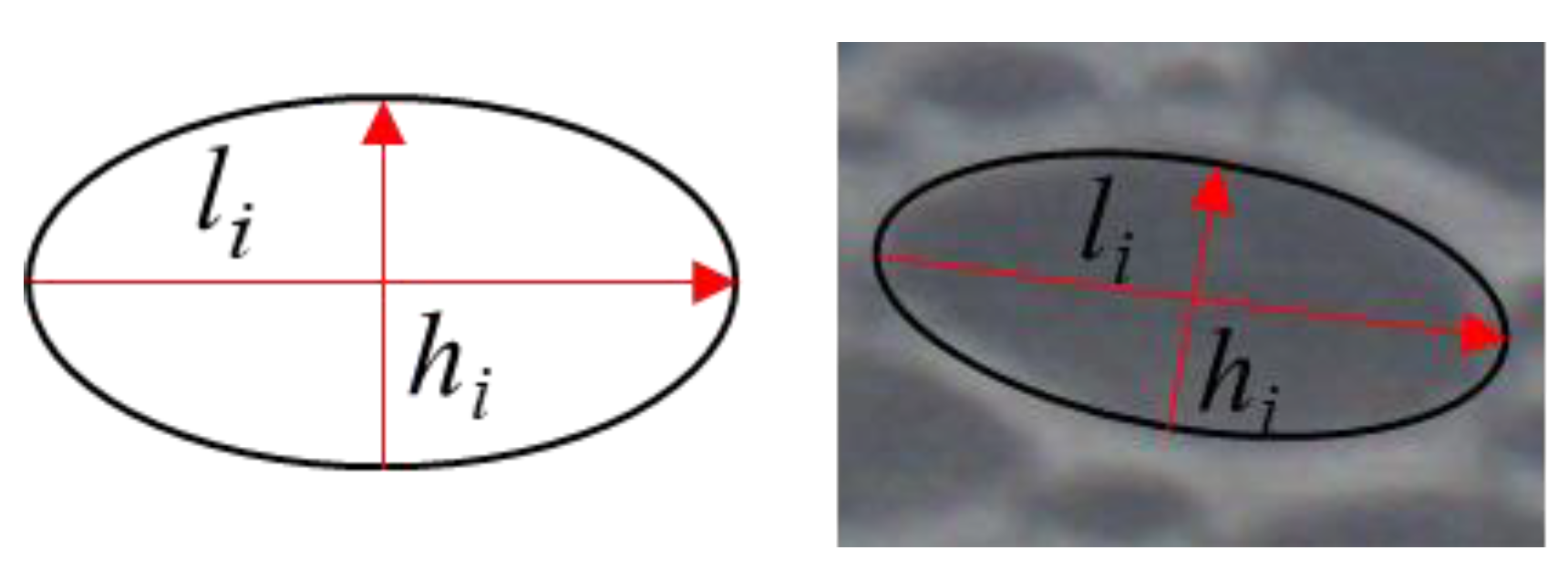
2.2. Experimental Design
3. Results
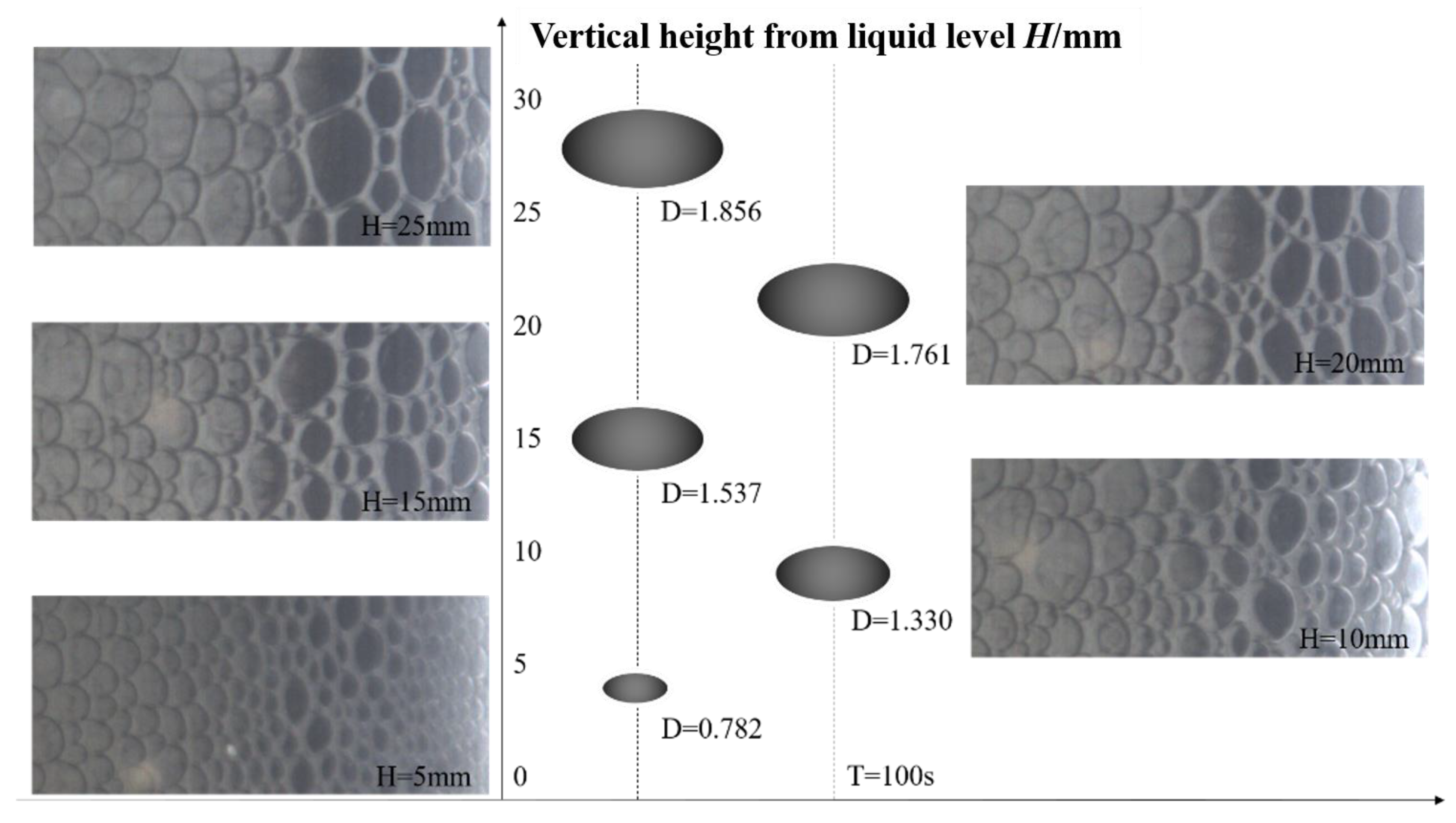
3.1. Vertical Distribution of Bubbles
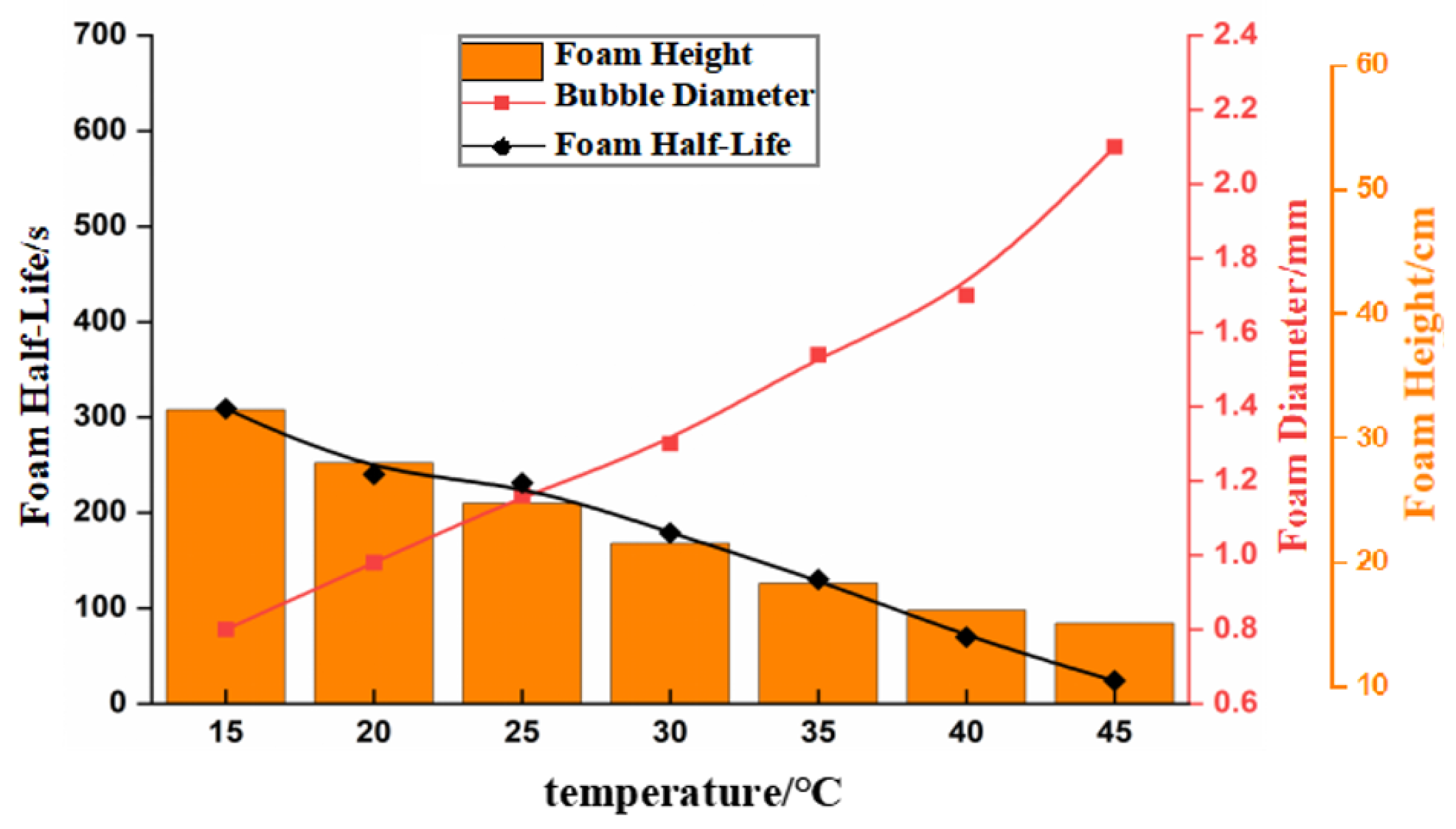
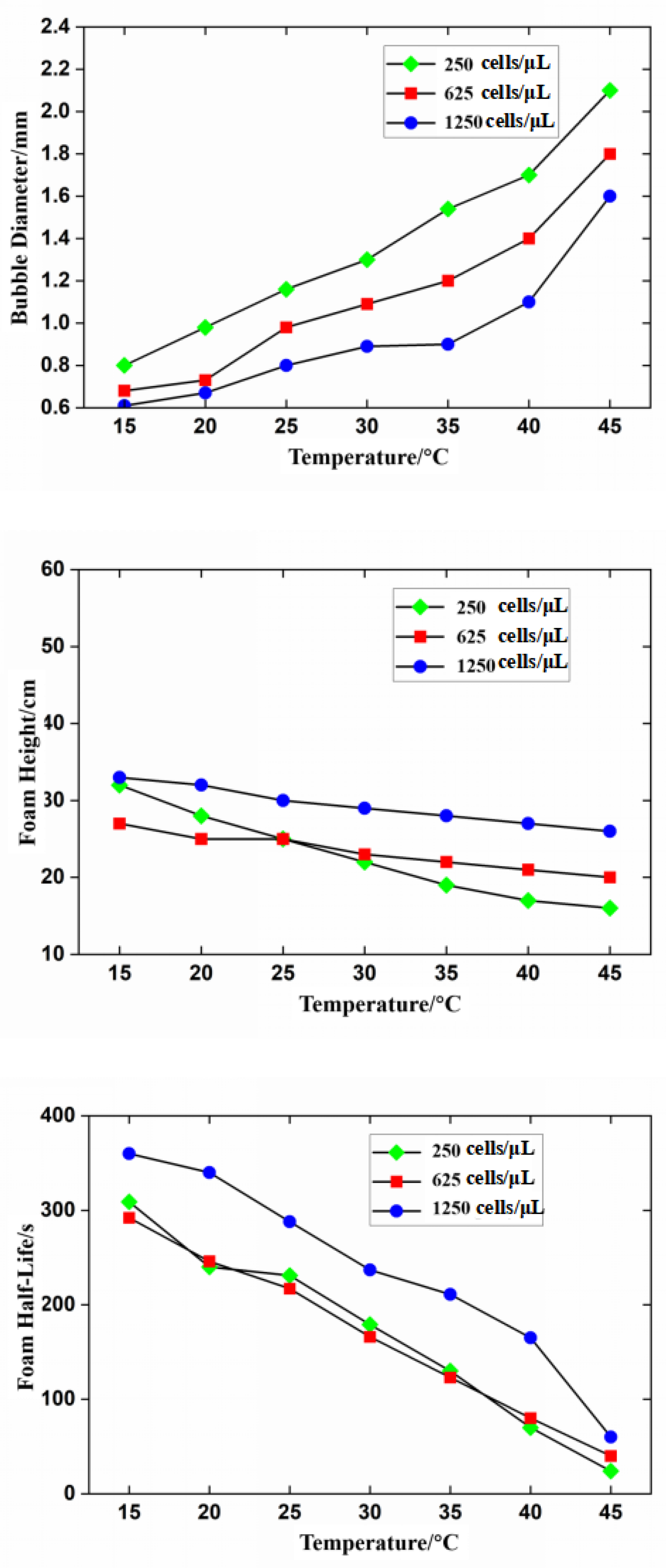
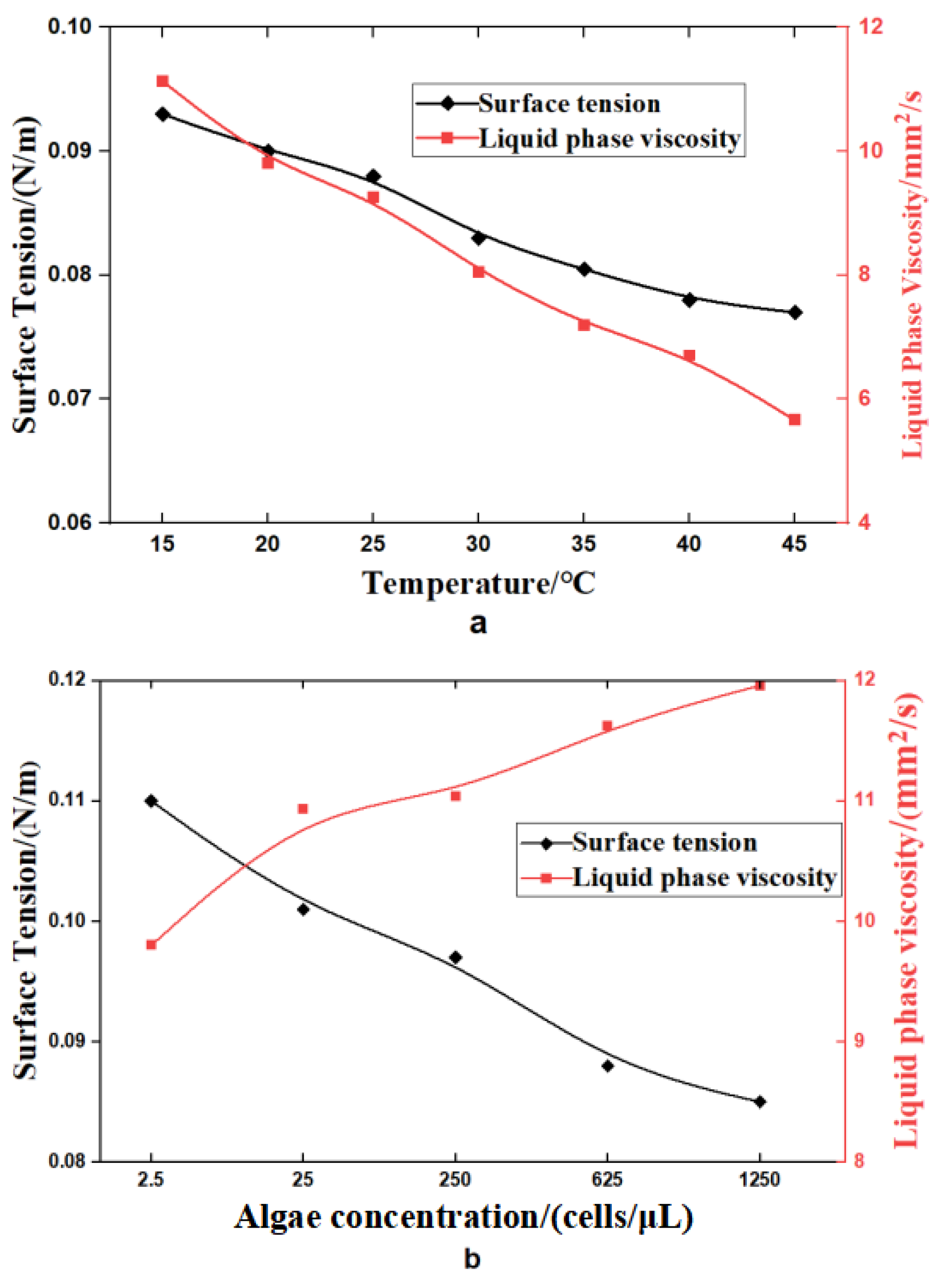
3.2. Effect of Temperature on Foam Stability
3.3. Effect of Algal Concentration on Foam Stability
3.4. Effect of Temperature on Foam Stability at Different Algal Concentrations
4. Discussion
5. Conclusions
- The effects of temperature and algal concentration on foam stability are mainly attributed to changes in the physicochemical properties of seawater. The viscosity of the seawater significantly decreases with increasing temperature, leading to a significant reduction in foam stability. Conversely, the surface tension significantly decreases with increasing algal concentration, which promotes the formation and stabilization of the foam.
- Foam stability decreases with increasing temperature. In summer, the high water temperature in the power plant hinders foam dissipation, indicating that temperature is not the main factor affecting foam stability. Instead, the high temperatures at the cooling water outlet promote the growth and reproduction of marine organisms. This indicates that the persistent and dense foam downstream of the outfall is mainly due to algal blooms and the release of surface-active substances.
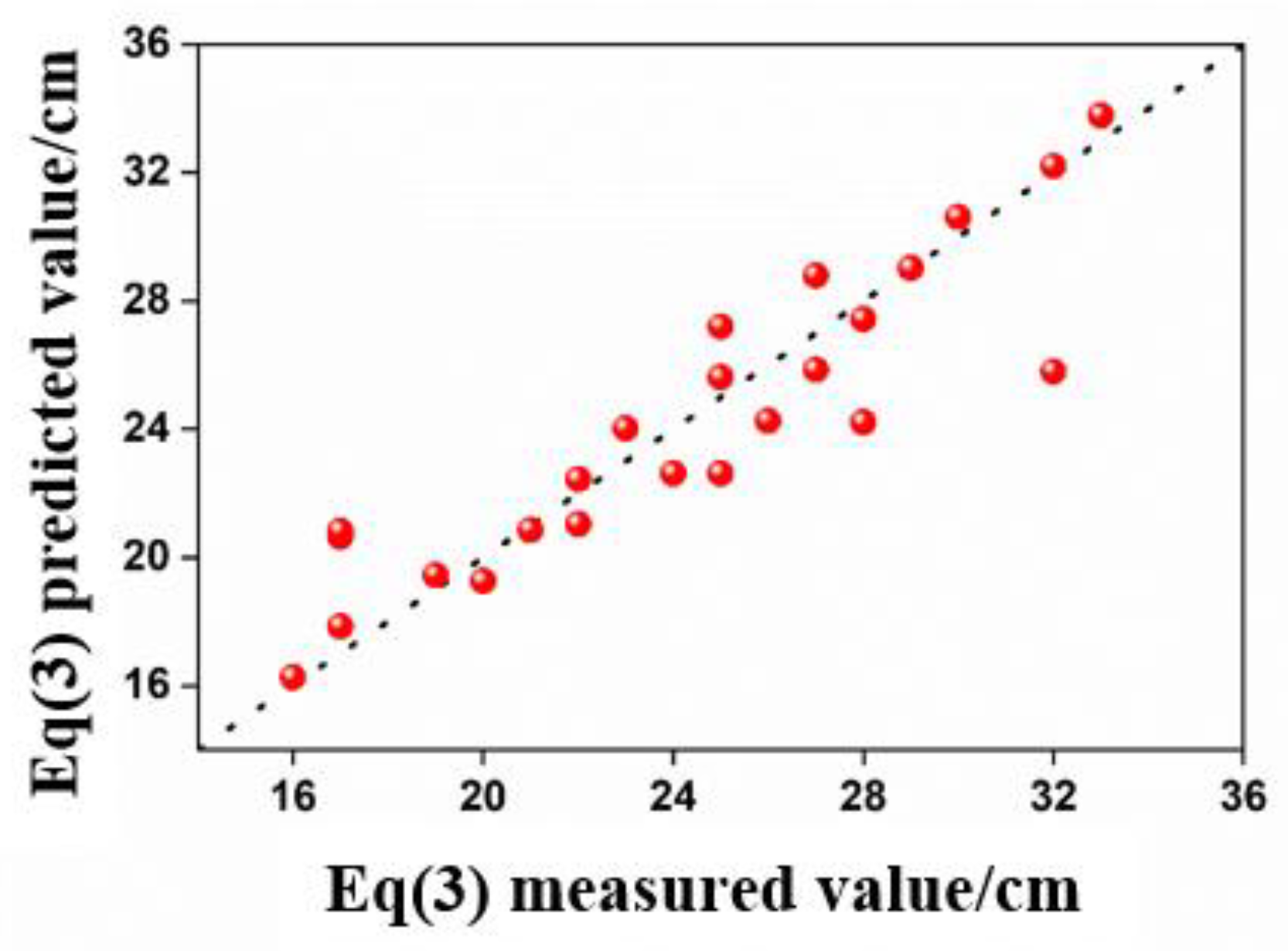
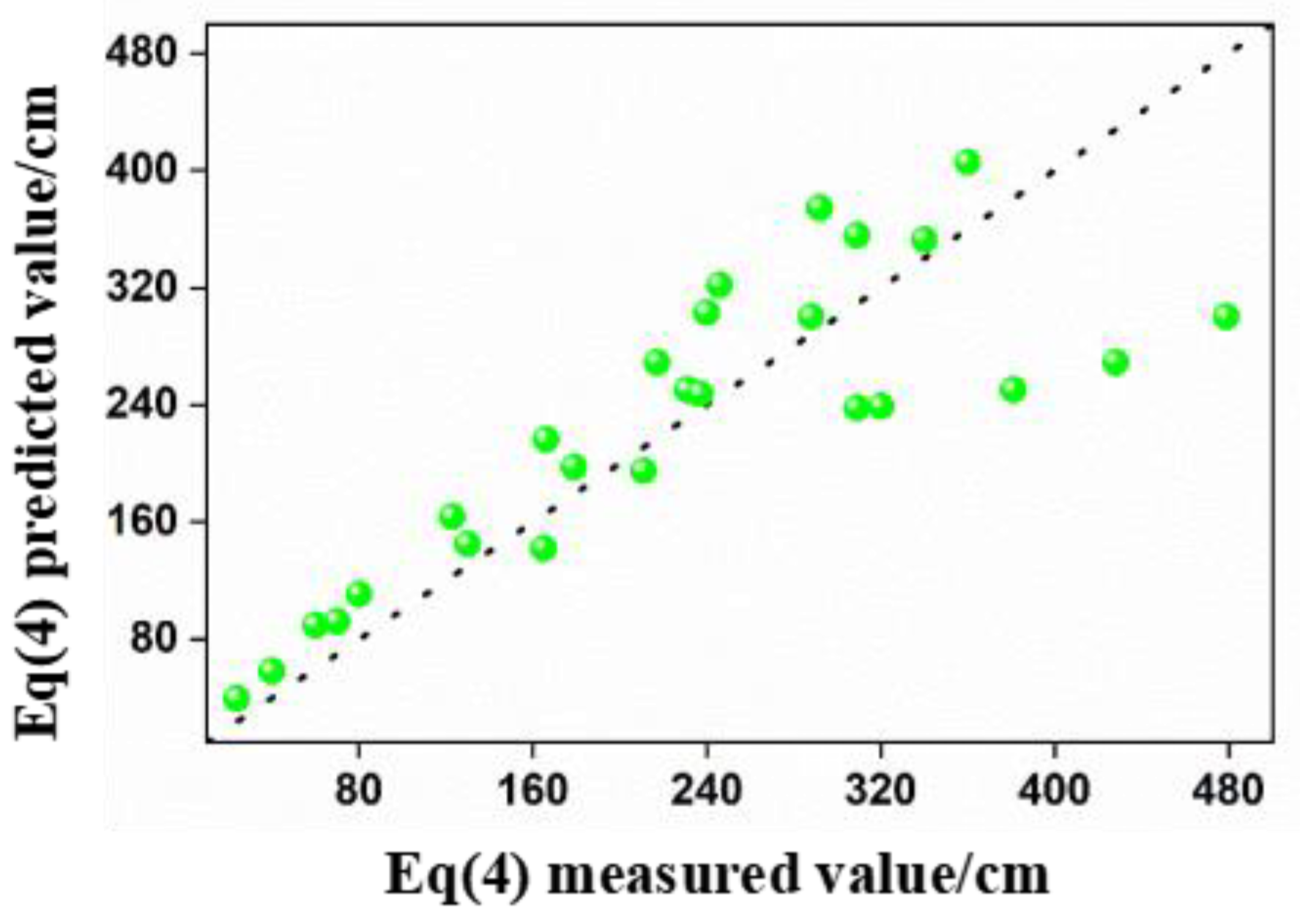
- The regression models for predicting foam layer height and bubble diameter exhibit high accuracy, while the model for predicting foam half-life exhibits moderate accuracy. This study provides a strong basis for further research and prediction and presents methods for mitigating the adverse environmental impacts of foams on the surrounding sea area.
- By adding experimental conditions such as pH, ionic strength, and different types of protein polysaccharides, a more comprehensive understanding of the mechanism of bubble generation can be achieved, providing more accurate foam suppression optimization solutions for future engineering practices.
- The existing simulation experimental equipment cannot express the complex marine environment (climate, lighting, ocean currents, etc.) of the discharge sea area. Therefore, it is considered to introduce more environmental parameters, such as water quality, meteorology, to more realistically simulate the impact of coastal power plant drainage on the surrounding sea area.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Zhu, X.; Zuo, S.; Mei, M. Application of Defoaming Agent in Seawater Circulating Cooling System of Coastal NPPs. Radiat. Prot. Bull. 2015, 35, 21–24. [Google Scholar]
- Hill, C.; Eastoe, J. Foams: From Nature to Industry. Adv. Colloid Interface Sci. 2017, 247, 496–513. [Google Scholar] [CrossRef]
- Stefani, F.; Salerno, F.; Copetti, D.; Rabuffetti, D.; Guidetti, L.; Torri, G.; Naggi, A.; Iacomini, M.; Morabito, G.; Guzzella, L. Endogenous Origin of Foams in Lakes: A Long-Term Analysis for Lake Maggiore (Northern Italy). Hydrobiologia 2016, 767, 249–265. [Google Scholar] [CrossRef]
- Kesaulya, I.; Leterme, S.C.; Mitchell, J.G.; Seuront, L. The Impact of Turbulence and Phytoplankton Dynamics on Foam Formation, Seawater Viscosity and Chlorophyll Concentration in the Eastern English Channel. Oceanologia 2008, 50, 167–182. [Google Scholar]
- Schippers, P.; Lürling, M.; Scheffer, M. Increase of Atmospheric CO2 Promotes Phytoplankton Productivity. Ecol. Lett. 2004, 7, 446–451. [Google Scholar] [CrossRef]
- Schilling, K.; Zessner, M. Foam in the Aquatic Environment. Water Res. 2011, 45, 4355–4366. [Google Scholar] [CrossRef]
- Oh, S.-H.; Oh, Y.M.; Kim, J.-Y.; Kang, K.-S. A Case Study on the Design of Condenser Effluent Outlet of Thermal Power Plant to Reduce Foam Emitted to Surrounding Seacoast. Ocean Eng. 2012, 47, 58–64. [Google Scholar] [CrossRef]
- Kováts, P.; Thévenin, D.; Zähringer, K. Influence of Viscosity and Surface Tension on Bubble Dynamics and Mass Transfer in a Model Bubble Column. Int. J. Multiph. Flow 2020, 123, 103174. [Google Scholar] [CrossRef]
- Huang, X.; Sun, Q.; Chen, X.; Wang, Z.; Mao, J. Research Progress on the Enhancement of Protein Foaming Properties by Plant Polyphenols. J. Food Saf. Qual. Insp. 2024, 15, 220–228. [Google Scholar]
- Tan, B.; Wang, Q.; Zheng, X.; Liu, J.; Yang, B.; Shen, W. Foam motion modeling simulation and stability experiments of printing paste foam. Wool Text. J. 2024, 52, 26–32. [Google Scholar]
- Li, X. Structural Characterization and Ultrasonic Modification of a Wheat-Derived Protein Affecting Beer Foam Properties. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2024. [Google Scholar]
- Zhao, Y. Study on Stability Regulation and Application of Buckwheat Protein—Based Pickering Foam. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2024. [Google Scholar]
- Luo, H.; Gao, J.; Liu, J.; Sun, T. Chemical Process and Application of Continuous Foam Drainage Gas Production in Low Production Gas Wells. Contemp. Chem. Ind. 2024, 53, 1585–1589. [Google Scholar]
- Xu, J.; Zhu, Y.; Xu, X.; Bao, H. Study on multidimensional evaluation method of foam performance of daily chemicals. China Surfactant Deterg. Cosmet. 2022, 52, 506–513. [Google Scholar]
- He, Z. Study on Foam Stabilization Mechanism of Janus Nanoparticles in Porous Media. Master’s Thesis, Xi’an Shiyou University, Xi’an, China, 2022. [Google Scholar]
- Narsimhan, G.; Xiang, N. Role of proteins on formation, drainage, and stability of liquid food foams. Annu. Rev. Food Sci. Technol. 2018, 9, 45–63. [Google Scholar] [CrossRef]
- Li, L.; Ma, F.; Zhai, X.; Yang, W.; Ye, P.; Liu, Y. Analysis on the key factors for the population evolution and early warning of harmful algal blooms based on an algal bloom in Shenzhen bay, the South China Sea. Ecol. Sci. 2022, 41, 82–89. [Google Scholar]
- Wu, Y.; Fu, Y.; Zhang, Y.; Pu, X.; Zhou, C. Phytoplankton distribution and its relation to the runoff in the YANGZTE estuary. Oceanol. Limnol. Sin. 2004, 246–251. [Google Scholar]
- Wang, J.; Nguyen, A.V.; Farrokhpay, S. A critical review of the growth, drainage and collapse of foams. Adv. Colloid Interface Sci. 2016, 228, 55–70. [Google Scholar] [CrossRef]
- Han, J.; Li, Y.; Chen, P. Research progress on phenomenon and mechanism in flotation froth phase. Nonferrous Met. Miner. Process. Sect. 2023, 17–28. [Google Scholar]
- Nguyen, A.V.; Phan, C.M.; Evans, G.M. Effect of the Bubble Size on the Dynamic Adsorption of Frothers and Collectors in Flotation. Int. J. Miner. Process. 2006, 79, 18–26. [Google Scholar] [CrossRef]
- Lin, H.; Liu, D.; Huang, B.; Cheng, X.; Sun, L.; Yin, X. Study of siphon well drainage foam in coastal power plant. China Water Transp. 2023, 23, 64–66. [Google Scholar]
- Wang, H.; Guo, W.; Zheng, C.; Wang, D.; Zhan, H. Effect of Temperature on Foaming Ability and Foam Stability of Typical Surfactants Used for Foaming Agent. J. Surfactants Deterg. 2017, 20, 615–622. [Google Scholar] [CrossRef]
- Oetjen, K.; Bilke-Krause, C.; Madani, M.; Willers, T. Temperature Effect on Foamability, Foam Stability, and Foam Structure of Milk. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 280–285. [Google Scholar] [CrossRef]
- Schwenzfeier, A.; Lech, F.; Wierenga, P.A.; Eppink, M.H.; Gruppen, H. Foam Properties of Algae Soluble Protein Isolate: Effect of pH and Ionic Strength. Food Hydrocoll. 2013, 33, 111–117. [Google Scholar] [CrossRef]
- Tang, S.; Shang, Z.; Huang, H.; Zhang, Y. Ecological impacts of coastal power plants associated with once-through cooling in China and mitigation: A preliminary evaluation. J. Appl. Oceanogr. 2020, 39, 590–599. [Google Scholar]
- Li, J. Influence factors and control measures to the foam at drainage outlet of cooling water in coastal power plant. Guangdong Chem. Ind. 2021, 48, 128, 150. [Google Scholar]
- Wang, Q.; Xi, H.; Zuo, Y. Review on measurement techniques of performance and influence factors of stability for foam. Energy Chem. Ind. 2007, 25–30. [Google Scholar]
- Zhao, X. Summary of foam stability. Drill. Fluid Complet. Fluid 1992, 9, 7–14+23+2. [Google Scholar]
- Wang, M.; Chang, Z.; Xi, H.; Zuo, Y.; Liu, H.; Li, W. Advances of Research and Measurement of Stability of Aqueous Foam. Chem. Ind. Eng. Prog. 2005, 24, 723–728. [Google Scholar]





| Experiment No. | Temperature (°C) | Algae Concentration (cells/μL) | Foam Layer Height (cm) | Foam Half-Life (s) | Bubble Diameter (mm) |
|---|---|---|---|---|---|
| 1 | 15 | 250 | 32 | 309 | 0.8 |
| 2 | 20 | 250 | 28 | 240 | 0.98 |
| 3 | 25 | 250 | 25 | 231 | 1.16 |
| 4 | 30 | 250 | 22 | 179 | 1.3 |
| 5 | 35 | 250 | 19 | 130 | 1.54 |
| 6 | 40 | 250 | 17 | 70 | 1.7 |
| 7 | 45 | 250 | 16 | 24 | 2.1 |
| 8 | 25 | 2.5 | 17 | 309 | 1.3 |
| 9 | 25 | 25 | 17 | 320 | 1.23 |
| 10 | 25 | 250 | 24 | 381 | 1.16 |
| 11 | 25 | 625 | 25 | 428 | 0.98 |
| 12 | 25 | 1250 | 30 | 479 | 0.8 |
| 13 | 15 | 625 | 27 | 292 | 0.68 |
| 14 | 20 | 625 | 25 | 246 | 0.73 |
| 15 | 25 | 625 | 25 | 217 | 0.98 |
| 16 | 30 | 625 | 23 | 166 | 1.09 |
| 17 | 35 | 625 | 22 | 123 | 1.2 |
| 18 | 40 | 625 | 21 | 80 | 1.4 |
| 19 | 45 | 625 | 20 | 40 | 1.8 |
| 20 | 15 | 1250 | 33 | 360 | 0.55 |
| 21 | 20 | 1250 | 32 | 340 | 0.67 |
| 22 | 25 | 1250 | 30 | 288 | 0.8 |
| 23 | 30 | 1250 | 29 | 237 | 0.89 |
| 24 | 35 | 1250 | 28 | 211 | 0.9 |
| 25 | 40 | 1250 | 27 | 165 | 1.1 |
| 26 | 45 | 1250 | 26 | 60 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Lin, H.; Cheng, X.; Sun, L.; Guo, L.; Hong, C. Experimental Study on Foam Stability in Tailwater Discharge from Coastal Power Plants in Marine Environments. Coasts 2025, 5, 9. https://doi.org/10.3390/coasts5010009
Liu D, Lin H, Cheng X, Sun L, Guo L, Hong C. Experimental Study on Foam Stability in Tailwater Discharge from Coastal Power Plants in Marine Environments. Coasts. 2025; 5(1):9. https://doi.org/10.3390/coasts5010009
Chicago/Turabian StyleLiu, Da, Hui Lin, Xiangju Cheng, Lubin Sun, Lei Guo, and Changhong Hong. 2025. "Experimental Study on Foam Stability in Tailwater Discharge from Coastal Power Plants in Marine Environments" Coasts 5, no. 1: 9. https://doi.org/10.3390/coasts5010009
APA StyleLiu, D., Lin, H., Cheng, X., Sun, L., Guo, L., & Hong, C. (2025). Experimental Study on Foam Stability in Tailwater Discharge from Coastal Power Plants in Marine Environments. Coasts, 5(1), 9. https://doi.org/10.3390/coasts5010009





