Forty Years of No-Take Protection Preserves Local Fish Diversity in a Small Urban Marine Protected Area
Abstract
1. Introduction
2. Materials and Methods
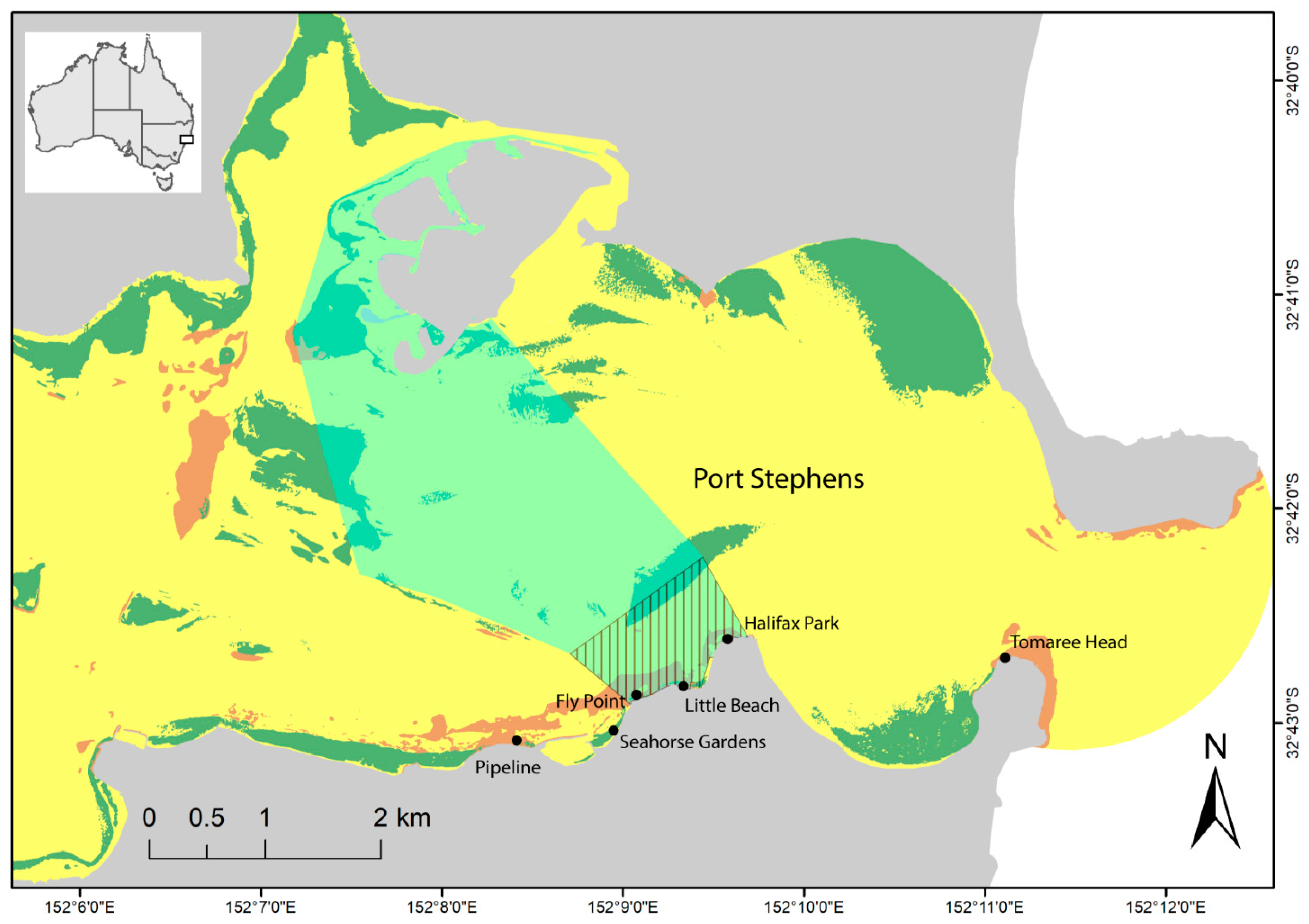
2.1. Study Area
2.2. Sampling Methods
2.3. Statistical Analysis
3. Results
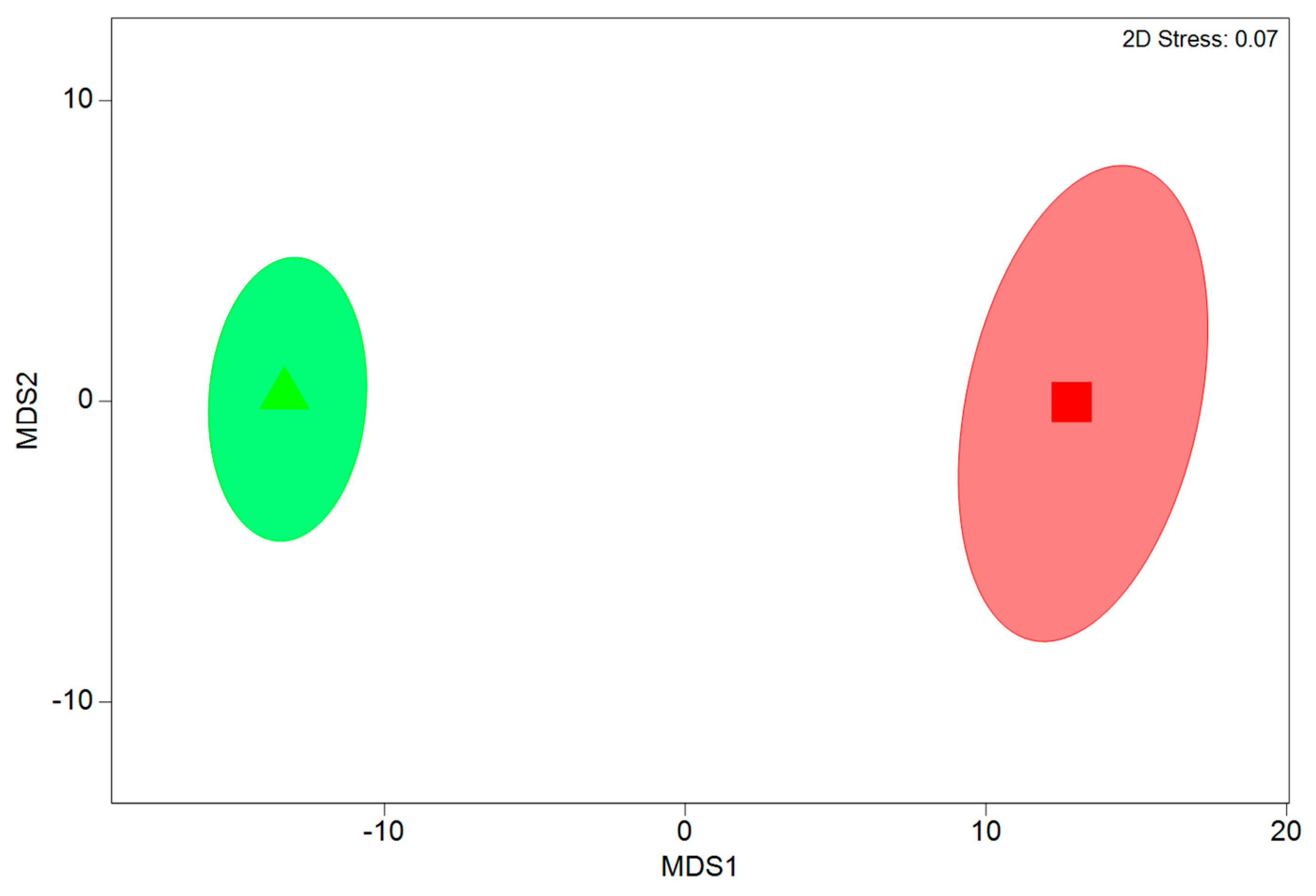
3.1. Variations in Fish Assemblages among Area Types
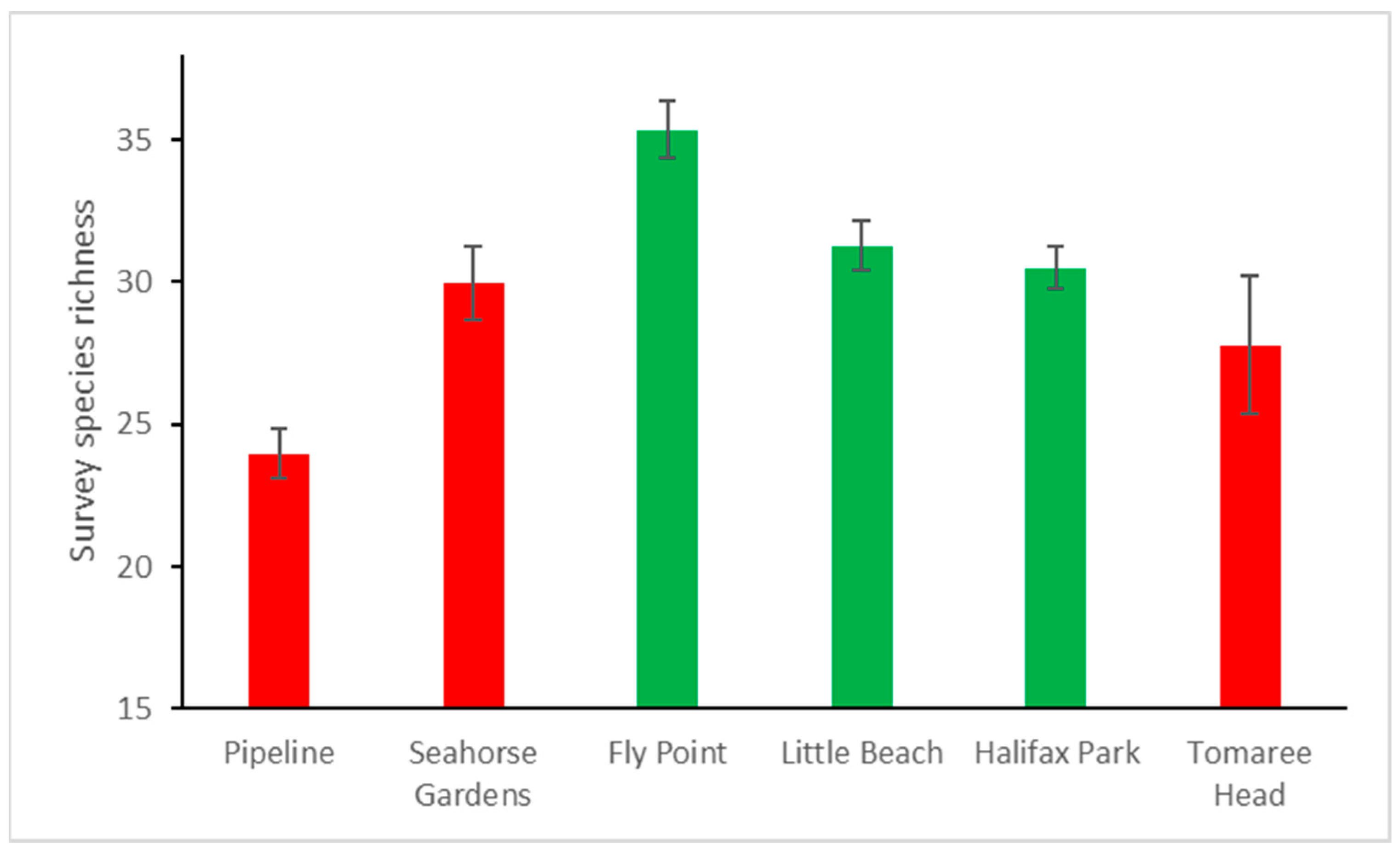
3.2. Variations in Survey Species Richness among Areas and Sites
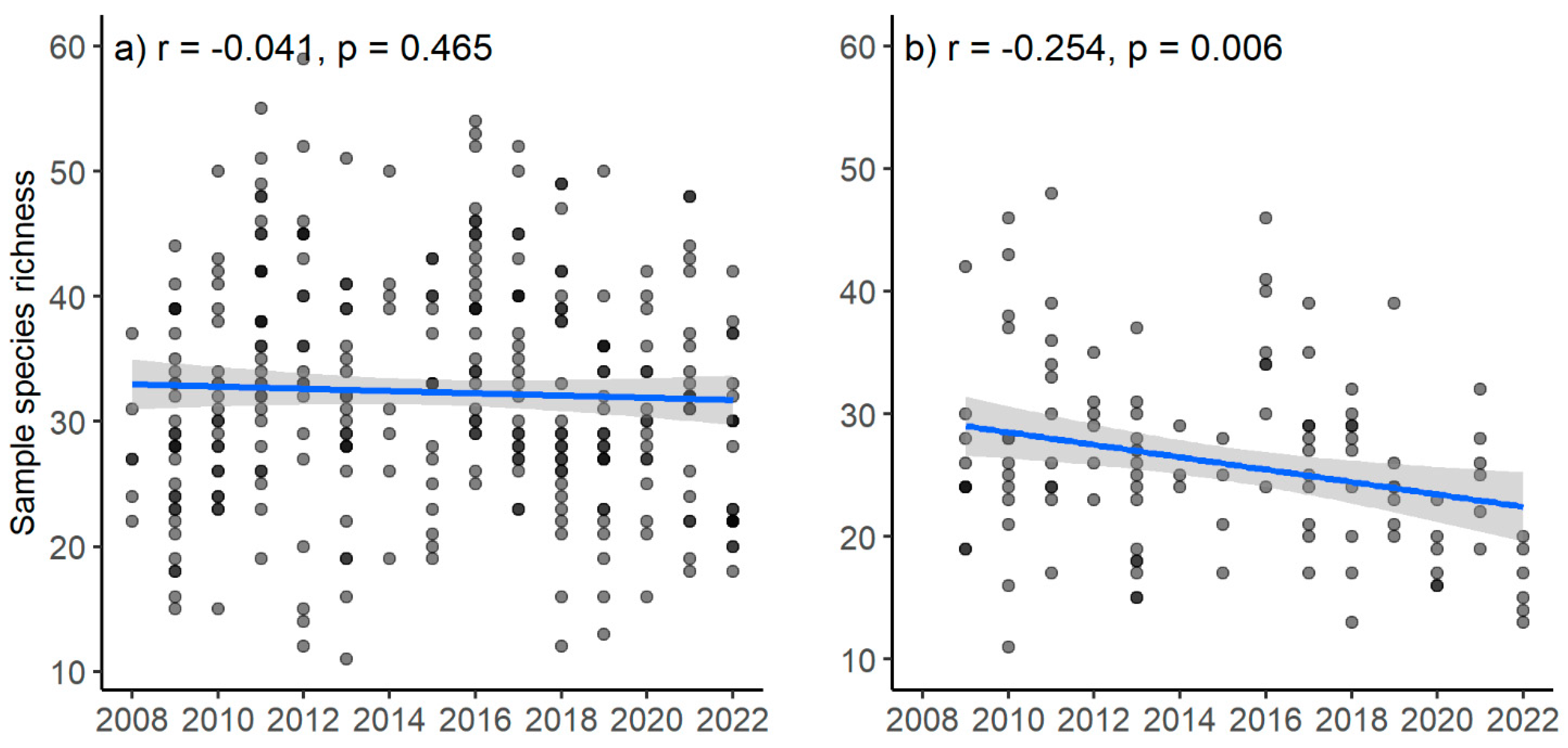
3.3. Temporal Trends in Survey-Species Richness
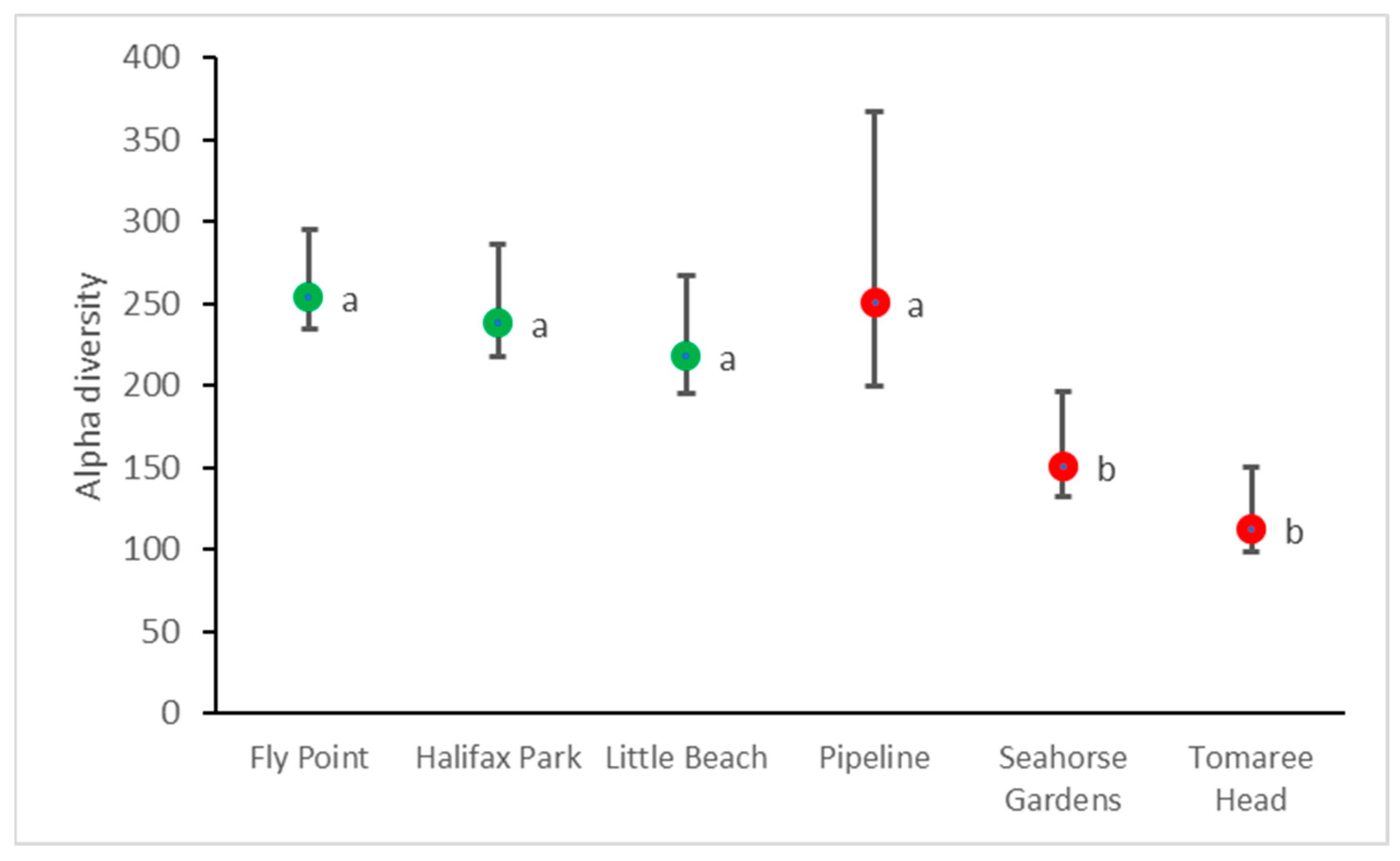
3.4. Fish Regional (Gamma) Diversity and Site (Alpha) Diversity
4. Discussion
4.1. MPA Age and No-Take Effects
4.2. MPA Isolation and Enforcement Effects
4.3. MPA Size and Habitat Effects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doney, S.C.; Ruckelshaus, M.; Emmett Duffy, J.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N. Climate Change Impacts on Marine Ecosystems. Ann. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Tanaka, M. Impacts of Pollution on Coastal and Marine Ecosystems Including Coastal and Marine Fisheries and Approach for Management: A Review and Synthesis. Mar. Pollut. Bull. 2004, 48, 624–649. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.N.K.; Krawchuk, M.A.; Dulvy, N.K. Why Have Global Shark and Ray Landings Declined: Improved Management or Overfishing? Fish Fish. 2016, 17, 438–458. [Google Scholar] [CrossRef]
- Limburg, K.E.; Hughes, R.M.; Jackson, D.C.; Czech, B. Human Population Increase, Economic Growth, and Fish Conservation: Collision Course or Savvy Stewardship? Fisheries 2011, 36, 27–35. [Google Scholar] [CrossRef]
- Gray, J.S. Marine Biodiversity: Patterns, Threats and Conservation Needs. Biodivers. Conserv. 1997, 6, 153–175. [Google Scholar] [CrossRef]
- Kroodsma, D.A.; Mayorga, J.; Hochberg, T.; Miller, N.A.; Boerder, K.; Ferretti, F.; Wilson, A.; Bergman, B.; White, T.D.; Block, B.A. Tracking the Global Footprint of Fisheries. Science 2018, 359, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Grorud-Colvert, K.; Sullivan-Stack, J.; Roberts, C.; Constant, V.; Horta e Costa, B.; Pike, E.P.; Kingston, N.; Laffoley, D.; Sala, E.; Claudet, J. The MPA Guide: A Framework to Achieve Global Goals for the Ocean. Science 2021, 373, eabf0861. [Google Scholar] [CrossRef]
- Edgar, G.J.; Stuart-Smith, R.D.; Willis, T.J.; Kininmonth, S.; Baker, S.C.; Banks, S.; Barrett, N.S.; Becerro, M.A.; Bernard, A.T.F.; Berkhout, J. Global Conservation Outcomes Depend on Marine Protected Areas with Five Key Features. Nature 2014, 506, 216. [Google Scholar]
- Di Cintio, A.; Niccolini, F.; Scipioni, S.; Bulleri, F. Avoiding “Paper Parks”: A Global Literature Review on Socioeconomic Factors Underpinning the Effectiveness of Marine Protected Areas. Sustainability 2023, 15, 4464. [Google Scholar] [CrossRef]
- Iacarella, J.C.; Clyde, G.; Bergseth, B.J.; Ban, N.C. A Synthesis of the Prevalence and Drivers of Non-Compliance in Marine Protected Areas. Biol. Conserv. 2021, 255, 108992. [Google Scholar] [CrossRef]
- Rife, A.N.; Erisman, B.; Sanchez, A.; Aburto-Oropeza, O. When Good Intentions Are Not Enough… Insights on Networks of “Paper Park” Marine Protected Areas. Conserv. Lett. 2013, 6, 200–212. [Google Scholar]
- Agardy, T.; Di Sciara, G.N.; Christie, P. Mind the Gap: Addressing the Shortcomings of Marine Protected Areas through Large Scale Marine Spatial Planning. Mar. Policy 2011, 35, 226–232. [Google Scholar] [CrossRef]
- Harasti, D.; Davis, T.R.T.R.; Jordan, A.; Erskine, L.; Moltschaniwskyj, N. Illegal Recreational Fishing Causes a Decline in a Fishery Targeted Species (Snapper: Chrysophrys auratus) within a Remote No-Take Marine Protected Area. PLoS ONE 2019, 14, e0209926. [Google Scholar] [CrossRef]
- Novaczek, E.; Howse, V.; Pretty, C.; Devillers, R.; Edinger, E.; Copeland, A. Limited Contribution of Small Marine Protected Areas to Regional Biodiversity: The Example of a Small Canadian No-Take MPA. Front. Mar. Sci. 2017, 4, 174. [Google Scholar]
- Harasti, D.; Williams, J.; Mitchell, E.; Lindfield, S.; Jordan, A. Increase in Relative Abundance and Size of Snapper Chrysophrys auratus within Partially-Protected and No-Take Areas in a Temperate Marine Protected Area. Front. Mar. Sci. 2018, 5, 20. [Google Scholar] [CrossRef]
- Harasti, D.; Davis, T.R.; Mitchell, E.; Lindfield, S.; Smith, S.D.A. A Tale of Two Islands: Decadal Changes in Rocky Reef Fish Assemblages Following Implementation of No-Take Marine Protected Areas in New South Wales, Australia. Reg. Stud. Mar. Sci. 2018, 18, 229–236. [Google Scholar]
- Pollard, D.A. A Review of Marine and Estuarine Protected Areas in NSW, with Emphasis on Research and Monitoring as a Basis for Their Scientific Management; New South Wales Fisheries: Cronulla, Australia, 1997.
- Navarro, M.; Kragt, M.E.; Hailu, A.; Langlois, T.J. Recreational Fishers’ Support for No-Take Marine Reserves Is High and Increases with Reserve Age. Mar. Policy 2018, 96, 44–52. [Google Scholar] [CrossRef]
- Martin, C.L.; Momtaz, S.; Jordan, A.; Moltschaniwskyj, N.A. Exploring Recreational Fishers’ Perceptions, Attitudes, and Support towards a Multiple-Use Marine Protected Area Six Years after Implementation. Mar. Policy 2016, 73, 138–145. [Google Scholar]
- Byers, J.E.; Noonburg, E.G. Poaching, Enforcement, and the Efficacy of Marine Reserves. Ecol. Appl. 2007, 17, 1851–1856. [Google Scholar]
- Davis, T.R.R.; Harasti, D.; Smith, S.D.A.D.A. Developing a Habitat Classification Typology for Subtidal Habitats in a Temperate Estuary in New South Wales, Australia. Mar. Freshw. Res. 2016, 67, 1186–1195. [Google Scholar] [CrossRef]
- Davis, T.R.R.; Harasti, D.; Kelaher, B.; Smith, S.D.A.D.A. Diversity Surrogates for Estuarine Fish Assemblages in a Temperate Estuary in New South Wales, Australia. Reg. Stud. Mar. Sci. 2016, 7, 55–62. [Google Scholar] [CrossRef]
- NSWMPA. Port Stephens-Great Lakes Marine Park Zoning Plan 2007; New South Wales Marine Parks Authority: Sydney, Australia, 2007.
- Edgar, G.J.; Stuart-Smith, R.D. Ecological Effects of Marine Protected Areas on Rocky Reef Communities-a Continental-Scale Analysis. Mar. Ecol. Prog. Ser. 2009, 388, 51–62. [Google Scholar]
- Edgar, G.J.; Cooper, A.; Baker, S.C.; Barker, W.; Barrett, N.S.; Becerro, M.A.; Bates, A.E.; Brock, D.; Ceccarelli, D.M.; Clausius, E. Reef Life Survey: Establishing the Ecological Basis for Conservation of Shallow Marine Life. Biol. Conserv. 2020, 252, 108855. [Google Scholar]
- Edgar, G.J.; Stuart-Smith, R.D. Systematic Global Assessment of Reef Fish Communities by the Reef Life Survey Program. Sci. Data 2014, 1, 140007. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2015. [Google Scholar]
- Clarke, K.R. Non-Parametric Multivariate Analyses of Changes in Community Structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 9. 2013. Available online: http://purl.oclc.org/estimates (accessed on 18 May 2023).
- Chao, A. Nonparametric Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Davis, T.R.; Smith, S.D.A. Proximity Effects of Natural and Artificial Reef Walls on Fish Assemblages. Reg. Stud. Mar. Sci. 2017, 9, 17–23. [Google Scholar] [CrossRef]
- Jonas, H.D.; MacKinnon, K.; Marnewick, D.; Wood, P. Site-Level Tool for Identifying Other Effective Area-Based Conservation Measures (OECMs); IUCN (International Union for Conservation of Nature): Gland, Switzerland, 2023. [Google Scholar]
- Worm, B.; Hilborn, R.; Baum, J.K.; Branch, T.A.; Collie, J.S.; Costello, C.; Fogarty, M.J.; Fulton, E.A.; Hutchings, J.A.; Jennings, S. Rebuilding Global Fisheries. Science 2009, 325, 578–585. [Google Scholar]
- Davis, T.R.; Larkin, M.F.; Forbes, A.; Veenhof, R.J.; Scott, A.; Coleman, M.A. Extreme Flooding and Reduced Salinity Causes Mass Mortality of Nearshore Kelp Forests. Estuar. Coast. Shelf Sci. 2022, 275, 107960. [Google Scholar]
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime Shifts, Resilience, and Biodiversity in Ecosystem Management. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 557–581. [Google Scholar]
- Gill, D.A.; Mascia, M.B.; Ahmadia, G.N.; Glew, L.; Lester, S.E.; Barnes, M.; Craigie, I.; Darling, E.S.; Free, C.M.; Geldmann, J. Capacity Shortfalls Hinder the Performance of Marine Protected Areas Globally. Nature 2017, 543, 665–669. [Google Scholar]
- Stuart-Smith, R.D.; Edgar, G.J.; Barrett, N.S.; Bates, A.E.; Baker, S.C.; Bax, N.J.; Becerro, M.A.; Berkhout, J.; Blanchard, J.L.; Brock, D.J. Assessing National Biodiversity Trends for Rocky and Coral Reefs through the Integration of Citizen Science and Scientific Monitoring Programs. Bioscience 2017, 67, 134–146. [Google Scholar]
- Wolfenden, J.; Cram, F.; Kirkwood, B. Marine Reserves in New Zealand: A Survey of Community Reactions. Ocean Coast. Manag. 1994, 25, 31–51. [Google Scholar]
- Rossiter, J.S.; Levine, A. What Makes a “Successful” Marine Protected Area? The Unique Context of Hawaii′ s Fish Replenishment Areas. Mar. Policy 2014, 44, 196–203. [Google Scholar]
- Chaigneau, T.; Brown, K. Challenging the Win-Win Discourse on Conservation and Development: Analyzing Support for Marine Protected Areas. Ecol. Soc. 2016, 21, 36. [Google Scholar]
- Edgar, G.J.; Barrett, N.S.; Stuart-Smith, R.D. Exploited Reefs Protected from Fishing Transform over Decades into Conservation Features Otherwise Absent from Seascapes. Ecol. Appl. 2009, 19, 1967–1974. [Google Scholar]
- Balbar, A.C.; Metaxas, A. The Current Application of Ecological Connectivity in the Design of Marine Protected Areas. Glob. Ecol. Conserv. 2019, 17, e00569. [Google Scholar]
- Lee, K.A.; Huveneers, C.; Macdonald, T.; Harcourt, R.G. Size Isn’t Everything: Movements, Home Range, and Habitat Preferences of Eastern Blue Gropers (Achoerodus viridis) Demonstrate the Efficacy of a Small Marine Reserve. Aquat. Conserv. 2015, 25, 174–186. [Google Scholar]
- Afonso, P.; Fontes, J.; Santos, R.S. Small Marine Reserves Can Offer Long Term Protection to an Endangered Fish. Biol. Conserv. 2011, 144, 2739–2744. [Google Scholar] [CrossRef]
- Rojo, I.; Anadón, J.D.; García-Charton, J.A. Exceptionally High but Still Growing Predatory Reef Fish Biomass after 23 Years of Protection in a Marine Protected Area. PLoS ONE 2021, 16, e0246335. [Google Scholar]
- Giakoumi, S.; Scianna, C.; Plass-Johnson, J.; Micheli, F.; Grorud-Colvert, K.; Thiriet, P.; Claudet, J.; Di Carlo, G.; Di Franco, A.; Gaines, S.D. Ecological Effects of Full and Partial Protection in the Crowded Mediterranean Sea: A Regional Meta-Analysis. Sci. Rep. 2017, 7, 8940. [Google Scholar]
- Harasti, D.; Lee, K.A.; Gallen, C.; Hughes, J.M.; Stewart, J. Movements, Home Range and Site Fidelity of Snapper (Chrysophrys auratus) within a Temperate Marine Protected Area. PLoS ONE 2015, 10, e0142454. [Google Scholar] [CrossRef]
- Ferguson, A.M.; Harvey, E.S.; Taylor, M.D.; Knott, N.A. A Herbivore Knows Its Patch: Luderick, Girella Tricuspidata, Exhibit Strong Site Fidelity on Shallow Subtidal Reefs in a Temperate Marine Park. PLoS ONE 2013, 8, e65838. [Google Scholar]
- Quaas, Z.; Harasti, D.; Gaston, T.F.; Platell, M.E.; Fulton, C.J. Influence of Habitat Condition on Shallow Rocky Reef Fish Community Structure around Islands and Headlands of a Temperate Marine Protected Area. Mar. Ecol. Prog. Ser. 2019, 626, 1–13. [Google Scholar]
- Pereira, T.J.; Silva, A.F.; de Almeida, P.R.; Belo, A.F.; Costa, J.L.; Castro, N.; Quintella, B.R. Assessing the Size Adequacy of a Small No-Take Marine Protected Area (MPA) for Mediterranean Moray and European Conger. Mar. Ecol. Prog. Ser. 2017, 584, 213–227. [Google Scholar]
- Rojo, I.; Sánchez-Meca, J.; García-Charton, J.A. Small-Sized and Well-Enforced Marine Protected Areas Provide Ecological Benefits for Piscivorous Fish Populations Worldwide. Mar. Environ. Res. 2019, 149, 100–110. [Google Scholar]
- Harasti, D.; Martin-Smith, K.; Gladstone, W. Does a No-Take Marine Protected Area Benefit Seahorses? PLoS ONE 2014, 9, e105462. [Google Scholar] [CrossRef]
- Komyakova, V.; Munday, P.L.; Jones, G.P. Relative Importance of Coral Cover, Habitat Complexity and Diversity in Determining the Structure of Reef Fish Communities. PLoS ONE 2013, 8, e83178. [Google Scholar]
- Darling, E.S.; Graham, N.A.J.; Januchowski-Hartley, F.A.; Nash, K.L.; Pratchett, M.S.; Wilson, S.K. Relationships between Structural Complexity, Coral Traits, and Reef Fish Assemblages. Coral Reefs 2017, 36, 561–575. [Google Scholar]
| Protection/Site | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fished | -- | 9 | 13 | 11 | 6 | 16 | 4 | 4 | 8 | 11 | 10 | 7 | 6 | 6 | 6 | 117 |
| Pipeline | -- | 7 | 6 | 3 | 3 | 6 | 3 | 4 | 3 | 9 | 8 | 7 | 6 | 3 | 6 | 74 |
| Seahorse Gardens | -- | 1 | 4 | 6 | 2 | 5 | -- | -- | 4 | 2 | 2 | -- | -- | 3 | -- | 29 |
| Tomaree Head | -- | 1 | 3 | 2 | 1 | 5 | 1 | -- | 1 | -- | -- | -- | -- | -- | -- | 14 |
| No-take | 6 | 30 | 23 | 30 | 21 | 23 | 8 | 16 | 28 | 24 | 27 | 23 | 17 | 22 | 19 | 317 |
| Fly Point | 4 | 14 | 4 | 5 | 5 | 11 | 3 | 4 | 11 | 10 | 7 | 6 | 6 | 9 | 8 | 107 |
| Halifax Park | 2 | 9 | 16 | 21 | 11 | 7 | 3 | 7 | 9 | 8 | 10 | 10 | 5 | 6 | 4 | 128 |
| Little Beach | -- | 7 | 3 | 4 | 5 | 5 | 2 | 5 | 8 | 6 | 10 | 7 | 6 | 7 | 7 | 82 |
| Grand Total | 6 | 39 | 36 | 41 | 27 | 39 | 12 | 20 | 36 | 35 | 37 | 30 | 23 | 28 | 25 | 434 |
| Species | Avg. Abundance Fished | Avg. Abundance No-Take | Ratio No-Take/Fished | Contribution to Differences (%) |
|---|---|---|---|---|
| Atypichthys strigatus | 149.8 | 99.6 | 0.7 | 16.0 |
| Trachurus novaezelandiae | 202.9 | 72.7 | 0.4 | 8.4 |
| Acanthopagrus australis | 4.3 | 47.5 | 11.0 | 6.6 |
| Scorpis lineolata | 14.7 | 36.6 | 2.5 | 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, T.R.; Harasti, D. Forty Years of No-Take Protection Preserves Local Fish Diversity in a Small Urban Marine Protected Area. Coasts 2023, 3, 401-413. https://doi.org/10.3390/coasts3040024
Davis TR, Harasti D. Forty Years of No-Take Protection Preserves Local Fish Diversity in a Small Urban Marine Protected Area. Coasts. 2023; 3(4):401-413. https://doi.org/10.3390/coasts3040024
Chicago/Turabian StyleDavis, Tom Robert, and David Harasti. 2023. "Forty Years of No-Take Protection Preserves Local Fish Diversity in a Small Urban Marine Protected Area" Coasts 3, no. 4: 401-413. https://doi.org/10.3390/coasts3040024
APA StyleDavis, T. R., & Harasti, D. (2023). Forty Years of No-Take Protection Preserves Local Fish Diversity in a Small Urban Marine Protected Area. Coasts, 3(4), 401-413. https://doi.org/10.3390/coasts3040024






