Use of Sorbitan to Extract Capsaicinoids and Bioactive Compounds: Condition Optimization Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Plant Material
2.3. Experimental Process
- 1.
- Use of sorbitan as a capsaicinoid-extracting agent
- 2.
- Optimization of capsaicinoid extraction in sorbitan
2.4. Experimental Design for Optimization
2.5. Capsaicinoid Quantification
2.6. Evaluation of Antioxidant Capacity (ABTS and FRAP) and Total Polyphenolic Content
2.7. Statistical Analysis
3. Results and Discussion
3.1. Sorbitan as a Capsaicinoid Extractor
3.2. Optimization of Capsaicinoid Extraction in Sorbitan
3.2.1. Analysis of the Design and Model Fitting
3.2.2. Capsaicin and Dihydrocapsaicin
3.3. Effect of Particle Size, Temperature, and Concentration of Sorbitan on the Antioxidant Capacity and Polyphenols in Freeze-Dried Serrano Pepper Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moazzen, A.; Öztinen, N.; Ak-Sakalli, E.; Koşar, M. Structure-antiradical activity relationships of 25 natural antioxidant phenolic compounds from different classes. Heliyon 2022, 8, e10467. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, A.; Vasamsetti, B.M.K.; Park, J.-H. A comprehensive review of capsaicin: Biosynthesis, industrial productions, processing to applications, and clinical uses. Heliyon 2024, 10, e39721. [Google Scholar] [CrossRef]
- Thongin, S.; Den-udom, T.; Uppakara, K.; Sriwantana, T.; Sibmooh, N.; Laolob, T.; Boonthip, C.; Wichai, U.; Muta, K.; Ketsawatsomkron, P. Beneficial effects of capsaicin and dihydrocapsaicin on endothelial inflammation, nitric oxide production and antioxidant activity. Biomed. Pharmacother. 2022, 154, 113521. [Google Scholar] [CrossRef]
- Kaur, M.; Verma, B.R.; Zhou, L.; Lak, H.M.; Kaur, S.; Sammour, Y.M.; Kapadia, S.R.; Grimm, R.A.; Griffin, B.P.; Xu, B. Association of pepper intake with all-cause and specific cause mortality—A systematic review and meta-analysis. Am. J. Prev. Cardiol. 2022, 9, 100301. [Google Scholar] [CrossRef] [PubMed]
- Janyou, A.; Wicha, P.; Seechamnanturakit, V.; Bumroongkit, K.; Tocharus, C.; Suksamrarn, A.; Tocharus, J. Dihydrocapsaicin-induced angiogenesis and improved functional recovery after cerebral ischemia and reperfusion in a rat model. J. Pharmacol. Sci. 2020, 143, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Balasubramanian, A.; Pedersen, S.E.; Romero, J.; Pautler, R.G.; Marrelli, S.P. TRPV1-mediated Pharmacological Hypothermia Promotes Improved Functional Recovery Following Ischemic Stroke. Sci. Rep. 2017, 7, 17685. [Google Scholar] [CrossRef]
- Janyou, A.; Wicha, P.; Jittiwat, J.; Suksamrarn, A.; Tocharus, C.; Tocharus, J. Dihydrocapsaicin Attenuates Blood Brain Barrier and Cerebral Damage in Focal Cerebral Ischemia/Reperfusion via Oxidative Stress and Inflammatory. Sci. Rep. 2017, 7, 10556. [Google Scholar] [CrossRef]
- Wu, D.; Zhi, X.; Duan, Y.; Zhang, M.; An, H.; Wei, W.; Dong, K.; Zhang, Y.; Shi, J.; He, X.; et al. Inflammatory cytokines are involved in dihydrocapsaicin (DHC) and regional cooling infusion (RCI)-induced neuroprotection in ischemic rat. Brain Res. 2019, 1710, 173–180. [Google Scholar] [CrossRef]
- Ávila, D.L.; Fernandes-Braga, W.; Silva, J.L.; Santos, E.A.; Campos, G.; Leocádio, P.C.L.; Capettini, L.S.A.; Aguilar, E.C.; Alvarez-Leite, J.I. Capsaicin Improves Systemic Inflammation, Atherosclerosis, and Macrophage-Derived Foam Cells by Stimulating PPAR Gamma and TRPV1 Receptors. Nutrients 2024, 16, 3167. [Google Scholar] [CrossRef]
- Sharma, A.; Devi, L.; Swamy, M.K.; Pandey, D.K. Extraction of Capsaicin and Related Compounds by Using Conventional and Contemporary Technologies. In Capsaicinoids: From Natural Sources to Biosynthesis and Their Clinical Applications; Swamy, M.K., Kumar, A., Eds.; Springer Nature: Singapore, 2024; pp. 113–128. [Google Scholar]
- Castro-Muñoz, R.; Gontarek-Castro, E.; Jafari, S.M. Up-to-date strategies and future trends towards the extraction and purification of Capsaicin: A comprehensive review. Trends Food Sci. Technol. 2022, 123, 161–171. [Google Scholar] [CrossRef]
- Hussain, M.; Qamar, M.T.; Ahmed, D. Microwave- and ultrasound-assisted extraction of capsaicin from Capsicum annuum using deep eutectic solvents. Int. J. Veg. Sci. 2022, 28, 312–319. [Google Scholar] [CrossRef]
- Muvva, S.; M, S.P.; P, P.; N, R.R. A Review on Capsaicin-Methods of Extraction, Estimation and Therapeutic Effects. Int. J. Pharm. Sci. Nanotechnol. (IJPSN) 2023, 16, 6888–6893. [Google Scholar] [CrossRef]
- Li, Z.-h.; Cai, M.; Liu, Y.-s.; Sun, P.-l. Development of finger citron (Citrus medica L. var. sarcodactylis) essential oil loaded nanoemulsion and its antimicrobial activity. Food Control 2018, 94, 317–323. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, N.K.; Srivastava, A.; Kataria, A.; Dubey, S.; Sharma, S.; Kundu, B. Clove and lemongrass oil based non-ionic nanoemulsion for suppressing the growth of plant pathogenic Fusarium oxysporum f.sp. lycopersici. Ind. Crops Prod. 2018, 123, 353–362. [Google Scholar] [CrossRef]
- Akbas, E.; Soyler, B.; Oztop, M.H. Formation of capsaicin loaded nanoemulsions with high pressure homogenization and ultrasonication. LWT 2018, 96, 266–273. [Google Scholar] [CrossRef]
- Marhamati, M.; Ranjbar, G.; Rezaie, M. Effects of emulsifiers on the physicochemical stability of Oil-in-water Nanoemulsions: A critical review. J. Mol. Liq. 2021, 340, 117218. [Google Scholar] [CrossRef]
- Li, F.; Raza, A.; Wang, Y.W.; Xu, X.Q.; Chen, G.H. Optimization of Surfactant-Mediated, Ultrasonic-assisted Extraction of Antioxidant Polyphenols from Rattan Tea (Ampelopsis grossedentata) Using Response Surface Methodology. Pharmacogn. Mag. 2017, 13, 446–453. [Google Scholar] [CrossRef]
- Skrypnik, L.; Novikova, A. Response Surface Modeling and Optimization of Polyphenols Extraction from Apple Pomace Based on Nonionic Emulsifiers. Agronomy 2020, 10, 92. [Google Scholar] [CrossRef]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, energy efficient and green cloud point extraction: Technology and applications in food processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef]
- Azooz, E.A.; Ridha, R.K.; Abdulridha, H.A. The Fundamentals and Recent Applications of Micellar System Extraction for Nanoparticles and Bioactive Molecules: A Review. Nano Biomed. Eng. 2021, 13, 264–278. [Google Scholar] [CrossRef]
- More, P.R.; Arya, S.S. A novel, green cloud point extraction and separation of phenols and flavonoids from pomegranate peel: An optimization study using RCCD. J. Environ. Chem. Eng. 2019, 7, 103306. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; Souza, C.R.F.; Oliveira, W.P. Surfactant Mediated Extraction of Antioxidants from Syzygium aromaticum. Sep. Sci. Technol. 2015, 50, 207–213. [Google Scholar] [CrossRef]
- Haldar, S.; Mishra, H.N.; Majumdar, G.C. Optimization of Oleoresin Extraction from Curcuma longa L. Using RSM and Determination of Equilibrium Constant. J. Food Process. Preserv. 2016, 40, 1188–1198. [Google Scholar] [CrossRef]
- Mokhtar, M.; Russo, M.; Cacciola, F.; Donato, P.; Giuffrida, D.; Riazi, A.; Farnetti, S.; Dugo, P.; Mondello, L. Capsaicinoids and Carotenoids in Capsicum annuum L.: Optimization of the Extraction Method, Analytical Characterization, and Evaluation of its Biological Properties. Food Anal. Methods 2016, 9, 1381–1390. [Google Scholar] [CrossRef]
- Sepúlveda, M.; Costa, J.; Cayún, Y.; Gallardo, V.; Barría, E.; Rigotto Caruso, G.; von Zeska Kress, M.R.; Cornejo, P.; Santos, C. Chemical composition and antifungal activity of Capsicum pepper aqueous extracts against plant pathogens and food spoilage fungi. Front. Cell. Infect. Microbiol. 2024, 14, 1451287. [Google Scholar] [CrossRef]
- Waqas, M.; Ahmed, D.; Qamar, M.T. Surfactant-mediated extraction of capsaicin from Capsicum annuum L. fruit in various solvents. Heliyon 2022, 8, e10273. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Fast determination of capsaicinoids from peppers by high-performance liquid chromatography using a reversed phase monolithic column. Food Chem. 2008, 107, 1276–1282. [Google Scholar] [CrossRef]
- Chinn, M.S.; Sharma-Shivappa, R.R.; Cotter, J.L. Solvent extraction and quantification of capsaicinoids from Capsicum chinense. Food Bioprod. Process. 2011, 89, 340–345. [Google Scholar] [CrossRef]
- Carballo, D.E.; Torres-Hermosillo, S.; Piñón-Gómez, L.; Mendoza, J.; Sánchez-Ramírez, B.; Quíntero-Ramos, A.; Chávez-Flores, D.; Gutiérrez-Méndez, N. Development of food-grade microemulsions (F–G μEms) for green extraction of bioactive compounds from Jalapeño waste (Capsicum annum var. Hot). Sustain. Chem. Pharm. 2025, 43, 101912. [Google Scholar] [CrossRef]
- Gan, C.-Y.; Latiff, A.A. Extraction of antioxidant pectic-polysaccharide from mangosteen (Garcinia mangostana) rind: Optimization using response surface methodology. Carbohydr. Polym. 2011, 83, 600–607. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Rosselló, C.; Teissedre, P.L. Proanthocyanidin composition and antioxidant potential of the stem winemaking byproducts from 10 different grape varieties (Vitis vinifera L.). J. Agric. Food Chem. 2012, 60, 11850–11858. [Google Scholar] [CrossRef]
- ICH. Validation of Analytical Procedures Q2(R2). Complete Revision of Guideline. 2022. Available online: https://database.ich.org/sites/default/files/ICH_Q2-R2_Document_Step2_Guideline_2022_0324.pdf (accessed on 27 January 2025).
- Alvarez-Parrilla, E.; de la Rosa, L.A.; Amarowicz, R.; Shahidi, F. Antioxidant Activity of Fresh and Processed Jalapeño and Serrano Peppers. J. Agric. Food Chem. 2011, 59, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Hayman, M.; Kam, P.C.A. Capsaicin: A review of its pharmacology and clinical applications. Curr. Anaesth. Crit. Care 2008, 19, 338–343. [Google Scholar] [CrossRef]
- Ornelas-Paz, J.d.J.; Martínez-Burrola, J.M.; Ruiz-Cruz, S.; Santana-Rodríguez, V.; Ibarra-Junquera, V.; Olivas, G.I.; Pérez-Martínez, J.D. Effect of cooking on the capsaicinoids and phenolics contents of Mexican peppers. Food Chem. 2010, 119, 1619–1625. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of capsaicinoids from peppers. Talanta 2008, 75, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Ananthan, R.; Subhash, K.; Longvah, T. Capsaicinoids, amino acid and fatty acid profiles in different fruit components of the world hottest Naga king chilli (Capsicum chinense Jacq). Food Chem. 2018, 238, 51–57. [Google Scholar] [CrossRef]
- Sugiyama, R. Capsaicinoids Production and Accumulation in Epidermal Cells on the Internal Side of the Fruit Pericarp in ‘Bhut Jolokia’ (Capsicum chinense). Cytologia 2017, 82, 303–306. [Google Scholar] [CrossRef]
- Cho, H.-S.; Olawuyi, I.F.; Park, J.-J.; Said, N.S.; Na, Y.-j.; Lee, W.-Y. Extraction and recovery of capsaicin from scotch bonnet by alkaline-based deep eutectic solvent. J. Food Meas. Charact. 2024, 18, 1372–1381. [Google Scholar] [CrossRef]
- Gammoudi, N.; Mabrouk, M.; Bouhemda, T.; Nagaz, K.; Ferchichi, A. Modeling and optimization of capsaicin extraction from Capsicum annuum L. using response surface methodology (RSM), artificial neural network (ANN), and Simulink simulation. Ind. Crops Prod. 2021, 171, 113869. [Google Scholar] [CrossRef]
- Nagy, B.; Simándi, B. Effects of particle size distribution, moisture content, and initial oil content on the supercritical fluid extraction of paprika. J. Supercrit. Fluids 2008, 46, 293–298. [Google Scholar] [CrossRef]
- Jahongir, H.; Miansong, Z.; Amankeldi, I.; Yu, Z.; Changheng, L. The influence of particle size on supercritical extraction of dog rose (Rosa canina) seed oil. J. King Saud Univ.-Eng. Sci. 2019, 31, 140–143. [Google Scholar] [CrossRef]
- Shah, N.A.; Prasad, R.V.; Patel, B.B. Optimization of Supercritical Fluid Extraction of Paprika (cv. Reshampatti) Oil, Capsaicin and Pigments. Flavour Fragr. J. 2020, 35, 469–477. [Google Scholar] [CrossRef]
- Santos, P.; Aguiar, A.C.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Supercritical carbon dioxide extraction of capsaicinoids from malagueta pepper (Capsicum frutescens L.) assisted by ultrasound. Ultrason. Sonochemistry 2015, 22, 78–88. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Zaini, A.S.; Yunus, M.A.C.; Machmudah, S.; Idham, Z.b.; Hazwan Ruslan, M.S. Effect of particle size on yield extract and antioxidant activity of peanut skin using modified supercritical carbon dioxide and soxhlet extraction. J. Food Process. Preserv. 2018, 42, e13689. [Google Scholar] [CrossRef]
- Eman, N.A.; Muhamad, K.N.S. Comparison of Moringa Oleifera seeds oil characterization produced chemically and mechanically. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012063. [Google Scholar] [CrossRef]
- Peña-Alvarez, A.; Ramírez-Maya, E.; Alvarado-Suárez, L.Á. Analysis of capsaicin and dihydrocapsaicin in peppers and pepper sauces by solid phase microextraction–gas chromatography–mass spectrometry. J. Chromatogr. A 2009, 1216, 2843–2847. [Google Scholar] [CrossRef]
- Othman, Z.A.A.; Ahmed, Y.B.H.; Habila, M.A.; Ghafar, A.A. Determination of Capsaicin and Dihydrocapsaicin in Capsicum Fruit Samples using High Performance Liquid Chromatography. Molecules 2011, 16, 8919–8929. [Google Scholar] [CrossRef]
- Torstensen, J.; Ottesen, V.; Rodríguez-Fabià, S.; Syverud, K.; Johansson, L.; Lervik, A. The influence of temperature on cellulose swelling at constant water density. Sci. Rep. 2022, 12, 20736. [Google Scholar] [CrossRef]
- Lindman, B.; Karlström, G. Nonionic polymers and surfactants: Temperature anomalies revisited. Comptes Rendus Chim. 2009, 12, 121–128. [Google Scholar] [CrossRef]
- Liang, W.; Lan, Y.; Chen, C.; Song, M.; Xiao, J.; Huang, Q.; Cao, Y.; Ho, C.-T.; Lu, M. Modulating effects of capsaicin on glucose homeostasis and the underlying mechanism. Crit. Rev. Food Sci. Nutr. 2023, 63, 3634–3652. [Google Scholar] [CrossRef]
- Erol, Ü.H.; Gümüş, P.; Arpacı, B.B. Comparative analysis of fatty acid profiles, phytochemical and mineral contents of pepper spice types in Türkiye. Mustafa Kemal Üniversitesi Tarım Bilim. Derg. 2024, 29, 133–147. [Google Scholar] [CrossRef]
- Wang, F.; Xue, Y.; Fu, L.; Wang, Y.; He, M.; Zhao, L.; Liao, X. Extraction, purification, bioactivity and pharmacological effects of capsaicin: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 5322–5348. [Google Scholar] [CrossRef]
- Topuz, A.; Ozdemir, F. Assessment of carotenoids, capsaicinoids and ascorbic acid composition of some selected pepper cultivars (Capsicum annuum L.) grown in Turkey. J. Food Compos. Anal. 2007, 20, 596–602. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Mizobuchi, T.; Kajikawa, R.; Kawashima, H.; Miyabe, F.; Terao, J.; Takamura, H.; Matoba, T. Radical-Scavenging Activity of Vegetables and the Effect of Cooking on Their Activity. Food Sci. Technol. Res. 2001, 7, 250–257. [Google Scholar] [CrossRef]
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Tunchaiyaphum, S.; Eshtiaghi, M.; Yoswathana, N. Extraction of Bioactive Compounds from Mango Peels Using Green Technology. Int. J. Chem. Eng. Appl. 2013, 4, 194–198. [Google Scholar] [CrossRef]
- Li, C.; Meng, X.; Tian, M.; Li, S.; Tian, Y.; Wang, T.; Zhao, C. A Novel Method to Extract Juglone from Juglans mandshurica Waste Branches Using a Water-in-Oil Microemulsion. Waste Biomass Valorization 2022, 13, 1547–1563. [Google Scholar] [CrossRef]
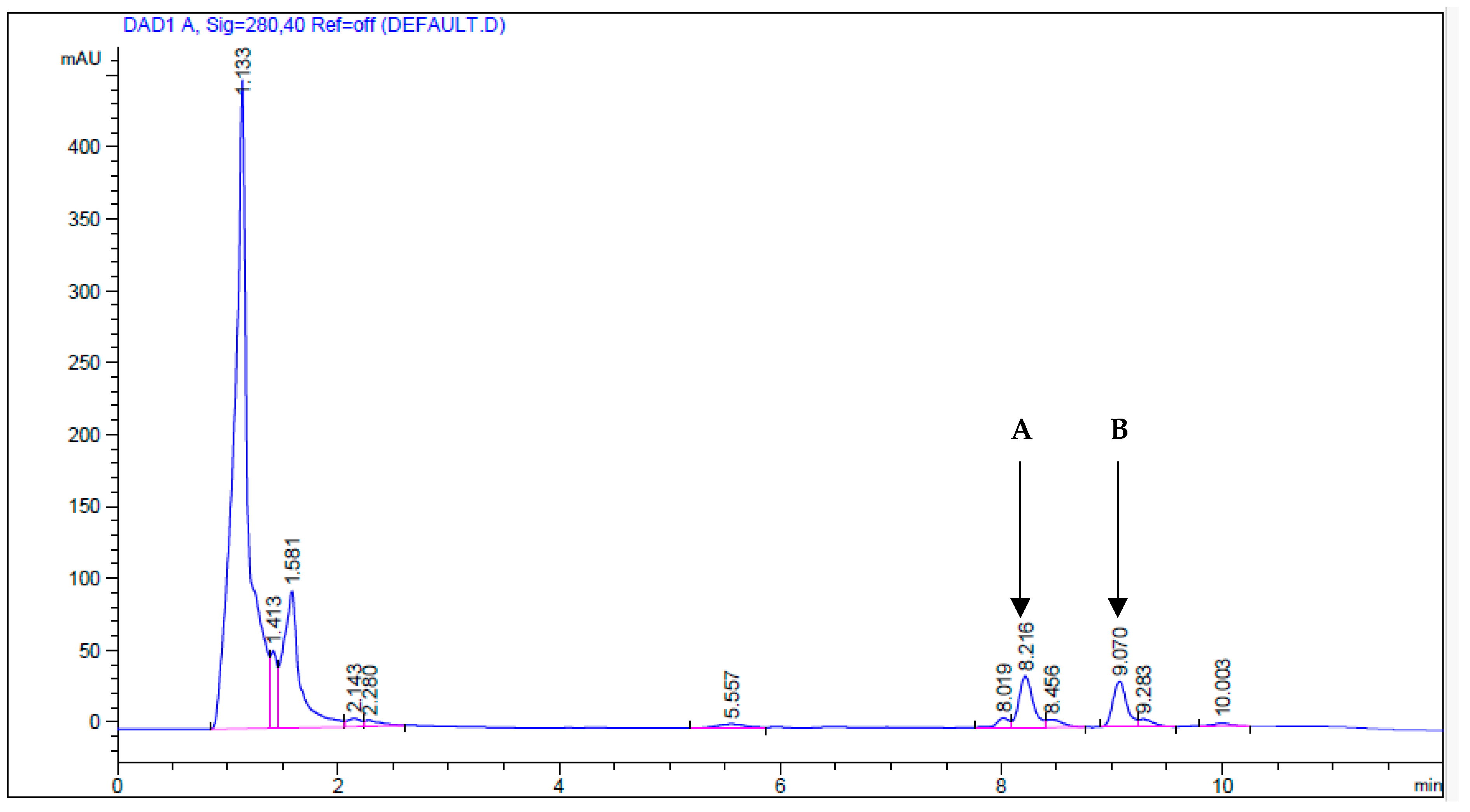
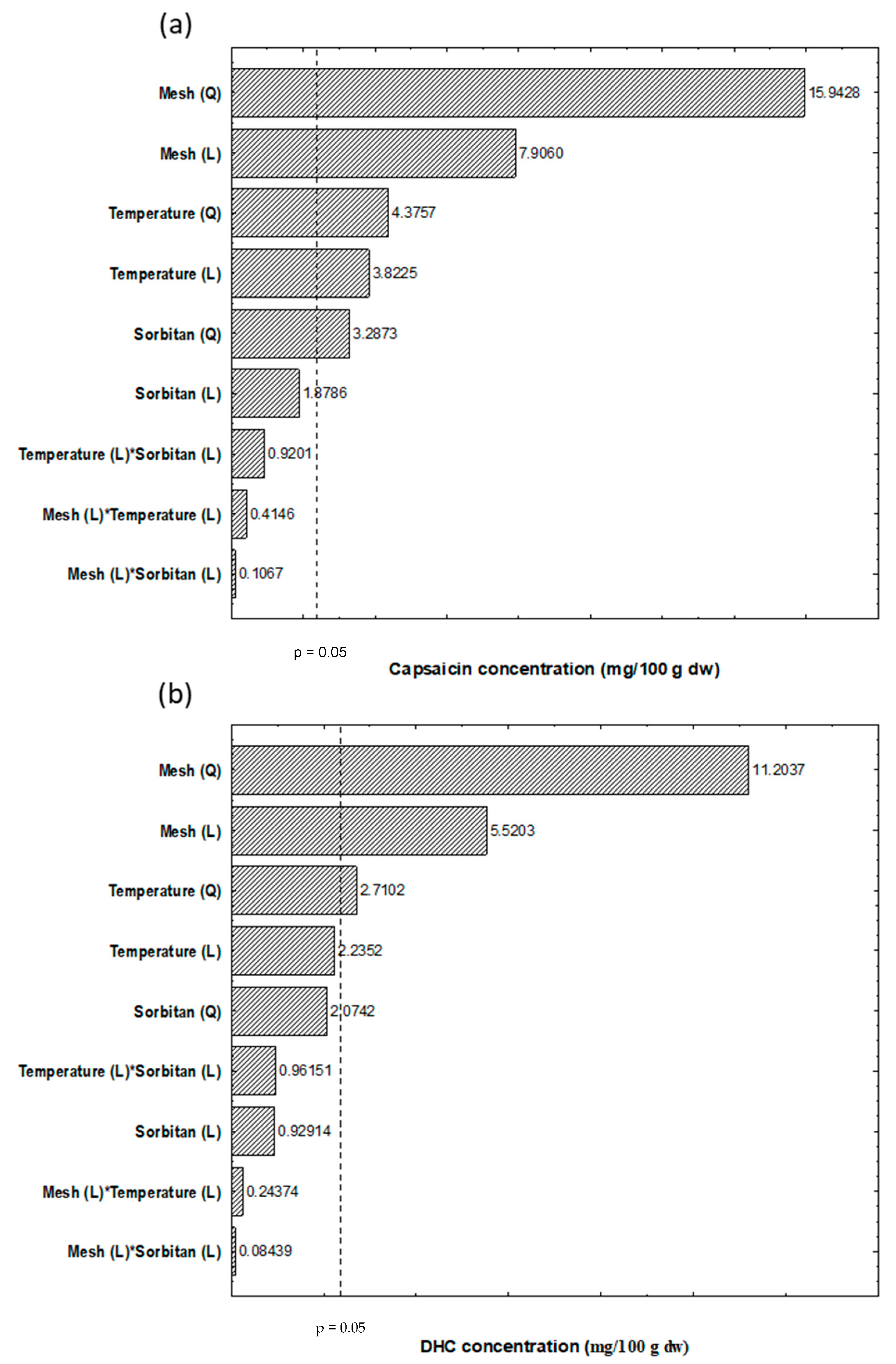

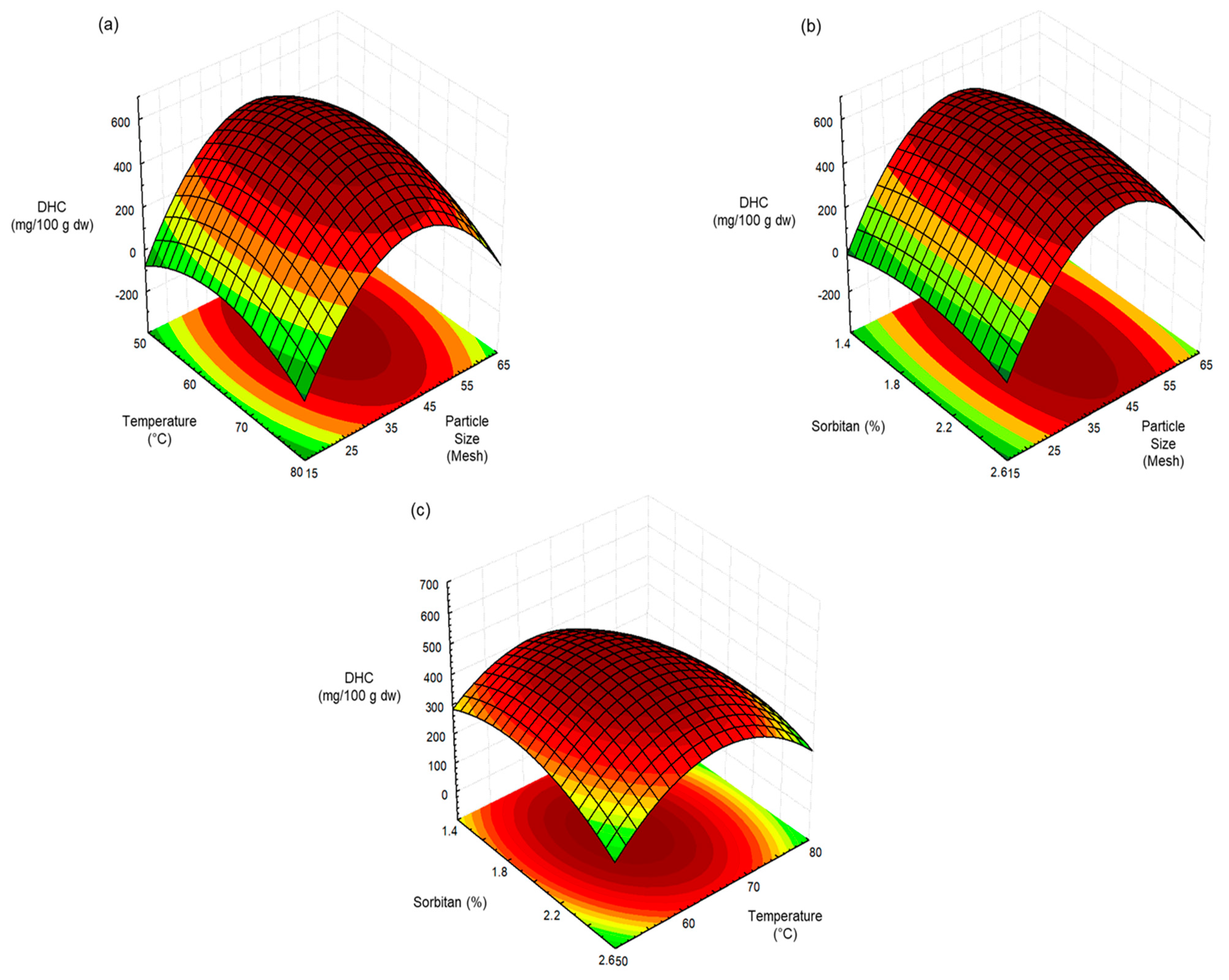
| Capsaicin | Dihydrocapsaicin | |
|---|---|---|
| Correlation coefficient | 0.999 | 0.999 |
| Precision | 8.09% | 7.81% |
| Relative recoveries | 95–105% | 93–102% |
| Detection limit * | 2.4 mg/L | 2.7 mg/L |
| Quantification limit * | 7.2 mg/L | 7.6 mg/L |
| Capsaicin | Dihydrocapsaicin | |
|---|---|---|
| Sorbitan * | 595.01 ± 30.12 b | 553.13 ± 20.50 a |
| Acetonitrile | 558.40 ± 16.37 bc | 494.99 ± 27.69 b |
| Methanol | 686.36 ± 5.010 a | 561.78 ± 23.21 a |
| Acetone | 531.59 ± 21.55 c | 466.13 ± 13.93 b |
| Hexane | 192.33 ± 26.25 d | 121.66 ± 10.39 c |
| Soxhlet ** | 612.26 ± 17.83 b | 564.58 ± 21.67 a |
| Uncoded (Xi) and Coded (xi) Value | Capsaicin | DHC | |||||
|---|---|---|---|---|---|---|---|
| Run | Mesh | Temperature | Sorbitan | Observed | Predicted | Observed | Predicted |
| 1 | 20 (−1) | 55 (−1) | 2.0 (0) | 243.24 | 233.06 | 215.23 | 205.39 |
| 2 | 60 (1) | 55 (−1) | 2.0 (0) | 371.29 | 387.26 | 330.00 | 346.61 |
| 3 | 40 (0) | 55 (−1) | 1.5 (−1) | 590.00 | 566.25 | 553.13 | 528.71 |
| 4 | 40 (0) | 55 (−1) | 2.5 (1) | 514.34 | 532.29 | 456.32 | 473.97 |
| 5 | 20 (−1) | 65 (0) | 1.5 (−1) | 206.46 | 240.39 | 175.22 | 209.47 |
| 6 | 60 (1) | 65 (0) | 1.5 (−1) | 366.60 | 374.38 | 326.27 | 334.08 |
| 7 | 20 (−1) | 65 (0) | 2.5 (1) | 245.74 | 237.97 | 206.96 | 199.15 |
| 8 | 60 (1) | 65 (0) | 2.5 (1) | 414.17 | 380.23 | 366.56 | 332.30 |
| 9 | 40 (0) | 75 (1) | 1.5 (−1) | 503.56 | 485.61 | 451.77 | 434.12 |
| 10 | 20 (−1) | 75 (1) | 2.0 (0) | 220.14 | 204.16 | 188.45 | 171.84 |
| 11 | 60 (1) | 75 (1) | 2.0 (0) | 316.04 | 326.22 | 278.53 | 288.37 |
| 12 | 40 (0) | 75 (1) | 2.5 (1) | 499.24 | 523.00 | 452.36 | 476.77 |
| 13 | 40 (0) | 65 (0) | 2.0 (0) | 651.25 | 671.57 | 580.45 | 596.49 |
| 14 | 40 (0) | 65 (0) | 2.0 (0) | 732.69 | 671.57 | 665.98 | 596.49 |
| 15 | 40 (0) | 65 (0) | 2.0 (0) | 674.72 | 671.57 | 606.10 | 596.49 |
| 16 | 40 (0) | 65 (0) | 2.0 (0) | 634.06 | 671.57 | 511.29 | 596.49 |
| 17 | 40 (0) | 65 (0) | 2.0 (0) | 665.14 | 671.57 | 618.63 | 596.49 |
| Mesh | Temperature (° C) | Sorbitan (%) | TPC | ABTS | FRAP |
|---|---|---|---|---|---|
| 20 | 55 | 2 | 877 ± 108.8 d | 5124 ± 175 f | 3906 ± 94 ef |
| 20 | 65 | 1.5 | 926 ± 240.5 d | 4457 ± 296 g | 3641 ± 481 f |
| 20 | 65 | 2.5 | 1041 ± 233.4 cd | 5262 ± 225 ef | 3699 ± 110 f |
| 20 | 75 | 2 | 1706 ± 101.5 ab | 5715 ± 97 e | 3538 ± 371 f |
| 40 | 55 | 1.5 | 1322 ± 166.5 bc | 7355 ± 127 ab | 5929 ± 206 ab |
| 40 | 55 | 2.5 | 797 ± 18.1 d | 7760 ± 87 a | 4793 ± 860 d |
| 40 | 65 | 2 | 1487 ± 30.0 ab | 7418 ± 103 ab | 5667 ± 524 bc |
| 40 | 75 | 1.5 | 1715 ± 94.2 a | 6427 ± 126 cd | 5577 ± 293 bc |
| 40 | 75 | 2.5 | 1501 ± 223.2 ab | 5712 ± 165 e | 6108 ± 566 ab |
| 60 | 55 | 2 | 935 ± 40.2 d | 7039 ± 155 bc | 5140 ± 378 cd |
| 60 | 65 | 1.5 | 1348 ± 236.2 abc | 6230 ± 152 de | 4255 ± 795 df |
| 60 | 65 | 2.5 | 835 ± 33.9 d | 5980 ± 181 de | 4537 ± 510 de |
| 60 | 75 | 2 | 1374 ± 84.2 abc | 5943 ± 719 de | 4814 ± 219 d |
| Methanol * | 1029 ± 62 cd | 6847 ± 259 bc | 6511 ± 428 a |
| Source | DF | SS | MS | F-Value | p-Value | R2 | Adeq. Precision |
|---|---|---|---|---|---|---|---|
| Capsaicin | |||||||
| Regression | 9 | 496,268 | 55,141 | 36.69 | 0.001 | 0.979 | 27.240 |
| Linear | 3 | 42,210 | 14,070 | 9.36 | 0.008 | ||
| Quadratic | 3 | 452,510 | 150,837 | 100.35 | 0.000 | ||
| Cross-point | 3 | 1548 | 516 | 0.34 | 0.795 | ||
| Lack of fit | 3 | 4915 | 1638 | 1.17 | 0.426 | ||
| DHC | |||||||
| Regression | 9 | 410,979 | 45,664.00 | 17.80 | 0.011 | 0.958 | 41.365 |
| Linear | 3 | 37,503 | 12,501 | 4.87 | 0.039 | ||
| Quadratic | 3 | 370,933 | 123,644 | 48.2 | 0.000 | ||
| Cross-point | 3 | 2542 | 847 | 0.33 | 0.804 | ||
| Lack of fit | 3 | 5029 | 1676 | 0.52 | 0.690 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos-Badillo, A.d.J.; Luna-Zapién, E.A.; Ascacio-Valdés, J.A.; Marszalek, J.E.; Minjares-Fuentes, R.; Quintero-Ramos, A.; Meza-Velázquez, J.A. Use of Sorbitan to Extract Capsaicinoids and Bioactive Compounds: Condition Optimization Study. AppliedChem 2025, 5, 7. https://doi.org/10.3390/appliedchem5020007
Campos-Badillo AdJ, Luna-Zapién EA, Ascacio-Valdés JA, Marszalek JE, Minjares-Fuentes R, Quintero-Ramos A, Meza-Velázquez JA. Use of Sorbitan to Extract Capsaicinoids and Bioactive Compounds: Condition Optimization Study. AppliedChem. 2025; 5(2):7. https://doi.org/10.3390/appliedchem5020007
Chicago/Turabian StyleCampos-Badillo, Andrea de J., Edén A. Luna-Zapién, Juan A. Ascacio-Valdés, Jolanta E. Marszalek, Rafael Minjares-Fuentes, Armando Quintero-Ramos, and Jorge A. Meza-Velázquez. 2025. "Use of Sorbitan to Extract Capsaicinoids and Bioactive Compounds: Condition Optimization Study" AppliedChem 5, no. 2: 7. https://doi.org/10.3390/appliedchem5020007
APA StyleCampos-Badillo, A. d. J., Luna-Zapién, E. A., Ascacio-Valdés, J. A., Marszalek, J. E., Minjares-Fuentes, R., Quintero-Ramos, A., & Meza-Velázquez, J. A. (2025). Use of Sorbitan to Extract Capsaicinoids and Bioactive Compounds: Condition Optimization Study. AppliedChem, 5(2), 7. https://doi.org/10.3390/appliedchem5020007








