Effects of Li Salt and Additive Content on the Electrochemical Performance of [C4C1mim]-Based Ionic Liquid Electrolytes
Abstract
1. Introduction
2. Materials and Methods
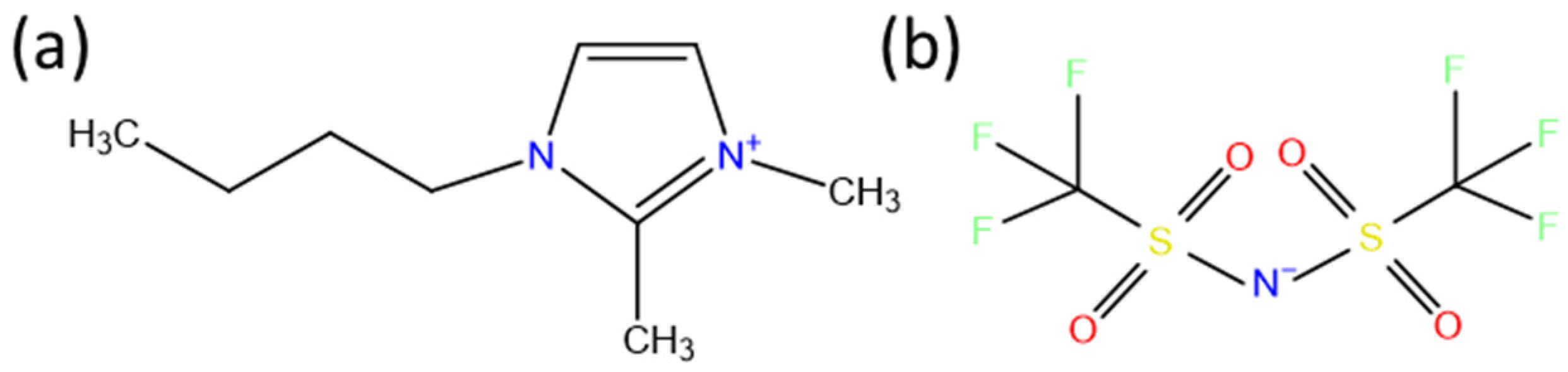
2.1. Synthesis of [C4C1mim][TFSA] IL
2.2. Synthesis of IL-Based Electrolytes
2.3. Physicochemical and Electrochemical Characterization
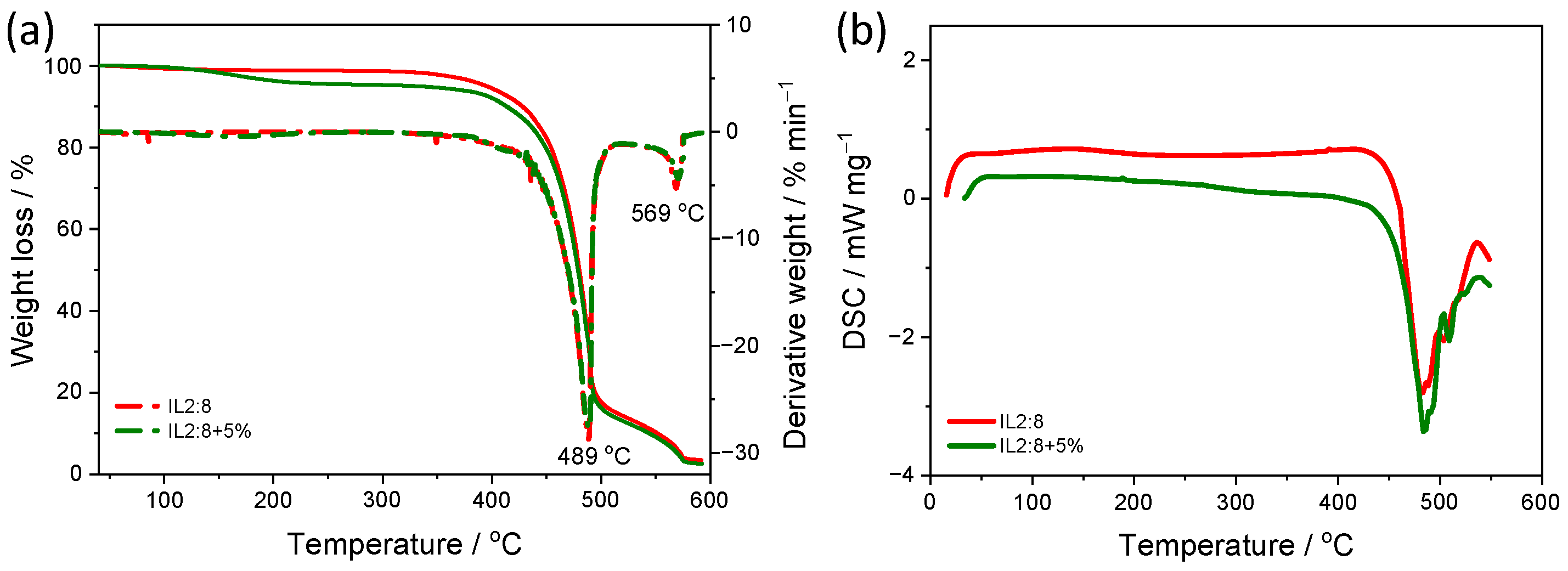
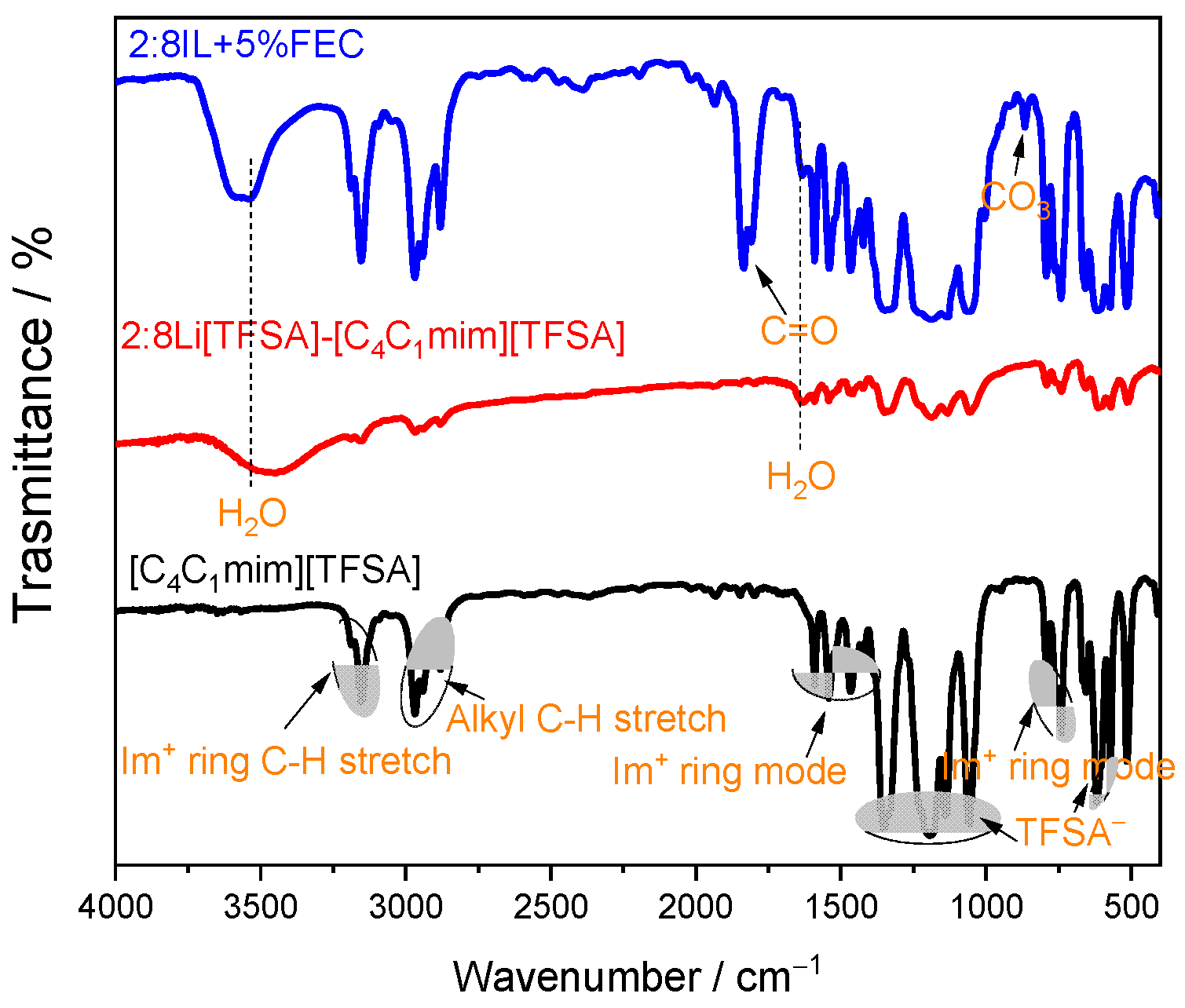
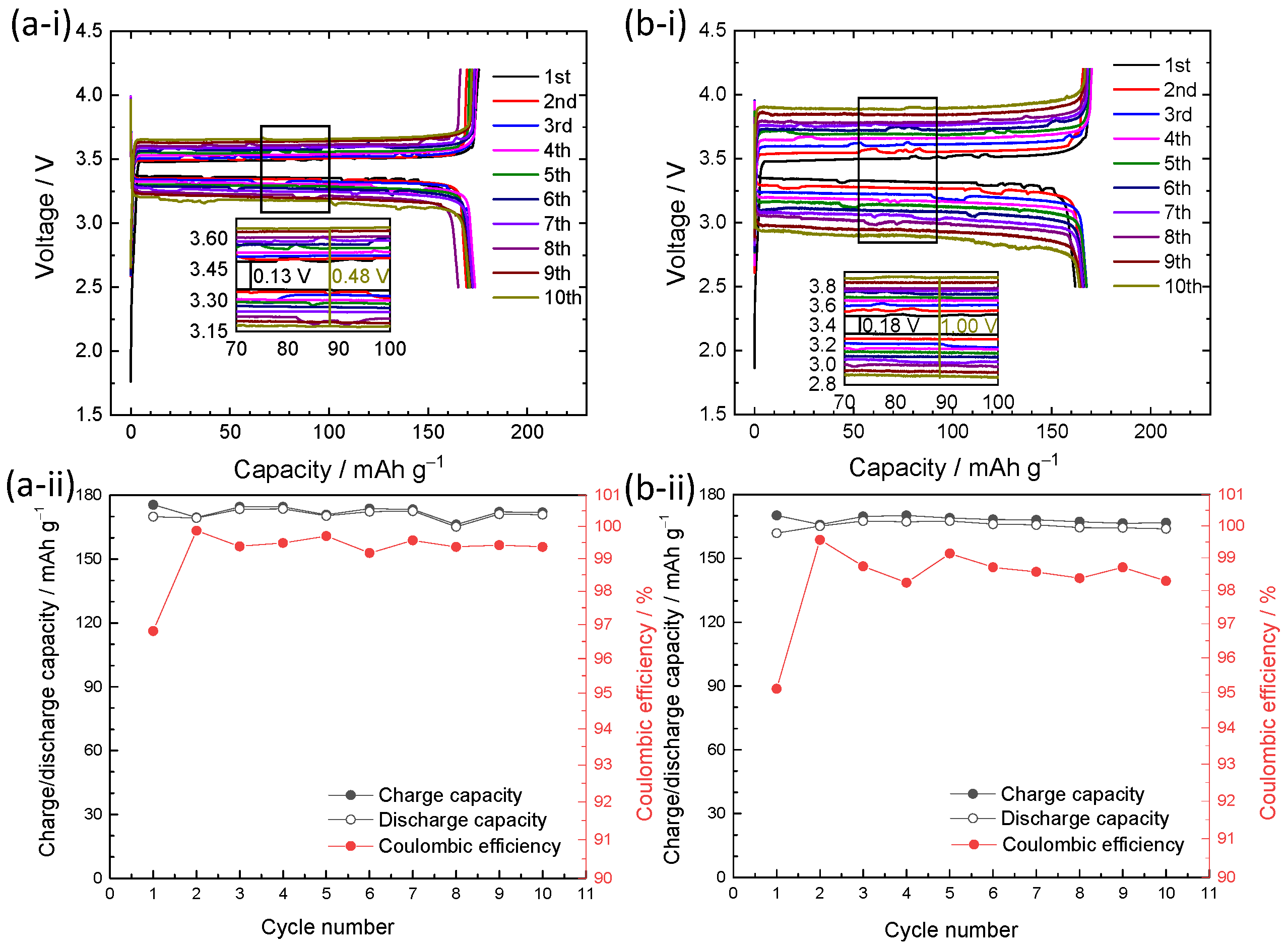
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matuszek, K.; Piper, S.L.; Brzęczek-Szafran, A.; Roy, B.; Saher, S.; Pringle, J.M.; MacFarlane, D.R. Unexpected Energy Applications of Ionic Liquids. Adv. Mater. 2024, 36, 2313023. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, D.; Kaushik, S.; Zhang, S.; Wada, T.; Hwang, J.; Matsumoto, K.; Hagiwara, R. Ionic Liquid Electrolytes for Next-generation Electrochemical Energy Devices. EnergyChem 2022, 4, 100075. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Feng, X.; Wang, L.; Ouyang, M.; Zhang, Q. Thermal stability of ionic liquids for lithium-ion batteries: A review. Renew. Sustain. Energ. Rev. 2025, 207, 114949. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Bi, Z.; Li, Z.; Zhou, F.; Shi, R.; Mu, T. Reviewing electrochemical stability of ionic liquids-/deep eutectic solvents-based electrolytes in lithium-ion, lithium-metal and post-lithium-ion batteries for green and safe energy. Green Energy Environ. 2024, 9, 966–991. [Google Scholar] [CrossRef]
- He, T.; Han, Y.; Shi, B.; Wang, J.; Yang, H. Electrode–electrolyte interphases in lithium-based rechargeable batteries with ionic liquid electrolytes: Recent advances and future perspectives. J. Mater. Chem. A 2024, 12, 32593–32612. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Z.; Shi, L.; Jungsuttiwong, S.; Yuan, S. Ionic liquids for high performance lithium metal batteries. J. Energy Chem. 2021, 59, 320–333. [Google Scholar] [CrossRef]
- Sun, X.-G.; Liao, C.; Shao, N.; Bell, J.R.; Guo, B.; Luo, H.; Jiang, D.-e.; Dai, S. Bicyclic imidazolium ionic liquids as potential electrolytes for rechargeable lithium ion batteries. J. Power Sources 2013, 237, 5–12. [Google Scholar] [CrossRef]
- Gu, J.; Jia, Y.; Ren, X.; Li, S.; Yan, T. The effects of C2-methylation of imidazolium-based ionic liquid electrolytes on the lithium-ion transport. J. Mol. Liq. 2023, 369, 120815. [Google Scholar] [CrossRef]
- Minami, I. Ionic Liquids in Tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef]
- Tang, X.; Lv, S.; Jiang, K.; Zhou, G.; Liu, X. Recent development of ionic liquid-based electrolytes in lithium-ion batteries. J. Power Sources 2022, 542, 231792. [Google Scholar] [CrossRef]
- Mandai, T.; Masu, H.; Imanari, M.; Nishikawa, K. Comparison between Cycloalkyl- and n-Alkyl-Substituted Imidazolium-Based Ionic Liquids in Physicochemical Properties and Reorientational Dynamics. J. Phys. Chem. B 2012, 116, 2059–2064. [Google Scholar] [CrossRef]
- Bazito, F.F.C.; Kawano, Y.; Torresi, R.M. Synthesis and characterization of two ionic liquids with emphasis on their chemical stability towards metallic lithium. Electrochim. Acta 2007, 52, 6427–6437. [Google Scholar] [CrossRef]
- Noorhisham, N.A.; Amri, D.; Mohamed, A.H.; Yahaya, N.; Ahmad, N.M.; Mohamad, S.; Kamaruzaman, S.; Osman, H. Characterisation techniques for analysis of imidazolium-based ionic liquids and application in polymer preparation: A review. J. Mol. Liq. 2021, 326, 115340. [Google Scholar] [CrossRef]
- Bazito, F.F.C.; Silveira, L.T.; Torresi, R.M.; Córdoba de Torresi, S.I. Spectroelectrochemical study of a soluble derivative of poly(aniline) in a room temperature ionic liquid. Electrochim. Acta 2007, 53, 1217–1224. [Google Scholar] [CrossRef]
- Wang, Y.; Zaghib, K.; Guerfi, A.; Bazito, F.F.C.; Torresi, R.M.; Dahn, J.R. Accelerating rate calorimetry studies of the reactions between ionic liquids and charged lithium ion battery electrode materials. Electrochim. Acta 2007, 52, 6346–6352. [Google Scholar] [CrossRef]
- Schmitz, P.; Kolek, M.; Pyschik, M.; Jalkanen, K.; Nowak, S.; Winter, M.; Bieker, P. Modified Imidazolium-Based Ionic Liquids With Improved Chemical Stability Against Lithium Metal. ChemistrySelect 2017, 2, 6052–6056. [Google Scholar] [CrossRef]
- Sutto, T.E. The Electrochemical Behavior of Trialkylimidazolium Imide Based Ionic Liquids and Their Polymer Gel Electrolytes. J. Electrochem. Soc. 2007, 154, P130–P135. [Google Scholar] [CrossRef]
- Nadherna, M.; Dominko, R.; Hanzel, D.; Reiter, J.; Gaberscek, M. Electrochemical Behavior of Li2FeSiO4 with Ionic Liquids at Elevated Temperature. J. Electrochem. Soc. 2009, 156, A619–A626. [Google Scholar] [CrossRef]
- Borges, R.S.; Ribeiro, H.; Lavall, R.L.; Silva, G.G. Temperature stable supercapacitors based on ionic liquid and mixed functionalized carbon nanomaterials. J. Solid State Electrochem. 2012, 16, 3573–3580. [Google Scholar] [CrossRef]
- Wang, G.; Xiong, X.; Xie, D.; Fu, X.; Lin, Z.; Yang, C.; Zhang, K.; Liu, M. A Scalable Approach for Dendrite-Free Alkali Metal Anodes via Room-Temperature Facile Surface Fluorination. ACS Appl. Mater. Interfaces 2019, 11, 4962–4968. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Huang, X.; He, X. Lithium Bis(Trifluoromethanesulfonyl)Imide (LiTFSI): A Prominent Lithium Salt in Lithium-Ion Battery Electrolytes—Fundamentals, Progress, and Future Perspectives. Adv. Funct. Mater. 2024, 34, 2408319. [Google Scholar] [CrossRef]
- Seki, S.; Ohno, Y.; Kobayashi, Y.; Miyashiro, H.; Usami, A.; Mita, Y.; Tokuda, H.; Watanabe, M.; Hayamizu, K.; Tsuzuki, S.; et al. Imidazolium-Based Room-Temperature Ionic Liquid for Lithium Secondary Batteries: Effects of Lithium Salt Concentration. J. Electrochem. Soc. 2007, 154, A173–A177. [Google Scholar] [CrossRef]
- Haregewoin, A.M.; Wotango, A.S.; Hwang, B.-J. Electrolyte additives for lithium ion battery electrodes: Progress and perspectives. Energy Environ. Sci. 2016, 9, 1955–1988. [Google Scholar] [CrossRef]
- Mousa, A.O.; Chuang, C.-H.; Kuo, S.-W.; Mohamed, M.G. Strategic Design and Synthesis of Ferrocene Linked Porous Organic Frameworks toward Tunable CO2 Capture and Energy Storage. Int. J. Mol. Sci. 2023, 24, 12371. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.M.; Awad, W.H.; Gilman, J.W.; Maupin, P.H.; De Long, H.C.; Trulove, P.C. Flammability, thermal stability, and phase change characteristics of several trialkylimidazolium salts. Green Chem. 2003, 5, 724–727. [Google Scholar] [CrossRef]
- Rajkumar, T.; Ranga Rao, G. Synthesis and characterization of hybrid molecular material prepared by ionic liquid and silicotungstic acid. Mater. Chem. Phys. 2008, 112, 853–857. [Google Scholar] [CrossRef]
- Agafonov, A.V.; Ramenskaya, L.M.; Grishina, E.P.; Kudryakova, N.O. Cation effects on the properties of halloysite-confined bis(trifluoromethylsulfonyl)imide based ionic liquids. RSC Adv. 2021, 11, 38605–38615. [Google Scholar] [CrossRef] [PubMed]
- Rathika, R.; Suthanthiraraj, S.A. Influence of 1-ethyl-3-methylimidazolium bis (trifluoromethyl sulfonyl) imide plasticization on zinc-ion conducting PEO/PVdF blend gel polymer electrolyte. J. Mater. Sci. Mater. Electron. 2018, 29, 19632–19643. [Google Scholar] [CrossRef]
- Tang, W.-J.; Peng, W.-J.; Yan, G.-C.; Guo, H.-J.; Li, X.-H.; Zhou, Y. Effect of fluoroethylene carbonate as an electrolyte additive on the cycle performance of silicon-carbon composite anode in lithium-ion battery. Ionics 2017, 23, 3281–3288. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Li, H.; Chen, J.; Lei, D.; Wang, C. A dual-function liquid electrolyte additive for high-energy non-aqueous lithium metal batteries. Nat. Commun. 2022, 13, 1297. [Google Scholar] [CrossRef]
- Chan, K.L.A.; Shalygin, A.S.; Martyanov, O.N.; Welton, T.; Kazarian, S.G. High throughput study of ionic liquids in controlled environments with FTIR spectroscopic imaging. J. Mol. Liq. 2021, 337, 116412. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nishiwaki, E.; Hosokawa, T.; Tawa, S.; Nohira, T.; Hagiwara, R. Thermal, Physical, and Electrochemical Properties of Li[N(SO2F)2]-[1-Ethyl-3-methylimidazolium][N(SO2F)2] Ionic Liquid Electrolytes for Li Secondary Batteries Operated at Room and Intermediate Temperatures. J. Phys. Chem. C 2017, 121, 9209–9219. [Google Scholar] [CrossRef]
- Bandara, T.M.W.J.; Fernando, H.D.N.S.; Furlani, M.; Albinsson, I.; Ratnasekera, J.L.; DeSilva, L.A.; Dissanayake, M.A.K.L.; Mellander, B.E. Combined effect of alkaline cations and organic additives for iodide ion conducting gel polymer electrolytes to enhance efficiency in dye sensitized solar cells. Electrochim. Acta 2017, 252, 208–214. [Google Scholar] [CrossRef]
- Kufian, M.Z.; Majid, S.R. Performance of lithium-ion cells using 1 M LiPF6 in EC/DEC (v/v = 1/2) electrolyte with ethyl propionate additive. Ionics 2009, 16, 409–416. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Lin, W.; Wei, B.; Chen, D. A Concentrated Electrolyte of LiTFSI and Dimethyl Carbonate for High-Voltage Li Batteries. ACS Appl. Energy Mater. 2023, 6, 9337–9346. [Google Scholar] [CrossRef]
- Deshpande, A.; Kariyawasam, L.; Dutta, P.; Banerjee, S. Enhancement of Lithium Ion Mobility in Ionic Liquid Electrolytes in Presence of Additives. J. Phys. Chem. C 2013, 117, 25343–25351. [Google Scholar] [CrossRef]
- Bayley, P.M.; Lane, G.H.; Rocher, N.M.; Clare, B.R.; Best, A.S.; MacFarlane, D.R.; Forsyth, M. Transport properties of ionic liquid electrolytes with organic diluents. Phys. Chem. Chem. Phys. 2009, 11, 7202–7208. [Google Scholar] [CrossRef]
- Qi, X.; Cai, D.; Wang, X.; Xia, X.; Gu, C.; Tu, J. Ionic Liquid-Impregnated ZIF-8/Polypropylene Solid-like Electrolyte for Dendrite-free Lithium-Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 6859–6868. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, L.; Li, Y.; An, Y.; Zhu, M.; Dai, B. Mechanism studies of LiFePO4 cathode material: Lithiation/delithiation process, electrochemical modification and synthetic reaction. RSC Adv. 2014, 4, 54576–54602. [Google Scholar] [CrossRef]
- Kumar, S.; Chand, P.; Kumar, A.; Anand, H. Effect of different aqueous electrolytes on electrochemical behavior of LiFePO4 as a cathode material: Lithium ion battery and renewable energy nexus. Energy Nexus 2021, 1, 100005. [Google Scholar] [CrossRef]
- Zane, D.; Carewska, M.; Scaccia, S.; Cardellini, F.; Prosini, P.P. Factor affecting rate performance of undoped LiFePO4. Electrochim. Acta 2004, 49, 4259–4271. [Google Scholar] [CrossRef]
- Berhaut, C.L.; Lemordant, D.; Porion, P.; Timperman, L.; Schmidt, G.; Anouti, M. Ionic association analysis of LiTDI, LiFSI and LiPF6in EC/DMC for better Li-ion battery performances. RSC Adv. 2019, 9, 4599–4608. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wei, S.; Li, S.; Li, Q.; Lu, Y. Recent Progress of the Solid-State Electrolytes for High-Energy Metal-Based Batteries. Adv. Energy Mater. 2018, 8, 1702657. [Google Scholar] [CrossRef]
- Ubong, E.U.; Dimitrov, B. Regression of the Response Variable of a High Temperature PEMFC–PBI Membrane. J. Electrochem. Soc. 2010, 157, B1059–B1067. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Zhou, W.; Cheng, K.; Chen, Z. Effects of Li Salt and Additive Content on the Electrochemical Performance of [C4C1mim]-Based Ionic Liquid Electrolytes. AppliedChem 2025, 5, 6. https://doi.org/10.3390/appliedchem5010006
Zheng Y, Zhou W, Cheng K, Chen Z. Effects of Li Salt and Additive Content on the Electrochemical Performance of [C4C1mim]-Based Ionic Liquid Electrolytes. AppliedChem. 2025; 5(1):6. https://doi.org/10.3390/appliedchem5010006
Chicago/Turabian StyleZheng, Yayun, Wenbin Zhou, Kui Cheng, and Zhengfei Chen. 2025. "Effects of Li Salt and Additive Content on the Electrochemical Performance of [C4C1mim]-Based Ionic Liquid Electrolytes" AppliedChem 5, no. 1: 6. https://doi.org/10.3390/appliedchem5010006
APA StyleZheng, Y., Zhou, W., Cheng, K., & Chen, Z. (2025). Effects of Li Salt and Additive Content on the Electrochemical Performance of [C4C1mim]-Based Ionic Liquid Electrolytes. AppliedChem, 5(1), 6. https://doi.org/10.3390/appliedchem5010006





