Abstract
The Pharmaceutical Strategy for Europe addresses the environmental implications at all stages of the life cycle of pharmaceuticals, from design and production through use to disposal. In the last decade, “green chemistry” has transformed pharmaceuticals by promoting sustainability and reducing environmental impact. This review discusses the latest developments in green chemistry approaches, which are applied in drug design and production, including the concepts, innovative techniques, and methodologies. This review is notably built on over 80 documents and demonstrates the practical application of green chemistry principles in pharmaceutical synthesis, emphasizing successful implementation and the environmental benefits achieved. Therefore, this review discusses the positive changes brought by green chemistry to pharmaceutical production and highlights the need for further research in designing and manufacturing “greener” substances, as well as in pollution abatement.
1. Introduction
European industry persists in the production and consumption of substantial quantities of dangerous chemical chemicals, which are subsequently discharged into the environment and may present health concerns. Simultaneously, Europe has some of the most stringent chemical regulations and the most ambitious programs worldwide, exemplified by the EU Chemicals Strategy [1] and the Zero Pollution Action Plan [2]. Industrial chemicals are regulated through the REACH regulations [3]. Other laws exist on specific products, such as biocides, pesticides, cosmetics, and pharmaceuticals [4].
The European Pharmaceutical Strategy seeks to bolster the sector in advancing research and technology that meet patients’ treatment demands while rectifying market failures. The plan complements the European Green Deal, particularly the Zero Pollution objective, which targets the environmental effect of medicinal chemicals. The pharmaceutical strategy is essential for allowing the sector to aid the EU’s climate neutrality objective by concentrating on the reduction of greenhouse gas emissions throughout the supply chain. The strategy further supports the action plan for executing the European Pillar of Social Rights and the strategic frameworks aimed at establishing a Union of Equality. It endorses the plan for defining Europe’s digital future, the European data strategy, the initiative to establish a European health data space, the European One Health Action Plan addressing antibiotic resistance, and the new European industrial strategy.
The manufacturing, use, and disposal of pharmaceuticals have environmental ramifications, since residues and debris may infiltrate the ecosystem. This approach is harmful to the environment and may produce trash and residues with endocrine-disrupting qualities, as well as others that might elevate the likelihood of antibiotic resistance. The presence of antimicrobial agents in soil and water might expedite the formation of resistant microbes. Actions must be implemented across the whole lifespan of pharmaceuticals to optimize resource consumption and emissions while decreasing the concentration of pharmaceutical residues in the environment. Overall exposure to such residues should be minimized to the greatest extent feasible. A considerable volume of trash continues to be produced due to unused pharmaceuticals, and the manufacturing, use, and disposal of medications have environmental consequences, since residues and waste materials may infiltrate the ecosystem. The European Commission recently adopted new guidelines on the separate collection of household hazardous waste, which includes pharmaceuticals [5]. Further waste-limiting measures should also be considered. The pharmaceutical industry should be driven by innovation to develop environmentally sustainable and climate-neutral pharmaceuticals and manufacturing. It should apply the best available techniques at the manufacturing level to reduce emissions and contribute to the EU’s climate ambition throughout its value chain.
Green chemistry is becoming more popular in the scientific community, due to its ability to tackle chemical innovation and achieve both economic and environmental objectives at the same time.
The concept of “green chemistry” arose in the early 1990s and was defined by Paul Anastas and John Warner [6]. Thus, green chemistry is interpreted as the “design of chemical products and processes that reduce or eliminate the use and generation of hazardous substances” [7]. Green chemistry is also known as sustainable chemistry and focuses on technological methods to reduce pollution and the consumption of non-renewable resources; even though it is primarily committed to a more sustainable approach to the environment, it also works on low-cost methods that have better results and greater effectiveness [8].
The development of the green chemistry concept was prompted by the progressive pollution of the environment, to which the chemical industry contributes significantly. To counteract this negative trend, in 1998, Paul Anastas and John Warner formulated 12 principles of green chemistry to make chemical production less harmful to nature and humans. Consequently, “green chemistry” is not a branch of chemistry, it is a code of conduct based on 12 dedicated principles, aiming to reduce the environmental impact of any chemical process at all stages of the life cycle of pharmaceuticals, from design and production through use to disposal.
Today, the development of green chemistry has grown significantly in various industry sectors: aerospace, automobiles, cosmetics, electronics, energy, household products, agriculture, and pharmaceuticals [9].
According to the concept of the E-factor introduced by Roger Sheldon [10], pharmaceutical industries have some of the highest E-Factors, often ranging from 25 to over 100, meaning that for every 1 kg of drug produced, 25 to 100 kg of waste is generated. It was shown that the pharmaceutical industry produces a lot of waste because of its use of solvents. In the pharmaceutical industry, solvents make up between 80 and 90 percent of the total mass used in the manufacturing processes of fine chemicals and pharmaceuticals [11].
2. Materials and Methods
A systematic approach was applied to identify those academic publications that are relevant to green chemistry and sustainable synthesis. The study encompasses over 80 publications from 2000 to 2024, incorporating both early foundational works and contemporary advancements in the field, with a few notable exceptions for older works that present noteworthy data. This period of time has resulted in substantial advancements following the formalization of the 12 principles of green chemistry and the increasing worldwide focus on sustainability in chemical processes.
A thorough exploration of academic databases, including Scopus, the Web of Science, PubMed, and ScienceDirect, was conducted. These sites were chosen for their extensive coverage of environmental research, chemical sciences, and multidisciplinary sustainability studies. The search was performed using a variety of keywords and phrases. The green chemistry terminology sought in these studies included “sustainable synthesis”, “green solvents”, “catalyzed atom economy”, “atom economy”, “biobased chemicals”, and “renewable feedstocks”. Boolean operators were used in conjunction with truncation and phrase searching. This guaranteed complete retrieval, permitting the incorporation of variations in terminology and spelling.
A two-stage screening process was implemented, where the articles were first searched using databases, analyzing their titles and abstracts to identify relevant data. Afterward, in the second stage, full-text analysis was conducted to ensure that all selection criteria were met.
Studies meeting the following criteria were selected: written in English and published in peer-reviewed journals, and with direct relevance to green pharmaceutical synthesis. The articles that were eliminated were ones that only briefly mentioned these subjects or exclusively focused on traditional synthesis without addressing sustainability or environmental effects. The findings of the research unequivocally revealed the development of, and ongoing challenges in, the area of sustainable chemical practices, thereby providing a clear foundation for further research and implementation.
3. The 12 Principles of Green Chemistry
Back in 1998, the first suggestions for moving toward green synthetic processes were announced by John Warner, president of the Warner Babcock Institute for Green Chemistry, and Paul Anastas of the US Environmental Protection Agency. They called these the 12 principles of green chemistry. The framework for designing new chemical products and processes is now based on these principles. All aspects of the processing life cycle are covered, from the raw materials used to the toxicity and biodegradability of products and reagents [12].
These 12 principles (Table 1), which have remained relevant over the years, include the following requirements for newly developed chemical processes and products.

Table 1.
The 12 principles of green chemistry.
4. Green Chemistry Approaches in Pharmaceutical Synthesis
Green chemistry approaches (Figure 1) are essential in pharmaceutical synthesis because they reduce production costs and use energy more efficiently while making processes sustainable for the environment.
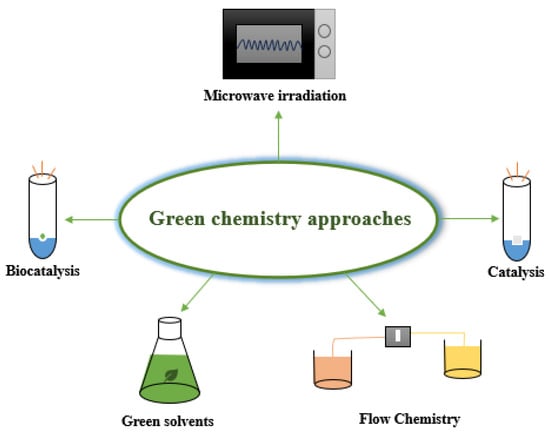
Figure 1.
Green chemistry approaches.
4.1. Microwave-Assisted Technique
The microwave-assisted technology for green synthesis is a new and promising economical and energy-efficient method that is becoming more and more popular in pharmaceutical settings [26].
By using microwave irradiation as an alternative energy source, various organic reactions can be finished in minutes rather than taking hours or even days. Microwave heating is the process of converting microwave energy into heat energy by the interaction of molecules with alternating electromagnetic radiation. This radiation has wavelengths ranging from 0.001 to 1 m and frequencies between 0.3 and 300 GHz. The process of heating can be facilitated by two primary mechanisms: ionic conduction and dipole polarization [27,28,29].
Drug substance synthesis under microwave irradiation is primarily contingent upon the reaction medium’s capacity to absorb microwave energy effectively and on the solvent system chosen to carry out the synthetic reaction [30].
In the field of organic synthesis, polar organic solvents such as DMF, DMA, DMSO, NMP, methanol, ethanol, and acetic acid are preferred due to their polarity. Also, solvents with a high boiling point are preferred for this type of reaction, due to the possibility of a significant increase in reaction rate [31]. Conversely, the use of non-polar solvents, including toluene, dioxane, and THF, is only feasible under conditions where other components in the reaction mixture react to microwave energy, the reason being that nonpolar molecules are inert to MW dielectric loss [32].
The benefits of utilizing MW heating methods for chemical synthesis have been documented in numerous studies, including “rapid volumetric heating, fast reaction rate, selectivity, low time, low cost, and good product yield” [32,33,34,35,36].
In the study conducted by Gopinadh Meera and her colleagues, five-membered nitrogen heterocycles, namely, pyrroles, pyrrolidines, fused pyrazoles, fused isoxazoles, and indoles, were synthesized using the microwave-assisted synthesis method. The results showed that in comparison with other synthetic techniques, microwave-assisted protocols were found to produce cleaner results with a shorter reaction time, higher purity to the final compound, and a higher yield [37].
Bimal Krishna Banik and his colleagues reported that synthesizing different heterocyclic compounds, such as oxadiazole derivatives, under microwave irradiation offers several benefits, including a remarkably short reaction time, high product yields, and an easy purification procedure, when compared to traditional synthetic methods. Additionally, the volume of solvents used during a chemical reaction is reduced, which makes it an ecologically sustainable synthetic approach [31].
In the study conducted by Ivan Ristić and his team, a microwave-assisted method was effectively used to manufacture sodium alginate-chitosan hydrogels, indicating an environmentally benign and sustainable approach [38].
Even though the microwave-assisted technique is more commonly used in pharmaceutical synthesis, it also plays an important role in the management of pharmaceutical waste. Microwave radiation with special energy excitation was utilized by Tang’s group to post-process a succession of MnOx samples to create oxygen vacancies. These vacancies increase the oxygen’s reducibility and storage capacity, which speeds up the breakdown of air contaminants. They reported that the microwave-synthesized MnCo2O4.5 had higher activity than the samples made using the traditional hydrothermal process because more active areas with lots of oxygen vacancies were produced by the microwave irradiation’s hotspot effect [39].
Mn-based catalysts with oxygen vacancies are very useful for managing pharmaceutical waste sustainably because they can efficiently degrade contaminants in the air via sophisticated oxidation processes (such as catalytic ozonation and Fenton-like reactions). As a result, the microwave-assisted approach is effective not only for medicine manufacturing but also for the safe degradation of pharmaceuticals.
Based on these findings, microwave heating is one of the most straightforward, affordable, and useful techniques used in applied medicinal chemistry. This environmentally friendly method increases efficiency while reducing waste and chemical use. In addition to saving energy, this green technology encourages new sustainable, high-throughput approaches for novel drug production, as well as quicker and more selective transformations [26]. Although this microwave-assisted technique comes with scale-up issues due to the particular limitations of microwave heating in large-scale experiments, numerous studies have demonstrated successful strategies for overcoming these problems [40,41] This makes microwave technology a valuable tool for enhancing pharmaceutical synthesis processes.
4.2. Catalysis
Catalysis is a fundamental component of chemical production, steering the synthesis of complex compounds and promoting environmentally friendly production methods [42].
Catalytic reagents allow for site-specific transformations and the diastereomeric control of multifunctional compounds, which can enhance product selectivity. Additionally, catalysts enable reactions to proceed under milder conditions, and catalytic techniques can avoid the need to pre-functionalize the starting materials with directing or activating groups that would subsequently need to be eliminated from the finished molecule.
In the pharmaceutical industry, transition metal-catalyzed cross-coupling reactions are frequently employed for both drug production and medicinal chemistry [43,44,45].
However, a lot of catalytic processes depend on elements that are costly, potentially toxic, or erratic in supply, or those that may be hazardous or have a large environmental impact during extraction and isolation [46]; palladium-catalyzed cross-coupling reactions could be used as an example [47,48,49,50].
These reactions show great results; for example, in one study, abemaciclib, a CDK 4/6 inhibitor used to treat metastatic breast cancer with the particular mutation HR+/HER2 (triple-positive breast cancer) [51], was synthesized utilizing a Suzuki cross-coupling reaction [52].
Regardless, because platinum is a rare and crucial raw material with concerns over supply chain stability, other elements could be used. Strategies employing non-precious metal catalysts might offer a supplementary approach.
Nickel [53,54,55], copper [56,57], and iron [58,59] catalysts are especially appealing for the following reasons: cost, toxicity, reactivity, and environmental sustainability. With the aim of using nickel-based catalysts that are cost-effective and ecologically benign for these processes, Garg and colleagues have demonstrated the efficiency of the Suzuki–Miyaura cross-coupling reaction of aryl halides and phenolic derivatives catalyzed by nicotinamide in green solvents. This reaction yields biaryl products with excellent synthetic utility [60].
In the last century, the identification of β-lactams, a widely used class of antibiotics, has prompted the development of numerous synthesis strategies.
Recent studies have indicated that photo- and transition metal catalysis processes are emerging as novel strategies for the synthesis of β-lactams [61].
In a study conducted by Oddy et al., a methodology was proposed in which the transfer of visible light energy is utilized to facilitate the process of intramolecular hydrogen atom transfer, thereby resulting in the formation of β-lactam from acrylamide precursors. This approach offers a mild, efficient, and atom-economical route to β-lactam production [62].
Another relevant structural component of pharmaceuticals is N-heterocycles. In a previous study, green catalytic methods, including metal-catalyzed acceptorless coupling and one-pot reactions, were presented for the synthesis of N-heterocycles. These methods used recyclable catalysts, prevented hazardous waste, and improved atom economy [63].
4.3. Biocatalysis
In recent years, biocatalysis (enzymatic catalysis) has become prominent as a sustainable technique [64,65].
Biocatalysis is defined as the use of natural molecules, such as enzymes derived from biological sources or complete cells, to accelerate chemical reactions [66]. This technology’s key features include the use of green reaction media, such as water, higher selectivity, and no product contamination by trace metals [67]. This technique has been used effectively in the industrial synthesis of active pharmaceutical ingredients (API) [64,68].
For instance, the production of several valuable chiral medicines can be achieved through biocatalysis. Examples include sitagliptin (Januvia), atorvastatin (Lipitor), rosuvastatin (Crestor), and montelukast (Singulair) [69]. The economically viable use of biocatalysis on an industrial scale is exemplified by the enzyme-catalyzed synthesis of sitagliptin, a medication marketed by Merck for the treatment of type II diabetes [70,71].
This approach resulted in a 10% increase in yield and a 53% increase in productivity. This significant outcome eliminates the need for a rare heavy metal-based catalyst, which requires purification and specialized equipment for high-pressure operation [72]. In another study, Codexis and Merck invested significantly in research to scale up a monoamine oxidase (MAO)-catalyzed process for the enantioselective desymmetrization of a bicyclic proline intermediate. This intermediate is an important precursor in the synthesis of boceprevir, a non-structural protein 3 (NS3) protease inhibitor used to treat chronic hepatitis C [73]. This method was shown to be a good alternative, reducing operation time and waste generation [74]. Another biocatalytic approach is the biosynthetic method of manufacturing simvastatin, an important drug for lowering cholesterol levels in adults [75].
The key advantages of this process are continuous processing via reactors, which keep the compounds for a short time, the reduction of risk and waste, and affordability [76].
Its use has also significantly reduced the manufacturing footprint, allowing for the efficient processing of sensitive compounds [77].
Recently, in one study, a KRED (ketoreductases) process that was part of the synthesis of the Akt inhibitor ipatasertib (a potential drug) was reported [78]. For this transformation, a commercially available enzyme was found that could execute the extremely diastereoselective reduction needed to access the intermediate while also regenerating the cofactor NADPH from i-PrOH as a terminal reductant. This approach demonstrated excellent efficiency, achieving a high yield of 86%, and its environmentally friendly design was further enhanced by the recycling of NADPH [79].
There are some challenges with biocatalysis, since enzymes can be expensive, and it remains the exception rather than the norm in the organic synthesis of pharmaceuticals [80]. However, adopting biocatalysis as a mainstream technology in chemical production promotes greener production processes, reduces pollution, lowers costs, and creates greater sustainability.
4.4. Green Solvents
Pharmaceutical industries increasingly employ a range of green solvents to reduce environmental impact and enhance the sustainability of drug manufacturing processes. Among the most commonly used eco-friendly solvents are glycerol, ethanol, and ethyl lactate, the latter of which is typically derived from renewable resources such as corn. These solvents are incorporated at various stages of drug synthesis, including reaction, extraction, purification, and formulation. The pharmaceutical industry is one of the primary users of solvents for its active pharmaceutical ingredient (API) purification and refinement operations [81]. Due to the fact that solvents account for more than 60% of all processed materials or waste in the pharmaceutical industry, the careful selection of solvents is needed. For this purpose, a number of green solvents have been proposed, including water, supercritical carbon dioxide (scCO2), and ionic liquids (ILs) [82,83,84].
4.4.1. Water
Using water as a solvent in pharmaceutical synthesis is challenging since many organic reaction components have poor solubility in water.
However, this does not diminish its importance as one of the key solvents in green chemistry [85]. Water has several advantages as a solvent: it is non-flammable, non-toxic, cost-effective, and abundantly available. One example of water being used as a solvent is demonstrated in the synthesis of ABT-546. In the final steps of production, water was used as a co-solvent. ABT-546 was obtained at an excellent yield (96%) without the need for solvent removal and extraction [77]. In another study, Takeda Pharmaceuticals’ process chemists and engineers created a second-generation process for producing the TAK-954 active pharmaceutical ingredient (API), which is almost water-based and includes many controlled separation steps.
In comparison to the first-generation solvent-based method, the second-generation water-based process consumes 77% less material inputs, 94% less organic solvent, and 48% less water, while increasing overall yield from 35% to 56% [86].
4.4.2. Supercritical Carbon Dioxide (scCO2)
The use of carbon dioxide instead of standard solvents is gaining attraction in pharmaceutical processes [87] due to its beneficial properties: it is non-toxic, non-flammable, non-corrosive, widely available, and easy to manage [88]. Studies have demonstrated that scCO2 can influence the crystallization behavior of drugs, leading to different polymorphic forms [89]. Superficial CO2 is also used for fluid extraction in the pharmaceutical industry. This innovative technique utilizes supercritical carbon dioxide to selectively isolate compounds, ensuring the purity and efficacy of medication. G. N. Sapkale and colleagues demonstrated the feasibility of the supercritical fluid extraction method to extract lipid compounds such as tocopherols, phytosterols, and free fatty acids from sorghum and the preventive roles of these compounds in many diseases [88].
4.4.3. Ionic Liquids
Ionic liquids, composed solely of ions (organic or inorganic anions and organic cations), have melting temperatures below 100 °C and can exist in liquid form at normal temperatures [90].
Ionic liquids are molten salts with extremely low vapor pressures. Furthermore, many of them exhibit low combustibility, superior thermal stability, and solvating properties, and are also electrically conductive. They serve as solvents or reaction media for many separation or catalytic processes because ionic liquids dissolve well in a wide range of organic, inorganic, and polymeric compounds [91].
Despite facing challenges such as side effects, impurities, biocompatibility issues, and stability problems, ionic liquids have significant potential to increase drug solubility, overcome physiological barriers, and improve particle stability [92]. ILs can be utilized to generate stable dispersions, significantly enhancing the solubility and bioavailability of poorly water-soluble medications [93]. This enhancement optimizes the absorption and distribution of pharmaceuticals, ultimately resulting in improved therapeutic outcomes. The efficacy of ionic liquids in facilitating transcellular and paracellular transport has been demonstrated through the compromise of cell integrity, the fluidization of lipids, the establishment of diffusional routes, and the removal of lipid components from the stratum corneum (SC). The properties of ionic liquids have the potential to enhance the efficiency of transdermal drug delivery, which may result in more effective treatments and enhanced patient outcomes.
Moreover, ILs facilitate the incorporation of pre-existing ionic cores into pharmaceutical molecules, thereby enhancing drug solubility, stability, and bioavailability. For example, N-acetyl amino acid N-alkyl cholinium-based ILs have been shown to increase the water solubility of paracetamol and sodium diclofenac by up to four times compared to pure water [94].
Also, Stefano Sangiorgi and colleagues demonstrated that ionic liquids (ILs) and deep eutectic solvents (DESs) improve the oral bioavailability of poorly soluble medicines, which proves that the integration of ionic liquids in pharmaceuticals is a viable and scalable approach to making pharmaceutical synthesis and formulation more sustainable and safer [95].
This process also enables the development of novel pharmaceuticals and physiologically active compounds. This facilitates the synthesis of custom-designed pharmaceutical molecules that are characterized by increased therapeutic efficacy, reduced toxicity, and enhanced controlled release.
Although green solvents represent a promising approach to green, sustainable chemistry, solvent-free synthesis methods are also studied for their reduced waste and minimal environmental impact. In a recent study, researchers have developed a new method for synthesizing indolizines via a copper-catalyzed reaction involving pyridine, acetophenone, and nitroalkenes under solvent-free conditions, achieving high yields [96].
A highly efficient and neat mechanochemical grinding procedure for the facile synthesis of N-substituted amines has been optimized in another study. This procedure uses easily available substituted halides and amines. Advantages of this strategy include a short reaction time at room temperature, benign reaction conditions, and the ability to isolate products in high yields without the need for column chromatography [97].
4.5. Flow Chemistry
Flow chemistry, also known as continuous flow chemistry, is the practice of carrying out chemical reactions in a continually flowing stream rather than by the conventional batch approach [82].
Continuous flow synthetic techniques can also be integrated easily with other enabling technologies. Examples of these technologies include microwave irradiation, supported reagents or catalysts, inductive heating, photochemistry, electrochemistry, 3D printing, novel solvent systems, or microreactor technology. These combinations may enable the development of fully automated processes that are more efficient and, in many cases, more sustainable [98].
A multitude of studies have emphasized the potential of flow chemistry in the sustainable synthesis of active pharmaceutical ingredients [99,100].
Imatinib, a chemical compound utilized in the treatment of chronic myeloid leukemia and gastrointestinal stromal tumors, represents a notable example of a major active pharmaceutical ingredient (API) that has been synthesized by employing flow devices in the past decade. The use of an in-line solvent-switching technique facilitates the modification of reaction solvents as part of a continuous process, underscoring the significance of flow chemistry techniques in the synthesis of challenging and poorly soluble compounds [101]. Artemisinin is another significant API. A continuous-flow process for converting dihydroartemisinic acid into artemisinin has been shown to be a cost-effective and scalable method, thereby ensuring a stable and affordable supply [102]. Another example is the synthesis of efavirenz. A distinctive semi-continuous technique has been demonstrated to yield rac-efavirenz at a yield of 45% across a series of four steps [103]. In the last study, the efficacious two-step continuous flow synthesis of diazepam, an agent listed on the World Health Organization’s (WHO) list of essential drugs, was outlined. It was stated that a telescoped flow synthesis using two microreactors organized in series at 0 °C and 60 °C, respectively, obtained a 96% yield of 91% pure diazepam within a 15-minute timeframe by applying an NH4Br/NH4OH solution in the secondary stage [104].
5. Conclusions
The analysis carried out in this review clearly indicates that the green chemistry paradigm formulated by its founders, Paul Anastas and John Warner, comprising 12 principles that have already become classic, greatly influences the strategy and trends of modern organic synthesis. The most obvious consequence of this impact is the extensive use of catalysts and catalytic methods in numerous studies carried out by chemists all over the world in various areas of synthetic chemistry and related fields, with most of the other environmental recommendations being addressed.
The achievements in green chemistry to date are remarkable and are attributable to scientists in universities, businesses, and research institutions around the world. The achievements attained so far serve as a precursor to the significant difficulties that remain in the field.
The 12 Principles of Green Chemistry framework has catalyzed several advancements in the discipline. Nonetheless, the principles were not conceived as 12 distinct objectives, but rather as a cohesive, coherent design framework. One may only aspire to develop a truly sustainable process by concurrently using all principles. Identifying the mutually reinforcing elements of the principles fosters sustainable systemic design and may promote revolutionary innovation instead of incremental enhancement. The notion of safer chemicals is a fundamental aspect of sustainable development.
The concepts of green chemistry, aimed at minimizing toxicity and environmental effects, guide the creation of chemicals that are safer for human health and the ecosystem. Scientists are making substantial progress in this field by using cleaner solvents and other synthesis processes. Ongoing governmental support and business dedication are essential to establish safer chemicals as the norm, fostering a healthier and more sustainable future for everyone.
Author Contributions
Conceptualization, A.S. and I.I.L.; methodology, A.M. and G.-A.M.; software, A.M.; validation, A.-M.M., A.M.M. and F.C.; formal analysis, D.L.; investigation, M.B.; resources, O.C.; data curation, D.L.; writing—original draft preparation, A.M.; writing—review and editing, G.-A.M.; visualization, O.C.; supervision, M.H.; project administration, M.H.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| UEM | Unused or expired medicines |
| PLA | Polylactic acid |
| DPM | Diphenylmethane |
| (N(P)E) | Nor(pseudo)ephedrine |
| PAT | Process analytical technology |
| MW | Microwave |
| CDK 4/6 | Cyclin-dependent kinase 4 and 6 |
| HR | Hormone receptor |
| API | Active pharmaceutical ingredient |
| MAO | Monoamine oxidases |
| KRED | Ketoreductases |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| ILs | Ionic liquids |
| DMA | Dimethoxyamphetamine |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| NMP | N-methyl pyrrolidone |
| THF | Tetrahydrofuran |
| scCO2 | supercritical carbon dioxide |
| SC | Stratum corneum |
References
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, Chemicals Strategy for Sustainability Towards a Toxic-Free Environment; 14.10.2020, COM(2020) 667 Final; Publications Office of the European Union: Luxembourg, 2003. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, Pathway to a Healthy Planet for All; 12.5.2021, COM(2021) 400 Final; Publications Office of the European Union: Luxembourg, 2003. [Google Scholar]
- European Union. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH); OJ L 396, 30.12.2006; European Union: Brussels, Belgium, 2006; pp. 1–849. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, Pharmaceutical Strategy for Europe; 25.11.2020, COM(2020) 761 Final; Publications Office of the European Union: Luxembourg, 2003. [Google Scholar]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/post-authorisation/pharmacovigilance-post-authorisation/referral-procedures-human-medicines (accessed on 2 April 2025).
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- EEA Glossary. Green Chemistry. Available online: https://www.eea.europa.eu/help/glossary/eea-glossary/green-chemistry (accessed on 1 April 2025).
- Anastas, P.T.; Williamson, T.C. Green Chemistry: Designing Chemistry for the Environment; American Chemical Series Books; Amer Chemical Society: Washington, DC, USA, 1996; pp. 1–20. [Google Scholar]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Roschangar, F.; Sheldon, R.; Senanayake, C. Overcoming Barriers to Green Chemistry in the Pharmaceutical Industry—The Green Aspiration LevelTM Concept. Green Chem. 2014, 17, 752–768. [Google Scholar] [CrossRef]
- Raymond, C.; Slater, S.; Savelski, M.J. LCA Approach to the Analysis of Solvent Waste Issues in the Pharmaceutical Industry. Green Chem. 2010, 12, 1826–1834. [Google Scholar] [CrossRef]
- Andraos, J.; Matlack, A.S. Introduction to Green Chemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Bekker, C.; van den Bemt, B.J.F.; Egberts, A.C.G.; Bouvy, M.L.; Gardarsdottir, H. Patient and Medication Factors Associated with Preventable Medication Waste and Possibilities for Redispensing. Int. J. Clin. Pharm. 2018, 40, 704–711. [Google Scholar] [CrossRef]
- Patel, K.R.; Sen, D.J.; Jatakiya, V.P. Atom Economy in Drug Synthesis Is a Playground of Functional Groups. Am. J. Adv. Drug Deliv. 2013, 1, 73–83. [Google Scholar]
- Ha, M.-W.; Paek, S.-M. Recent Advances in the Synthesis of Ibuprofen and Naproxen. Molecules 2021, 26, 4792. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A Review on Poly Lactic Acid (PLA) as a Biodegradable Polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Sonavane, S.; Pagire, R.; Patil, D.; Pujari, U.; Nikam, R.; Pradhan, N. Improved, Solvent-Free, Atom Efficient Commercial Process for the Synthesis of Diphenhydramine Hydrochloride. Curr. Green Chem. 2017, 4, 161–165. [Google Scholar] [CrossRef]
- Huang, H.; Kang, J.Y. Mitsunobu Reaction Using Basic Amines as Pronucleophiles. J. Org. Chem. 2017, 82, 6604–6614. [Google Scholar] [CrossRef]
- Ravelo, M.; Gallardo, M.E.; Ladero, M.; Garcia-Ochoa, F. Synthesis of Ibuprofen Monoglyceride Using Novozym®435: Biocatalyst Activation and Stabilization in Multiphasic Systems. Catalysts 2022, 12, 1531. [Google Scholar] [CrossRef]
- Park, J.; Evans, C.; Maier, J.; Hatzell, M.; France, S.; Sievers, C.; Bommarius, A.S. Renewables-Based Routes to Paracetamol: A Green Chemistry Analysis. ACS Sustain. Chem. Eng. 2024, 12, 16271–16282. [Google Scholar] [CrossRef]
- Sehl, T.; Hailes, H.C.; Ward, J.M.; Menyes, U.; Pohl, M.; Rother, D. Efficient 2-Step Biocatalytic Strategies for the Synthesis of All Nor(Pseudo)ephedrine Isomers. Green Chem. 2014, 16, 3341. [Google Scholar] [CrossRef]
- Savile, C.K.; Janey, J.M.; Mundorff, E.C.; Moore, J.C.; Tam, S.; Jarvis, W.R.; Colbeck, J.C.; Krebber, A.; Fleitz, F.J.; Brands, J.; et al. Biocatalytic Asymmetric Synthesis of Chiral Amines from Ketones Applied to Sitagliptin Manufacture. Science 2010, 329, 305–309. [Google Scholar] [CrossRef]
- Babazadeh, M.; Sheidaei, M.; Abbaspour, S.; Edjlali, L. Synthesis, Characterization, and In Vitro Evaluation of New Ibuprofen Polymeric Prodrugs Based on 2-Hydroxypropyl Methacrylate. Sci. Pharm. 2013, 81, 281–296. [Google Scholar] [CrossRef]
- Dalvi, H.; Langlet, A.; Colbert, M.-J.; Cournoyer, A.; Guay, J.-M.; Abatzoglou, N.; Gosselin, R. In-Line Monitoring of Ibuprofen during and after Tablet Compression Using Near-Infrared Spectroscopy. Talanta 2019, 195, 87–96. [Google Scholar] [CrossRef]
- Golemac, L.; Kondža, M. Synthesis of Acetylsalicylic Acid—An Environmentally Friendly Approach. Ann. Biomed. Clin. Res. 2023, 2, 100–108. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Sowa-Kasprzak, K.; Olender, D.; Zaprutko, L. Microwave (MW), Ultrasound (US) and Combined Synergic MW-US Strategies for Rapid Functionalization of Pharmaceutical Use Phenols. Molecules 2018, 23, 2360. [Google Scholar] [CrossRef] [PubMed]
- Lidstrom, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave Assisted Organic Synthesis—A Review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Sweygers, N.; Alewaters, N.; Dewil, R.; Appels, L. Microwave Effects in the Dilute Acid Hydrolysis of Cellulose to 5-Hydroxymethylfurfural. Sci. Rep. 2018, 8, 7719. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Shanab, K.; Neudorfer, C.; Schirmer, E.; Spreitzer, H. Green Solvents in Organic Synthesis: An Overview. Curr. Org. Chem. 2013, 17, 1179. [Google Scholar] [CrossRef]
- Banik, B.K.; Sahoo, B.M.; Kumar, B.V.V.R.; Panda, K.C.; Jena, J.; Mahapatra, M.K.; Borah, P. Green Synthetic Approach: An Efficient Eco-Friendly Tool for Synthesis of Biologically Active Oxadiazole Derivatives. Molecules 2021, 26, 1163. [Google Scholar] [CrossRef] [PubMed]
- Varma, R. Solvent-Free Accelerated Organic Syntheses Using Microwaves. Pure Appl. Chem. 2001, 73, 193–198. [Google Scholar] [CrossRef]
- Kumar, A.; Kuang, Y.; Liang, Z.; Sun, X. Microwave Chemistry, Recent Advancements and Eco-Friendly Microwave-Assisted Synthesis of Nanoarchitectures and Their Applications: A Review. Mater. Today Nano 2020, 11, 100076. [Google Scholar] [CrossRef]
- Nain, S.; Singh, R.; Ravichandran, S. Importance of Microwave Heating in Organic Synthesis. Adv. J. Chem. Sect. A 2019, 2, 94–104. [Google Scholar] [CrossRef]
- Gabano, E.; Ravera, M. Microwave-Assisted Synthesis: Can Transition Metal Complexes Take Advantage of This “Green” Method? Molecules 2022, 27, 4249. [Google Scholar] [CrossRef]
- Patil, A.B.; Phole, P.M.; Charde, M.S.; Chakole, R.D.; Mali, N.S. Microwave Assisted Organic Synthesis: A Green Chemistry Approach. Int. J. Nov. Res. Dev. 2023, 8, C310–C321. Available online: https://ijnrd.org/papers/IJNRD2306234.pdf (accessed on 1 April 2025).
- Gopinadh, M.; Rohit, K.; Saranya, S.; Gopinathan, A. Microwave Assisted Synthesis of Five Membered Nitrogen Heterocycles. RSC Adv. 2020, 10, 36031–36041. [Google Scholar] [CrossRef]
- Ristić, I.; Nikolić, L.; Cakić, S.; Nikolić, V.; Tanasić, J.; Zvezdanović, J.; Krstić, M. Eco-Friendly Microwave Synthesis of Sodium Alginate-Chitosan Hydrogels for Effective Curcumin Delivery and Controlled Release. Gels 2024, 10, 637. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, J.; Zhao, Q.; Ma, X.; Du, X.; Hao, X.; Tang, B.; Abudula, A.; Guan, G. Microwave-Assisted Synthesis of Manganese Oxide Catalysts for Total Toluene Oxidation. J. Colloid Interface Sci. 2022, 607, 100–110. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, X.; Zhang, Z.; Zhang, W.; Wang, J. Large-Scale and Fast Synthesis of Nano-Hydroxyapatite Powder by a Microwave-Hydrothermal Method. RSC Adv. 2019, 9, 13623–13630. [Google Scholar] [CrossRef]
- Gonzalez-Moragas, L.; Yu, S.M.; Murillo-Cremaes, N.; Laromaine, A.; Roig, A. Scale-Up Synthesis of Iron Oxide Nanoparticles by Microwave-Assisted Thermal Decomposition. Chem. Eng. J. 2015, 281, 87–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, B.; Zheng, C.; Zhou, J.; Su, T.; Guo, J.; Chen, J.; Chen, Y.; Zhang, J.; Dang, H.; et al. Research on the Resistance of Catalysts for Selective Catalytic Reduction: Current Progresses and Future Perspectives. J. Clean. Prod. 2024, 434, 139920. [Google Scholar] [CrossRef]
- Brown, D.; Boström, J. Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone? J. Med. Chem. 2015, 59, 4443–4458. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.P.; Weix, D.J. The Once and Future Catalysts: How the Challenges of First-Row Transition-Metal Catalysis Grew to Become Strengths. Acc. Chem. Res. 2024, 57, 2451–2452. [Google Scholar] [CrossRef]
- Crabtree, R.H. The Organometallic Chemistry of the Transition Metals, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Bullock, R.M.; Helm, M.L. Molecular Electrocatalysts for Oxidation of Hydrogen Using Earth-Abundant Metals: Shoving Protons Around with Proton Relays. Acc. Chem. Res. 2015, 48, 2015–2023. [Google Scholar] [CrossRef]
- Aghahosseini, H.; Saadati, M.R.; Rezaei, S.J.T.; Ramazani, A.; Asadi, N.; Yahiro, H.; Mori, M.; Shajari, N.; Kazemizadeh, A.R. A Robust Polyfunctional Pd(II)-Based Magnetic Amphiphilic Nanocatalyst for the Suzuki–Miyaura Coupling Reaction. Sci. Rep. 2021, 11, 89424. [Google Scholar] [CrossRef]
- Lin, H.; Gao, X.; Yao, H.; Luo, Q.; Xiang, B.; Liu, C.; Ouyang, Y.; Zhou, N.; Xiang, D. Immobilization of a Pd(II)-Containing N-Heterocyclic Carbene Ligand on Porous Organic Polymers: Efficient and Recyclable Catalysts for Suzuki–Miyaura Reactions. Catal. Sci. Technol. 2021, 11, 3676–3680. [Google Scholar] [CrossRef]
- Kiani, M.; Rabiee, N.; Bagherzadeh, M.; Ghadiri, A.M.; Fatahi, Y.; Dinarvand, R.; Webster, T.J. High-Gravity Assisted Green Synthesis of Palladium Nanoparticles: The Flowering of Nanomedicine. Nanomedicine 2020, 30, 102297. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, J.; Jin, X.; Sun, J.; Huang, J.; Li, R. Magnetically recyclable Fe@Pd/C as a highly active catalyst for Suzuki coupling reaction in aqueous solution. Catal. Commun. 2014, 43, 75–78. [Google Scholar] [CrossRef]
- Corona, S.P.; Generali, D. Abemaciclib: A CDK4/6 inhibitor for the treatment of HR+/HER2- advanced breast cancer. Drug Des. Dev. Ther. 2018, 12, 321–330. [Google Scholar] [CrossRef]
- Frederick, M.; Kjell, D. A Synthesis of Abemaciclib Utilizing a Leuckart–Wallach Reaction. Tetrahedron Lett. 2015, 56, 10. [Google Scholar] [CrossRef]
- Soliev, S.B.; Astakhov, A.V.; Pasyukov, D.V.; Chernyshev, V.M. Nickel(II) N-heterocyclic carbene complexes as efficient catalysts for the Suzuki–Miyaura reaction. Russ. Chem. Bull. 2020, 69, 683–690. [Google Scholar] [CrossRef]
- Muthu Tamizh, M.; Karvembu, R. Synthesis of triethylphosphite complexes of nickel(II) and palladium(II) with tridentate Schiff base ligand for catalytic application in carbon–carbon coupling reactions. Inorg. Chem. Commun. 2012, 25, 30–34. [Google Scholar] [CrossRef]
- Xiang, J.; Li, P.; Chong, H.; Feng, L.; Fu, F.; Wang, Z.; Zhang, S.; Zhu, M. Bimetallic Pd–Ni core–shell nanoparticles as effective catalysts for the Suzuki reaction. Nano Res. 2014, 7, 1337–1343. [Google Scholar] [CrossRef]
- Lungu, I.I.; Cioanca, O.; Mircea, C.; Tuchilus, C.; Stefanache, A.; Huzum, R.; Hancianu, M. Insights into Catechin–Copper Complex Structure and Biologic Activity Modulation. Molecules 2024, 29, 4969. [Google Scholar] [CrossRef]
- Rabiee, N.; Bagherzadeh, M.; Kiani, M.; Ghadiri, A.M.; Etessamifar, F.; Jaberizadeh, A.H.; Shakeri, A. Biosynthesis of Copper Oxide Nanoparticles with Potential Biomedical Applications. Int. J. Nanomed. 2020, 15, 3983–3999. [Google Scholar] [CrossRef]
- Turan, N.; Buldurun, K.; Colak, N.; Ozdemir, I. Preparation and spectroscopic studies of Fe(II), Ru(II), Pd(II) and Zn(II) complexes of Schiff base containing terephthalaldehyde and their transfer hydrogenation and Suzuki–Miyaura coupling reaction. Open Chem. 2019, 17, 571–580. [Google Scholar] [CrossRef]
- Reckling, A.; Martin, D.; Dawe, L.; Decken, A.; Kozak, C. Structure and C–C cross-coupling reactivity of iron(III) complexes of halogenated amine-bis(phenolate) ligands. J. Organomet. Chem. 2011, 696, 787–794. [Google Scholar] [CrossRef]
- Ramgren, S.D.; Hie, L.; Ye, Y.; Garg, N.K. Nickel-catalyzed Suzuki–Miyaura couplings in green solvents. Org. Lett. 2013, 15, 3950–3953. [Google Scholar] [CrossRef]
- Hosseyni, S.; Jarrahpour, A. Recent advances in β-lactam synthesis. Org. Biomol. Chem. 2018, 16, 6840–6852. [Google Scholar] [CrossRef]
- Oddy, M.J.; Kusza, D.A.; Epton, R.G.; Lynam, J.M.; Unsworth, W.P.; Petersen, W.F. Visible-Light-Mediated Energy Transfer Enables the Synthesis of β-Lactams via Intramolecular Hydrogen Atom Transfer. Angew. Chem. Int. Ed. 2022, 61, e202213086. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.S.; Raydan, D.; Cunha, J.C.; Viduedo, N.; Silva, A.M.S.; Marques, M.M.B. Advances in Green Catalysis for the Synthesis of Medicinally Relevant N-Heterocycles. Catalysts 2021, 11, 1108. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Benítez-Mateos, A.; Paradisi, F. Perspectives on flow biocatalysis: The engine propelling enzymatic reactions. J. Flow Chem. 2023, 14, 211–218. [Google Scholar] [CrossRef]
- Pandeeti, E.; Sangeetha, V.; Deepika, R. Emerging Trends in the Industrial Production of Chemical Products by Microorganisms. In Microbial Diversity in the Genomic Era; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Sharma, S.; Das, J.; Braje, W.M.; Dash, A.K.; Handa, S. A Glimpse into Green Chemistry Practices in the Pharmaceutical Industry. ChemSusChem 2020, 13, 2859–2875. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M. Green Chemistry in the Fine Chemicals and Pharmaceutical Industries. Org. Process Res. Dev. 2013, 17, 1479–1484. [Google Scholar] [CrossRef]
- Williams-Herman, D.; Round, E.; Swern, A.S.; Musser, B.; Davies, M.J.; Stein, P.P.; Kaufman, K.D.; Amatruda, J.M. Safety and tolerability of sitagliptin in patients with type 2 diabetes: A pooled analysis. BMC Endocr. Disord. 2008, 8, 14. [Google Scholar] [CrossRef]
- Kendall, D.M.; Cuddihy, R.M.; Bergenstal, R.M. Clinical application of incretin-based therapy: Therapeutic potential, patient selection and clinical use. Am. J. Med. 2009, 122 (Suppl. S6), S37–S50. [Google Scholar] [CrossRef]
- Desai, A.A. Sitagliptin manufacture: A compelling tale of green chemistry, process intensification, and industrial asymmetric catalysis. Angew. Chem. Int. Ed. 2011, 50, 1974–1976. [Google Scholar] [CrossRef]
- Kjellin, M.; Wesslén, T.; Löfblad, E.; Lennerstrand, J.; Lannergård, A. The effect of the first-generation HCV-protease inhibitors boceprevir and telaprevir and the relation to baseline NS3 resistance mutations in genotype 1: Experience from a small Swedish cohort. Ups. J. Med. Sci. 2018, 123, 50–56. [Google Scholar] [CrossRef]
- Li, T.; Liang, J.; Ambrogelly, A.; Brennan, T.; Gloor, G.; Huisman, G.; Lalonde, J.; Lekhal, A.; Mijts, B.; Muley, S.; et al. Efficient, chemoenzymatic process for manufacture of the Boceprevir bicyclic [3.1.0]proline intermediate based on amine oxidase-catalyzed desymmetrization. J. Am. Chem. Soc. 2012, 134, 6467–6472. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Tang, Y. Efficient synthesis of simvastatin by use of whole-cell biocatalysis. Appl. Environ. Microbiol. 2007, 73, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Veleva, V.; Cue, B. Benchmarking green chemistry adoption by “big pharma” and generics manufacturers. Benchmarking Int. J. 2017, 24, 1414–1436. [Google Scholar] [CrossRef]
- Mishra, M.; Sharma, M.; Dubey, R.; Kumari, P.; Ranjan, V.; Pandey, J. Green synthesis interventions of pharmaceutical industries for sustainable development. Curr. Res. Green Sustain. Chem. 2021, 4, 100174. [Google Scholar] [CrossRef]
- Han, C.; Savage, S.; Al-Sayah, M.; Yajima, H.; Remarchuk, T.; Reents, R.; Wirz, B.; Iding, H.; Bachmann, S.; Fantasia, S.M.; et al. Asymmetric synthesis of Akt kinase inhibitor Ipatasertib. Org. Lett. 2017, 19, 4806–4809. [Google Scholar] [CrossRef]
- France, S.P.; Lewis, R.D.; Martinez, C.A. The evolving nature of biocatalysis in pharmaceutical research and development. JACS Au 2023, 3, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.P.; Eichhorn, E.J.; Hanlon, S.P.; Lütz, S.; Schürmann, M.; Wohlgemuth, R.; Coppolecchia, R. The use of enzymes in organic synthesis and the life sciences: Perspectives from the Swiss Industrial Biocatalysis Consortium (SIBC). Catal. Sci. Technol. 2013, 3, 29–40. [Google Scholar] [CrossRef]
- Aboagye, E.A.; Chea, J.D.; Yenkie, K.M. Systems level roadmap for solvent recovery and reuse in industries. iScience 2021, 24, 103114. [Google Scholar] [CrossRef]
- Ahmad, S.; Jaiswal, R.; Yadav, R.; Verma, S. Recent advances in green chemistry approaches for pharmaceutical synthesis. Sustain. Chem. One World 2024, 4, 100029. [Google Scholar] [CrossRef]
- Kumar, R.; Maurya, A. Green Chemistry Techniques for Sustainable Pharmaceutical Synthesis. J. Drug Discov. Health Sci. 2024, 1, 187–200. [Google Scholar] [CrossRef]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Green Chemistry in the Synthesis of Pharmaceuticals. Chem. Rev. 2021, 122, 3637–3710. [Google Scholar] [CrossRef] [PubMed]
- Cioanca, O.; Lungu, I.-I.; Mita-Baciu, I.; Robu, S.; Burlec, A.F.; Hancianu, M.; Crivoi, F. Extraction and Purification of Catechins from Tea Leaves: An Overview of Methods, Advantages, and Disadvantages. Separations 2024, 11, 171. [Google Scholar] [CrossRef]
- Bailey, J.D.; Helbling, E.; Mankar, A.; Stirling, M.; Hicks, F.; Leahy, D.K. Beyond organic solvents: Synthesis of a 5-HT4 receptor agonist in water. Green Chem. 2021, 23, 788–795. [Google Scholar] [CrossRef]
- Subramaniam, B.; Rajewski, R.A.; Snavely, K. Pharmaceutical processing with supercritical carbon dioxide. J. Pharm. Sci. 1997, 86, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Sapkale, G.N.; Patil, S.M.; Surwase, U.S.; Bhatbhage, P.K. Supercritical fluid extraction: A review. Int. J. Chem. Sci. 2010, 8, 729–743. [Google Scholar]
- Rodrigues, M.A.; Tiago, J.M.; Duarte, A.; Geraldes, V.; Matos, H.A.; Gomes Azevedo, E. Polymorphism in pharmaceutical drugs by supercritical CO2 processing: Clarifying the role of the antisolvent effect and atomization enhancement. Crystal Growth Des. 2016, 16, 6222–6229. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Shanab, K.; Neudorfer, C.; Spreitzer, H. Green solvents in organic synthesis: An overview II. Curr. Org. Chem. 2016, 20, 1576–1583. [Google Scholar] [CrossRef]
- Jain, A.; Shakya, A.K.; Prajapati, S.K.; Eldesoqui, M.; Mody, N.; Jain, S.K.; Naik, R.R.; Patil, U.K. An insight into pharmaceutical challenges with ionic liquids: Where do we stand in transdermal delivery? Front. Bioeng. Biotechnol. 2024, 12, 1454247. [Google Scholar] [CrossRef]
- Kapre, S.; Palakurthi, S.S.; Jain, A.; Palakurthi, S. DES-igning the future of drug delivery: A journey from fundamentals to drug delivery applications. J. Mol. Liq. 2024, 400, 124517. [Google Scholar] [CrossRef]
- Jesus, A.R.; Soromenho, M.R.C.; Raposo, L.R.; Esperança, J.M.S.S.; Baptista, P.V.; Fernandes, A.R.; Reis, P.M. Enhancement of Water Solubility of Poorly Water-Soluble Drugs by New Biocompatible N-Acetyl Amino Acid N-Alkyl Cholinium-Based Ionic Liquids. Eur. J. Pharm. Biopharm. 2019, 137, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Sangiorgi, S.; Albertini, B.; Bertoni, S.; Passerini, N. An Overview on the Role of Ionic Liquids and Deep Eutectic Solvents in Oral Pharmaceuticals. Pharmaceutics 2025, 17, 300. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Liu, Z.; Bi, W.; Shen, J.; Li, G. Efficient Solvent-Free Synthesis of Indolizines Using CuBr Catalyst from Pyridine, Acetophenone, and Electron-Deficient Alkenes. Molecules 2024, 29, 2061. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, H.; Kumar, M.; Grishina, M.; Pandit, U.; Poonam; Rathi, B. Solvent-Free Mechanochemical Grinding Facilitates Clean Synthesis of N-Substituted Amines. Org. Biomol. Chem. 2022, 20, 6673–6679. [Google Scholar] [CrossRef]
- Porta, R.; Benaglia, M.; Puglisi, A. Flow chemistry: Recent developments in the synthesis of pharmaceutical products. Org. Process Res. Dev. 2015, 20, 2–25. [Google Scholar] [CrossRef]
- Gutmann, B.; Cantillo, D.; Kappe, C.O. Continuous-flow technology—A tool for the safe manufacturing of active pharmaceutical ingredients. Angew. Chem. Int. Ed. 2015, 54, 6688–6728. [Google Scholar] [CrossRef]
- Baumann, M.; Baxendale, I.R. The synthesis of active pharmaceutical ingredients (APIs) using continuous flow chemistry. Beilstein J. Org. Chem. 2015, 11, 1194–1219. [Google Scholar] [CrossRef]
- Hopkin, M.D.; Baxendale, I.R.; Ley, S.V. A flow-based synthesis of imatinib: The API of Gleevec. Chem. Commun. 2010, 46, 2450–2452. [Google Scholar] [CrossRef]
- Lévesque, F.; Seeberger, P.H. Continuous-flow synthesis of the anti-malaria drug artemisinin. Angew. Chem. Int. Ed. 2012, 51, 1706–1709. [Google Scholar] [CrossRef]
- Correia, C.; Gilmore, K.; McQuade, D.; Seeberger, P. A concise flow synthesis of Efavirenz. Angew. Chem. Int. Ed. 2015, 54, 4945–4948. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, R.; McGuire, M.; Hyun, S.-H.; Cullison, M.; Thompson, D. Development of an efficient, high purity continuous flow synthesis of diazepam. Front. Chem. Eng. 2022, 4, 877498. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).