Plasticized Polylactide Film Coating Formation from Redispersible Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
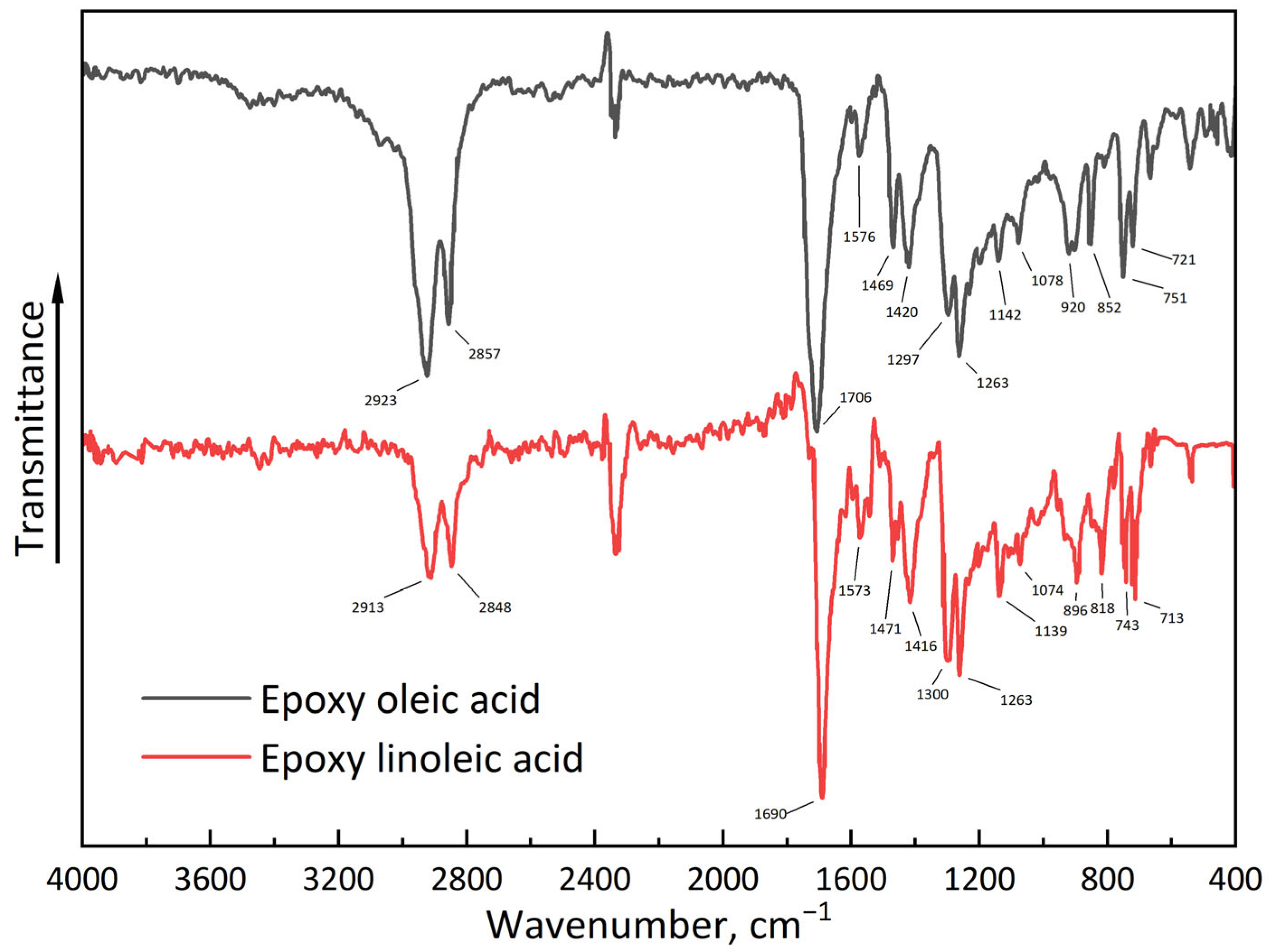
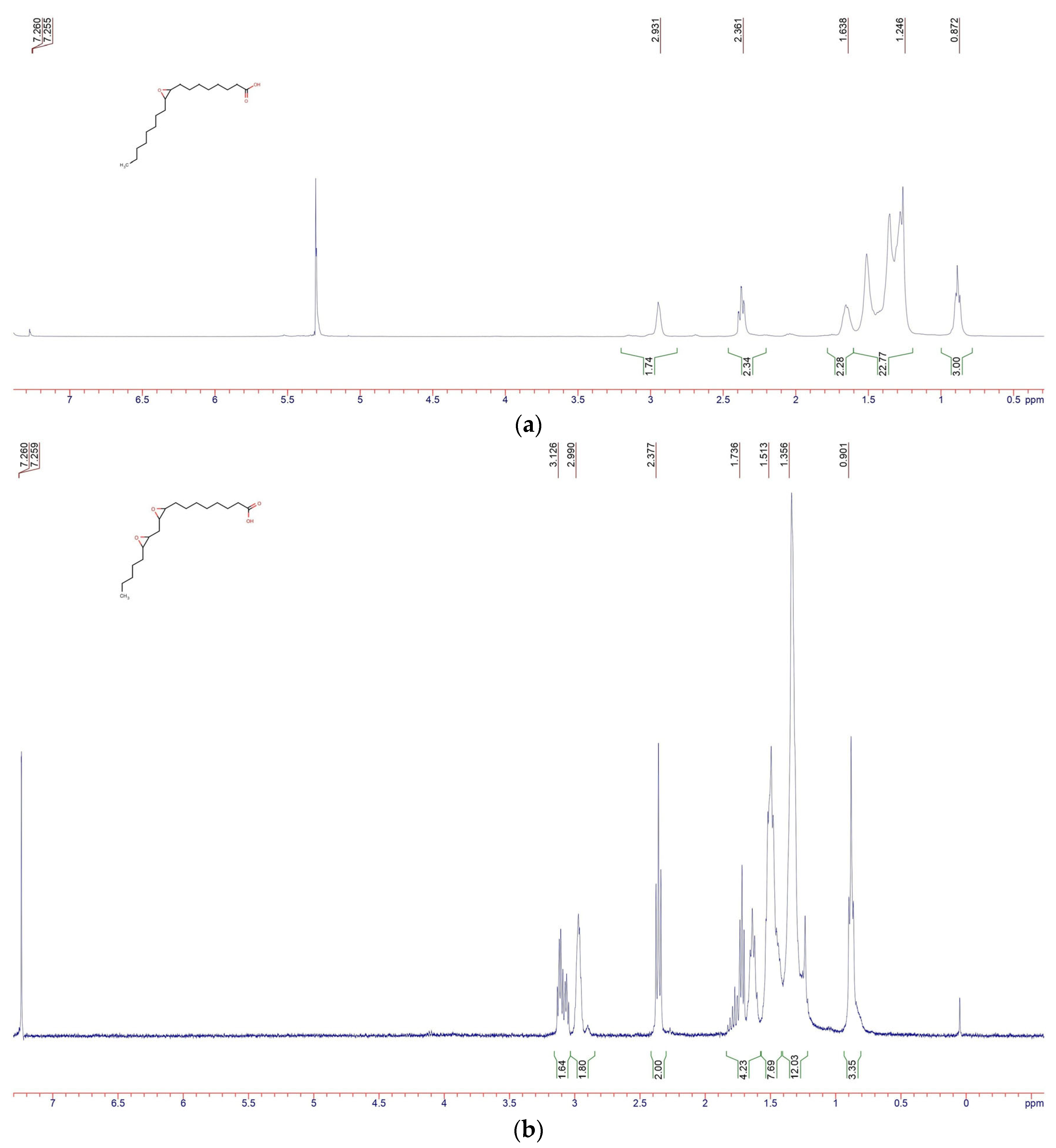
2.2. Epoxidized Plasticizers Synthesis and Characterization
2.3. Obtaining Polylactide Dispersions
2.4. Characterization Methods
3. Results and Discussion
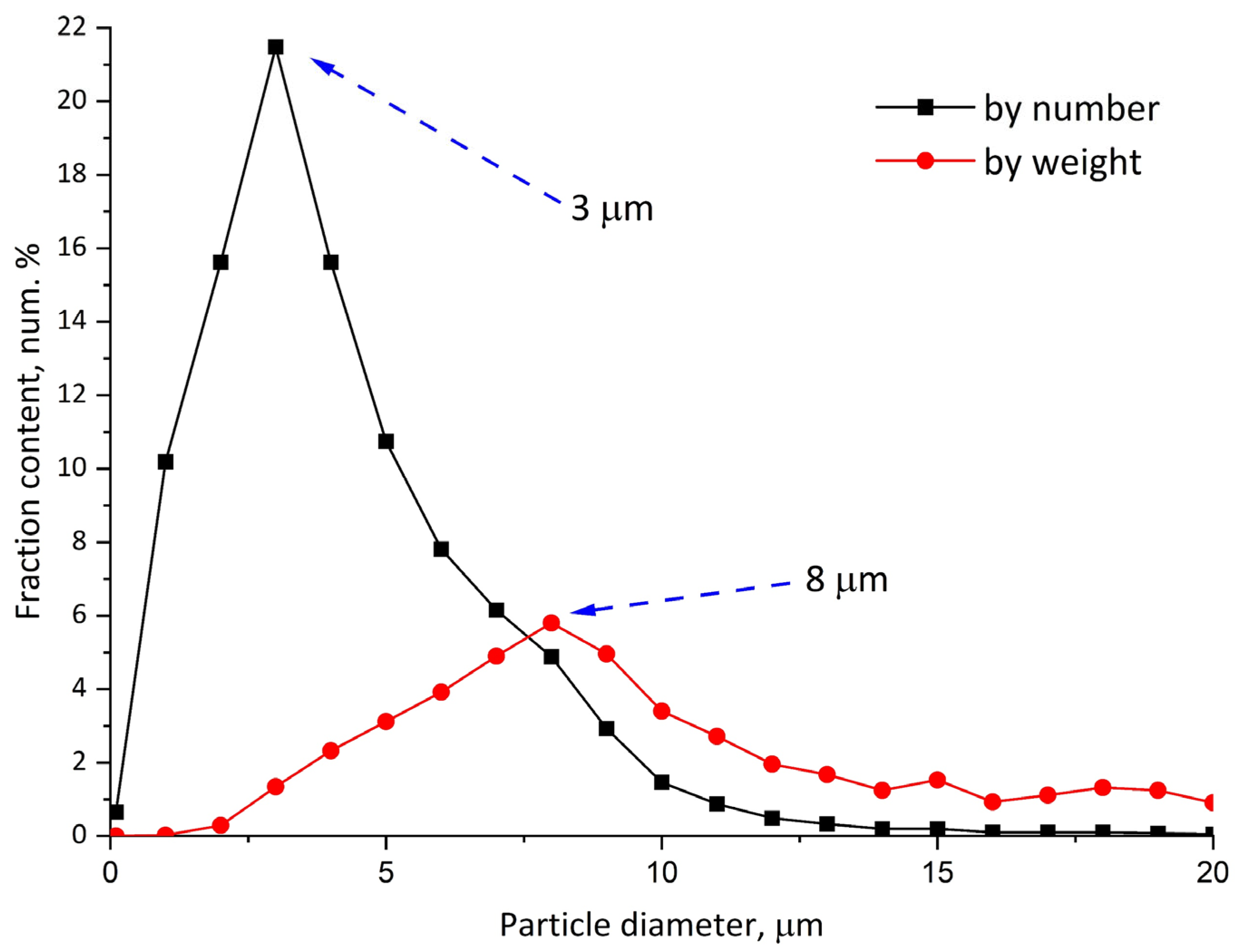
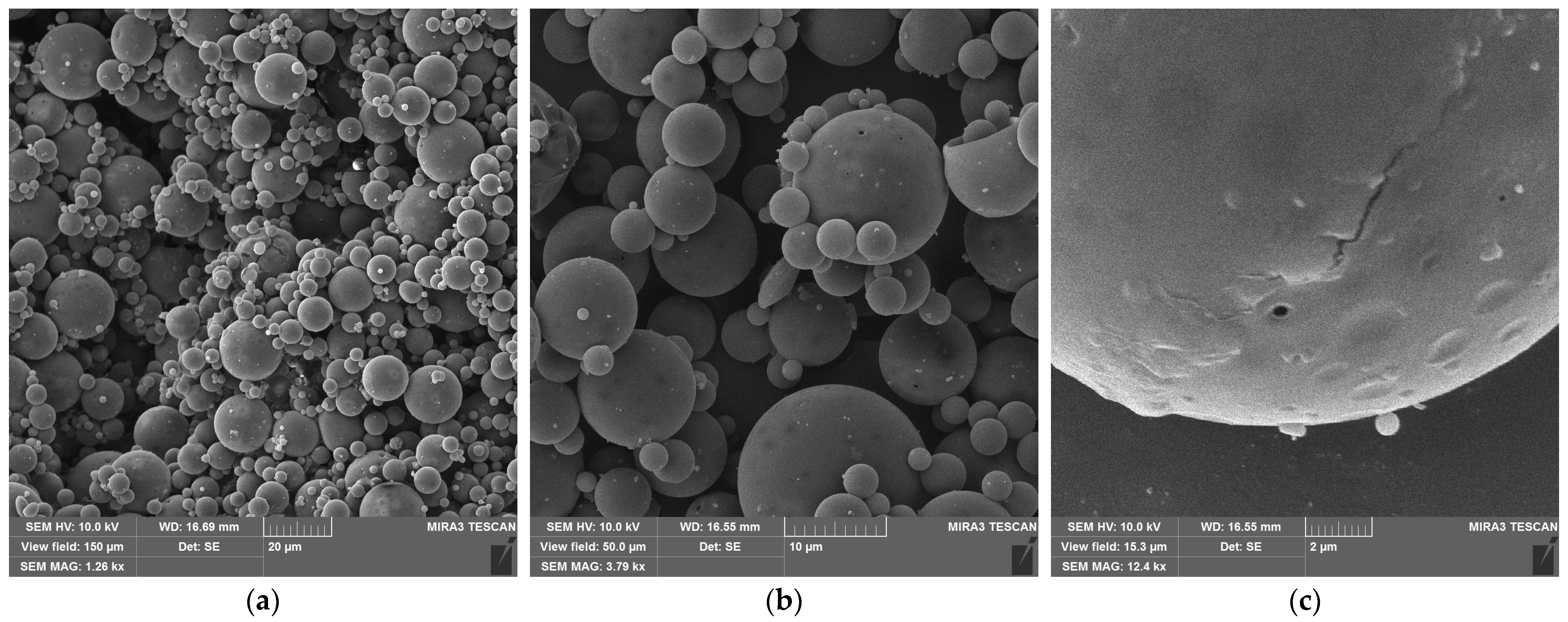
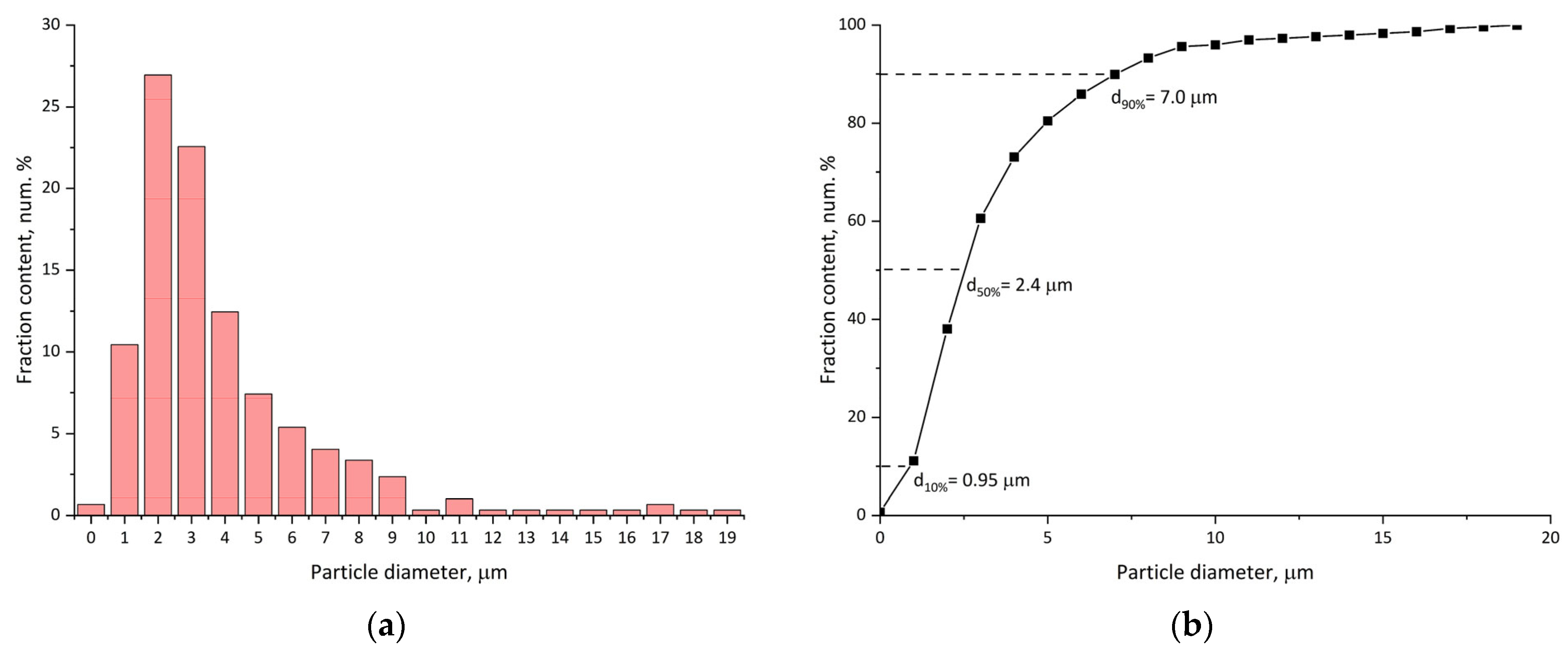
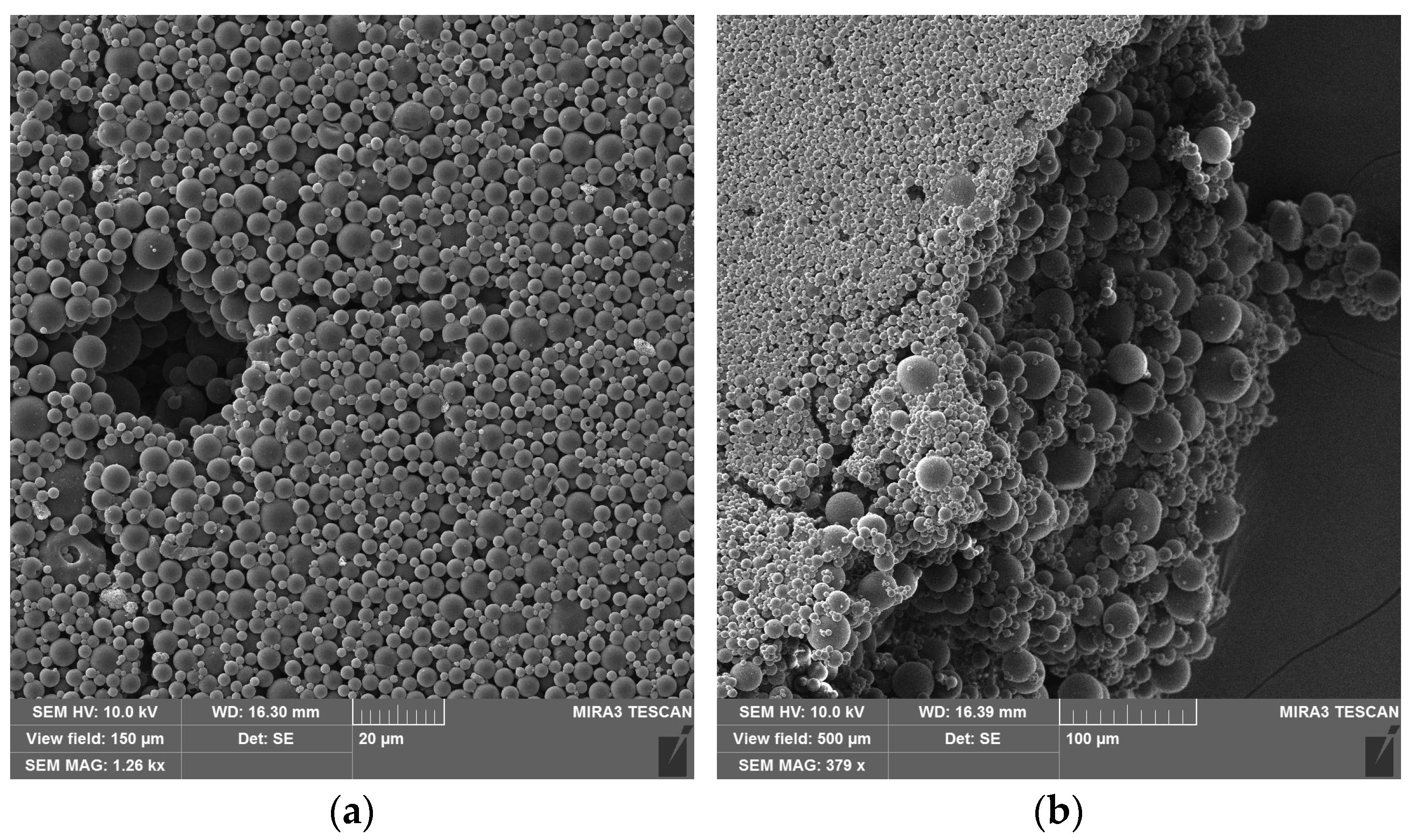
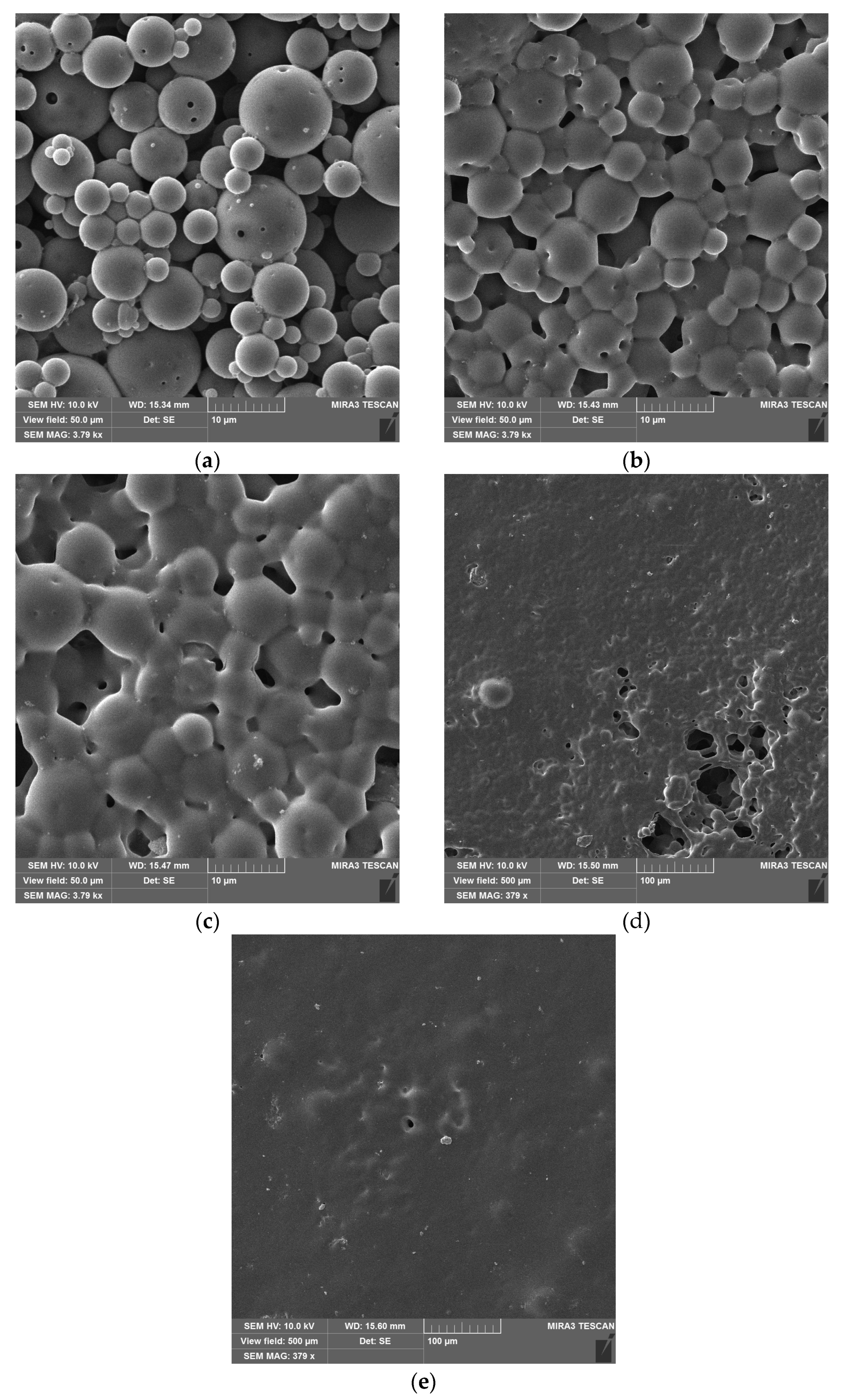
3.1. Re-Dispersable PLA Particle Characterization
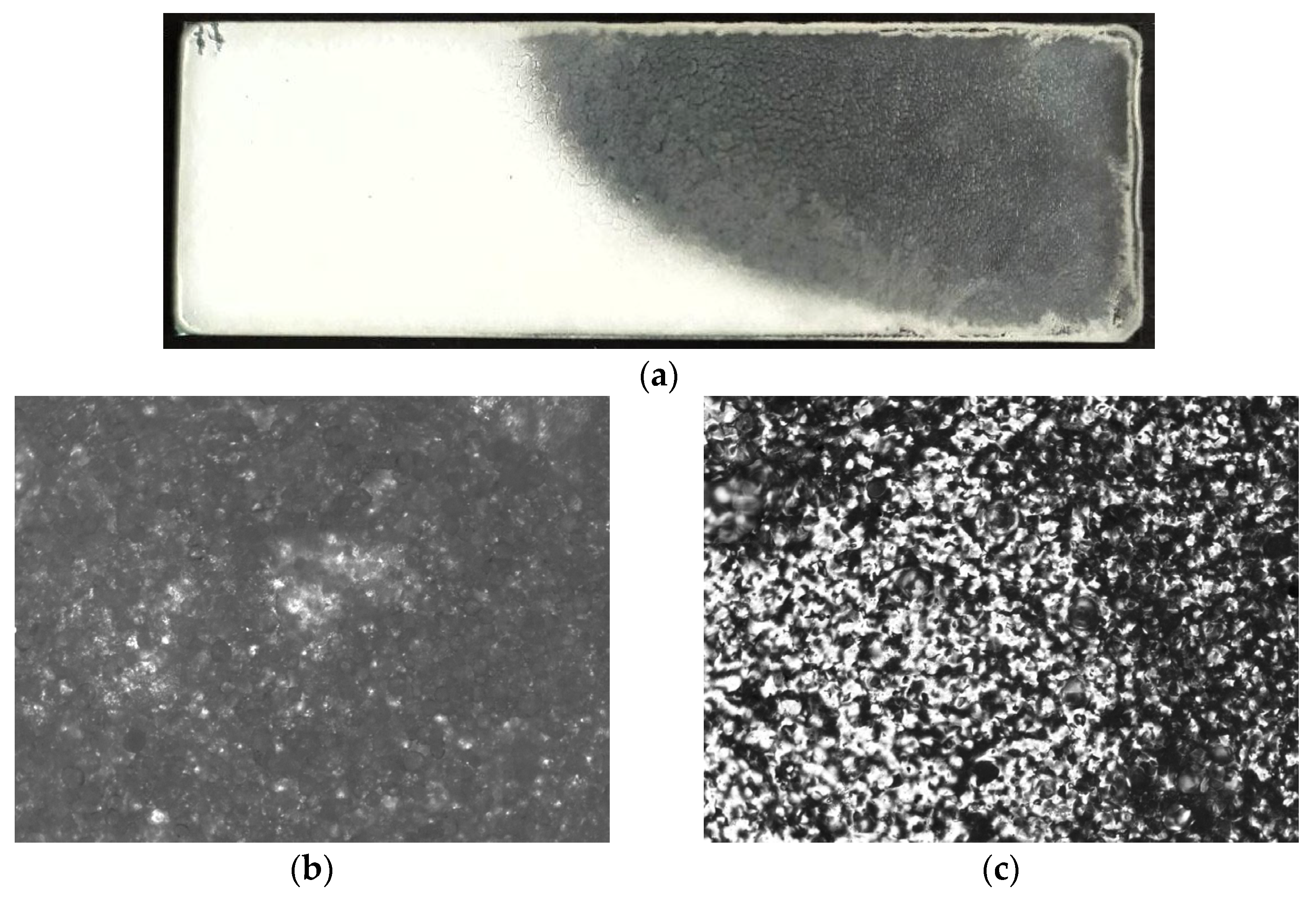
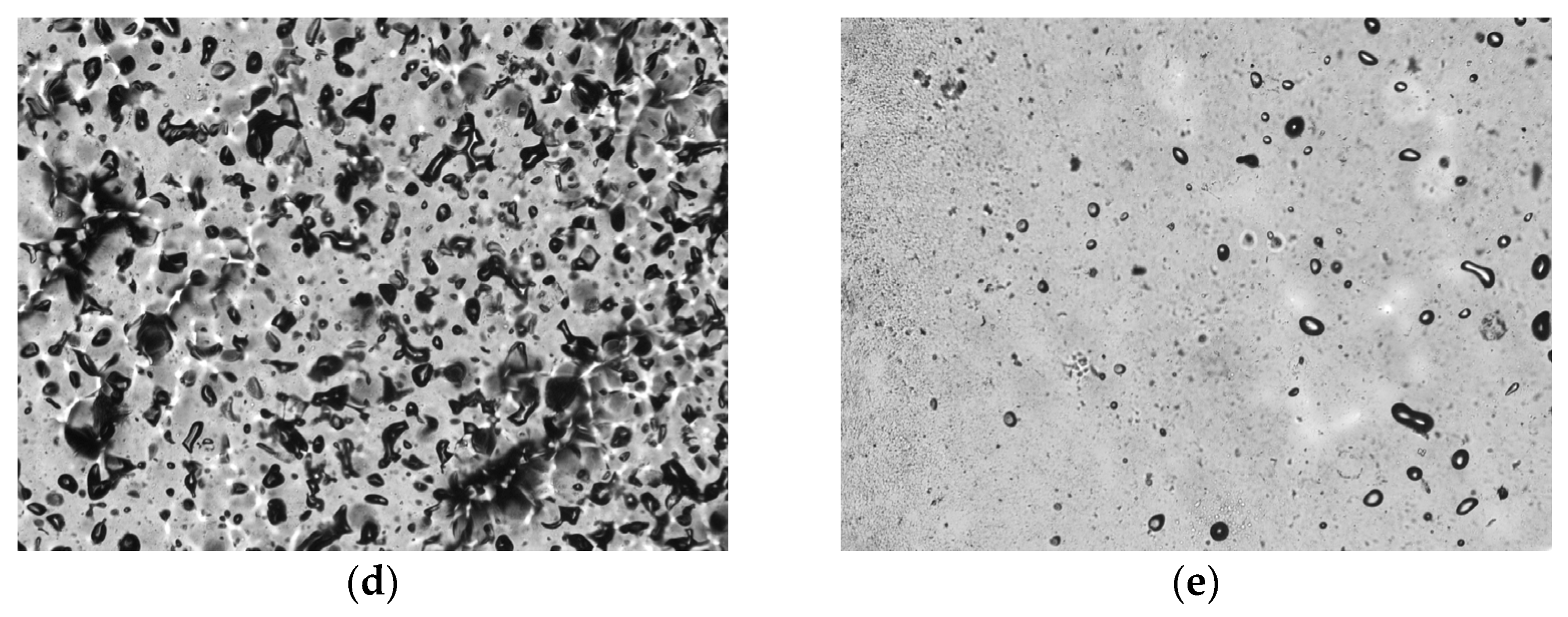
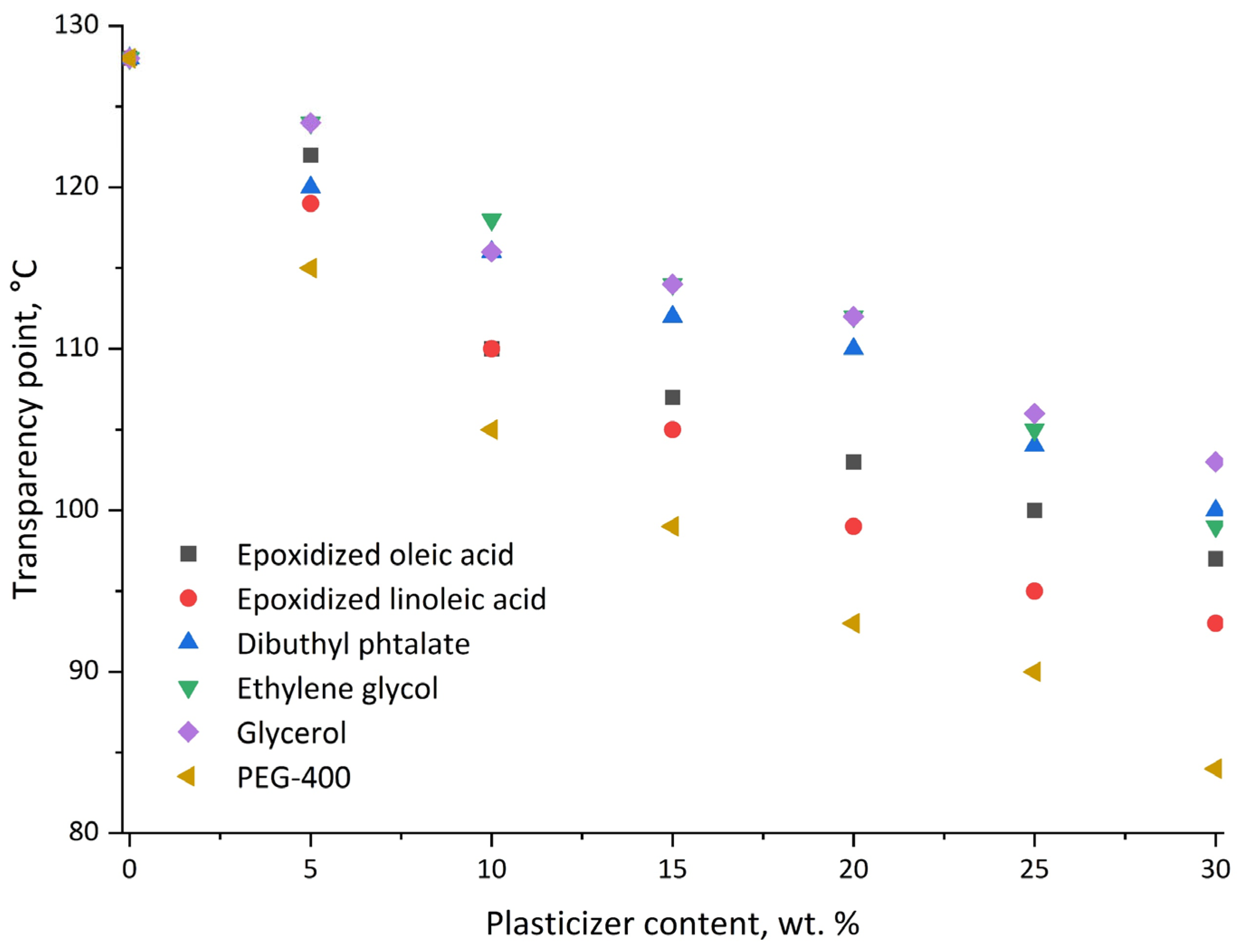
3.2. Film Formation
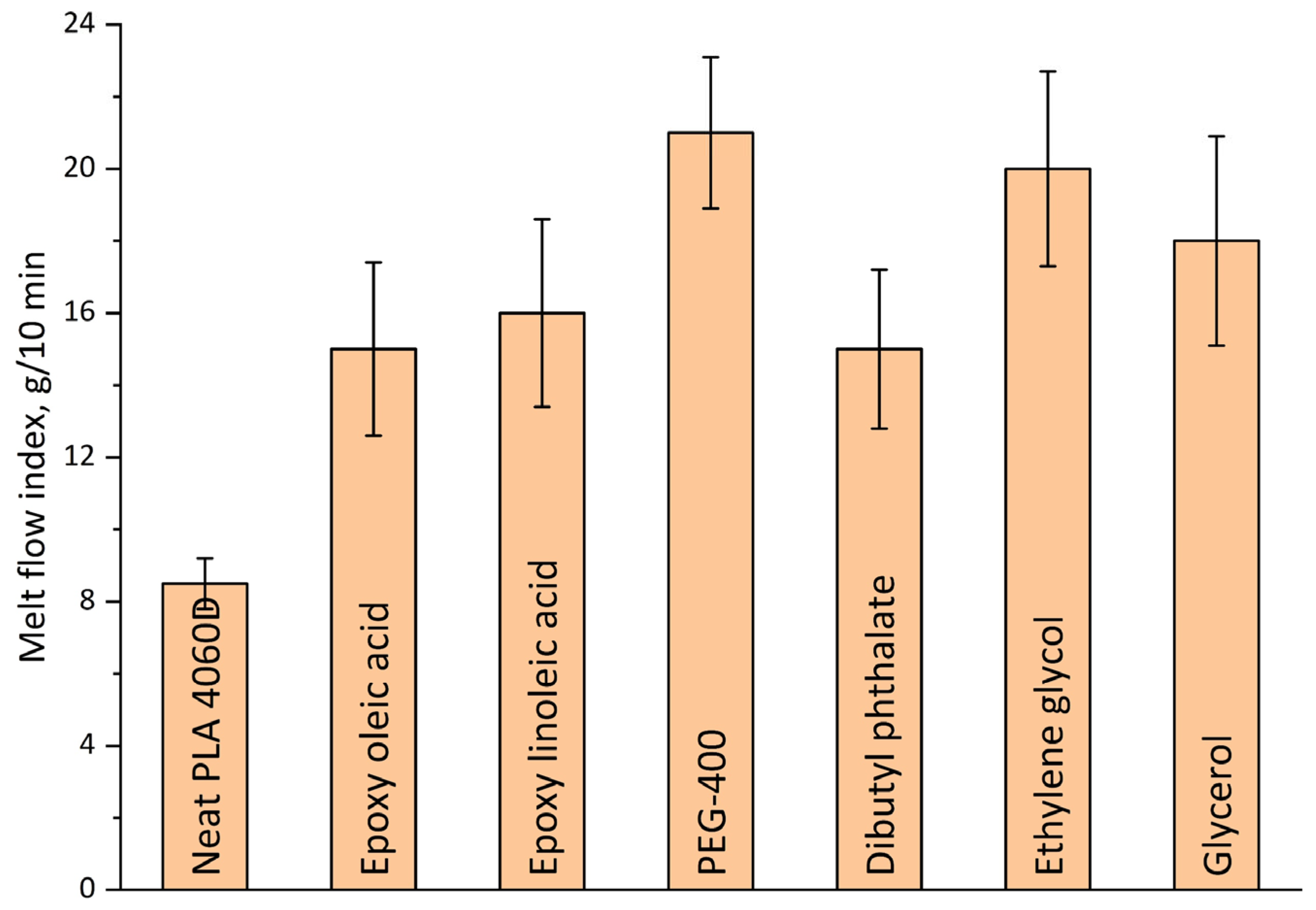
3.3. Plasticizer Effectiveness Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PLA | Polylactide |
| PEG | Minimum film forming temperature |
| DBP | Dibutyl phthalate |
| EG | Ethylene glycol |
| m-CPBA | Meta-chloroperoxybenzoic acid |
| SDS | Sodium dodecyl sulfate |
| RED | Relative energy difference |
| MFI | Melt flow index |
Appendix A
References
- Ghomi, E.R.R.; Khosravi, F.; Ardahaei, A.S.S.; Dai, Y.; Neisiany, R.E.; Foroughi, F.; Wu, M.; Das, O.; Ramakrishna, S. The Life Cycle Assessment for Polylactic Acid (PLA) to Make It a Low-Carbon Material. Polymers 2021, 13, 1854. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(Lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef]
- Teixeira, L.V.; Bomtempo, J.V.; De Almeida Oroski, F.; De Andrade Coutinho, P.L. The Diffusion of Bioplastics: What Can We Learn from Poly(Lactic Acid)? Sustainability 2023, 15, 4699. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Fernandes, I.; Barreiro, F.; Corcuera, M.A.; Eceiza, A. Advances in Waterborne Polyurethane and Polyurethane-Urea Dispersions and Their Eco-Friendly Derivatives: A Review. Polymers 2021, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ge, S.; Wang, J.; Zhang, X.; Zhang, T.; Lin, J.; Zhao, C.X.; Wang, B.; Zhu, G.; Guo, Z. Waterborne Acrylic Resin Modified with Glycidyl Methacrylate (GMA): Formula Optimization and Property Analysis. Polymer 2018, 143, 155–163. [Google Scholar] [CrossRef]
- Bandera, D.; Meyer, V.; Prevost, D.; Zimmermann, T.; Boesel, L. Polylactide/Montmorillonite Hybrid Latex as a Barrier Coating for Paper Applications. Polymers 2016, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiang, S.; Luan, Q.; Bao, Y.; Deng, Q.; Zheng, M.; Liu, S.; Song, J.; Tang, H.; Huang, F. Development of Poly (Lactic Acid) Microspheres and Their Potential Application in Pickering Emulsions Stabilization. Int. J. Biol. Macromol. 2017, 108, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Kishore, S.R.; Tomy, A.T.; Sugavaneswaran, M.; Scholz, S.G.; Elkaseer, A.; Wilson, V.H.; Rajan, A.J. Vapour Polishing of Fused Deposition Modelling (FDM) Parts: A Critical Review of Different Techniques, and Subsequent Surface Finish and Mechanical Properties of the Post-Processed 3D-Printed Parts. Prog. Addit. Manuf. 2023, 8, 1161–1178. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Gache, C.C.L.; Cascolan, H.M.S.; Cancino, L.T.; Advincula, R.C. Post-Processing of 3D-Printed Polymers. Technologies 2021, 9, 61. [Google Scholar] [CrossRef]
- Baker, G.; Vogel, E.; Smith, M. Glass Transitions in Polylactides. Polym. Rev. 2008, 48, 64–84. [Google Scholar] [CrossRef]
- Fekete, I.; Ronkay, F.; Lendvai, L. Highly Toughened Blends of Poly(Lactic Acid) (PLA) and Natural Rubber (NR) for FDM-Based 3D Printing Applications: The Effect of Composition and Infill Pattern. Polym. Test. 2021, 99, 107205. [Google Scholar] [CrossRef]
- Naddeo, M.; Sorrentino, A.; Pappalardo, D. Thermo-Rheological and Shape Memory Properties of Block and Random Copolymers of Lactide and Ε-Caprolactone. Polymers 2021, 13, 627. [Google Scholar] [CrossRef] [PubMed]
- Rafie, M.; Marsilla, K.K.; Hamid, Z.; Rusli, A.; Abdullah, M. Enhanced Mechanical Properties of Plasticized Polylactic Acid Filament for Fused Deposition Modelling: Effect of in Situ Heat Treatment. Prog. Rubber Plast. Recycl. Technol. 2019, 36, 131–142. [Google Scholar] [CrossRef]
- Gálvez, J.; Aguirre, J.C.; Salazar, M.H.; Mondragón, B.V.; Wagner, E.; Caicedo, C. Effect of Extrusion Screw Speed and Plasticizer Proportions on the Rheological, Thermal, Mechanical, Morphological and Superficial Properties of PLA. Polymers 2020, 12, 2111. [Google Scholar] [CrossRef] [PubMed]
- Shirai, M.A.; Grossmann, M.V.E.; Mali, S.; Yamashita, F.; Garcia, P.S.; Müller, C.M.O. Development of Biodegradable Flexible Films of Starch and Poly(Lactic Acid) Plasticized with Adipate or Citrate Esters. Carbohydr. Polym. 2012, 92, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Burgos, N.; Martino, V.P.; Jiménez, A. Characterization and Ageing Study of Poly(Lactic Acid) Films Plasticized with Oligomeric Lactic Acid. Polym. Degrad. Stab. 2012, 98, 651–658. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Y.; Ren, J.; Cao, E.; Fu, X.; Guo, W. Plasticizing Effect of Poly(Ethylene Glycol)s with Different Molecular Weights in Poly(Lactic Acid)/Starch Blends. J. Appl. Polym. Sci. 2015, 132, 41808. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Panariello, L.; Gigante, V.; Coltelli, M.-B.; Lazzeri, A. Sustainable Micro and Nano Additives for Controlling the Migration of a Biobased Plasticizer from PLA-Based Flexible Films. Polymers 2020, 12, 1366. [Google Scholar] [CrossRef]
- Ivorra-Martinez, J.; Gomez-Caturla, J.; Garcia-Sanoguera, D.; Moreno, V.; Dominici, F.; Puglia, D.; Torre, L. Effect of Reactive Extrusion in Plasticized Polylactide Formulations with Biobased Dibutyl Itaconate. Key Eng. Mater. 2023, 957, 81–86. [Google Scholar] [CrossRef]
- Xu, M.; Sun, W.; Li, C.; Li, J.; Tan, J.; Zhu, X. Facile Approach to Synthesis of Full Bio-Based Cardanol-Derived Plasticizer for Polylactic Acid with Excellent Plasticization and Water Vapor Resistance. Int. J. Biol. Macromol. 2025, 306, 141428. [Google Scholar] [CrossRef]
- Choi, K.-M.; Choi, M.-C.; Han, D.-H.; Park, T.-S.; Ha, C.-S. Plasticization of Poly(Lactic Acid) (PLA) through Chemical Grafting of Poly(Ethylene Glycol) (PEG) via in Situ Reactive Blending. Eur. Polym. J. 2013, 49, 2356–2364. [Google Scholar] [CrossRef]
- Sun, S.; Weng, Y.; Zhang, C. Recent Advancements in Bio-Based Plasticizers for Polylactic Acid (PLA): A Review. Polym. Test. 2024, 140, 108603. [Google Scholar] [CrossRef]
- Septevani, A.A.; Bhakri, S. Plasticization of Poly(Lactic Acid) Using Different Molecular Weight of Poly(Ethylene Glycol). AIP Conf. Proc. 2017, 1904, 020038. [Google Scholar] [CrossRef]
- Belyamani, I.; Najemi, L.; Wilson, K.; Abdullah, M.; Al-Badi, N. Influence of Glycerol and Clove Essential Oil on the Properties and Biodegradability of Poly(Lactic Acid)/Poly(Hydroxybutyrate-Co-Hydroxyvalerate) Blends. Int. J. Biol. Macromol. 2025, 308, 142698. [Google Scholar] [CrossRef]
- Maia, D.L.H.; Fernandes, A.N. Influence of Carboxylic Acid in the Production of Epoxidized Soybean Oil by Conventional and Ultrasound-Assisted Methods. Biomass Convers. Biorefinery 2020, 12, 5861–5868. [Google Scholar] [CrossRef]
- Rad, N.G.; Karami, Z.; Zohuriaan-Mehr, M.J.; Salimi, A.; Kabiri, K. Linseed Oil-based Reactive Diluents Preparation to Improve Tetra-functional Epoxy Resin Properties. Polym. Adv. Technol. 2019, 30, 2361–2369. [Google Scholar] [CrossRef]
- ISO 527-3:2019; Plastics-Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets. ISO: Geneva, Switzerland, 2019.
- ISO 1133-1:2022; Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics. ISO: Geneva, Switzerland, 2022.
- De Los Ríos, M.D.; Belmonte, R.M. Extending Microsoft Excel and Hansen Solubility Parameters Relationship to Double Hansen’s Sphere Calculation. SN Appl. Sci. 2022, 4, 185. [Google Scholar] [CrossRef]
- De Los Ríos, M.D.; Ramos, E.H. Determination of the Hansen Solubility Parameters and the Hansen Sphere Radius with the Aid of the Solver Add-in of Microsoft Excel. SN Appl. Sci. 2020, 2, 676. [Google Scholar] [CrossRef]
- Pulingam, T.; Foroozandeh, P.; Chuah, J.-A.; Sudesh, K. Exploring Various Techniques for the Chemical and Biological Synthesis of Polymeric Nanoparticles. Nanomaterials 2022, 12, 576. [Google Scholar] [CrossRef]
- Ramli, R.A. Hollow Polymer Particles: A Review. RSC Adv. 2017, 7, 52632–52650. [Google Scholar] [CrossRef]
- Fuji, M.; Han, Y.S.; Takai, C. Synthesis and Applications of Hollow Particles. KONA Powder Part J. 2013, 30, 47–68. [Google Scholar] [CrossRef]
- Raichur, A.; Nakajima, Y.; Nagaoka, Y.; Maekawa, T.; Kumar, D.S. Hollow Polymeric (PLGA) Nano Capsules Synthesized Using Solvent Emulsion Evaporation Method for Enhanced Drug Encapsulation and Release Efficiency. Mater. Res. Express 2014, 1, 045407. [Google Scholar] [CrossRef]
- Li, G.; Yu, Y.; Han, W.; Zhu, L.; Si, T.; Wang, H.; Li, K.; Sun, Y.; He, Y. Solvent Evaporation Self-Motivated Continual Synthesis of Versatile Porous Polymer Microspheres via Foaming-Transfer. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126239. [Google Scholar] [CrossRef]
- Sivadas, B.O.; Ashcroft, I.; Khlobystov, A.N.; Goodridge, R.D. Laser Sintering of Polymer Nanocomposites. Adv. Ind. Eng. Polym. Res. 2021, 4, 277–300. [Google Scholar] [CrossRef]
- Shen, F.; Yuan, S.; Chua, C.K.; Zhou, K. Development of Process Efficiency Maps for Selective Laser Sintering of Polymeric Composite Powders: Modeling and Experimental Testing. J. Mater. Process. Technol. 2017, 254, 52–59. [Google Scholar] [CrossRef]
- Bahloul, A.; Doghri, I.; Adam, L. Mesoscale Modelling of Polymer Powder Densification Due to Thermal Sintering. Appl. Math. Model. 2022, 114, 408–422. [Google Scholar] [CrossRef]
- Alqarni, M.H.; Haq, N.; Alam, P.; Abdel-Kader, M.S.; Foudah, A.I.; Shakeel, F. Solubility Data, Hansen Solubility Parameters and Thermodynamic Behavior of Pterostilbene in Some Pure Solvents and Different (PEG-400 + Water) Cosolvent Compositions. J. Mol. Liq. 2021, 331, 115700. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Litauszki, K.; Petrény, R.; Haramia, Z.; Mészáros, L. Combined Effects of Plasticizers and D-Lactide Content on the Mechanical and Morphological Behavior of Polylactic Acid. Heliyon 2023, 9, e14674. [Google Scholar] [CrossRef]
- Li, F.-J.; Liang, J.-Z.; Zhang, S.-D.; Zhu, B. Tensile Properties of Polylactide/Poly(Ethylene Glycol) Blends. J. Polym. Environ. 2015, 23, 407–415. [Google Scholar] [CrossRef]
- Petchwattana, N.; Sanetuntikul, J.; Narupai, B. Plasticization of Biodegradable Poly(Lactic Acid) by Different Triglyceride Molecular Sizes: A Comparative Study with Glycerol. J. Polym. Environ. 2017, 26, 1160–1168. [Google Scholar] [CrossRef]
- Belletti, G.; Buoso, S.; Ricci, L.; Guillem-Ortiz, A.; Aragón-Gutiérrez, A.; Bortolini, O.; Bertoldo, M. Preparations of Poly(Lactic Acid) Dispersions in Water for Coating Applications. Polymers 2021, 13, 2767. [Google Scholar] [CrossRef] [PubMed]
- Brito, E.L.; Ballard, N. Film Formation of Two-Stage Acrylic Latexes: Toward Soft-Core/Hard-Shell Systems. Macromol. Mater. Eng. 2023, 309, 2300287. [Google Scholar] [CrossRef]
- Pashley, R.M.; Karaman, M.E.; Ninham, B.W. A New Approach to the Measurement of the Minimum Film Formation Temperature of Latex Dispersions. Colloids Surf. A Physicochem. Eng. Asp. 2002, 211, 285–293. [Google Scholar] [CrossRef]
- Dron, S.M.; Paulis, M. Tracking Hydroplasticization by DSC: Movement of Water Domains Bound to Poly(Meth)Acrylates during Latex Film Formation. Polymers 2020, 12, 2500. [Google Scholar] [CrossRef]
- Kwon, Y.R.; Moon, S.K.; Kim, H.C.; Kim, J.S.; Kwon, M.; Kim, D.H. Effects of Grafting Degree on the Formation of Waterborne Polyurethane-Acrylate Film with Hard Core–Soft Shell Structure. Polymers 2023, 15, 3765. [Google Scholar] [CrossRef]
- Xu, C.-A.; Yang, Z. Progress in Polyurethane and Composites. Polymers 2024, 16, 2031. [Google Scholar] [CrossRef] [PubMed]
- De Santis, F.; Volpe, V.; Pantani, R. Effect of Molding Conditions on Crystallization Kinetics and Mechanical Properties of Poly(Lactic Acid). Polym. Eng. Sci. 2016, 57, 306–311. [Google Scholar] [CrossRef]
- Tábi, T.; Ageyeva, T.; Kovács, J.G. The Influence of Nucleating Agents, Plasticizers, and Molding Conditions on the Properties of Injection Molded PLA Products. Mater. Today Commun. 2022, 32, 103936. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Functional Olive Pit Powders: The Role of the Bio-Based Filler in Reducing the Water Uptake Phenomena of the Waterborne Paint. Coatings 2023, 13, 442. [Google Scholar] [CrossRef]
- Antil, B.; Elkasabi, Y.; Strahan, G.D.; Wal, R.L.V. Development of Graphitic and Non-Graphitic Carbons Using Different Grade Biopitch Sources. Carbon 2024, 232, 119770. [Google Scholar] [CrossRef]
- Cunha, I.L.C.; Rosa, F.; Kulay, L. Green Coalescent Synthesis Based on the Design for Environment (DFE) Principles: Brazilian Experience. Sustainability 2021, 13, 12802. [Google Scholar] [CrossRef]


| Sample | δD | δP | δH | R0 | Ra | RED | Reference |
|---|---|---|---|---|---|---|---|
| PLA 4060D | 16.5 | 9.9 | 6.4 | 8.5 | |||
| Epoxy oleic acid | 16.6 | 11.1 | 9.8 | 3.6 | 0.42 | ||
| Epoxy linoleic acid | 16.6 | 11.4 | 10.5 | 4.4 | 0.51 | ||
| PEG-400 | 14.6 | 7.5 | 9.4 | 5.4 | 0.64 | [39] | |
| Dibutyl phthalate | 17.8 | 8.6 | 4.1 | 3.7 | 0.44 | [40] | |
| Ethylene glycol | 17.0 | 11.0 | 26.0 | 19.7 | 2.31 | [40] | |
| Glycerol | 17.4 | 12.1 | 29.3 | 23.1 | 2.71 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myronyuk, O.; Baklan, D.; Bilousova, A.; Smalii, I.; Vorobyova, V.; Halysh, V.; Trus, I. Plasticized Polylactide Film Coating Formation from Redispersible Particles. AppliedChem 2025, 5, 14. https://doi.org/10.3390/appliedchem5030014
Myronyuk O, Baklan D, Bilousova A, Smalii I, Vorobyova V, Halysh V, Trus I. Plasticized Polylactide Film Coating Formation from Redispersible Particles. AppliedChem. 2025; 5(3):14. https://doi.org/10.3390/appliedchem5030014
Chicago/Turabian StyleMyronyuk, Oleksiy, Denys Baklan, Anna Bilousova, Illia Smalii, Victoria Vorobyova, Vita Halysh, and Inna Trus. 2025. "Plasticized Polylactide Film Coating Formation from Redispersible Particles" AppliedChem 5, no. 3: 14. https://doi.org/10.3390/appliedchem5030014
APA StyleMyronyuk, O., Baklan, D., Bilousova, A., Smalii, I., Vorobyova, V., Halysh, V., & Trus, I. (2025). Plasticized Polylactide Film Coating Formation from Redispersible Particles. AppliedChem, 5(3), 14. https://doi.org/10.3390/appliedchem5030014






