Kinetic Modeling, Comparative Investigations, and a New Approach to Quantifying the Global Extraction Yield of Algerian Pomegranate Peel Phenolic Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bioactive Extraction of Pomegranate Peel
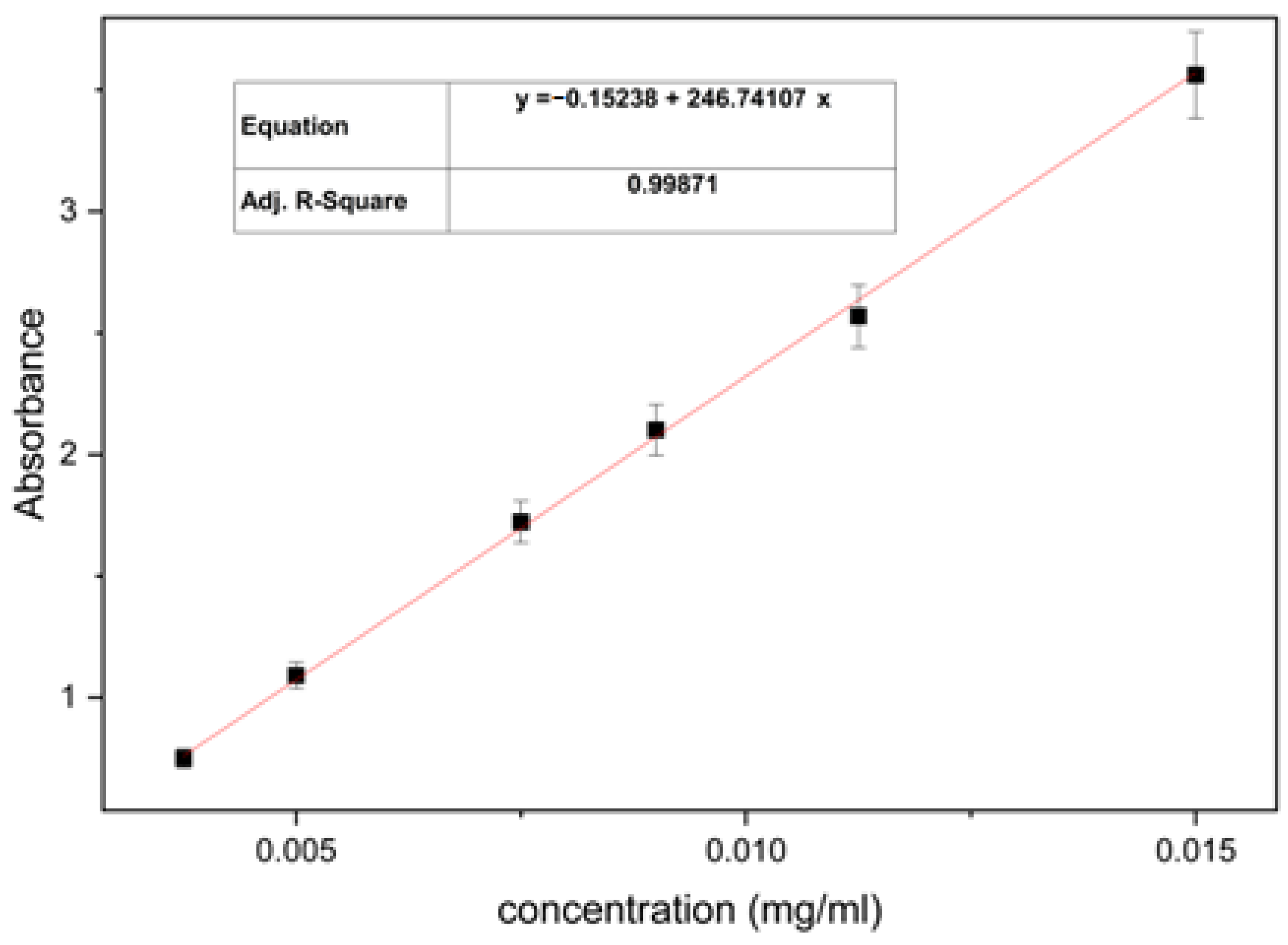
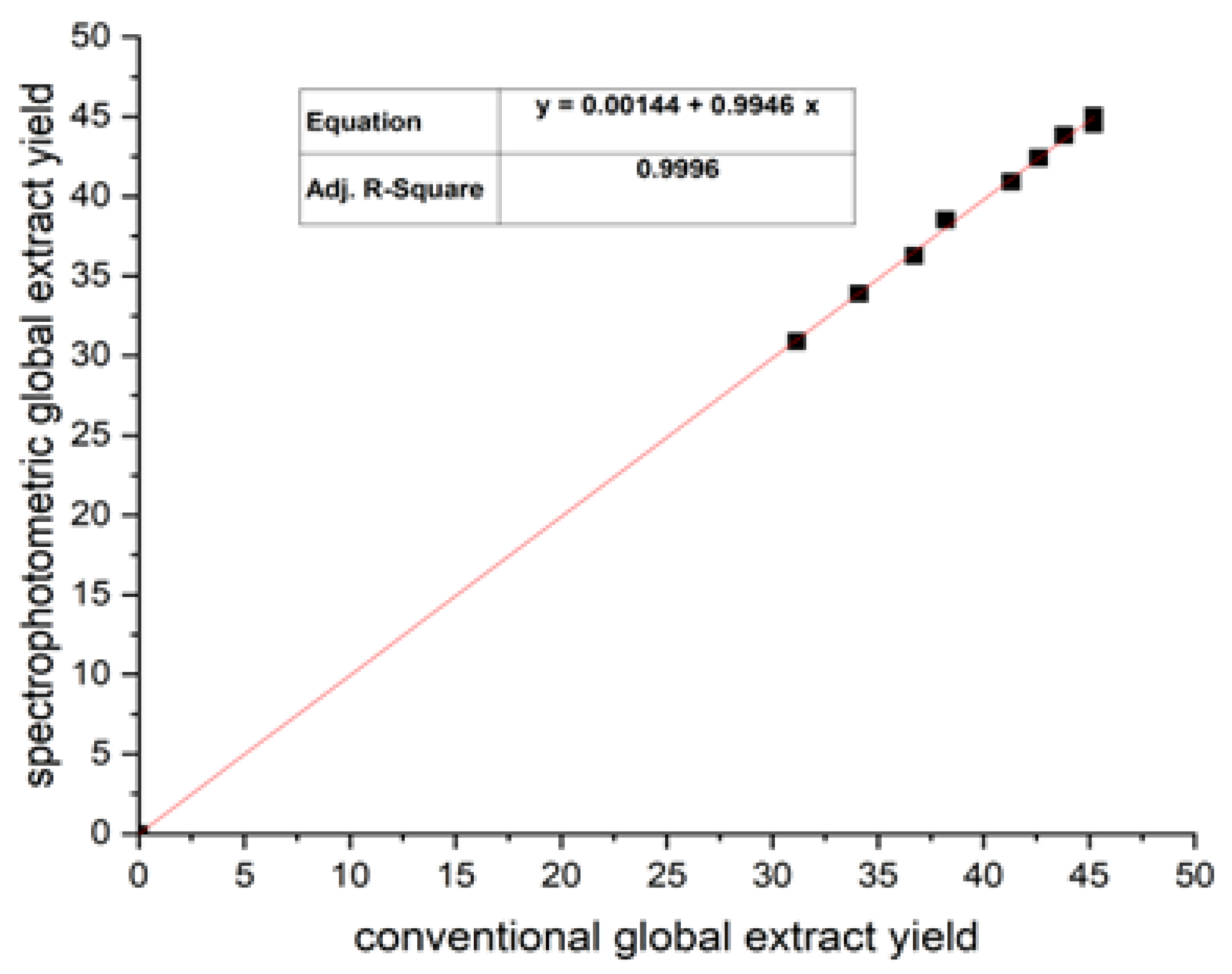
2.3. Quantification of Global Extraction Yield Without Evaporation by Spectrophotometry
- W1: weight of sample extraction.
- W2: initial weight of plant material.
2.4. Quantification of the Total Phenolic Compounds
2.5. The Quantification of the Total Flavonoid Compounds
2.6. Quantification of Condensed Tannin Compounds
2.7. Statistical Analysis
3. Results and Discussion
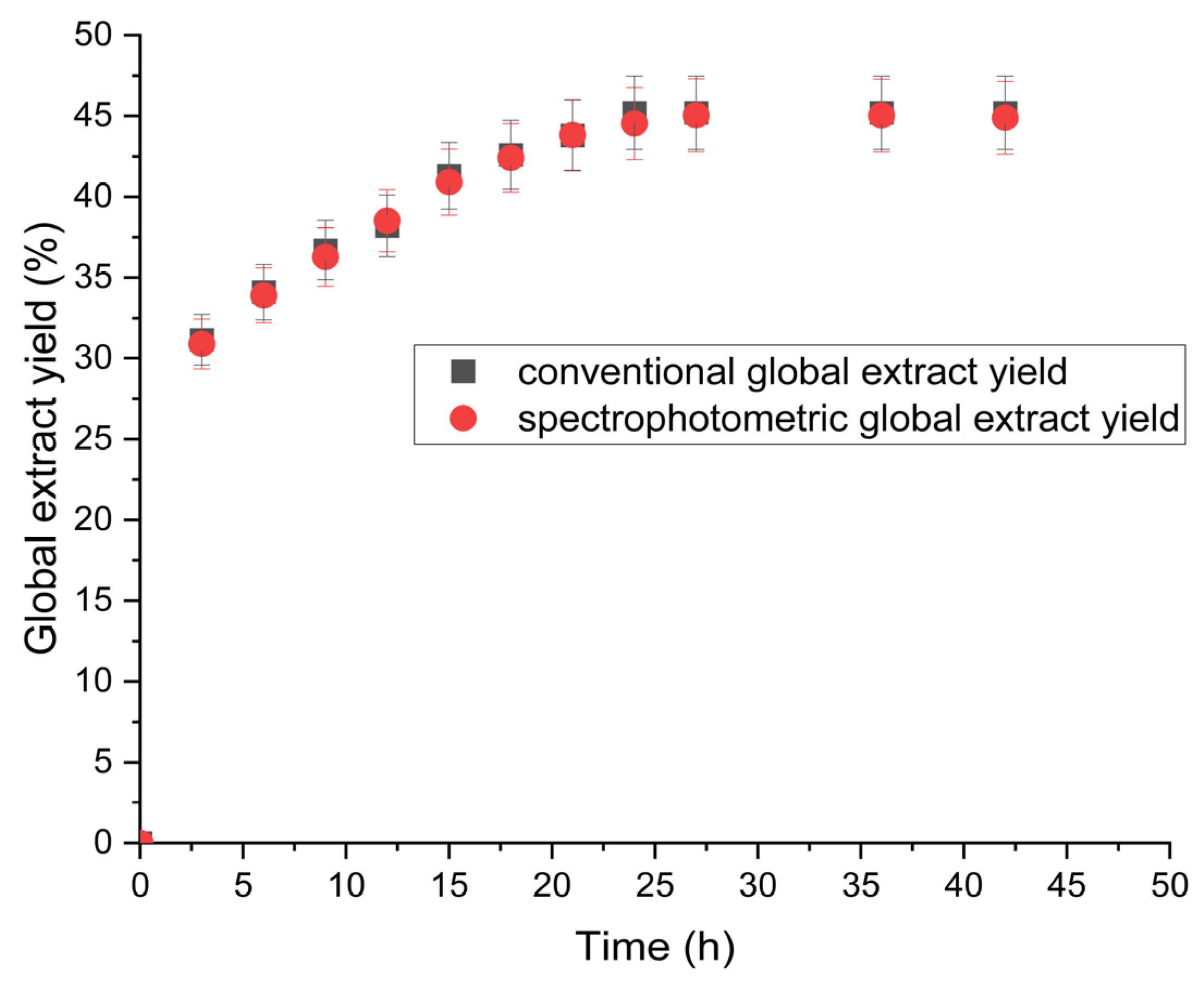
3.1. Quantification of Global Extract Yield Using the Spectrophotometry Method
3.2. Quantification of Total Phenolic Compounds, Total Flavonoids, and Condensed Tannins
3.3. Kinetic Study of TPC, TFC and CTC Contents
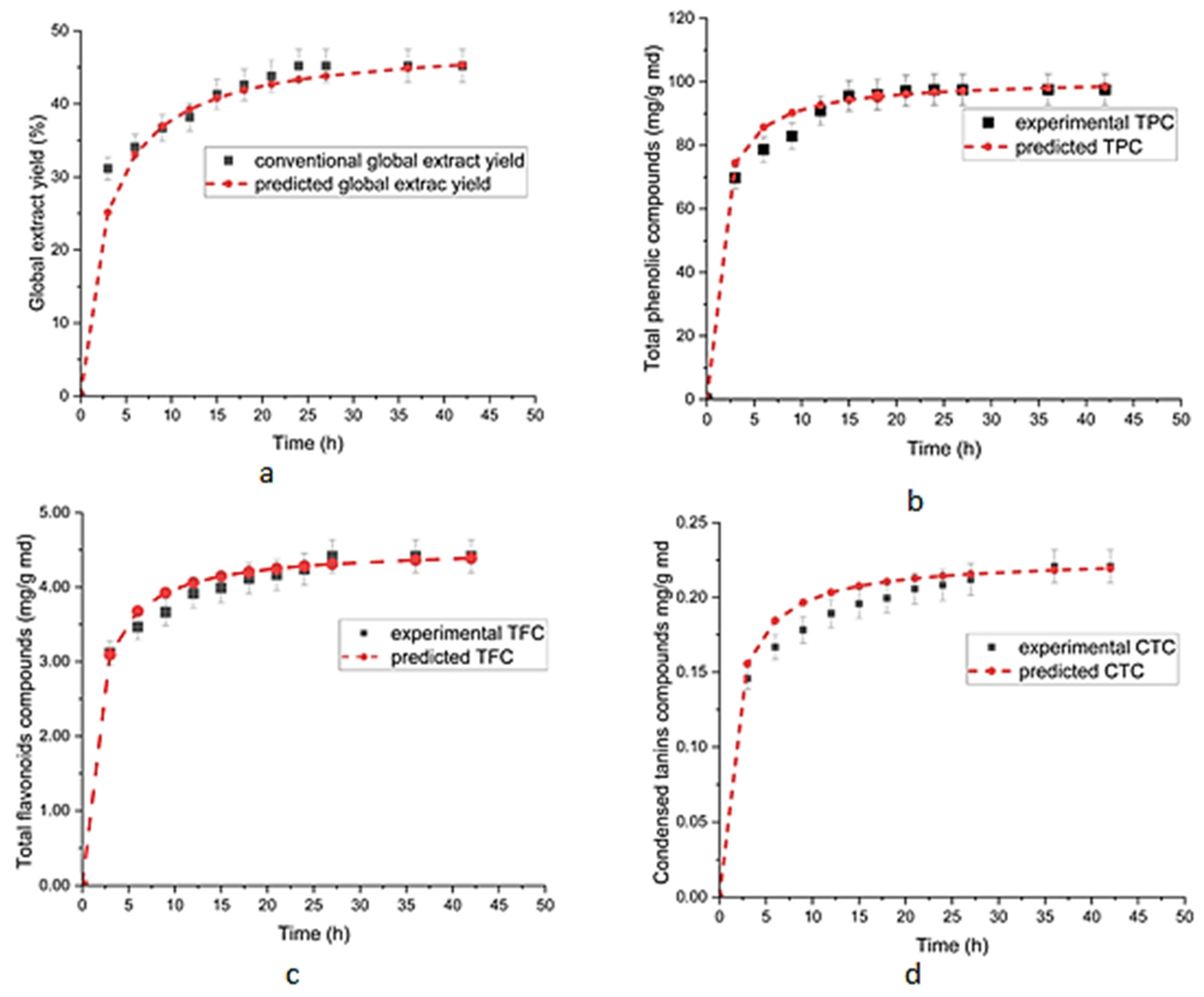
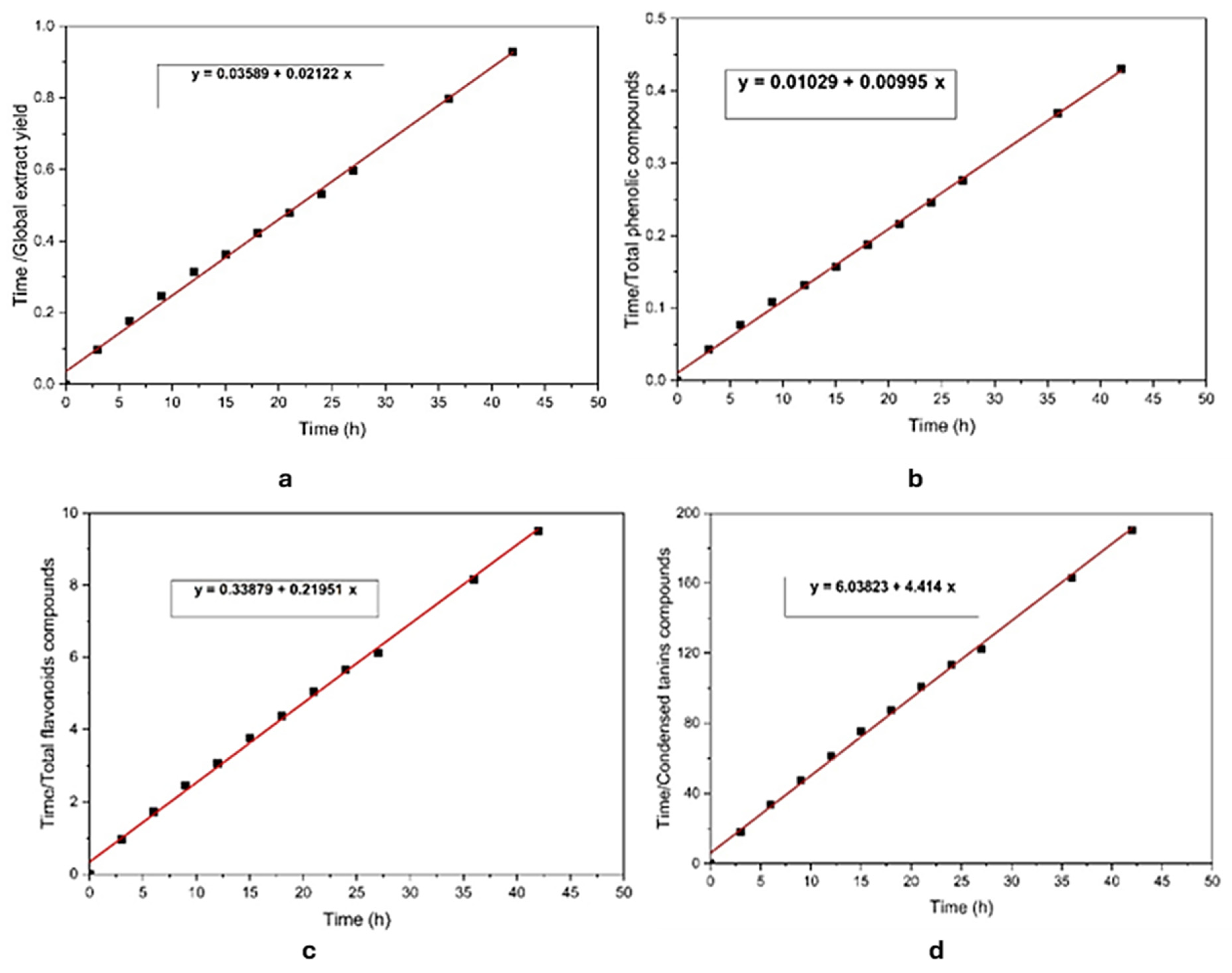
3.4. Kinetic Modeling Study of GEY, TPCs, TFCs, and CTCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luna-Guevara, M.L.; Luna-Guevara, J.J.; Hernández-Carranza, P.; Ruíz-Espinosa, H.; Ochoa-Velasco, C.E. Chapter 3—Phenolic Compounds: A Good Choice Against Chronic Degenerative Diseases. In Studies in Natural Products Chemistry; Atta-ur-Rahman, F.R.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 79–108. [Google Scholar]
- Teterovska, R.; Sile, I.; Paulausks, A.; Kovalcuka, L.; Koka, R.; Mauriņa, B.; Bandere, D. The Antioxidant Activity of Wild-Growing Plants Containing Phenolic Compounds in Latvia. Plants 2023, 12, 4108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Saini, N.; Anmol, A.; Kumar, S.; Wani, A.W.; Bakshi, M.; Dhiman, Z. Exploring Phenolic Compounds as Natural Stress Alleviators in Plants—A Comprehensive Review. Physiol. Mol. Plant Pathol. 2024, 133, 102383. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Ng, M.-S.; Cheng, S.-S.; Lo, A.W.-Y.; Xiao, Z.; Shin, T.-S.; Chung, G.; Lam, H.-M. Understanding the Composition, Biosynthesis, Accumulation and Transport of Flavonoids in Crops for the Promotion of Crops as Healthy Sources of Flavonoids for Human Consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef]
- Zaki, S.A.; Abdelatif, S.H.; Abdelmohsen, N.R.; Ismail, F.A. Phenolic Compounds and Antioxidant Activities of Pomegranate Peels. ETP Int. J. Food Eng. 2015, 1, 73–76. [Google Scholar] [CrossRef]
- Molino, S.; Pilar Francino, M.; Ángel Rufián Henares, J. Why Is It Important to Understand the Nature and Chemistry of Tannins to Exploit Their Potential as Nutraceuticals? Food Res. Int. 2023, 173, 113329. [Google Scholar] [CrossRef]
- De Melo, L.F.M.; Aquino-Martins, V.G.d.Q.; da Silva, A.P.; Oliveira Rocha, H.A.; Scortecci, K.C. Biological and Pharmacological Aspects of Tannins and Potential Biotechnological Applications. Food Chem. 2023, 414, 135645. [Google Scholar] [CrossRef]
- Moradnia, M.; Mohammadkhani, N.; Azizi, B.; Mohammadi, M.; Ebrahimpour, S.; Tabatabaei-Malazy, O.; Mirsadeghi, S.; Ale-Ebrahim, M. The Power of Punica granatum: A Natural Remedy for Oxidative Stress and Inflammation; a Narrative Review. J. Ethnopharmacol. 2024, 330, 118243. [Google Scholar] [CrossRef]
- Asgary, S.; Keshvari, M.; Sahebkar, A.; Sarrafzadegan, N. Pomegranate Consumption and Blood Pressure: A Review. Curr. Pharm. Des. 2017, 23, 1042–1050. [Google Scholar] [CrossRef]
- Cordiano, R.; Gammeri, L.; Di Salvo, E.; Gangemi, S.; Minciullo, P.L. Pomegranate (Punica granatum L.) Extract Effects on Inflammaging. Molecules 2024, 29, 4174. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Merma, P.R.; Cornejo-Figueroa, M.H.; Crisosto-Fuster, A.d.R.; Strieder, M.M.; Chañi-Paucar, L.O.; Náthia-Neves, G.; Rodríguez-Papuico, H.; Rostagno, M.A.; Meireles, M.A.A.; Alcázar-Alay, S.C. Phenolic Compounds Recovery from Pomegranate (Punica granatum L.) By-Products of Pressurized Liquid Extraction. Foods 2022, 11, 1070. [Google Scholar] [CrossRef] [PubMed]
- Mungwari, C.P.; King’ondu, C.K.; Sigauke, P.; Obadele, B.A. Conventional and Modern Techniques for Bioactive Compounds Recovery from Plants: Review. Sci. Afr. 2025, 27, e02509. [Google Scholar] [CrossRef]
- Mao, Y.; Robinson, J.; Binner, E. Understanding Heat and Mass Transfer Processes during Microwave-Assisted and Conventional Solvent Extraction. Chem. Eng. Sci. 2021, 233, 116418. [Google Scholar] [CrossRef]
- Moussa, H.; Dahmoune, F.; Hentabli, M.; Remini, H.; Mouni, L. Optimization of ultrasound-assisted extraction of phenolic-saponin content from Carthamus caeruleus L. rhizome and predictive model based on support vector regression optimized by dragonfly algorithm. Chemom. Intell. Lab. Syst. 2022, 222, 104493. [Google Scholar] [CrossRef]
- Lemes, A.C.; Egea, M.B.; de Oliveira Filho, J.G.; Gautério, G.V.; Ribeiro, B.D.; Coelho, M.A.Z. Biological Approaches for Extraction of Bioactive Compounds From Agro-Industrial By-Products: A Review. Front. Bioeng. Biotechnol. 2022, 9, 802543. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A Review of Modern and Conventional Extraction Techniques and Their Applications for Extracting Phytochemicals from Plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hobbi, P.; Okoro, O.V.; Delporte, C.; Alimoradi, H.; Podstawczyk, D.; Nie, L.; Bernaerts, K.V.; Shavandi, A. Kinetic Modelling of the Solid–Liquid Extraction Process of Polyphenolic Compounds from Apple Pomace: Influence of Solvent Composition and Temperature. Bioresour. Bioprocess. 2021, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Galgano, F.; Tolve, R.; Scarpa, T.; Caruso, M.C.; Lucini, L.; Senizza, B.; Condelli, N. Extraction Kinetics of Total Polyphenols, Flavonoids, and Condensed Tannins of Lentil Seed Coat: Comparison of Solvent and Extraction Methods. Foods 2021, 10, 1810. [Google Scholar] [CrossRef]
- Villamil-Galindo, E.; Piagentini, A.M. Kinetic Modeling of Valuable Phenolic Compounds Extraction from Strawberry and Apple Agro-Industrial by-Products. J. Food Process Eng. 2024, 47, e14573. [Google Scholar] [CrossRef]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A Powerful and Greener Alternative to the Latest Solid-Liquid Extraction Techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef]
- Susanti, D.Y.; Sediawan, W.B.; Fahrurrozi, M.; Hidayat, M. A Mechanistic Model of Mass Transfer in the Extraction of Bioactive Compounds from Intact Sorghum Pericarp. Processes 2019, 7, 837. [Google Scholar] [CrossRef]
- Pourhakkak, P.; Taghizadeh, A.; Taghizadeh, M.; Ghaedi, M.; Haghdoust, S. Chapter 1—Fundamentals of Adsorption Technology. In Interface Science and Technology; Ghaedi, M., Ed.; Adsorption: Fundamental Processes and Applications; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 1–70. [Google Scholar]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.-V.N.; Prabhakar, S. Techniques and Modeling of Polyphenol Extraction from Food: A Review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Xu, H.; Fei, Q.; Manickam, S.; Li, D.; Xiao, H.; Han, Y.; Show, P.L.; Zhang, G.; Tao, Y. Mechanistic Study of the Solid-Liquid Extraction of Phenolics from Walnut Pellicle Fibers Enhanced by Ultrasound, Microwave and Mechanical Agitation Forces. Chemosphere 2022, 309, 136451. [Google Scholar] [CrossRef]
- Shen, Z.; Ji, X.; Yao, S.; Zhang, H.; Xiong, L.; Li, H.; Chen, X.; Chen, X. Solid-Liquid Extraction of Chlorogenic Acid from Eucommia Ulmoides Oliver Leaves: Kinetic and Mass Transfer Studies. Ind. Crops Prod. 2023, 205, 117544. [Google Scholar] [CrossRef]
- Tran, H.N. Applying Linear Forms of Pseudo-Second-Order Kinetic Model for Feasibly Identifying Errors in the Initial Periods of Time-Dependent Adsorption Datasets. Water 2023, 15, 1231. [Google Scholar] [CrossRef]
- Kostoglou, M.; Karapantsios, T.D. Why Is the Linearized Form of Pseudo-Second Order Adsorption Kinetic Model So Successful in Fitting Batch Adsorption Experimental Data? Colloids Interfaces 2022, 6, 55. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Maghat, H. Critical of Linear and Nonlinear Equations of Pseudo-First Order and Pseudo-Second Order Kinetic Models. Karbala Int. J. Mod. Sci. 2018, 4, 244–254. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; du Toit, W. The Role of UV-Visible Spectroscopy for Phenolic Compounds Quantification in Winemaking. In Frontiers and New Trends in the Science of Fermented Food and Beverages; IntechOpen: London, UK, 2018; ISBN 978-1-78985-496-1. [Google Scholar]
- Mihaly Cozmuta, A.; Peter, A.; Nicula, C.; Jastrzębska, A.; Jakubczak, M.; Purbayanto, M.A.K.; Bunea, A.; Bora, F.; Uivarasan, A.; Szakács, Z.; et al. The Impact of Visible Light Component Bands on Polyphenols from Red Grape Seed Extract Powder Encapsulated in Alginate–Whey Protein Matrix. Food Chem. X 2024, 23, 101758. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stănilă, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Tian, W.; Chen, G.; Gui, Y.; Zhang, G.; Li, Y. Rapid Quantification of Total Phenolics and Ferulic Acid in Whole Wheat Using UV–Vis Spectrophotometry. Food Control 2021, 123, 107691. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin–Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. In Measurement of Antioxidant Activity & Capacity; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 107–115. ISBN 978-1-119-13538-8. [Google Scholar]
- Ali-Rachedi, F.; Meraghni, S.; Touaibia, N.; Mesbah, S. Analyse quantitative des composés phénoliques d’une endémique algérienne Scabiosa Atropurpurea sub. Maritima L. Bull. Soc. R. Sci. Liège 2018, 87, 13–21. [Google Scholar] [CrossRef]
- Thitz, P.; Mehtätalo, L.; Välimäki, P.; Randriamanana, T.; Lännenpää, M.; Hagerman, A.E.; Andersson, T.; Julkunen-Tiitto, R.; Nyman, T. Phytochemical Shift from Condensed Tannins to Flavonoids in Transgenic Betula Pendula Decreases Consumption and Growth but Improves Growth Efficiency of Epirrita Autumnata Larvae. J. Chem. Ecol. 2020, 46, 217–231. [Google Scholar] [CrossRef]
- Bergamin, E.; Fabris, G.; Neffat, M.; Toaldo, P.B.; Luchi, D.D.; Bonomi, R. Multiple Solid–Liquid Extraction: Determination of Extraction Yield through UV–Vis Spectroscopy. J. Chem. Educ. 2023, 100, 875–879. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the Extraction Method on the Recovery of Bioactive Phenolic Compounds from Food Industry By-Products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Ramesh, M.M.; Shankar, N.S.; Venkatappa, A.H. Driving/Critical Factors Considered During Extraction to Obtain Bioactive Enriched Extracts. Pharmacogn. Rev. 2024, 18, 68–81. [Google Scholar] [CrossRef]
- Bennour, N.; Mighri, H.; Eljani, H.; Zammouri, T.; Akrout, A. Effect of Solvent Evaporation Method on Phenolic Compounds and the Antioxidant Activity of Moringa Oleifera Cultivated in Southern Tunisia. S. Afr. J. Bot. 2020, 129, 181–190. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Benguiar, R.; Yahla, I.; Benaraba, R.; Bouamar, S.; Riazi, A. Phytochemical Analysis, Antibacterial and Antioxidant Activities of Pomegranate (Punica granatum L.) Peel Extracts. Int. J. Biosci. IJB 2020, 16, 35–44. [Google Scholar] [CrossRef]
- Ghasemi, R.; Akrami Mohajeri, F.; Heydari, A.; Yasini, S.A.; Dehghani Tafti, A.; Khalili Sadrabad, E. Application of Pomegranate Peel Extract, a Waste Agricultural Product, as a Natural Preservative in Tahini. Int. J. Food Sci. 2023, 2023, e8860476. [Google Scholar] [CrossRef]
- Hadjadj, S.; Benyahkem, M.; Lamri, K.; Ould El Hadj-Khelil, A. Potential Assessment of Pomegranate (Punica granatum L.) Fruit Peels as A Source of Natural Antioxidants. Pharmacophore 2018, 9, 29–34. [Google Scholar]
- Hasnaoui, N.; Wathelet, B.; Jiménez-Araujo, A. Valorization of Pomegranate Peel from 12 Cultivars: Dietary Fibre Composition, Antioxidant Capacity and Functional Properties. Food Chem. 2014, 160, 196–203. [Google Scholar] [CrossRef]
- Sabraoui, T.; Khider, T.; Nasser, B.; Eddoha, R.; Moujahid, A.; Benbachir, M.; Essamadi, A. Determination of Punicalagins Content, Metal Chelating, and Antioxidant Properties of Edible Pomegranate (Punica granatum L) Peels and Seeds Grown in Morocco. Int. J. Food Sci. 2020, 2020, 8885889. [Google Scholar] [CrossRef]
- Chaabna, N.; Naili, O.; Ziane, N.; Bensouici, C.; Dahamna, S.; Harzallah, D. In Vitro Antioxidant, Anti-Alzheimer and Antibacterial Activities of Ethyl Acetate and n-Butanol Fractions of Punica granatum Peel from Algeria. J. Nat. Prod. Res. TJNPR 2023, 7, 3470–3477. [Google Scholar] [CrossRef]
- Salim, A.; Deiana, P.; Fancello, F.; Molinu, M.G.; Santona, M.; Zara, S. Antimicrobial and Antibiofilm Activities of Pomegranate Peel Phenolic Compounds: Varietal Screening through a Multivariate Approach. J. Bioresour. Bioprod. 2023, 8, 146–161. [Google Scholar] [CrossRef]
- Abid, M.; Yaich, H.; Cheikhrouhou, S.; Khemakhem, I.; Bouaziz, M.; Attia, H.; Ayadi, M.A. Antioxidant Properties and Phenolic Profile Characterization by LC-MS/MS of Selected Tunisian Pomegranate Peels. J. Food Sci. Technol. 2017, 54, 2890–2901. [Google Scholar] [CrossRef]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant Activity and Total Phenolic Content of Ethanolic Extract of Pomegranate Peels, Juice and Seeds. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 114, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Hlima, H.B.; Bohli, T.; Kraiem, M.; Ouederni, A.; Mellouli, L.; Michaud, P.; Abdelkafi, S.; Smaoui, S. Combined Effect of Spirulina Platensis and Punica granatum Peel Extacts: Phytochemical Content and Antiphytophatogenic Activity. Appl. Sci. 2019, 9, 5475. [Google Scholar] [CrossRef]
- Mirzaeva, S.U.; Muxamadiev, B.T.; Mirzaeva, S.U.; Muxamadiev, B.T. Perspective Chapter: Theoretical Foundations of the Extraction Process; IntechOpen: London, UK, 2024; ISBN 978-0-8a5466-739-0. [Google Scholar]
- Ponphaiboon, J.; Krongrawa, W.; Aung, W.W.; Chinatangkul, N.; Limmatvapirat, S.; Limmatvapirat, C. Advances in Natural Product Extraction Techniques, Electrospun Fiber Fabrication, and the Integration of Experimental Design: A Comprehensive Review. Molecules 2023, 28, 5163. [Google Scholar] [CrossRef]
- Shibasaki-Kitakawa, N.; Iizuka, Y.; Takahashi, A.; Yonemoto, T. A Kinetic Model for Flavonoid Production in Tea Cell Culture. Bioprocess. Biosyst. Eng. 2017, 40, 211–219. [Google Scholar] [CrossRef]
- Agu, C.M.; Menkiti, M.C.; Ohale, P.E.; Ugonabo, V.I. Extraction Modeling, Kinetics, and Thermodynamics of Solvent Extraction of Irvingia Gabonensis Kernel Oil, for Possible Industrial Application. Eng. Rep. 2021, 3, e12306. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H. Kinetics Studies on Effects of Extraction Techniques on Bioactive Compounds from Vernonia Cinerea Leaf. J. Food Sci. Technol. 2019, 56, 580–588. [Google Scholar] [CrossRef]
| Country | Solvent | Extraction Time (h) | TPC | TFC | CTC | References |
|---|---|---|---|---|---|---|
| Morocco | Methanol | 24 | 63.34 (mg GAE/g) | 2.11 (mg RE/g) | NM | [51] |
| Algeria | Ethanol/water 70/30 | 24 | 677.55 µg GAE/mg | 93.48 µg QE/mg | 106.95 (µg TAE/mg) | [52] |
| Italy | Ultrapure water | 4 | 127.0 mg/g | 20.8 mg/g | 7.5 (mg/g) | [53] |
| Tunisia | Acetone | 1 | 304.60 mg GAE/g | 15.46 mg Quer/g | 292.23 (mg GAE/g) | [54] |
| Iran | Ethanol | 48 | 276 mg GAE/g | 36 mg rutin/g | NM | [55] |
| Tunisia | Ethanol | 24 | 131.14 (mg GAE/g) | 6.75 (mg QE/g) | NM | [56] |
| Algeria | Methanol/water 75/25 | 21 | 97.17 mg/g md | 4.115 mg | 411.75 µg | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherdaoui, D.; Bouazza, F.; Ihadadene, S.; Yahoum, M.M.; Lefnaoui, S.; Amrane, A.; Mouni, L. Kinetic Modeling, Comparative Investigations, and a New Approach to Quantifying the Global Extraction Yield of Algerian Pomegranate Peel Phenolic Compounds. AppliedChem 2025, 5, 11. https://doi.org/10.3390/appliedchem5020011
Gherdaoui D, Bouazza F, Ihadadene S, Yahoum MM, Lefnaoui S, Amrane A, Mouni L. Kinetic Modeling, Comparative Investigations, and a New Approach to Quantifying the Global Extraction Yield of Algerian Pomegranate Peel Phenolic Compounds. AppliedChem. 2025; 5(2):11. https://doi.org/10.3390/appliedchem5020011
Chicago/Turabian StyleGherdaoui, Dehbiya, Fatma Bouazza, Samira Ihadadene, Madiha Melha Yahoum, Sonia Lefnaoui, Abdeltif Amrane, and Lotfi Mouni. 2025. "Kinetic Modeling, Comparative Investigations, and a New Approach to Quantifying the Global Extraction Yield of Algerian Pomegranate Peel Phenolic Compounds" AppliedChem 5, no. 2: 11. https://doi.org/10.3390/appliedchem5020011
APA StyleGherdaoui, D., Bouazza, F., Ihadadene, S., Yahoum, M. M., Lefnaoui, S., Amrane, A., & Mouni, L. (2025). Kinetic Modeling, Comparative Investigations, and a New Approach to Quantifying the Global Extraction Yield of Algerian Pomegranate Peel Phenolic Compounds. AppliedChem, 5(2), 11. https://doi.org/10.3390/appliedchem5020011







