Controlled Nickel Nanoparticles: A Review on How Parameters of Synthesis Can Modulate Their Features and Properties
Abstract
1. Introduction
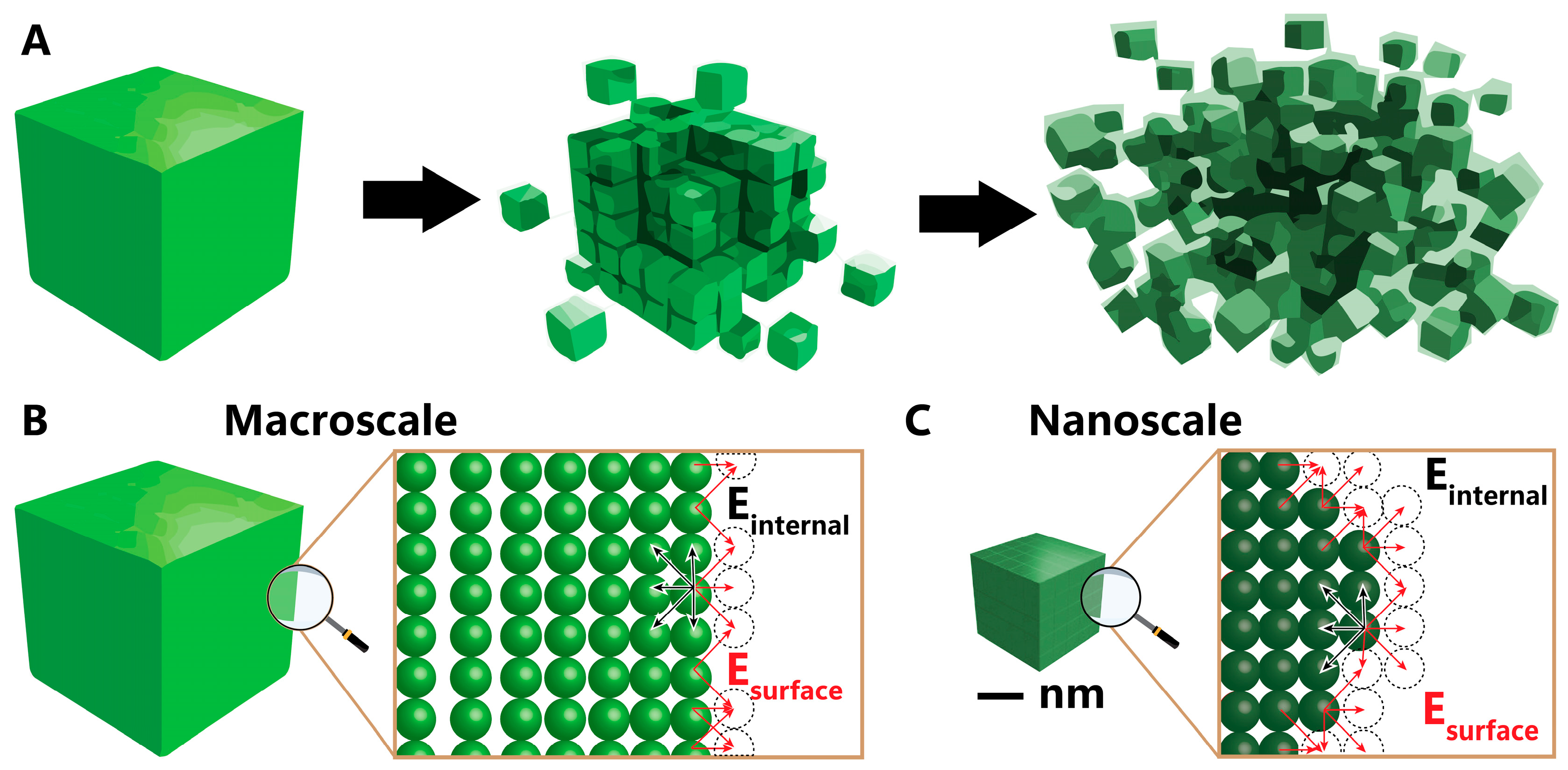
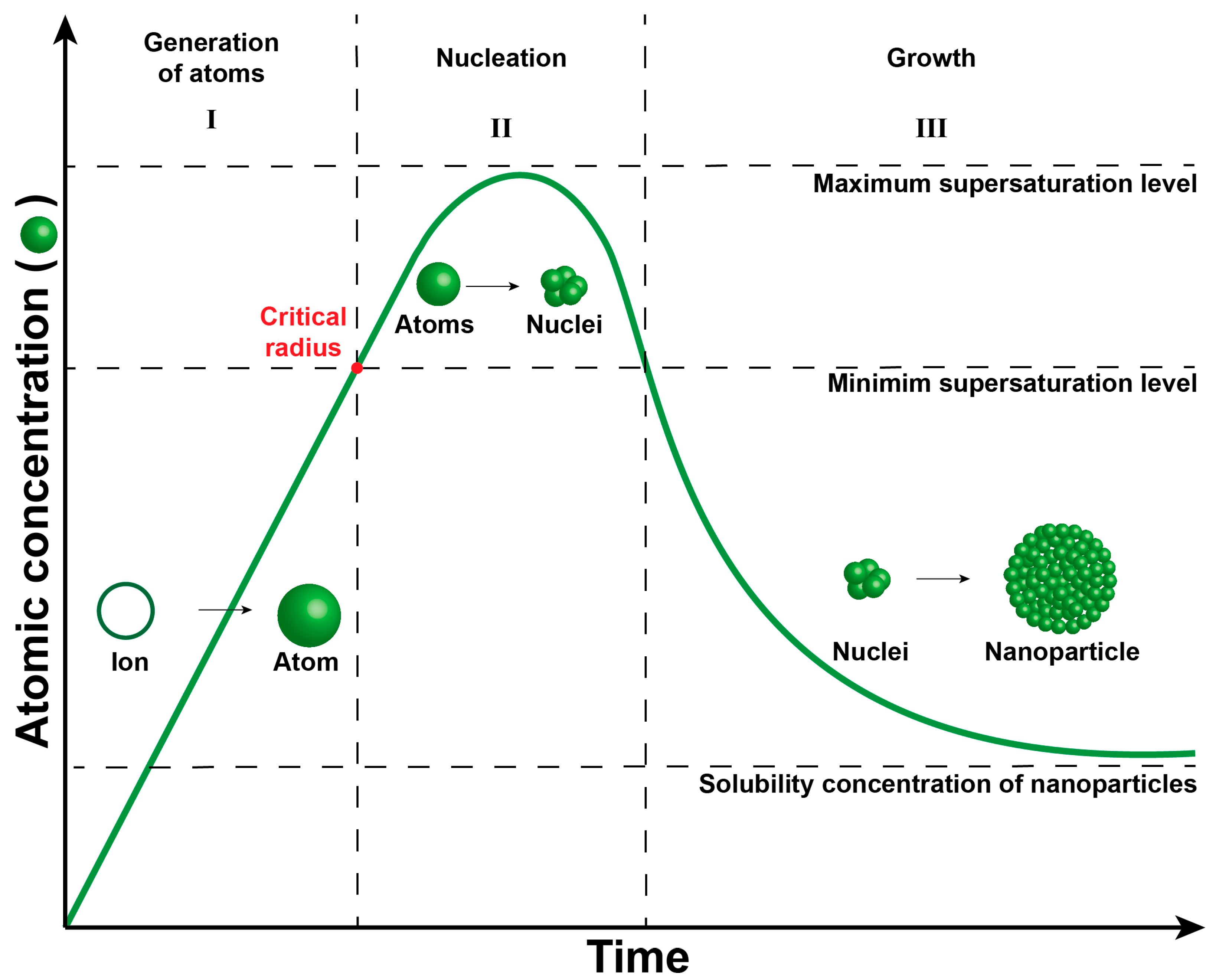
2. Nickel Nanoparticle Synthesis
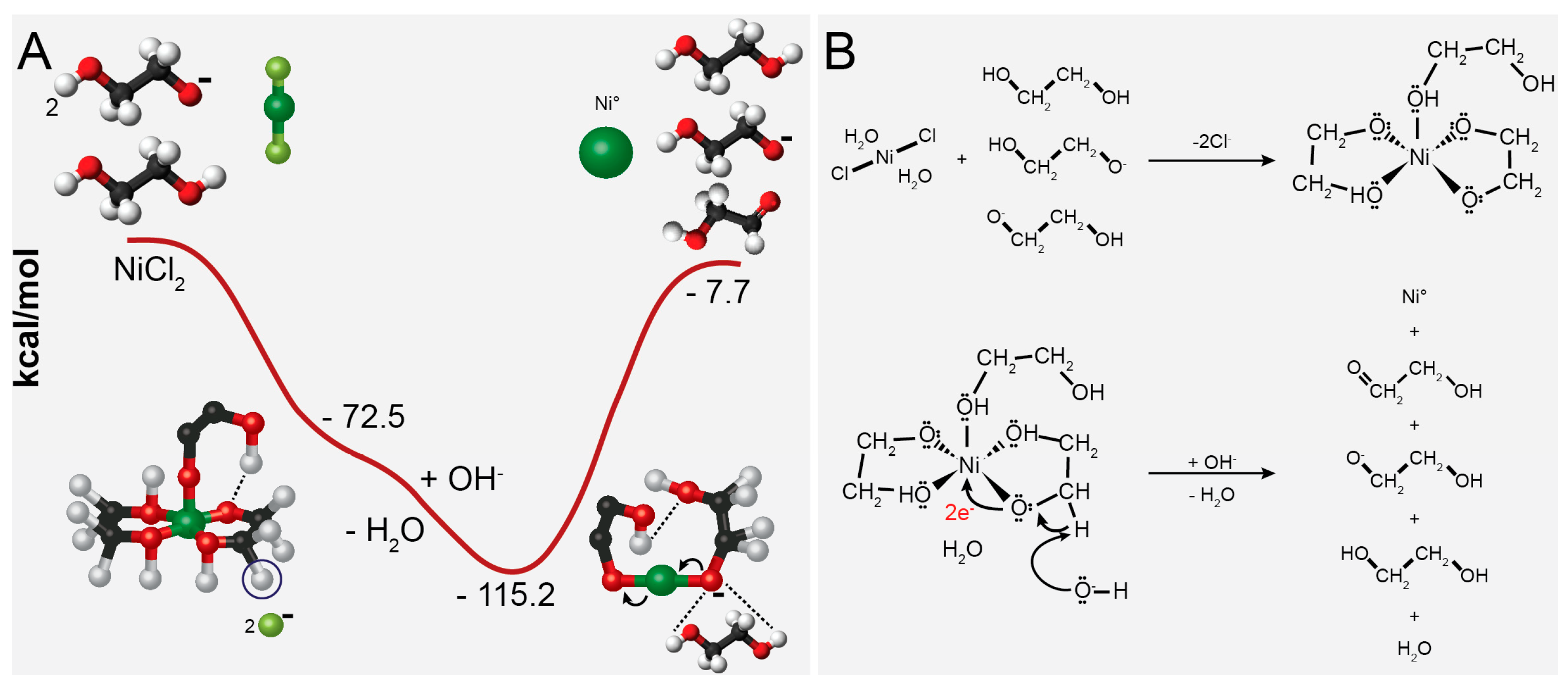
2.1. Reducing Agent
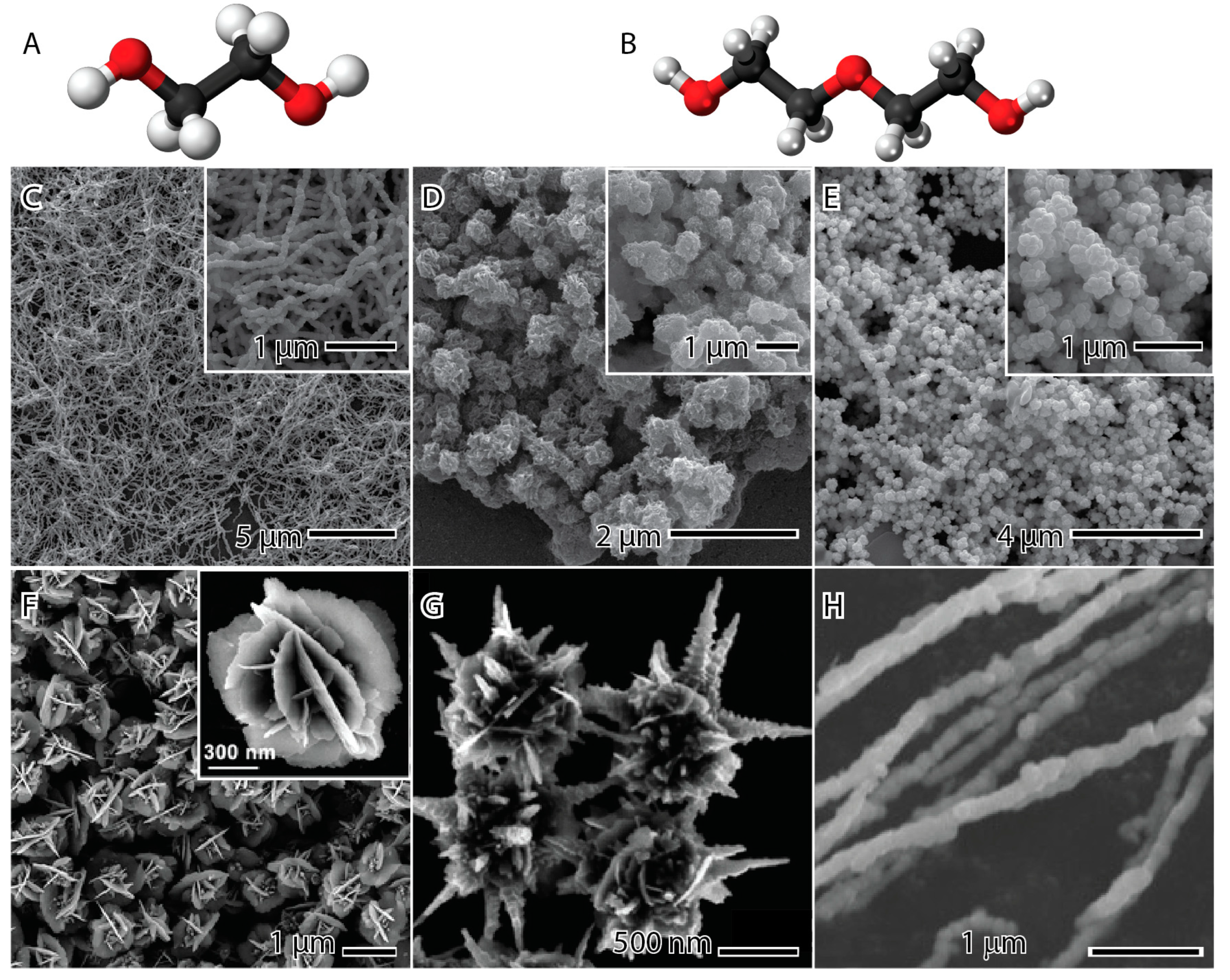
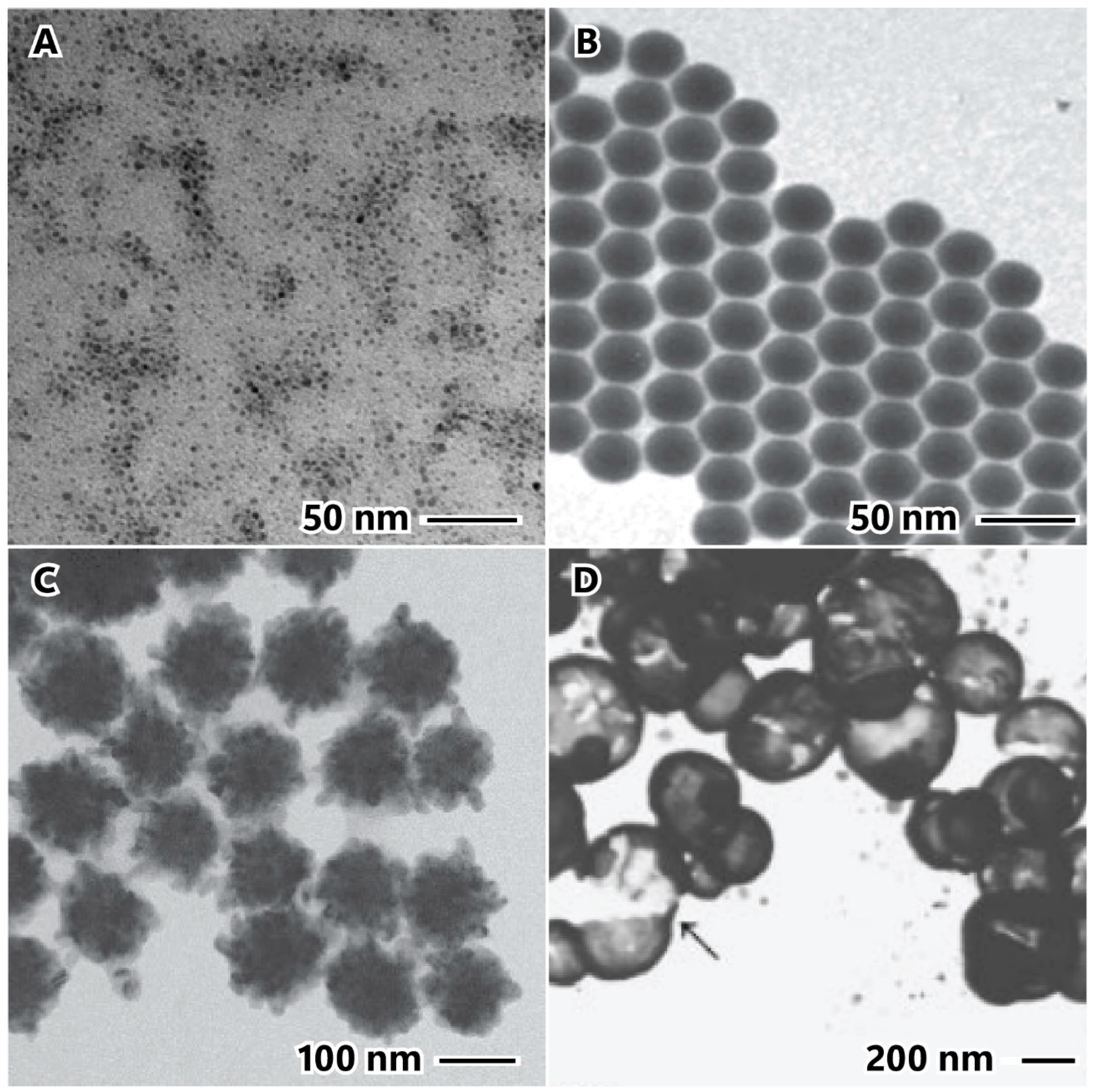
2.2. Stabilizing Agent
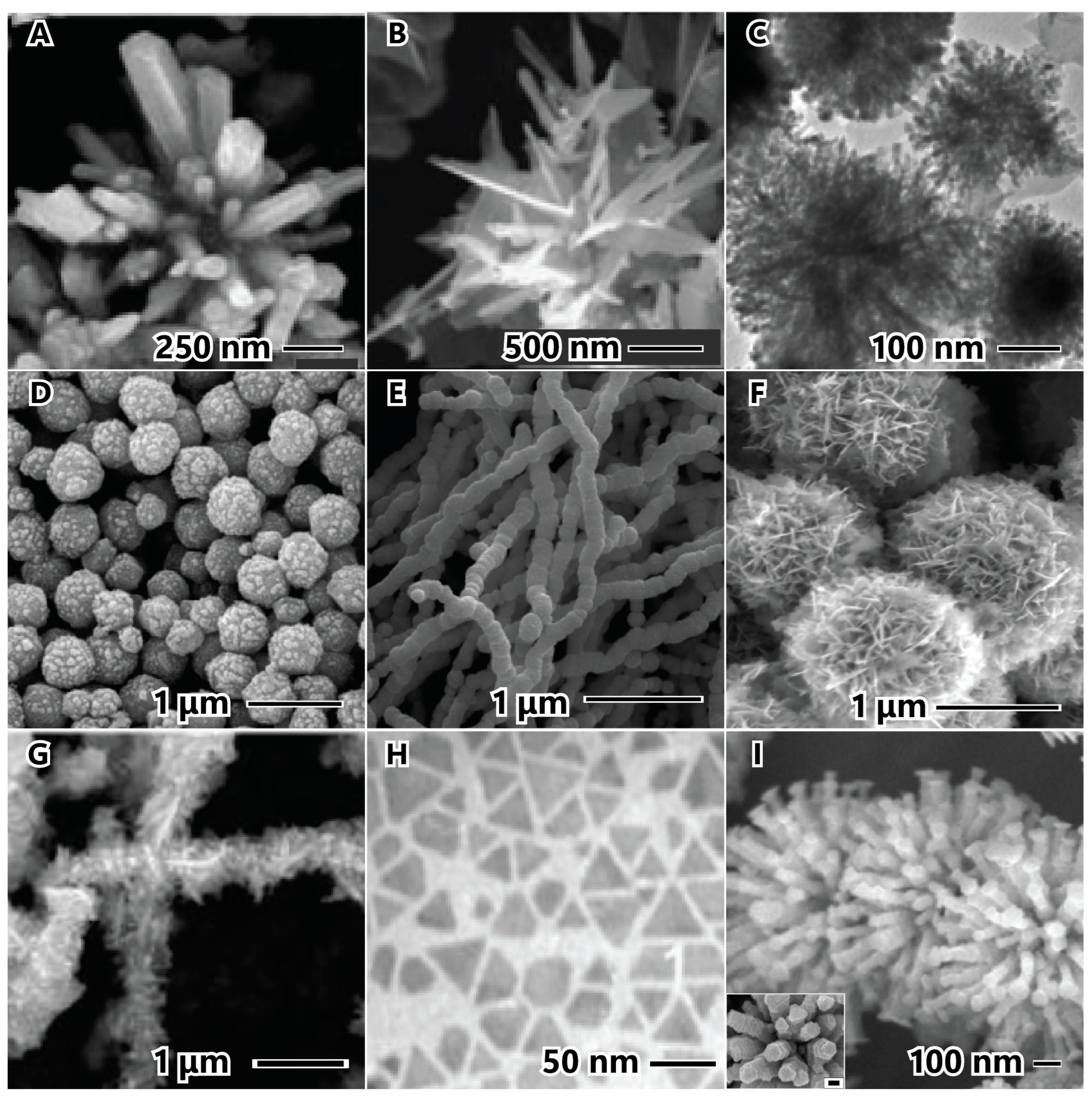
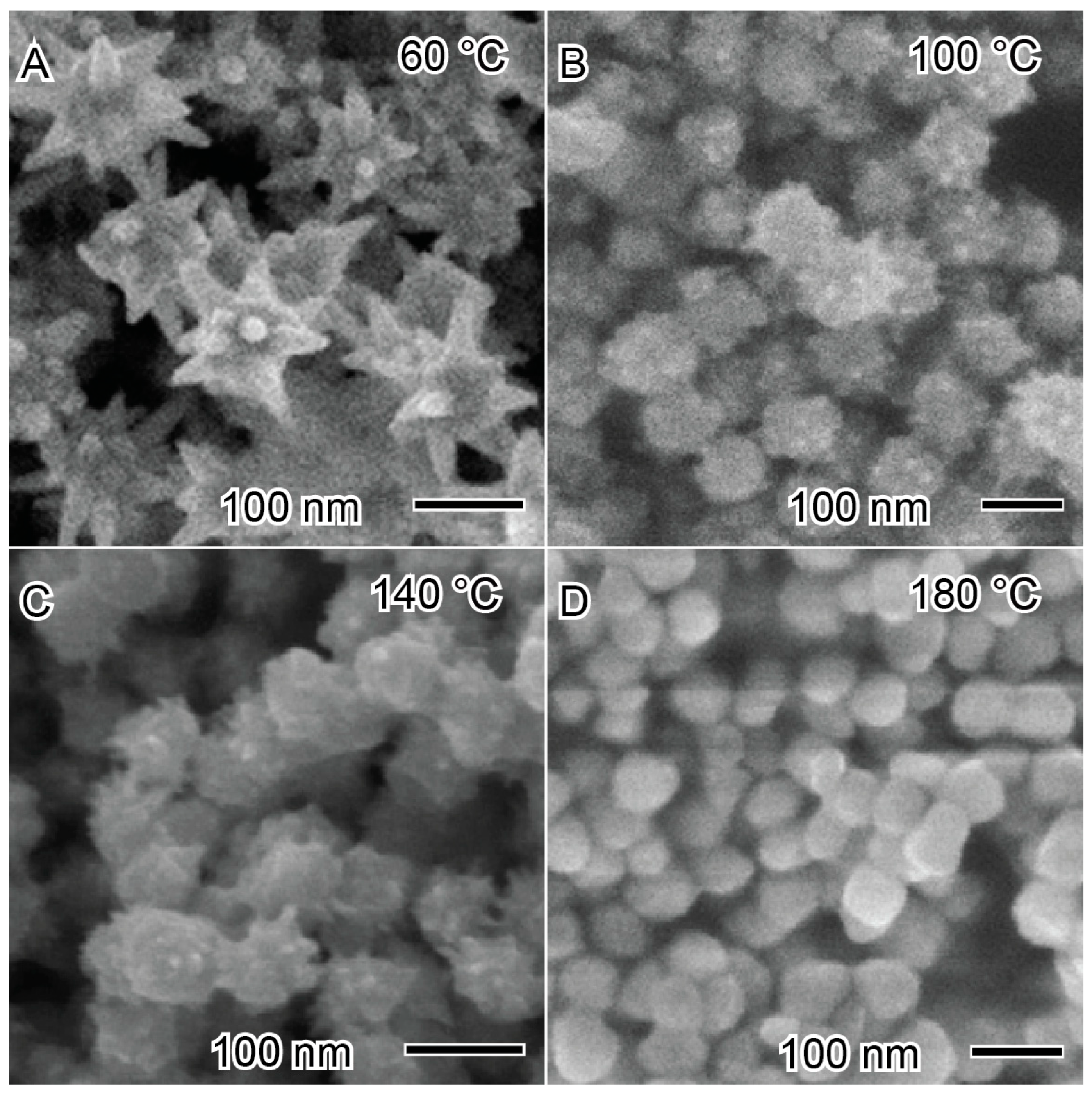
2.3. Reaction Temperature
3. Purification
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kazuhi, Q.Z.Y.K. Differences in the Extent of Inflammation caused by Intratracheal Exposure to Three Ultrafine Metals: Role of Free Radicals. J. Toxicol. Environ. Health A 1998, 53, 423–438. [Google Scholar] [CrossRef]
- Zhang, Q.; Kusaka, Y.; Zhu, X.; Sato, K.; Mo, Y.; Kluz, T.; Donaldson, K. Comparative Toxicity of Standard Nickel and Ultrafine Nickel in Lung after Intratracheal Instillation. J. Occup. Health 2003, 45, 23–30. [Google Scholar] [CrossRef]
- Zhu, F.Q.; Chern, G.W.; Tchernyshyov, O.; Zhu, X.C.; Zhu, J.G.; Chien, C.L. Magnetic Bistability and Controllable Reversal of Asymmetric Ferromagnetic Nanorings. Phys. Rev. Lett. 2006, 96, 27205. [Google Scholar] [CrossRef]
- Ban, I.; Stergar, J.; Drofenik, M.; Ferk, G.; Makovec, D. Synthesis of Copper–Nickel Nanoparticles Prepared by Mechanical Milling for Use in Magnetic Hyperthermia. J. Magn. Magn. Mater. 2011, 323, 2254–2258. [Google Scholar] [CrossRef]
- Maynard, A.D.; Kuempel, E.D. Airborne Nanostructured Particles and Occupational Health. J. Nanopart. Res. 2005, 7, 587–614. [Google Scholar] [CrossRef]
- Wang, S.-F.; Xie, F.; Hu, R.-F. Carbon-Coated Nickel Magnetic Nanoparticles Modified Electrodes as a Sensor for Determination of Acetaminophen. Sens. Actuators B Chem. 2007, 123, 495–500. [Google Scholar] [CrossRef]
- Lei, D.; Lee, D.-C.; Magasinski, A.; Zhao, E.; Steingart, D.; Yushin, G. Performance Enhancement and Side Reactions in Rechargeable Nickel–Iron Batteries with Nanostructured Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 2088–2096. [Google Scholar] [CrossRef]
- Bajpai, R.; Roy, S.; Kulshrestha, N.; Rafiee, J.; Koratkar, N.; Misra, D.S. Graphene Supported Nickel Nanoparticle as a Viable Replacement for Platinum in Dye Sensitized Solar Cells. Nanoscale 2012, 4, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Ashar, A.; Iqbal, M.; Bhatti, I.A.; Ahmad, M.Z.; Qureshi, K.; Nisar, J.; Bukhari, I.H. Synthesis, Characterization and Photocatalytic Activity of ZnO Flower and Pseudo-Sphere: Nonylphenol Ethoxylate Degradation under UV and Solar Irradiation. J. Alloys Compd. 2016, 678, 126–136. [Google Scholar] [CrossRef]
- Kashid, S.B.; Raut, R.W.; Malghe, Y.S. Microwave Assisted Synthesis of Nickel Nanostructures by Hydrazine Reduction Route: Effect of Solvent and Capping Agent on Morphology and Magnetic Properties. Mater. Chem. Phys. 2016, 170, 24–31. [Google Scholar] [CrossRef]
- Kisukuri, C.M.; Palmeira, D.J.; Rodrigues, T.S.; Camargo, P.H.C.; Andrade, L.H. Bimetallic Nanoshells as Platforms for Metallo- and Biometallo-Catalytic Applications. ChemCatChem 2016, 8, 171–179. [Google Scholar] [CrossRef]
- Da Silva, A.G.M.; Rodrigues, T.S.; Wang, J.; Yamada, L.K.; Alves, T.V.; Ornellas, F.R.; Ando, R.A.; Camargo, P.H.C. The Fault in Their Shapes: Investigating the Surface-Plasmon-Resonance-Mediated Catalytic Activities of Silver Quasi-Spheres, Cubes, Triangular Prisms, and Wires. Langmuir 2015, 31, 10272–10278. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.G.M.; Rodrigues, T.S.; Parussulo, A.L.A.; Candido, E.G.; Geonmonond, R.S.; Brito, H.F.; Toma, H.E.; Camargo, P.H.C. Controlled Synthesis of Nanomaterials at the Undergraduate Laboratory: Cu(OH)2 and CuO Nanowires. J. Chem. Educ. 2017, 94, 743–750. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; da Silva, A.G.M.; de Oliveira, L.C.; da Silva, A.M.; Teixeira, R.R.; Camargo, P.H.C. Cu2O Spheres as an Efficient Source of Catalytic Cu(I) Species for Performing Azide-Alkyne Click Reactions. Tetrahedron Lett. 2017, 58, 590–595. [Google Scholar] [CrossRef]
- Estournés, C.; Lutz, T.; Happich, J.; Quaranta, T.; Wissler, P.; Guille, J.L. Nickel Nanoparticles in Silica Gel: Preparation and Magnetic Properties. J. Magn. Magn. Mater. 1997, 173, 83–92. [Google Scholar] [CrossRef]
- Park, J.; Joo, J.; Kwon, S.G.; Jang, Y.; Hyeon, T. Synthesis of Monodisperse Spherical Nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 4630–4660. [Google Scholar] [CrossRef]
- You, H.; Fang, J. Particle-Mediated Nucleation and Growth of Solution-Synthesized Metal Nanocrystals: A New Story beyond the LaMer Curve. Nano Today 2016, 11, 145–167. [Google Scholar] [CrossRef]
- LaMer, V.K.; Dinegar, R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Jahangirian, H.; Kalantari, K.; Izadiyan, Z.; Rafiee-Moghaddam, R.; Shameli, K.; Webster, T.J. A Review of Small Molecules and Drug Delivery Applications Using Gold and Iron Nanoparticles. Int. J. Nanomed. 2019, 14, 1633–1657. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, X.; Chen, D. Monodispersed Nickel Nanoparticles with Tunable Phase and Size: Synthesis, Characterization, and Magnetic Properties. J. Phys. Chem. C 2008, 112, 18793–18797. [Google Scholar] [CrossRef]
- Deepa, E.; Therese, H.A. Hierarchical Nickel Nanowire Synthesis Using Polysorbate 80 as Capping Agent. Appl. Surf. Sci. 2018, 449, 48–54. [Google Scholar] [CrossRef]
- Başkaya, G.; Yıldız, Y.; Savk, A.; Okyay, T.O.; Eriş, S.; Sert, H.; Şen, F. Rapid, Sensitive, and Reusable Detection of Glucose by Highly Monodisperse Nickel Nanoparticles Decorated Functionalized Multi-Walled Carbon Nanotubes. Biosens. Bioelectron. 2017, 91, 728–733. [Google Scholar] [CrossRef]
- Carenco, S.; Boissière, C.; Nicole, L.; Sanchez, C.; Le Floch, P.; Mézailles, N. Controlled Design of Size-Tunable Monodisperse Nickel Nanoparticles. Chem. Mater. 2010, 22, 1340–1349. [Google Scholar] [CrossRef]
- Liu, X.; Liang, X.; Zhang, N.; Qiu, G.; Yi, R. Selective Synthesis and Characterization of Sea Urchin-like Metallic Nickel Nanocrystals. Mater. Sci. Eng. B 2006, 132, 272–277. [Google Scholar] [CrossRef]
- Sidhaye, D.S.; Bala, T.; Srinath, S.; Srikanth, H.; Poddar, P.; Sastry, M.; Prasad, B.L.V. Preparation of Nearly Monodisperse Nickel Nanoparticles by a Facile Solution Based Methodology and Their Ordered Assemblies. J. Phys. Chem. C 2009, 113, 3426–3429. [Google Scholar] [CrossRef]
- Wang, A.; Yin, H.; Lu, H.; Xue, J.; Ren, M.; Jiang, T. Catalytic Activity of Nickel Nanoparticles in Hydrogenation of P-Nitrophenol to p-Aminophenol. Catal. Commun. 2009, 10, 2060–2064. [Google Scholar] [CrossRef]
- Mathew, A.; Munichandraiah, N.; Rao, G.M. Synthesis and Magnetic Studies of Flower-like Nickel Nanocones. Mater. Sci. Eng. B 2009, 158, 7–12. [Google Scholar] [CrossRef]
- Wang, N.; Cao, X.; Kong, D.; Chen, W.; Guo, L.; Chen, C. Nickel Chains Assembled by Hollow Microspheres and Their Magnetic Properties. J. Phys. Chem. C 2008, 112, 6613–6619. [Google Scholar] [CrossRef]
- Wang, D.-P.; Sun, D.-B.; Yu, H.-Y.; Meng, H.-M. Morphology Controllable Synthesis of Nickel Nanopowders by Chemical Reduction Process. J. Cryst. Growth 2008, 310, 1195–1201. [Google Scholar] [CrossRef]
- Bao, J.; Liang, Y.; Xu, Z.; Si, L. Facile Synthesis of Hollow Nickel Submicrometer Spheres. Adv. Mater. 2003, 15, 1832–1835. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Putri, R.A.K.; Abukhadra, M.R.; Ko, Y.G. Recent Experimental and Theoretical Advances in the Design and Science of High-Entropy Alloy Nanoparticles. Nano Energy 2023, 110, 108362. [Google Scholar] [CrossRef]
- Guan, J.; Liu, L.; Xu, L.; Sun, Z.; Zhang, Y. Nickel Flower-like Nanostructures Composed of Nanoplates: One-Pot Synthesis, Stepwise Growth Mechanism and Enhanced Ferromagnetic Properties. CrystEngComm 2011, 13, 2636. [Google Scholar] [CrossRef]
- Tang, S.; Vongehr, S.; Ren, H.; Meng, X. Diameter-Controlled Synthesis of Polycrystalline Nickel Nanowires and Their Size Dependent Magnetic Properties. CrystEngComm 2012, 14, 7209. [Google Scholar] [CrossRef]
- Wang, H.; Kou, X.; Zhang, L.; Li, J. Size-Controlled Synthesis, Microstructure and Magnetic Properties of Ni Nanoparticles. Mater. Res. Bull. 2008, 43, 3529–3536. [Google Scholar] [CrossRef]
- Khanna, P.K.; More, P.V.; Jawalkar, J.P.; Bharate, B.G. Effect of Reducing Agent on the Synthesis of Nickel Nanoparticles. Mater. Lett. 2009, 63, 1384–1386. [Google Scholar] [CrossRef]
- Chandra, S.; Kumar, A.; Tomar, P.K. Synthesis of Ni Nanoparticles and Their Characterizations. J. Saudi Chem. Soc. 2014, 18, 437–442. [Google Scholar] [CrossRef]
- Eluri, R.; Paul, B. Microwave Assisted Greener Synthesis of Nickel Nanoparticles Using Sodium Hypophosphite. Mater. Lett. 2012, 76, 36–39. [Google Scholar] [CrossRef]
- Soumare, Y.; Dakhlaoui-Omrani, A.; Schoenstein, F.; Mercone, S.; Viau, G.; Jouini, N. Nickel Nanofibers and Nanowires: Elaboration by Reduction in Polyol Medium Assisted by External Magnetic Field. Solid. State Commun. 2011, 151, 284–288. [Google Scholar] [CrossRef]
- Metin, Ö.; Mazumder, V.; Özkar, S.; Sun, S. Monodisperse Nickel Nanoparticles and Their Catalysis in Hydrolytic Dehydrogenation of Ammonia Borane. J. Am. Chem. Soc. 2010, 132, 1468–1469. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.T.; Moon, J.Y.; Lee, G.H.; Park, J.; Chang, Y. Comparison of the Magnetic Properties of Metastable Hexagonal Close-Packed Ni Nanoparticles with Those of the Stable Face-Centered Cubic Ni Nanoparticles. J. Phys. Chem. B 2006, 110, 1187–1191. [Google Scholar] [CrossRef]
- Railsback, J.G.; Johnston-Peck, A.C.; Wang, J.; Tracy, J.B. Size-Dependent Nanoscale Kirkendall Effect During the Oxidation of Nickel Nanoparticles. ACS Nano 2010, 4, 1913–1920. [Google Scholar] [CrossRef]
- Chen, D.-H.; Hsieh, C.-H. Synthesis of Nickel Nanoparticles in Aqueous Cationic Surfactant Solutions. J. Mater. Chem. 2002, 12, 2412–2415. [Google Scholar] [CrossRef]
- Huang, G.; Xu, S.; Xu, G.; Li, L.; Zhang, L. Preparation of Fine Nickel Powders via Reduction of Nickel Hydrazine Complex Precursors. Trans. Nonferrous Met. Soc. China 2009, 19, 389–393. [Google Scholar] [CrossRef]
- Duan, X.; Qian, G.; Liu, Y.; Ji, J.; Zhou, X.; Chen, D.; Yuan, W. Structure Sensitivity of Ammonia Decomposition over Ni Catalysts: A Computational and Experimental Study. Fuel Process. Technol. 2013, 108, 112–117. [Google Scholar] [CrossRef]
- Park, J.W.; Chae, E.H.; Kim, S.H.; Lee, J.H.; Kim, J.W.; Yoon, S.M.; Choi, J.J.-Y. Preparation of Fine Ni Powders from Nickel Hydrazine Complex. Mater. Chem. Phys. 2006, 97, 371–378. [Google Scholar] [CrossRef]
- Guo, L.; Liu, C.; Wang, R.; Xu, H.; Wu, Z.; Yang, S. Large-Scale Synthesis of Uniform Nanotubes of a Nickel Complex by a Solution Chemical Route. J. Am. Chem. Soc. 2004, 126, 4530–4531. [Google Scholar] [CrossRef]
- Gao, C.; Lu, Z.; Yin, Y. Gram-Scale Synthesis of Silica Nanotubes with Controlled Aspect Ratios by Templating of Nickel-Hydrazine Complex Nanorods. Langmuir 2011, 27, 12201–12208. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.C.; Ruberu, T.P.A.; Reichert, M.D.; Vela, J. Templated Synthesis and Chemical Behavior of Nickel Nanoparticles within High Aspect Ratio Silica Capsules. J. Phys. Chem. C 2013, 117, 25826–25836. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, T.-H.; Zhao, M.; Xia, Y. Quantitative Analysis of the Reduction Kinetics of a Pt(II) Precursor in the Context of Pt Nanocrystal Synthesis. Chin. J. Chem. Phys. 2018, 31, 370–374. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; Zhao, M.; Yang, T.; Gilroy, K.D.; da Silva, A.G.M.; Camargo, P.H.C.; Xia, Y. Synthesis of Colloidal Metal Nanocrystals: A Comprehensive Review on the Reductants. Chem. A Eur. J. 2018, 24, 16944–16963. [Google Scholar] [CrossRef]
- Carroll, K.J.; Reveles, J.U.; Shultz, M.D.; Khanna, S.N.; Carpenter, E.E. Preparation of Elemental Cu and Ni Nanoparticles by the Polyol Method: An Experimental and Theoretical Approach. J. Phys. Chem. C 2011, 115, 2656–2664. [Google Scholar] [CrossRef]
- Song, H.-J.; Jia, X.-H.; Yang, X.-F.; Tang, H.; Li, Y.; Su, Y.-T. Controllable Synthesis of Monodisperse Polyhedral Nickelnanocrystals. CrystEngComm 2012, 14, 405–410. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Wiley, B.J.; Kim, M.; Formo, E.V.; Xia, Y. On the Polyol Synthesis of Silver Nanostructures: Glycolaldehyde as a Reducing Agent. Nano Lett. 2008, 8, 2077–2081. [Google Scholar] [CrossRef]
- Fievet, F.; Lagier, J.P.; Figlarz, M. Preparing Monodisperse Metal Powders in Micrometer and Submicrometer Sizes by the Polyol Process. MRS Bull. 1989, 14, 29–34. [Google Scholar] [CrossRef]
- Ying, Z.; Shengming, J.; Guanzhou, Q.; Min, Y. Preparation of Ultrafine Nickel Powder by Polyol Method and Its Oxidation Product. Mater. Sci. Eng. B 2005, 122, 222–225. [Google Scholar] [CrossRef]
- Couto, G.G.; Klein, J.J.; Schreiner, W.H.; Mosca, D.H.; de Oliveira, A.J.A.; Zarbin, A.J.G. Nickel Nanoparticles Obtained by a Modified Polyol Process: Synthesis, Characterization, and Magnetic Properties. J. Colloid. Interface Sci. 2007, 311, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Roselina, N.R.N.; Azizan, A. Ni Nanoparticles: Study of Particles Formation and Agglomeration. Procedia Eng. 2012, 41, 1620–1626. [Google Scholar] [CrossRef]
- Wu, S.-H.; Chen, D.-H. Synthesis and Characterization of Nickel Nanoparticles by Hydrazine Reduction in Ethylene Glycol. J. Colloid. Interface Sci. 2003, 259, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Guo, L.; Wang, R.-M.; Deng, Y.; Xu, H.-B.; Yang, S. Magnetic Nanochains of Metal Formed by Assembly of Small Nanoparticles. Chem. Commun. 2004, 2726. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Guo, L.; He, L.; Chen, C.P. Synthesis, Characterization, and Magnetic Properties of Flower-like Nickel Materials. Phys. Status Solidi (A) 2008, 205, 1109–1112. [Google Scholar] [CrossRef]
- Tzitzios, V.; Basina, G.; Gjoka, M.; Alexandrakis, V.; Georgakilas, V.; Niarchos, D.; Boukos, N.; Petridis, D. Chemical Synthesis and Characterization of Hcp Ni Nanoparticles. Nanotechnology 2006, 17, 3750–3755. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.C. High-Yield Synthesis of Nickel and Nickel Phosphide Nanowires via Microwave-Assisted Processes. Chem. Mater. 2008, 20, 6743–6749. [Google Scholar] [CrossRef]
- Biacchi, A.J.; Schaak, R.E. The Solvent Matters: Kinetic versus Thermodynamic Shape Control in the Polyol Synthesis of Rhodium Nanoparticles. ACS Nano 2011, 5, 8089–8099. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, H.-C.; Liu, J.; Huang, C.Z.; Xia, Y. Use of Reduction Rate as a Quantitative Knob for Controlling the Twin Structure and Shape of Palladium Nanocrystals. Nano Lett. 2015, 15, 1445–1450. [Google Scholar] [CrossRef]
- Niu, W.; Li, Z.-Y.; Shi, L.; Liu, X.; Li, H.; Han, S.; Chen, J.; Xu, G. Seed-Mediated Growth of Nearly Monodisperse Palladium Nanocubes with Controllable Sizes. Cryst. Growth Des. 2008, 8, 4440–4444. [Google Scholar] [CrossRef]
- O’Brien, W.L.; Tonner, B.P. Transition to the Perpendicular Easy Axis of Magnetization in Ni Ultrathin Films Found by X-ray Magnetic Circular Dichroism. Phys. Rev. B 1994, 49, 15370–15373. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Chen, Q.; Zhu, H.; Lin, Y.; Zhang, X. Magnetic Field-Induced Growth and Self-Assembly of Cobalt Nanocrystallites. J. Mater. Chem. 2003, 13, 1803. [Google Scholar] [CrossRef]
- Yang, H.G.; Zeng, H.C. Preparation of Hollow Anatase TiO2 Nanospheres via Ostwald Ripening. J. Phys. Chem. B 2004, 108, 3492–3495. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Sugawara, K. Magnetic-Field-Assisted Synthesis of Ni Nanostructures: Selective Control of Particle Shape. Chem. Phys. Lett. 2009, 477, 184–188. [Google Scholar] [CrossRef]
- Heidari, N.; Ghiasvand, A. A Review on Magnetic Field-Assisted Solid-Phase Microextraction Techniques. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 75–82. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, W.; Liu, Y.; Hu, M.; Zhao, K. Synthesis of High-Aspect-Ratio Nickel Nanowires by Dropping Method. Nanoscale Res. Lett. 2016, 11, 118. [Google Scholar] [CrossRef]
- Ni, X.; Zhao, Q.; Zhang, D.; Zhang, X.; Zheng, H. Novel Hierarchical Nanostructures of Nickel: Self-Assembly of Hexagonal Nanoplatelets. J. Phys. Chem. C 2007, 111, 601–605. [Google Scholar] [CrossRef]
- Shevchenko, E.V.; Talapin, D.V.; Schnablegger, H.; Kornowski, A.; Festin, Ö.; Svedlindh, P.; Haase, M.; Weller, H. Study of Nucleation and Growth in the Organometallic Synthesis of Magnetic Alloy Nanocrystals: The Role of Nucleation Rate in Size Control of CoPt3 Nanocrystals. J. Am. Chem. Soc. 2003, 125, 9090–9101. [Google Scholar] [CrossRef]
- Omrani, A.D.; Bousnina, M.A.; Smiri, L.S.; Taibi, M.; Leone, P.; Schoenstein, F.; Jouini, N. Elaboration of Nickel Nanoparticles by Modified Polyol Process and Their Spark Plasma Sintering, Characterization and Magnetic Properties of the Nanoparticles and the Dense Nano-Structured Material. Mater. Chem. Phys. 2010, 123, 821–828. [Google Scholar] [CrossRef]
- Ni, Y.; Tao, A.; Hu, G.; Cao, X.; Wei, X.; Yang, Z. Synthesis, Characterization and Properties of Hollow Nickel Phosphide Nanospheres. Nanotechnology 2006, 17, 5013–5018. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, H.; Han, M.; Zhu, J.; Liang, Y.; Xu, Z.; Song, Y. Nanometer-Sized Nickel Hollow Spheres. Adv. Mater. 2005, 17, 1995–1999. [Google Scholar] [CrossRef]
- Yu, L.; Banerjee, I.A.; Shima, M.; Rajan, K.; Matsui, H. Size-Controlled Ni Nanocrystal Growth on Peptide Nanotubes and Their Magnetic Properties. Adv. Mater. 2004, 16, 709–712. [Google Scholar] [CrossRef]
- Abedini, A.; Daud, A.R.; Hamid, M.A.A.; Othman, N.K.; Saion, E. A Review on Radiation-Induced Nucleation and Growth of Colloidal Metallic Nanoparticles. Nanoscale Res. Lett. 2013, 8, 474. [Google Scholar] [CrossRef]
- Alex, S.; Tiwari, A. Functionalized Gold Nanoparticles: Synthesis, Properties and Applications—A Review. J. Nanosci. Nanotechnol. 2015, 15, 1869–1894. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Kondoh, H.; Ohta, T.; Gao, S. Size-Controlled Synthesis of Nickel Nanoparticles. Appl. Surf. Sci. 2005, 241, 218–222. [Google Scholar] [CrossRef]
- Salim, M.; Minamikawa, H.; Sugimura, A.; Hashim, R. Amphiphilic Designer Nano-Carriers for Controlled Release: From Drug Delivery to Diagnostics. Med. Chem. Commun. 2014, 5, 1602–1618. [Google Scholar] [CrossRef]
- Singla, M.L.; Negi, A.; Mahajan, V.; Singh, K.C.; Jain, D.V.S. Catalytic Behavior of Nickel Nanoparticles Stabilized by Lower Alkylammonium Bromide in Aqueous Medium. Appl. Catal. A Gen. 2007, 323, 51–57. [Google Scholar] [CrossRef]
- Huang, G.; Xu, S.; Li, L.; Wang, X. Effect of Surfactants on Dispersion Property and Morphology of Nano-Sized Nickel Powders. Trans. Nonferrous Met. Soc. China 2014, 24, 3739–3746. [Google Scholar] [CrossRef]
- Wang, A.; Yin, H.; Lu, H.; Xue, J.; Ren, M.; Jiang, T. Effect of Organic Modifiers on the Structure of Nickel Nanoparticles and Catalytic Activity in the Hydrogenation of p-Nitrophenol to p-Aminophenol. Langmuir 2009, 25, 12736–12741. [Google Scholar] [CrossRef]
- Ramírez-Meneses, E.; Torres-Huerta, A.M.; Domínguez-Crespo, M.A.; Ponce-Varela, M.G.; Hernández-Pérez, M.A.; Betancourt, I.; Palacios-González, E. Synthesis and Electrochemical Characterization of Ni Nanoparticles by Hydrazine Reduction Using Hydroxyethyl Cellulose as Capping Agent. Electrochim. Acta 2014, 127, 228–238. [Google Scholar] [CrossRef]
- Ni, X.; Zhao, Q.; Zhang, D.; Yang, D.; Zheng, H. Large Scaled Synthesis of Chainlike Nickel Wires Assisted by Ligands. J. Cryst. Growth 2005, 280, 217–221. [Google Scholar] [CrossRef]
- Singh, K.; Kate, K.H.; Chilukuri, V.V.S.; Khanna, P.K. Glycerol Mediated Low Temperature Synthesis of Nickel Nanoparticles by Solution Reduction Method. J. Nanosci. Nanotechnol. 2011, 11, 5131–5136. [Google Scholar] [CrossRef]
- Zhang, J.; Ohara, S.; Umetsu, M.; Naka, T.; Hatakeyama, Y.; Adschiri, T. Colloidal Ceria Nanocrystals: A Tailor-Made Crystal Morphology in Supercritical Water. Adv. Mater. 2007, 19, 203–206. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Zhou, Y.C.; Pan, Y. Effects of Hydrogen Adsorption on the Surface-Energy Anisotropy of Nickel. Phys. B Condens. Matter. 2010, 405, 1335–1338. [Google Scholar] [CrossRef]
- Leng, Y.; Li, Y.; Li, X.; Takahashi, S. Improved Magnetic Anisotropy of Monodispersed Triangular Nickel Nanoplates. J. Phys. Chem. C 2007, 111, 6630–6633. [Google Scholar] [CrossRef]
- Blakely, J.M.; Mykura, H. The Effect of Impurity Adsorption on the Surface Energy and Surface Self Diffusion in Nickel. Acta Metall. 1961, 9, 595–599. [Google Scholar] [CrossRef]
- Fu, Y.; Pichon, B.; Devred, F.; Singleton, M.L.; Hermans, S. Synthesis of Spherical, Rod, or Chain Ni Nanoparticles and Their Structure–Activity Relationship in Glucose Hydrogenation Reaction. J. Catal. 2022, 415, 63–76. [Google Scholar] [CrossRef]
- Cho, H.; Lee, N.; Kim, B.H. Synthesis of Highly Monodisperse Nickel and Nickel Phosphide Nanoparticles. Nanomaterials 2022, 12, 3198. [Google Scholar] [CrossRef]
- He, M.; Ai, Y.; Hu, W.; Guan, L.; Ding, M.; Liang, Q. Recent Advances of Seed-Mediated Growth of Metal Nanoparticles: From Growth to Applications. Adv. Mater. 2023, 35, 2211915. [Google Scholar] [CrossRef]
- Jiang, Y.; Tao, R.; Zhang, H.; Wan, N.; Yang, Y.; Gu, D.; Zhang, T.; Rui, Y.; Xu, J. Separating Nucleation from Growth for High-Yield Synthesis of Thin Silver Nanowires. J. Mater. Sci. Mater. Electron. 2023, 34, 26. [Google Scholar] [CrossRef]
- Kuchkina, N.; Sorokina, S.; Torozova, A.; Bykov, A.; Shifrina, Z. Ni Nanoparticles Entrapped by a Functional Dendrimer as a Highly Efficient and Recyclable Catalyst for Suzuki-Miyaura Cross-Coupling Reactions. ChemistrySelect 2022, 7, e202202653. [Google Scholar] [CrossRef]
- Moreira, M.; Felix, L.C.; Cottancin, E.; Pellarin, M.; Ugarte, D.; Hillenkamp, M.; Galvao, D.S.; Rodrigues, V. Influence of Cluster Sources on the Growth Mechanisms and Chemical Composition of Bimetallic Nanoparticles. J. Phys. Chem. C 2023, 127, 1944–1954. [Google Scholar] [CrossRef]
- Bai, L.; Yuan, F.; Tang, Q. Synthesis of Nickel Nanoparticles with Uniform Size via a Modified Hydrazine Reduction Route. Mater. Lett. 2008, 62, 2267–2270. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Recent Advances on Protein Separation and Purification Methods. Adv. Colloid. Interface Sci. 2020, 284, 102254. [Google Scholar] [CrossRef]
- Robertson, J.D.; Rizzello, L.; Avila-Olias, M.; Gaitzsch, J.; Contini, C.; Magoń, M.S.; Renshaw, S.A.; Battaglia, G. Purification of Nanoparticles by Size and Shape. Sci. Rep. 2016, 6, 27494. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; e Silva, F.A.; Candido, E.G.; da Silva, A.G.M.; dos Geonmonond, R.S.; Camargo, P.H.C.; Linardi, M.; Fonseca, F.C. Ethanol Steam Reforming: Understanding Changes in the Activity and Stability of Rh/MxOy Catalysts as Function of the Support. J. Mater. Sci. 2019, 54, 11400–11416. [Google Scholar] [CrossRef]
- Stephens, J.R.; Beveridge, J.S.; Williams, M.E. Analytical Methods for Separating and Isolating Magnetic Nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 3280. [Google Scholar] [CrossRef] [PubMed]
- Bouremana, A.; Mouaci, S.; Berriah, A.; Boutebina, Z.; Manseri, A.; Bensouilah, A. High Yield Solvothermal Synthesis of Ni Nanoparticles: Structural, Microstructural, and Magnetic Properties. J. Nanopart. Res. 2022, 24, 204. [Google Scholar] [CrossRef]
- Le, T.-D.; Suttikhana, I.; Ashaolu, T.J. State of the Art on the Separation and Purification of Proteins by Magnetic Nanoparticles. J. Nanobiotechnology 2023, 21, 363. [Google Scholar] [CrossRef]
- Ikeda, K.; Shimoyama, Y.; Orita, Y. Efficient Purification of Surface Modified Nanoparticles from Its Nanosuspension by Using Supercritical CO2 Technology. J. Supercrit. Fluids 2023, 199, 105966. [Google Scholar] [CrossRef]
- Mouaci, S.; Bouremana, A.; Boutebina, Z.; Berriah, A.; Manseri, A.; Saidi, M.; Saidi-Amroun, N. Enhancing LDPE Performance Using Ni Nanoparticles: A Comprehensive Study of Structural, Magnetic, and Mechanical Properties. J. Polym. Res. 2023, 30, 374. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
e Silva, F.A.; Salim, V.M.M.; Rodrigues, T.S. Controlled Nickel Nanoparticles: A Review on How Parameters of Synthesis Can Modulate Their Features and Properties. AppliedChem 2024, 4, 86-106. https://doi.org/10.3390/appliedchem4010007
e Silva FA, Salim VMM, Rodrigues TS. Controlled Nickel Nanoparticles: A Review on How Parameters of Synthesis Can Modulate Their Features and Properties. AppliedChem. 2024; 4(1):86-106. https://doi.org/10.3390/appliedchem4010007
Chicago/Turabian Stylee Silva, Felipe Anchieta, Vera Maria Martins Salim, and Thenner Silva Rodrigues. 2024. "Controlled Nickel Nanoparticles: A Review on How Parameters of Synthesis Can Modulate Their Features and Properties" AppliedChem 4, no. 1: 86-106. https://doi.org/10.3390/appliedchem4010007
APA Stylee Silva, F. A., Salim, V. M. M., & Rodrigues, T. S. (2024). Controlled Nickel Nanoparticles: A Review on How Parameters of Synthesis Can Modulate Their Features and Properties. AppliedChem, 4(1), 86-106. https://doi.org/10.3390/appliedchem4010007




