Exercise and Dietary Factors in Skeletal Muscle Anabolism Across Aging: Inferences for the Insulin/IGF-1 Axis—A Narrative Review
Abstract
1. Introduction
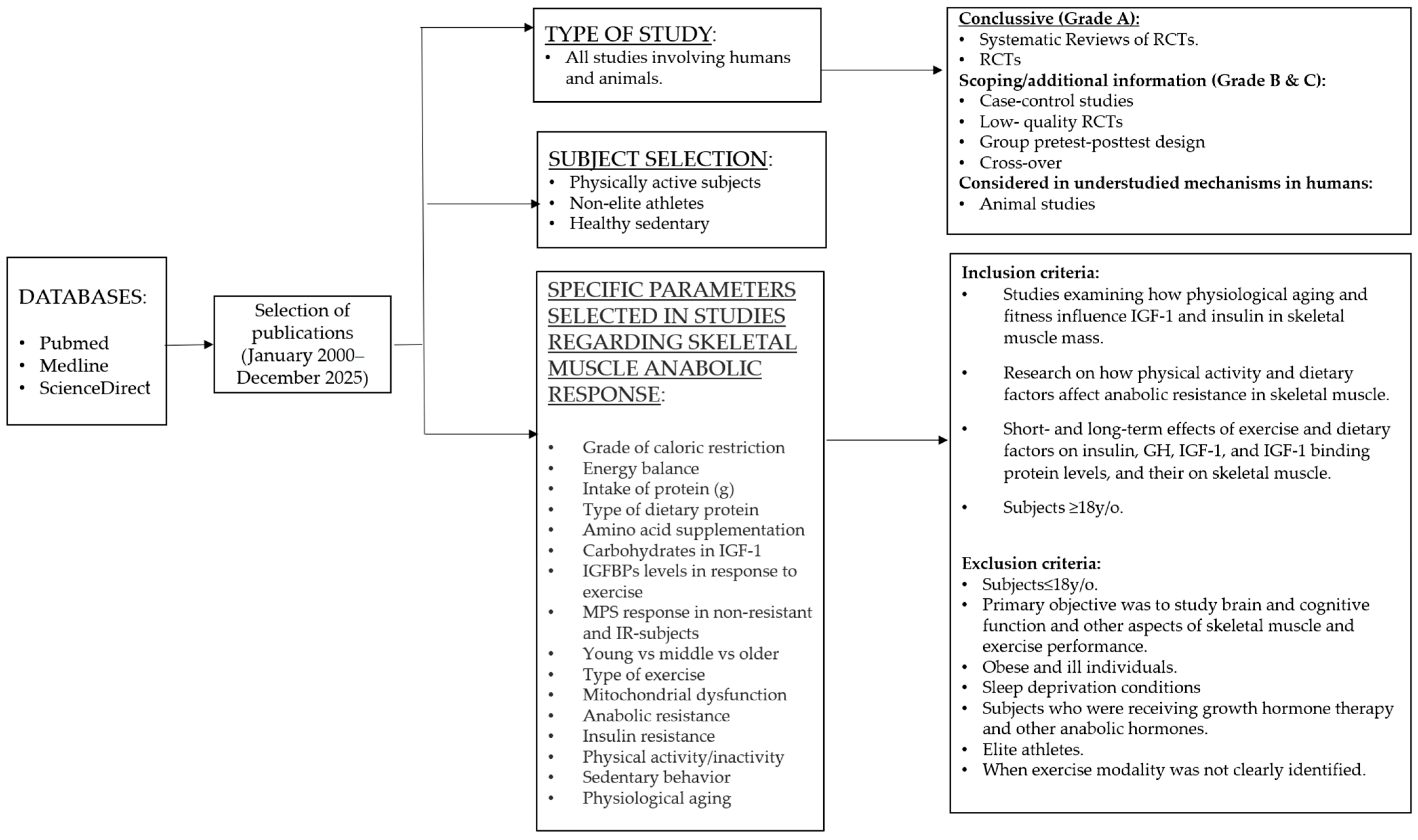
2. Methods
2.1. Review Questions
2.2. Search Strategy and Study Selection
2.3. Eligibility and Evidence Handling
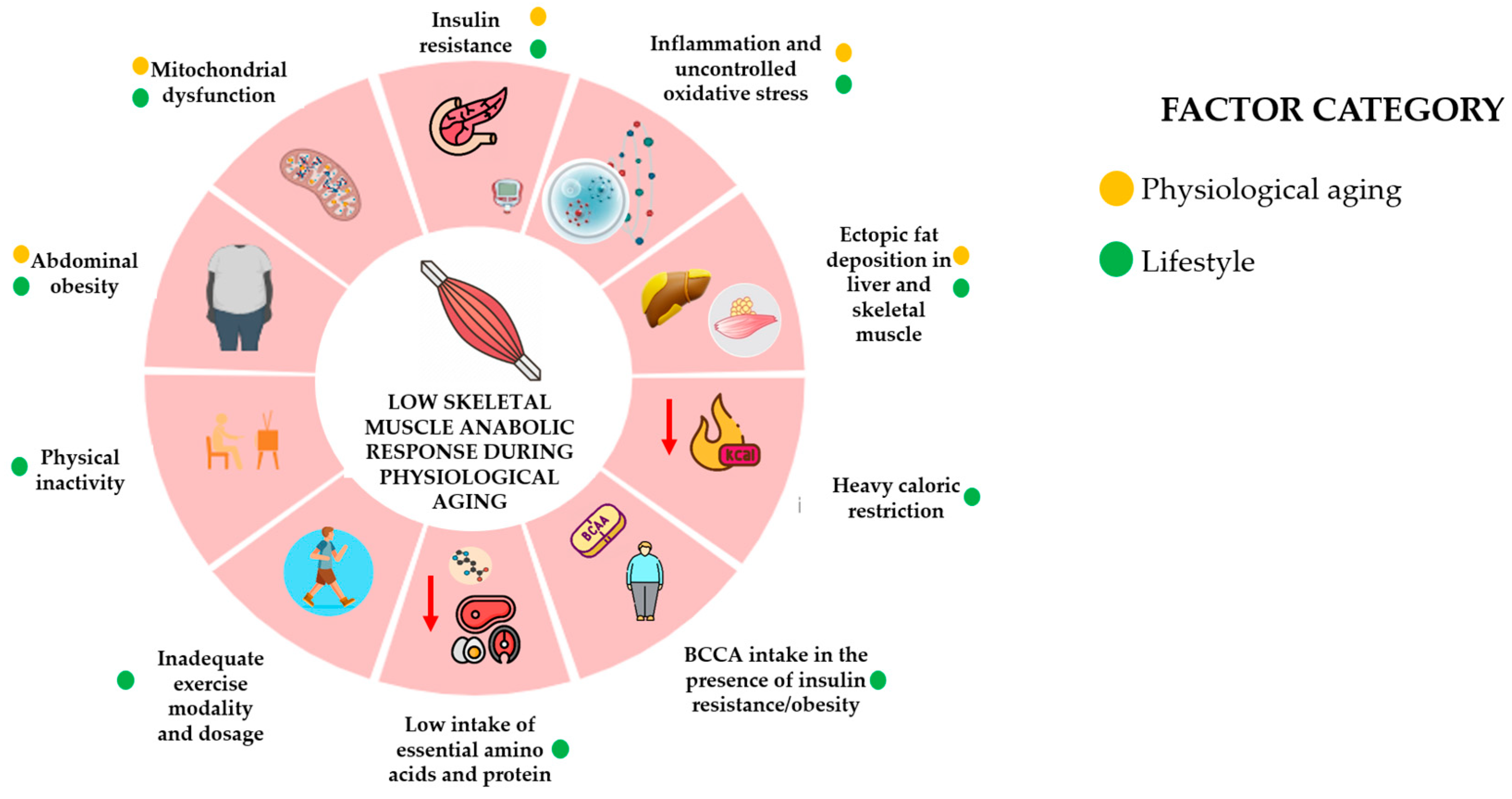
3. Physiological Aging, Skeletal Muscle Function, and the Anabolic Resistance Effect
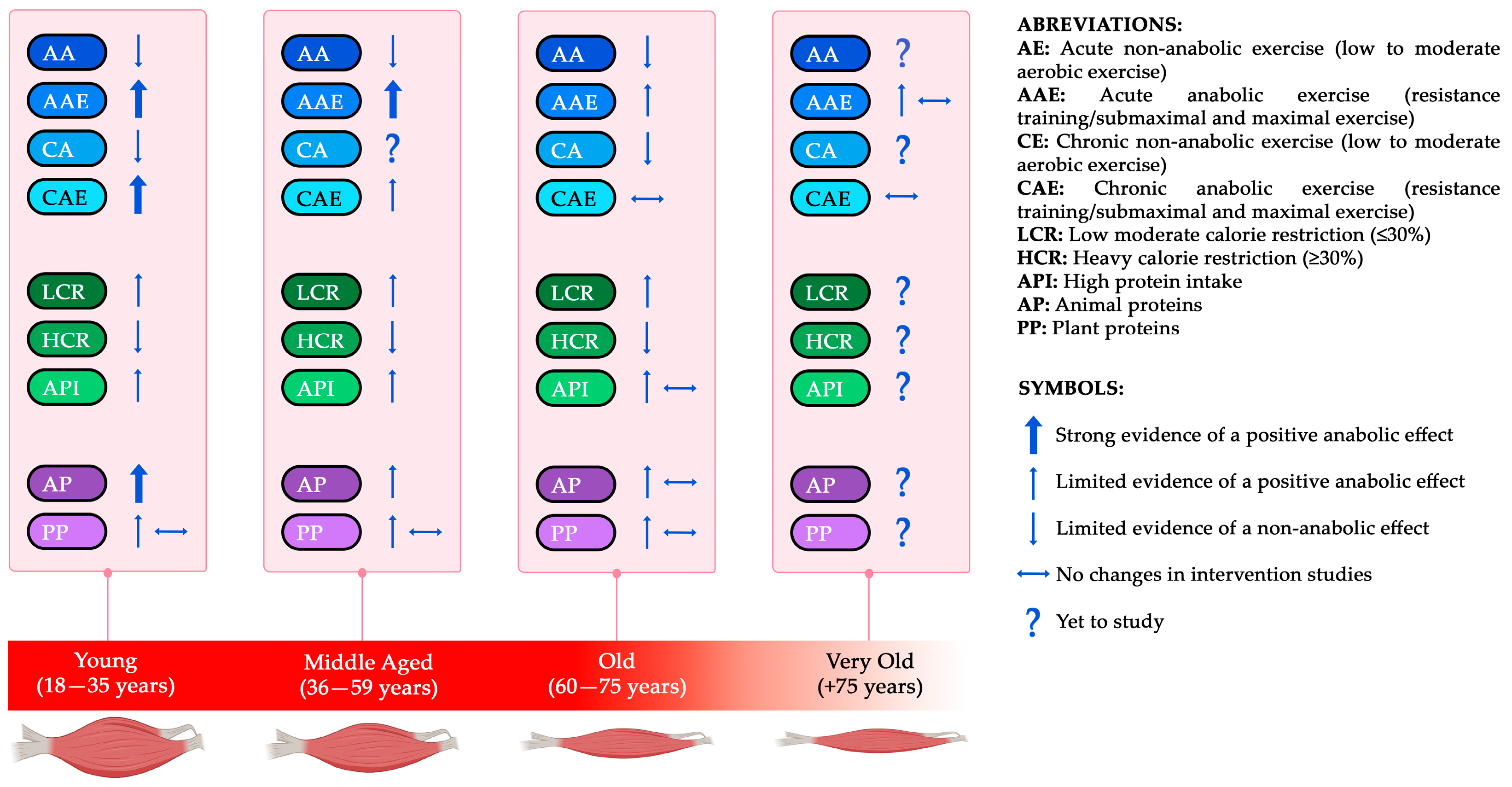
4. Effect of Physiological Aging on Anabolic Changes in Skeletal Muscle
5. Effect of Insulin, IGF-1, and IGFBPS on the Anabolic Response of Skeletal Muscle
6. Effects of Exercise on the Anabolic Response in Skeletal Muscle
6.1. Acute Exercise: RT/HIIT/SIT—Aerobic at Different Intensities
6.2. Chronic Effects of Exercise: RT/HIIT/SIT—Aerobic at Different Intensities
6.3. Effects of Exercise on Insulin Function
7. Effects of Dietary Factors on the Anabolic Response in Skeletal Muscle
7.1. Caloric Restriction and Protein Intake
7.2. Dietary Protein, Protein Supplements, and Amino Acids
7.2.1. Animal Protein and Animal Protein-Based Dietary Supplements
7.2.2. Plant Dietary Protein and Plant-Based Supplements
7.2.3. Anabolic Amino Acids
7.3. Carbohydrates
8. Limitations
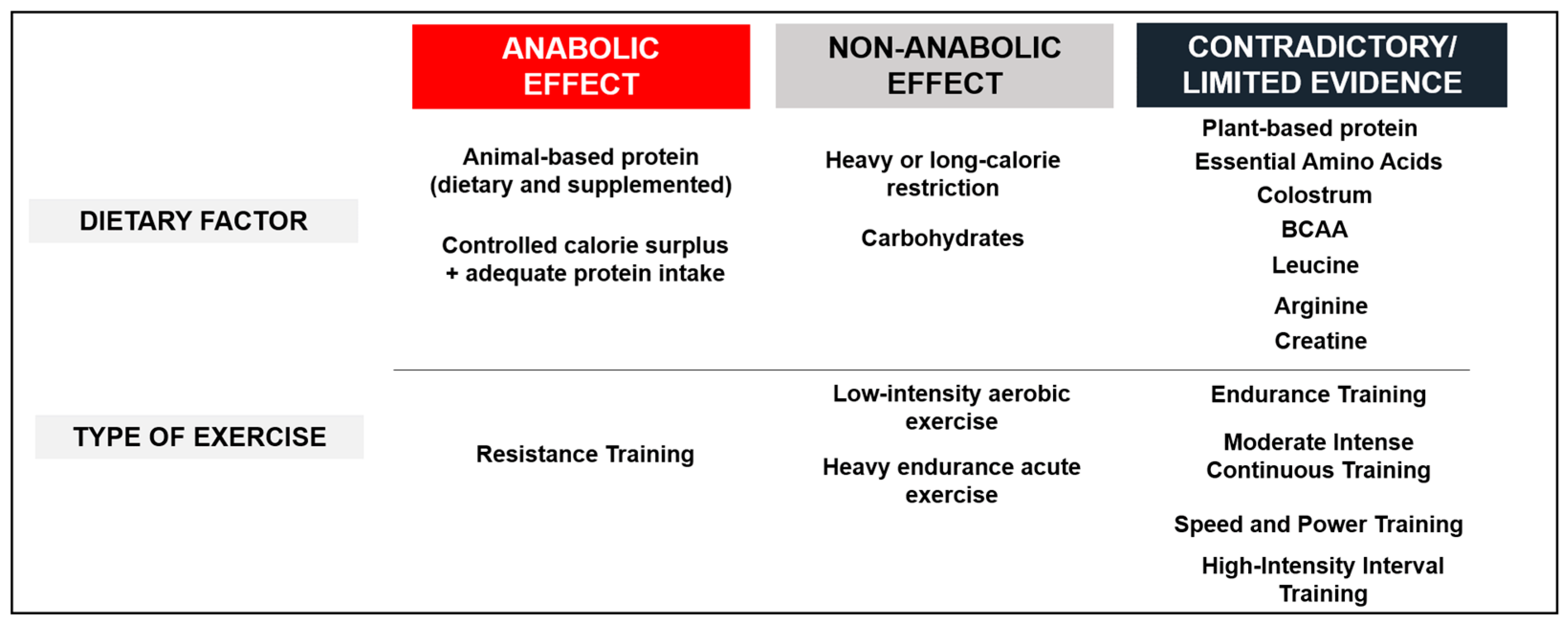
9. Conclusions and Practical Considerations
- Acute and chronic exposure to RT improves anabolic response, especially in young and middle-aged subjects;
- Acute heavy endurance exercise and chronic low-intensity exercise do not induce an anabolic response;
- Moderate CR (≤30%) does not seem to affect the anabolic response;
- Short- and long-term heavy CR (30–50%) seems to decrease anabolic response in SM;
- Low carbohydrate availability impairs IGF-1 and GH function;
- High-quality proteins stimulate MPS and IGF-1 in a dose-dependent manner.
- Local effects of any exercise modality on intramuscular anabolic signaling and hypertrophic adaptations;
- In general, all dietary factors and exercise modalities are considered in very old subjects, especially combined protocols (diet + exercise);
- Optimization of diet + exercise to combat AR in older subjects;
- Individualization of acute dose–response and chronic protein intake by age sub-groups (young, middle-aged, old, and very old);
- Optimal plant–protein combinations to achieve the same anabolic effect as AP;
- Appropriate dosage, timing, and periodization of CR;
- Proper dosage, periodization, and progression of exercise to ensure long-term benefits;
- Sex differences in the anabolic response of SM to dietary factors and exercise.
- Young and middle-aged individuals:
- RT frequency suggested ≥2–3 x/w plus additional sessions of SIT, HIIT, or moderate- to high-intensity aerobic exercise;
- Consider including portions of AP food sources or AP or PP dietary supplements that match in leucine content;
- Increasing protein intake during CR.
- Older adults:
- RT frequency recommended 2–3 x/w;
- High-intensity exercise mixed with low to moderate intensity aerobic exercise to stimulate both hormonal response in SM but also favor IS;
- Avoiding moderate to heavy CR (≥30%);
- It is suggested to increase protein intake (~1.2–1.8 g/kg/d) and consider the addition of protein supplements rich in leucine, including leucine-rich sources, in the absence of IR;
- Adjusting carbohydrate sources and timing to favor IS.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| ALS | Acid-labile subunit |
| AMPK | AMP-activated protein kinase |
| AP | Animal proteins |
| AR | Anabolic resistance |
| BCAA | Branched Chain Amino Acids |
| CR | Caloric restriction |
| EAA | Essential amino acids |
| FFM | Fat-free mass |
| FOXO1 | Transcription factor; Forkhead box protein O1 |
| FRS | Fractional Rate Protein Synthesis |
| HIIT | High-intensity interval training |
| IGF-1 | Insulin Growth Factor-1 |
| IGFBPs | Insulin Growth Factor Binding Proteins |
| INS | Insulin |
| IR | Insulin resistance |
| IS | Insulin sensitivity |
| MICT | Moderate-Intensity Continuous Training |
| MPB | Muscle protein breakdown |
| MPS | Muscle protein synthesis |
| MSTN | Myostatin |
| Mtor | Mechanistic target of rapamycin |
| P | Grams of protein |
| PAL | Physical Activity Level |
| PGC-1α | Peroxisome proliferator-activated receptor-γ (PPAR-γ)-coactivator-1α |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| PP | Plan proteins |
| RDA | Recommended Dietary Allowance |
| RT | Resistance training |
| SIRTs | Family of proteins, nicotine adenine dinucleotide(+)-dependent histone Deacetylases |
| SIT | Sprint Interval Training |
| SM | Skeletal muscle |
| UPP | Ubiquitin–proteasome pathway |
| VO2MAX | Maximum rate of oxygen consumption |
| W | Weeks |
References
- Kraemer, W.J.; Ratamess, N.A.; Hymer, W.C.; Nindl, B.C.; Fragala, M.S. Growth hormone(s), testosterone, insulin-like growth factors, and cortisol: Roles and integration for cellular development and growth with exercise. Front. Endocrinol. 2020, 11, 33. [Google Scholar] [CrossRef]
- Velloso, C.P. Regulation of muscle mass by growth hormone and IGF-1. Br. J. Pharmacol. 2008, 154, 557–568. [Google Scholar] [CrossRef]
- Barclay, R.D.; Burd, N.A.; Tyler, C.; Tillin, N.A.; Mackenzie, R.W. The role of the IGF-1 signaling cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Front. Nutr. 2019, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Syverud, B.C.; VanDusen, K.W.; Larkin, L.M. Growth factors for skeletal muscle tissue engineering. Cells Tissues Organs 2016, 202, 169–179. [Google Scholar] [CrossRef]
- Okuyama, T.; Kyohara, M.; Terauchi, Y.; Shirakawa, J. The roles of the IGF axis in the regulation of the metabolism: Interaction and difference between insulin receptor signaling and IGF-1 receptor signaling. Int. J. Mol. Sci. 2021, 22, 6817. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ji, Y.; Liu, R.; Zhu, X.; Wang, K.; Yang, X.; Liu, B.; Gao, Z.; Yan, Y.; Shen, Y.; et al. Mitochondrial Dysfunction: Roles in Skeletal Muscle Atrophy. J. Transl. Med. 2023, 21, 503. [Google Scholar] [CrossRef]
- Morton, R.W.; Traylor, D.A.; Weijs, P.J.; Phillips, S.M. Defining anabolic resistance: Implications for delivery of clinical care nutrition. Curr. Opin. Crit. Care 2018, 24, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Paulussen, K.J.M.; McKenna, C.F.; Beals, J.W.; Wilund, K.R.; Salvador, A.F.; Burd, N.A. Anabolic resistance of muscle protein turnover comes in various shapes and sizes. Front. Nutr. 2021, 8, 615849. [Google Scholar] [CrossRef]
- Burd, N.A.; Gorissen, S.H.; Van Loon, L.J. Anabolic resistance of muscle protein synthesis with aging. Exerc. Sport Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef]
- Haran, P.H.; Rivas, D.A.; Fielding, R.A. Role and potential mechanisms of anabolic resistance in sarcopenia. J. Cachexia Sarcopenia Muscle 2012, 3, 157–162. [Google Scholar] [CrossRef]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.; Rueda, R.; Rodriguez-Mañas, L. Skeletal muscle regulates metabolism via interorgan crosstalk: Roles in health and disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef]
- Boros, K.; Freemont, T. Physiology of ageing of the musculoskeletal system. Best Pract. Res. Clin. Rheumatol. 2017, 31, 203–217. [Google Scholar] [CrossRef]
- Angulo, J.; Assar, M.E.; Álvarez-Bustos, A.; Rodríguez-Mañas, L. Physical activity and exercise: Strategies to manage frailty. Redox Biol. 2020, 35, 101513. [Google Scholar] [CrossRef]
- Shur, N.F.; Creedon, L.; Skirrow, S.; Atherton, P.J.; MacDonald, I.A.; Lund, J.; Greenhaff, P.L. Age-Related Changes in Muscle Architecture and Metabolism in Humans: The Likely Contribution of Physical Inactivity to Age-Related Functional Decline. Ageing Res. Rev. 2021, 68, 101344. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.N.; Jung, C.H.; Hwang, Y. Sarcopenia in youth. Metabolism 2023, 144, 155557. [Google Scholar] [CrossRef] [PubMed]
- Gries, K.J.; Raue, U.; Perkins, R.K.; Lavin, K.M.; Overstreet, B.S.; D’Acquisto, L.J.; Graham, B.; Finch, W.H.; Kaminsky, L.A.; Trappe, T.A.; et al. Cardiovascular and Skeletal Muscle Health with Lifelong Exercise. J. Appl. Physiol. 2018, 125, 1636–1645. [Google Scholar] [CrossRef]
- Dardevet, D.; Rémond, D.; Peyron, M.-A.; Papet, I.; Savary-Auzeloux, I.; Mosoni, L. Muscle Wasting and Resistance of Muscle Anabolism: The “Anabolic Threshold Concept” for Adapted Nutritional Strategies during Sarcopenia. Sci. World J. 2012, 2012, 269531. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Brightwell, C.R.; Deer, R.R.; Graber, T.G.; Galvan, E.; Fry, C.S.; Volpi, E.; Rasmussen, B.B. Muscle protein anabolic resistance to essential amino acids does not occur in healthy older adults before or after resistance exercise training. J. Nutr. 2018, 148, 900–909. [Google Scholar] [CrossRef]
- Sherlala, R.A.; Kammerer, C.M.; Kuipers, A.L.; Wojczynski, M.K.; Ukraintseva, S.V.; Feitosa, M.F.; Mengel-From, J.; Zmuda, J.M.; Minster, R.L. Relationship between Serum IGF-1 and BMI Differs by Age. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 76, 1303–1308. [Google Scholar] [CrossRef]
- Schiaffino, S.; Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skelet. Muscle 2011, 1, 4. [Google Scholar] [CrossRef]
- Bidlingmaier, M.; Friedrich, N.; Emeny, R.T.; Spranger, J.; Wolthers, O.D.; Roswall, J.; Körner, A.; Obermayer-Pietsch, B.; Hübener, C.; Dahlgren, J.; et al. Reference Intervals for Insulin-like Growth Factor-1 (IGF-1) from Birth to Senescence: Results from a Multicenter Study Using a New Automated Chemiluminescence IGF-1 Immunoassay Conforming to Recent International Recommendations. J. Clin. Endocrinol. Metab. 2014, 99, 1712–1721. [Google Scholar] [CrossRef]
- Giovannini, S.; Marzetti, E.; Borst, S.E.; Leeuwenburgh, C. Modulation of GH/IGF-1 axis: Potential strategies to counteract sarcopenia in older adults. Mech. Ageing Dev. 2008, 129, 593–601. [Google Scholar] [CrossRef]
- Martín, A.I.; Priego, T.; Moreno-Ruperez, Á.; González-Hedström, D.; Granado, M.; López-Calderón, A. IGF-1 and IGFBP-3 in inflammatory cachexia. Int. J. Mol. Sci. 2021, 22, 9469. [Google Scholar] [CrossRef]
- Junnila, R.K.; List, E.O.; Berryman, D.E.; Murrey, J.W.; Kopchick, J.J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 2013, 9, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Eliakim, A.; Nemet, D. Exercise Training, Physical Fitness and the Growth Hormone-Insulin-Like Growth Factor-1 Axis and Cytokine Balance. Med. Sport Sci. 2010, 128, 128–140. [Google Scholar] [CrossRef]
- Sartorio, A.; Marazzi, N.; Agosti, F.; Faglia, G.; Corradini, C.; Palo, E.D.; Cella, S.; Rigamonti, A.; Muller, E.E. Elite Volunteer Athletes of Different Sport Disciplines May Have Elevated Baseline GH Levels Divorced from Unaltered Levels of Both IGF-1 and GH-Dependent Bone and Collagen Markers: A Study On-The-Field. J. Endocrinol. Investig. 2004, 27, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Huang, C.; Chen, S.; Yang, W. Serum reference value of two potential doping candidates—Myostatin and insulin-like growth factor-I in the healthy young male. J. Int. Soc. Sports Nutr. 2017, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Herbert, P.; Hayes, L.D.; Sculthorpe, N.; Grace, F.M. High-intensity interval training (HIIT) increases insulin-like growth factor-I (IGF-1) in sedentary aging men but not masters’ athletes: An observational study. Aging Male 2016, 20, 54–59. [Google Scholar] [CrossRef]
- Curiel-Cervantes, V.; Solis-Sainz, J.C.; Camacho-Barrón, M.; Aguilar-Galarza, A.; Valencia, M.E.; Anaya-Loyola, M.A. Systematic training in master swimmer athletes increases serum insulin growth factor-1 and decreases myostatin and irisin levels. Growth Factors 2022, 40, 1–12. [Google Scholar] [CrossRef]
- Yoo, T.K.; Oh, B.K.; Lee, M.Y.; Sung, K.C. Association between physical activity and insulin resistance using the homeostatic model assessment for insulin resistance independent of waist circumference. Sci. Rep. 2022, 12, 6002. [Google Scholar] [CrossRef]
- Ateia, S.; Rusu, E.; Cristescu, V.; Enache, G.; Cheța, D.M.; Radulian, G. Proinsulin and age in general population. J. Med. Life 2013, 6, 424–429. [Google Scholar] [PubMed]
- Bryhni, B.; Arnesen, E.; Jenssen, T.G. Associations of age with serum insulin, proinsulin and the proinsulin-to-insulin ratio: A cross-sectional study. BMC Endocr. Disord. 2010, 10, 21. [Google Scholar] [CrossRef]
- Loh, N.Y.; Noordam, R.; Christodoulides, C. Telomere length and metabolic syndrome traits: A Mendelian randomisation study. Aging Cell 2021, 20, e13445. [Google Scholar] [CrossRef]
- Tucker, L.A. Insulin resistance and biological aging: The role of body mass, waist circumference, and inflammation. Biomed. Res. Int. 2022, 2022, 2146596. [Google Scholar] [CrossRef]
- Tokarz, V.L.; MacDonald, P.E.; Klip, A. The cell biology of systemic insulin function. J. Cell Biol. 2018, 217, 2273–2289. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.J.; Kang, S.W.; Lockwood, G.P.; Couteur, D.G.L.; Cogger, V.C. Hallmarks of Aging in the Liver. Comput. Struct. Biotechnol. J. 2019, 17, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Asthana, P.; Gurung, S.; Zhang, S.; Wong, S.K.K.; Fallah, S.; Chow, C.F.W.; Che, S.; Zhai, L.; Wang, Z.; et al. Regulation of age-associated insulin resistance by MT1-MMP-mediated cleavage of insulin receptor. Nat. Commun. 2022, 13, 3749. [Google Scholar] [CrossRef]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and Metabolic Dysfunction in Ageing and Age-Related Diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Ritz, P.; Berrut, G. Mitochondrial function, energy expenditure, aging and insulin resistance. Diabetes Metab. 2005, 31, 5S67–5S73. [Google Scholar] [CrossRef]
- Thankamony, A.; Capalbo, D.; Marcovecchio, M.L.; Sleigh, A.; Jørgensen, S.W.; Hill, N.R.; Mooslehner, K.; Yeo, G.S.; Bluck, L.; Juul, A.; et al. Low circulating levels of IGF-1 in healthy adults are associated with reduced β-cell function, increased intramyocellular lipid, and enhanced fat utilization during fasting. J. Clin. Endocrinol. Metab. 2014, 99, 2198–2207. [Google Scholar] [CrossRef]
- Shou, J.; Chen, P.; Xiao, W. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, Y.; Pan, Y.; Li, M.; Niu, Y.; Zhang, T.; Sun, H.; Zhou, S.; Liu, M.; Zhang, Y.; et al. Fatty infiltration in the musculoskeletal system: Pathological mechanisms and clinical implications. Front. Endocrinol. 2024, 15, 1406046. [Google Scholar] [CrossRef]
- Conte, M.; Martucci, M.; Sandri, M.; Franceschi, C.; Salvioli, S. The Dual Role of the Pervasive “Fattish” Tissue Remodeling With Age. Front. Endocrinol. 2019, 10, 114. [Google Scholar] [CrossRef]
- Dondero, K.; Friedman, B.; Rekant, J.; Landers-Ramos, R.; Addison, O. The effects of myosteatosis on skeletal muscle function in older adults. Physiol. Rep. 2024, 12, e16042. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhang, P.; Chen, X.; Liu, W. Ubiquitin-proteasome pathway in skeletal muscle atrophy. Front. Physiol. 2023, 14, 1289537. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Ngu, M.C.; Hunt, N.J.; Brandon, A.E.; Simpson, S.J.; Cogger, V.C. Liver, ageing and disease. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 680–695. [Google Scholar] [CrossRef] [PubMed]
- Dehkhoda, F.; Lee, C.M.M.; Medina, J.; Brooks, A.J. The Growth Hormone Receptor: Mechanism of Receptor Activation, Cell Signaling, and Physiological Aspects. Front. Endocrinol. 2018, 9, 35. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.H.F.; da Silva, D.P.; de Alvarenga, R.L.; Terra, A.; Koch, A.; Machado, M.; Monteiro Baboia Pompeo, F.A. Skeletal muscle hypertrophy: Molecular and applied aspects of exercise physiology. Ger. J. Exerc. Sport Res. 2020, 50, 195–207. [Google Scholar] [CrossRef]
- Harridge, S.D.R. Ageing and local growth factors in muscle. Scand. J. Med. Sci. Sports 2003, 13, 34–39. [Google Scholar] [CrossRef]
- Vassilakos, G.; Barton, E.R. Insulin-like growth factor I regulation and its actions in skeletal muscle. Compr. Physiol. 2018, 9, 413–438. [Google Scholar] [CrossRef]
- Guan, X.; Yan, Q.; Wang, D.; Du, G.; Zhou, J. IGF-1 signaling regulates mitochondrial remodeling during myogenic differentiation. Nutrients 2022, 14, 1249. [Google Scholar] [CrossRef] [PubMed]
- Halling, J.F.; Pilegaard, H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020, 45, 927–936. [Google Scholar] [CrossRef]
- Ferreira, R.P.; Duarte, J.A. Protein turnover in skeletal muscle: Looking at molecular regulation towards an active lifestyle. Int. J. Sports Med. 2023, 44, 763–777. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Wideman, L.; Weltman, J.Y.; Hartman, M.L.; Veldhuis, J.D.; Weltman, A. Growth hormone release during acute and chronic aerobic and resistance exercise. Sports Med. 2002, 32, 987–1004. [Google Scholar] [CrossRef] [PubMed]
- Sterczala, A.J.; Pierce, J.R.; Barnes, B.R.; Urso, M.L.; Matheny, R.W.; Scofield, D.E.; Flanagan, S.D.; Maresh, C.M.; Zambraski, E.J.; Kraemer, W.J.; et al. Insulin-like growth factor-I biocompartmentalization across blood, interstitial fluid, and muscle, before and after 3 months of chronic resistance exercise. J. Appl. Physiol. 2022, 133, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Rose, A.J.; Richter, E.A. Regulatory mechanisms of skeletal muscle protein turnover during exercise. J. Appl. Physiol. 2009, 106, 1702–1711. [Google Scholar] [CrossRef]
- Widdowson, W.M.; Healy, M.; Sönksen, P.H.; Gibney, J. The physiology of growth hormone and sport. Growth Horm. IGF Res. 2009, 19, 308–319. [Google Scholar] [CrossRef]
- Arazi, H.; Damirchi, A.; Faraji, H.; Rahimi, R. Hormonal responses to acute and chronic resistance exercise in middle-age versus young men. Sport Sci. Health 2012, 8, 59–65. [Google Scholar] [CrossRef]
- Jones, T.W.; Eddens, L.; Kupusarevic, J.; Simoes, D.C.M.; Furber, M.J.W.; Van Someren, K.A.; Howatson, G. Aerobic exercise intensity does not affect the anabolic signaling following resistance exercise in endurance athletes. Sci. Rep. 2021, 11, 10785. [Google Scholar] [CrossRef]
- De Alcantara Borba, D.; Da Silva Alves, E.; Rosa, J.P.P.; Alves Facundo, L.; Magno Amaral Acosta, C.; Coelho Silva, A.; Veruska Narciso, F.; Silva, A.; Túlio de Mello, M. Can IGF-1 Serum Levels Really be Changed by Acute Physical Exercise? A Systematic Review and Meta-Analysis. J. Phys. Act. Health 2020, 17, 575–584. [Google Scholar] [CrossRef]
- Moore, D.R.; McKay, B.R.; Tarnopolsky, M.A.; Parise, G. Blunted satellite cell response is associated with dysregulated IGF-1 expression after exercise with age. Eur. J. Appl. Physiol. 2018, 118, 2225–2231. [Google Scholar] [CrossRef]
- Santos, P.A.; da Silva Aguiar, S.; Barbosa, L.D.M.P.F.; Dos Santos Rosa, T.; Sales, M.M.; Maciel, L.A.; Lopes de Araújo Leite, P.; Gutierrez, S.D.; Minuzzi, L.G.; Sousa, C.V.; et al. Relationship of Testosterone, LH, Estradiol, IGF-1, and SHBG with Physical Performance of Master Athletes. Res. Q. Exerc. Sport 2023, 95, 363–369. [Google Scholar] [CrossRef]
- Khalid, K.; Szewczyk, A.; Kiszałkiewicz, J.; Middalska-Sek, M.; Domańska-Senderowska, D.; Brzeziański, M.; Lulińska, E.; Jegier, A.; Brzeziańska-Lasota, E. Type of training has a significant influence on the GH/IGF-1 axis but not on regulating miRNAs. Biol. Sport 2020, 37, 217–228. [Google Scholar] [CrossRef]
- Arazi, H.; Babaei, P.; Moghimi, M.; Asadi, A. Acute effects of strength and endurance exercise on serum BDNF and IGF-1 levels in older men. BMC Geriatr. 2021, 21, 50. [Google Scholar] [CrossRef]
- Kliszczewicz, B.; Markert, C.D.; Bechke, E.; Williamson, C.; Clemons, K.N.; Snarr, R.L.; McKenzie, M.J. Acute effect of popular high-intensity functional training exercise on physiologic markers of growth. J. Strength Cond. Res. 2021, 35, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, T.; Kirschnick, F.; Kröger, L.; Beileke, P.; Rezepin, M.; Brigadski, T.; Leßmann, V.; Schega, L. Comparison of the effects of open vs. closed skill exercise on the acute and chronic BDNF, IGF-1 and IL-6 response in older healthy adults. BMC Neurosci. 2021, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Eryılmaz, S.K.; Aslankeser, Z.; Özdemir, Ç.; Özgünen, K.; Kurdak, S. The effect of 30-m repeated sprint exercise on muscle damage indicators, serum insulin-like growth factor-I and cortisol. Biomed. Hum. Kinet. 2019, 11, 151–157. [Google Scholar] [CrossRef]
- Taipale, R.; Gagnon, S.; Ahtiainen, J.; Häkkinen, K.; Kyröläinen, H.; Nindl, B. Active recovery shows favorable IGF-1 and IGF binding protein responses following heavy resistance exercise compared to passive recovery. Growth Horm. IGF Res. 2019, 48–49, 45–52. [Google Scholar] [CrossRef]
- Geesmann, B.; Gibbs, J.C.; Mester, J.; Koehler, K. Association between energy balance and metabolic hormone suppression during ultraendurance exercise. Int. J. Sports Physiol. Perform. 2017, 12, 984–989. [Google Scholar] [CrossRef]
- Kim, T.; Chang, J.S.; Kim, H.; Lee, K.H.; Kong, I.D. Intense walking exercise affects serum IGF-1 and IGFBP3. J. Lifestyle Med. 2015, 5, 21–25. [Google Scholar] [CrossRef]
- Hasani-Ranjbar, S.; Soleymani Far, E.; Heshmat, R.; Rajabi, H.; Kosari, H. Time course responses of serum GH, insulin, IGF-1, IGFBP1, and IGFBP3 concentrations after heavy resistance exercise in trained and untrained men. Endocrine 2012, 41, 144–151. [Google Scholar] [CrossRef]
- Borges Bastos, C.L.; Miranda, H.; Vale, R.G.; Portal Mde, N.; Gomes, M.T.; Novaes Jda, S.; Winchester, J.B. Chronic effect of static stretching on strength performance and basal serum IGF-1 levels. J. Strength Cond. Res. 2013, 27, 2465–2472. [Google Scholar] [CrossRef]
- Nascimento, M.A.D.; Gerage, A.M.; Silva, D.R.P.D.; Ribeiro, A.S.; Machado, D.G.D.S.; Pina, F.L.C.; Tomeleri, C.M.; Venturini, D.; Barbosa, D.S.; Mayhew, J.L.; et al. Effect of resistance training with different frequencies and subsequent detraining on muscle mass and appendicular lean soft tissue, IGF-1, and testosterone in older women. Eur. J. Sport Sci. 2019, 19, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Lou, K.; Hou, L.; Lu, Y.; Sun, L.; Tan, S.C.; Low, T.Y.; Kord-Varkaneh, H.; Pang, S. The effect of resistance training on serum insulin-like growth factor 1 (IGF-1): A systematic review and meta-analysis. Complement. Ther. Med. 2020, 50, 102360. [Google Scholar] [CrossRef]
- Fink, J.; Schoenfeld, B.J.; Nakazato, K. The role of hormones in muscle hypertrophy. Phys. Sportsmed. 2018, 46, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Reidy, P.T.; Bhattarai, N.; Sidossis, L.S.; Rasmussen, B.B. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med. Sci. Sports Exerc. 2015, 47, 1922–1931. [Google Scholar] [CrossRef]
- Groennebaek, T.; Vissing, K. Impact of resistance training on skeletal muscle mitochondrial biogenesis, content, and function. Front. Physiol. 2017, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Roldán, M.J.; Sañudo, B.; Carrasco Páez, L. Influence of High-Intensity Interval Training on IGF-1 Response, Brain Executive Function, Physical Fitness and Quality of Life in Sedentary Young University Women-Protocol for a Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 5327. [Google Scholar] [CrossRef]
- Yasuda, Y. Sex differences in salivary free insulin-like growth factor-1 levels in elderly outpatients. Cureus 2021, 13, e17553. [Google Scholar] [CrossRef]
- Maroto-Izquierdo, S.; Martín-Rivera, F.; Nosaka, K.; Beato, M.; González-Gallego, J.; De Paz, J.A. Effects of submaximal and supramaximal accentuated eccentric loading on mass and function. Front. Physiol. 2023, 14, 1176835. [Google Scholar] [CrossRef]
- Huschtscha, Z.; Parr, A.; Porter, J.; Costa, R.J.S. The Effects of a High-Protein Dairy Milk Beverage With or Without Progressive Resistance Training on Fat-Free Mass, Skeletal Muscle Strength and Power, and Functional Performance in Healthy Active Older Adults: A 12-Week Randomized Controlled Trial. Front. Nutr. 2021, 8, 644865. [Google Scholar] [CrossRef]
- Cunha, P.M.; Nunes, J.P.; Tomeleri, C.M.; Nascimento, M.A.; Schoenfeld, B.J.; Antunes, M.; Gobbo, L.A.; Teixeira, D.; Cyrino, E.S. Resistance training performed with single and multiple sets induces similar improvements in muscular strength, muscle mass, muscle quality, and IGF-1 in older women: A randomized controlled trial. J. Strength Cond. Res. 2020, 34, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Micielska, K.; Gmiat, A.; Zychowska, M.; Kozlowska, M.; Walentukiewicz, A.; Lysak-Radomska, A.; Jaworska, J.; Rodziewicz, E.; Duda-Biernacka, B.; Ziemann, E. The beneficial effects of 15 units of high-intensity circuit training in women is modified by age, baseline insulin resistance and physical capacity. Diabetes Res. Clin. Pract. 2019, 152, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Arazi, H.; Khanmohammadi, A.; Asadi, A.; Haff, G.G. The effect of resistance training set configuration on strength, power, and hormonal adaptation in female volleyball players. Appl. Physiol. Nutr. Metab. 2018, 43, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.; Slater, G.; Hackett, D.; Johnson, N.; O’Connor, H. Physiological implications of preparing for a natural male bodybuilding competition. Eur. J. Sport Sci. 2018, 18, 619–629. [Google Scholar] [CrossRef]
- Ojanen, T.; Kyröläinen, H.; Igendia, M.; Häkkinen, K. Effect of prolonged military field training on neuromuscular and hormonal responses and shooting performance in warfighters. Mil. Med. 2018, 183, e705–e712. [Google Scholar] [CrossRef]
- Ives, S.J.; Norton, C.; Miller, V.; Minicucci, O.; Robinson, J.; O’Brien, G.; Escudero, D.; Paul, M.; Sheridan, C.; Curran, K.; et al. Multi-modal exercise training and protein-pacing enhances physical performance adaptations independent of growth hormone and BDNF but may be dependent on IGF-1 in exercise-trained men. Growth Horm. IGF Res. 2017, 32, 60–70. [Google Scholar] [CrossRef]
- Sellami, M.; Dhahbi, W.; Hayes, L.D.; Padulo, J.; Rhibi, F.; Djemail, H.; Chaouachi, A. Combined sprint and resistance training abrogates age differences in somatotropic hormones. PLoS ONE 2017, 12, e0183184. [Google Scholar] [CrossRef]
- So, W.; Song, M.; Park, Y.; Cho, B.; Lim, J.; Kim, S.; Song, W. Body composition, fitness level, anabolic hormones, and inflammatory cytokines in the elderly: A randomized controlled trial. Aging Clin. Exp. Res. 2013, 25, 167–174. [Google Scholar] [CrossRef]
- Nindl, B.C.; McClung, J.P.; Miller, J.K.; Karl, J.P.; Pierce, J.R.; Scofield, D.E.; Young, A.J.; Lieberman, H.R. Bioavailable IGF-1 is associated with fat-free mass gains after physical training in women. Med. Sci. Sports Exerc. 2011, 43, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Matsubara, T.; Tobina, T.; Shindo, M.; Tokuyama, K.; Tanaka, K.; Tanaka, H. Effect of low-intensity aerobic exercise on insulin-like growth factor-I and insulin-like growth factor-binding proteins in healthy men. Int. J. Endocrinol. 2010, 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Jun, T.; Park, K.; Chang, H.; So, W.Y.; Song, W. Twelve weeks of combined exercise is better than aerobic exercise for increasing growth hormone in middle-aged women. Int. J. Sport Nutr. Exerc. 2010, 20, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Borst, S.E.; De Hoyos, D.V.; Garzarella, L.; Vincent, K.; Pollock, B.H.; Lowenthal, D.T.; Pollock, M.L. Effects of resistance training on insulin-like growth factor-I and IGF binding proteins. Med. Sci. Sports Exerc. 2001, 33, 648–653. [Google Scholar] [CrossRef]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2017, 2, e000143. [Google Scholar] [CrossRef]
- Jiahao, L.; Jiajin, L.; Yifan, L. Effects of resistance training on insulin sensitivity in the elderly: A meta-analysis of randomized controlled trials. J. Exerc. Sci. Fit. 2021, 19, 241–251. [Google Scholar] [CrossRef]
- Solomon, T.P.; Malin, S.K.; Karstoft, K.; Knudsen, S.H.; Haus, J.M.; Laye, M.J.; Kirwan, J.P. Association between cardiorespiratory fitness and the determinants of glycemic control across the entire glucose tolerance continuum. Diabetes Care 2015, 38, 921–929. [Google Scholar] [CrossRef]
- Iaccarino, G.; Franco, D.; Sorriento, D.; Strisciuglio, T.; Barbato, E.; Morisco, C. Modulation of Insulin Sensitivity by Exercise Training: Implications for Cardiovascular Prevention. J. Cardiovasc. Transl. Res. 2021, 14, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Kahn, D.; Perreault, L.; Macias, E.; Zarini, S.; Newsom, S.A.; Strauss, A.; Kerege, A.; Harrison, K.; Snell-Bergeon, J.; Bergman, B.C. Subcellular localization and composition of intramuscular triacylglycerol influence insulin sensitivity in humans. Diabetologia 2021, 64, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, N.; Tamura, Y.; Takeno, K.; Kakehi, S.; Someya, Y.; Funayama, T.; Furukawa, Y.; Kaga, H.; Suzuki, R.; Sugimoto, D.; et al. Both higher fitness level and higher current physical activity level may be required for intramyocellular lipid accumulation in non-athlete men. Sci. Rep. 2020, 10, 4102. [Google Scholar] [CrossRef]
- Zouhal, H.; Jayavel, A.; Parasuraman, K.; Hayes, L.D.; Tourny, C.; Rhibi, F.; Laher, I.; Abderrahman, A.B.; Hackney, A.C. Effects of Exercise Training on Anabolic and Catabolic Hormones with Advanced Age: A Systematic Review. Sports Med. 2022, 52, 1353–1368. [Google Scholar] [CrossRef]
- Michaelsen, K.F. Effect of protein intake from 6 to 24 months on insulin-like growth factor 1 (IGF-1) levels, body composition, linear growth velocity, and linear growth acceleration: What are the implications for stunting and wasting? Food Nutr. Bull. 2013, 34, 268–271. [Google Scholar] [CrossRef]
- Oxfeldt, M.; Phillips, S.M.; Andersen, O.E.; Johansen, F.T.; Bangshaab, M.; Risikesan, J.; McKendry, J.; Melin, A.K.; Hansen, M. Low energy availability reduces myofibrillar and sarcoplasmic muscle protein synthesis in trained females. J. Physiol. 2023, 601, 3481–3497. [Google Scholar] [CrossRef] [PubMed]
- Cupka, M.; Sedliak, M. Hungry runners–low energy availability in male endurance athletes and its impact on performance and testosterone: Mini-review. Eur. J. Transl. Myol. 2023, 33, 11104. [Google Scholar] [CrossRef]
- Chen, C.N.; Liao, Y.H.; Tsai, S.C.; Thompson, L.V. Age-dependent effects of caloric restriction on mTOR and ubiquitin-proteasome pathways in skeletal muscles. GeroScience 2019, 41, 871–880. [Google Scholar] [CrossRef]
- Sharples, A.P.; Hughes, D.C.; Deane, C.S.; Saini, A.; Selman, C.; Stewart, C.E. Longevity and skeletal muscle mass: The role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell 2015, 14, 511–523. [Google Scholar] [CrossRef]
- De Andrade, P.B.; Neff, L.A.; Strosova, M.K.; Arsenijevic, D.; Patthey-Vuadens, O.; Scapozza, L.; Montani, J.P.; Ruegg, U.T.; Dulloo, A.G.; Dorchies, O.M. Caloric restriction induces energy-sparing alterations in skeletal muscle contraction, fiber composition and local thyroid hormone metabolism that persist during catch-up fat upon refeeding. Front. Physiol. 2015, 6, 254. [Google Scholar] [CrossRef]
- Ham, D.J.; Börsch, A.; Chojnowska, K.; Lin, S.; Leuchtmann, A.B.; Ham, A.S.; Thürkauf, M.; Delezie, J.; Furrer, R.; Burri, D.; et al. Distinct and additive effects of calorie restriction and rapamycin in aging skeletal muscle. Nat. Commun. 2022, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Slater, G.J.; Dieter, B.P.; Marsh, D.J.; Helms, E.R.; Shaw, G.; Iraki, J. Is an energy surplus required to maximize skeletal muscle hypertrophy associated with resistance training? Front. Nutr. 2019, 6, 131. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Margolis, L.M.; Orr, J.S. Optimized dietary strategies to protect skeletal muscle mass during periods of unavoidable energy deficit. FASEB J. 2015, 29, 1136–1142. [Google Scholar] [CrossRef]
- Carbone, J.W.; McClung, J.P.; Pasiakos, S.M. Skeletal muscle responses to negative energy balance: Effects of dietary protein. Adv. Nutr. 2012, 3, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Bouhlel, E.; Zaouali, M.; Miled, A.; Tabka, Z.; Bigard, X.; Shephard, R.J. Ramadan fasting and the GH/IGF-1 axis of trained men during submaximal exercise. Ann. Nutr. Metab. 2008, 52, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Mercken, E.M.; Crosby, S.D.; Lamming, D.W.; JeBailey, L.; Krzysik-Walker, S.; Villareal, D.T.; Capri, M.; Franceschi, C.; Zhang, Y.; Becker, K.; et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell 2013, 12, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Bradley, J.S.; McCoski, S.R.; Gonzalez, J.M.; Ealy, A.D.; Johnson, S.E. Reduced skeletal muscle fiber size following caloric restriction is associated with calpain-mediated proteolysis and attenuation of IGF-1 signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R806–R815. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Koehler, K. Caloric restriction induces anabolic resistance to resistance exercise. Eur. J. Appl. Physiol. 2020, 120, 1155–1164. [Google Scholar] [CrossRef]
- Rahmani, J.; Kord Varkaneh, H.; Clark, C.; Zand, H.; Bawadi, H.; Ryan, P.M.; Fatahi, S.; Zhang, Y. The influence of fasting and energy restricting diets on IGF-1 levels in humans: A systematic review and meta-analysis. Ageing Res. Rev. 2019, 53, 100910. [Google Scholar] [CrossRef]
- Longland, T.M.; Oikawa, S.Y.; Mitchell, C.J.; Devries, M.C.; Phillips, S.M. Higher compared with lower dietary protein during an energy deficit combined with intense exercise promotes greater lean mass gain and fat mass loss: A randomized trial. Am. J. Clin. Nutr. 2016, 103, 738–746. [Google Scholar] [CrossRef]
- Ardavani, A.; Aziz, H.; Smith, K.; Atherton, P.J.; Phillips, B.E.; Idris, I. The Effects of Very Low Energy Diets and Low Energy Diets with Exercise Training on Skeletal Muscle Mass: A Narrative Review. Adv. Ther. 2021, 38, 149–163. [Google Scholar] [CrossRef]
- Das, J.K.; Banskota, N.; Candia, J.; Griswold, M.E.; Orenduff, M.; de Cabo, R.; Corcoran, D.L.; Das, S.K.; De, S.; Huffman, K.M.; et al. Calorie restriction modulates the transcription of genes related to stress response and longevity in human muscle: The CALERIE study. Aging Cell 2023, 22, e13963. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Kater, C.E. Hormonal aspects of overtraining syndrome: A systematic review. BMC Sports Sci. Med. Rehabil. 2017, 9, 14. [Google Scholar] [CrossRef]
- Wasserfurth, P.; Palmowski, J.; Hahn, A.; Krüger, K. Reasons for and consequences of low energy availability in female and male athletes: Social environment, adaptations, and prevention. Sports Med. Open 2020, 6, 44. [Google Scholar] [CrossRef]
- Joro, R.; Uusitalo, A.; DeRuisseau, K.C.; Atalay, M. Changes in cytokines, leptin, and IGF-1 levels in overtrained athletes during a prolonged recovery phase: A case-control study. J. Sports Sci. 2017, 35, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Zietz, B.; Schnabl, S.; Nerlich, M.; Schoelmerich, J.; Schaeffler, A. Nutritional Composition in Different Training Stages in Young Female Athletes (Swimming) and Association with Leptin, IGF-1 and Estradiol. Exp. Clin. Endocrinol. Diabetes 2008, 117, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Tamolienė, V.; Remmel, L.; Gruodyte-Raciene, R.; Jürimäe, J. Relationships of bone mineral variables with body composition, blood hormones and training volume in adolescent female athletes with different loading patterns. Int. J. Environ. Res. Public Health 2021, 18, 6571. [Google Scholar] [CrossRef] [PubMed]
- Koundouros, N.; Blenis, J. Targeting mTOR in the context of diet and whole-body metabolism. Endocrinology 2022, 163, bqac041. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tezze, C.; Sandri, M.; Tessari, P. Anabolic resistance in the pathogenesis of sarcopenia in the elderly: Role of nutrition and exercise in young and old people. Nutrients 2023, 15, 4073. [Google Scholar] [CrossRef]
- Arentson-Lantz, E.J.; Von Ruff, Z.; Connolly, G.; Albano, F.; Kilroe, S.P.; Wacher, A.; Campbell, W.W.; Paddon-Jones, D. Meals containing equivalent total protein from foods providing complete, complementary, or incomplete essential amino acid profiles do not differentially affect 24-h skeletal muscle protein synthesis in healthy, middle-aged women. J. Nutr. 2024, 154, 3626–3638. [Google Scholar] [CrossRef]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballèvre, O.; Beaufrère, B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E348. [Google Scholar] [CrossRef]
- Gulick, C.N.; Peddie, M.C.; Jowett, T.; Hackney, A.C.; Rehrer, N.J. Exercise, dietary protein, and combined effect on IGF-1. Int. J. Sci. Res. Methodol. 2020, 16, 61–77. [Google Scholar]
- Goi, A.; De Marchi, M.; Costa, A. Minerals and essential amino acids of bovine colostrum: Phenotypic variability and predictive ability of mid- and near-infrared spectroscopy. J. Dairy Sci. 2023, 106, 8341–8356. [Google Scholar] [CrossRef]
- Davison, G. The Use of Bovine Colostrum in Sport and Exercise. Nutrients 2021, 13, 1789. [Google Scholar] [CrossRef]
- Davison, G.; Jones, A.W.; Marchbank, T.; Playford, R.J. Oral bovine colostrum supplementation does not increase circulating insulin-like growth factor-1 concentration in healthy adults: Results from short- and long-term administration studies. Eur. J. Nutr. 2020, 59, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Duff, W.R.D.; Chilibeck, P.D.; Rooke, J.J.; Kaviani, M.; Krentz, J.R.; Haines, D.M. The Effect of Bovine Colostrum Supplementation in Older Adults During Resistance Training. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Khalil, D.A.; Lucas, E.A.; Juma, S.; Smith, B.J.; Payton, M.E.; Arjmandi, B.H. Soy protein supplementation increases serum insulin-like growth factor-I in young and old men but does not affect markers of bone metabolism. J. Nutr. 2002, 132, 2605–2608. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Feng, Y.; Feng, J.; Chen, X. The effect of soy intervention on insulin-like growth factor 1 levels: A meta-analysis of clinical trials. Phytother. Res. 2020, 34, 1570–1577. [Google Scholar] [CrossRef]
- Messina, M.; Magee, P. Does soy protein affect circulating levels of unbound IGF-1? Eur. J. Nutr. 2017, 57, 423–432. [Google Scholar] [CrossRef]
- Lim, C.; Janssen, T.A.H.; Currier, B.S.; Paramanantharajah, N.; McKendry, J.; Abou Sawan, S.; Phillips, S.M. Muscle Protein Synthesis in Response to Plant-Based Protein Isolates With and Without Added Leucine Versus Whey Protein in Young Men and Women. Curr. Dev. Nutr. 2024, 8, 103769. [Google Scholar] [CrossRef]
- Weijzen, M.E.; Holwerda, A.M.; Jetten, G.H.J.; Houben, L.H.P.; Kerr, A.; Davis, H.; Keogh, B.; Khaldi, N.; Verdijk, L.B.; Van Loon, L.J.C. Vicia faba peptide network supplementation does not differ from milk protein in modulating changes in muscle size during short-term immobilization and subsequent remobilization, but increases muscle protein synthesis rates during remobilization in healthy young men. J. Nutr. 2023, 153, 1718–1729. [Google Scholar] [CrossRef]
- Kerr, A.; Hart, L.; Davis, H.; Wall, A.; Lacey, S.; Franklyn-Miller, A.; Khaldi, N.; Keogh, B. Improved strength recovery and reduced fatigue with suppressed plasma myostatin following supplementation of a Vicia faba hydrolysate, in a healthy male population. Nutrients 2023, 15, 986. [Google Scholar] [CrossRef]
- Davies, R.W.; Kozior, M.; Lynch, A.E.; Bass, J.J.; Atherton, P.J.; Smith, K.; Jakeman, P.M. The Effect of Fava Bean (Vicia faba L.) Protein Ingestion on Myofibrillar Protein Synthesis at Rest and after Resistance Exercise in Healthy, Young Men and Women: A Randomised Control Trial. Nutrients 2022, 14, 3688. [Google Scholar] [CrossRef]
- Pinckaers, P.J.M.; Kouw, I.W.K.; Gorissen, S.H.M.; Houben, L.H.P.; Senden, J.M.; Wodzig, W.K.H.W.; de Groot, L.C.P.G.M.; Verdijk, L.B.; Snijders, T.; van Loon, L.J.C. The Muscle Protein Synthetic Response to the Ingestion of a Plant-Derived Protein Blend Does Not Differ from an Equivalent Amount of Milk Protein in Healthy Young Males. J. Nutr. 2023, 152, 2734–2743. [Google Scholar] [CrossRef]
- Tom, A.; Nair, K.S. Assessment of branched-chain amino acid status and potential for biomarkers. J. Nutr. 2006, 136, 324S–330S. [Google Scholar] [CrossRef]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A. Branched-chain amino acids: Catabolism in skeletal muscle and implications for muscle and whole-body metabolism. Front. Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ferreira, M.P.; Cooke, M.B.; La Bounty, P.; Campbell, B.; Greenwood, M.; Willoughby, D.S.; Kreider, R.B. Co-ingestion of carbohydrate with branched-chain amino acids or L-leucine does not preferentially increase serum IGF-1 and expression of myogenic-related genes in response to a single bout of resistance exercise. Amino Acids 2015, 47, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, D.L.; Delcastillo, K.; Van Every, D.W.; Tipton, K.D.; Aragon, A.A.; Schoenfeld, B.J. Isolated Leucine and Branched-Chain Amino Acid Supplementation for Enhancing Muscular Strength and Hypertrophy: A Narrative Review. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 292–301. [Google Scholar] [CrossRef]
- Bagheri, R.; Forbes, S.C.; Candow, D.G.; Wong, A. Effects of branched-chain amino acid supplementation and resistance training in postmenopausal women. Exp. Gerontol. 2021, 144, 111185. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; McGarrah, R.W.; Herman, M.A.; Bain, J.R.; Shah, S.H.; Newgard, C.B. Insulin action, type 2 diabetes, and branched-chain amino acids: A two-way street. Mol. Metab. 2021, 52, 101261. [Google Scholar] [CrossRef]
- Supruniuk, E.; Żebrowska, E.; Chabowski, A. Branched chain amino acids-friend or foe in the control of energy substrate turnover and insulin sensitivity? Crit. Rev. Food Sci. Nutr. 2023, 63, 2559–2597. [Google Scholar] [CrossRef]
- Nishi, H.; Uchida, K.; Saito, M.; Yamanaka, D.; Nagata, H.; Tomoshige, H.; Miyata, I.; Ito, K.; Toyoshima, Y.; Takahashi, S.I.; et al. Essential amino acid intake is required for sustaining serum insulin-like growth factor-I levels but is not necessarily needed for body growth. Cells 2022, 11, 1523. [Google Scholar] [CrossRef]
- Dillon, E.L.; Sheffield-Moore, M.; Paddon-Jones, D.; Gilkison, C.; Sanford, A.P.; Casperson, S.L.; Jiang, J.; Chinkes, D.L.; Urban, R.J. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J. Clin. Endocrinol. Metab. 2009, 94, 1630–1637. [Google Scholar] [CrossRef]
- Ispoglou, T.; Witard, O.C.; Duckworth, L.C.; Lees, M.J. The efficacy of essential amino acid supplementation for augmenting dietary protein intake in older adults: Implications for skeletal muscle mass, strength and function. Proc. Nutr. Soc. 2020, 80, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Farshidfar, F.; Pinder, M.A.; Myrie, S.B. Creatine supplementation and skeletal muscle metabolism for building muscle mass: Review of the potential mechanisms of action. Curr. Protein Pept. Sci. 2017, 18, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Eghbali, E.; Arazi, H.; Suzuki, K. Supplementing With Which Form of Creatine (Hydrochloride or Monohydrate) Alongside Resistance Training Can Have More Impacts on Anabolic/Catabolic Hormones, Strength and Body Composition? Physiol. Res. 2024, 73, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D.; Cornish, S.M.; Antonio, J.; Kreider, R.B. Variables influencing the effectiveness of creatine supplementation as a therapeutic intervention for sarcopenia. Front. Nutr. 2019, 6, 124. [Google Scholar] [CrossRef]
- Zajac, A.; Poprzecki, S.; Zebrowska, A.; Chalimoniuk, M.; Langfort, J. Arginine and ornithine supplementation increases growth hormone and insulin-like growth factor-1 serum levels after heavy-resistance exercise in strength-trained athletes. J. Strength Cond. Res. 2010, 24, 1082–1090. [Google Scholar] [CrossRef]
- Nejati, M.; Dehghan, P.; Safari, S.; Jamilian, P.; Zarezadeh, M. The influence of arginine supplementation on IGF-1: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2023, 55, 51–57. [Google Scholar] [CrossRef]
- Fujita, S.; Rasmussen, B.B.; Cadenas, J.G.; Grady, J.J.; Volpi, E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E745–E754. [Google Scholar] [CrossRef]
- Clemmons, D.R. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol. Metab. Clin. N. Am. 2012, 41, 425–443. [Google Scholar] [CrossRef]
- Abdulla, H.; Smith, K.; Atherton, P.J.; Idris, I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: A systematic review and meta-analysis. Diabetologia 2016, 59, 44–55. [Google Scholar] [CrossRef]
- Wilburn, D.T.; Machek, S.B.; Cardaci, T.D.; Willoughby, D.S. Carbohydrate-induced insulin signaling activates focal adhesion kinase: A nutrient and mechanotransduction crossroads. Nutrients 2020, 12, 3145. [Google Scholar] [CrossRef]
- Cholewa, J.M.; Newmire, D.E.; Zanchi, N.E. Carbohydrate restriction: Friend or foe of resistance-based exercise performance? Nutrition 2019, 60, 136–146. [Google Scholar] [CrossRef]
- Margolis, L.M.; Pasiakos, S.M. Low carbohydrate availability impairs hypertrophy and anaerobic performance. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; de Souto Barreto, P.; Arai, H.; Bischoff-Ferrari, H.A.; Cadore, E.L.; Cesari, M.; Chen, L.K.; Coen, P.M.; Courneya, K.S.; Duque, G.; et al. Global consensus on optimal exercise recommendations for enhancing healthy longevity in older adults (ICFSR). J. Nutr. Health Aging 2025, 29, 100401. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.T.; Witard, O.C.; Højfeldt, G.; Church, D.D.; Breen, L. Dietary protein recommendations to support healthy muscle ageing in the 21st century and beyond: Considerations and future directions. Proc. Nutr. Soc. 2025, 84, 245–258. [Google Scholar] [CrossRef] [PubMed]
| Author, y | Country | Age | Sex | Exercise Modality | Case–Control | Type of Diet Control | Outcomes |
|---|---|---|---|---|---|---|---|
| Santos et al., 2024 [65] | Brazil | 51.0 ± 6.8 (51.7 ± 9.4 y/o) | Men | SIT vs. endurance | MS: master sprint athletes (n = 34) ME: master endurance (n = 32) athletes | Not reported | MS exhibited a higher average IGF-1. Performance showed a significant correlation with IGF-1. |
| Curiel-Cervantes et al., 2022 [29] | Mexico | 42 (11.7) | Men/Women | SIT vs. HIIT vs. low intensity | Master swimmers with systematic training (ST, n = 30), physically active individuals with no systematic training (NST, n = 32) | Non-energy-deficient/adequate protein intake | ST group showed higher levels of FFM, SM, IGF-1, Testosterone, Irisin, and IGF-1/INS and IGF-1/Myostatin ratios. |
| Khalid et al., 2020 [66] | Poland | 25.4 (±4.1) | Men | RT vs. endurance | Control (non-athletes) vs. strength athletes (ST) and endurance athletes (EN). | Not reported. | IGF-1, insulin, and IGFBP3 levels are higher in athletes than in controls. |
| Author, y | Study Type | Age | Sex | Intervention Groups | Exercise Modality | Dosage | Intensity | Type of Diet Control | Outcomes | Outcome Direction |
|---|---|---|---|---|---|---|---|---|---|---|
| Arazi et al., 2021 [67] | RCT | 60.8 (±1.8 y/o) | Men | Strength, n = 10 Endurance, n = 10 Control: no exercise, n = 10 | RT vs. endurance | 1 session 45 min | Strength: 65–70% Endurance: 30 min (3 × 10 min with 120 s interval) at 65–70% | Not reported | IGF-1 levels increased in both exercises. | ↑ |
| Kliszczewicz et al., 2021 [68] | RCD | 28.1 ± 5.4 y/o | Men | Cross-over Short bout and long bout, n = 10 | HIITs + RT with different durations | 1 session, 30 min | Short bout: 30 power clean and jerks, 30 reps within 5 min Long bout: 15 min reps as possible. Circuit (250 m row, 20 kettlebell swings, 15 dumbbell thrusters) | Fasting, eating 3 h after exercise | No time-dependent changes in IGF-1 levels, IGFBP-1, and IGFBP-2. GH increased after exercise. | ↔ |
| Behrendt et al., 2021 [69] | Cross over | 55–75 y/o | Men/ Women | N = 38 aOSE: badminton/table tennis/hockey, n = 12 aCSE: cycling, n = 12 Control: reading, n = 12 | Low to moderate dynamic sports vs. MICT | 1 session, 30 min | aOSE: 30 min of continuous playing aCSE: 30 min of continuous cycling at 60 ± 5% | Not reported | Both aOSE and aCSE increased levels of BDNFS, BDNFP, IGF-1, and IL-6. | ↑ |
| Eryilmaz et al., 2019 [70] | OGPPD | 23.3 (±3.3) | Men | University-level athletes, n = 9 | SIT vs. HIIT | 1 session | 2 sets of 10 reps of 30 m all-out sprints plus active and passive recovery | Not reported | IGF-1 increased after the exercise. | ↑ |
| Taipale et al., 2019 [71] | RCT | 26 (±4 y/o | Men | PR: passive recovery, n = 11 AR: active recovery, n = 7. | RT at different intensities | 1 session | 10 × 10 RM sets of bilateral leg press, starting from 70%RM | CR 24 h before. Balanced energy bar prior to exercise | IGF-1 levels increased. IGFBP-1 decreased in the AR group. | ↑ |
| Geesmann et al., 2017 [72] | OGPPD | 43.6 (±7.8 y/o) | Men | Well-trained amateur cyclists in the Paris-Brest-Paris event (n = 14) | Endurance | 1 sports event | 1230 km event in 54 h, net cycling time: 42:48 h; average speed: 28.7 km/h | Energy balance | Leptin, testosterone, and IGF-1 decreased significantly after the competition. | ↓ |
| Kim et al., 2015 [73] | OGPPD | 41(±6.78 y/o) | Men | Healthy middle-aged participants of the 100 K walk festival in W city (n = 14) | Endurance | 1 sports event | 100 km walking race, 9 h average | Ad libitum | Insulin, IGF-1, IGFBP3, and the IGF-1/IGFBP3 ratio decreased after the event. | ↓ |
| Hasani-Ranjbar et al., 2012 [74] | RCT | 20–30 | Men | Trained group: regular RT. Untrained group: regular exercisers. | RT | 1 session | 70–80% of RM, 10–12 reps, 4 sets. Upper and lower body exercises | Balanced breakfast | The increase in IGF-1 levels was fitness-dependent. | ↑ |
| Author, y | Study Type | Age | Sex | Intervention Groups | Exercise Modality | Dosage | Intensity | Type of Diet Control | Outcomes | Outcome Direction |
|---|---|---|---|---|---|---|---|---|---|---|
| Maroto-Izquierdo et al., 2023 [83] | RCT | 20.1 ± (2.1) | Men | N = 27 SUB: submaximal load, n = 14. SUPRA: supramaximal load, n = 13 | RT at different intensities | 10 w, 2 s/w of eccentric unilateral leg press training program | SUB: 90% 1 RM SUPRA: 120% 1 RM | Not reported | Both groups showed similar increases in IGF-1 and GH compared to pre-training. | ↑ |
| Behrendt et al., 2021 [69] | RCT | 55–75 | Men/ Women | N = 22 aOSE: sports, n = 11 aCSE: cycling, n = 11. Control: reading | Low to moderate dynamic sports vs. MICT | 12/w, 1 s/w 50 min. aOSE: 40 min of badminton/hockey/table tennis. aCSE: 20 min RT + 20 min cycling at 60 ± 5%. | aCSE: 7 of perceived exertion scale | Not reported | BDNFS, BDNFP, and IL-6 levels increased whilst IGF-1 decreased after intervention. | ↑ |
| Huschtscha et al., 2021 [84] | RCT | ≥50 | Men | N = 51 DM: high-protein dairy milk beverage (HPMB), n = 9 EX + DM: RT + HPMB, n = 11 EX: RT, n = 11 CON: control, n = 10 | RT | 12/w; 3 s/w. Full-body RT rotational workouts. | First 2 w: 3 sets of 10–15 reps at 50–60%. 3–6 w: 3 sets of 8–12 reps at 60–75%. 7–12 w: 80–95% 3 sets of 6–8 reps. | 2x/day of HPMB regular diet | The EX + DM group showed the highest increase in IGF-1 at 6 w. | ↑ |
| Cunha et al., 2020 [85] | RCT | ≥60 | Women | SS: single-set group, n = 23 MS: multiple-set group, n = 23 CC: control group, n = 23 | RT | 12 w whole-body RT 3 d/w, 8 exercises, 10–15 reps. 3 sets in the MS. | Load increased 2 to 5% for upper-limb and 5–10% for lower-limb exercises. | Not reported | Muscle quality, lean soft tissue, strength, and IGF-1 increased in the intervention groups. | ↑ |
| Micielska et al., 2019 [86] | RCT | 38 (±12) | Women | N = 33 HICT: high-intensity circuit training, n = 20 CON: control, n = 13 | HIIT | 5 w, 3 d/w. 15 HICT sessions 3 t/w. HICT session: 3 circuits with 9 exercises with one’s body as a workload. | 80% to 90%. | Not reported | IGF-1 increased in the HICT group. INS/IGF-1 ratio is higher in the HICT group. | ↑ |
| Nascimento et al., 2019 [76] | RCT | >60 | Women | N = 52 G2X: 2 t/w RT, n = 26 G3X: 3 t/w, n = 26 | RT vs. detraining | 32 w, 2 w: familiarization 12 w RT 1 set; 10–15 of 8 exercises for upper and lower extremities; 12 w detraining. | Progressive weight increase: 2–10%. | Balanced diet | ASM, SM, IGF-1, and testosterone were similar in G2X and G3X. Testosterone levels decreased after detraining. | ↔ |
| Arazi et al., 2018 [87] | RCT | 18 (±2.4) | Women | CRT: cluster sets, n = 10; TRT: traditional sets, n = 10; CON: control, n = 10 | RT | CRT AND TRT took 3 sessions of resistance training | Progressive load until 90%. | Not reported | CRT had higher vertical jump and higher IGF-1 increases post-intervention than the other groups. | ↑ |
| Mitchell et al., 2018 [88] | OGPPD | >18 | Men | natural bodybuilding training, n = 9 | RT + aerobic exercise | PRE16: 16 w before competition; PRE8: 8 w before competition; PRE1: 1 w before competition; POST4: 4 w post-competition | Not reported | High-P, low carbohydrates | Testosterone, insulin, and IGF-1 are lower in PRE16 compared to PRE1. All parameters returned to baseline in POST4. | ↓ |
| Ojanen et al., 2018 [89] | OGPPD | 20 (±1) | Men | N = 49. PRE: 1 w before Military Field Training (MFT); MID: MFT, 12 days; POST: MFT and RECO: 4 d after MFT. | RT + military skills | MFT | MFT | Not reported | MFT decreased testosterone and IGF-1. Military abilities were reduced. | ↓ |
| Ives et al., 2017 [90] | RCT | 25–55 | Men | PRISE: multimodal exercise (RT, stretching, endurance) + high-protein diet (2 g/kg) RISE: multimodal training + normal protein intake (1 g/kg) | RT + HIIT + endurance | 4 d/w multimodal progressive training. Each exercise was performed for 60 min 1 d/w. | RT1: upper body + core. RT2: lower body + core. Interval: 35 min of 7 sets of 30-s or 60-s. Stretching: Pilates + yoga. | Isocaloric, 5–6 meals/day, different P content | GH increased in the RISE group, and IGF-1 increased in the PRISE group. | ↑ |
| Sellami et al., 2017 [91] | RCT | Young: 21.6 (±1.8) Middle-age: 40.5(±1.8) | Men | YT: young training, n = 10; YC: young control, n = 9; MAT: middle-aged training, n = 10 MAT: middle-aged control, n = 9 | RT + SIT | 3 t/w/13 w 70 min 13 sprint running sessions, 13 RT sessions, 13 sprint cycling sessions | Maximal sprint bouts of running and cycling. RT at 50%, 70% and failure. | Balanced breakfast before exercise | MAT showed higher levels of GH/IGF-1 axis markers post-training. YT and MAC improved peak power output. | ↑ |
| Herbert et al., 2016 [28] | TGPPD | LEX: 60 (±5) Control (62 ± 2) | Men | SED: lifelong sedentary, n = 22 LEX: lifelong exercisers, n = 17 | HIIT | Phase 1: conditioning phase in SED (12 w) Phase 2: 9HIIT sessions Phase 3: testing | 6 × 30 s cycloergometer sprints at 40% peak power | Not reported | LEX had higher baseline levels of IGF-1. After HIIT, IGF-1 levels increased only in SED. | ↑↔ |
| Borges Bastos et al., 2013 [75] | RCT | 25–35 | Men | SBST (n = 10): stretching SDST (n = 10): stretching + RT RT (n = 10): RT | RT vs. RT + stretching | 3 d/w/10 w | RT: progressive load until reps. Stretching: 4 exercises before RT. | Balanced diet with 1.2–1.4 g/kg/gP | IGF-1 levels increased only in the RT. | ↑ |
| So et al., 2013 [92] | RCT | 65–82 | Men/Women | exercise group, n = 18 control group, n = 22 | RT | 3 d/w/11 w elastic band exercise | 40 min, 15–25 reps, 2–3 sets elastic band. | Not reported | GH, IGF-1, and IGFBF-3 did not differ. | ↔ |
| Nindl et al., 2011 [93] | OGPPD | 21 ± 5 | Women | Progressive basic combat military training (BCT) | RT + endurance | 8 w: RT + basic technical military training. | Progressive load. | Ad libitum | IGF-1 increased after BCT and was associated with FFM. | ↑ |
| Nishida et al., 2010 [94] | OGPPD | 22.6 ± 0.5 | Men | healthy sedentary mean | Very low aerobic | 5 d/w/6 w | 60 min cycle ergometer at lactate threshold. | Not reported | IGF-1 levels decreased, while IGFBP-1 levels increased. | ↓ |
| Seo et al., 2010 [95] | RCT | 50–65 | Women | AEG, n = 7: walking + aerobics CEG, n = 8: walking + RT | Aerobic vs. aerobic + RT | 3 d/w/12 w | AEG: 60–80%. CEG: 50–70%. | Not reported | GH increased in both exercise groups. IGF-1 did not increase. GH increased in CEG. | ↑↔ |
| Borst et al., 2001 [96] | RCT | 37 (±7) | Men/ Women | 1-SET: single-set RT, n = 11 3-SETs: multiple-set RT, n = 11 control, n = 9 | RT at different set load | 3 d/w/25 w | Progressive: 60–70% to failure, 8–12 reps. | Not reported | IGF-1 from w 13 until the end in both groups. No change in IGFBP-1 levels throughout the intervention. | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Curiel-Cervantes, V. Exercise and Dietary Factors in Skeletal Muscle Anabolism Across Aging: Inferences for the Insulin/IGF-1 Axis—A Narrative Review. Physiologia 2026, 6, 5. https://doi.org/10.3390/physiologia6010005
Curiel-Cervantes V. Exercise and Dietary Factors in Skeletal Muscle Anabolism Across Aging: Inferences for the Insulin/IGF-1 Axis—A Narrative Review. Physiologia. 2026; 6(1):5. https://doi.org/10.3390/physiologia6010005
Chicago/Turabian StyleCuriel-Cervantes, Vianney. 2026. "Exercise and Dietary Factors in Skeletal Muscle Anabolism Across Aging: Inferences for the Insulin/IGF-1 Axis—A Narrative Review" Physiologia 6, no. 1: 5. https://doi.org/10.3390/physiologia6010005
APA StyleCuriel-Cervantes, V. (2026). Exercise and Dietary Factors in Skeletal Muscle Anabolism Across Aging: Inferences for the Insulin/IGF-1 Axis—A Narrative Review. Physiologia, 6(1), 5. https://doi.org/10.3390/physiologia6010005







