Abstract
Background: We tested whether gene polymorphisms for angiotensin-converting enzyme (ACE, rs1799752) and tenascin-C (TNC, rs2104772) are associated with variability in fatigue resistance and metabolic strain during static lumbar exercise through interactions with chronic nonspecific lower back pain and habitual physical exercise levels (PA). Methods: Forty-eight patients and matched controls performed an isometric endurance test for lumbar extensors. Metabolic strain to longissimus muscle (oxygen saturation, lactate) and cardiovascular system (muscle hemoglobin, blood pressure) and holding time were monitored. Subjects were genotyped for rs1799752 (II, ID, DD) and rs2104772 (AA, AT, TT). Associations of variance with group, genotype, and PA were analyzed under a 5% false discovery rate. Results: The holding time was lower in patients than in controls (150.9 vs. 188.6 s). This difference was associated with both genotypes, as patients with DD-rs1799752-genotype (p = 0.007) and TT-rs2104772-genotype (p = 0.041) showed lower fatigue resistance. Muscle deoxygenation during exercise varied in positive association with the rs2104772-genotype and PA (p = 0.010, η2 = 0.236). Mean arterial blood pressure (p = 0.028, η2 = 0.108) and recovery of hemoglobin concentration (p = 0.003, η2 = 0.907) demonstrated complex group x rs2104772 interactions. Conclusions: Polymorphisms rs1799752 and rs2104772 influence back pain-related variability in lumbar fatigue resistance. rs2104772 was linked to cardiovascular strain during isometric exercise and recovery via muscle perfusion.
Keywords:
personalized medicine; genetic; physical activity; exercise; muscle; NIRS; oxygen; aerobic; perfusion; cardiovasculature; Sørensen; pain 1. Introduction
Nonspecific lower back pain is a relatively frequent condition for which there is evidence of impaired recruitment and endurance capacity of lumbar muscle groups during static exercise [1,2,3] and possibly decreased systemic aerobic capacity [4]. Impaired muscle perfusion and oxygenation have been found to be related to a reduced endurance capacity of the erector spinae muscle during repetitive exercise for the back in chronic low back pain patients [5,6]. Training-induced improvements in lumbar muscle oxygenation in patients with back pain during dynamic exercise and the influence of physical exercise (PA) on the occurrence and persistence of lower back pain [7] indicate that the mechanism underlying nonspecific lower back pain is affected by habitual PA [6].
A typical clinical sign of chronic lower back pain is a patient’s reduced fatigue resistance in a Biering-Sørensen test for isometric endurance capacity of lumbar extensors. During this maneuver, participants are required to lie prone on a table with the upper body flexed at the waist and off the table in order to lift and hold their upper body stable in an extended position to tolerance [1,2,3]. An indifferent decrease in the oxygen saturation of the lumbar extensor muscles, musculus (m.) longissimus and m. multifidus, between patients and controls during the Biering-Sørensen test [8] indicates that the functional deficit in the lumbar muscle extensors of nonspecific lower back pain comprises factors other than local metabolic factors.
The general syndrome of back pain is equally understood to share a genetic predisposition, with estimates of heritability ranging from 30 to 68% [9,10]. Several gene polymorphisms have been identified as risk factors for the general syndrome of lower back pain [11]. Gene variants associated with differences in endurance-related muscle performance have not been investigated for their association with chronic nonspecific lower back pain. Based on an interpretative hypothesis, we proposed that the occurrence of nonspecific lower back pain may be associated with specific polymorphisms in the genes for angiotensin-converting enzyme and tenascin-C because the encoded factors affect microvascular perfusion and inflammation of skeletal muscle and modulate pain sensation [12]. This also affects metabolic aspects of endurance performance, as shown formerly for locomotor muscle [13,14].
The presence of a silencer region in the gene for angiotensin-converting enzyme (ACE), the insertion allele (ACE-I), is a prominent gene polymorphism, rs1799752, that is associated with variability in improvements in endurance performance with physical training and cardiorehabilitation [13,15]. This influence is largely mediated by the inhibitory impact of the ACE-I allele on the expression of the encoded dipeptidyl carboxypeptidase compared to noncarriers of the ACE-I allele, i.e., ACE-DD genotypes, which are characterized by the absence or deletion of the I allele (reviewed by [16,17]). The active ACE enzyme cleaves the circulating hormones angiotensin 1 and bradykinin, both of which modulate the contractility of smooth and cardiac muscle but in opposing ways via receptor-mediated influences on intracellular calcium signaling [18,19,20,21]. Thus, due to the action of ACE, angiotensin 1-derived angiotensin 2 levels are elevated when bradykinin levels decrease. Both exert myogenic effects on smooth and cardiac muscle, increasing constriction [22,23]. Intriguingly, the ACE insertion/deletion gene polymorphism is associated with differences in muscle pain in different diseases [24], including inflammatory back pain [25].
Metabolically, the association of the rs1799752 gene polymorphism with variability in physical training-induced improvements in endurance performance is explained by the counteracting influences of the ACE-I allele and the D-allele on the aerobic capacity of skeletal muscle and the cardiovascular system. Therefore, the ACE-I allele exerts a positive influence on contraction-induced vasodilatation, improving the blood-borne transport of oxygen to skeletal muscle [26], and contributes to enhancing muscle aerobic metabolism with repeated physical exercise in training via increases in mitochondrial volume density (reviewed by [27,28]). This manifests as an exaggerated reduction in muscle oxygen saturation during dynamic exercise [29], reflecting enhanced mitochondrial respiration [30]. In contrast, ACE-D allele carriers typically demonstrate functional improvements within the cardiovascular system, such as more pronounced left ventricular hypertrophy and heart rate variability [31,32,33,34,35], which may enhance the perfusion of skeletal muscle contracting under high muscle tension [34]. Collectively, observable influences on mitochondrial and cardiovascular parameters corroborate the view that I- and D-allele carriers of ACE influence muscle performance during dynamic exercise in different physical fitness and muscle tension-related ways by affecting hemoglobin concentration (tHb) and oxygen saturation (SmO2) [27].
Similarly, a main association with physical endurance-related metabolic traits, including maximal oxygen uptake and aerobic peak power output, was revealed for the frequent polymorphism rs2104772 in the adhesion-modulatory protein tenascin-C (TNC) gene [14,36]. The rs2104772 polymorphism is associated with the nonsynonymous exchange of thymidine (T)-to-adenosine (A) in amino acid codon 1677 of TNC, altering its molecular elasticity [14,37]. In addition, rs2104772 influences the expression of the TNC protein, which is part of the ACE-I/D-modulated expression response of skeletal muscle to strenuous PA [17]. TNC orchestrates the growth response of cardiovascular structures and the regenerative response of muscle fibers and associated structures in response to tissue damage. Its aberrant expression is associated with preferential atrophy of fast-type muscle fibers [38,39,40,41,42]. Consistent with this notion, the rs2104772 gene polymorphism is associated with considerable variability in the volumetric adjustment of structural components of the energy supply in skeletal muscle. This concerns, for instance, alterations in capillarization, mitochondrial volume density, and the cross-sectional area of muscle fibers during systematic physical training of moderate to high intensity [43,44], all of which may affect fatigue resistance owing to influences on metabolic strain [45].
Both the rs2104772 and rs1799752 polymorphisms demonstrate PA-related differences in muscle perfusion, such as an acute reduction in muscle tHb concentration during intense cycling endurance and interval exercise [34,43]. These influences reflect genotype-dependent elevations in peripheral vascular resistance during PA and carry over to metabolic effects in working muscle. Specifically, the TT-rs2104771 and II-rs1799752 genotypes demonstrate, compared to the complementary genotype variants, a reduced local aerobic capacity and underperfusion of working muscle groups, both of which depend on habitual levels of PA. This reflects the contribution of ACE- and TNC gene polymorphism-modulated pathways in conditioning aerobic muscle and cardiovascular metabolism by repeated PA [28,43,46,47].
The implications of genetically modulated ACE and TNC gene and protein activity in the setting of fatigue resistance in lumbar muscle groups and its association with chronic pain in physically capable populations have not been characterized. Several hypotheses have been proposed. First, we expected that variability in isometric fatigue resistance of lumbar extensors during the Biering-Sørensen test would differ between rs1799752 and rs2104772 genetic variants and would be related to differences in metabolic strain in the lumbar muscles and the cardiovascular system. Second, we hypothesized that genotype-related variability in isometric endurance and metabolic strain would be associated with nonspecific lower back pain. Third, we assumed that genetic and back pain-related influences on isometric fatigue resistance and metabolic strain during the Biering-Sørensen test would best resolve in physically active subjects. The measures, therefore, included the holding time when the horizontal position could not be held any longer during the Biering-Sørensen test, parameters of aerobic muscle metabolism (SmO2 and blood lactate), and cardiovascular parameters [tHb, peak blood pressure (PP), mean arterial pressure versus baseline (MAP), heart rate (HR)] during the Biering-Sørensen test and subsequent recovery. We specifically hypothesized that I-allele carriers of rs1799752 and T-allele carriers of rs2104772 would demonstrate a greater reduction in SmO2, i.e., deoxygenation, during the static endurance test for lumbar extensors and an exaggerated influence on cardiovascular parameters, including tHb and blood pressure [34,43,44].
2. Results
2.1. Subjects
Data from the 48 patients and 48 matched controls were considered for statistical analysis. The genotype distribution was consistent with the Hardy–Weinberg equilibrium for the rs2104772 and rs1799752 gene polymorphisms, irrespective of whether they were assessed separately for either group or combined over both groups (rs2104772 patients, p = 0.692; rs2104772 controls, p = 0.085; rs1799752 patients, p = 0.108; rs799752 controls, p = 0.248). Patients with chronic, nonspecific low-back pain demonstrated considerably more back pain than controls (p < 0.001) and patients with other pain (p = 0.043). Table 1 summarizes the anthropometric data of the study participants. No differences were observed between the patient and control groups for age, body mass index (BMI), height, fat thickness of musculus (m.) longissimus, or reported PA. Similarly, hand grip strength did not differ between controls and patients.

Table 1.
Biometric data of the studied chronic nonspecific lower-back pain patients and controls.
2.2. Metabolic Characteristics of the Isometric Fatigue Test
During the Biering-Sørensen test, the subjects were able to hold their upper body stable in a horizontal position for an average duration of less than three minutes (Table 2). The task of isometric lumbar exercise produced moderate metabolic strain on the lumbar extensor muscles and the cardiovascular system. This was reflected in a reduction in the area under the curve (AUC) for SmO2 (AUC ΔSmO2) and tHb (AUC ΔtHb) in m. longissimus and an increase in the blood lactate concentration to 2.9 ± 1.3 mM. HR, mean MAP, and PP were equally increased during the isometric endurance test for lumbar extensors (Table 2).

Table 2.
Parameters of metabolic strain to lumbar extensor muscle and the cardiovascular system during the isometric endurance test.
2.3. Back Pain and Gene Polymorphisms Affect Fatigue Resistance during the Isometric Endurance Test
Table 3 reports the overall assessed effects. The holding time during the isometric endurance test of lumbar extensors demonstrated differences between the patient and control groups. On average, patients achieved a holding time that was 21.4 s shorter than the matched controls (p = 0.004).

Table 3.
Association of variability in the influence of the isometric endurance test on metabolic strain with nonspecific chronic back pain and the rs1799752 and rs2104772 gene polymorphisms.
The variability of the holding time demonstrated a complex interaction of the ‘study group’ with the rs1799752 genotype (p = 0.005). Therefore, the holding time was shorter in patients than controls for the DD-genotype (−61.6 s) and tended to be shorter for the II-genotype (−39.8 s, p = 0.071) but not the ID-genotype (p = 0.751; Figure 1). For the patients, only some ID genotypes completed the 4 min cap of the isometric endurance test, differing from the frequency observed for II and DD genotypes (p = 0.020, Chi2-test).
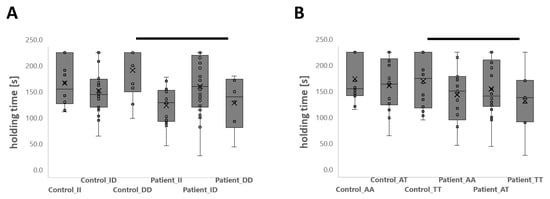
Figure 1.
Genotype-associated isometric endurance capacity of lumbar muscle in patients and controls. Box-whisker plots of the interaction effect of genotypes rs1799752 (A) and rs2104772 (B) on the holding time during the Biering-Sørensen test in the patient and control groups. The following designations apply: line, median; cross, mean; box, data from 1st to 3rd quartile; whiskers, ±1.5 × interquartile range); circles, individual data points. Thick bars connect differences that were considered significant (p < 0.05). ANOVA with a post hoc test of least significance.
The rs2104772 genotype (p = 0.097, η2 = 0.060) and its interaction with the rs1799752 genotype (p = 0.071, η2 = 0.100) demonstrated medium-sized trends of interaction with the study group for the holding time. For the TT-rs2104772 genotype, the holding time was 50.8 s shorter in patients than in the controls. For the AA (29.7 s, p = 0.114) and AT (25.8 s, p = 0.246) genotypes of rs2104772, study group differences in the holding time were not significant.
PA, neither alone nor in interaction with the group or genotype, affected the holding time (Table 4).

Table 4.
Association of physical activity levels with variability in the assessed metabolic factors of fatigue resistance and its interaction with genotype and study group.
2.4. Group Effects on Metabolic Strain during the Isometric Endurance Test
Three indices of metabolic strain, baseline-related alterations in blood lactate concentration (Δlactate), the increase in heart rate (AUC ΔHR), and the time to recovery of oxygen saturation (Trec_tHb), differed between the patient and control groups during the isometric endurance test (Table 3, Table S1). Δlactate and AUC ΔHR after the isometric endurance test were 0.89 mM (p = 0.035) and 25.4 beats lower (p = 0.034), respectively, in the patients than the controls. Trec_tHb after the isometric endurance test was 26.6 s longer in the controls than in the patients (p = 0.004). The reductions and their time-related averages in oxygenation (i.e., AUC ΔSmO2) and total hemoglobin concentration (i.e., AUC ΔtHb) in m. longissimus and the time-related indices of the average cardiovascular strain (i.e., AUC ΔMAP, AUC ΔPP) did not differ between controls and patients.
2.5. Genotype × Group Effects on Metabolic Strain during the Isometric Endurance Test
The variability in the time-related delta of the average MAP during the isometric lumbar endurance test (i.e., AUC ΔMAP/time) demonstrated a complex interaction between the rs2104772 gene polymorphism and the study group (p = 0.028, η2 = 0.108). At the post hoc level, the delta for the average MAP was greater for controls with a homozygous TT genotype than for patients with a TT genotype (+8.5 mmHg) or controls with an AA-genotype (+5.2 mmHg, Figure 2). The accrued and time-related average reduction in SmO2 and tHb in m. longissimus during the test for isometric lumbar endurance did not demonstrate an association with the studied genotypes alone or interaction with the study group (Table 3; Table S1). Trec_tHb in the m. longissimus demonstrated a complex interaction effect between the study group and the rs2104772 gene polymorphism (p = 0.003, η2 = 0.907). This resolved multiple post hoc differences showing positive associations of Trec_tHb in the TT-genotype in controls, which reversed to a negative association in patients, as shown in Figure 2. At the post hoc level, Δlactate during the isometric endurance test differed between patients and controls for the DD (p = 0.046) and ID genotypes (p = 0.046) but not for the II genotype (p = 0.933) of rs1799752. Similarly, Δlactate differed between patients and controls for the AT genotype of rs2104772 (p = 0.007) but not for the AA (p = 0.199) or TT genotype (p = 0.350).
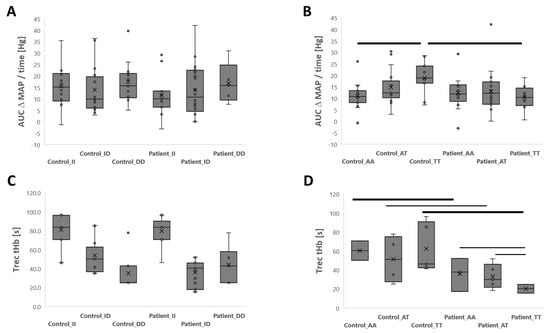
Figure 2.
Genotype x group interactions for cardiovascular strain during and recovery of tHb after the isometric lumbar endurance test. Box-whisker plots of the average increase in MAP (AUC ΔMAP/time; (A,B) and Trec for tHb after (C,D) the isometric endurance test of lumbar muscles as a function of the rs1799752 (A,C) or rs2104772 (B,D) polymorphism in patients and matched controls. ANOVA with post hoc differences with a test of least significant difference. Thin and thick bars connect differences that met a significance level of p < 0.05 and <0.01, respectively.
Variability in the baseline concentration of blood lactate before the isometric lumbar endurance test was associated with the rs2104772 gene polymorphism but not the rs1799752 polymorphism (Table 3). Therefore, blood lactate concentrations were elevated in TT genotypes compared to AT and AA genotypes of rs2104772 (1.96 vs. 1.29 and 1.35 mM, respectively; Figure 3).
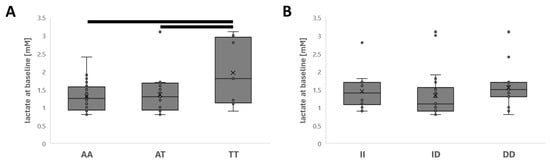
Figure 3.
Effects of genotype on blood lactate concentration. Box whisker plots of the baseline blood lactate concentration before the Biering-Sørensen test for the rs2104772 (A) and rs1799752 genotypes (B). Bars connect differences that met a significance level of p < 0.01.
2.6. Metabolic Strain during the Isometric Test Is Affected by Physical Activity and Genotype
Table 4 presents the interaction effects between the habitual level of PA and the studied gene polymorphisms for the assessed parameters. PA affected the variability in the average and accrued reduction in SmO2 of m. longissimus (i.e., deoxygenation) and alterations in blood lactate concentration, respectively, during the isometric endurance test for lumbar muscles in a positive direction with an increase in physical activity levels (Table S2 and Table 4). The effect size of PA on the AUC ΔSmO2 was further pronounced when the complex interaction with the rs2104772 gene polymorphism was considered. Thereby, larger reductions for the average AUC ΔSmO2 were revealed in the physically most active T-allele carriers than homozygous A-allele carriers of gene polymorphism rs2104772, which was not seen in more sedentary subjects (Figure S1). Reductions of tHb (AUC ΔtHb) in m. longissimus during the isometric endurance test were not significantly associated with any of the investigated factors.
Furthermore, PA affected the variability of Trec_tHb in m. longissimus in complex interaction with either gene polymorphism. For rs2104772, the latter parameter also demonstrated a complex interaction with the study group. The observed main effects were localized to differences at the post hoc level (Figures S1 and S2). Thereby, higher values for Trec_tHb resolved for physically active than inactive AT-rs2104772 genotypes but not for AA or TT genotypes (Figure S2).
2.7. Group Differences in Anthropometric Parameters According to Physical Activity and Genotype
Variability in two gross metabolic parameters was associated with the assessed gene polymorphisms in interaction with the study group. Body height demonstrated a complex interaction effect between rs1799752 and the study group (p = 0.042, η2 = 0.069; Table S3). BMI (p = 0.028, η2 = 0.135) and fat thickness of m. longissimus (p = 0.040, η2 = 0.123) demonstrated a complex interaction effect between PA, rs2104772, and group.
3. Discussion
Chronic back pain is a frequent human affliction with a variety of etiologies from multiple interdependent origins [48], including disruption of motor control for key stabilizing muscles in the lumbar spine in conjunction with behavioral and anthropometric factors that chronically alleviate postural strain to the lumbar spine and attached muscles [49,50,51,52]. The Biering-Sørensen test allows us to assess a typical clinical sign of chronic lower back pain, which is the reduced tolerance to resist fatigue of lumbar extensors during a maneuver involving static tension to the back [1,2,3].
Here, we addressed the hypothesis that genetic variability in two functionally related genes, owing to the insertion/deletion polymorphism rs1799752 in the ACE gene and the nonsynonymous nucleotide polymorphism rs2104772 in the TNC gene, would affect isometric fatigue resistance of lumbar muscles in patients with chronic lower back pain compared to controls. Additionally, we tested whether levels of physical activity, as hypothesized [12], based on the influences of dynamic physical exercise on locomotor muscle [17,27,28,29,43,44,53] and the activity-related manifestation of lower back pain [49,54], modify the genetic influence on lumbar muscle and cardiovascular metabolism.
Our data confirm the reduced fatigue resistance of patients with lower back pain and indicate that this is accompanied by a reduced increase in the blood lactate concentration (Table 2). Furthermore, the results revealed an association for the rs1799752 gene polymorphism (p = 0.002) and a medium-sized trend for the rs2104772 genotype (p = 0.097, η2 = 0.060) with variability in the holding time between lower back pain patients and controls. As expected, variability in rs2104772 genotype-associated group differences in the former index of fatigue resistance was reflected by complex group x rs2104772-genotype interactions for the metabolic parameter AUC ΔMAP, which could likewise be localized to the TT-rs2104772 genotype. Therefore, a greater average MAP was detected during the fatiguing lumbar endurance test for controls than for patients with the TT genotype (+138%, p = 0.005) but not for those with the AA (p = 0.430) or AT genotype (p = 0.657). Higher values were also detected for controls with the TT genotype than for those with the AA genotype (+85%, p = 0.006). These observations indicate that relative hypertension in healthy controls with the TT-rs2104772 genotype (Figure 2B) may support blood flow under the condition of a restrained static contraction of lumbar extensors. Our observation aligns with our assumption and our initial hypothesis [12], indicating that differences in cardiovascular strain contribute to rs2104772-genotype-associated variability in the isometric endurance capacity of lumbar muscle in association with nonspecific lower back pain.
To the best of our knowledge, the present study is the first to report rs2104772 genotype-associated differences in blood pressure regulation. However, we reported that rs2104772 affects the capillarization of muscle fibers in the knee extensor muscle [43], with a higher capillary-to-fiber ratio being identified for A-allele carriers. If this is a generalized muscle phenomenon, we would expect that rs2104772 affects mean arterial pressure during physical exercise with large muscle groups, such as the deployed isometric endurance test for lumbar extensors, via an increase in peripheric vascular resistance [55]. Indeed, the accentuated increase in mean arterial pressure in TT- compared to AA-genotypes of the control group during the imposed endurance test aligns with the former contention. The further observed lower values for the average AUC ΔMAP in patients with the TT-genotype compared with controls (Figure 2B) support the view of possible deficits in muscle capillarization and connected cardiovascular function as contributing factors to the reduced fatigue resistance in the investigated patients with nonspecific lower back pain.
In contrast to our hypothesis [12], no main effect was identified for rs1799752 or its interaction with a patient group or PA on the assessed metabolic parameters during the isometric endurance test for lumbar extensors (Table 3 and Table 4). This included the absence of rs1799752-genotype effects on increases in blood lactate concentration and the reduction in oxygen saturation of the investigated lumbar extensor, which both indicate aerobic metabolic strain in working muscle groups [56]. Additionally, the tHb concentration during the isometric endurance test was not affected by rs1799752. Thus, the assessed metabolic parameters cannot explain the rs1799752-gene polymorphism-associated group effect on the holding time (i.e., η2 = 0.120), which is localized to the rs1799752-DD genotype. Actions of the encoded angiotensin-converting enzyme on musculoskeletal and metabolic parameters and on pain could be the subject of future studies investigations [25,38,57,58,59,60]. In this respect, it is noteworthy that anthropometric parameters that may affect the manifestation of lower back pain [50], such as body height (p = 0.042, η2 = 0.069) and body mass (p = 0.069, η2 = 0.058), demonstrated borderline (non-)significant interaction effects between rs1799752 and the study group (Table S3). Another intriguing aspect was that only patients with the ID genotype completed the 4 min cap of the isometric endurance test and showed a similar holding time as the controls with the ID genotype. In contrast, a reduced holding time was identified in patients with the II and DD genotypes compared to the respective controls. This effect resembles the overdominant influence of the rs1799752 ID-genotype on contractile parameters of cardiac and skeletal muscle function in sedentary populations, which are opposingly affected by the ACE I and D allele (reviewed by [27,28,29,31,32,33,34,35,61]).
Reductions in oxygen saturation in single muscle fibers reflect mitochondrial respiration rates and the capillarization of muscle fibers [30,62]. The lack of a difference in the reduction in oxygen saturation in m. longissimus between the patient and control group indicates that local aerobic capacity was not the main limiting factor for resisting fatigue in the static endurance test for lumbar extensors. This contention is supported by the moderate increase in blood lactate concentration to approximately 3 mM and a maximal heart rate close to 144 bpm with the completion of the endurance test. Both values fall within an intensity zone where submaximal aerobic metabolism dominates during exercises involving large muscle groups [27]. Specifically, with the isometric endurance test used, the lumbar extensors and some lower extremity muscles are actively engaged but remain locked in position, resulting in minimal contractile work. We also observed a decrease in the tHb concentration during isometric contraction (Table 2). As this parameter indicates changes in tissue blood volume, which in the short term is influenced only by perfusion [8], our observations imply that the primary metabolic challenge of the deployed isometric test consisted of the stalling of blood flow to contracting lumbar extensors.
Interestingly, differences in the recovery time of the muscle hemoglobin concentration after the isometric endurance test were resolved between patients and controls. This group difference in Trec_tHb was also associated with the rs2104772 gene polymorphism and could be attributed to differences between the AA and AT- versus TT-genotypes in back pain patients (Figure 2C,D). Thus, our observations indicate that gene polymorphism rs2104772-associated processes contribute to differences between back pain patients and controls in the recovery of blood vessel perfusion in lumbar extensor muscle after their occlusion during the isometric endurance test. The extent to which these effects possibly also apply to the reported differences in fatigue resistance with a repetitive type of protocol involving lumbar extension and flexion remains to be addressed [5].
Variability in Trec_tHb in m. longissimus was also associated with the complex relationship between the two gene polymorphisms, rs2104772 and rs1799752, and habitual PA levels (Table 4; Figure S2). The latter observation concurs with the implication of exercise intensity-modulated blood flow in the occurrence of lower back pain [7,63] and that rs2104772-genotype and physically associated variability in Trec_tHb further interacted with the study group. These observations suggest that TNC-modulated muscle perfusion contributes to the PA-modulated manifestation of lower back pain.
Support for the implication of a TNC-related pathway in exercise-modulated aspects of lumbar muscle endurance is provided by the negative interaction effect between the level of PA and rs2104772 on the parameter AUC ΔSmO2 in m. longissimus. This effect resolved to a greater degree of deoxygenation during the endurance test in AT and TT genotypes than in the AA genotype for the more active subjects with a sports score of 4 (Figure S1A,B). This negative association in lumbar muscle’s average oxygen saturation in physically active subjects with the T-allele of rs2104772 resembles the greater increase in the volume density of mitochondria and capillary-to-fiber ratio in knee extensor muscle of AA compared to AT and TT genotypes of polymorphism rs2104772 with endurance training [43,46]. Collectively, the present novel observations support the interpretation that the m. longissimus of physically active T-allele carriers of rs2104772 is more reliant on aerobic respiration to fuel the isomeric contraction during the endurance test for lumbar extensors.
In contrast to our expectation, the level of PA did not affect fatigue resistance during lumbar extensors in the tested isometric task, either alone (p = 0.407) or in interaction with the patient group (p = 0.350). Interestingly, however, variability in parameters reflecting aerobic metabolic strain during the isometric endurance test, i.e., the average reduction in the oxygen saturation of m. longissimus and delta in blood lactate, were associated with PA levels. This indicates that a high level of PA increased the contribution of aerobic metabolism to the energetic requirements of the lumbar muscle being recruited during the task of holding the body position in a horizontal position. This observation aligns with the reported improvements in the oxygenation of erector spinae muscle with a PA-based training program [6].
Intriguingly, the rs2104772 polymorphism was associated with variability in lactate levels at baseline, where the highest levels were observed in the TT genotype (Figure 3A). This influence extended to complex interactions between PA and rs2104772, as well as between PA and the study group. The blood lactate concentration is a metabolic marker for pain in patients with discogenic back pain [64]. It may derive from invertebrate discs rather than from skeletal muscle and may affect pain reception via influences on the concentration of hydrogen ions [65]. With the present experiments, we cannot resolve whether the observed effects on baseline lactate levels reflect variability in the clearance rate of this metabolite in tissue due to, for instance, differences in muscle capillarization and PA.
The de-adhesive extracellular TNC protein plays an important mechanomodulatory role in the physiological and pathological plasticity of the musculoskeletal and cardiovascular systems [38,39,40,42,58]. The A/T gene polymorphism rs2104772 studied here affects the growth and remodeling of the connective compartment [43,66,67,68] by producing the exchange of isoleucine by leucine in amino acid codon 1677 of tenascin-C [37]. This finding suggested that the associations of rs2104772 with variability in the AUC ΔSmO2, baseline lactate, Trec_tHb, and AUC_MAP reflect altered molecular effects of the resulting variants of the TNC protein on the metabolic supply lines for lumbar extensors. Collectively, our observations motivate further research to integrate the lactate hypothesis of pain reception with physical activity and TNC-modulated tissue in otherwise physically able patients with nonspecific lower back pain.
We identify several limitations in our investigation that need to be considered when weighing our interpretations in future investigations and applications. First, we highlighted that we matched the controls to the patients based on PA levels, age, and BMI. This was motivated by the knowledge of the influential impact of PA on assessed metabolic parameters in skeletal muscle and the cardiovasculature, the variability of which is affected by the investigated genotypes [27,29,43,46,58,69,70]. Our investigation also relied on the isolated procedure of a static endurance test, which may not depict all aspects that are relevant for the fatigue resistance of lumbar extensors during dynamic types of exercise. This choice was based on the fact that the deployed test of Biering-Sørensen is the gold standard for assessing the isometric endurance capacity of back muscles, and we established its compatibility with NIRS-based measurements of muscle oxygenation and perfusion, along with cardiovascular parameters [8]. This allowed us to identify hypothesized main effects on fatigue-related parameters and/or muscle oxygen saturation. As well, our study extended the previously identified interdependent association of BMI and the fat thickness of m. longissimus with chronic lower back pain [8,54,71] to a back pain-related relationship with the TNC genotype rs2104772 and PA (Table S3). We also note that upon a post hoc inspection (Table S4), one effect alone was identified for the biological factor of sex, concerning a complex interaction between sex and the study group for the variability in the accrued difference in tHb concentration of m. longissimus (p = 0.013, η2 = 0.083). Finally, the identified association of rs2104772 with the variability in the average mean arterial pressure supports the view of a reasonable biological resolution of the selected approach to identify novel and disease-relevant associations of TNC with cardiovascular parameters, as shown previously for atherogenic disease and stenosis [68,72].
4. Materials and Methods
Ethics—This study reports results from an explorative observational investigation of chronic nonspecific lower back pain patients compared to controls. The investigation was carried out with the approval of the ethics committee of the canton of Zurich, Switzerland (2016-00647) and was registered at clinicaltrials.gov (NCT02955407).
Design—48 patients and 74 controls were included in this study to assess the isometric endurance of lumbar muscle groups with the Beiring-Sorenson test. Concomitantly, the effects of the strain on aerobic metabolism in m. longissimus and cardiovascular metabolism were monitored. For aerobic metabolism, SmO2 was measured in major dorsal muscle groups (longissimus, multifidus, iliocostalis) using near-infrared spectroscopy, as this method serves as a proxy for mitochondrial respiration [30]. This was accompanied by the measurement of the blood lactate concentration. Cardiovascular measurements included heart rate, blood pressure, and the NIRS-based assessment of tHb in the longissimus muscle as a proxy of perfusion [30]. Hand grip strength was assessed, and a general measure of strength was taken. Subcutaneous fat was evaluated using a caliper (GPM, Caliper, DKSH Switzerland Ltd., Zurich, Switzerland). Pain and habitual PA levels were estimated based on a numerical rating scale and the Baecke physical activity questionnaire (of Sports Scores) as previously described [8]. rs1799752 and rs2104772 gene polymorphisms were determined on mucosal samples. For the statistical analysis, the data from patients were matched 1:1 to those of the controls based on biometric parameters, leading to the exclusion of data for 26 control subjects.
Biering-Sørensen test—This test for static endurance of the lumbar muscles was carried out as described by the subject lying prone on a treatment table with the pelvic bone on the table’s edge and the arms folded across the chest and the upper part of the body. The position of the subjects during the test was controlled with a 2D inclinometer (Noraxon U.S.A. INC, Scottsdale, AZ, USA) placed at the level of vertebra T7. The subjects were instructed to hold the upper part of the body horizontally as long as possible. Deviations of more than five degrees from the horizontal testing position were signaled by a tone from the inclinometer. The test was stopped if the subject deviated from the horizontal position more than twice. The respective duration was noted as the ‘holding time’, and the reason for the termination was written down. After 240 s, the test was terminated by the investigator, and this duration was set as the ‘holding time’.
Genotyping—Typing of the insertion/deletion polymorphism of the ACE gene, rs1799752, and the single gene polymorphism rs2104772 in the TNC gene was carried out via polymerase chain reaction (PCR). Genomic deoxyribonucleic acid (DNA) was isolated from mucosal swabs. DNA was subjected to incubation with specific combinations of primers (200 nanomolar) and KAPA HRM FAST reaction mix (KAPA BIOSYSTEMS, Labgene Scientific, Châtel-St-Denis, Switzerland) in an Illumina ECO 48 Real-Time PCR System (Labgene Scientific, Châtel-St-Denis, Switzerland; Eco Real-Time PCR System Software v4.1) using thermal settings as described previously [14]. For amplification and detection of the ACE-I/D gene polymorphism, two different oligonucleotide primer pairs were used. A primer ACE2 (5′-tgggattacaggcgtgatacag-3′) and a primer ACE3 (5′ atttcagagctggaataaaatt-3′) were deployed to amplify the 66-base pair (bp)-long amplicon corresponding to the I-allele. For the 83-bp-long amplicon, the D-allele primer ACE1 (5′-catcctttctcccatttctc-3′) and the primer ACE3 (5′-atttcagagctggaataaaatt-3′) were used. The amplification and HRM-based detection of sequence variation in an 85-bp-long DNA fragment for rs2104772 was conducted with primers 5-caaaaagcagtctgagccac-3′ and 5-ttcagtagcctctctgagac-3′).
For the detection of the respective alleles, specific references, genotypic variants, and negative controls were included in each PCR run to identify the amplified alleles based on the thermal properties of the respective melting curves. For the gene polymorphism rs1799752, II, ID, and DD genotypes were declared based on the presence of the I-allele and/or D-allele. For the rs2104772 gene polymorphism, the AA, AT, and TT genotypes were determined based on software-based criteria that verified the correspondence of the respective melting curve of a sample with a respective genotypic variant based on normalized data. The genotype identities of the references and selected samples were validated by commercial microsequencing (Microsynth, Balgach, Switzerland).
Near-infrared spectroscopy—Probes of a portable near-infrared spectroscopy device [Muscle Oxygen Monitor (Moxy) (Fortiori Design LLC, MN, USA)] were placed at defined positions on the right side of the major dorsal muscle groups (longissimus, multifidus, iliocostalis) as described previously [7]. For the m. longissimus, the probe was placed at a two-finger width lateral to the spinous process of the first lumbar vertebra (L1). For M. multifidus, the electrode was placed on a line from the caudal tip of the posterior spina iliaca superior to the space between L1 and L2, at the level of the L5 spinous process (i.e., approximately 2–3 cm from the midline). For the iliocostalis muscle, the probe was placed one finger width medial to the line from the posterior spina iliaca superior to the lowest point of the lower rib at the level of L2. The positions of the probes were palpated and marked while the subjects were standing in an upright position. Data for the absolute SmO2 and tHb were sampled at 2 Hz during the test and the subsequent recovery period.
Cardiovascular measures—Systolic and diastolic blood pressure (BP) and HR were measured noninvasively throughout the experiment with a SOMNOtouch device (NIBP SOMNOMedics GmbH, Randersacker, Germany) that was attached to the fingertip and wrist. Measures were used to calculate the PP and MAP.
Blood lactate measurements—Blood was collected via a finger prick before and in the first two minutes after termination of the Biering-Sørensen test, essentially as described previously [73].
Data handling—Data were collected and stored via the application ‘research electronic data capture’ (REDCap Version 9.6.1; https://projectredcap.org/resources/ (accessed on 14 August 2018)). SmO2 and tHb data of m. longissimus from near-infrared spectroscopy were processed, and cardiovascular parameters were integrated as AUCs based on changes relative to baseline. The data sampled with a near-infrared spectroscopy device were processed by first interpolating missing values and resampling values using locally weighted regression fitting. Subsequently, the AUC of the changes from the start until the end of the Beiring-Sorenson test and the time-related AUC were calculated for SmO2 and tHb. This served to describe the accrued and time-related average influence as a delta relative to the baseline for the respective parameter. The measures were corrected for subcutaneous tissue thickness by linear regression with NIRS measurements as the dependent variable and subcutaneous tissue thickness as the independent variable, keeping the unstandardized residuals. Trec was determined via nonlinear regression analysis for a fitted exponential function and corresponded to the time at which 63.2% (corresponding to the exponential constant 1/e) of the maximal maximum value after the Beiring-Sorenson test was reached. Due to too many missing cases, all measurements of near-infrared spectroscopy in M. multifidus and M. iliocostalis were not further considered for the analyses. Lactate measurements were strongly associated with muscle performance and with the rs1799752 and rs2104772 genotypes and were separately considered despite a few missing values. The lactate response to the isometric endurance test for lumbar muscles was calculated as the delta between the highest value for the blood lactate concentration during the first 2 min after termination of the test and the values at baseline.
Statistics—Data were curated in MS Excel, and the patient cases were matched 1:1 to controls based on reported PA levels, age, and BMI. This amounted to 48 patients and their controls. In the second instance, matched pairs were balanced for the biological factor of ‘sex’, which was possible except for five of the 48 pairs. The values from the additional control cases were excluded. Statistical analysis was performed using the IBM SPSS Statistics (Version 28) statistical software package (SPSS, Inc., Chicago, IL, USA). The Hardy–Weinberg equilibrium was calculated using a Chi2-test and was rejected at a p-value of 5%. The study group × genotype × PA-related differences were identified using ANOVA or multivariate ANOVA, where the respective variables of the parameters were as follows: patients vs. controls for the ‘study group’, II, ID, and DD for the rs1799752 ‘genotype’ or AA, AT, and TT for the rs2104772 ‘genotype’, respectively, and scores of 2, 3, 4, or 5 for ‘habitual PA.’ The potential main effects of sex were separately assessed. Table S5 shows the distribution of the genotype data. The respective homogeneity of variance was described using a Levene test. When p-values from two ANOVAs (for either of the two genotypes) were available, the average value was used for display. The frequency of genotypes in either group according to the Biering-Sørensen test was assessed in contingency tables using a chi2-test (MS-Excel, Microsoft 365 Insider, version 2497, Kildare, Ireland). Post hoc differences were localized based on a test for the least significant differences.
Effect sizes were interpreted based on the calculated values for η2, where effects were called small if η2 = 0.01; medium if η2 = 0.06; and large if η2 = 0.14 [74]. The cutoff for statistically significant differences was set to 5% and adjusted for false discovery rates for hypothesized effects as described, based on the fact that the assessed parameters represented five intercorrelated (and dependent) ontologies (Figure S3) [75]. Interaction effects were qualified based on the r-value of the linear correlation over all cases for the factor and dependent variable and the unstandardized regression coefficients, b, for the, respectively, unfolded parameters of a predictor variable (i.e., factor) for the deployed ANOVA model. Interrelationships between ontologies were assessed based on the strength of Pearson-based linear correlations and assessed for differences in calculated R values for relationships to the different parameters using a paired t-test. Additionally, linear relationships were assessed using the expression correlation network plugin of Cytoscape software (3.8.2) as described previously [76]. Effects where false discovery rate adjusted p-values meet 5% ≤ p < 10% and an effect size η2 for a medium effect were considered to represent a trend. The data are presented as the mean ± standard deviation (SD) or standard error (SE) or graphically as box-whisker plots representing the mean, median, first and third quartiles, minima, maxima, and individual data points.
5. Conclusions
The present novel observations connect prominent sequence variations in the ACE and TNC genes, i.e., the gene polymorphisms rs1799752 and rs2104772, to differences in the isometric fatigue resistance of the lumbar extensors between patients with chronic lower back pain and matched controls. Genetic effects on fatigue resistance in back pain patients were associated with variability in the deficit in mean arterial pressure for the TT-genotypes of rs2104772. In contrast, for rs1799752, the absence of effects on the assessed metabolic parameters suggests the contribution of other factors, including anthropometric parameters and ACE-modulated inflammatory/neurohumoral factors of pain reception [51,52,54,60,71], as an explanation. Moreover, group differences in the recovery of total hemoglobin in the longissimus muscle after the fatiguing isometric test support the contribution of perfusion-related processes to the manifestation of nonspecific lower back pain. Rs2104772 is recognized to modulate the contribution of aerobic metabolism in positive interaction with PA in m. longissimus to the task of holding body position isometrically in a horizontal position in the strenuous maneuver of a Biering-Sørensen test. More research is required to determine the cellular mechanism underlying the contributions of the TNC gene polymorphism, physical activity, and ACE polymorphism rs1799752 to back-pain-related metabolic alterations in lumbar muscle groups.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/physiologia4030017/s1, Figure S1: Genotype differences in physically active subjects for aerobic back muscle metabolism during the test for isometric lumbar endurance; Figure S2: Genotype differences in physically active subjects for back muscle perfusion during the test for isometric lumbar endurance; Figure S3: Physical activity-associated genetic differences for recovery of perfusion after the isometric test for lumbar endurance; Table S1: Association of variability in the influence of the isometric endurance test on the accrued metabolic strain with nonspecific chronic back pain and gene polymorphisms rs1799752 and rs2104772; Table S2: Variability in auxiliary metabolic factors of lumbar muscle fatigue resistance in association with physical activity, gene polymorphisms, and study group; Table S3: Association of variability in anthropometric parameters with the factors, study group, gene polymorphisms, and physical activity; Table S4: Association of variability in anthropometric parameters with the factor of sex; Table S5: Distribution of cases.
Author Contributions
Conceptualization, M.F. and B.K.H.; methodology, M.F. and P.V.; software, M.-N.G. and M.F.; validation, P.V.; formal analysis, P.V. and M.F.; investigation, P.V.; resources, B.K.H. and M.-N.G.; data curation, M.F.; writing—original draft preparation, M.F.; writing—review and editing, M.F., M.-N.G. and B.K.H.; visualization, M.F.; supervision, B.K.H.; funding acquisition, B.K.H. All authors have read and agreed to the published version of the manuscript.
Funding
The salary for P.V. was supported by the University of Zurich.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the canton of Zurich, Switzerland (2016-00647) and was registered at clinicaltrials.gov (NCT02955407).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets presented in this article are not readily available because subjects are vulnerable individuals who did not consent to the public release of individual information that may allow them to identify them. Requests to access the datasets should be directed to the corresponding author.
Acknowledgments
The authors thank Aurélien Frobert for the technical assistance with software installations.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Demoulin, C.; Vanderthommen, M.; Duysens, C.; Crielaard, J.M. Spinal muscle evaluation using the Sorensen test: A critical appraisal of the literature. Jt. Bone Spine 2006, 73, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.; Bruce-Low, S.; Smith, D. A reappraisal of the deconditioning hypothesis in low back pain: Review of evidence from a triumvirate of research methods on specific lumbar extensor deconditioning. Curr. Med. Res. Opin. 2014, 30, 865–911. [Google Scholar] [CrossRef] [PubMed]
- Danneels, L.A.; Coorevits, P.L.; Cools, A.M.; Vanderstraeten, G.G.; Cambier, D.C.; Witvrouw, E.E.; De, C.H. Differences in electromyographic activity in the multifidus muscle and the iliocostalis lumborum between healthy subjects and patients with sub-acute and chronic low back pain. Eur. Spine J. 2002, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Duque, I.; Parra, J.H.; Duvallet, A. Maximal aerobic power in patients with chronic low back pain: A comparison with healthy subjects. Eur. Spine J. 2011, 20, 87–93. [Google Scholar] [CrossRef]
- Kell, R.T.; Bhambhani, Y. In vivo erector spinae muscle blood volume and oxygenation measures during repetitive incremental lifting and lowering in chronic low back pain participants. Spine 2006, 31, 2630–2637. [Google Scholar] [CrossRef]
- Olivier, N.; Thevenon, A.; Berthoin, S.; Prieur, F. An exercise therapy program can increase oxygenation and blood volume of the erector spinae muscle during exercise in chronic low back pain patients. Arch. Phys. Med. Rehabil. 2013, 94, 536–542. [Google Scholar] [CrossRef]
- Heneweer, H.; Staes, F.; Aufdemkampe, G.; van Rijn, M.; Vanhees, L. Physical activity and low back pain: A systematic review of recent literature. Eur. Spine J. 2011, 20, 826–845. [Google Scholar] [CrossRef] [PubMed]
- Langenfeld, A.; Wirth, B.; Scherer-Vrana, A.; Riner, F.; Gaehwiler, K.; Valdivieso, P.; Humphreys, B.K.; Scholkmann, F.; Flueck, M.; Schweinhardt, P. No alteration of back muscle oxygenation during isometric exercise in individuals with non-specific low back pain. Sci. Rep. 2022, 12, 8306. [Google Scholar] [CrossRef]
- Battie, M.C.; Videman, T.; Levalahti, E.; Gill, K.; Kaprio, J. Heritability of low back pain and the role of disc degeneration. Pain 2007, 131, 272–280. [Google Scholar] [CrossRef]
- Junqueira, D.R.; Ferreira, M.L.; Refshauge, K.; Maher, C.G.; Hopper, J.L.; Hancock, M.; Carvalho, M.G.; Ferreira, P.H. Heritability and lifestyle factors in chronic low back pain: Results of the Australian twin low back pain study (The AUTBACK study). Eur. J. Pain 2014, 18, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Freidin, M.B.; Tsepilov, Y.A.; Palmer, M.; Karssen, L.C.; Suri, P.; Aulchenko, Y.S.; Williams, F.M.K.; Group, C.M.W. Insight into the genetic architecture of back pain and its risk factors from a study of 509,000 individuals. Pain 2019, 160, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso, P.; Franchi, M.V.; Gerber, C.; Fluck, M. Does a Better Perfusion of Deconditioned Muscle Tissue Release Chronic Low Back Pain? Front. Med. 2018, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.; Brogioli, M.; Maier, T.; White, A.; Waldron, S.; Rittweger, J.; Toigo, M.; Wettstein, J.; Laczko, E.; Fluck, M. The Angiotensin Converting Enzyme Insertion/Deletion Polymorphism Modifies Exercise-Induced Muscle Metabolism. PLoS ONE 2016, 11, e0149046. [Google Scholar] [CrossRef]
- Gasser, B.; Fluck, M.; Frey, W.O.; Valdivieso, P.; Sporri, J. Association of Gene Variants for Mechanical and Metabolic Muscle Quality with Cardiorespiratory and Muscular Variables Related to Performance in Skiing Athletes. Genes 2022, 13, 1798. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Kim, C.H.; Park, D.S.; Choi, S.Y.; Lee, D.H.; Nam, H.S.; Hur, J.G.; Woo, J.H. The Impacts of ACE Activity according to ACE I/D Polymorphisms on Muscular Functions of People Aged 65. Ann. Rehabil. Med. 2012, 36, 433–446. [Google Scholar] [CrossRef]
- Rigat, B.; Hubert, C.; Alhenc-Gelas, F.; Cambien, F.; Corvol, P.; Soubrier, F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J. Clin. Investig. 1990, 86, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso, P.; Vaughan, D.; Laczko, E.; Brogioli, M.; Waldron, S.; Rittweger, J.; Fluck, M. The Metabolic Response of Skeletal Muscle to Endurance Exercise Is Modified by the ACE-I/D Gene Polymorphism and Training State. Front. Physiol. 2017, 8, 993. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Ushio-Fukai, M.; Lassegue, B.; Alexander, R.W. Angiotensin II signaling in vascular smooth muscle. New concepts. Hypertension 1997, 29, 366–373. [Google Scholar] [CrossRef]
- Dostal, D.E.; Hunt, R.A.; Kule, C.E.; Bhat, G.J.; Karoor, V.; McWhinney, C.D.; Baker, K.M. Molecular mechanisms of angiotensin II in modulating cardiac function: Intracardiac effects and signal transduction pathways. J. Mol. Cell Cardiol. 1997, 29, 2893–2902. [Google Scholar] [CrossRef]
- Borsodi, K.; Balla, H.; Molnar, P.J.; Lenart, A.; Kenessey, I.; Horvath, A.; Keszthelyi, A.; Romics, M.; Majoros, A.; Nyirady, P.; et al. Signaling Pathways Mediating Bradykinin-Induced Contraction in Murine and Human Detrusor Muscle. Front. Med. 2021, 8, 745638. [Google Scholar] [CrossRef]
- Dell’Italia, L.J.; Oparil, S. Bradykinin in the heart: Friend or foe? Circulation 1999, 100, 2305–2307. [Google Scholar] [CrossRef][Green Version]
- Koeppen, K.; Stanton, B.A. Glomerular Filtration and Renal Blood Flow. In Renal Physiology, 5th ed.; Mosby, Elsevier: Maryland Heights, MO, USA, 2013; pp. 27–43. [Google Scholar]
- Nurkiewicz, T.R.; Boegehold, M.A. Reinforcement of arteriolar myogenic activity by endogenous ANG II: Susceptibility to dietary salt. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H269–H278. [Google Scholar] [CrossRef]
- De Filippi, P.; Ravaglia, S.; Bembi, B.; Costa, A.; Moglia, A.; Piccolo, G.; Repetto, A.; Dardis, A.; Greco, G.; Ciana, G.; et al. The angiotensin-converting enzyme insertion/deletion polymorphism modifies the clinical outcome in patients with Pompe disease. Genet. Med. 2010, 12, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Shehab, D.K.; Al-Jarallah, K.F.; Al-Awadhi, A.M.; Al-Herz, A.; Nahar, I.; Haider, M.Z. Association of angiotensin-converting enzyme (ACE) gene insertion-deletion polymorphism with spondylarthropathies. J. Biomed. Sci. 2008, 15, 61–67. [Google Scholar] [CrossRef]
- Van Ginkel, S.; de Haan, A.; Woerdeman, J.; Vanhees, L.; Serne, E.; de Koning, J.; Fluck, M. Exercise intensity modulates capillary perfusion in correspondence with ACE I/D modulated serum angiotensin II levels. Appl. Transl. Genom. 2015, 4, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Gasser, B.; Frei, A.; Niederseer, D.; Catuogno, S.; Frey, W.O.; Fluck, M. Variability in the Aerobic Fitness-Related Dependence on Respiratory Processes During Muscle Work Is Associated With the ACE-I/D Genotype. Front. Sports Act. Living 2022, 4, 814974. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.; Huber-Abel, F.A.; Graber, F.; Hoppeler, H.; Fluck, M. The angiotensin converting enzyme insertion/deletion polymorphism alters the response of muscle energy supply lines to exercise. Eur. J. Appl. Physiol. 2013, 113, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Gasser, B.; Franchi, M.V.; Ruoss, S.; Frei, A.; Popp, W.L.; Niederseer, D.; Catuogno, S.; Frey, W.O.; Fluck, M. Accelerated Muscle Deoxygenation in Aerobically Fit Subjects During Exhaustive Exercise Is Associated With the ACE Insertion Allele. Front. Sports Act. Living 2022, 4, 814975. [Google Scholar] [CrossRef]
- Ryan, T.E.; Brophy, P.; Lin, C.T.; Hickner, R.C.; Neufer, P.D. Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: A comparison with in situ measurements. J. Physiol. 2014, 592, 3231–3241. [Google Scholar] [CrossRef]
- Hernandez, D.; de la Rosa, A.; Barragan, A.; Barrios, Y.; Salido, E.; Torres, A.; Martin, B.; Laynez, I.; Duque, A.; De Vera, A.; et al. The ACE/DD genotype is associated with the extent of exercise-induced left ventricular growth in endurance athletes. J. Am. Coll. Cardiol. 2003, 42, 527–532. [Google Scholar] [CrossRef]
- Hagberg, J.M.; McCole, S.D.; Brown, M.D.; Ferrell, R.E.; Wilund, K.R.; Huberty, A.; Douglass, L.W.; Moore, G.E. ACE insertion/deletion polymorphism and submaximal exercise hemodynamics in postmenopausal women. J. Appl. Physiol. 2002, 92, 1083–1088. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Busjahn, A.; Voss, A.; Knoblauch, H.; Knoblauch, M.; Jeschke, E.; Wessel, N.; Bohlender, J.; McCarron, J.; Faulhaber, H.D.; Schuster, H.; et al. Angiotensin-converting enzyme and angiotensinogen gene polymorphisms and heart rate variability in twins. Am. J. Cardiol. 1998, 81, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Gasser, B.; Niederseer, D.; Frey, W.O.; Catuogno, S.; Fluck, M. ACE-I/D Allele Modulates Improvements of Cardiorespiratory Function and Muscle Performance with Interval-Type Exercise. Genes 2023, 14, 1100. [Google Scholar] [CrossRef] [PubMed]
- Puthucheary, Z.; Skipworth, J.R.; Rawal, J.; Loosemore, M.; Van Someren, K.; Montgomery, H.E. The ACE gene and human performance: 12 years on. Sports Med. 2011, 41, 433–448. [Google Scholar] [CrossRef]
- Gasser, B.; Frey, W.O.; Valdivieso, P.; Scherr, J.; Sporri, J.; Fluck, M. Association of Gene Variants with Seasonal Variation in Muscle Strength and Aerobic Capacity in Elite Skiers. Genes 2023, 14, 1165. [Google Scholar] [CrossRef]
- Matsuda, A.; Hirota, T.; Akahoshi, M.; Shimizu, M.; Tamari, M.; Miyatake, A.; Takahashi, A.; Nakashima, K.; Takahashi, N.; Obara, K.; et al. Coding SNP in tenascin-C Fn-III-D domain associates with adult asthma. Hum. Mol. Genet. 2005, 14, 2779–2786. [Google Scholar] [CrossRef]
- Jarvinen, T.A.; Kannus, P.; Jarvinen, T.L.; Jozsa, L.; Kalimo, H.; Jarvinen, M. Tenascin-C in the pathobiology and healing process of musculoskeletal tissue injury. Scand. J. Med. Sci. Sports 2000, 10, 376–382. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K.; Aoki, H. Tenascin-C and mechanotransduction in the development and diseases of cardiovascular system. Front. Physiol. 2014, 5, 283. [Google Scholar] [CrossRef] [PubMed]
- Fluck, M.; Mund, S.I.; Schittny, J.C.; Klossner, S.; Durieux, A.C.; Giraud, M.N. Mechano-regulated tenascin-C orchestrates muscle repair. Proc. Natl. Acad. Sci. USA 2008, 105, 13662–13667. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, W.; Cai, G.; Ding, Y.; Wei, C.; Li, S.; Yang, Y.; Qin, J.; Liu, D.; Zhang, H.; et al. Myofiber necroptosis promotes muscle stem cell proliferation via releasing Tenascin-C during regeneration. Cell Res. 2020, 30, 1063–1077. [Google Scholar] [CrossRef]
- Matsumoto, K.I.; Aoki, H. The Roles of Tenascins in Cardiovascular, Inflammatory, and Heritable Connective Tissue Diseases. Front. Immunol. 2020, 11, 609752. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso, P.; Toigo, M.; Hoppeler, H.; Fluck, M. T/T homozygosity of the tenascin-C gene polymorphism rs2104772 negatively influences exercise-induced angiogenesis. PLoS ONE 2017, 12, e0174864. [Google Scholar] [CrossRef]
- Fluck, M.; Kramer, M.; Fitze, D.P.; Kasper, S.; Franchi, M.V.; Valdivieso, P. Cellular Aspects of Muscle Specialization Demonstrate Genotype—Phenotype Interaction Effects in Athletes. Front. Physiol. 2019, 10, 526. [Google Scholar] [CrossRef]
- Green, H.J. Mechanisms of muscle fatigue in intense exercise. J. Sports Sci. 1997, 15, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Fluck, M.; Hoppeler, H. Molecular basis of skeletal muscle plasticity--from gene to form and function. Rev. Physiol. Biochem. Pharmacol. 2003, 146, 159–216. [Google Scholar] [CrossRef] [PubMed]
- Mathes, S.; van Ginkel, S.; Vaughan, D.; Valdivieso, P.; Flück, M. Gene-Pharmacologial Effects on Exercise-Induced Muscle Gene Expression in Healthy Men. Anat. Physiol. 2015, S5, 005. [Google Scholar] [CrossRef]
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, J.; Liu, N.; Liu, Z.; Wei, X.; Yan, F.; Yu, S. Low back pain among taxi drivers: A cross-sectional study. Occup. Med. 2017, 67, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Heuch, I.; Heuch, I.; Hagen, K.; Zwart, J.A. A Comparison of Anthropometric Measures for Assessing the Association between Body Size and Risk of Chronic Low Back Pain: The HUNT Study. PLoS ONE 2015, 10, e0141268. [Google Scholar] [CrossRef] [PubMed]
- Teodorczyk-Injeyan, J.A.; Triano, J.J.; Injeyan, H.S. Nonspecific Low Back Pain: Inflammatory Profiles of Patients With Acute and Chronic Pain. Clin. J. Pain 2019, 35, 818–825. [Google Scholar] [CrossRef]
- Russo, M.; Deckers, K.; Eldabe, S.; Kiesel, K.; Gilligan, C.; Vieceli, J.; Crosby, P. Muscle Control and Non-specific Chronic Low Back Pain. Neuromodulation 2018, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bray, M.S.; Hagberg, J.M.; Perusse, L.; Rankinen, T.; Roth, S.M.; Wolfarth, B.; Bouchard, C. The human gene map for performance and health-related fitness phenotypes: The 2006–2007 update. Med. Sci. Sports Exerc. 2009, 41, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Matsudaira, K.; Sawada, S.S.; Gando, Y.; Kawakami, R.; Sloan, R.A.; Kinugawa, C.; Okamoto, T.; Tsukamoto, K.; Miyachi, M.; et al. Association between objectively measured physical activity and body mass index with low back pain: A large-scale cross-sectional study of Japanese men. BMC Public Health 2018, 18, 341. [Google Scholar] [CrossRef]
- Joyner, M.J.; Casey, D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, S.; Dapp, C.; Wittwer, M.; Durieux, A.C.; Mueller, M.; Weinstein, F.; Vogt, M.; Hoppeler, H.; Fluck, M. A hypoxia complement differentiates the muscle response to endurance exercise. Exp. Physiol. 2010, 95, 723–735. [Google Scholar] [CrossRef]
- Sierra, A.P.R.; Lima, G.H.O.; da Silva, E.D.; Maciel, J.F.S.; Benetti, M.P.; de Oliveira, R.A.; Martins, P.F.O.; Kiss, M.A.P.; Ghorayeb, N.; Newsholme, P.; et al. Angiotensin-Converting Enzyme Related-Polymorphisms on Inflammation, Muscle and Myocardial Damage after a Marathon Race. Front. Genet. 2019, 10, 984. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K. Tenascin-C in Heart Diseases-The Role of Inflammation. Int. J. Mol. Sci. 2021, 22, 5828. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.J.; van der Merwe, L.; Posthumus, M.; Cook, J.; Handley, C.J.; Collins, M.; September, A.V. Investigation of variants within the COL27A1 and TNC genes and Achilles tendinopathy in two populations. J. Orthop. Res. 2013, 31, 632–637. [Google Scholar] [CrossRef]
- Nemoto, W.; Yamagata, R.; Nakagawasai, O.; Tan-No, K. Angiotensin-Related Peptides and Their Role in Pain Regulation. Biology 2023, 12, 755. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.R.; Hwang, I.W.; Kim, H.J.; Kang, Y.D.; Park, J.W.; Jin, H.J. Genetic Association of Angiotensin-Converting Enzyme (ACE) Gene I/D Polymorphism with Preterm Birth in Korean Women: Case-Control Study and Meta-Analysis. Medicina 2019, 55, 264. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.M.; Adami, A.; Mazzolari, R.; Brocca, L.; Crea, E.; Zuccarelli, L.; Pellegrino, M.A.; Bottinelli, R.; Grassi, B.; Rossiter, H.B.; et al. Near-infrared spectroscopy estimation of combined skeletal muscle oxidative capacity and O2 diffusion capacity in humans. J. Physiol. 2022, 600, 4153–4168. [Google Scholar] [CrossRef]
- Gordon, R.; Bloxham, S. A Systematic Review of the Effects of Exercise and Physical Activity on Non-Specific Chronic Low Back Pain. Healthcare 2016, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Keshari, K.R.; Lotz, J.C.; Link, T.M.; Hu, S.; Majumdar, S.; Kurhanewicz, J. Lactic acid and proteoglycans as metabolic markers for discogenic back pain. Spine 2008, 33, 312–317. [Google Scholar] [CrossRef]
- Liang, C.Z.; Li, H.; Tao, Y.Q.; Zhou, X.P.; Yang, Z.R.; Li, F.C.; Chen, Q.X. The relationship between low pH in intervertebral discs and low back pain: A systematic review. Arch. Med. Sci. 2012, 8, 952–956. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K.; Yoshida, T.; Miyagawa-Tomita, S. Tenascin-C in development and disease of blood vessels. Anat. Rec. 2014, 297, 1747–1757. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K.; Tawara, I.; Yoshida, T. Tenascin-C in cardiac disease: A sophisticated controller of inflammation, repair, and fibrosis. Am. J. Physiol. Cell Physiol. 2020, 319, C781–C796. [Google Scholar] [CrossRef] [PubMed]
- Satta, J.; Melkko, J.; Pollanen, R.; Tuukkanen, J.; Paakko, P.; Ohtonen, P.; Mennander, A.; Soini, Y. Progression of human aortic valve stenosis is associated with tenascin-C expression. J. Am. Coll. Cardiol. 2002, 39, 96–101. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K. Tenascin-C in cardiovascular tissue remodeling: From development to inflammation and repair. Circ. J. 2012, 76, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, M., Jr.; Angelopoulos, T.J.; Gordon, P.; Moyna, N.; Visich, P.; Zoeller, R.; Seip, R.; Bilbie, S.; Thompson, P.; Devaney, J.; et al. The angiotensin-converting enzyme insertion/deletion polymorphism rs4340 associates with habitual physical activity among European American adults. Mol. Genet. Genomic Med. 2017, 5, 524–530. [Google Scholar] [CrossRef]
- Nitecki, M.; Shapiro, G.; Orr, O.; Levitin, E.; Sharshevsky, H.; Tzur, D.; Twig, G.; Shapira, S. Association Between Body Mass Index and Nonspecific Recurrent Low Back Pain in Over 600,000 Healthy Young Adults. Am. J. Epidemiol. 2023, 192, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, J.; Ni, H.; Shi, H.; Qi, Z.; Zhu, S.; Hao, C.; Xie, Q.; Luo, X.; Xie, K. Tenascin C: A Potential Biomarker for Predicting the Severity of Coronary Atherosclerosis. J. Atheroscler. Thromb. 2019, 26, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Vrana, A.; Scholkmann, F.; Wirth, B.; Flueck, M.; Humphreys, B.K. Changes in Spinal Muscle Oxygenation and Perfusion During the Biering-Sorensen Test: Preliminary Results of a Study Employing NIRS-Based Muscle Oximetry. Adv. Exp. Med. Biol. 2018, 1072, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Glickman, M.E.; Rao, S.R.; Schultz, M.R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol. 2014, 67, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Fluck, M.; Ruoss, S.; Mohl, C.B.; Valdivieso, P.; Benn, M.C.; von Rechenberg, B.; Laczko, E.; Hu, J.; Wieser, K.; Meyer, D.C.; et al. Genomic and lipidomic actions of nandrolone on detached rotator cuff muscle in sheep. J. Steroid Biochem. Mol. Biol. 2017, 165 Pt B, 382–395. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).