The Past, Present, Future: Pathophysiology, Diagnosis, and Treatment of Human Skin Diseases
Abstract
1. Introduction
2. Overview of Human Skin Diseases
2.1. Skin Cancers
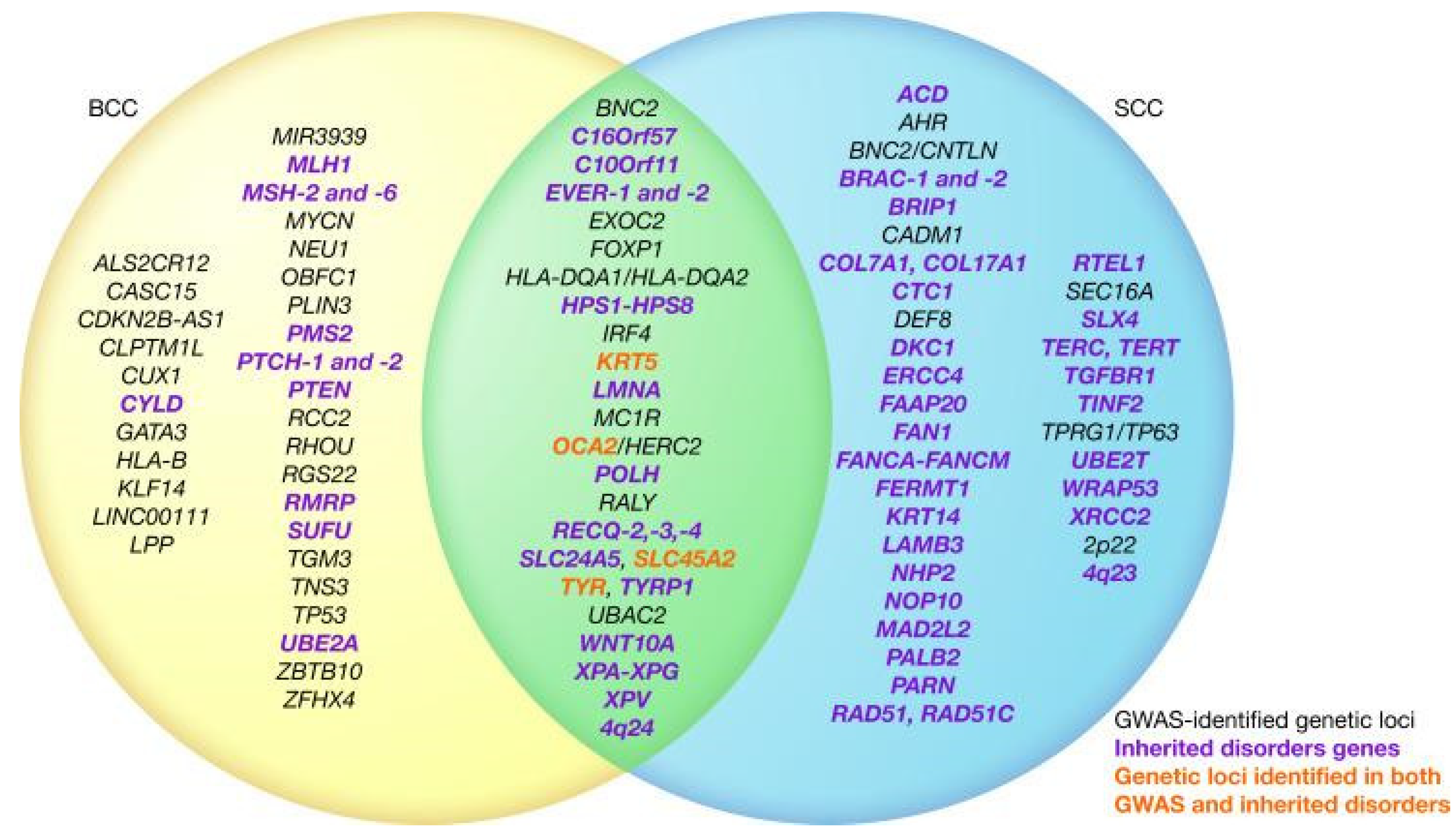
2.1.1. Basal Cell Cancer
2.1.2. Squamous Cell Cancer
2.1.3. Melanoma Skin Cancer
2.1.4. Merkel Cell Skin Cancer
2.1.5. Lymphoma
2.1.6. Kaposi Sarcoma
2.2. Benign Skin Disorders
2.2.1. Acne
2.2.2. Alopecia Areata
2.2.3. Atopic Dermatitis
2.2.4. Epidermolysis Bullosa
2.2.5. Hidradenitis Suppurativa
2.2.6. Ichthyosis
2.2.7. Pachyonychia Congenita
2.2.8. Pemphigus
2.2.9. Psoriasis
2.2.10. Raynaud’s Phenomenon
2.2.11. Rosacea
2.2.12. Scleroderma
2.2.13. Vitiligo
3. Pathophysiology of Human Skin Diseases
3.1. Common Pathophysiological Factors
3.1.1. Inflammation
3.1.2. Immune System Dysregulation
3.1.3. Environmental Triggers
3.2. Biomarkers by Skin Disease
3.2.1. Skin Cancers (Basal Cell Cancer, Squamous Cell Carcinoma, Melanoma)
Melanoma
Basal Cell Cancer
Squamous Cell Carcinoma
3.2.2. Benign Skin Disorders (Atopic Dermatitis, Pemphigus Vulgaris, Psoriasis, Rosacea, Vitiligo)
Atopic Dermatitis
Pemphigus Vulgaris
Psoriasis
Rosacea
Vitiligo
4. Diagnosis of Human Skin Diseases
4.1. Clinical Examination Methods
4.2. Histopathology
- Hematoxylin and eosin;
- Immunohistochemistry;
- Immunofluorescence (direct and indirect);
- In situ hybridization and fluorescence in situ hybridization.
5. Standard Treatment Approaches for Skin Diseases
5.1. Topical and Oral Medications
5.1.1. Topical Preparations Overview
5.1.2. Oral Preparations Overview
5.1.3. Topical and Oral Antibiotics
5.1.4. Topical and Oral Retinoids
5.1.5. Topical and Oral Antifungals
5.1.6. Topical and Oral Corticosteroids
5.1.7. Antiviral Medications
5.1.8. Immunosuppressants and Immunotherapy
5.2. Mechanical Therapy
5.2.1. UV Light Therapy
5.2.2. Laser Therapy
5.2.3. Mohs Micrographic Surgery
5.2.4. Cryotherapy
5.2.5. Dermabrasion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis; StatPearls Publishing: Treasure Island, FL, USA, 2022; Available online: https://www.ncbi.nlm.nih.gov/books/NBK470464/ (accessed on 25 September 2023).
- National Center for Chronic Disease Prevention and Health Promotion. Health and Economic Benefits of Skin Cancer Interventions. Center for Disease Control and Prevention. 2022. Available online: https://www.cdc.gov/chronicdisease/programs-impact/pop/skin-cancer.htm (accessed on 27 September 2023).
- National Institute of Arthritis and Musculoskeletal and Skin Diseases. Skin Diseases, National Institutes of Health. 2023. Available online: https://www.niams.nih.gov/health-topics/skin-diseases (accessed on 27 September 2023).
- Segall, A. The Sick Role Concept: Understanding Illness Behavior. J. Health Soc. Behav. 1976, 17, 162–169. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer; Office of the Surgeon General (US): Washington, DC, USA, 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK247178/ (accessed on 4 November 2023).
- Laughter, M.R.; Maymone, M.B.C.; Karimkhani, C. The Burden of Skin and Subcutaneous Diseases in the United States From 1990 to 2017. JAMA Dermatol. 2020, 156, 874–881. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Madan, V.; Lear, J.T.; Szeimies, R.M. Non-melanoma skin cancer. Lancet 2010, 375, 673–685. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, B.; Badri, T.; Steele, R.B. Basal Cell Carcinoma; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tanese, K. Diagnosis and Management of Basal Cell Carcinoma. Curr. Treat. Options Oncol. 2019, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.Y.; Ramsey, M.L. Squamous Cell Skin Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441939/ (accessed on 4 November 2023).
- Guo, W.; Wang, H.; Li, C. Signal pathways of melanoma and targeted therapy. Signal Transduct. Target. Ther. 2021, 6, 424. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Hussain, K. Merkel cell carcinoma. Clin. Exp. Dermatol. 2021, 46, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Mistry, K.; Levell, N.J.; Hollestein, L. Trends in incidence, treatment and survival of Merkel cell carcinoma in England 2004–2018: A cohort study. Br. J. Dermatol. 2023, 188, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Vermeer, M.H.; Scarisbrick, J.J. Cutaneous T cell lymphoma. Nat. Rev. Dis. Primers 2021, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Goodlad, J.R.; Cerroni, L.; Swerdlow, S.H. Recent advances in cutaneous lymphoma-implications for current and future classifications. Virchows Arch. 2023, 482, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; LeBlanc, R.E.; Carter, J.B. Cutaneous B-Cell Lymphoma. Hematol. Oncol. Clin. N. Am. 2019, 33, 149–161. [Google Scholar] [CrossRef]
- Agaimy, A.; Mueller, S.K.; Harrer, T.; Bauer, S.; Thompson, L.D.R. Head and Neck Kaposi Sarcoma: Clinicopathological Analysis of 11 Cases. Head Neck Pathol. 2018, 12, 511–516. [Google Scholar] [CrossRef]
- Carrilho, C.; Lunet, N. Global trends in Kaposi sarcoma incidence and mortality: The need for action to reduce inequalities. Lancet Glob. Health 2023, 11, e1479. [Google Scholar] [CrossRef]
- Hussein, H.A.M.; Okafor, I.B.; Walker, L.R.; Abdel-Raouf, U.M.; Akula, S.M. Cellular and viral oncogenes: The key to unlocking unknowns of Kaposi’s sarcoma-associated herpesvirus pathogenesis. Arch. Virol. 2018, 163, 2633–2643. [Google Scholar] [CrossRef]
- Claudel, J.P.; Auffret, N.; Leccia, M.T.; Poli, F.; Dréno, B. Acne and nutrition: Hypotheses, myths and facts. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1631–1637. [Google Scholar] [CrossRef]
- Karadağ, A.S.; Balta, İ.; Saricaoğlu, H. The effect of personal, familial, and environmental characteristics on acne vulgaris: A prospective, multicenter, case controlled study. G. Ital. Dermatol. Venereol. 2019, 154, 177–185. [Google Scholar] [CrossRef]
- Bakry, O.; Shoeib, M.; Soliman, S.; Kamal, L. Neutrophil Cytosolic Factor-1 Genotyping in Acne Vulgaris. Skin Pharmacol. Physiol. 2021, 34, 51–56. [Google Scholar] [CrossRef]
- Heng, A.H.S.; Say, Y.H.; Sio, Y.Y.; Ng, Y.T.; Chew, F.T. Gene variants associated with acne vulgaris presentation and severity: A systematic review and meta-analysis. BMC Med. Genom. 2021, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.A.; Salem, R.M.; El-Shimi, O.S.; Baghdady, S.M.A.; Hussein, S. IL1A (-889) gene polymorphism is associated with the effect of diet as a risk factor in Acne Vulgaris. J. Cosmet. Dermatol. 2019, 18, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Teder-Laving, M.; Kals, M.; Reigo, A. Genome-wide meta-analysis identifies novel loci conferring risk of acne vulgaris. Eur. J. Hum. Genet. 2023, 31, 1–8, Erratum in Eur. J. Hum. Genet. 2023. [Google Scholar] [CrossRef]
- Fujii, H.; Endo, Y.; Dainichi, T. Predictive factors of response to pulse methylprednisolone therapy in patients with alopecia areata: A follow-up study of 105 Japanese patients. J. Dermatol. 2019, 46, 522–525. [Google Scholar] [CrossRef]
- Jacobsen, E.W.; Pedersen, O.B.; Andorsdóttir, G.; Jemec, G.B.E.; Bryld, L.E. Family recurrence risk of alopecia areata in the Faroe Islands. Clin. Exp. Dermatol. 2019, 44, e224–e229. [Google Scholar] [CrossRef]
- Moravvej, H.; Tabatabaei-Panah, P.S.; Abgoon, R. Genetic variant association of PTPN22, CTLA4, IL2RA, as well as HLA frequencies in susceptibility to alopecia areata. Immunol. Investig. 2018, 47, 666–679. [Google Scholar] [CrossRef]
- Kinoshita-Ise, M.; Martinez-Cabriales, S.A.; Alhusayen, R. Chronological association between alopecia areata and autoimmune thyroid diseases: A systematic review and meta-analysis. J. Dermatol. 2019, 46, 702–709. [Google Scholar] [CrossRef]
- Tsakok, T.; Woolf, R.; Smith, C.H.; Weidinger, S.; Flohr, C. Atopic dermatitis: The skin barrier and beyond. Br. J. Dermatol. 2019, 180, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.L.; Chang, L.I.; Wu, W.F. The prevalence and risk factors of atopic dermatitis in 6-8 year-old first graders in Taipei. Pediatr. Neonatol. 2019, 60, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Belugina, I.N.; Yagovdik, N.Z.; Belugina, O.S.; Belugin, S.N. Outdoor environment, ozone, radionuclide-associated aerosols and incidences of infantile eczema in Minsk, Belarus. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1977–1985. [Google Scholar] [CrossRef]
- Nishijima, H.; Suzuki, S.; Kondo, K.; Yamasoba, T.; Yanagimoto, S. Environmental factors associated with allergic rhinitis symptoms in Japanese university students: A cross-sectional. Auris Nasus Larynx 2018, 45, 1006–1013, Erratum in Auris Nasus Larynx 2019, 46, 485. [Google Scholar] [CrossRef]
- Prodinger, C.; Bauer, J.W.; Laimer, M. Translational perspectives to treat Epidermolysis bullosa-Where do we stand? Exp. Dermatol. 2020, 29, 1112–1122. [Google Scholar] [CrossRef]
- Yenamandra, V.K.; Vellarikkal, S.K.; Chowdhury, M.R. Genotype-Phenotype Correlations of Dystrophic Epidermolysis Bullosa in India: Experience from a Tertiary Care Centre. Acta Derm. Venereol. 2018, 98, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Diaconescu, S.; Strat, S.; Balan, G.G. Dermatological Manifestations in Pediatric Inflammatory Bowel Disease. Medicina 2020, 56, 425. [Google Scholar] [CrossRef] [PubMed]
- Abdollahimajd, F.; Youssefian, L.; Pourani, M.R.; Vahidnezhad, H.; Uitto, J. Coronavirus disease 2019 and epidermolysis bullosa: Report of three cases. Dermatol. Ther. 2020, 33, e14194. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.S.; Rosenberg, A.Z.; Shipman, W.D. Hidradenitis suppurativa in Black and White patients—A clinical study. Eur. Rev. Med. Pharmacol. Sci. 2023, 27 (Suppl. S3), 92–98. [Google Scholar] [CrossRef] [PubMed]
- De, D.R.; Rick, J.W.; Shih, T.; Hsiao, J.L.; Hamzavi, I.; Shi, V.Y. COVID-19 Infection in Hidradenitis Suppurativa Patients: A Retrospective Study. Skin Appendage Disord. 2023, 9, 203–206. [Google Scholar] [CrossRef]
- Gierek, M.; Niemiec, P.; Szyluk, K.; Ochala-Gierek, G.; Bergler-Czop, B. Hidradenitis suppurativa and squamous cell carcinoma: A systematic review of the literature. Postepy Dermatol. Alergol. 2023, 40, 350–354. [Google Scholar] [CrossRef]
- Mokos, Z.B.; Čagalj, A.M.; Marinović, B. Epidemiology of Hidradenitis Suppurativa. Clin Dermatol 2023. ahead of print. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, H.S.; Jung, H.M.; Kim, G.M.; Bae, J.M. Prevalence and comorbidities associated with hidradenitis suppurativa in Korea: A nationwide population-based study. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Wertenteil, S.; Baltz, R.; Strunk, A.; Finelt, N. Prevalence Estimates for Hidradenitis Suppurativa among Children and Adolescents in the United States: A Gender- and Age-Adjusted Population Analysis. J. Investig. Dermatol. 2018, 138, 2152–2156. [Google Scholar] [CrossRef]
- Shrestha, A.B.; Biswas, P.; Shrestha, S. Harlequin ichthyosis: A case report and literature review. Clin. Case Rep. 2022, 10, e6709. [Google Scholar] [CrossRef]
- Kaushik, H.; Mahajan, R.; Dabas, G. A cross-sectional study to find association of VDR gene polymorphism with non-syndromic congenital ichthyosis and with vitamin D deficiency. Arch. Dermatol. Res. 2023, 315, 551–557. [Google Scholar] [CrossRef]
- Smith, F.J.D.; Hansen, C.D.; Hull, P.R.; Kaspar, R.L.; McLean, I.; O’Toole, E.; Sprecher, E. Pachyonychia Congenita. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Eds.; University of Washington: Seattle, WA, USA, 2006; pp. 1993–2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1280/ (accessed on 15 October 2023).
- Chovatiya, R.; Silverberg, J.I. Association of pemphigus and pemphigoid with osteoporosis and pathological fractures. Arch. Dermatol. Res. 2020, 312, 263–271. [Google Scholar] [CrossRef]
- Kang, M.; Bilgic, A.; Radjenovic, M.; Murrell, D.F. Osteoporosis and bone health in autoimmune blistering skin disease-an evidenced based review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2745–2756. [Google Scholar] [CrossRef]
- De Medeiros, V.L.S.; Monteiro-Neto, A.U.; França, D.D.T.; Castelo Branco, R.; de Miranda Coelho, É.O.; Takano, D.M. Pemphigus Vulgaris After COVID-19: A Case of Induced Autoimmunity. SN Compr. Clin. Med. 2021, 3, 1768–1772. [Google Scholar] [CrossRef]
- Siddig, O.; Mustafa, M.B.; Kordofani, Y.; Gibson, J.; Suleiman, A.M. The epidemiology of autoimmune bullous diseases in Sudan between 2000 and 2016. PLoS ONE 2021, 16, e0254634. [Google Scholar] [CrossRef]
- Lin, N.; Li, X.; Lang, Y.; Han, J. Case Report: Pemphigus in Young Patients With Thymic Anomalies. Front. Med. 2022, 9, 844223. [Google Scholar] [CrossRef] [PubMed]
- Seifollahi, A.; Fazl, M.R.; Setayesh, L. The Association Between Dietary Diversity Score and Cardiovascular Risk Factors Among Patients With Pemphigus Vulgaris: A Cross Sectional Study. Clin. Nutr. Res. 2022, 11, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Namazi, N.; Ariaeenejad, S.; Azad, M.E.; Pishgahi, M. Risk of Atrial Fibrillation in Pemphigus Vulgaris. Indian J. Dermatol. 2022, 67, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Cozzani, E.; Rosa, G.M.; Burlando, M.; Parodi, A. Psoriasis as a cardiovascular risk factor: Updates and algorithmic approach. G. Ital. Dermatol. Venereol. 2018, 153, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Kadian-Dodov, D. Cold Hands or Feet: Is It Raynaud’s or Not? Med. Clin. N. Am. 2023, 107, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Lomanta, J.M.J.; Atienza, M.A.; Gonzales, J.R.M. Erasmus Syndrome: A Case Report and Literature Review. Am. J. Case Rep. 2022, 23, e937061. [Google Scholar] [CrossRef] [PubMed]
- Nobeyama, Y.; Aihara, Y.; Asahina, A. Characteristics of Rosacea and Similar Diseases in Patients Wearing Face Masks. Skin Appendage Disord. 2022, 8, 462–468. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Zhao, Z. Excessive cleansing: An underestimating risk factor of rosacea in Chinese population. Arch. Dermatol. Res. 2021, 313, 225–234. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Chiang, Y.Y.; Huang, Y.C. Cardiovascular Risk and Comorbidities in Patients with Rosacea: A Systematic Review and Meta-analysis. Acta Derm. Venereol. 2020, 100, adv00300. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Y.; Jiang, P. Association between rosacea and cardiovascular disease: A systematic review and meta-analysis. J. Cosmet. Dermatol. 2021, 20, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xue, Y.; Chen, Y. Alcohol consumption and the risk of rosacea: A systematic review and meta-analysis. J. Cosmet. Dermatol. 2022, 21, 2954–2961. [Google Scholar] [CrossRef]
- Bütikofer, L.; Varisco, P.A.; Distler, O. ACE inhibitors in SSc patients display a risk factor for scleroderma renal crisis-a EUSTAR analysis. Arthritis Res. Ther. 2020, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Hesselstrand, R.; Scheja, A.; Wuttge, D.M. Scleroderma renal crisis in a Swedish systemic sclerosis cohort: Survival, renal outcome, and RNA polymerase III antibodies as a risk factor. Scand. J. Rheumatol. 2012, 41, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Chen, X.; Assassi, S. Cigarette smoking is not a risk factor for systemic sclerosis. Arthritis Rheum. 2011, 63, 3098–3102. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.M.; Hammad, N.M.; Sharaf, A.L.; Khattab, F. Hepatitis C virus infection could be a risk factor for adult-onset vitiligo in Egyptian patients: A cross-sectional study. J. Cosmet. Dermatol. 2022, 21, 4983–4989. [Google Scholar] [CrossRef] [PubMed]
- Al-Harthi, F.; Zouman, A.; Arfin, M.; Tariq, M.; Al-Asmari, A. Tumor necrosis factor-α and -β genetic polymorphisms as a risk factor in Saudi patients with vitiligo. Genet. Mol. Res. 2013, 12, 2196–2204. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.R.; Eleftheriadou, V.; Nesnas, J. The mental health associations of vitiligo: UK population-based cohort study. BJPsych. Open 2022, 8, e190. [Google Scholar] [CrossRef]
- Kussainova, A.; Kassym, L.; Akhmetova, A.; Glushkova, N.; Sabirov, U.; Adilgozhina, S.; Tuleutayeva, R.; Semenova, Y. Vitiligo and anxiety: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241445. [Google Scholar] [CrossRef]
- Yang, Y.T.; Hsu, C.H.; Wang, Y.F.; Chang, Y.J.; Yang, H.J.; Ko, J.L.; Yang, K.C. Worsening Quality of Life in Young Adult, Highly Educated, and Married Female Patients with Vitiligo: A Hospital-Based Case Control Study in Taiwan. Int. J. Environ. Res. Public Health 2022, 19, 6741. [Google Scholar] [CrossRef]
- Parsad, D.; Dogra, S.; Kanwar, A.J. Quality of life in patients with vitiligo. Health Qual. Life Outcomes 2003, 1, 58. [Google Scholar] [CrossRef]
- Naik, S.; Fuchs, E. Inflammatory memory and tissue adaptation in sickness and in health. Nature 2022, 607, 249–255. [Google Scholar] [CrossRef]
- Marek-Jozefowicz, L.; Nedoszytko, B.; Grochocka, M. Molecular Mechanisms of Neurogenic Inflammation of the Skin. Int. J. Mol. Sci. 2023, 24, 5001. [Google Scholar] [CrossRef]
- Jakovija, A.; Chtanova, T. Skin immunity in wound healing and cancer. Front. Immunol. 2023, 14, 1060258. [Google Scholar] [CrossRef]
- Novak, N.; Tordesillas, L.; Cabanillas, B. Diversity of T cells in the skin: Novel insights. Int. Rev. Immunol. 2023, 42, 185–198. [Google Scholar] [CrossRef]
- Castillo-González, R.; Cibrian, D.; Sánchez-Madrid, F. Dissecting the complexity of γδ T-cell subsets in skin homeostasis, inflammation, and malignancy. J. Allergy Clin. Immunol. 2021, 147, 2030–2042. [Google Scholar] [CrossRef]
- Venanzi Rullo, E.; Maimone, M.G.; Fiorica, F. Non-Melanoma Skin Cancer in People Living with HIV: From Epidemiology to Clinical Management. Front. Oncol. 2021, 11, 689789. [Google Scholar] [CrossRef]
- Hasche, D.; Akgül, B. Prevention and Treatment of HPV-Induced Skin Tumors. Cancers 2023, 15, 1709. [Google Scholar] [CrossRef]
- Choquet, H.; Ashrafzadeh, S.; Kim, Y.; Asgari, M.M.; Jorgenson, E. Genetic and environmental factors underlying keratinocyte carcinoma risk. JCI Insight 2020, 5, e134783. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- Liu, Y.; Sheikh, M.S. Melanoma: Molecular Pathogenesis and Therapeutic Management. Mol. Cell. Pharmacol. 2014, 6, 228. [Google Scholar]
- Bataille, V. It’s Not All Sunshine: Non-sun-related Melanoma Risk-factors. Acta Derm. Venereol. 2020, 100, adv00137. [Google Scholar] [CrossRef]
- Newton-Bishop, J.; Bishop, D.T.; Harland, M. Melanoma Genomics. Acta Derm. Venereol. 2020, 100, adv00138. [Google Scholar] [CrossRef]
- Krakowski, A.C.; Hafeez, F.; Westheim, A.; Pan, E.Y.; Wilson, M. Advanced basal cell carcinoma: What dermatologists need to know about diagnosis. J. Am. Acad. Dermatol. 2022, 86, S1–S13. [Google Scholar] [CrossRef]
- Basset-Seguin, N.; Herms, F. Update in the Management of Basal Cell Carcinoma. Acta Derm. Venereol. 2020, 100, adv00140. [Google Scholar] [CrossRef]
- Droll, S.; Bao, X. Oh, the Mutations You’ll Acquire! A Systematic Overview of Cutaneous Squamous Cell Carcinoma. Cell Physiol. Biochem. 2021, 55, 89–119. [Google Scholar] [CrossRef]
- Løset, M.; Brown, S.J.; Saunes, M.; Hveem, K. Genetics of Atopic Dermatitis: From DNA Sequence to Clinical Relevance. Dermatology 2019, 235, 355–364. [Google Scholar] [CrossRef]
- Bocheva, G.S.; Slominski, R.M.; Slominski, A.T. Immunological Aspects of Skin Aging in Atopic Dermatitis. Int. J. Mol. Sci. 2021, 22, 5729. [Google Scholar] [CrossRef]
- Malik, A.M.; Tupchong, S.; Huang, S.; Are, A.; Hsu, S.; Motaparthi, K. An Updated Review of Pemphigus Diseases. Medicina 2021, 57, 1080. [Google Scholar] [CrossRef]
- Amber, K.T.; Valdebran, M.; Grando, S.A. Non-Desmoglein Antibodies in Patients With Pemphigus Vulgaris. Front. Immunol. 2018, 9, 1190. [Google Scholar] [CrossRef]
- Parab, S.; Doshi, G. An update on emerging immunological targets and their inhibitors in the treatment of psoriasis. Int. Immunopharmacol. 2022, 113 Pt A, 109341. [Google Scholar] [CrossRef]
- Yan, B.; Liu, N.; Li, J. The role of Langerhans cells in epidermal homeostasis and pathogenesis of psoriasis. J. Cell. Mol. Med. 2020, 24, 11646–11655. [Google Scholar] [CrossRef]
- Marek-Jozefowicz, L.; Czajkowski, R.; Borkowska, A. The Brain-Skin Axis in Psoriasis-Psychological, Psychiatric, Hormonal, and Dermatological Aspects. Int. J. Mol. Sci. 2022, 23, 669. [Google Scholar] [CrossRef]
- Daou, H.; Paradiso, M.; Hennessy, K.; Seminario-Vidal, L. Rosacea and the Microbiome: A Systematic Review. Dermatol. Ther. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Van Zuuren, E.J.; Arents, B.W.M.; van der Linden, M.M.D.; Vermeulen, S.; Fedorowicz, Z.; Tan, J. Rosacea: New Concepts in Classification and Treatment. Am. J. Clin. Dermatol. 2021, 22, 457–465. [Google Scholar] [CrossRef]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A Review. Dermatology 2020, 236, 571–592. [Google Scholar] [CrossRef]
- Frisoli, M.L.; Essien, K.; Harris, J.E. Vitiligo: Mechanisms of Pathogenesis and Treatment. Annu. Rev. Immunol. 2020, 38, 621–648. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, Y. Advances in vitiligo: Update on therapeutic targets. Front. Immunol. 2022, 13, 986918. [Google Scholar] [CrossRef]
- Benedetti, J. Diagnosis of Skin Disorders. In MSD Manual Consumer Version; Merck & Co., Inc.: Rahway, NJ, USA, 2022; Available online: https://www.msdmanuals.com/home/skin-disorders/biology-of-the-skin/diagnosis-of-skin-disorders (accessed on 1 December 2023).
- Ladoyanni, E. Histopathology of the Skin: General Principles. In Atlas of Dermatology, Dermatopathology and Venereology; Smoller, B., Bagherani, N., Eds.; Springer Nature: Cham, Switzerland, 2022; pp. 145–160. [Google Scholar]
- Schneider, S.L.; Kohli, I.; Hamzavi, I.H.; Council, M.L.; Rossi, A.M.; Ozog, D.M. Emerging imaging technologies in dermatology: Part I: Basic principles. J. Am. Acad. Dermatol. 2019, 80, 1114–1120. [Google Scholar] [CrossRef]
- Keri, J.E. Treatment of Skin Disorders. In Merck Manual Consumer Version; Merck & Co., Inc.: Rahway, NJ, USA, 2022; Available online: https://www.merckmanuals.com/home/skin-disorders/treatment-of-skin-disorders/treatment-of-skin-disorders (accessed on 1 December 2023).
- Dallo, M.; Patel, K.; Hebert, A.A. Topical Antibiotic Treatment in Dermatology. Antibiotics 2023, 12, 188. [Google Scholar] [CrossRef]
- Jo, J.H.; Harkins, C.P.; Schwardt, N.H. Alterations of human skin microbiome and expansion of antimicrobial resistance after systemic antibiotics. Sci. Transl. Med. 2021, 13, eabd8077. [Google Scholar] [CrossRef]
- Nagler, A.R.; Del Rosso, J. The Use of Oral Antibiotics in the Management of Rosacea. J. Drugs Dermatol. 2019, 18, 506. [Google Scholar]
- Baldwin, H. Oral Antibiotic Treatment Options for Acne Vulgaris. J. Clin. Aesthet. Dermatol. 2020, 13, 26–32. [Google Scholar]
- Rotta, I.; Sanchez, A.; Gonçalves, P.R.; Otuki, M.F.; Correr, C.J. Efficacy and safety of topical antifungals in the treatment of dermatomycosis: A systematic review. Br. J. Dermatol. 2012, 166, 927–933. [Google Scholar] [CrossRef]
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The Skin Microbiome: A New Actor in Inflammatory Acne. Am. J. Clin. Dermatol. 2020, 21 (Suppl. S1), 18–24. [Google Scholar] [CrossRef]
- Callender, V.D.; Baldwin, H.; Cook-Bolden, F.E.; Alexis, A.F.; Stein Gold, L.; Guenin, E. Effects of Topical Retinoids on Acne and Post-inflammatory Hyperpigmentation in Patients with Skin of Color: A Clinical Review and Implications for Practice. Am. J. Clin. Dermatol. 2022, 23, 69–81. [Google Scholar] [CrossRef]
- Gupta, A.K.; Stec, N.; Summerbell, R.C. Onychomycosis: A review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1972–1990. [Google Scholar] [CrossRef]
- Kovitwanichkanont, T.; Chong, A.H. Superficial fungal infections. Aust. J. Gen. Pract. 2019, 48, 706–711. [Google Scholar] [CrossRef]
- Gupta, A.K.; Talukder, M.; Venkataraman, M. Review of the alternative therapies for onychomycosis and superficial fungal infections: Posaconazole, fosravuconazole, voriconazole, oteseconazole. Int. J. Dermatol. 2022, 61, 1431–1441. [Google Scholar] [CrossRef]
- Goa, K.L. Clinical pharmacology and pharmacokinetic properties of topically applied corticosteroids. A review. Drugs 1998, 36 (Suppl. S5), 51–61. [Google Scholar] [CrossRef]
- Stacey, S.K.; McEleney, M. Topical Corticosteroids: Choice and Application. Am. Fam. Physician. 2021, 103, 337–343. [Google Scholar]
- Zhao, W.; Wang, J.; Zhu, H.; Pan, M. Comparison of Guidelines for Management of Pemphigus: A Review of Systemic Corticosteroids, Rituximab, and Other Immunosuppressive Therapies. Clin. Rev. Allergy Immunol. 2021, 61, 351–362. [Google Scholar] [CrossRef]
- Aromolo, I.F.; Maronese, C.A.; Avallone, G.; Beretta, A.; Boggio, F.L.; Murgia, G.; Marletta, D.A.; Barei, F.; Carrera, C.G.; Ramoni, S.; et al. Clinical spectrum of human monkeypox: An Italian single-centre case series. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e368–e371. [Google Scholar] [CrossRef]
- Maronese, C.A.; Avallone, G.; Aromolo, I.F.; Spigariolo, C.B.; Quattri, E.; Ramoni, S.; Carrera, C.G.; Marzano, A.V. Mpox: An updated review of dermatological manifestations in the current outbreak. Br. J. Dermatol. 2023, 189, 260–270. [Google Scholar] [CrossRef]
- Koelzer, V.H.; Sirinukunwattana, K.; Rittscher, J.; Mertz, K.D. Precision immunoprofiling by image analysis and artificial intelligence. Virchows Arch. 2019, 474, 511–522. [Google Scholar] [CrossRef]
- Zelin, E.; Maronese, C.A.; Dri, A.; Toffoli, L.; Di Meo, N.; Nazzaro, G.; Zalaudek, I. Identifying Candidates for Immunotherapy among Patients with Non-Melanoma Skin Cancer: A Review of the Potential Predictors of Response. J. Clin. Med. 2022, 11, 3364. [Google Scholar] [CrossRef]
- Ajina, R.; Zamalin, D.; Weiner, L.M. Functional genomics: Paving the way for more successful cancer immunotherapy. Brief. Funct. Genom. 2019, 18, 86–98. [Google Scholar] [CrossRef]
- Wang, L.L.; Lin, S.K.; Stull, C.M. Cutaneous Oncology in the Immunosuppressed. Dermatol. Clin. 2023, 41, 141–162. [Google Scholar] [CrossRef]
- Griffith, C.F. Skin cancer in immunosuppressed patients. JAAPA 2022, 35, 19–27. [Google Scholar] [CrossRef]
- Kreher, M.A.; Konda, S.; Noland, M.M.B.; Longo, M.I.; Valdes-Rodriguez, R. Risk of melanoma and nonmelanoma skin cancer with immunosuppressants, part II: Methotrexate, alkylating agents, biologics, and small molecule inhibitors. J. Am. Acad. Dermatol. 2023, 88, 534–542. [Google Scholar] [CrossRef]
- Vieyra-Garcia, P.A.; Wolf, P. A deep dive into UV-based phototherapy: Mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol. Ther. 2021, 222, 107784. [Google Scholar] [CrossRef] [PubMed]
- Mayo Clinic. Available online: https://www.mayoclinic.org/tests-procedures/laser-resurfacing/about/pac-20385114 (accessed on 20 October 2023).
- Braun, S.A.; Schrumpf, H.; Buhren, B.A.; Homey, B.; Gerber, P.A. Laser-assisted drug delivery: Mode of action and use in daily clinical practice. J. Dtsch. Dermatol. Ges. 2016, 14, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Stanford Healthcare. Available online: https://stanfordhealthcare.org/medical-treatments/p/pulsed-dye-laser-treatment.html (accessed on 15 November 2023).
- Labadie, J.G.; Ibrahim, S.A.; Worley, B. Evidence-Based Clinical Practice Guidelines for Laser-Assisted Drug Delivery. JAMA Dermatol. 2022, 158, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Swanson, N.A. Mohs Surgery: Technique, Indications, Applications, and the Future. Arch. Dermatol. 1983, 119, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Robins, P.; Ebede, T.L.; Hale, E.K. The Evolution of Mohs Surgery. Available online: https://www.skincancer.org/treatment-resources/mohs-surgery/history-of-mohs/ (accessed on 15 November 2023).
- Elgash, M.; Young, J.; White, K.; Leitenberger, J.; Bar, A. An Update and Review of Clinical Outcomes Using Immunohistochemical Stains in Mohs Micrographic Surgery for Melanoma. Dermatol. Surg 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Swetter, S.M.; Tsao, H.; Bichakjian, C.K.; Curiel-Lewandrowski, C.; Elder, D.E.; Gershenwald, J.E.; Guild, V.; Grant-Kels, J.M.; Halpern, A.C.; Johnson, T.M.; et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad. Dermatol. 2019, 80, 208–250. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Omar, G.A.B.; Hamdino, M. Long-pulsed Nd: YAG laser (1064 nm) versus intralesional botulinum toxin type (A) in acne vulgaris therapy: A split face study. Int. J. Dermatol. 2023, 62, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Mallat, F.; Chaaya, C.; Aoun, M.; Soutou, B.; Helou, J. Adverse Events of Light-Assisted Hair Removal: An Updated Review. J. Cutan. Med. Surg. 2023, 27, 375–387. [Google Scholar] [CrossRef]
- Kao, Y.C.; Lin, D.Z.; Kang, Y.N.; Chang, C.J.; Chiu, W.K.; Chen, C. Efficacy of Laser in Hair Removal: A Network Meta-analysis. J. Cosmet. Laser Ther. 2023, 25, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Brodland, D.G. Mohs Micrographic Surgery for Melanoma: Evidence, Controversy, and a Critical Review of Excisional Margin Guidelines. Dermatol. Clin. 2023, 41, 79–88. [Google Scholar] [CrossRef]
- Crum, O.M.; Campbell, E.H.; Chelf, C.J.; Demer, A.M.; Brewer, J.D. Disease-specific survival of malignant melanoma after Mohs micrographic surgery is not impacted by initial margins: A systematic review and meta-analysis. JAAD Int. 2023, 13, 140–149. [Google Scholar] [CrossRef]
- Beal, B.T.; Udkoff, J.; Aizman, L.; Etzkorn, J.; Zitelli, J.A.; Miller, C.J.; Shin, T.M.; Sobanko, J.F.; Brodland, D.G. Outcomes of invasive melanoma of the head and neck treated with Mohs micrographic surgery—A multicenter study. J. Am. Acad. Dermatol. 2023, 89, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Neill, B.C.; Siscos, S.M.; Bar, A.A.; Seger, E.W.; Latour, E.; Tolkachjov, S.N. Factors Influencing General Dermatologists When Referring Patients with Head and Neck Melanoma for Mohs Micrographic Surgery: A Nationwide Cross-Sectional Survey. Dermatol. Surg. 2023, 49, 451–455. [Google Scholar] [CrossRef]
- Ashique, K.T.; Kaliyadan, F.; Jayasree, P. Cryotherapy: Tips and Tricks. J. Cutan. Aesthet. Surg. 2021, 14, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Sabel, M.S. Cryo-immunology: A review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology 2009, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Chen, Y.; Liu, S.; Wang, W.; Fu, S.; Wu, J. Low-dose total body irradiation enhances systemic anti-tumor immunity induced by local cryotherapy. J. Cancer Res. Clin. Oncol. 2023, 149, 10053–10063. [Google Scholar] [CrossRef]
- Goberdhan, L.T.; Schneider, K.; Makino, E.T.; Mehta, R.C. Combining Diamond-Tip Dermabrasion Treatments and Topical Skincare in Participants with Dry, Hyperpigmented, Photodamaged or Acne-Prone/Oily Facial Skin: A Clinical Usage Study. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2645–2657. [Google Scholar] [CrossRef]
| Vehicle | Benefits | Drawbacks |
|---|---|---|
| Ointments | Stronger drug delivery, low irritancy | Greasy feel and difficult to wash off |
| Creams | Easy to apply, relatively nonirritating | Evaporate easily, resulting in weak moisture and skin barrier formation; water content can allow for microbial growth |
| Lotions | Easy application | Low drug delivery, see cream drawbacks |
| Foams | Absorbed rapidly, can be used in hairy areas | Can leave skin dry due to alcohol and drying agents |
| Solutions | Easy to apply | Requires additional agents to ease irritation caused by solvent |
| Powders | Effective at drying skin | Tricky application, inhalation danger, limited effectiveness with oily skin types, cannot form protective barrier on skin |
| Gels | Easy application, useful for cleaning debris that cannot be washed with water | Low moisturization capability |
| Skin Disease | Common Antibiotics |
|---|---|
| Rosacea | Azelaic acid, minocycline, metronidazole |
| Acne vulgaris | Azelaic acid, benzoyl peroxide, clindamycin, dapsone, minocycline |
| Hidradenitis suppurativa | Doxycycline, clindamycin, TNF Inhibitors |
| Inflammatory skin conditions | Immunosuppresents or immunomodulating therapies such steroids |
| Skin and soft-tissue infection | Amikacin, clindamycin, fusidic acid, mupirocin, ozenoxacin, retapamulicin |
| Virus | Skin Disease State | Vaccine Availability |
|---|---|---|
| Herpes simplex virus | Herpes labialis, herpetic whitlow | No, No |
| Varicella–zoster virus | Chickenpox, shingles | Yes, Yes |
| Human papillomavirus virus | Common warts, plantar warts, genital warts | No, No, Yes |
| Measles virus | Measles | Yes |
| Coxsackievirus | Hand, foot, and mouth disease | No |
| Human immunodeficiency virus | Induced rash | No |
| Molluscum contagiosum virus | Warts | No |
| Dengue virus | Dengue fever | Yes |
| Mpox virus | Mpox | Yes |
| Antibody | Skin Cancer Target | Clinical Trials |
|---|---|---|
| Ipilimumab | SCC, BCC | NCT03521830 |
| Cemiplimab | SCC, BCC | NCT04428671, NCT03565783, |
| NCT03969004, NCT04428671, NCT04632433, NCT031326 | ||
| Pembrolizumab | SCC, MCC | NCT02883556, NCT02721732 |
| NCT02964559, NCT03082534 | ||
| NCT03833167, NCT02690948 | ||
| NCT04323202, NCT03712605 | ||
| Nivolumab | Melanoma, neck SCC, BCC, MCC | NCT03834233, NCT04204837, NCT02978625, NCT04620200 |
| NCT03521830, NCT03816332, NCT02834013, NCT04570683, | ||
| NCT03071406, NCT02978625, | ||
| NCT02196961, NCT03798639 | ||
| Avelumab | MCC, SCC | NCT02155647, NCT02584829 |
| NCT04393753, NCT04261855 | ||
| NCT03853317, NCT03271372, NCT04291885, NCT03944941 | ||
| NCT03737721 |
| Laser | Target | Reference |
|---|---|---|
| CO2 laser | Scars, warts, wrinkles, skin outgrowths (benign and cancerous) | Mayo Clinic [128], Braun et al. (2016) [129] |
| Erbium YAG laser | See above | Braun et al. (2016) [129], Ibrahim (2023) [126] |
| Pulsed Dye laser | Vascular skin conditions like rosacea, scar tissue, hemangioma | Stanford Healthcare [130], Mallat et al. (2023) [35] |
| Alexandrite and diode laser | Unwanted hair and pigmentation disorders | Kao et al. (2023) [131], Mallat et al. (2023) [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimnejad, N.; Jaafar, D.; Goodarzi, H. The Past, Present, Future: Pathophysiology, Diagnosis, and Treatment of Human Skin Diseases. Physiologia 2024, 4, 81-99. https://doi.org/10.3390/physiologia4010005
Ebrahimnejad N, Jaafar D, Goodarzi H. The Past, Present, Future: Pathophysiology, Diagnosis, and Treatment of Human Skin Diseases. Physiologia. 2024; 4(1):81-99. https://doi.org/10.3390/physiologia4010005
Chicago/Turabian StyleEbrahimnejad, Niki, Duaa Jaafar, and Heidi Goodarzi. 2024. "The Past, Present, Future: Pathophysiology, Diagnosis, and Treatment of Human Skin Diseases" Physiologia 4, no. 1: 81-99. https://doi.org/10.3390/physiologia4010005
APA StyleEbrahimnejad, N., Jaafar, D., & Goodarzi, H. (2024). The Past, Present, Future: Pathophysiology, Diagnosis, and Treatment of Human Skin Diseases. Physiologia, 4(1), 81-99. https://doi.org/10.3390/physiologia4010005





