Gravitational Ischemia in the Brain: How Interfering with Its Release May Predispose to Either Alzheimer’s- or Parkinson’s-like Illness, Treatable with Hyperbaric Oxygen
Abstract
:1. Introduction
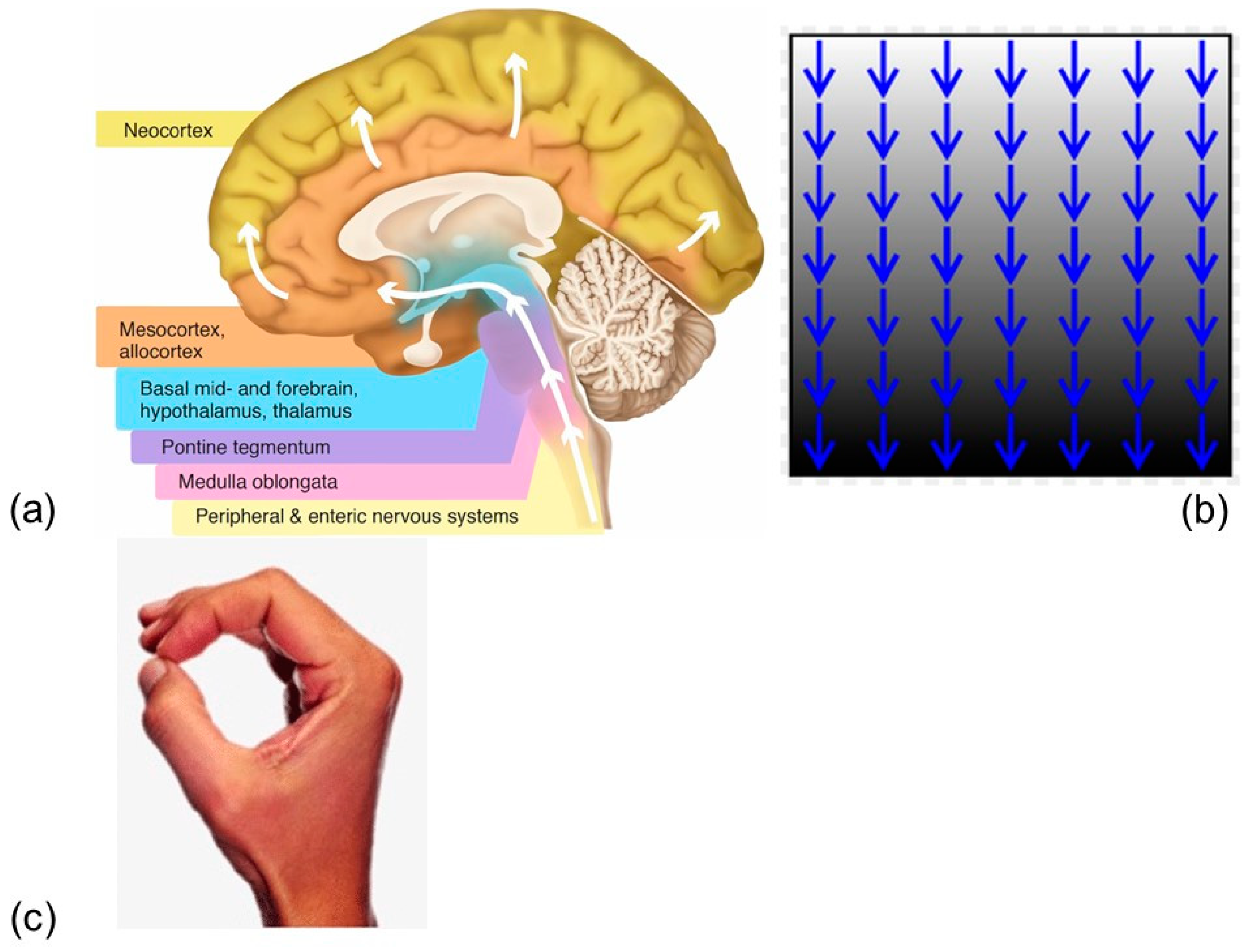
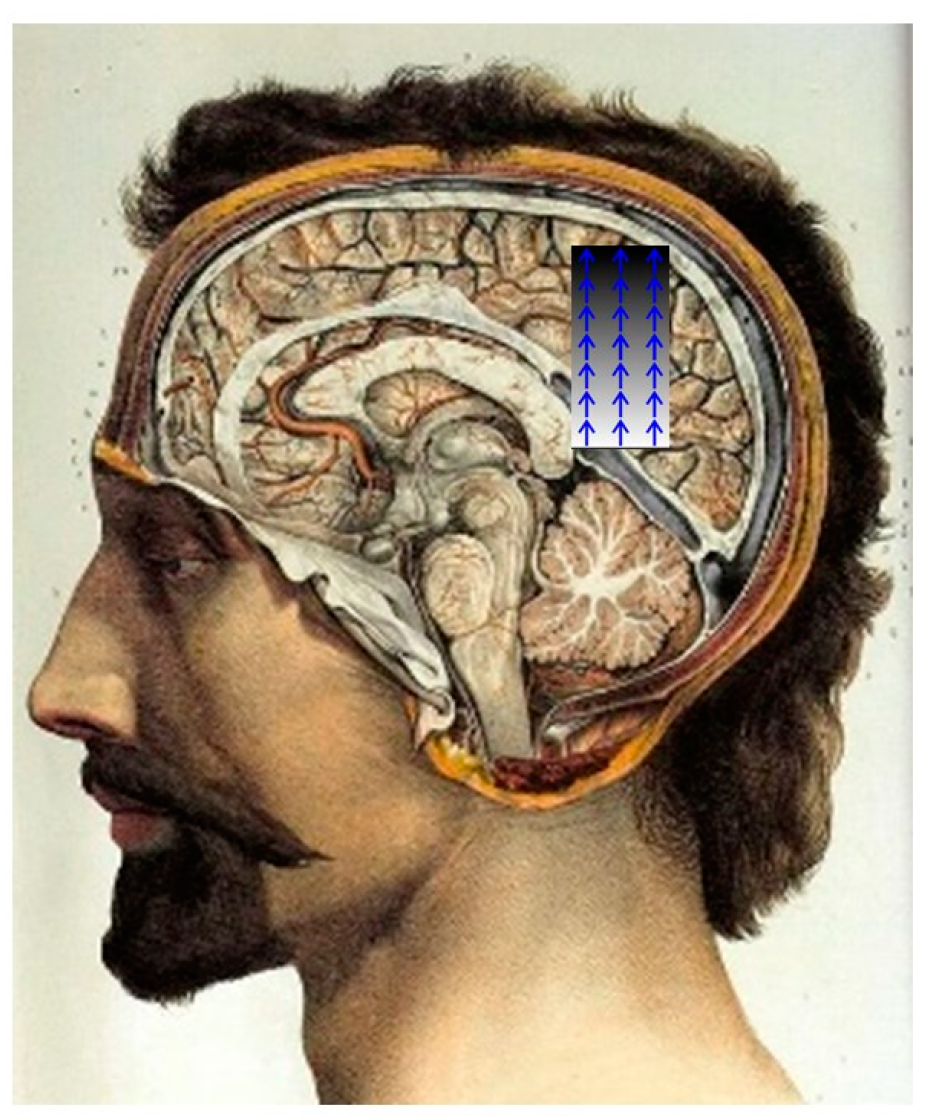
1.1. Neurodegenerative Diseases and Gravity in the Brain
1.1.1. Ischemia
1.1.2. Space Flight
1.1.3. Infectious Agents (Influenza)
1.2. Hyperbaric Oxygen
2. Results
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shannon, K.M. Infections and Changes in Commensal Bacteria and the Pathogenesis of Parkinson’s Disease. J. Park. Dis. 2022, 12, S45–S51. [Google Scholar] [CrossRef] [PubMed]
- Jaster, J.H.; Ottaviani, G. Acute Heart Failure, 90-Day Mortality, and Gravitational Ischemia in the Brain. Diagnostics 2022, 12, 1473. [Google Scholar] [CrossRef]
- Jaster, J.H.; Ottaviani, G. Late Seizures Following Cerebral Venous Thrombosis-May Be a Maladaptive Attempt to Release Gravitational Ischemia in the Brain. Neurol. Sci. 2022, 43, 6573–6574. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Yamada, Y.; Kosugi, K.; Yamada, M.; Narita, K.; Nakahara, T.; Fujiwara, H.; Toda, M.; Jinzaki, M. Effect of Gravity on Brain Structure as Indicated on Upright Computed Tomography. Sci. Rep. 2021, 11, 392. [Google Scholar] [CrossRef]
- Roberts, D.R.; Albrecht, M.H.; Collins, H.R.; Asemani, D.; Chatterjee, A.R.; Spampinato, M.V.; Zhu, X.; Chimowitz, M.I.; Antonucci, M.U. Effects of Spaceflight on Astronaut Brain Structure as Indicated on MRI. N. Engl. J. Med. 2017, 377, 1746–1753. [Google Scholar] [CrossRef]
- Ong, J.; Tarver, W.; Brunstetter, T.; Mader, T.H.; Gibson, C.R.; Mason, S.S.; Lee, A. Spaceflight Associated Neuro-Ocular Syndrome: Proposed Pathogenesis, Terrestrial Analogues, and Emerging Countermeasures. Br. J. Ophthalmol. 2023, 107, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Mekari, S.; Murphy, R.J.L.; MacKinnon, A.R.S.; Hollohan, Q.; Macdougall, S.C.; Courish, M.K.; Kimmerly, D.S.; Neyedli, H.F. The Impact of a Short-Period Head-down Tilt on Executive Function in Younger Adults. Sci. Rep. 2022, 12, 20888. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Kurt, M.; Zeng, N.; Ozkaya, E.; Marcuz, F.; Wu, L.; Laksari, K.; Camarillo, D.B.; Pauly, K.B.; Wang, Z.; et al. MR Elastography Frequency-Dependent and Independent Parameters Demonstrate Accelerated Decrease of Brain Stiffness in Elder Subjects. Eur. Radiol. 2020, 30, 6614–6623. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, N.; Xiang, B.; Ding, N.; Liu, J.; Huang, J.; Zhao, M.; Zhao, Y.; Wang, Y.; Ma, Z. In Vivo Characterization of Cerebrovascular Impairment Induced by Amyloid β Peptide Overload in Glymphatic Clearance System Using Swept-Source Optical Coherence Tomography. Neurophotonics 2023, 10, 015005. [Google Scholar] [CrossRef] [PubMed]
- Scott-Massey, A.; Boag, M.K.; Magnier, A.; Bispo, D.P.C.F.; Khoo, T.K.; Pountney, D.L. Glymphatic System Dysfunction and Sleep Disturbance May Contribute to the Pathogenesis and Progression of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 12928. [Google Scholar] [CrossRef]
- Hsu, J.-L.; Wei, Y.-C.; Toh, C.H.; Hsiao, I.-T.; Lin, K.-J.; Yen, T.-C.; Liao, M.-F.; Ro, L.-S. Magnetic Resonance Images Implicate That Glymphatic Alterations Mediate Cognitive Dysfunction in Alzheimer Disease. Ann. Neurol. 2023, 93, 164–174. [Google Scholar] [CrossRef]
- Demura, T.; Okuno, T.; Miwa, T.; Iritani, O.; Nakano, H.; Yamamoto, J.; Shiga, H.; Kodera, K.; Morimoto, C.; Demura, N.; et al. Sarcopenia and Decline in Appendicular Skeletal Muscle Mass Are Associated with Hypoperfusion in Key Hubs of Central Autonomic Network on 3DSRT in Older Adults with Progression of Normal Cognition to Alzheimer’s Disease. Geriatr. Gerontol. Int. 2023, 23, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Brouillette, M. Gravity Could Be the Cause of Your IBS, According to This Doctor. Available online: https://www.popularmechanics.com/science/health/a42552346/gravity-may-cause-irritable-bowel-syndrome/ (accessed on 20 July 2023).
- Yang, C.; Yang, Q.; Xiang, Y.; Zeng, X.-R.; Xiao, J.; Le, W.-D. The Neuroprotective Effects of Oxygen Therapy in Alzheimer’s Disease: A Narrative Review. Neural Regen. Res. 2023, 18, 57–63. [Google Scholar] [CrossRef]
- Mensah-Kane, P.; Sumien, N. The Potential of Hyperbaric Oxygen as a Therapy for Neurodegenerative Diseases. GeroScience 2023, 45, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.Y.; Wu, C.-Y.; Yu, D.; Kim, E.; Wong, M.; Elez, R.; Zebarth, J.; Ouk, M.; Tan, J.; Liao, J.; et al. Biofluid Markers of Blood-Brain Barrier Disruption and Neurodegeneration in Lewy Body Spectrum Diseases: A Systematic Review and Meta-Analysis. Park. Relat. Disord. 2022, 101, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Hourfar, H.; Aliakbari, F.; Aqdam, S.R.; Nayeri, Z.; Bardania, H.; Otzen, D.E.; Morshedi, D. The Impact of α-Synuclein Aggregates on Blood-Brain Barrier Integrity in the Presence of Neurovascular Unit Cells. Int. J. Biol. Macromol. 2023, 229, 305–320. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Wu, S.-M.; Kuan, Y.-C.; Lin, Y.-T.; Hsu, C.-R.; Hsu, W.-H.; Liu, Y.-S.; Majumdar, A.; Stettler, M.; Yang, C.-M.; et al. Associations between Risk of Alzheimer’s Disease and Obstructive Sleep Apnea, Intermittent Hypoxia, and Arousal Responses: A Pilot Study. Front. Neurol. 2022, 13, 1038735. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, F.; Zhao, L.; Cheng, C.; Zhong, R.; Dong, C.; Le, W. Hyperbaric Oxygen Ameliorates Cognitive Impairment in Patients with Alzheimer’s Disease and Amnestic Mild Cognitive Impairment. Alzheimer’s Dement. 2020, 6, e12030. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Callejón-Peláez, E.; Sáez, M.A.; Álvarez-Mon, M.A.; García-Honduvilla, N.; Monserrat, J.; Álvarez-Mon, M.; Bujan, J.; et al. A General Overview on the Hyperbaric Oxygen Therapy: Applications, Mechanisms and Translational Opportunities. Medicina (Kaunas) 2021, 57, 864. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Jia, Y.; Wang, T.; Meng, D. Adverse Effects of Hyperbaric Oxygen Therapy: A Systematic Review and Meta-Analysis. Front. Med. 2023, 10, 1160774. [Google Scholar] [CrossRef]
- Gupta, U.; Baig, S.; Majid, A.; Bell, S.M. The Neurology of Space Flight; How Does Space Flight Effect the Human Nervous System? Life Sci. Sp. Res. 2023, 36, 105–115. [Google Scholar] [CrossRef]
- Levine, K.S.; Leonard, H.L.; Blauwendraat, C.; Iwaki, H.; Johnson, N.; Bandres-Ciga, S.; Ferrucci, L.; Faghri, F.; Singleton, A.B.; Nalls, M.A. Virus Exposure and Neurodegenerative Disease Risk across National Biobanks. Neuron 2023, 111, 1086–1093. [Google Scholar] [CrossRef]
- Nair, A.K.; Van Hulle, C.A.; Bendlin, B.B.; Zetterberg, H.; Blennow, K.; Wild, N.; Kollmorgen, G.; Suridjan, I.; Busse, W.W.; Dean, D.C.; et al. Impact of Asthma on the Brain: Evidence from Diffusion MRI, CSF Biomarkers and Cognitive Decline. Brain Commun. 2023, 5, fcad180. [Google Scholar] [CrossRef]
- McGregor, H.R.; Hupfeld, K.E.; Pasternak, O.; Beltran, N.E.; De Dios, Y.E.; Bloomberg, J.J.; Wood, S.J.; Mulavara, A.P.; Riascos, R.F.; Reuter-Lorenz, P.A.; et al. Impacts of Spaceflight Experience on Human Brain Structure. Sci. Rep. 2023, 13, 7878. [Google Scholar] [CrossRef] [PubMed]
- Adelaide-Born Astronaut Reveals He Has Parkinson’s Disease. Available online: https://www.facebook.com/7NEWSAdelaide/videos/adelaide-born-astronaut-reveals-he-has-parkinsons-disease/878348489458878/ (accessed on 20 July 2023).
- SA Astronaut Andy Thomas Reveals Parkinson’s Diagnosis. Available online: https://newslettercollector.com/newsletter/morning-sa-astronaut-andy-thomas-revea-ls-parkinson-s-diagnosis/ (accessed on 20 July 2023).
- Presley, K.F.; Falcucci, T.; Shaidani, S.; Fitzpatrick, V.; Barry, J.; Ly, J.T.; Dalton, M.J.; Grusenmeyer, T.A.; Kaplan, D.L. Engineered Porosity for Tissue-Integrating, Bioresorbable Lifetime-Based Phosphorescent Oxygen Sensors. Biomaterials 2023, 301, 122286. [Google Scholar] [CrossRef] [PubMed]
- Matarrese, M.A.G.; Loppini, A.; Fabbri, L.; Tamilia, E.; Perry, M.S.; Madsen, J.R.; Bolton, J.; Stone, S.S.D.; Pearl, P.L.; Filippi, S.; et al. Spike Propagation Mapping Reveals Effective Connectivity and Predicts Surgical Outcome in Epilepsy. Brain 2023, 146, 3898–3912. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Taylor, A.J.; Himmelbach, M.; Hagberg, G.E.; Scheffler, K.; Ress, D. Characterization of the Blood Oxygen Level Dependent Hemodynamic Response Function in Human Subcortical Regions with High Spatiotemporal Resolution. Front. Neurosci. 2022, 16, 1009295. [Google Scholar] [CrossRef]
- Sousani, M.; Rojas, R.F.; Preston, E.; Ghahramani, M. Towards a Multi-Modal Brain-Body Assessment in Parkinson’s Disease: A Systematic Review in FNIRS (February 2023). IEEE J. Biomed. Health Inform. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaster, J.H.; Ottaviani, G. Gravitational Ischemia in the Brain: How Interfering with Its Release May Predispose to Either Alzheimer’s- or Parkinson’s-like Illness, Treatable with Hyperbaric Oxygen. Physiologia 2023, 3, 510-521. https://doi.org/10.3390/physiologia3040037
Jaster JH, Ottaviani G. Gravitational Ischemia in the Brain: How Interfering with Its Release May Predispose to Either Alzheimer’s- or Parkinson’s-like Illness, Treatable with Hyperbaric Oxygen. Physiologia. 2023; 3(4):510-521. https://doi.org/10.3390/physiologia3040037
Chicago/Turabian StyleJaster, J. Howard, and Giulia Ottaviani. 2023. "Gravitational Ischemia in the Brain: How Interfering with Its Release May Predispose to Either Alzheimer’s- or Parkinson’s-like Illness, Treatable with Hyperbaric Oxygen" Physiologia 3, no. 4: 510-521. https://doi.org/10.3390/physiologia3040037
APA StyleJaster, J. H., & Ottaviani, G. (2023). Gravitational Ischemia in the Brain: How Interfering with Its Release May Predispose to Either Alzheimer’s- or Parkinson’s-like Illness, Treatable with Hyperbaric Oxygen. Physiologia, 3(4), 510-521. https://doi.org/10.3390/physiologia3040037






