Decrease in Leptin Expression in the Liver after Prolonged Every-Other-Day Feeding in C57Bl/6 Male Mice
Abstract
1. Introduction
2. Results
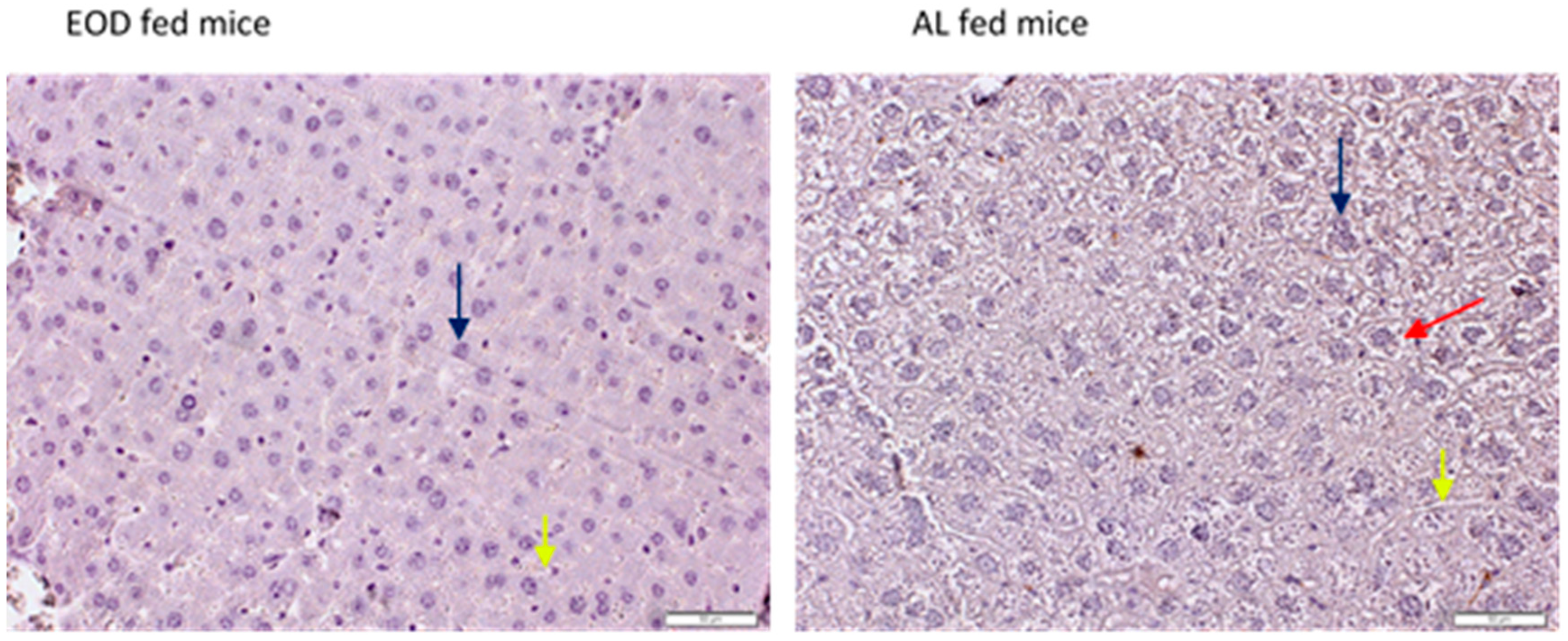
2.1. General Morphology of the Liver Tissue
2.2. Expression of Adipokines and Their Receptors in the Liver Tissue
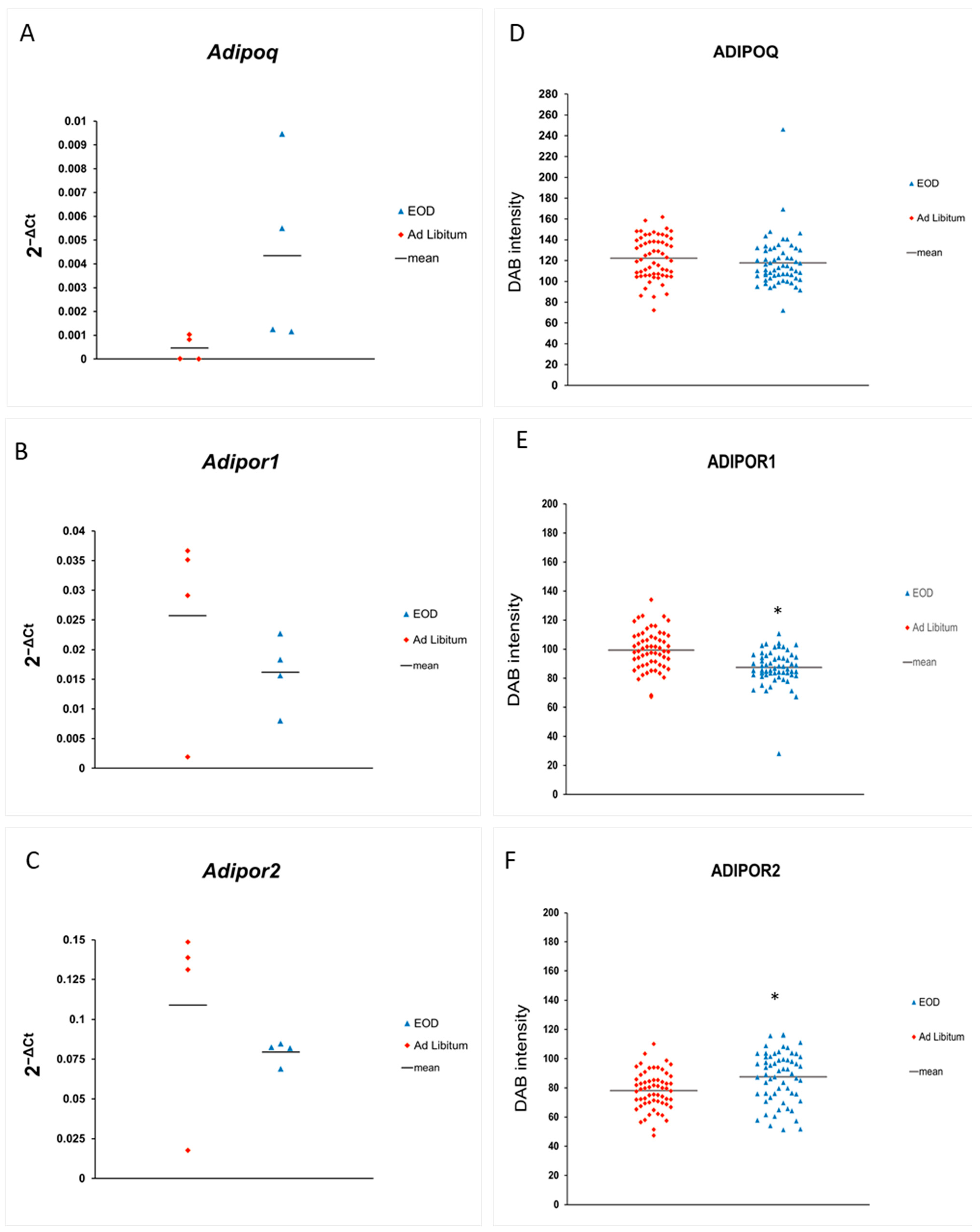
2.2.1. Expression of ADIPOQ and Receptors in Liver Tissue
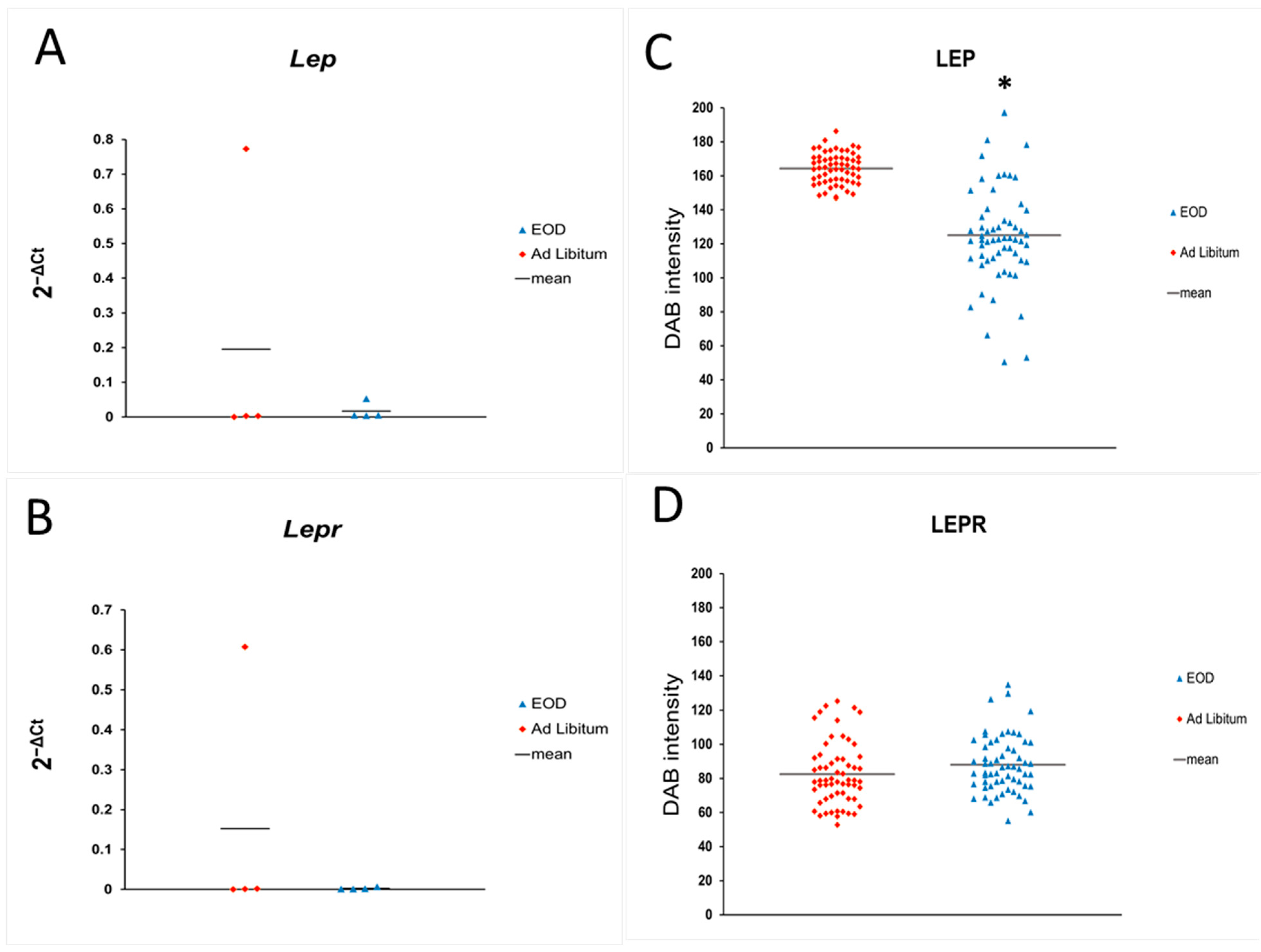
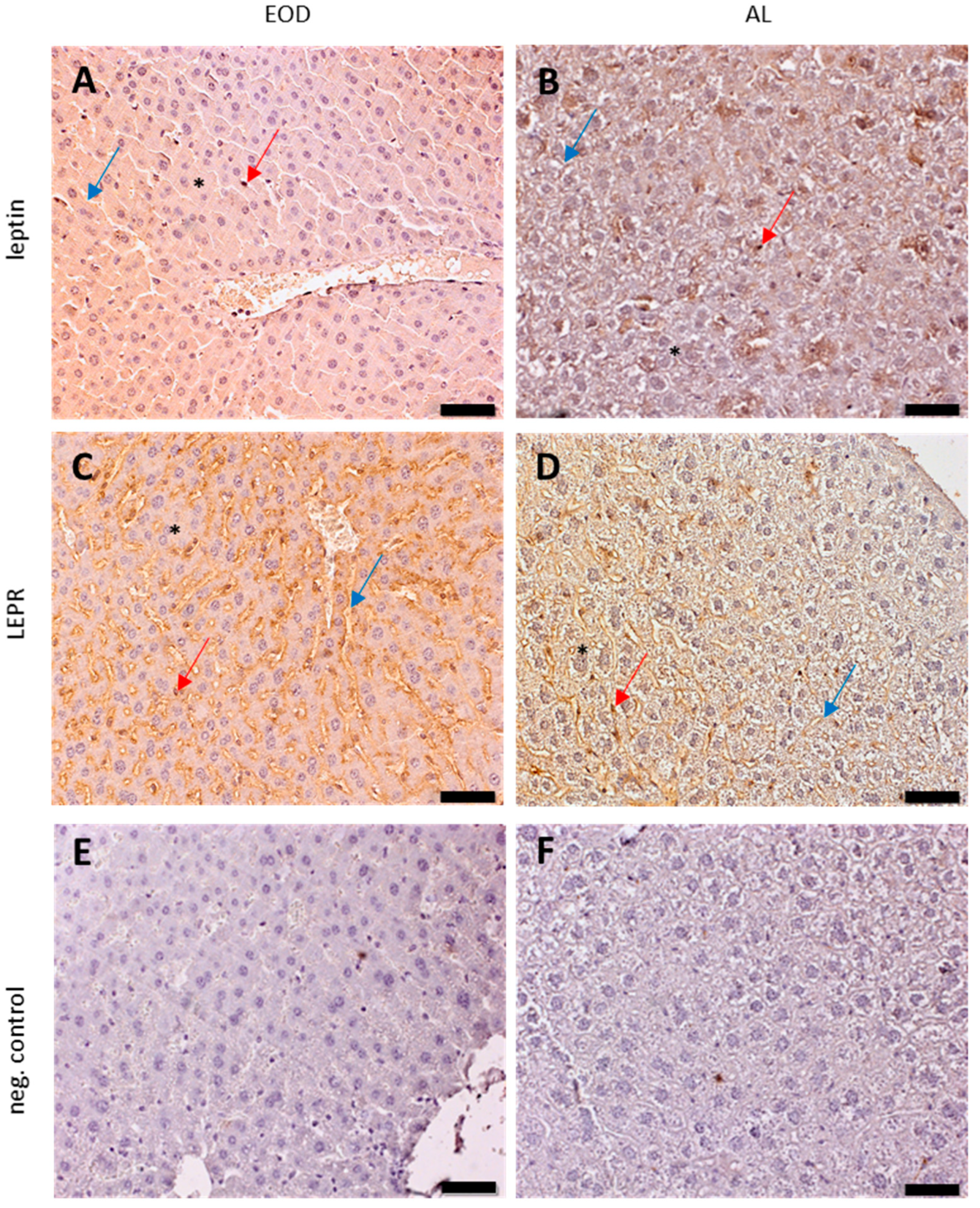
2.2.2. Leptin Expression in Liver Tissue
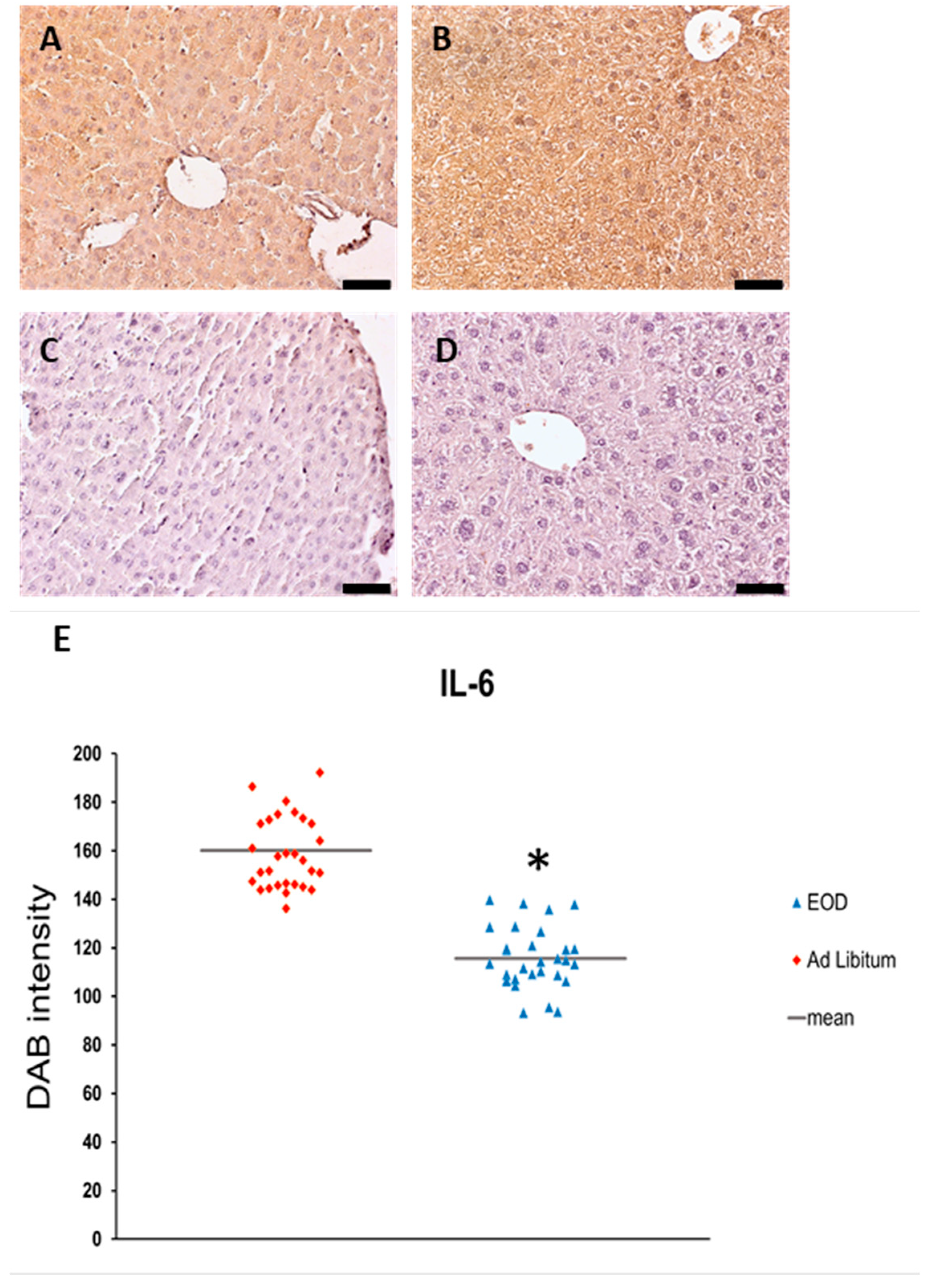
2.3. IL-6 Expression in the Liver
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tissue Preparation
4.3. Immunohistochemical Staining of Liver Samples
4.3.1. Immunolocalization of Adipokines and Receptors on IHC-Stained Liver Slides
4.3.2. ImageJ Semi-Quantitative Estimation of Protein Expression on IHC Images
4.4. Real-Time Quantitative Reverse-Transcription PCR (RQ-PCR)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anson, R.M.; Jones, B.; de Cabod, R. The diet restriction paradigm: A brief review of the effects of every-other-day feeding. Age 2005, 27, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Neff, F.; Markert, A.; Rozman, J.; Aguilar-Pimentel, J.A.; Amarie, O.V.; Becker, L.; Brommage, R.; Garrett, L.; Henzel, K.S.; et al. Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice. Nat. Commun. 2017, 8, 155. [Google Scholar] [CrossRef]
- Westbrook, R.; Bonkowski, M.S.; Arum, O.; Strader, A.D.; Bartke, A. Metabolic alterations due to caloric restriction and every other day feeding in normal and growth hormone receptor knockout mice. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Rogozina, O.P.; Bonorden, M.J.; Seppanen, C.N.; Grande, J.P.; Cleary, M.P. Effect of chronic and intermittent calorie restriction on serum adiponectin and leptin and mammary tumorigenesis. Cancer Prev. Res. 2011, 4, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.J.; Caldwell, J.L.; van der Merwe, M.; Sharma, S.; Butawan, M.; Puppa, M.; Bloomer, R.J. A Comparison of Dietary and Caloric Restriction Models on Body Composition, Physical Performance, and Metabolic Health in Young Mice. Nutrients 2019, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.G.; Turner, D.L.; Swoap, S.J. Health Effects of Alternate Day Fasting Versus Pair-Fed Caloric Restriction in Diet-Induced Obese C57Bl/6J Male Mice. Front. Physiol. 2021, 12, 641532. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, K.; Tarnowski, M.; Zgutka, K.; Pawlik, A. Gender Differences in Response to Prolonged Every-Other-Day Feeding on the Proliferation and Apoptosis of Hepatocytes in Mice. Nutrients 2016, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Sanz, M.J.; Real, J.T.; Marques, P.; Capuozzo, M.; Ait Eldjoudi, D.; Gualillo, O. Adipokines in Non-Alcoholic Fatty Liver Disease: Are We on the Road toward New Biomarkers and Therapeutic Targets? Biology 2022, 11, 1237. [Google Scholar] [CrossRef]
- Ristic-Medic, D.; Bajerska, J.; Vucic, V. Crosstalk between dietary patterns, obesity and nonalcoholic fatty liver disease. World J. Gastroenterol. 2022, 28, 3314–3333. [Google Scholar] [CrossRef]
- Marchisello, S.; Di Pino, A.; Scicali, R.; Urbano, F.; Piro, S.; Purrello, F.; Rabuazzo, A.M. Pathophysiological, Molecular and Therapeutic Issues of Nonalcoholic Fatty Liver Disease: An Overview. Int. J. Mol. Sci. 2019, 20, 1948. [Google Scholar] [CrossRef]
- Yamaza, H.; Komatsu, T.; To, K.; Toyama, H.; Chiba, T.; Higami, Y.; Shimokawa, I. Involvement of insulin-like growth factor-1 in the effect of caloric restriction: Regulation of plasma adiponectin and leptin. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 27–33. [Google Scholar] [CrossRef]
- Ding, Q.; Ash, C.; Mracek, T.; Merry, B.; Bing, C. Caloric restriction increases adiponectin expression by adipose tissue and prevents the inhibitory effect of insulin on circulating adiponectin in rats. J. Nutr. Biochem. 2012, 23, 867–874. [Google Scholar] [CrossRef]
- Miller, K.N.; Burhans, M.S.; Clark, J.P.; Howell, P.R.; Polewski, M.A.; DeMuth, T.M.; Eliceiri, K.W.; Lindstrom, M.J.; Ntambi, J.M.; Anderson, R.M. Aging and caloric restriction impact adipose tissue, adiponectin, and circulating lipids. Aging Cell 2017, 16, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Kaser, S.; Moschen, A.; Cayon, A.; Kaser, A.; Crespo, J.; Pons-Romero, F.; Ebenbichler, C.F.; Patsch, J.R.; Tilg, H. Adiponectin and its receptors in non-alcoholic steatohepatitis. GUT 2005, 54, 117–121. [Google Scholar] [CrossRef]
- Cho, J.; Koh, Y.; Han, J.; Kim, D.; Kim, T.; Kang, H. Adiponectin mediates the additive effects of combining daily exercise with caloric restriction for treatment of non-alcoholic fatty liver. Int. J. Obes. 2016, 40, 1760–1767. [Google Scholar] [CrossRef]
- Jové, M.; Naudí, A.; Ramírez-Núñez, O.; Portero-Otín, M.; Selman, C.; Withers, D.J.; Pamplona, R. Caloric restriction reveals a metabolomic and lipidomic signature in liver of male mice. Aging Cell 2014, 13, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Jung, Y.; Min, S.; Nam, M.; Heo, R.W.; Jeon, B.T.; Song, D.H.; Yi, C.O.; Jeong, E.A.; Kim, H.; et al. Caloric restriction of db/db mice reverts hepatic steatosis and body weight with divergent hepatic metabolism. Sci. Rep. 2016, 6, 30111. [Google Scholar] [CrossRef]
- Cabezas, J.; Mayorga, M.; Crespo, J. Nonalcoholic Fatty Liver Disease: A Pathological View. In Liver Biopsy—Indications, Procedures, Results; InTech: Berlin, Germany, 2012; Available online: https://www.intechopen.com/chapters/41027 (accessed on 2 October 2023).
- Kiernan, K.; MacIver, N.J. The Role of the Adipokine Leptin in Immune Cell Function in Health and Disease. Front. Immunol. 2021, 11, 622468. [Google Scholar] [CrossRef]
- Brown-Borg, H.M.; Rakoczy, S. Metabolic adaptations to short-term every-other-day feeding in long-living Ames dwarf mice. Exp. Gerontol. 2013, 48, 905–919. [Google Scholar] [CrossRef][Green Version]
- Combs, T.P.; Marliss, E.B. Adiponectin signaling in the liver. Rev. Endocr. Metab. Disord. 2014, 15, 137–147. [Google Scholar] [CrossRef]
- Łączna, M.; Kopytko, P.; Tkacz, M.; Zgutka, K.; Czerewaty, M.; Tarnowski, M.; Larysz, D.; Tkacz, R.; Kotrych, D.; Piotrowska, K.; et al. Adiponectin Is a Component of the Inflammatory Cascade in Rheumatoid Arthritis. J. Clin. Med. 2022, 11, 2740. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, R.; Iglesias, M.J.; Gallego, R.; Raghay, K.; Eiras, S.; Rubio, J.; Diéguez, C.; Gualillo, O.; González-Juanatey, J.R.; Lago, F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005, 579, 5163–5169. [Google Scholar] [CrossRef] [PubMed]
- Katsiougiannis, S.; Kapsogeorgou, E.K.; Manoussakis, M.N.; Skopouli, F.N. Salivary gland epithelial cells: A new source of the immunoregulatory hormone adiponectin. Arthritis Rheum. 2006, 54, 2295–2299. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Woo, J.G.; Geraghty, S.R.; Altaye, M.; Davidson, B.S.; Banach, W.; Dolan, L.M.; Ruiz-Palacios, G.M.; Morrow, A.L. Adiponectin is present in human milk and is associated with maternal factors. Am. J. Clin. Nutr. 2006, 83, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, H.; Xiang, G.; Zhang, L.; Li, L.; Cao, Y.; Zhang, J. Adiponectin and its receptors in chronic hepatitis B patients with steatosis in China. Hepat. Mon. 2013, 13, e6065. [Google Scholar] [CrossRef] [PubMed]
- Heydari, M.; Cornide-Petronio, M.E.; Jiménez-Castro, M.B.; Peralta, C. Data on Adiponectin from 2010 to 2020: Therapeutic Target and Prognostic Factor for Liver Diseases? Int. J. Mol. Sci. 2020, 21, 5242. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Saxena, N.K.; Lin, S.; Xu, A.; Srinivasan, S.; Anania, F.A. The roles of leptin and adiponectin: A novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am. J. Pathol. 2005, 166, 1655–1669. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Iwabu, M.; Okada-Iwabu, M.; Kadowaki, T. Adiponectin receptors: A review of their structure, function and how they work. Best. Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 15–23. [Google Scholar] [CrossRef]
- Rhoads, T.W.; Burhans, M.S.; Chen, V.B.; Hutchins, P.D.; Rush, M.J.P.; Clark, J.P.; Stark, J.L.; McIlwain, S.J.; Eghbalnia, H.R.; Pavelec, D.M.; et al. Caloric Restriction Engages Hepatic RNA Processing Mechanisms in Rhesus Monkeys. Cell Metab. 2018, 27, 677–688.e5. [Google Scholar] [CrossRef]
- Mendoza-Herrera, K.; Florio, A.A.; Moore, M.; Marrero, A.; Tamez, M.; Bhupathiraju, S.N.; Mattei, J. The Leptin System and Diet: A Mini Review of the Current Evidence. Front. Endocrinol. 2021, 12, 749050. [Google Scholar] [CrossRef]
- Martínez-Uña, M.; López-Mancheño, Y.; Diéguez, C.; Fernández-Rojo, M.A.; Novelle, M.G. Unraveling the Role of Leptin in Liver Function and Its Relationship with Liver Diseases. Int. J. Mol. Sci. 2020, 21, 9368. [Google Scholar] [CrossRef]
- Ikejima, K.; Takei, Y.; Honda, H.; Hirose, M.; Yoshikawa, M.; Zhang, Y.J.; Lang, T.; Fukuda, T.; Yamashina, S.; Kitamura, T.; et al. Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology 2002, 122, 1399–1410. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef]
- Cortegana- Jiménes, C.; García-Galey, A.; Tami, M.; del Pino, P.; Carmona, I.; López, S.; Alba, G.; Sánchez-Margalet, V. Role of Leptin in Non-Alcoholic Fatty Liver Disease. Biomedicines 2021, 9, 762. [Google Scholar] [CrossRef]
- Yang, W.H.; Liu, S.C.; Tsai, C.H.; Fong, Y.C.; Wang, S.J.; Chang, Y.S.; Tang, C.H. Leptin induces IL-6 expression through OBR1 receptor signalling pathway in human synovial fibroblasts. PLoS ONE 2013, 8, e75551. [Google Scholar]
- Cohen, P.; Yang, G.; Yu, X.; Soukas, A.A.; Wolfish, C.S.; Friedman, J.M.; Li, C. Induction of leptin receptor expression in the liver by leptin and food deprivation. J. Biol. Chem. 2005, 280, 10034–10039. [Google Scholar] [CrossRef]
- Schanton, M.; Maymó, J.L.; Pérez-Pérez, A.; Sánchez-Margalet, V.; Varone, C.L. Involvement of leptin in the molecular physiology of the placenta. Reproduction 2018, 155, R1–R12. [Google Scholar] [CrossRef]
- Shen, J.; Sakaida, I.; Uchida, K.; Terai, S.; Okita, K. Leptin enhances TNF-alpha production via p38 and JNK MAPK in LPS-stimulated Kupffer cells. Life Sci. 2005, 77, 1502–1515. [Google Scholar] [CrossRef]
- Wang, J.; Leclercq, I.; Brymora, J.M.; Xu, N.; Ramezani-Moghadam, M.; London, R.M.; Brigstock, D.; George, J. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology 2009, 137, 713–723. [Google Scholar] [CrossRef]
- Mandal, P.; Park, P.H.; McMullen, M.R.; Pratt, B.T.; Nagy, L.E. The anti-inflammatory effects of adiponectin are mediated via a heme oxygenase-1-dependent pathway in rat Kupffer cells. Hepatology 2010, 51, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Semmler, G.; Datz, C.; Trauner, M. Eating, diet, and nutrition for the treatment of non-alcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S244–S260. [Google Scholar] [CrossRef] [PubMed]
- Crowe, A.R.; Yue, W. Semi-quantitative Determination of Protein Expression using Immunohistochemistry Staining and Analysis: An Integrated Protocol. Bio-Protocol 2019, 9, e3465. [Google Scholar] [CrossRef] [PubMed]

| EOD | AL | |
|---|---|---|
| ADIPOQ | 117.80 ± 3.09 | 113.33 ± 2.63 |
| ADIPOR1 | 87.37 ± 1.56 * | 99.50 ± 1.74 |
| ADIPOR2 | 87.50 ± 2.25 * | 78.19 ± 1.67 |
| Leptin | 125.06 ± 3.84 * | 164.43 ± 1.18 |
| LEPR | 88.09 ± 2.15 * | 82.60 ± 2.39 |
| IL-6 | 115.59 ± 2.29 * | 154.10 ± 5.32 |
| Antibody | Antibody Host | Dilution | Manufacturer/Cat No. |
|---|---|---|---|
| Adiponectin | Mouse | 1:100 | ThermoFisher Scientific, Waltham, MA, USA/(MA1-054) |
| Leptin | Rabbit | 1:100 | ThermoFisher Scientific, Waltham, MA, USA/(PA1-051) |
| ADIPOR1 | Mouse | 1:200 | Santa Cruz Biotechnology Inc., Dallas, TX, USA/(sc-518030) |
| ADIPOR2 | Mouse | 1:200 | Santa Cruz Biotechnology Inc., Dallas, TX, USA/(sc-514045) |
| LEPR | Rabbit | 1:200 | Santa Cruz Biotechnology Inc., Dallas, TX, USA/(sc-8391) |
| IL-6 | Rat | 1:100 | ThermoFisher Scientific, Waltham, MA, USA/(MP520F3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowska, K.; Tarnowski, M.; Tomasiak, P.; Czerewaty, M.; Zgutka, K.; Pawlik, A. Decrease in Leptin Expression in the Liver after Prolonged Every-Other-Day Feeding in C57Bl/6 Male Mice. Physiologia 2024, 4, 100-114. https://doi.org/10.3390/physiologia4010006
Piotrowska K, Tarnowski M, Tomasiak P, Czerewaty M, Zgutka K, Pawlik A. Decrease in Leptin Expression in the Liver after Prolonged Every-Other-Day Feeding in C57Bl/6 Male Mice. Physiologia. 2024; 4(1):100-114. https://doi.org/10.3390/physiologia4010006
Chicago/Turabian StylePiotrowska, Katarzyna, Maciej Tarnowski, Patrycja Tomasiak, Michał Czerewaty, Katarzyna Zgutka, and Andrzej Pawlik. 2024. "Decrease in Leptin Expression in the Liver after Prolonged Every-Other-Day Feeding in C57Bl/6 Male Mice" Physiologia 4, no. 1: 100-114. https://doi.org/10.3390/physiologia4010006
APA StylePiotrowska, K., Tarnowski, M., Tomasiak, P., Czerewaty, M., Zgutka, K., & Pawlik, A. (2024). Decrease in Leptin Expression in the Liver after Prolonged Every-Other-Day Feeding in C57Bl/6 Male Mice. Physiologia, 4(1), 100-114. https://doi.org/10.3390/physiologia4010006






