Abstract
This study examined the effects of two different acute bouts of treadmill running (e.g., steady-state moderate-intensity exercise (SSE) and high-intensity interval exercise (HIIE)) on adipokine (e.g., adiponectin, leptin, and omentin) concentrations in individuals with moderate stages of chronic kidney disease (CKD). Fourteen participants (8 females and 6 males) (age 58.7 ± 9.7, height (cm) 168.5 ± 9.7, weight (kg) 83.5 ± 18.2) were classified as having moderate stages of CKD (stages G3a and G3b; estimated glomerular filtration rate (eGFR) between 59 and 30 mL/min/1.73 m2). Participants completed 30 min of either SSE at 65% oxygen consumption reserve (VO2R) or HIIE for 3 min at a 90% VO2R separated by 2 min of slow walking (20% VO2R) in a randomized, crossover design on a treadmill. Venous blood samples were obtained at baseline, 1 h, and 24 h post exercise. Data were analyzed using a two by three repeated measures ANOVA (p < 0.05). There were no significant differences in adiponectin (p < 0.353), leptin (p < 0.084), and omentin (p < 0.235) concentrations between SSE and HIIE. Similarly, no significant differences were seen between the sampled time points for either aerobic exercise condition. In conclusion, this study demonstrated there were no changes in adiponectin, leptin, or omentin concentrations when performing an acute bout of HIIE or SSE for 30 min, at 1 or 24 h post exercise. Future studies should seek to either increase the duration of the exercise bout or identify different adipokines to examine for patients experiencing moderate stages of CKD.
1. Introduction
The worldwide prevalence of chronic kidney disease (CKD) is estimated to be 10 to 16% and holds a Medicare cost burden of USD 60 billion in the United States [1,2]. The high morbidity and mortality rates of CKD pose a significant health concern to millions of people across the globe, and identifying potential therapeutics is a growing focus [1,2,3]. CKD is commonly characterized by a decline in renal filtration, endothelial dysfunction, and both local and systemic inflammation [4]. The damage seen on the renal and vascular endothelium of the body is likely the result of the chronic systemic inflammation typically seen with CKD [4,5,6]. Several comorbidities and diseases, such as hypertension, diabetes, and obesity, are thought to be primary causes of CKD [6]. Typically, increases in adipose tissue accompany these comorbidities and diseases that exacerbate the early development of CKD via damage to the vascular and renal endothelium [7]. Injury to the endothelium is largely due to the various inflammatory cytokines that are released from adipose tissue, known as adipokines, which may further promote the development and progression of CKD [8,9].
Adipokines are hormones released from adipose tissue that directly influence blood pressure, metabolism, appetite, angiogenesis, and immune function [9,10,11]. They are synthesized from normal or dysregulated, premature, and mature adipocytes within adipose tissue and can be classified as either pro- or anti-inflammatory. Of the more commonly studied pro-inflammatory adipokines, leptin, resistin, IL-6, and TNFα, have all been implicated as mediators of various chronic diseases (e.g., T2D, CVDs, metabolic syndrome, insulin resistance) [12,13], while anti-inflammatory adipokines, such as adiponectin, omentin-1, and TNF-related factors have been shown to play positive roles in the reduction of oxidative stress and systemic inflammation [13]. One adipokine in particular, omentin-1, has previously been shown to have suppressive effects on inflammation through AMP-activated protein kinase, serine/threonine kinase (Akt), and nuclear factor-kappa beta (NF-kβ) intracellular signaling pathways [14,15]. Moreover, there appears to be a balancing cascade where anti-inflammatory adipokines may aid in the reduction of pro-inflammatory adipokines [13]. A dysregulation in the secretion of these pro- and anti-inflammatory adipokines from adipose tissue accumulation may lead to the progression of obesity-related diseases and CKD through increases in low-grade systemic inflammation [9,16]. Therefore, identifying methods to mitigate adipokine dysregulation would be highly valuable to patients coping with CKD.
Various therapeutic interventions (e.g., physical activity, resistance training (RT), aerobic exercise (AE), and nutrition) have been examined to identify a purposeful way to reduce the harmful effects of an over-production of pro- and anti-inflammatory adipokines in various clinical populations [17,18,19,20]. While the literature is still limited, some articles have acknowledged that exercise (both RT and AE) may be a powerful adipokine mediator in overweight and obese individuals suffering from excess adipose tissue [18,21,22]. Parastesh et al. [23] previously conducted a study that examined the effects of 12 weeks of aerobic exercise on leptin levels in obese males and found that leptin levels decreased significantly preceding the 12-week exercise program. This likely could be due to the loss of fat mass the participants experienced, which has previously been shown to have a relationship with leptin concentrations [9,24]. However, while it is generally hypothesized that an increase in adipokines is associated with the increase of adipose tissue that an individual carries in their body, it is still unclear if there are any acute changes from single bouts of aerobic exercise training in various clinical populations [17,25,26].
The beneficial effects from acute high-intensity interval exercise (HIIE) training or moderate intensity steady-state exercise (SSE) in adipokine concentrations of patients suffering from moderate stages of CKD remain unknown. Identifying the acute changes of these adipokines from HIIE and SSE may provide more insight into how we can better address the chronic adipokine dysregulation in those suffering from CKD. Thus, the purpose of this study was to examine the effects on the concentrations of several pro- and anti-inflammatory adipokines (e.g., adiponectin, leptin, omentin) after patients with moderate stages (stages G3a and G3b) of CKD performed two separate acute bouts of aerobic exercise (e.g., SSE, HIIE).
2. Materials and Methods
The study protocol was approved by the Institutional Review Board for Human Subjects at Baylor University in accordance with the Helsinki Code of ethics. Each participant was informed of the benefits and risks of the study both written and verbally, prior to signing an informed consent document. Participants were free to withdraw from the study at any point, without penalty.
2.1. Experimental Design
Upon arrival at the laboratory for the preliminary visit, participants fasted for 8 to 10 h. Resting blood pressure, a baseline blood sample, height, and weight were all measured at the beginning of the preliminary visit. Subsequently, body composition was measured using dual-energy X-ray absorptiometry (DEXA) (Hologic Discovery Series W, Waltham, MA, USA). Participants then performed a standardized maximal graded exercise test (e.g., Bruce Protocol) on a treadmill to determine their current cardiorespiratory fitness (e.g., peak oxygen consumption, ventilation, heart rate, rate of perceived exertion). The speed and incline of the treadmill were increased every 3 min until the participant reached volitional fatigue. The test was terminated at the participant’s request, at observed signs or symptoms that warranted test termination, at 85% of age-predicted maximal HR, or attainment of a respiratory exchange ratio (RER) of 1.15 or greater. Based on the graded exercise test, participants’ oxygen consumption reserve (VO2R) was used to calculate the work rate equating to the treadmill speed and grade for the subsequent experimental sessions.
2.2. Participants
Fourteen participants (8 females and 6 males) were classified as having moderate stages of CKD (stages G3a and G3b; estimated glomerular filtration rate (eGFR) between 59 and 30 mL/min/1.73 m2) and were recruited to participate in this study. eGFR was determined by using the modification of diet in renal disease (MDRD) formula and verified by a baseline blood sample during recruitment, along with voluntary consent to release a previous blood profile record to further confirm CKD stage. The sample size needed for this study was determined to be a minimum of 14 participants. An a priori power analysis was performed using a two-tail test with α = 0.05, an effect size of 0.80, and power set at 90% for anticipated differences in concentrations of omentin-1 (G*Power 3.1.9.7, Universität Kiel, Germany) [15]. Participants were eligible to participate in this study if they were (1) aged between 40 and 75, (2) overweight or moderately obese (body mass index (BMI) between 25 and 35 kg/m2), (3) physically active (>90 min of physical activity per week in the previous 3 months), (4) non-smoker, (5) free from cardiovascular or pulmonary complications that would inhibit safe exercise in a non-medical setting, and (6) no musculoskeletal injuries that would inhibit walking or jogging on a treadmill.
2.3. Baseline Demographics
Participants’ height and weight were measured using a calibrated stadiometer scale (Seca, Chino, CA, USA). Recorded measurements were rounded to the nearest 0.10 cm or 0.01 kg. Body composition parameters (i.e., fat mass (FM), fat-free mass (FFM), body fat percentage (BFP)) were taken using a DEXA [27]. Blood pressure was manually obtained using a standard sphygmomanometer (American Diagnostic Corporation, Hauppauge, NY, USA). Heart rate was recorded using the Polar H7 heart rate monitor (Polar, Bethpage, NY, USA). Both measures were recorded in a seated resting position by experienced exercise physiologists.
2.4. Exercise Protocol
Experimental data collection was conducted on two consecutive days and was preceded by 48 h of physical inactivity, standardized use of medication, and the consumption of a stable diet with no use of supplements. In a randomized crossover design fashion, participants completed two conditions on a treadmill (30 min per condition; matched workloads) after a 5 minute warmup of a self-selected walking pace: (1) SSE at 60–65% of participants’ VO2R and (2) HIIE for 3 min at 90% VO2R separated by 2 min of slow walking (20% VO2R) [6,28]. The HIIE session began with 60–65% of VO2R for 2.5 min and then transitioned into five high (90%) and five low (20%) intervals, ending with another 2.5 min of 60–65% (See Figure 1). The speed and incline were based on predetermined values from the Bruce protocol performed during the preliminary visit.
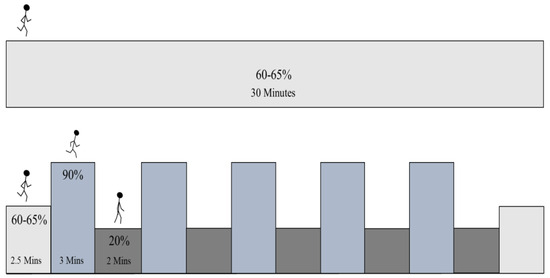
Figure 1.
Exercise protocols. Steady-state exercise (SSE) was set at 60–65% of VO2R. High-intensity interval exercise (HIIE) set at 90% of VO2R for 3 min for the upper limit and 20% of VO2R for 2 min.
2.5. Blood Sample Procedures
Venous blood samples were taken at baseline and the following exercise testing sessions (e.g., baseline, 1 h post exercise, 24 h post exercise). Serum samples were allowed to clot for 10 min at room temperature, followed by centrifugation at 3330× g RPM for 10 min. Plasma samples were collected via K-EDTA (purple top) tubes and then centrifuged. All samples were aliquoted into 2 mL microtubules and stored in an ultra-low temperature freezer at −80 °C. A single serum sample was sent to a CLIA-certified lab (Quest Diagnostics, Secaucus, NJ, USA) to evaluate basic biomarkers (e.g., metabolic and lipid profile, complete blood count) and to ensure patients were classified as having moderate stages of chronic kidney disease.
2.6. Biochemical Analyses
All biochemical analyses were performed on plasma samples following cessation of the study. Adipokine concentrations were assessed in duplicate using enzyme-linked immunosorbent assays (ELISA) with a microplate reader (iMark, Bio-Rad, Hercules, CA, USA) and the corresponding data reduction software (Microplate Manager, Bio-Rad, Hercules, CA, USA). Several adipokines were measured in this study, including adiponectin (RayBiotech Life, Inc., Peachtree Corners, GA, USA), leptin (RayBiotech Life, Inc., Peachtree Corners, GA, USA), and omentin-1 (MyBioSource, Inc., San Diego, CA, USA). ELISA kits had intra-assay coefficient of variabilities of 4%, 3%, and 1%, respectively.
2.7. Statistical Analysis
Data collected from this study were analyzed using SPSS Version 28 (IBM SPSS, Chicago, IL, USA). All data were assessed for normality prior to conducting statistical tests. A paired sample t-test showed there were no sex differences within our participants (Table 1). Subsequently, a two-by-three repeated measures ANOVA (Condition X Time) was employed to assess significant interactions in adipokine concentrations (e.g., adiponectin, leptin, omentin-1) between SSE and HIIE and several time intervals (e.g., baseline, 1 h, 24 h) following the bout of aerobic exercise. In addition, a two-by-three repeated measures ANOVA (Gender X Condition X Time) was performed to determine gender differences. If any significant interactions or main effects were found, a pairwise comparisons test with a Bonferroni correction was used to assess mean differences. The level of significance was set at α = 0.05.

Table 1.
Participant demographics.
3. Results
All demographic, anthropometric, and biochemical measurements can be seen in Table 1 and Table 2. The paired samples t-test showed there were no differences between sexes. Moreover, a two-by-three repeated measures ANOVA demonstrated there were no differences in adiponectin, leptin, and omentin-1 concentrations between either acute aerobic exercise conditions (e.g., SSE, HIIE) (See Figure 2). No significant differences were seen at baseline, 1 h post-exercise, and 24 h post-exercise (Table 2) for either condition. Likewise, no significant interactions were seen between time points and conditions. There were no gender differences observed between conditions and time.

Table 2.
Differences in adipokine concentrations between SSE and HIIE.
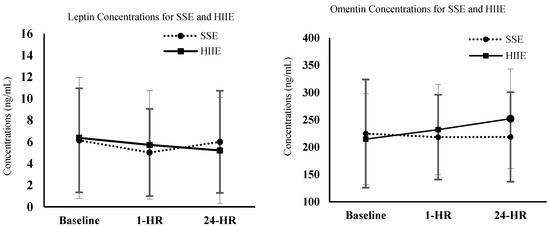
Figure 2.
Concentrations of leptin and omentin for SSE and HIIE at baseline, 1 h, and 24 h post exercise.
4. Discussion
This study aimed to identify the differences seen in adipokine concentrations between SSE and HIIE in patients with mid-spectrum CKD. Based on the results of this study, there were no acute changes seen in adiponectin, leptin, or omentin-1 concentrations in either modality of acute aerobic exercise (e.g., SSE, HIIE).
Several studies have previously shown adiponectin may respond to acute exercise bouts in healthy and athletic populations [25,29]. In one study conducted on 10 male elite rowers, participants rowed maximally for a total of 6000 m (~20 min), and plasma adiponectin concentrations were sampled. The adiponectin concentrations of these rowers significantly rose 30 min following the bout of aerobic exercise [29]. On the contrary, another study performed on nine overweight males, determined there were no changes in adiponectin (e.g., immediately after, 24 h, 48 h) after performing a 45-minute submaximal bout of aerobic exercise (65% VO2max). The results of our study demonstrated no changes in adiponectin in either SSE or HIIE in moderate-stage CKD patients. This adds additional support to the scarce literature on acute changes in adiponectin from different intensities of acute bouts of aerobic exercise.
Some studies have also examined acute changes in leptin from bouts of aerobic exercise [25,30,31]. Jürimäe and Jürimäe [30] conducted a study on 13 collegiate male rowers, who performed a 30-min maximal rowing ergometer assessment. They assessed plasma leptin levels preceding the exercise bout and found that there was a positive change (significant decrease) in leptin. The authors concluded that leptin may be sensitive to short-term intense exercise [30]. Likewise, in a study assessing 12 inactive subjects running for 20 min at 70% VO2max, leptin levels decreased following the exercise bout [31].
While these studies show positive changes in leptin levels from short-term bouts of aerobic exercise, some have shown no effect on leptin following aerobic exercise [25,32]. One study in particular examined 23 obese women who performed a 45-minute walking session at 60–80% of maximum heart rate. At the end of the initial walking session, plasma leptin levels remained unchanged [32]. The leptin concentrations from our study also saw no significant changes in leptin concentrations between either SSE (60–65% VO2R) and HIIE (90% VO2R). This potentially identifies a need to examine if there are certain intensities, in combination with specific durations, that are required to affect leptin concentrations on an acute level.
While the more commonly assessed adipokines are adiponectin, leptin, visfatin, resistin, and TNF-α, there are more novel anti- and pro-inflammatory adipokines that have recently been examined in terms of resistance training exercises. More specifically, omentin-1 has shown anti-inflammatory properties when performing resistance training in healthy individuals and aerobic training in male smokers [33,34]. While our study did not show statistical significance, omentin concentrations appeared to trend upward 24 h post HIIE compared to baseline and SSE. To our knowledge, this is the first study to assess changes in omentin-1 concentrations in patients with moderate stages of CKD. If future studies aim to examine this further, it may be interesting to identify if performing longer durations of HIIE would have protective benefits towards different populations through an upregulated release of the anti-inflammatory adipokine, omentin-1.
There was an interesting finding from this study that was seen in the changes of adipokines based on the examined population. In our study, we demonstrated there were no changes in adipokine concentrations (e.g., adiponectin, leptin, and omentin-1) from acute bouts of aerobic exercise in a sample of patients with CKD who are also considered obese. Similarly, various studies have reported there were no changes in adipokines concentrations in obese populations performing acute bouts of aerobic exercise [32,35]. On the contrast, several studies in healthy, active populations showed there were positive changes in adipokine concentrations (e.g., adiponectin, leptin) from acute bouts of aerobic exercise [29,30,36]. This may imply the ability of adipose tissue and its secretion of adipokines could potentially respond to acute bouts of aerobic exercise differently in obese populations than in healthy populations. However, as previously discussed, Legakis et al. [31] assessed inactive adults (BMI: 26.1 ± 8 kg/m2) performing a 20-minute run at 70% VO2max and found that at the cessation of exercise there was a rapid decrease in leptin concentrations. While this is conflicting evidence towards this potential implication, to our knowledge this is the only study that was found to be in contradiction of the proposed theory. Thus, future research should aim to examine the differences between obese and healthy populations and how adipokines would respond to acute bouts of aerobic exercise.
Several limitations in this study should be taken into consideration when considering the results of this manuscript. First, our study only assessed three time points (e.g., baseline, 1 h, 24 h), and this may not be completely representative of what happened post-exercise. Future studies should aim at assessing more time points following the acute bouts of aerobic exercise. Moreover, this study only assessed differences between acute bouts (30 min) of HIIE and SSE. It is possible that this may not have been enough time to experience a response and a lengthier time-period for an acute bout of aerobic exercise may be more conducive towards eliciting a change in adipokine concentrations. Though our study was determined to be statistically powered, there potentially could be differences observed with a higher number of participants.
5. Conclusions
Various adipokines are involved in the systemic inflammation seen in patients with chronic kidney disease. This study effectively demonstrated there were no beneficial changes in adipokine levels of patients with mid-spectrum CKD from either HIIE or SSE. Future studies should aim at identifying other adipokines in CKD patients and their response to acute bouts of aerobic exercise training. In addition, future studies may be needed to examine differences in the adipokine response between obese and healthy populations when performing acute bouts of aerobic exercise.
Author Contributions
Conceptualization, J.S.F.; methodology, J.S.F.; software, J.S.F. and T.J.C.-L.; validation, J.S.F., T.J.C.-L. and D.W.; formal analysis, J.S.F., T.J.C.-L., D.W. and J.K.T.; investigation, J.S.F. and D.C.A.; resources, J.S.F. and J.K.T.; data curation, J.S.F. and D.C.A.; writing—original draft preparation, T.J.C.-L., D.W. and J.S.F.; writing—review and editing, J.S.F., T.J.C.-L., D.W., R.T., J.K.T. and L.K.F.; visualization, T.J.C.-L.; supervision, J.S.F.; project administration, J.S.F.; funding acquisition, J.S.F. and J.K.T. All authors have read and agreed to the published version of the manuscript.
Funding
Baylor University and Auburn University Internal Faculty Grant.
Institutional Review Board Statement
The Baylor University Institutional Review Board approved the research study (617014) with human subjects. To ensure the safety of all participants, the ethical guidelines of the 1975 Declaration of Helsinki were employed by all research personnel in accordance with the institution’s human research committee.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available upon request.
Acknowledgments
Thank you to all the participants for their willingness to be involved in the research project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharaf El Din, U.A.A.; Salem, M.M.; Abdulazim, D.O. Stop chronic kidney disease progression: Time is approaching. World J. Nephrol. 2016, 5, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Yang, H.-C.; Fogo, A.B. A perspective on chronic kidney disease progression. Am. J. Physiol. Renal Physiol. 2017, 312, F375–F384. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, Z.; Grandjean, P.W.; Forsse, J.S. Effects of Acute Exercise on Cardiac Autonomic Response and Recovery in Non-Dialysis Chronic Kidney Disease Patients. Res. Q. Exerc. Sport 2022, 94, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis—From experimental insights to the clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef]
- Forsse, J.S.; Papadakis, Z.; Peterson, M.N.; Taylor, J.K.; Hess, B.W.; Schwedock, N.; Allison, D.C.; Griggs, J.O.; Wilson, R.L.; Grandjean, P.W. The Influence of an Acute Bout of Aerobic Exercise on Vascular Endothelial Function in Moderate Stages of Chronic Kidney Disease. Life 2022, 12, 91. [Google Scholar] [CrossRef]
- Forsse, J.; Papadakis, Z.; Bane, A.; Marroquín, F.M.; Grandjean, P. Brachial Artery FMD and Endothelial Responses to High-Intensity Interval and Steady-State Moderate-Intensity Exercise. Int. J. Exerc. Sci. Conf. Proc. 2016, 2, 10. [Google Scholar] [CrossRef][Green Version]
- Miricescu, D.; Balan, D.G.; Tulin, A.; Stiru, O.; Vacaroiu, I.A.; Mihai, D.A.; Popa, C.C.; Enyedi, M.; Nedelea, A.S.; Nica, A.E.; et al. Impact of adipose tissue in chronic kidney disease development (Review). Exp. Ther. Med. 2021, 21, 1–8. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Scherer, P.E. Adipose tissue: From lipid storage compartment to endocrine organ. Diabetes 2006, 55, 1537–1545. [Google Scholar] [CrossRef]
- Das, E.; Moon, J.H.; Lee, J.H.; Thakkar, N.; Pausova, Z.; Sung, H.-K. Adipose Tissue and Modulation of Hypertension. Curr. Hypertens. Rep. 2018, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Freitas Lima, L.C.; de Braga, V.A.; de França Silva, M.d.S.; de Cruz, J.C.; Sousa Santos, S.H.; de Oliveira Monteiro, M.M.; de Balarini, C.M. Adipokines, diabetes and atherosclerosis: An inflammatory association. Front. Physiol. 2015, 6, 304. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P. The role of adipokines in chronic inflammation. ImmunoTargets Ther. 2016, 5, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Watanabe-Kominato, K.; Takahashi, Y.; Kojima, M.; Watanabe, R. Adipose Tissue-Derived Omentin-1 Function and Regulation. Compr. Physiol. 2017, 7, 765–781. [Google Scholar] [CrossRef]
- Wilms, B.; Ernst, B.; Gerig, R.; Schultes, B. Plasma Omentin-1 Levels are Related to Exercise Performance in Obese Women and Increase Upon Aerobic Endurance Training. Exp. Clin. Endocrinol. Diabetes 2015, 123, 187–192. [Google Scholar] [CrossRef]
- Pereira, S.S.; Alvarez-Leite, J.I. Adipokines: Biological functions and metabolically healthy obese profile. J. Recept. Ligand Channel Res. 2014, 7, 15–25. [Google Scholar] [CrossRef]
- Chapman-Lopez, T.; Wilburn, D.; Fletcher, E.; Adair, K.; Ismaeel, A.; Heileson, J.; Gallucci, A.; Funderburk, L.; Koutakis, P.; Forsse, J.S. The influence of resistance training on adipokines in post-menopausal women: A brief review. Sports Med. Health Sci. 2022, 4, 219–224. [Google Scholar] [CrossRef]
- Golbidi, S.; Laher, I. Exercise induced adipokine changes and the metabolic syndrome. J. Diabetes Res. 2014, 2014, 726861. [Google Scholar] [CrossRef]
- Kashino, I.; Nanri, A.; Kurotani, K.; Akter, S.; Yasuda, K.; Sato, M.; Hayabuchi, H.; Mizoue, T. Association of dietary patterns with serum adipokines among Japanese: A cross-sectional study. Nutr. J. 2015, 14, 58. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. NMCD 2014, 24, 929–939. [Google Scholar] [CrossRef]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of Exercise on Fatty Acid Metabolism and Adipokine Secretion in Adipose Tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Park, S.C.; Kang, S. Effects of resistance exercise on adipokine factors and body composition in pre- and postmenopausal women. J. Exerc. Rehabil. 2019, 15, 676–682. [Google Scholar] [CrossRef]
- Parastesh, M.; Alibakhshi, E.; Saremi, A.; Shavandi, N. The effect of aerobic exercise training on leptin and pulmonary function tests during weight loss in men with visceral obesity. J. Shahrekord Univ. Med. Sci. 2020, 22, 96–101. [Google Scholar] [CrossRef]
- Marshall, J.A.; Grunwald, G.K.; Donahoo, W.T.; Scarbro, S.; Shetterly, S.M. Percent body fat and lean mass explain the gender difference in leptin: Analysis and interpretation of leptin in Hispanic and non-Hispanic white adults. Obes. Res. 2000, 8, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Bouassida, A.; Chamari, K.; Zaouali, M.; Feki, Y.; Zbidi, A.; Tabka, Z. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br. J. Sports Med. 2010, 44, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Lehr, S.; Hartwig, S.; Sell, H. Adipokines: A treasure trove for the discovery of biomarkers for metabolic disorders. Proteom. Clin. Appl. 2012, 6, 91–101. [Google Scholar] [CrossRef]
- Forsse, J.S.; Richardson, K.A.; Chapman-Lopez, T.J.; Torres, R.; Heileson, J.L.; Ismaeel, A.; Funderburk, L.; Gallucci, A.R.; Allison, D.C.; Koutakis, P. The Utilization of Body Composition to Predict Cardiorespiratory Fitness and Determine Association with CKD Stage in Individuals with Mid-Spectrum CKD: A Pilot Study. Kidney Dial. 2023, 3, 265–273. [Google Scholar] [CrossRef]
- Forsse, J.S.; Peterson, M.; Papadakis, Z.; Taylor, J.K.; Hess, B.W.; Schwedock, N.; Allison, C.D.; Griggs, J.O.; Wilson, R.L.; Grandjean, P.W. The Effect of Acute Aerobic Exercise on Biomarkers of Renal Health and Filtration in Moderate-CKD. Res. Q. Exerc. Sport 2022. [Google Scholar] [CrossRef]
- Jürimäe, J.; Purge, P.; Jürimäe, T. Adiponectin is altered after maximal exercise in highly trained male rowers. Eur. J. Appl. Physiol. 2005, 93, 502–505. [Google Scholar] [CrossRef]
- Jürimäe, J.; Jürimäe, T. Leptin responses to short term exercise in college level male rowers. Br. J. Sports Med. 2005, 39, 6–9. [Google Scholar] [CrossRef]
- Legakis, I.N.; Mantzouridis, T.; Saramantis, A.; Lakka-Papadodima, E. Rapid decrease of leptin in middle-aged sedentary individuals after 20 minutes of vigorous exercise with early recovery after the termination of the test. J. Endocrinol. Investig. 2004, 27, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Sari, R.; Balci, M.K.; Balci, N.; Karayalcin, U. Acute effect of exercise on plasma leptin level and insulin resistance in obese women with stable caloric intake. Endocr. Res. 2007, 32, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Atashak, S.; Stannard, S.R.; Daraei, A.; Soltani, M.; Saeidi, A.; Moradi, F.; Laher, I.; Hackney, A.C.; Zouhal, H. High-intensity Interval Training Improves Lipocalin-2 and Omentin-1 Levels in Men with Obesity. Int. J. Sports Med. 2022, 43, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Heidarianpour, A.; Tavassoli, H. Aerobic Exercise Training Effects on Omentin-1, Insulin Resistance, and Lipid Profile Among Male Smokers. Res. Q. Exerc. Sport 2022, 94, 880–885. [Google Scholar] [CrossRef]
- Jamurtas, A.Z.; Theocharis, V.; Koukoulis, G.; Stakias, N.; Fatouros, I.G.; Kouretas, D.; Koutedakis, Y. The effects of acute exercise on serum adiponectin and resistin levels and their relation to insulin sensitivity in overweight males. Eur. J. Appl. Physiol. 2006, 97, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, R.R.; Aboudehen, K.S.; Carruth, A.K.; Durand, R.T.J.; Acevedo, E.O.; Hebert, E.P.; Johnson, L.G.; Castracane, V.D. Adiponectin responses to continuous and progressively intense intermittent exercise. Med. Sci. Sports Exerc. 2003, 35, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).