Abstract
Soybeans are vulnerable to drought and temperature increases potentially induced by climate change. Hydraulic dysfunction and stomatal closure to avoid excessive transpiration are the main problems caused by drought. The vulnerability of soybeans to drought will depend on the intensity and duration of water stress. The purpose of this study was to determine if the use of cover crops (CCs) can influence the gas exchange potential of glyphosate-tolerant soybeans when the vapor pressure deficit (Vpd) increases. This two-year study was conducted in an open experimental field comprising direct seeding plots with or without CCs. Stomatal conductance (Gs) was measured five times on the same identified leaves following glyphosate-based herbicide application. These leaves were then collected in order to observe the stomata and foliar traits with a scanning electron microscope. The Vpd was calculated concomitantly to Gs measurements at the leaf surface. The results suggest that the use of CCs promotes phenotypic change in soybean leaves (more elaborate venation and a higher abaxial stomatal density), which in turn may enhance their tolerance to drier conditions. In 2019, Gs could be up to 29% higher in plots with CCs compared to those without CCs with similar Vpd values. This study shows that the benefits of using CCs can be observed via the morphological development strategies of the crop plants and their higher tolerance to drought.
1. Introduction
Over recent years, alternative cropping systems have been proposed to challenge the negative environmental impacts of conventional field cropping caused by intensive mechanical soil disturbance and the use of synthetic pesticides and fertilizers [1,2,3,4,5,6]. Direct seeding (DS) systems have been put forward to reduce mechanical tillage and more than 70% [7] of the incidence of soil erosion. DS systems allow for a reduction in carbon and nitrate leaching from soils or emissions to the atmosphere and maintaining soil organic carbon (SOC) and soil functions [5,8,9]. The use of glyphosate-based herbicides (GBH) in combination with glyphosate-tolerant plants has promoted the adoption of DS systems on a larger scale [8,9,10]. During 1998 and 2008, the DS area increased by 71.6% in soybean production in the United States of America [9]. Although DS systems aim to maintain SOC and soil functions, they tend to provoke surface soil compaction, in turn limiting water infiltration, seed germination, and the development of crop plants [5]. Reduced water infiltration into the soil can result in water limitation for crops, which can influence their physiological activities and their gas exchange potential [11,12]. Stomata present on leaves constitute the main sites for CO2 assimilation by the plants [13,14]. Stomata also play an important role in plant transpiration since the uptake of CO2 corresponds to water release, i.e., a significant trade-off for the metabolic management of the plant [12,15]. Along with climate change, the occurrence of drier periods will be more frequent and will have a considerable influence on the water content of soils during key periods of crop plant growth.
Direct seeding on cover crops (DSCC) implies implementing catch crops between field crop production periods or intercropping during the field crop production periods [16,17]. The addition of cover crops (CCs) may bring agronomic benefits such as limiting surface soil compaction, increasing soil porosity and water infiltration, and limiting soil water evaporation [16,18,19,20,21]. We hypothesize that CCs can influence gas exchange potential and enable plants to maintain their activity during stressful drought episodes. We also hypothesize that plants from plots without CCs will be more sensitive to an increase in Vpd, which will induce stomatal closing and reduce stomatal conductance. C3 plants such as soybeans are sensitive to abiotic factors such as temperature, air humidity, and light intensity, in turn influencing the gas exchange potential that contributes to water management and photosynthesis of the plant [12,22]. In the coming years, climate change will have strong repercussions on the temperature and air humidity that will cause more frequent and longer drought periods [23,24]. It has been reported that the increase in vapor pressure deficit (Vpd) has a negative impact on field crop production [23,24,25]. It is estimated that an increase in Vpd will reduce crop yields by more than 30% over the next 50 years, with a more drastic impact from 2050 onwards [25]. Vpd represents the difference between the amount of water vapor that air can contain at saturation and the actual vapor pressure in the air. Vpd can thus be considered as a direct measurement of the atmospheric desiccation power, an important factor influencing plants productivity and enhancing evapotranspiration and sensitivity to other abiotic stressors [26,27]. Because Vpd is highly dependent on temperature, it will increase with global warming and thus raise questions regarding field crops’ water management. To our knowledge, few studies have yet reported the influence of CCs on stomatal conductance while Vpd values are raised. It has been observed that higher Vpd values seemed to have a particular influence on soybean stomatal conductance [28]. This 1-year study also pointed out the potential mitigation effect of CCs on crop plants experiencing higher Vpd values [28]. However, there is an urgent need to understand the processes that may impact cash crop production and to put in place practices that will increase the plasticity and resilience of crops in the face of future climate change. This study aims to determine if the use of CCs in row crops may represent a clue for reducing soybean sensitivity to variation in Vpd and to drought periods. In addition, it also aims at integrating physiological activity data by complementing them with leaf drought tolerance traits data (ex. foliar size and vein architecture).
2. Materials and Methods
2.1. Description of the Experimental Design
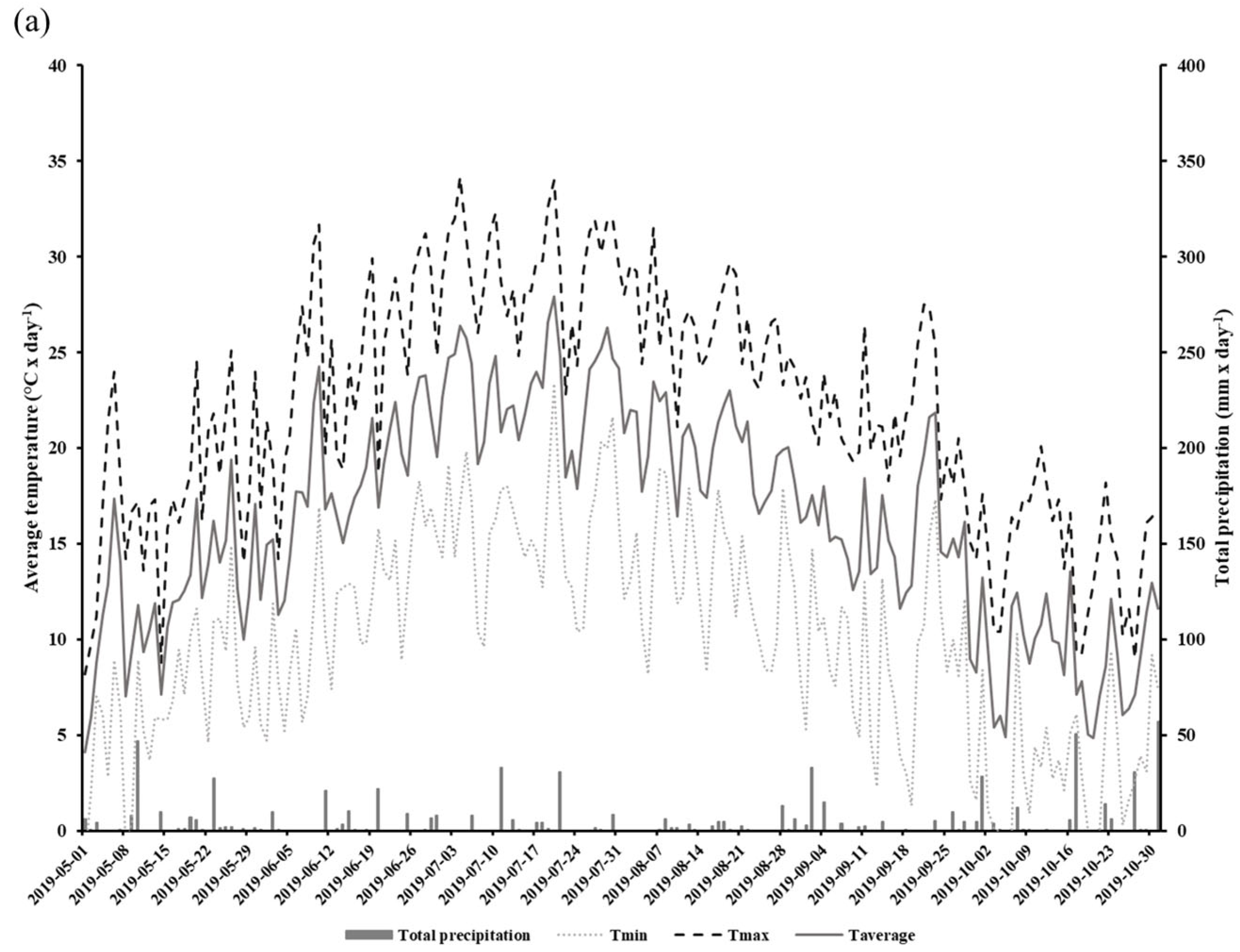
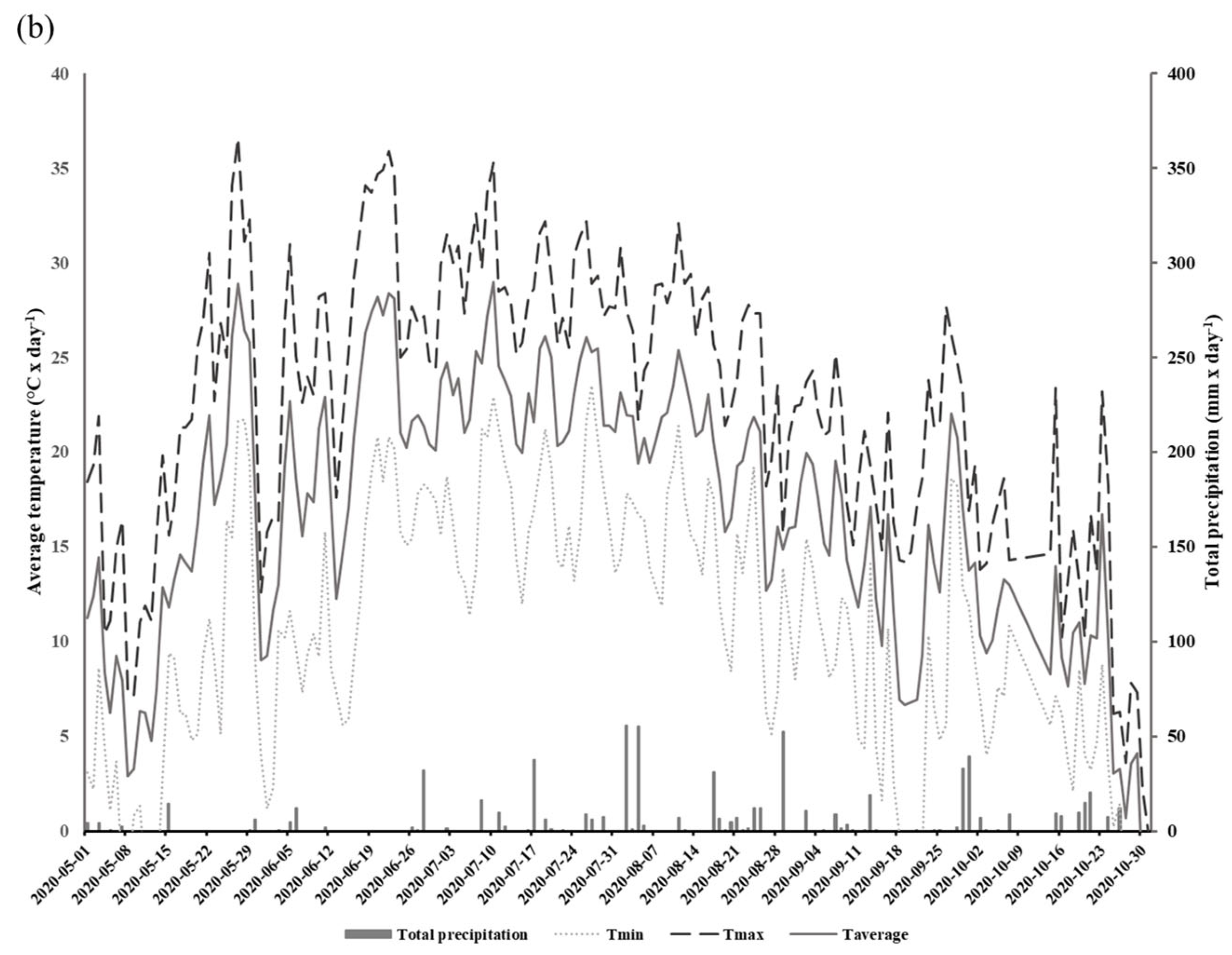
We conducted a two-year field study aimed at determining how the implementation of CCs may influence the gas exchange potential of glyphosate-tolerant soybeans in DS systems. Experiments were conducted in 2019 and 2020 in an open field located at the Grain Research Center (CEROM) in St-Mathieu-de-Beloeil (Quebec, Canada, 45.5828 N, −73.2374 W). Soil cores collected with an auger were taken from the experimental site in order to obtain a characterization of the soils prior to the implementation of the different plots. The experimental plots were established in 2018 on a humic Gleysol (AAFC, 1998) with a heavy clay texture (average and standard deviation percentage of clay: 72.6 0.9%, loam: 27.4 ± 0.9%, and sand: 0%). The soil mineral total content of the 0–20 cm horizon is presented in Table 1. The Mehlich-3 extraction method was used to obtain the metal element contained [29]. The size of each plot was 9 m × 20 m, and each treatment was replicated four times. The experimental design relied on the use of glyphosate-tolerant soybeans (Altitude R2®) on two direct seeding practices: direct seeding without CCs (DS) and with CCs (DSCC). The choice to work with glyphosate-tolerant soybeans is based on the desire to reproduce as closely as possible the conditions of direct seeding production in North and South America where this type of crops is widely used [30]. In addition, the choice of cultivar is based on the suggestions of agronomists from the Ministry of Agriculture, Fisheries and Food of Quebec (MAPAQ), and on regional specificities. In each plot, soybeans were seeded on the previous year’s corn residues at a rate of 90.8 kg ha−1, 18 May 2019 and 26 May 2020. During the 2018 autumn, winter rye (200 kg ha−1) was also sown as cover crops in those plots between annual productions. No cover crops were sown in the DS plots. Herbicide treatment (Roundup WheaterMax® with glyphosate a.i. at 540 g L−1) was applied twice at rates of 902 g ha−1 of glyphosate in DS and DSCC plots. The first application was conducted on 18 May 2019 and 2 June 2020. The second application was conducted post-emergence on 24 June 2019 and 3 July 2020 at the V2 soybean growth stage. Soybeans were harvested on 15 October 2019 and 31 October 2020. The field meteorological data, including total daily precipitation (mm) and minimum, maximum, and average daily temperatures, were recorded for the 2019 and 2020 production period with a weather station located on the CEROM main building (Figure 1a,b).

Table 1.
Soil mineral total content at the depth 0–20 cm of the experimental site.
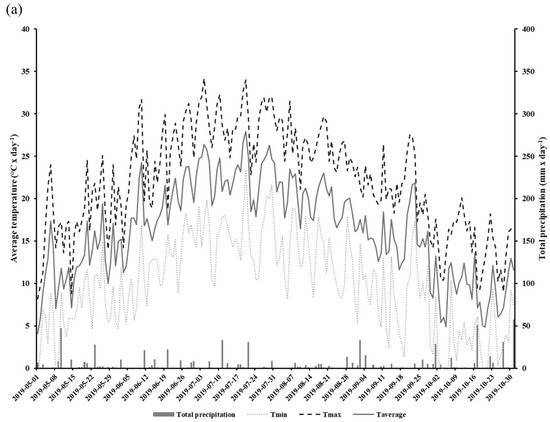
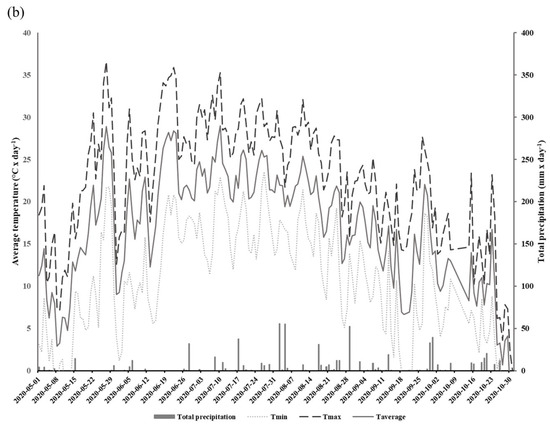
Figure 1.
Total daily precipitation (mm) and minimum, maximum, and average daily temperatures (°C) at the experimental field during the growth period (May to October) in 2019 (a) and 2020 (b).
2.2. Sampling and Measurements
Stomatal Conductance and Stomatal Traits
At each plot, stomatal conductance (Gs expressed as mmol m−2 s−1) was measured with a steady-state diffusion porometer (SC-1 Leaf porometer, Decacon Devices®, Pullman, WA, United-Sates) using one leaflet from three different plants similarly arranged and with initially the same growth stage (V2). All plants and leaves were identified in the V2 growth stage in order to follow the same plants and leaflets throughout the study period (from the V2 to the R2-R3 growth stages). The stomatal conductance was measured around midday on abaxial foliar surfaces during five field sampling campaigns (48 h and 7, 14, 21, and 28 days following the second GBH application). In situ calibration of the porometer was carried out on all sampling days before measurements began. The measurements began at 11 a.m. and ended around 2 p.m. Leaves’ temperature and air relative humidity were also recorded using a portable psychrometer (REED instrument©, model#8706) at the leaf surface. The corresponding Vpd at the leaf surface was calculated according to the August-Roche Magnus formula, where Vpd = 6.109417.625×T/T+243.04 [31].
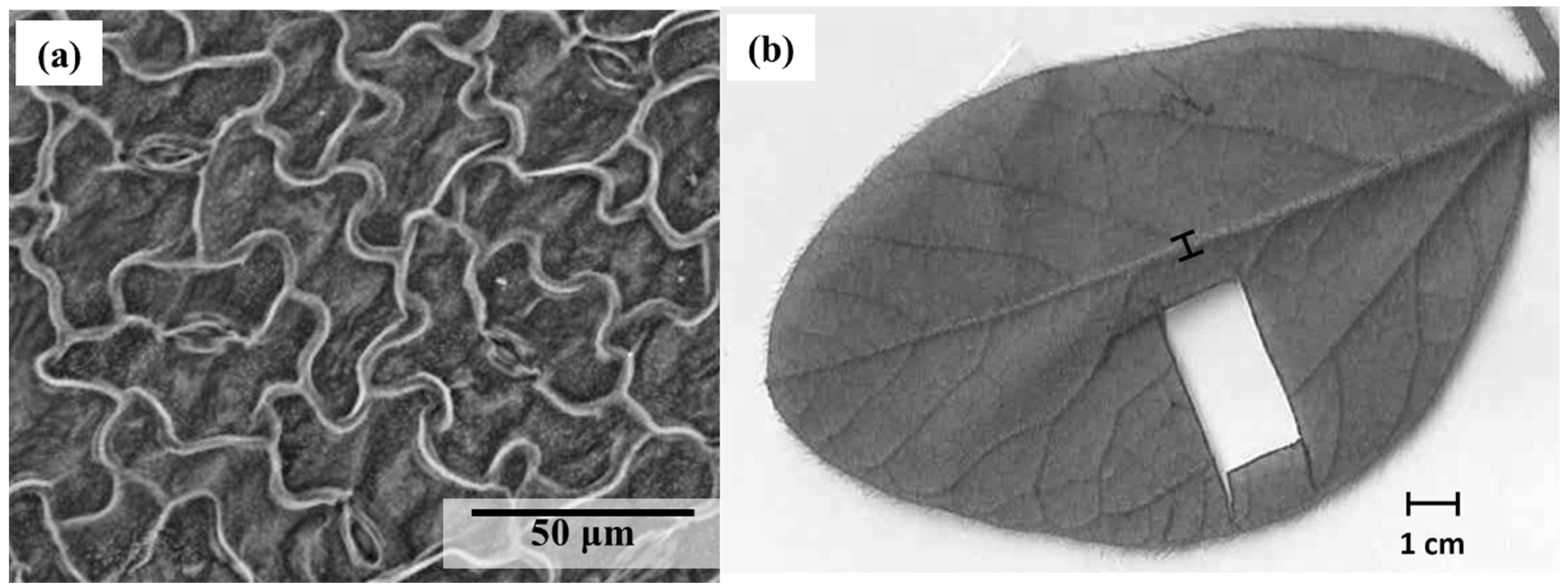
After measuring the stomatal conductance 28 days after the second GBH application, each identified leaf (R2-R3 growth stage) was collected in order to calculate the stomatal size, density, and index. On each leaf, three locations on one fresh leaflet were observed for the stomatal density (StoDen) calculation on the abaxial surface with a scanning electron microscope (Hitachi S-3400N, Hitachi®, Chiyoda, Tokyo, Japan) at a magnification of 400× (Figure 2a). The three observed locations came from a section of the fresh leaflet (±1 cm × 2.5 cm), which was mounted on a microscope slide using transparent double-sided adhesive tape (Figure 2b). Pictures of those observations with the SEM were taken, and the stomatal sizes (StoSize), width (StoWidth), and length (StoLength) were measured with ImageJ© software version 1.52v (National Institutes of Health, NIH). StoSize represents the total area of the stomata, StoWidth is the distance between the two opposite outer thin walls of the guard cell, and StoLength is the maximum length of the guard cell. The stomatal index (StoIndex) was calculated by multiplying the stomatal density by the stomatal size [32]. An average of these measurements was calculated following observation of the three locations on the abaxial surface, providing the information needed to interpret the results.
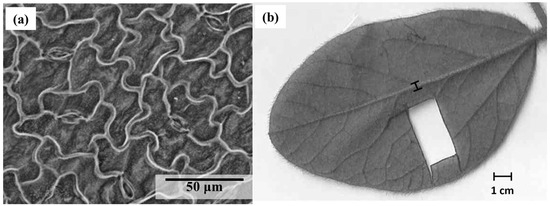
Figure 2.
Observation of (a) soybean stomata with a scanning electron microscope SEM at magnification of 400× and (b) leaflet trait with imagery software.
2.3. Foliar Trait
One of the leaflets from each leaf collected in the field for stomatal trait measurements (28 days after the second GBH application) was stored in a decolorizing solution (70% aqueous ethanol solution [15]. Once decolored, these leaves were dipped in a safranin solution (4% v/v) for 30 min or until we obtained sufficient staining of the foliar veins. This vein staining increased color contrast and allowed better accuracy of leaf trait measurements. The colored leaflets were then scanned, and the measurements were made using imagery software (ImageJ© software).
In this study, the distance between veins was used to obtain a proxy of the venation density [33]. An average of 11 measurements was taken between secondary veins for each leaflet. The midrib width and the leaf size of that leaflet was also measured with the imagery software (ImageJ© software) (Figure 2b)
2.4. Statistical Analyses
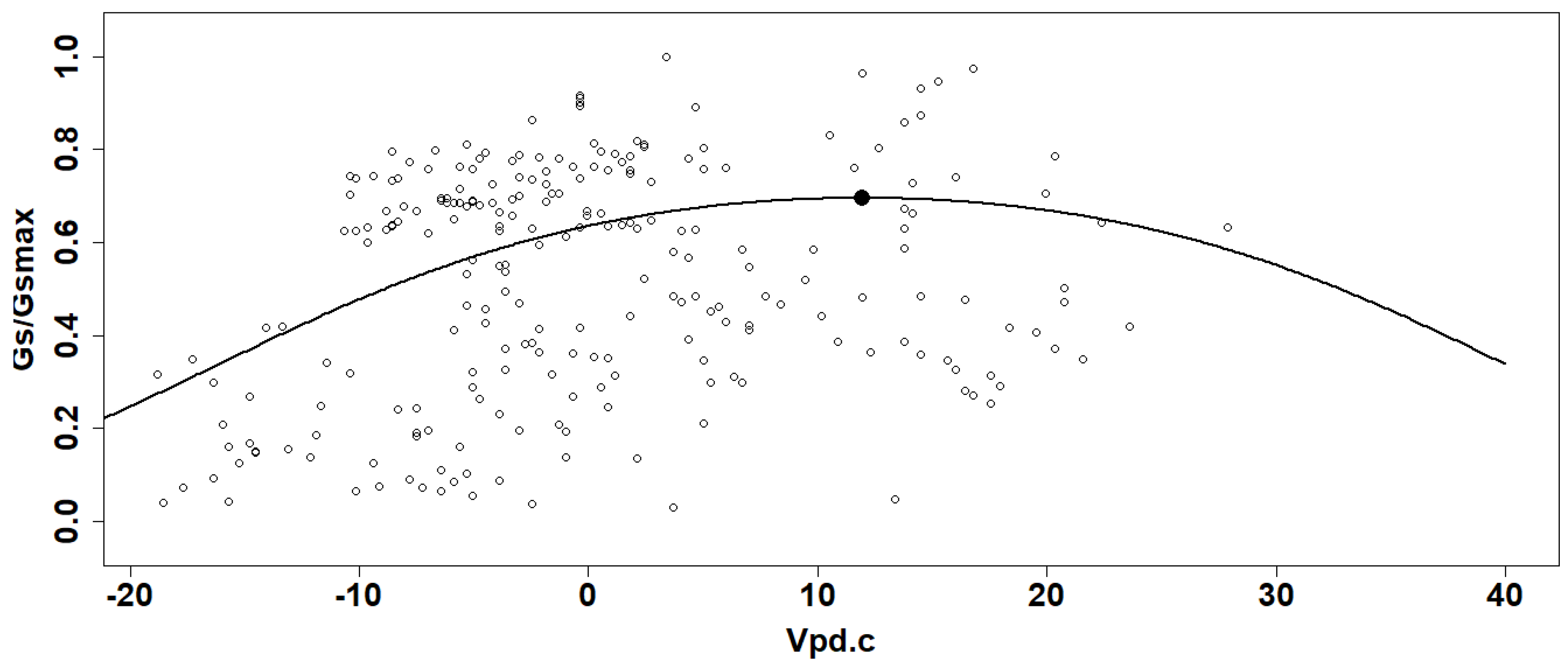
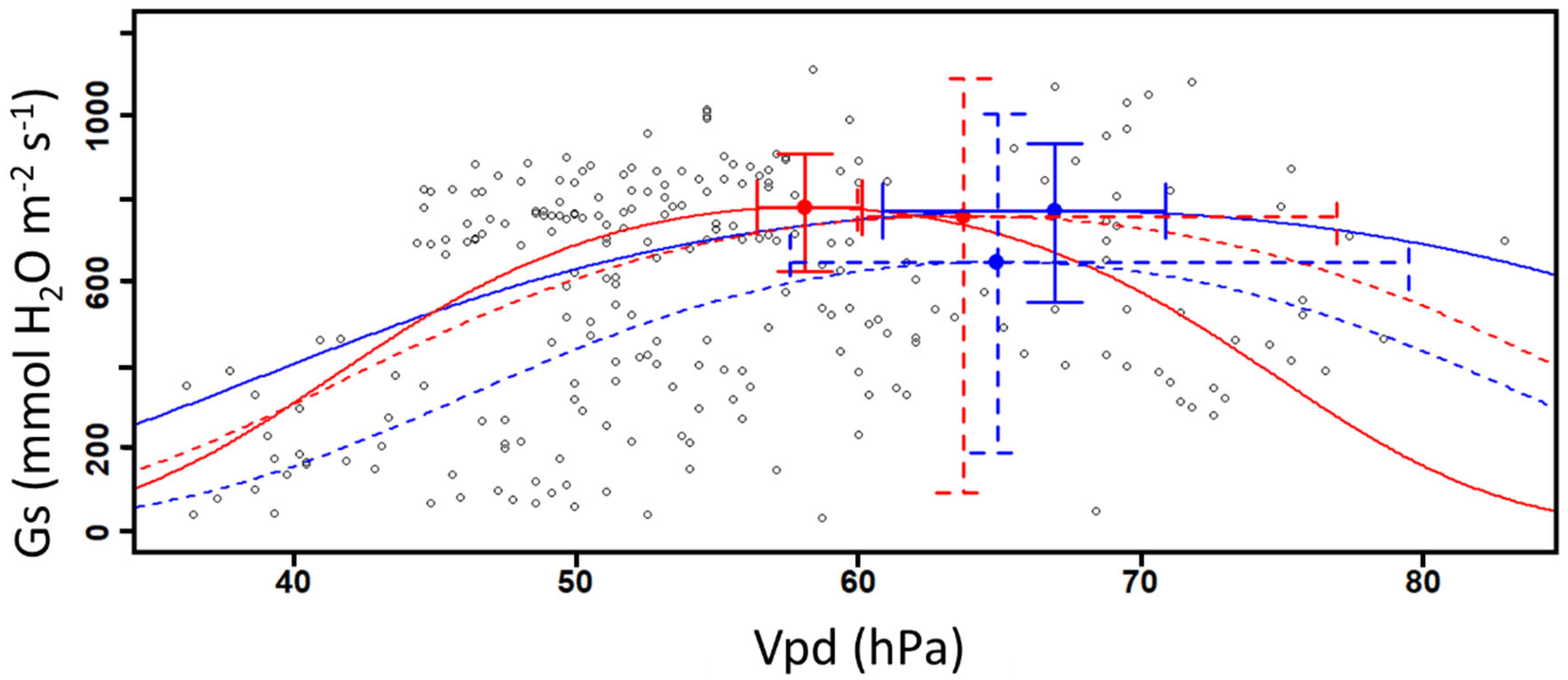
The Gs/Gsmax values from all plants and for both sampled years were used to obtain a generalized linear model (GLM) in function of the corresponding Vpd with beta distribution using a logit link function) (Figure 3) [15,28]. Here, Gsmax represents the highest Gs value measured during the two years, which was 1110.5 mmol m−2 s−1. The inflexion point was calculated for each curve and considered the optimal condition for gas exchange [15,28]. The Vpd values for these optimum points were calculated to determine if there is a difference in plant sensitivity to Vpd between DS systems with or without CCs. Figure 3 shows an example of the fitted curves from the GLM as a function of Vpd centered value for each system and for the years 2019 and 2020. Since the logit function has only one rising point of inflection, the optimal gas exchange points were calculated from the second derivative for each curve. The confidence interval (95% CI) was calculated for those points to take into consideration the interval on the values for the stomatal conductance (the y-axis interval) and the Vpd (the x-axis interval).
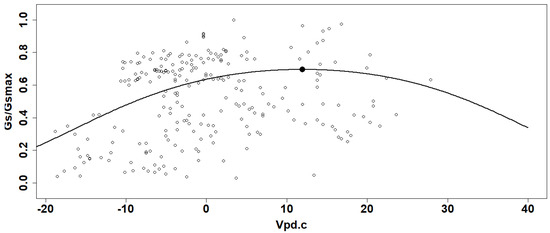
Figure 3.
Example of Gs/Gsmax data (curve and point of inflexion) generated by the general linear model in function on centered values of vapor pressure deficit (Vpd.c).
An ANOVA analysis was carried out to assess whether there is a significant difference (p ≤ 0.05) in Gs values between years for the stomatal traits and foliar traits from different cultivation systems. Also, a Chi-square test analysis was also carried out to evaluate the influence of both the year of production and the combination of year–agricultural management.
3. Results
3.1. Stomatal Conductance and Vapor Pressure Deficit
Combining DS and DSCC, significant differences in Gs between years are observed (p = 0.0006), where the means ± SE values were 635.15 ± 23.80 mmol m−2 s−1 for 2019 and 516.14 ± 24.51 mmol m−2 s−1 in 2020. On the opposite, the mean ± SE values of Vpd were significantly higher (p < 0.0001) in 2020 (58.68 ± 0.84 hPa) than in 2019 (51.33 ± 0.75 hPa).
By modeling the relation between Gs and the rising values of Vpd, we observe that the calculated inflexion points from Gs values in 2019 are similar between DS and DSCC plots (Figure 4 and Table 2). However, we observed a significant difference in 2019 between DSCC and DS Vpd values based on the 95% confidence interval (Figure 4 and Table 2), where DSCC has similar Gs values at higher Vpd values. No difference is observed in the calculated inflexion points from Gs values and on Vpd values in 2020 between DSCC and DS. The large variation around the inflexion points values in 2020 does not allow us to observe the difference with 2019.
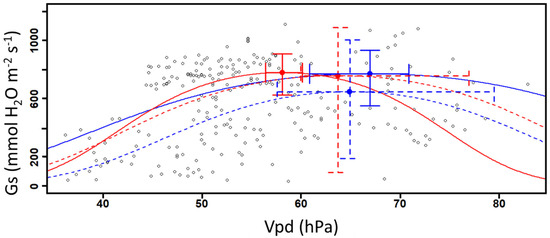
Figure 4.
Abaxial stomatal conductance as a function of raising vapor pressure deficit in soybeans (n = 240). The curves represent the DS (red) and DSCC (blue) plots for 2019 (solid line) and 2020 (dashed line). The optimal points are defined here as the mean Gs and Vpd values with their associated confidence interval (95% CI).

Table 2.
Calculated optimal gas exchange points following glyphosate-based herbicide application on soybean fields with DSCC and DS plots.
3.2. Stomatal Traits
Abaxial stomatal traits analysis (Table 3) shows that StoDen is significantly higher in DSCC plots compared to DS ones in both 2019 (p = 0.0581) and 2020 (p = 0.0247). StoLength, StoWidth, and StoSize have higher values in DS plots in 2019 when compared to DSCC, but no significant difference between the systems was observed in 2020. In the case of StoIndex, no significant difference between DS and DSCC is observed for either year. However, different results on the stomatal traits are observed between both years, where StoDen and StoIndex values for both systems are lower in 2020 compared to 2019. StoWidth and StoSize are significantly higher in DS plots in 2019 than in 2020, whereas no significant differences in those traits are observed in DSCC plots between 2019 and 2020.

Table 3.
Soybean stomatal traits measurements in direct seeding on cover crops (DSCC) and direct seeding (DS) for the years 2019 and 2020.
3.3. Foliar Traits
No difference in the leaf size values between DSCC and DS was observed in 2019 and 2020 (Table 4). Also, the average leaf sizes are similar between years. Concerning the midrib width, significant differences exist between DSCC and DS in 2019 but not in 2020 (Table 4). In 2019, the midrib width values were higher in DSCC compared to DS plots. Also, significantly smallest DistVein values are observed in DSCC for the years 2019 and 2020.

Table 4.
Soybean foliar traits measurements in direct seeding on cover crops (DSCC) and direct seeding (DS) for the years 2019 and 2020.
4. Discussion
The observed correlation between Vpd and Gs confirms that Vpd influences the physiological activity of plants, which is consistent with other publications [12,27,28]. Here, a positive relation between Vpd and Gs is observed until the gases exchange potential reaches an inflexion point defined as an optimal point (Figure 4). Once this value is reached, Gs values decrease along with higher Vpd values. Higher Vpd values promote the ascension of water in the xylem, enhancing water accumulation in the sub-stomatal cavities and its exit through plant transpiration [12,35]. The decreasing Gs values observed in this study can be explained by the fact that plants close their stomata in order to limit excessive water loss when their ambient environment becomes drier (higher Vpd values) [12,15,28]. Here, it was not possible to observe significantly higher abaxial Gs values in plants present in plots without CCs compared with those in plots with CCs, which invalidates our first hypothesis. However, DSCC plots in 2019 appear less sensitive to Vpd while plants maintained their physiological activities and stomata opening during less favorable periods (i.e., drought and hydric stress episodes). This result corroborated our second hypothesis, which stated that plants from plots without plant cover would be more sensitive to an increase in Vpd. Gs values can be up to 29% higher in DSCC plots compared to DS ones for the same Vpd values in 2019 (Figure 4 and Table 2).
Interestingly, we are able to observe differences in morphological traits of plants grown in DS and DSCC plots (Table 3 and Table 4). This observation corroborates other research, which proposed that a difference in physiological activity and gas exchange can be explained by different leaf morphological traits. Those differences in leaves can be explained by morphological plasticity to optimize plant performance according to growth conditions [36,37,38,39]. These morphological differences can occur in stomata and other foliar traits, such as the main vein structure [36,40]. The stomatal activity could be explained by the stomata density, considering that it can be positively correlated with stomatal conductance [12,13,22,41,42,43,44,45]. StoDen is a good drought proxy and is an indicator of the strategy that plants adopt to develop their stomata while reducing stressful conditions [12,28,44,46]. These strategies closely influence the number and size of stomata, which, in combination, represent the potential for foliar epidermal cells’ surface allocation for gas exchange and optimal stomatal conductance potential [37]. In this study, the epidermal cells surface allocation for gas exchange is represented by the StoIndex value, which shows no significant differences between agricultural management and years (Table 2). We observe that in 2019, the StoDen of soybeans growing in DSCC plots is higher than that of plants growing in DS plots (Table 3). This could explain the differences in stomatal behavior and gas exchange where DSCC StoDen values are significantly different from those for plants growing in DS plots in 2020 (p value = 0.0247) (Table 3). Moreover, the abaxial stomata of plants growing in DSCC plots were significantly smaller than those in DS plots (Table 3). This was expected, considering that it has been largely demonstrated that a negative relationship generally exists between stomatal size and number of stomata [37]. However, smaller and more numerous stomata allow soybeans to quickly adapt their stomatal aperture for optimal conductance or close them in order to avoid excessive transpiration [28,37,47]. This can be a significant short-term advantage, especially for crops that have to react quickly without allocated epidermal cells for the development of new stomata. For short-lived crops like soybeans, plants tend to optimize resource acquisition by minimizing construction cost [39,48]. In the case of DSCC plants in 2019, a higher StoDen allowed physiological plasticity, which allows the maintenance of gas exchange in a context where Vpd values were higher.
In addition, the different stomatal traits on the abaxial surface between agricultural management can also be explained by the morphological differences of the foliar veins, i.e., another indicator of drought tolerance of the plants [40]. In our case, it was observed that soybeans growing in DSCC plots in 2019 had a significantly wider midrib and a significantly lower DistVein, which represents a higher venation density. Higher venation density can be an indicator of the willingness and resilience of the plants growing in plots with CCs during high Vpd or drought episodes [36]. A more elaborated venation may be linked to better water management [33]. Scoffoni et al. (2011) proposed that a large midrib and a small distance between secondary veins allow a more important number of stomata, which is also consistent with our observations. Higher major vein density would thus have a lower hydraulic vulnerability, allowing a larger number of stomata [40]. Also, the presence of CCs can have a positive influence on soil functions, which can explain the willingness to facilitate the phenological plasticity of plants in DSCC plots. It has been shown that CCs can increase the number and diversity of root systems in the field, which can improve soil porosity, aggregation, and fertility [18,19]. These soil functions can facilitate the accessibility and the uptake of water by crop plants, in turn favoring gas exchange and transpiration with less restriction.
5. Conclusions
This study suggests that CCs contribute to maintaining gas exchange potential in the context of soybeans exposed to higher Vpd values. The implementation of CCs would thus favor a higher resilience to the potential combined stress of drought and GBH application by increasing crop plasticity in glyphosate-tolerant soybean field crops. At similar Vpd values, the stomatal conductance on the abaxial leaf surface of plants growing in DSCC plots was significantly higher than that of plants growing in DS plots. This can be explained by a higher tolerance under conditions that can cause water limitation to plants. This tolerance is expressed by a more elaborate venation and higher StoDen in plants growing in DSCC plots. Via the response of the plants and their development strategy, the benefits of CCs on crops could be observed in the short term in this study. Finally, CCs seem to represent, in part, a sustainable solution to fight against drought and future climate changes. CCs also seem to be a promising alternative to minimize the reduction in the gas exchange of soybeans triggered by herbicide spraying during a drought period.
Author Contributions
Conceptualization, J.B.B. and M.L.; methodology, J.B.B., M.M. and M.L.; software, J.B.B.; validation, J.B.B., M.M. and M.L.; formal analysis, J.B.B.; investigation, J.B.B. and M.L.; resources, M.M. and M.L.; data curation, J.B.B.; writing—original draft preparation, J.B.B.; writing—review and editing, J.B.B., M.M. and M.L.; visualization, J.B.B. and M.L.; supervision, M.M. and M.L.; project administration, M.L.; funding acquisition, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Strategic Partnership Program by the Natural Sciences and Engineering Research Council of Canada (NSERC). The grant number is STPGP 506291-17.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in FigShare at https://doi.org/10.6084/m9.figshare.24431173.
Acknowledgments
We wish to acknowledge the implication of the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ), and Le Centre de recherche sur les grains (CEROM) on this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carlson, S.; Stockweel, R. Research priorities for advancing adoption of cover crops in agriculture-intensive regions. J. Agric. Food Syst. Community Dev. 2013, 3, 125–129. [Google Scholar] [CrossRef]
- DeLonge, M.S.; Miles, A.; Carlisle, L. Investing in the transition to sustainable agriculture. Environ. Sci. Policy 2016, 55, 266–273. [Google Scholar] [CrossRef]
- FAO; ITPS. Status of the World’s Soil Ressources; Food and Agricultue Organization of the United Nations: Rome, Italy, 2015; pp. 1–94. [Google Scholar]
- Magdoff, F. Ecological agriculture: Principles, practices, and constraints. Renew. Agric. Food Syst. 2007, 22, 109–117. [Google Scholar] [CrossRef]
- Triplett, G.B.; Dick, W.A. No-tillage crop production: A revolution in agriculture! Agron. J. 2008, 100, 153–166. [Google Scholar] [CrossRef]
- Oerke, E.-C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Lee, S.; Chua, M.L.; Guzmana, J.A.; Botero-Acostab, A. A comprehensive modeling framework to evaluate soil erosion by water and tillage. J. Environ. Manag. 2021, 279, 111631. [Google Scholar] [CrossRef]
- Kassam, A.; Friedrich, T.; Derpsch, R. Global spread of Conservation Agriculture. Int. J. Environ. Stud. 2019, 76, 29–51. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, C.; Hennessy, D.A.; Feng, H.; Tian, H. Impacts of tillage practices on soil carbon stocks in the US corn-soybean cropping system during 1998 to 2016. Environ. Res. Lett. 2020, 15, 014008. [Google Scholar] [CrossRef]
- Kassam, A.; Friedrich, T.; Derpsch, R. Successful experiences and lessons from conservation agriculture worldwide. Agronomy 2022, 12, 769. [Google Scholar] [CrossRef]
- Domec, J.-C.; Noormets, A.; Gavazzi, M.J.; Bogg, J.L.; King, J.S.; Sun, G.E.; Treasure, E.A. Decoupling the influence of leaf and root hydraulic conductances on stomatal conductance and its sensitivity to vapour pressure deficit as soil dries in a drained loblolly pine plantation. Plant Cell Environ. 2009, 32, 980–991. [Google Scholar] [CrossRef]
- Driesen, E.; Van den Ende, W.; De Proft, M.; Saeys, W. Influence of Environmental Factors Light, CO2, Temperature, and Relative Humidity on Stomatal Opening and Development: A Review. Agronomy 2020, 10, 1975. [Google Scholar] [CrossRef]
- Tanaka, Y.; Fujii, K.; Shiraiwa, T. Variability of leaf morphology and stomatal conductance in soybean [Glycine max (L.) Merr.] cultivars. Crop Sci. 2010, 50, 2525–2532. [Google Scholar] [CrossRef]
- Zeiger, E.; Farquhar, G.D.; Cowan, I.R. Stomatal Function. In The Evolution of Stomata; Stanford University Press: Redwood City, CA, USA, 1987. [Google Scholar]
- Krober, W.; Bruelheide, H. Transpiration and stomatal control: A cross-species study of leaf traits in 39 evergreen and deciduous broadleaved subtropical tree species. Trees 2014, 28, 901–914. [Google Scholar] [CrossRef]
- Hartwig, N.L.; Ammon, H.U. Cover Crops and Living Mulches. Weed Sci. 2002, 50, 688–699. [Google Scholar] [CrossRef]
- Woolford, A.R.; Jarvis, P.E. Cover, catch and companion crops: Benefits, challenges and economics for Uk growers. Agricology 2017, 1, 1–28. [Google Scholar]
- Amsili, J.P.; Kaye, J.P. Root traits of cover crops and carbon inputs in an organic grain rotation. Renew. Agric. Food Syst. 2021, 36, 182–191. [Google Scholar] [CrossRef]
- Liu, A.; Ma, B.L.; Bomke, A.A. Effects of cover crops on soil aggregate stability, total organic carbon, and polysaccharides. Soil Sci. Soc. Am. J. 2005, 69, 2041–2048. [Google Scholar] [CrossRef]
- Robertson, G.P.; Gross, K.L.; Hamilton, S.K.; Landis, D.A.; Schmidt, T.M.; Snapp, S.S.; Swinton, S.M. Farming for ecosystem services: An ecological approach to production agriculture. BioScience 2014, 64, 404–415. [Google Scholar] [CrossRef]
- Wagg, C.; van Erk, A.; Fava, E.; Comeau, L.-P.; Mitterboeck, T.F.; Goyer, C.; Li, S.; McKenzie-Gopsill, A.; Mills, A. Full-season cover crops and their traits that promote agroecosystem services. Agriculture 2021, 11, 830. [Google Scholar] [CrossRef]
- Roche, D. Stomatal conductance is essential for higher yield potential of C3 crops. Crit. Rev. Plant Sci. 2015, 34, 429–453. [Google Scholar] [CrossRef]
- Seager, R.; Hooks, A.; Parkwilliams, A.; Cook, B.; Nakamura, J.; Henderson, N. Climatology, variability, and trends in the U.S. vapor pressure deficit, an important fire-related meteorological quantity. J. Appl. Meteorol. Climatol. 2015, 54, 1121–1141. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Roberts, M.J.; Schlenker, W.; Braun, N.; Little, B.B.; Rejesus, R.M.; Hammer, G.L. Greater sensitivity to drought accompanies maize yield Increase in the U.S. midwest. Science 2014, 344, 516–519. [Google Scholar] [CrossRef]
- Grossiord, C.; Buckley, T.N.; Novick, K.A.; Poulter, B.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [PubMed]
- Ocheltree, T.W.; Nippert, J.B.; Prasad, P.V.V. Stomatal responses to changes in vapor pressure deficit reflect tissue-specific differences in hydraulic conductance. Plant Cell Environ. 2014, 37, 132–139. [Google Scholar] [CrossRef]
- Bernier Brillon, J.; Moingt, M.; Lucotte, M. Direct seeding under cover crops: A solution to optimize the potential for adaptation of transgenic field crops to water stress in a context of glyphosate exposure. J. Agric. Crop Res. 2022, 10, 85–97. [Google Scholar]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Sims, B.; Corsi, S.; Gbehounou, G.; Kienzle, J.; Taguchi, M.; Friedrich, T. Sustainable weed management for conservation agriculture: Options for smallholder farmers. Agriculture 2018, 8, 118. [Google Scholar] [CrossRef]
- Alduchov, O.A.; Eskridge, R.E. Improved Magnus form approximation of saturation vapor pressure. J. Appl. Meteorol. 1996, 35, 601–609. [Google Scholar] [CrossRef]
- Kim, L.; Balani, S.; Edelberg, M.; Macke, N. Effects of various environmental factors on stomatal density, area, and potential conductance index. J. Emerg. Investig. 2021, 4, 1–8. [Google Scholar] [CrossRef]
- Uhl, D.; Mosbrugger, V. Leaf venation density as a climate and environmental proxy: A critical review and new data. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1999, 149, 15–26. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Devi, J.; Shekoofa, A.; Choudhary, S.; Sadok, W.; Vadez, V.; Riar, M.; Rufty, T. Limited-transpiration response to high vapor pressure deficit in crop species. Plant Sci. 2017, 260, 109–118. [Google Scholar] [CrossRef]
- Carins Murphy, M.R.; Jordan, G.J.; Brodribb, T.J. Acclimation to humidity modifies the link between leaf size and the density of veins and stomata. Plant Cell Environ. 2014, 37, 124–131. [Google Scholar] [CrossRef]
- Franks, P.J.; Drake, P.L.; Beerling, D.J. Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density: An analysis using Eucalyptus globulus. Plant Cell Environ. 2009, 32, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Flexas, J.; Yu, T.; Peng, S.; Huang, J. Leaf anatomy mediates coordination of leaf hydraulic conductance and mesophyll conductance to CO2 in Oryza. New Phytol. 2017, 213, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, G.; Catoni, R.; Spoletini, A.; Varone, L.; Gratani, L. Short-term physiological plasticity: Trade-off between drought and recovery responses in three Mediterranean Cistus species. Ecol. Evol. 2017, 7, 10880–10889. [Google Scholar] [CrossRef] [PubMed]
- Scoffoni, C.; Rawls, M.; McKown, A.; Cochard, H.; Lawren Sack, L. Decline of leaf hydraulic conductance with dehydration: Relationship to leaf size and venation architecture. Plant Physiol. 2011, 156, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, H.; Zou, Y.; Wen, Y. Stomatal behaviors reflect enantioselective phytotoxicity of chiral herbicide dichlorprop in Arabidopsis thaliana. Sci. Total Environ. 2016, 562, 73–80. [Google Scholar] [CrossRef]
- Gaskell, M.L.; Pearce, R.B. Stomatal frequency and stomatal resistance of maize hybrids differing in photosynthetic capability. Crop Sci. 1983, 23, 176–177. [Google Scholar] [CrossRef]
- Qi, X.; Torii, K.U. Hormonal and environmental signals guiding stomatal development. BMC Biol. 2018, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Shiraiwa, T. Stem growth habit affects leaf morphology and gas exchange traits in soybean. Ann. Bot. 2009, 104, 1293–1299. [Google Scholar] [CrossRef]
- Tanaka, Y.; Shiraiwa, T.; Nakajima, A.; Sato, J.; Nakazaki, T. Leaf gas exchange activity in soybean as related to leaf traits and stem growth habit. Crop Sci. 2008, 48, 1925–1932. [Google Scholar] [CrossRef]
- Bernier Brillon, J.; Lucotte, M.; Tremblay, G.; Smedbol, E.; Paquet, S. Impacts of glyphosate-based herbicide on leaf stomatal density and biomass production of transgenic soybean (Glycine max [L.] Merr.) and corn (Zea mays L.). Acta Physiol. Plant. 2023, 45, 68. [Google Scholar] [CrossRef]
- Aasama, K.; Sober, A.; Rahi, M. Leaf anatomical characteristics associated with shoot hydraulic conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Aust. J. Plant Physiol. 2001, 28, 765–774. [Google Scholar] [CrossRef]
- Correia, O.; Ascensão, L. Summer semi-deciduous species of the Mediterranean landscape: A winning strategy of Cistus species to face the predicted changes of the Mediterranean climate. Plant Biodivers. Monit. Assess. Conserv. 2017, 195–217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).