Bacterial Inactivation by Common Food Industry Sanitizers
Abstract
1. Introduction
2. Materials and Methods
2.1. Efficacy of Different Sanitizers Used in the Food Industry
2.2. Bacterial Strains and Maintenance of Reference Microorganisms
2.3. Bacterial Suspension of Work
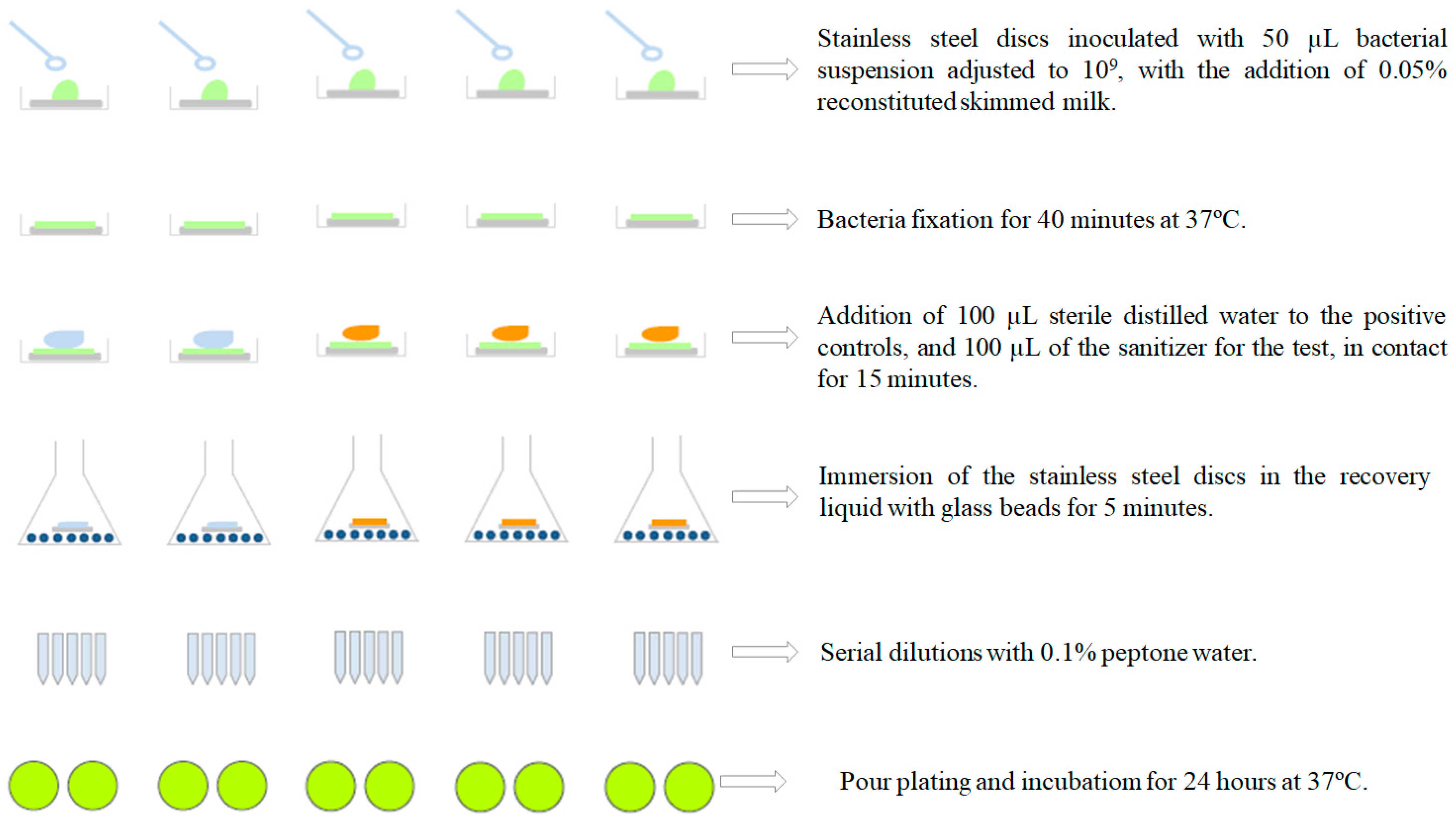
2.4. Evaluation of the Bactericidal Activity of Commercial Sanitizers
2.5. Plating and Counting Bacteria
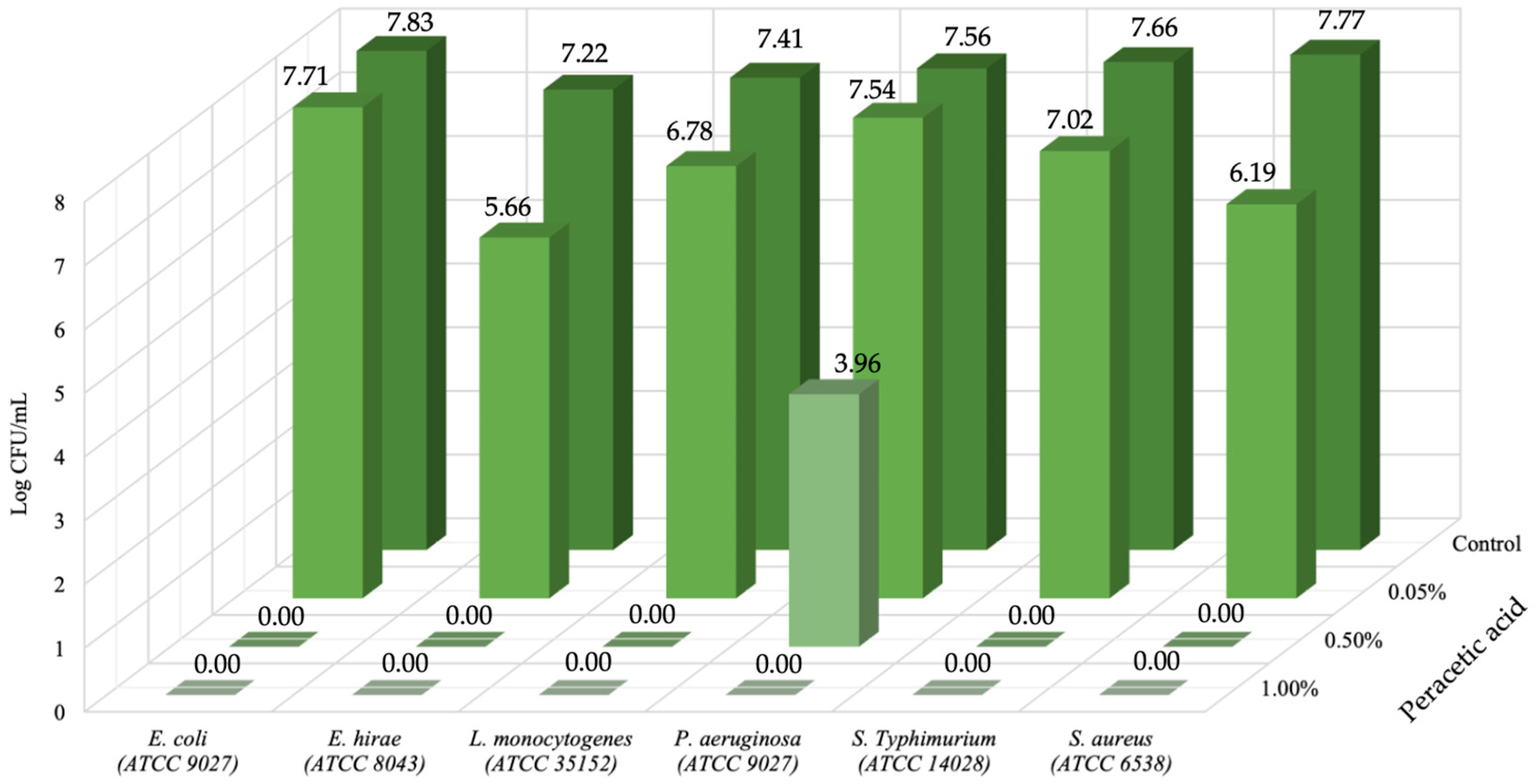
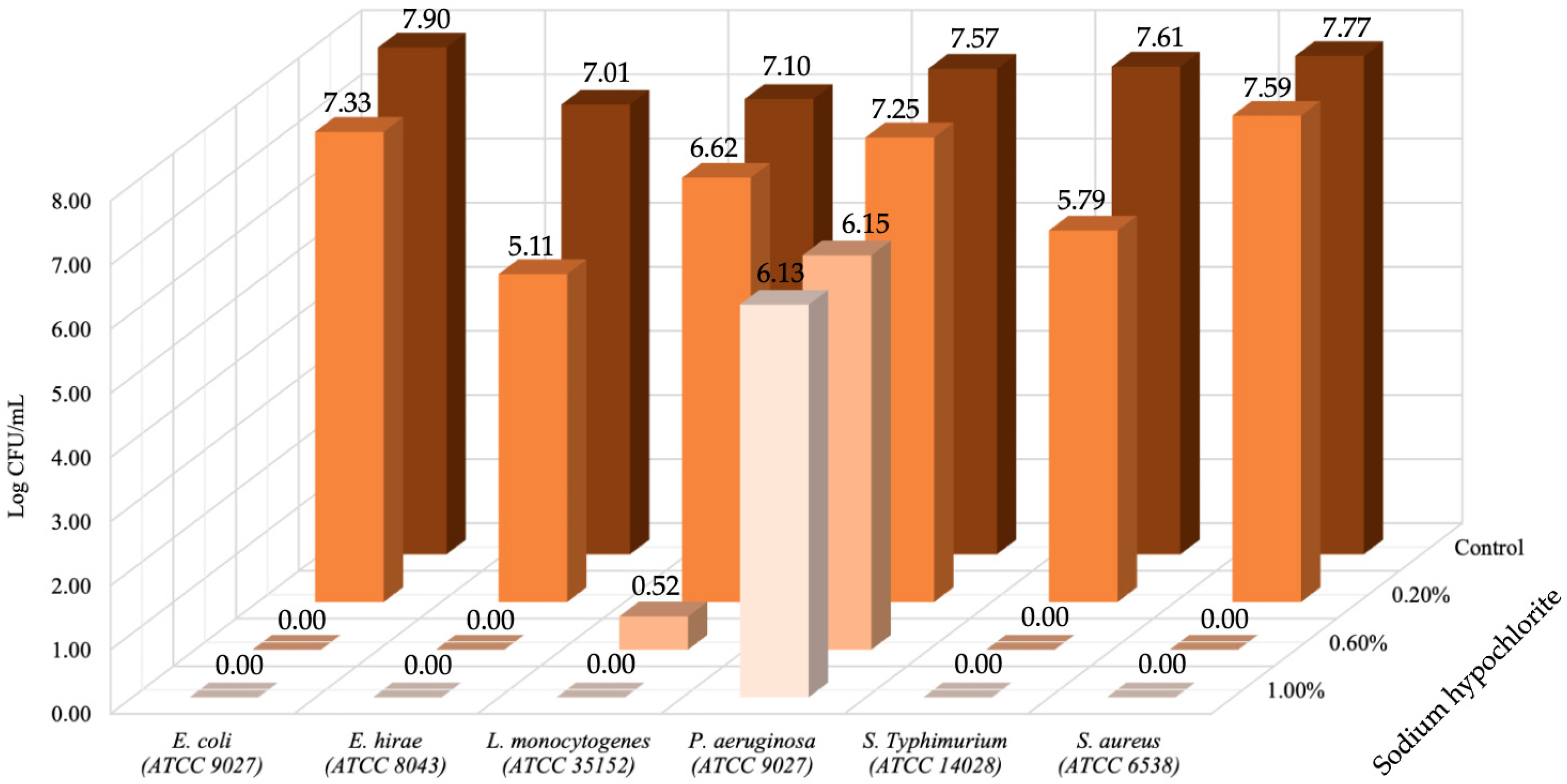
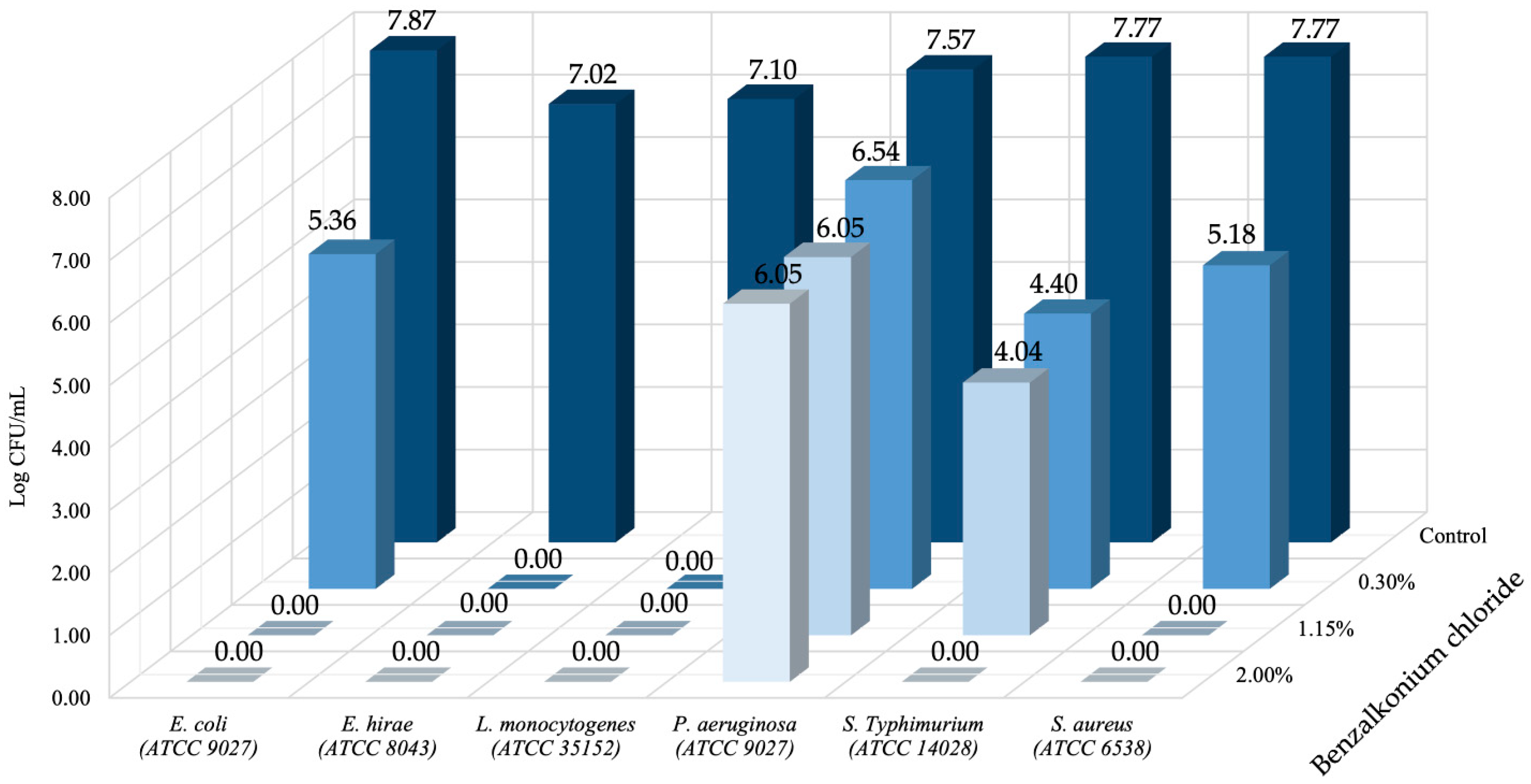
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- FAO; WHO. Codex Alimentarius: Princípios Gerais de Higiene dos Alimentos–CXC 1-1969; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2020; Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%3A%2F%2Fworkspace.fao.org%2Fsites%2Fcodex%2FStandards%2FCXC%2B1-1969%2FCXC_001e.pdf (accessed on 1 July 2025).
- Falcó, I.; Verdeguer, M.; Aznar, R.; Sánchez, G.; Randazzo, W. Sanitizing food contact surfaces by the use of essential oils. Innov. Food Sci. Emerg. Technol. 2019, 51, 220–228. [Google Scholar] [CrossRef]
- Ziech, R.E.; Perin, A.P.; Lampugnani, C.; Sereno, M.J.; Viana, C.; Soares, V.M.; Pereira, J.G.; Pinto, J.P.A.N.; Bersot, L.S. Biofilm-producing ability and tolerance to industrial sanitizers in Salmonella spp. isolated from Brazilian poultry processing plants. LWT-Food Sci. Technol. 2016, 68, 85–90. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Zhao, T.; Doyle, M.P. Inactivation and induction of sublethal injury of Listeria monocytogenes in biofilm treated with various sanitizers. Food Control 2016, 70, 371–379. [Google Scholar] [CrossRef]
- Silva, S.; Stefanello, A.; Santos, B.; Fracari, J.; Leães, G.; Copetti, M.V. Factors that interfere in the action of sanitizers against ochratoxigenic fungi deteriorating dry-cured meat products. Fermentation 2023, 9, 83. [Google Scholar] [CrossRef]
- Chauret, C.P. Sanitization. In Encyclopedia of Food Microbiology, 2nd ed.; Carl, A.B., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 360–364. [Google Scholar]
- Fong, D.; Gaulin, C.; Le, M.L.; Shum, M. Disinfectants and Sanitizers for Use on Food Contact Surfaces: Evidence Review. 2011. Available online: https://ncceh.ca/sites/default/files/Food_Contact_Surface_Sanitizers_Aug_2011.pdf (accessed on 13 August 2025).
- Wang, R.; Schmidt, J.W.; Harhay, D.M.; Bosilevac, J.M.; King, D.A.; Arthur, T.M. Biofilm Formation, Antimicrobial Resistance, and Sanitizer Tolerance of Salmonella enterica Strains Isolated from Beef Trim. Foodborne Pathog. Dis. 2017, 14, 102–109. [Google Scholar] [CrossRef]
- Ortega Morente, E.; Fernández-Fuentes, M.A.; Grande Burgos, M.J.; Abriouel, H.; Pérez Pulido, R.; Gálvez, A. Biocide Resistance in Bacteria. Int. J. Food Microbiol. 2013, 162, 13–25. [Google Scholar] [CrossRef]
- Andreoletti, O.; Budka, H.; Buncic, S.; Colin, P.; Collins, J.; De Koeijer, A.; Griffin, J.; Havelaar, A.; Hope, G.; Klein, G.; et al. Foodborne antimicrobial resistance as a biological hazard. EFSA J. 2008, 6, 2. Available online: https://www.bezpecnostpotravin.cz/UserFiles/File/Kvasnickova/EFSA_BIOHAZ_rezistence.pdf (accessed on 1 July 2025).
- Brasil. Resolução—RDC N° 774. Diário Oficial da União, De 15 De Fevereiro De 2023. Available online: https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-774-de-15-de-fevereiro-de-2023-465487847 (accessed on 1 July 2025).
- Jaenisch, F.R.F.; Kuchiishi, S.S.; Coldebella, A. Atividade antibacteriana de desinfetantes para uso na produção orgânica de aves. Cienc. Rural 2010, 40, 384–388. [Google Scholar] [CrossRef]
- EN 13697:2023; Chemical Disinfectants and Antiseptics—Quantitative Non-Porous Surface Test for the Evaluation of Bactericidal and/or Fungicidal Activity. European Standard: Brussels, Belgium, 2023.
- Stefanello, A.; Fracari, J.C.; Silva, M.; Lemos, J.G.; Garcia, M.V.; Santos, B.A.D.; Copetti, M.V. Influence of type, concentration, exposure time, temperature, and presence of organic load on the antifungal efficacy of industrial sanitizers against Aspergillus brasiliensis (ATCC 16404). Food Microbiol. 2021, 97, 103740. [Google Scholar] [CrossRef]
- Watson, S.C.; Neujahr, A.C.; Chaves, B.D.; Fernando, S.C.; Sullivan, G.A. Environmental monitoring of Nebraska ready-to-eat meat processing establishments resulted in the isolation of Listeria alongside Pseudomonas highly resistant to quaternary ammonia sanitizer. J. Food Prot. 2024, 87, 100391. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, Y.-S.; Cho, Y.H.; Park, H.-K.; Park, B.-K.; Lee, K.-H.; Kang, K.-J.; Jeon, D.-H.; Park, K.-H.; Ha, S.-D. Evaluation of efficacy of sanitizers and disinfectants marketed in Korea. Korean J. Food Sci. Technol. 2005, 37, 671–677. [Google Scholar]
- Iñiguez-Moreno, M.; Avila-Novoa, M.G.; Gutiérrez-Lomelí, M. Resistance of pathogenic and spoilage microorganisms to disinfectants in the presence of organic matter and their residual effect on stainless steel and polypropylene. J. Glob. Antimicrob. Resist. 2018, 14, 197–201. [Google Scholar] [CrossRef]
- Barbosa, J.; Cuppini, M.; Flach, J.; Steffens, C. Removal of Escherichia coli in boning knives with different sanitizers. LWT-Food Sci. Technol. 2016, 71, 309–315. [Google Scholar] [CrossRef]
- Lee, S.H.; Barancelli, G.V.; de Camargo, T.M.; Corassin, C.H.; Rosim, R.E.; da Cruz, A.G.; Cappato, L.P.; de Oliveira, C.A.F. Biofilm-producing ability of Listeria monocytogenes isolates from Brazilian cheese processing plants. Food Res. Int. 2017, 91, 88–91. [Google Scholar] [CrossRef]
- Riazi, S.; Matthews, K.R. Failure of foodborne pathogens to develop resistance to sanitizers following repeated exposure to common sanitizers. Int. Biodeterior. Biodegrad. 2011, 65, 374–378. [Google Scholar] [CrossRef]
- Rosado, M.; Castro, D.; Fernandes, S.; Yorika, D.; Yoshiteru, A. Biofilm formation on stainless steel as a function of time and temperature and control through sanitizers. Int. Dairy J. 2017, 68, 9–16. [Google Scholar] [CrossRef]
- Kohler, A.T.; Rodloff, A.C.; Labahn, M.; Reinhardt, M.; Truyen, U.; Speck, S. Efficacy of sodium hypochlorite against multidrug-resistant Gram-negative bacteria. J. Hosp. Infect. 2018, 100, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Lineback, C.B.; Nkemngong, C.A.; Wu, S.T.; Li, X.; Teska, P.J.; Oliver, H.F. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrob. Resist. Infect. Control 2018, 7, 154. [Google Scholar] [CrossRef]
- Poimenidou, V.; Chrysadakou, M.; Tzakoniati, A.; Bikouli, V.C.; Nychas, G.; Skandamis, P.N. Variability of Listeria monocytogenes strains in biofilm formation on stainless steel and polystyrene materials and resistance to peracetic acid and quaternary ammonium compounds. Int. J. Food Microbiol. 2016, 237, 164–171. [Google Scholar] [CrossRef]
- Du, W.X.; Danyluk, M.D.; Harris, L.J. Efficacy of aqueous and alcohol-based quaternary ammonium sanitizers for reducing Salmonella in dusts generated in almond hulling and shelling facilities. J. Food Sci. 2010, 75, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, H.M.; Delgado, D.A.; Filomena, I. Avaliação da aplicação de agentes sanitizantes como controladores do crescimento microbiano na indústria alimentícia. Rev. Ceciliana 2010, 2, 11–13. [Google Scholar]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Available online: https://pubmed.ncbi.nlm.nih.gov/9880479/ (accessed on 1 July 2025).
- Berkelman, R.L.; Godley, J.; Weber, J.A.; Anderson, R.L.; Lerner, A.M.; Paterson, N.J.; Allen, J.R. Pseudomonas cepacia peritonitis associated with contamination of automatic peritoneal dialysis machines. Ann. Intern. Med. 1982, 94, 456–458. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, H.F.G.; Verbrugh, H.A.; Abee, T.; Andriessen, J.W.; ter Kuile, B.H.; Mevius, D.J.; Montforts, M.H.M.; van Schaik, W.; Schmitt, H.; Smidt, H.; et al. Resisting disinfectants. Commun. Med. 2022, 2, 6. [Google Scholar] [CrossRef]
- Bernardi, A.O.; Stefanello, A.; Garcia, M.V.; Copetti, M.V. The control of cheese and meat product spoilage fungi by sanitizers: In vitro testing and food industry usage. LWT 2021, 144, 111204. [Google Scholar] [CrossRef]
| Sanitizers | Active Principle | Recommended Concentration | Neutralizing |
|---|---|---|---|
| Peracetic acid | Peracetic acid 14%; hydrogen peroxide 23%; glacial acetic acid | 0.05–1% | Nutrient broth with 0.6% sodium thiosulfate |
| Sodium hypochlorite | Sodium hypochlorite 8% active chlorine | 0.2–1% | Nutrient broth with 0.5% Tween |
| Benzalkonium chloride | Alkyl dimethyl benzyl ammonium chloride 22% | 0.3–2% | Nutrient broth with 0.6% sodium thiosulfate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, V.d.A.; Bernardi, A.O.; Tagliapietra, B.L.; Escalona, M.; da Silva, M.N.; Fracari, J.C.; Copetti, M.V.; Richards, N.S.P.d.S. Bacterial Inactivation by Common Food Industry Sanitizers. Hygiene 2025, 5, 36. https://doi.org/10.3390/hygiene5030036
Flores VdA, Bernardi AO, Tagliapietra BL, Escalona M, da Silva MN, Fracari JC, Copetti MV, Richards NSPdS. Bacterial Inactivation by Common Food Industry Sanitizers. Hygiene. 2025; 5(3):36. https://doi.org/10.3390/hygiene5030036
Chicago/Turabian StyleFlores, Vinícius do Amaral, Angélica Olivier Bernardi, Bruna Lago Tagliapietra, Maximiliano Escalona, Maritiele Naissinger da Silva, Juliana Copetti Fracari, Marina Venturini Copetti, and Neila Silvia Pereira dos Santos Richards. 2025. "Bacterial Inactivation by Common Food Industry Sanitizers" Hygiene 5, no. 3: 36. https://doi.org/10.3390/hygiene5030036
APA StyleFlores, V. d. A., Bernardi, A. O., Tagliapietra, B. L., Escalona, M., da Silva, M. N., Fracari, J. C., Copetti, M. V., & Richards, N. S. P. d. S. (2025). Bacterial Inactivation by Common Food Industry Sanitizers. Hygiene, 5(3), 36. https://doi.org/10.3390/hygiene5030036






