Evaluation of Water Safety Plan Compliance in Italian Hospitals According to Legislative Decree 18/23 and Directive EU 2020/2184: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. The Questionnaire
- General data on the location and characteristics of the hospital (8 questions): year of construction, number of beds, source of water supply, usual water disinfection treatments, number of operating theaters, ornamental fountains, and functional rehabilitation and water birth pools;
- Management of the water network (33 questions): knowledge of the new Dir. 2020/2184 and Italian Lgs.D. 18/23; implementation of a WSP; and chemical–physical and microbiological controls routinely performed to monitor the water network and disinfect water systems;
- Control and prevention of legionellosis (19 questions): frequency of sampling and presence of Legionella in the water network during routine monitoring, level of contamination usually found, identification at genus and species level, and actions taken in case of suspected/confirmed nosocomial legionellosis.
2.3. Statistical Analyses
3. Results
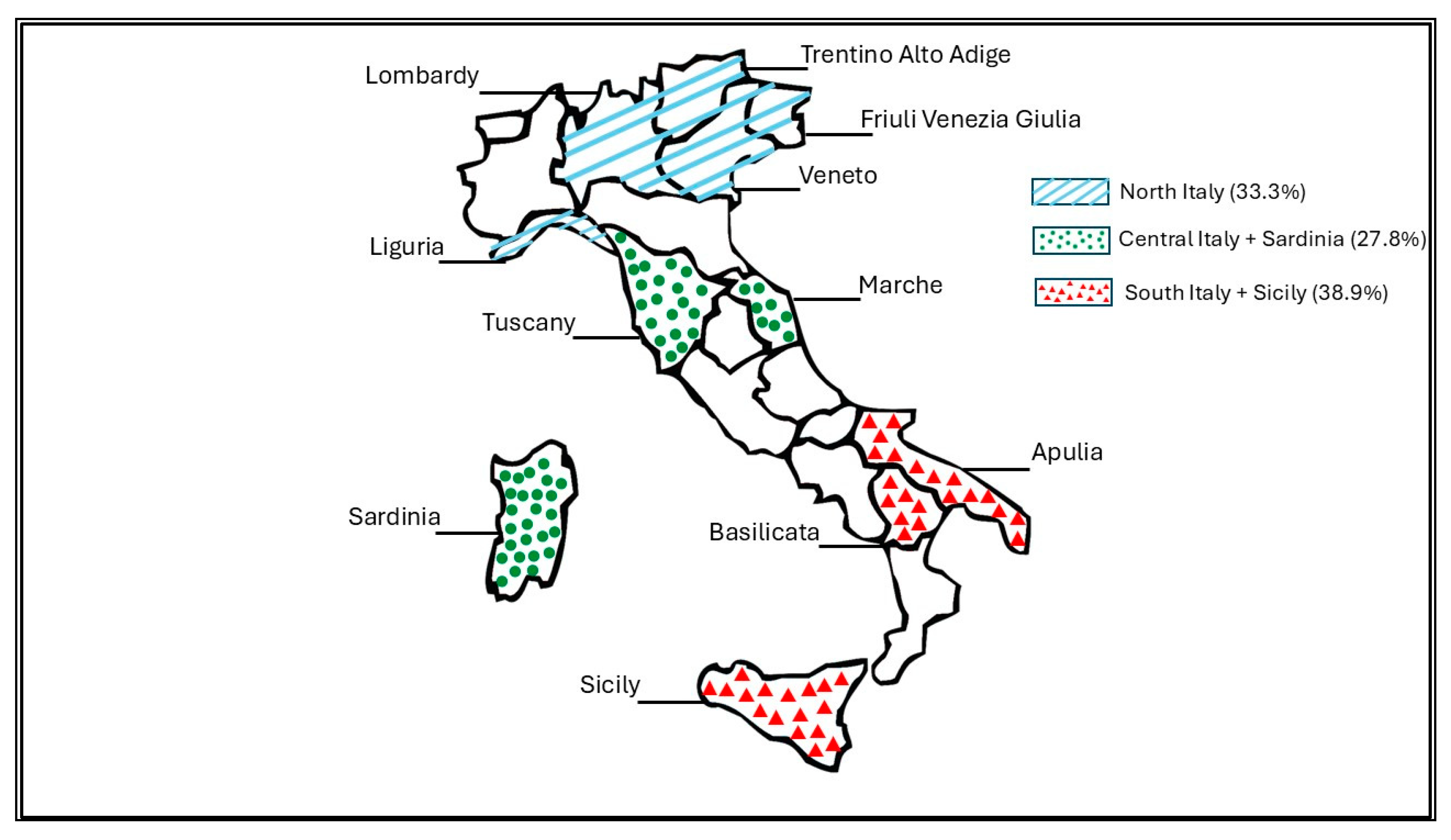
3.1. General Data on Hospital Location and Characteristics
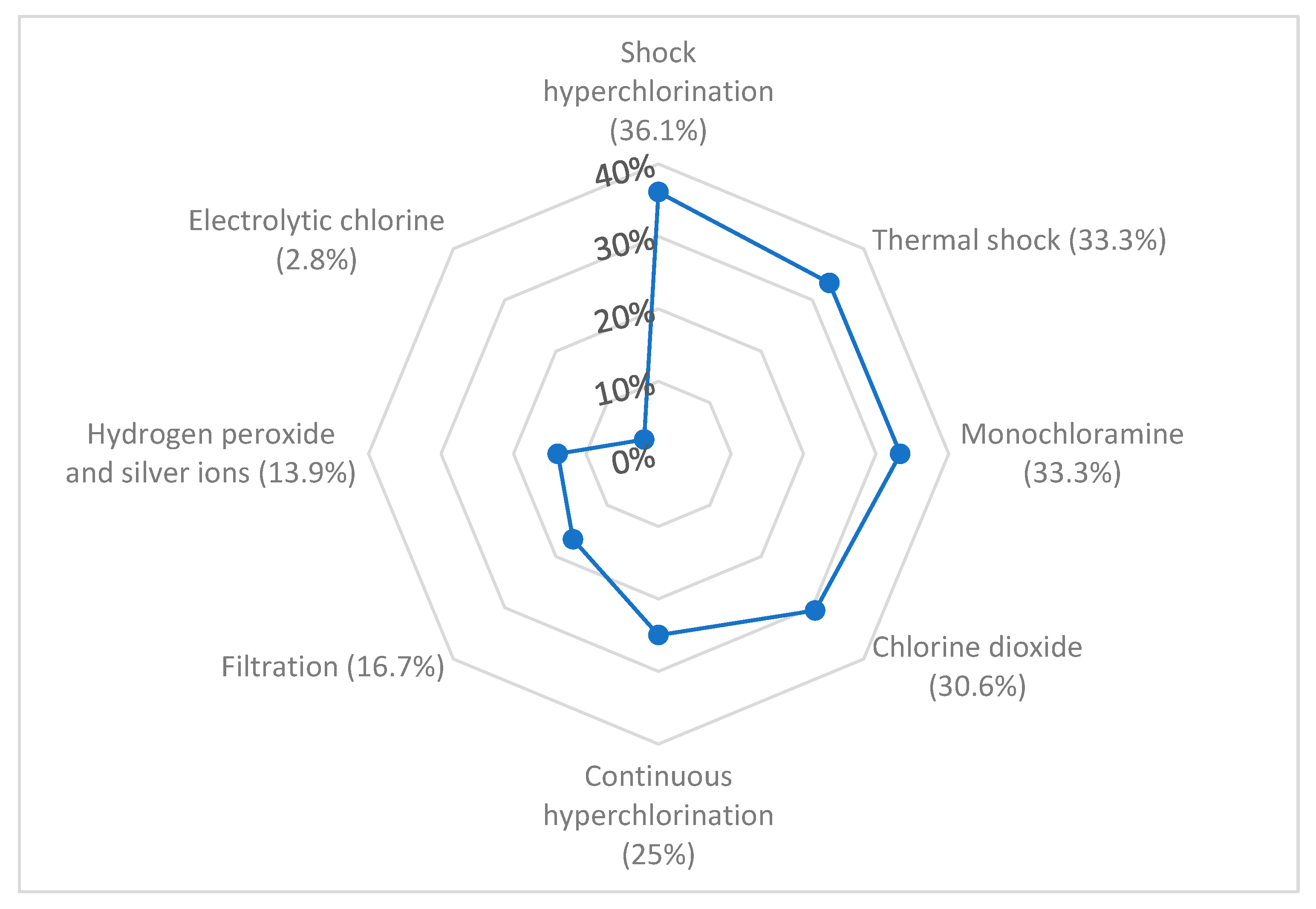
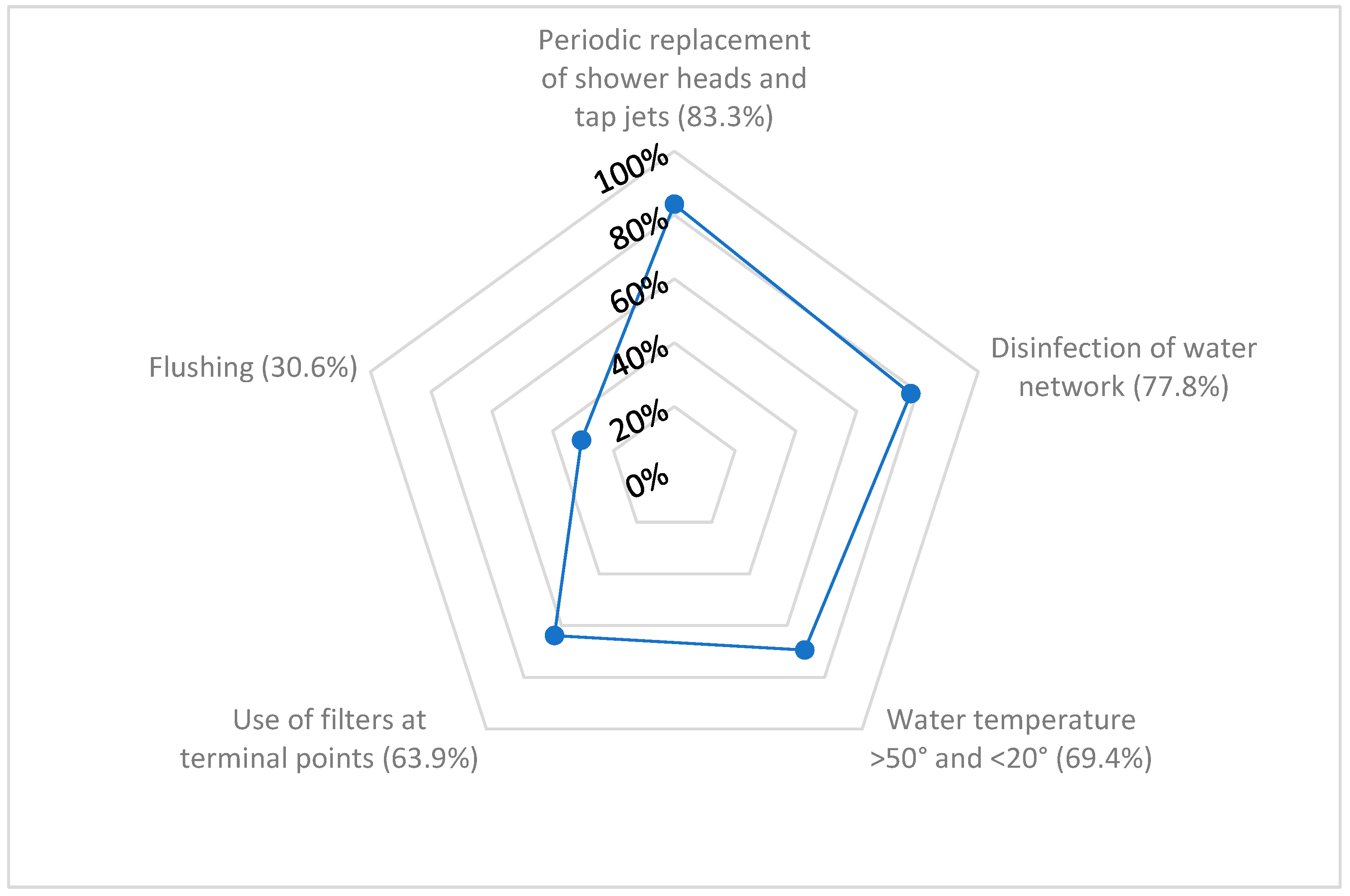
3.2. Management of the Water Network
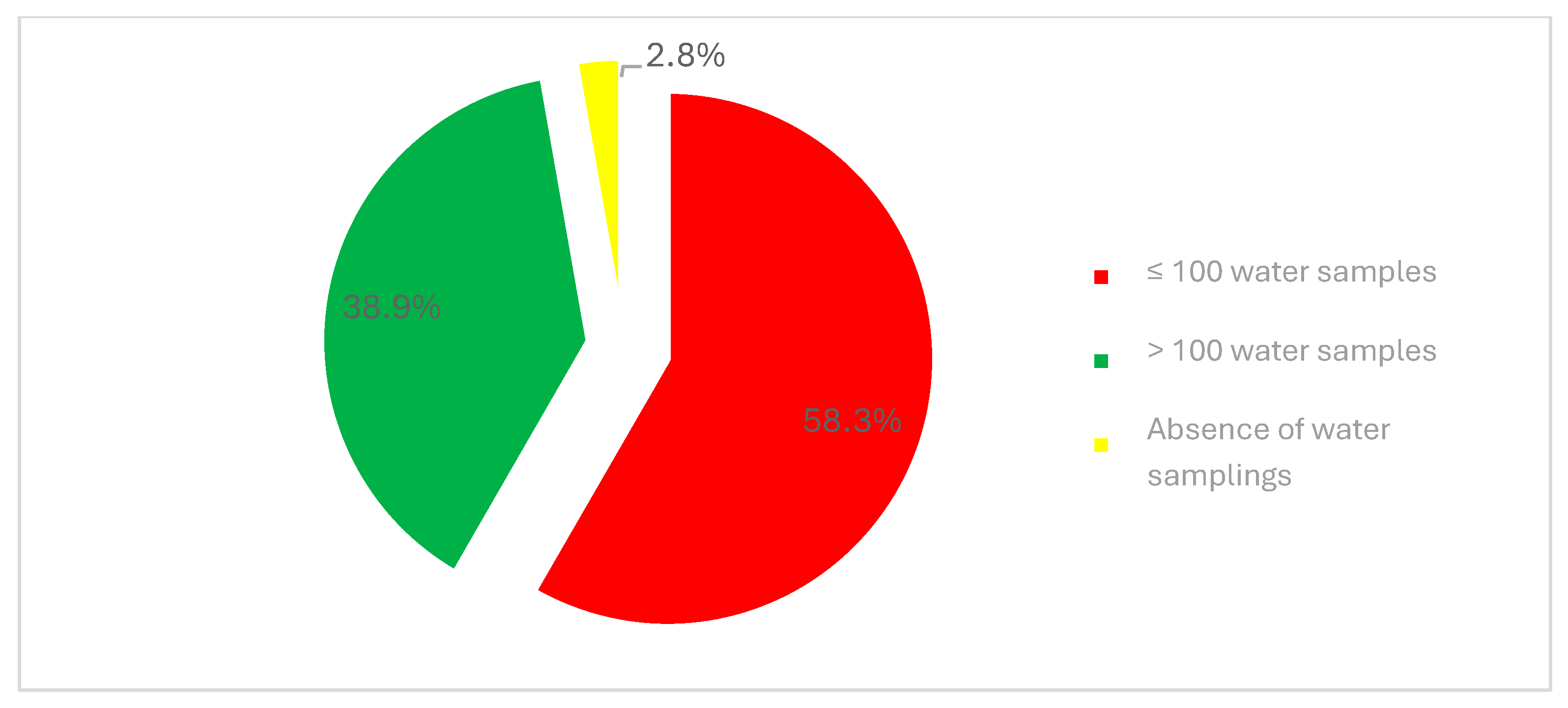
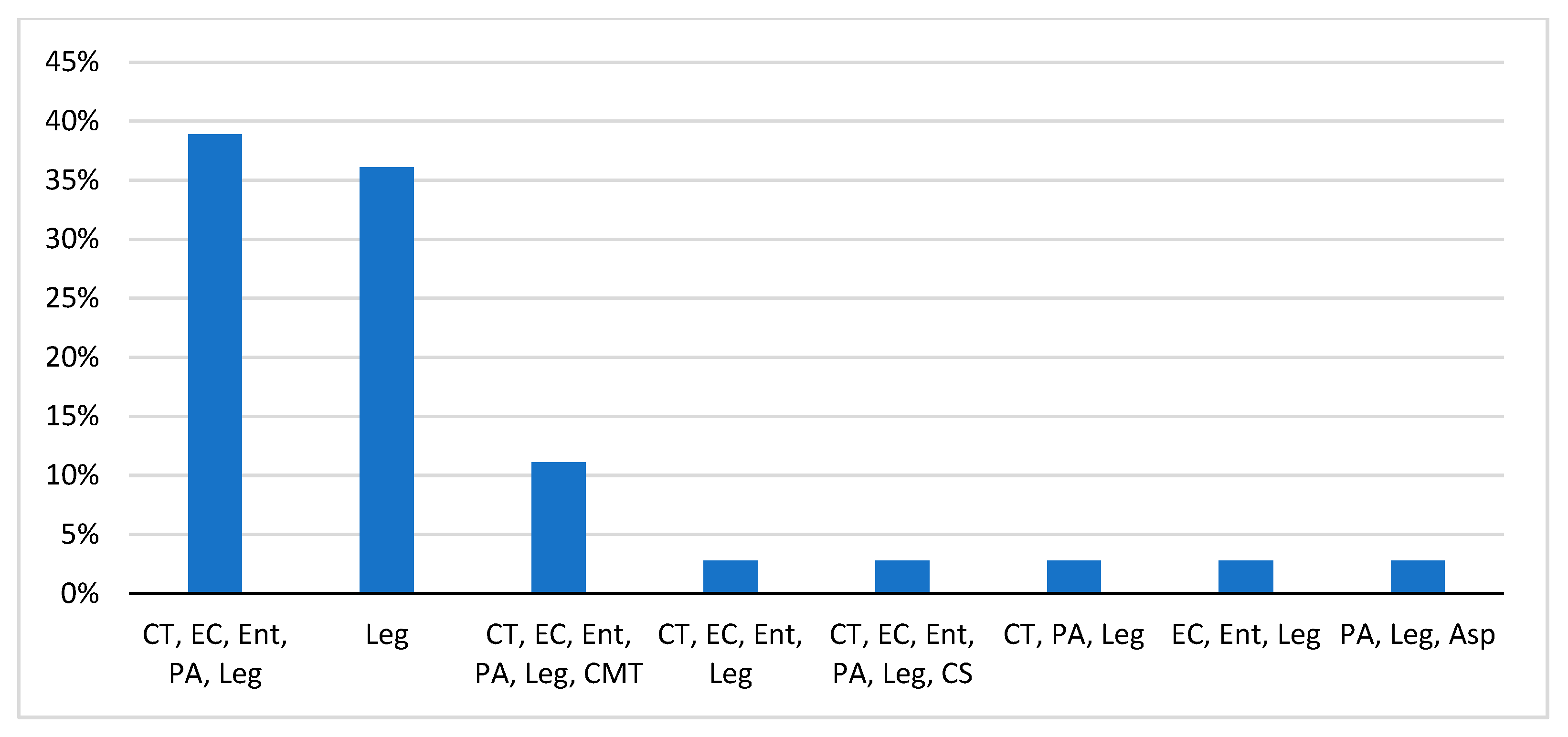
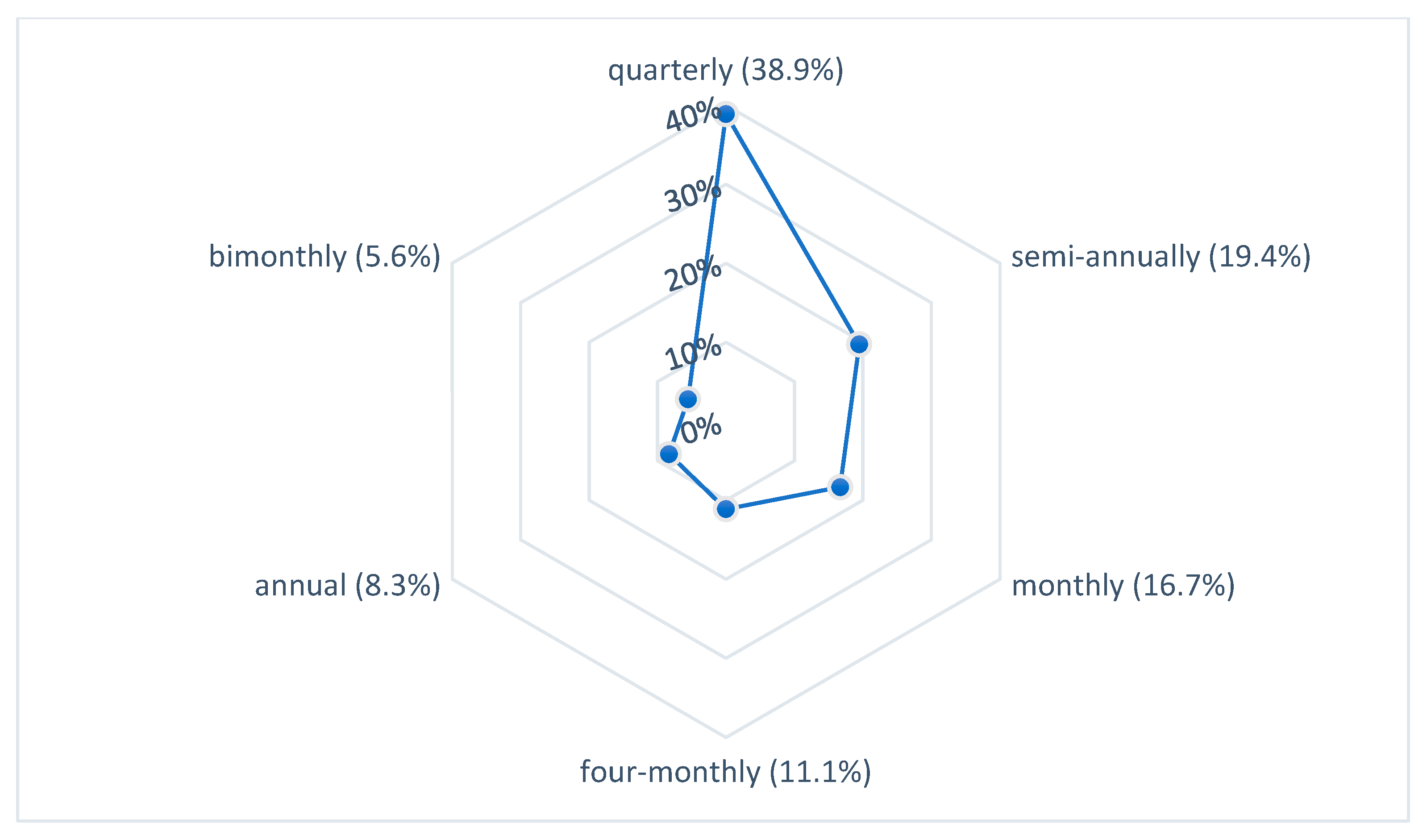
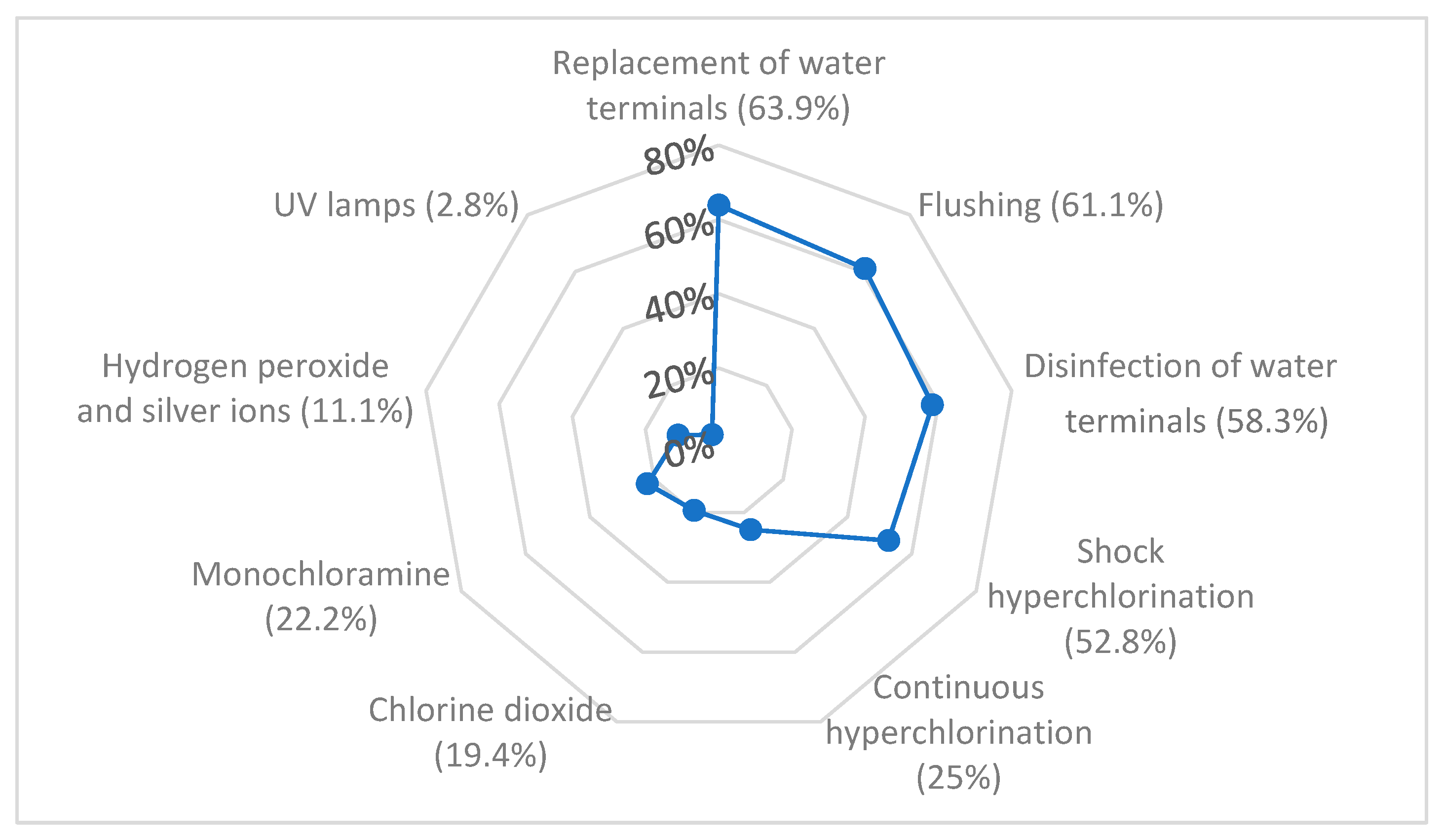
3.3. Control and Prevention of Legionellosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dufour, A.; Snozzi, M.; Koster, W.; Bartram, J.; Ronchi, E.; Fewtrell, L. Assessing Microbial Safety of Drinking Water: Improving Approaches and Methods, World Health Organization and Organisation for Economic Co-Operation; IWA Publishing: London, UK, 2003. [Google Scholar]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 3rd ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast). O.J. Europe Union L 435/1, 23 December 2020. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/HTML/?uri=CELEX:32020L2184 (accessed on 28 December 2024).
- World Health Organization; Regional Office for Europe. Drinking Water Parameter Cooperation Project. Support to the Revision of Annex I Council Directive 98/83/EC on the Quality of Water Intended for Human Consumption (Drinking Water Directive). Recommendations. Bonn, 11 September 2017. Available online: https://circabc.europa.eu/d/a/workspace/SpacesStore/7d664fea-50ed-4f6b-8eaf-8179900de47b/WHO%20Parameter%20Report.pdf (accessed on 28 December 2024).
- Legislative Decree No. 18 of 23 February 2023. Implementation of Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. O.J. n. 55 of 06 March 2023—General Series. Available online: https://www.gazzettaufficiale.it/eli/id/2023/03/06/23G00025/SG (accessed on 28 December 2024).
- World Health Organization. Burden of Disease Attributable to Unsafe Drinking-Water, Sanitation and Hygiene, 2019 Update; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Falkinham, J.O., 3rd; Hilborn, E.D.; Arduino, M.J.; Pruden, A.; Edwards, M.A. Epidemiology and Ecology of Opportunistic Premise Plumbing Pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environ. Health Perspect. 2015, 123, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Montagna, M.T.; De Giglio, O.; Napoli, C.; Diella, G.; Rutigliano, S.; Agodi, A.; Auxilia, F.; Baldovin, T.; Bisetto, F.; Arnoldo, L.; et al. Control and prevention measures for legionellosis in hospitals: A cross-sectional survey in Italy. Environ. Res. 2018, 166, 55–60. [Google Scholar] [CrossRef]
- Wittler, R.R. Foodborne and Waterborne Illness. Pediatr. Rev. 2023, 44, 81–91. [Google Scholar] [CrossRef]
- Inkster, T.; Walker, J.; Weinbren, M. Waterborne infections in haemato-oncology units—A narrative review. J. Hosp. Infect. 2023, 138, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Linee Guida per la Valutazione e la Gestione del Rischio per la Sicurezza dell’acqua nei Sistemi di Distribuzione Interni Degli Edifici Prioritari e non Prioritari e in Talune Navi ai Sensi Della Direttiva (UE) 2020/2184; Rapporti ISTISAN 22/32; Istituto Superiore di Sanità: Roma, Italy, 2022. Available online: https://www.iss.it/documents/20126/6682486/22-32+web.pdf/4a06c43b-f2c7-0f9e-5f08-7d390a46092c?t=1678695895185 (accessed on 3 January 2025).
- Linee Guida Nazionali per L’implementazione dei Piani di Sicurezza Dell’acqua; Rapporti ISTISAN 22/33; Istituto Superiore di Sanità: Roma, Italy, 2022. Available online: https://www.iss.it/en/-/rapporto-istisan-22/33-linee-guida-nazionali-per-l-implementazione-dei-piani-di-sicurezza-dell-acqua.-gruppo-nazionale-di-lavoro-per-la-redazione-delle-linee-guida-nazionali-per-l-implementazione-dei-psa (accessed on 3 January 2025).
- Anikeeva, O.; Hansen, A.; Varghese, B.; Borg, M.; Zhang, Y.; Xiang, J.; Bi, P. The impact of increasing temperatures due to climate change on infectious diseases. BMJ 2024, 387, e079343. [Google Scholar] [CrossRef]
- Scanlon, M.M.; Gordon, J.L.; Reynolds, K.A. Building Water Quality Commissioning in Healthcare Settings: Reducing Legionella and Water Contaminants Utilizing a Construction Scheduling Method. Buildings 2023, 13, 2533. [Google Scholar] [CrossRef]
- Bonadonna, L.; Cannarozzi de Grazia, M.; Capolongo, S.; Casini, B.; Cristina, M.L.; Daniele, G.; D’Alessandro, D.; De Giglio, O.; Di Benedetto, A.; Di Vittorio, G.; et al. Water safety in healthcare facilities. The Vieste Charter. Ann. Ig. 2017, 29, 92–100. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- UNI CEI EN ISO/IEC 17025:2018; General Requirements for the Competence of Testing and Calibration Laboratories. ISO: Geneva, Switzerland, 2018.
- Gelting, R.J.; Delea, K.; Medlin, E. A conceptual framework to evaluate the outcomes and impacts of water safety plans. J. Water Sanit. Hyg. Dev. 2012, 22, 103–111. [Google Scholar] [CrossRef]
- Water Safety Plan Manual: Step-by-Step Risk Management for Drinking-Water Suppliers, 2nd ed. World Health Organization: Geneva, Switzerland, 2023. Available online: https://www.who.int/publications/i/item/9789240067691 (accessed on 5 June 2025).
- World Health Statistics 2017: Monitoring Health for the SDGs, Sustainable Development Goals. World Health Organization: Geneva, Switzerland, 2017. Available online: https://www.who.int/publications/i/item/9789241565486 (accessed on 5 June 2025).
- Herschan, J.; Rickert, B.; Mkandawire, T.; Okurut, K.; King, R.; Hughes, S.J.; Lapworth, D.J.; Pond, K. Success Factors for Water Safety Plan Implementation in Small Drinking Water Supplies in Low- and Middle-Income Countries. Resources 2020, 9, 126. [Google Scholar] [CrossRef]
- Bufa-Dőrr, Z.; Sebestyén, Á.; Izsák, B.; Schmoll, O.; Pándics, T.; Vargha, M. Dual system of water safety plan auditing in Hungary: Benefits and lessons learnt. J. Water Health 2023, 21, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.M. A strategic approach for Water Safety Plans implementation in Portugal. J. Water Health 2011, 9, 107–116. [Google Scholar] [CrossRef]
- Bross, L.; Bäumer, J.; Voggenreiter, I.; Wienand, I.; Fekete, A. Public health without water? Emergency water supply and minimum supply standards of hospitals in high-income countries using the example of Germany and Austria. Water Policy 2021, 23, 205–220. [Google Scholar] [CrossRef]
- Muzzi, A.; Cutti, S.; Bonadeo, E.; Lodola, L.; Monzillo, V.; Corbella, M.; Scudeller, L.; Novelli, V.; Marena, C. Prevention of nosocomial legionellosis by best water management: Comparison of three decontamination methods. J. Hosp. Infect. 2020, 105, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Orsi, G.B.; Vitali, M.; Marinelli, L.; Ciorba, V.; Tufi, D.; Del Cimmuto, A.; Ursillo, P.; Fabiani, M.; De Santis, S.; Protano, C.; et al. Legionella control in the water system of antiquated hospital buildings by shock and continuous hyperchlorination: 5 years experience. BMC Infect. Dis. 2014, 14, 394. [Google Scholar] [CrossRef]
- Wang, S.; Shao, Z.; Chen, G.; Lin, B.; Li, D.; Chen, J. Assessment of chlorine and hydrogen peroxide on airborne bacteria: Disinfection efficiency and induction of antibiotic resistance. J. Hazard Mater. 2024, 474, 134697. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, L.; Liu, Z.; Han, J.; Zhao, Y.; Jin, Y.; Sheng, Y.; Zhu, L.; Hu, B. Excessive disinfection aggravated the environmental prevalence of antimicrobial resistance during COVID-19 pandemic. Sci. Total Environ. 2023, 882, 163598. [Google Scholar] [CrossRef]
- Federigi, I.; Bonetta, S.; Tesauro, M.; De Giglio, O.; Oliveri Conti, G.; Atomsa, N.T.; Bagordo, F.; Bonetta, S.; Consonni, M.; Diella, G.; et al. A systematic scoping review of antibiotic-resistance in drinking tap water. Environ. Res. 2024, 263, 120075. [Google Scholar] [CrossRef]
- Farina, C.; Cacciabue, E.; Averara, F.; Ferri, N.; Vailati, F.; Del Castillo, G.; Serafini, A.; Fermi, B.; Doniselli, N.; Pezzoli, F. Water Safety Plan, Monochloramine Disinfection and Extensive Environmental Sampling Effectively Control Legionella and Other Waterborne Pathogens in Nosocomial Settings: The Ten-Year Experience of an Italian Hospital. Microorganisms 2023, 11, 1794. [Google Scholar] [CrossRef]
- Kanamori, H.; Weber, D.J.; Rutala, W.A. Healthcare Outbreaks Associated With a Water Reservoir and Infection Prevention Strategies. Clin. Infect. Dis. 2016, 62, 1423–1435. [Google Scholar] [CrossRef]
- Decker, B.K.; Palmore, T.N. The role of water in healthcare-associated infections. Curr. Opin. Infect. Dis. 2013, 26, 345–351. [Google Scholar] [CrossRef]
- World Health Organization. Water Safety in Distribution Systems. 2014. Available online: https://iris.who.int/handle/10665/204422 (accessed on 28 December 2024).
- Rota, M.C.; Caporali, M.G.; Giannitelli, S.; Urciuoli, R.; Scaturro, M.; Ricci, M.L. I risultati del sistema di sorveglianza della legionellosi nel 2021. Boll. Epidemiol. Naz. 2023, 4, 25–32. [Google Scholar] [CrossRef]
- Montagna, M.T.; Ricci, M.L.; Napoli, C.; Tatò, D.; Scaturro, M.; Barbuti, G.; Pierucci, G.; Castellani Pastoris, M. Legionella pneumophila serogroup 5 infection in the presence of multiple environmental contamination. The importance of a bacteriological diagnosis. Ital. J. Public Health 2007, 4, 71–74. [Google Scholar] [CrossRef]
- De Giglio, O.; D’Ambrosio, M.; Calia, C.; Spagnuolo, V.; Oliva, M.; Lopuzzo, M.; Apollonio, F.; Triggiano, F.; Diella, G.; Scaturro, M.; et al. Case series study of nosocomial Legionnaires’ disease in Apulia region (southern Italy): The role of different molecular methods in identifying the infection source. Acta Biomed 2023, 94, e2023217. [Google Scholar] [CrossRef]
- Crook, B.; Young, C.; Rideout, C.; Smith, D. The Contribution of Legionella anisa to Legionella Contamination of Water in the Built Environment. Int. J. Environ. Res. Public Health 2024, 21, 1101. [Google Scholar] [CrossRef]
- Mercante, J.W.; Winchell, J.M. Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin. Microbiol. Rev. 2015, 28, 95–133. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, W.J.; Ji, P.; Pruden, A.; Edwards, M.A. Water heater temperature set point and water use patterns influence Legionella pneumophila and associated microorganisms at the tap. Microbiome 2015, 3, 67. [Google Scholar] [CrossRef]
- Kuroki, T.; Amemura-Maekawa, J.; Ohya, H.; Furukawa, I.; Suzuki, M.; Masaoka, T.; Aikawa, K.; Hibi, K.; Morita, M.; Lee, K.I.; et al. Outbreak of Legionnaire’s Disease Caused by Legionella pneumophila Serogroups 1 and 13. Emerg. Infect. Dis. 2017, 23, 349–351. [Google Scholar] [CrossRef]
- Amemura-Maekawa, J.; Kura, F.; Chida, K.; Ohya, H.; Kanatani, J.I.; Isobe, J.; Tanaka, S.; Nakajima, H.; Hiratsuka, T.; Yoshino, S.; et al. Legionella pneumophila and Other Legionella Species Isolated from Legionellosis Patients in Japan between 2008 and 2016. Appl. Environ. Microbiol. 2018, 84, e00721-18. [Google Scholar] [CrossRef]
- Romano Spica, V.; Borella, P.; Bruno, A.; Carboni, C.; Exner, M.; Hartemann, P.; Gianfranceschi, G.; Laganà, P.; Mansi, A.; Montagna, M.T.; et al. Legionnaires’ Disease Surveillance and Public Health Policies in Italy: A Mathematical Model for Assessing Prevention Strategies. Water 2024, 16, 2167. [Google Scholar] [CrossRef]
- Linee Guida per la Prevenzione e il Controllo della Legionellosi. 2015. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2362_allegato.pdf (accessed on 15 December 2024).
- Bertolino, G.; Marras, L.; Coroneo, V. The Detection Limits of Legionella According to the EU Directive 2020/2184. Could That Be Too Permissive? In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2023; pp. 1–8. [Google Scholar]
- ISO 11731:2017; Water Quality—Enumeration of Legionella. International Organization for Standardization: Geneva, Switzerland, 2017.
- Yáñez, M.A.; Carrasco-Serrano, C.; Barberá, V.M.; Catalán, V. Quantitative detection of Legionella pneumophila in water samples by immunomagnetic purification and real-time PCR amplification of the dotA gene. Appl. Environ. Microbiol. 2005, 71, 3433–3441. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Benson, R.; Pelish, T.; Brown, E.; Winchell, J.M.; Fields, B. Dual detection of Legionella pneumophila and Legionella species by real-time PCR targeting the 23S–5S rRNA gene spacer region. Clin. Microbiol. Infect. 2010, 16, 255–261. [Google Scholar] [CrossRef]
- Lee, J.V.; Lai, S.; Exner, M.; Lenz, J.; Gaia, V.; Casati, S.; Hartemann, P.; Lück, C.; Pangon, B.; Ricci, M.L.; et al. An international trial of quantitative PCR for monitoring Legionella in artificial water systems. J. Appl. Microbiol. 2011, 110, 1032–1044. [Google Scholar] [CrossRef]
- Eble, D.; Gehrig, V.; Schubert-Ullrich, P.; Köppel, R.; Füchslin, H.P. Comparison of the culture method with multiplex PCR for the confirmation of Legionella spp. and Legionella pneumophila. J. Appl. Microbiol. 2021, 131, 2600–2609. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Stevenson, D.; Walker, J.; Bennett, A. Evaluation of Legionella real-time PCR against traditional culture for routine and public health testing of water samples. J. Appl. Microbiol. 2017, 122, 1692–1703. [Google Scholar] [CrossRef]
- Whiley, H.; Taylor, M. Legionella detection by culture and qPCR: Comparing apples and oranges. Crit. Rev. Microbiol. 2016, 42, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Montagna, M.T.; Brigida, S.; Fasano, F.; Leone, C.M.; D’Ambrosio, M.; Spagnuolo, V.; Lopuzzo, M.; Apollonio, F.; Triggiano, F.; Caringella, M.E.; et al. The role of air temperature in Legionella water contamination and legionellosis incidence rates in southern Italy (2018–2023). Ann. Ig. 2023, 35, 631–640. [Google Scholar] [CrossRef]
- Gavaldà, L.; Garcia-Nuñez, M.; Quero, S.; Gutierrez-Milla, C.; Sabrià, M. Role of hot water temperature and water system use on Legionella control in a tertiary hospital: An 8-year longitudinal study. Water Res. 2019, 149, 460–466. [Google Scholar] [CrossRef]
- Darelid, J.; Löfgren, S.; Malmvall, B.E. Control of nosocomial Legionnaires’ disease by keeping the circulating hot water temperature above 55 degrees C: Experience from a 10-year surveillance programme in a district general hospital. J. Hosp. Infect. 2002, 50, 213–219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montagna, M.T.; Moro, M.; Casini, B.; Mura, I.I.; Finzi, G.; Spagnuolo, V.; Savino, A.F.; Fasano, F.; Triggiano, F.; Bonadonna, L.; et al. Evaluation of Water Safety Plan Compliance in Italian Hospitals According to Legislative Decree 18/23 and Directive EU 2020/2184: A Cross-Sectional Study. Hygiene 2025, 5, 28. https://doi.org/10.3390/hygiene5030028
Montagna MT, Moro M, Casini B, Mura II, Finzi G, Spagnuolo V, Savino AF, Fasano F, Triggiano F, Bonadonna L, et al. Evaluation of Water Safety Plan Compliance in Italian Hospitals According to Legislative Decree 18/23 and Directive EU 2020/2184: A Cross-Sectional Study. Hygiene. 2025; 5(3):28. https://doi.org/10.3390/hygiene5030028
Chicago/Turabian StyleMontagna, Maria Teresa, Matteo Moro, Beatrice Casini, Ida Iolanda Mura, Gianfranco Finzi, Valentina Spagnuolo, Antonella Francesca Savino, Fabrizio Fasano, Francesco Triggiano, Lucia Bonadonna, and et al. 2025. "Evaluation of Water Safety Plan Compliance in Italian Hospitals According to Legislative Decree 18/23 and Directive EU 2020/2184: A Cross-Sectional Study" Hygiene 5, no. 3: 28. https://doi.org/10.3390/hygiene5030028
APA StyleMontagna, M. T., Moro, M., Casini, B., Mura, I. I., Finzi, G., Spagnuolo, V., Savino, A. F., Fasano, F., Triggiano, F., Bonadonna, L., & De Giglio, O. (2025). Evaluation of Water Safety Plan Compliance in Italian Hospitals According to Legislative Decree 18/23 and Directive EU 2020/2184: A Cross-Sectional Study. Hygiene, 5(3), 28. https://doi.org/10.3390/hygiene5030028










