Commercial Arthrospira platensis Extract Modifies the Photophysiology of Cladocopium goreaui, Coral Endosymbiont Microalgae
Abstract
1. Introduction
2. Materials and Methods
2.1. Arthrospira Platensis Extract and Concentration Exposure
2.2. Microalgal Culture
2.3. Physiological Measurements on Algae
2.3.1. Growth Analysis
2.3.2. PAM Fluorometry
2.4. Statistical Analyses
3. Results
3.1. Growth Rates
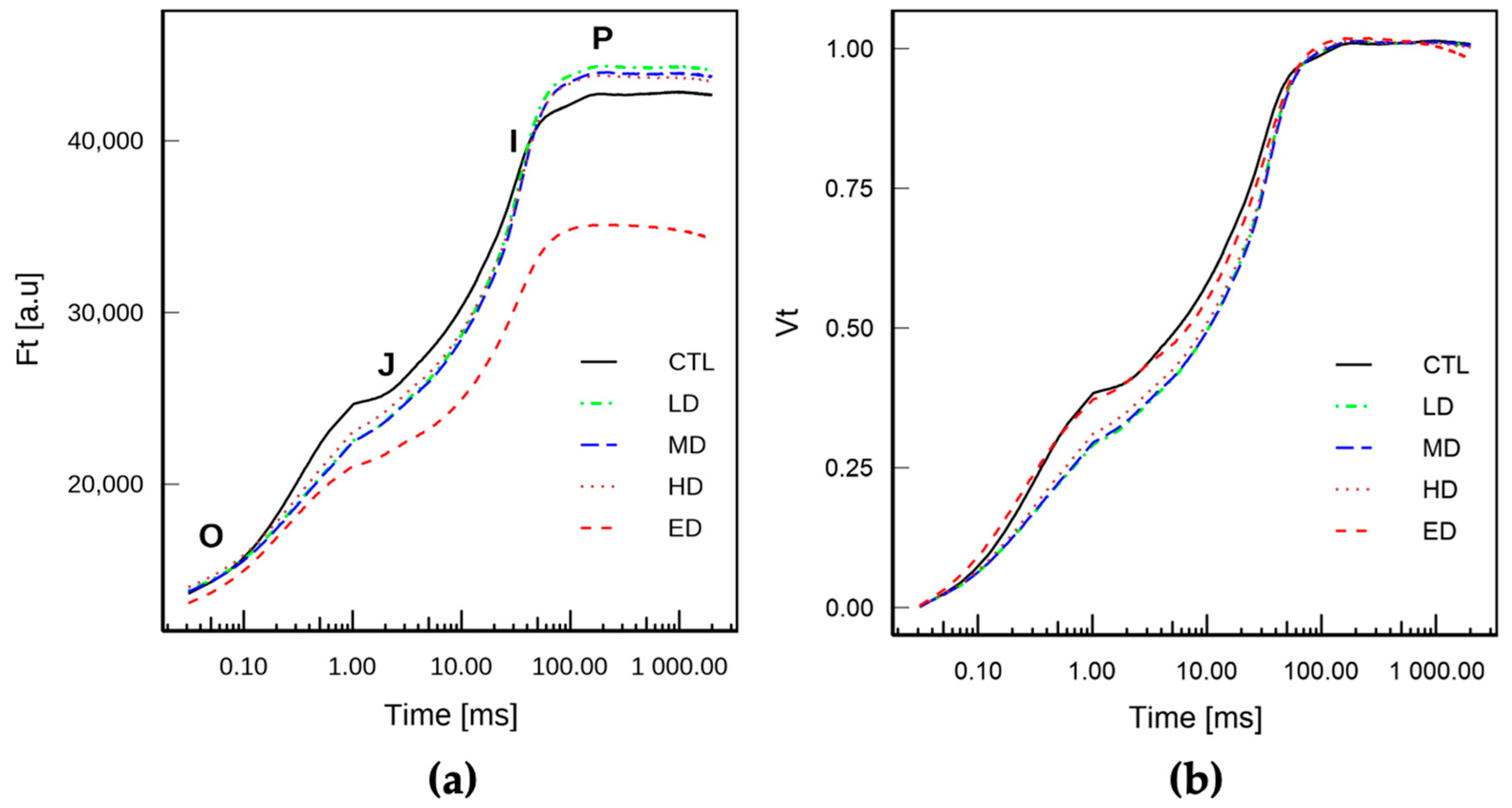
3.2. Chlorophyll Fluorescence Signal After 5 Days Exposure
3.2.1. Rapid Light Curves
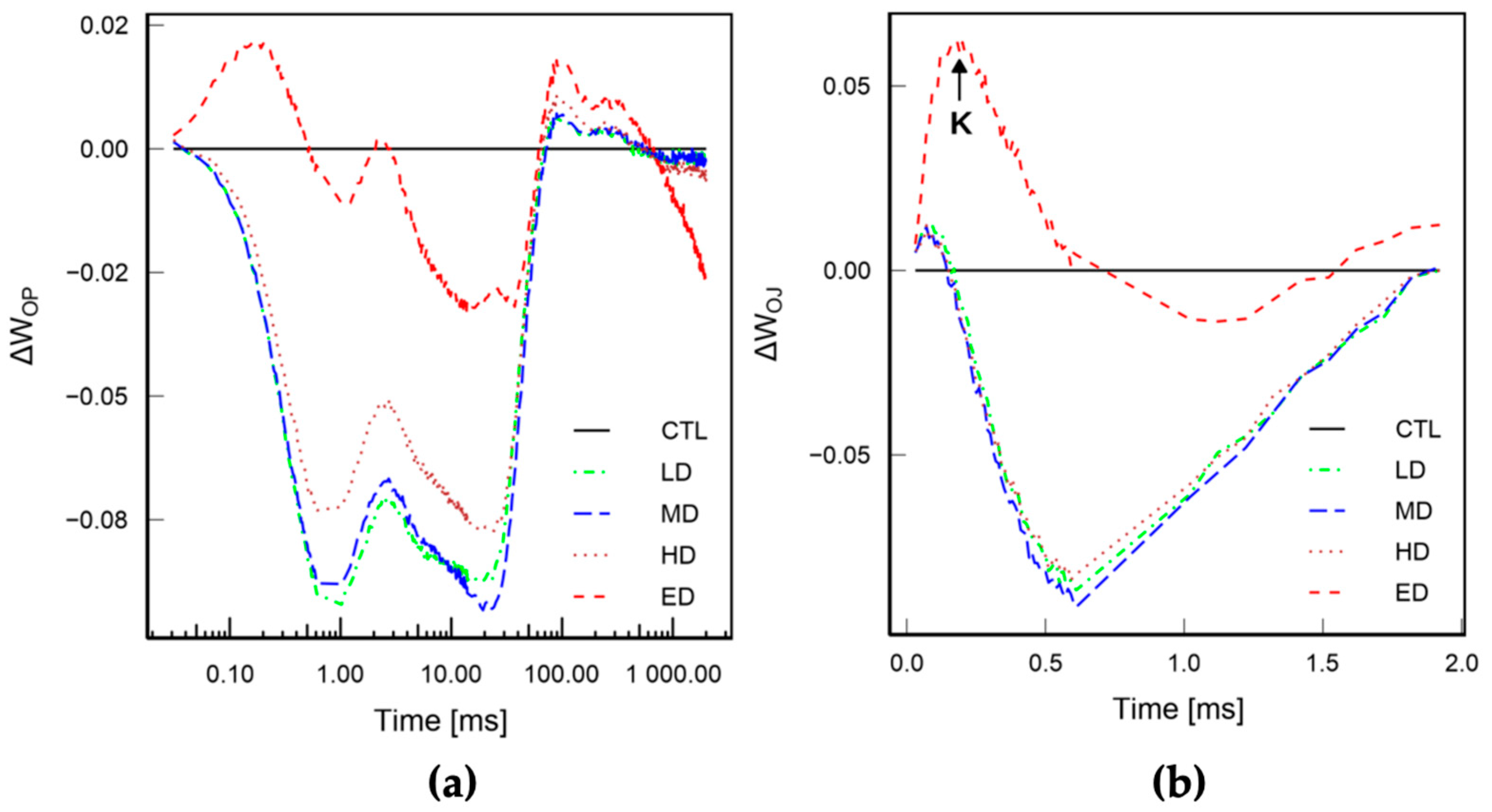
3.2.2. Rapid Chl a Fluorescence Transient Analyses
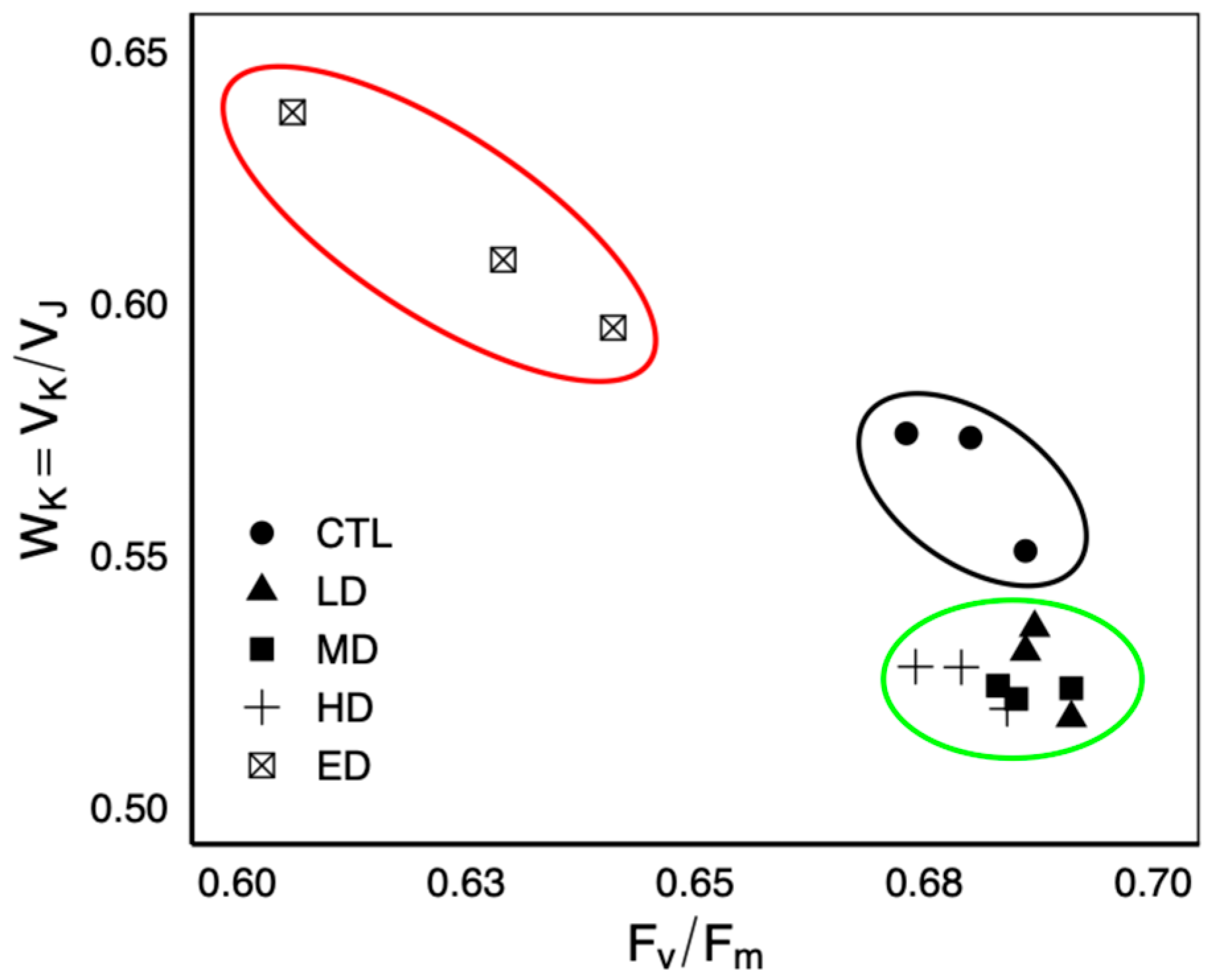
3.3. Parameters Extracted from JIP Transient
4. Discussion
4.1. Physiological Response of C. goreaui to the A. platensis Extract
4.2. Enhancement of the Photosynthetic Steps at Low Doses, Based on Absorption Basis
4.3. High-Dose Adverse Effects of Substances on Photophysiology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAM | Pulse Amplificated Fluorometry |
| OJIP | JIP-test |
| RLC | Rapid Light Curve |
| ETC | Electron Transport Chain |
| PSII | Photosystem II |
| OEC | Oxygen Evolving Complex |
| PCP | Personal Care Product |
| UVF | Ultra-Violet Filter |
| ROS | Reactive oxygen species |
| PC | Phycocyanin |
| NPQ | Non-Photochemical Quenching |
| PAR | Photosynthetically Active Radiation |
| NOAEL | No Observed Adverse Effect Level |
Appendix A
Appendix A.1
| Parameter | Definition | Formula | Meaning |
|---|---|---|---|
| Basic fluorescence data measured or calculated from the JIP-test | |||
| F0 | Minimal fluorescence after dark adaptation | F at 50 μs | Minimum fluorescence when all PSII RCs are open |
| Fm | Maximal fluorescence during a saturating flash on dark adapted sample | F at P-step (peak) | Maximal fluorescence when all PSII RCs are closed (can be attributed to Fp) |
| Fm’ | Maximum fluorescence in a light-adapted sample under a saturating flash | ||
| Ft | Fluorescence at time t | ||
| Fv | Variable fluorescence | Ft − F0 | |
| Fv/Fm | Maximum quantum yield for primary photochemistry | (Fm − F0)/Fm | Maximum light utilization efficiency of PSII |
| Vt | Relative variable fluorescence at time t | (Ft − F0)/(Fm − F0) | Relative fluorescence double normalized on Fm and F0 |
| M0 | Initial slope (in ms−1) of the fluorescent transcient normalized on the variable fluorescence (Vt) | M0 = [(ΔF/Δt)0]/(Fm − F0) | QA reduction rate |
| Biophysical parameters derived from the basic parameters by the JIP-test Specific energy fluxes (per active RC: QA-reducing PSII reducing center) | |||
| ABS/RC | Absorption flux at the antenna per RC | M0 × (1/VJ) × (1/ φP0) | PSII apparent antenna size. RC/ABS the reciprocal corresponds to the fraction of active RC per antenna. |
| TR0/RC | Trapped energy flux leading to QA reduction per active RC | M0 × (1/ VJ) | The rate of which an electron is trapped in RC resulting in reduction of QA to QA− |
| ET0/RC | Electron transport flux per active RC | M0 × (1/VJ) × (1 − VJ) | The rate by which an electron moves from QA− to PQ |
| DI0/RC | Dissipation flux into heat per active RC | ABS/RC − TR0/RC | |
| Quantum yields and efficiencies | |||
| φP0 | Maximum quantum yield for primary photochemistry | Fv/Fm | Quantum yield for primary chemistry |
| φE0 | Quantum yield for electron transport | [1 − (F0/Fm)] × (1 − Vj) | |
| ψE0 | (1 − Vj) | The probability that an electron moves further than QA− | |
| Performance index | |||
| Pi_Abs | Performance index for energy conservation | [RC/ABS] × [ φP0/(1 − φP0)] × [ψE0/(1 − ψE0)] | Performance index for energy conservation from a photon absorbed by PSII until the reduction of intersystem electron acceptors |
| RLC measured and extracted parameters | |||
| rETR | Relative electron transport rate | PAR × QY × 0.5 × 0.84 | |
| rETRmax | Maximum relative electron transport rate | rETR levelling at a maximum light-saturated rate | |
| α | Initial RLC slope, maximal light use coefficient for PSII | Ability to use low light intensities | |
| Ik | Light saturating index [μmol photon m−2 s−1] | Ik = rETRmax/α | Ability to use high light intensities |
| NPQind | Induced Non Photochemical Quenching during RLC experiment | (Fm − Ft)/(Ft) | Ability to dissipate energy into heat as a protective mechanism. Stern-Volmer coefficient |
References
- LaJeunesse, T.C.; Parkinson, J.E.; Gabrielson, P.W.; Jeong, H.J.; Reimer, J.D.; Voolstra, C.R.; Santos, S.R. Systematic Revision of Symbiodiniaceae Highlights the Antiquity and Diversity of Coral Endosymbionts. Curr. Biol. 2018, 28, 2570–2580.e6. [Google Scholar] [CrossRef] [PubMed]
- Muscatine, L. The Role of Symbiotic Algae in Carbon and Energy Flux in Reef Corals. Coral Reefs 1990, 25, 75. [Google Scholar]
- Burke, L.M. Reefs at Risk Revisited; World Resources Institute: Washington, DC, USA, 2011; ISBN 978-1-56973-762-0. [Google Scholar]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global Warming and Recurrent Mass Bleaching of Corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Andréfouët, S.; Fabricius, K.E.; Diaz-Pulido, G.; Lough, J.; Marshall, P.A.; Pratchett, M.S. Vulnerability of Coral Reefs in the Tropical Pacific to Climate Change. In Vulnerability of Tropical Pacific Fisheries and Aquaculture to Climate Change; Secretariat of the Pacific Community: Noumea, New Caledonia, 2011; Volume 49. [Google Scholar]
- Sánchez-Quiles, D.; Blasco, J.; Tovar-Sánchez, A. Sunscreen Components Are a New Environmental Concern in Coastal Waters: An Overview. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Gaw, S.; Thomas, K.V.; Hutchinson, T.H. Sources, Impacts and Trends of Pharmaceuticals in the Marine and Coastal Environment. Phil. Trans. R. Soc. B 2014, 369, 20130572. [Google Scholar] [CrossRef]
- Watkins, Y.S.D.; Sallach, J.B. Investigating the Exposure and Impact of Chemical UV Filters on Coral Reef Ecosystems: Review and Research Gap Prioritization. Integr. Envir Assess. Manag. 2021, 17, 967–981. [Google Scholar] [CrossRef]
- D’Amico, M.; Kallenborn, R.; Scoto, F.; Gambaro, A.; Gallet, J.C.; Spolaor, A.; Vecchiato, M. Chemicals of Emerging Arctic Concern in North-Western Spitsbergen Snow: Distribution and Sources. Sci. Total Environ. 2024, 908, 168401. [Google Scholar] [CrossRef]
- Apel, C.; Joerss, H.; Ebinghaus, R. Environmental Occurrence and Hazard of Organic UV Stabilizers and UV Filters in the Sediment of European North and Baltic Seas. Chemosphere 2018, 212, 254–261. [Google Scholar] [CrossRef]
- Downs, C.A.; Bishop, E.; Diaz-Cruz, M.S.; Haghshenas, S.A.; Stien, D.; Rodrigues, A.M.S.; Woodley, C.M.; Sunyer-Caldú, A.; Doust, S.N.; Espero, W.; et al. Oxybenzone Contamination from Sunscreen Pollution and Its Ecological Threat to Hanauma Bay, Oahu, Hawaii, U.S.A. Chemosphere 2022, 291, 132880. [Google Scholar] [CrossRef] [PubMed]
- Mitchelmore, C.L.; He, K.; Gonsior, M.; Hain, E.; Heyes, A.; Clark, C.; Younger, R.; Schmitt-Kopplin, P.; Feerick, A.; Conway, A.; et al. Occurrence and Distribution of UV-Filters and Other Anthropogenic Contaminants in Coastal Surface Water, Sediment, and Coral Tissue from Hawaii. Sci. Total Environ. 2019, 670, 398–410. [Google Scholar] [CrossRef]
- Mitchelmore, C.L.; Burns, E.E.; Conway, A.; Heyes, A.; Davies, I.A. A Critical Review of Organic Ultraviolet Filter Exposure, Hazard, and Risk to Corals. Environ. Toxicol. Chem. 2021, 40, 967–988. [Google Scholar] [CrossRef]
- Stien, D.; Suzuki, M.; Rodrigues, A.M.S.; Yvin, M.; Clergeaud, F.; Thorel, E.; Lebaron, P. A Unique Approach to Monitor Stress in Coral Exposed to Emerging Pollutants. Sci. Rep. 2020, 10, 9601. [Google Scholar] [CrossRef]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Fel, J.-P.; Lacherez, C.; Bensetra, A.; Mezzache, S.; Béraud, E.; Léonard, M.; Allemand, D.; Ferrier-Pagès, C. Photochemical Response of the Scleractinian Coral Stylophora Pistillata to Some Sunscreen Ingredients. Coral Reefs 2019, 38, 109–122. [Google Scholar] [CrossRef]
- He, T.; Tsui, M.M.P.; Tan, C.J.; Ng, K.Y.; Guo, F.W.; Wang, L.H.; Chen, T.H.; Fan, T.Y.; Lam, P.K.S.; Murphy, M.B. Comparative Toxicities of Four Benzophenone Ultraviolet Filters to Two Life Stages of Two Coral Species. Sci. Total Environ. 2019, 651, 2391–2399. [Google Scholar] [CrossRef]
- He, T.; Tsui, M.M.P.; Tan, C.J.; Ma, C.Y.; Yiu, S.K.F.; Wang, L.H.; Chen, T.H.; Fan, T.Y.; Lam, P.K.S.; Murphy, M.B. Toxicological Effects of Two Organic Ultraviolet Filters and a Related Commercial Sunscreen Product in Adult Corals. Environ. Pollut. 2019, 245, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Wijgerde, T.; van Ballegooijen, M.; Nijland, R.; van der Loos, L.; Kwadijk, C.; Osinga, R.; Murk, A.; Slijkerman, D. Adding Insult to Injury: Effects of Chronic Oxybenzone Exposure and Elevated Temperature on Two Reef-Building Corals. Sci. Total Environ. 2020, 733, 139030. [Google Scholar] [CrossRef]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens Cause Coral Bleaching by Promoting Viral Infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef]
- Muscatine, L.; Porter, J.W. Reef Corals: Mutualistic Symbioses Adapted to Nutrient-Poor Environments. BioScience 1977, 27, 454–460. [Google Scholar] [CrossRef]
- Downs, C.A.; Fauth, J.E.; Halas, J.C.; Dustan, P.; Bemiss, J.; Woodley, C.M. Oxidative Stress and Seasonal Coral Bleaching. Free Radic. Biol. Med. 2002, 33, 533–543. [Google Scholar] [CrossRef]
- Burns, E.E.; Davies, I.A. Coral Ecotoxicological Data Evaluation for the Environmental Safety Assessment of Ultraviolet Filters. Environ. Toxic. Chem. 2021, 40, 3441–3464. [Google Scholar] [CrossRef]
- Vuckovic, D.; Tinoco, A.I.; Ling, L.; Renicke, C.; Pringle, J.R.; Mitch, W.A. Conversion of Oxybenzone Sunscreen to Phototoxic Glucoside Conjugates by Sea Anemones and Corals. Science 2022, 376, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Salvi, L.; Niccolai, A.; Cataldo, E.; Sbraci, S.; Paoli, F.; Storchi, P.; Rodolfi, L.; Tredici, M.R.; Mattii, G.B. Effects of Arthrospira Platensis Extract on Physiology and Berry Traits in Vitis Vinifera. Plants 2020, 9, 1805. [Google Scholar] [CrossRef]
- Moeller, M.; Pawlowski, S.; Petersen-Thiery, M.; Miller, I.B.; Nietzer, S.; Heisel-Sure, Y.; Kellermann, M.Y.; Schupp, P.J. Challenges in Current Coral Reef Protection—Possible Impacts of UV Filters Used in Sunscreens, a Critical Review. Front. Mar. Sci. 2021, 8, 665548. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, Z.; Yang, W.; Guo, J.; Yan, Z.; Li, J.; Lin, J.; Cao, X.; Tang, J.; Liu, Z.; et al. Unraveling the Metabolic Effects of Benzophenone-3 on the Endosymbiotic Dinoflagellate Cladocopium Goreaui. Front. Microbiol. 2023, 13, 1116975. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.C.; Turnham, K.E.; Lewis, A.M.; Nitschke, M.R.; Warner, M.E.; Kemp, D.W.; Hoegh-Guldberg, O.; Fitt, W.K.; Van Oppen, M.J.H.; LaJeunesse, T.C. Formal Recognition of Host-generalist Species of Dinoflagellate (Cladocopium, Symbiodiniaceae) Mutualistic with Indo-Pacific Reef Corals. J. Phycol. 2023, 59, 698–711. [Google Scholar] [CrossRef]
- Alessi, C.; Lemonnier, H.; Camp, E.F.; Wabete, N.; Payri, C.; Rodolfo Metalpa, R. Algal Symbiont Diversity in Acropora Muricata from the Extreme Reef of Bouraké Associated with Resistance to Coral Bleaching. PLoS ONE 2024, 19, e0296902. [Google Scholar] [CrossRef]
- Quigley, K.M.; Alvarez Roa, C.; Beltran, V.H.; Leggat, B.; Willis, B.L. Experimental Evolution of the Coral Algal Endosymbiont, Cladocopium goreaui: Lessons Learnt across a Decade of Stress Experiments to Enhance Coral Heat Tolerance. Restor. Ecol. 2021, 29, e13342. [Google Scholar] [CrossRef]
- Marzonie, M.; Flores, F.; Sadoun, N.; Thomas, M.C.; Valada-Mennuni, A.; Kaserzon, S.; Mueller, J.F.; Negri, A.P. Toxicity Thresholds of Nine Herbicides to Coral Symbionts (Symbiodiniaceae). Sci. Rep. 2021, 11, 21636. [Google Scholar] [CrossRef]
- Liang, J.; Niu, T.; Zhang, L.; Yang, Y.; Li, Z.; Liang, Z.; Yu, K.; Gong, S. Polystyrene Microplastics Exhibit Toxic Effects on the Widespread Coral Symbiotic Cladocopium Goreaui. Environ. Res. 2025, 268, 120750. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. ISBN 978-1-4020-3217-2. [Google Scholar]
- Tsimilli-Michael, M. Special Issue in Honour of Prof. Reto J. Strasser—Revisiting JIP-Test: An Educative Review on Concepts, Assumptions, Approximations, Definitions and Terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]
- Labille, J.; Slomberg, D.; Catalano, R.; Robert, S.; Apers-Tremelo, M.-L.; Boudenne, J.-L.; Manasfi, T.; Radakovitch, O. Assessing UV Filter Inputs into Beach Waters during Recreational Activity: A Field Study of Three French Mediterranean Beaches from Consumer Survey to Water Analysis. Sci. Total Environ. 2020, 706, 136010. [Google Scholar] [CrossRef] [PubMed]
- Poiger, T.; Buser, H.-R.; Balmer, M.E.; Bergqvist, P.-A.; Müller, M.D. Occurrence of UV Filter Compounds from Sunscreens in Surface Waters: Regional Mass Balance in Two Swiss Lakes. Chemosphere 2004, 55, 951–963. [Google Scholar] [CrossRef]
- Milinkovitch, T.; Vacher, L.; Le Béguec, M.; Petit, E.; Dubillot, E.; Grimmelpont, M.; Labille, J.; Tran, D.; Ravier, S.; Boudenne, J.-L.; et al. Sunscreen Use during Recreational Activities on a French Atlantic Beach: Release of UV Filters at Sea and Influence of Air Temperature. Environ. Sci. Pollut. Res. 2024, 31, 41046–41058. [Google Scholar] [CrossRef] [PubMed]
- Ficheux, A.S.; Chevillotte, G.; Wesolek, N.; Morisset, T.; Dornic, N.; Bernard, A.; Bertho, A.; Romanet, A.; Leroy, L.; Mercat, A.C.; et al. Consumption of Cosmetic Products by the French Population Second Part: Amount Data. Food Chem. Toxicol. 2016, 90, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Branka, J.-E.; Darnis, E.; Lefeuvre, L. Telomere Protective Effects of a Cyanobacteria Phycocyanin against Blue Light and UV Irradiations: A Skin Anti-Aging and Photo-Protective Agent. J. Cosmet. Dermatol. Sci. Appl. 2019, 09, 336–345. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis Immobilis Is a Diatom, Not a Chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Chakravarti, L.J.; Beltran, V.H.; Van Oppen, M.J.H. Rapid Thermal Adaptation in Photosymbionts of Reef-building Corals. Glob. Change Biol. 2017, 23, 4675–4688. [Google Scholar] [CrossRef]
- Jauffrais, T.; Agogué, H.; Gemin, M.-P.; Beaugeard, L.; Martin-Jézéquel, V. Effect of Bacteria on Growth and Biochemical Composition of Two Benthic Diatoms Halamphora Coffeaeformis and Entomoneis Paludosa. J. Exp. Mar. Biol. Ecol. 2017, 495, 65–74. [Google Scholar] [CrossRef]
- Coulombier, N.; Blanchier, P.; Le Dean, L.; Barthelemy, V.; Lebouvier, N.; Jauffrais, T. The Effects of CO2-Induced Acidification on Tetraselmis Biomass Production, Photophysiology and Antioxidant Activity: A Comparison Using Batch and Continuous Culture. J. Biotechnol. 2021, 325, 312–324. [Google Scholar] [CrossRef]
- Sibat, M.; Mai, T.; Chomérat, N.; Bilien, G.; Lhaute, K.; Hess, P.; Séchet, V.; Jauffrais, T. Gambierdiscus Polynesiensis from New Caledonia (South West Pacific Ocean): Morpho-Molecular Characterization, Toxin Profile and Response to Light Intensity. Harmful Algae 2025, 145, 102859. [Google Scholar] [CrossRef]
- Jassby, A.D.; Platt, T. Mathematical Formulation of the Relationship between Photosynthesis and Light for Phytoplankton: Photosynthesis-Light Equation. Limnol. Oceanogr. 1976, 21, 540–547. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid Light Curves: A Powerful Tool to Assess Photosynthetic Activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Oukarroum, A.; Madidi, S.E.; Schansker, G.; Strasser, R.J. Probing the Responses of Barley Cultivars (Hordeum vulgare L.) by Chlorophyll a Fluorescence OLKJIP under Drought Stress and Re-Watering. Environ. Exp. Bot. 2007, 60, 438–446. [Google Scholar] [CrossRef]
- Tang, J.; Cai, W.; Yan, Z.; Zhang, K.; Zhou, Z.; Zhao, J.; Lin, S. Interactive Effects of Acidification and Copper Exposure on the Reproduction and Metabolism of Coral Endosymbiont Cladocopium Goreaui. Mar. Pollut. Bull. 2022, 177, 113508. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Yin, G.; Zhao, N.; Tan, X.; Wang, Y. A Sensitive Response Index Selection for Rapid Assessment of Heavy Metals Toxicity to the Photosynthesis of Chlorella Pyrenoidosa Based on Rapid Chlorophyll Fluorescence Induction Kinetics. Toxics 2023, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, I.; Mlinarić, S.; Tarkowská, D.; Oklestkova, J.; Novák, O.; Lepeduš, H.; Bok, V.V.; Brkanac, S.R.; Strnad, M.; Salopek-Sondi, B. Early Brassica Crops Responses to Salinity Stress: A Comparative Analysis Between Chinese Cabbage, White Cabbage, and Kale. Front. Plant Sci. 2019, 10, 450. [Google Scholar] [CrossRef]
- Antunović Dunić, J.; Mlinarić, S.; Pavlović, I.; Lepeduš, H.; Salopek-Sondi, B. Comparative Analysis of Primary Photosynthetic Reactions Assessed by OJIP Kinetics in Three Brassica Crops after Drought and Recovery. Appl. Sci. 2023, 13, 3078. [Google Scholar] [CrossRef]
- Mlinarić, S.; Žuna Pfeiffer, T.; Krstin, L.; Špoljarić Maronić, D.; Ožura, M.; Stević, F.; Varga, M. Adaptation of Amorpha Fruticosa to Different Habitats Is Enabled by Photosynthetic Apparatus Plasticity. Photosynthetica 2021, 59, 137–147. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. Probing Photosynth. Mech. Regul. Adapt. 2000, 25, 445–483. [Google Scholar]
- Kumar, D.; Singh, H.; Raj, S.; Soni, V. Chlorophyll a Fluorescence Kinetics of Mung Bean (Vigna radiata L.) Grown under Artificial Continuous Light. Biochem. Biophys. Rep. 2020, 24, 100813. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescence Parameters as Early Indicators of Light Stress in Barley. J. Photochem. Photobiol. B Biol. 2012, 112, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.; Blain, R. The Occurrence of Hormetic Dose Responses in the Toxicological Literature, the Hormesis Database: An Overview. Toxicol. Appl. Pharmacol. 2005, 202, 289–301. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Blain, R.B. The Hormesis Database: The Occurrence of Hormetic Dose Responses in the Toxicological Literature. Regul. Toxicol. Pharmacol. 2011, 61, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Ma, Y.; Ahammed, G.J.; Hao, B.; Chen, J.; Wan, W.; Zhao, Y.; Cui, H.; Xu, W.; Cui, J.; et al. Insights into Melatonin-Induced Photosynthetic Electron Transport under Low-Temperature Stress in Cucumber. Front. Plant Sci. 2022, 13, 1029854. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P. Production of Reactive Oxygen Species by Photosystem II. Biochim. Biophys. Acta (BBA)-Bioenerg. 2009, 1787, 1151–1160. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Cong, Y.; Zhu, P.; Xing, J.; Cui, J.; Xu, W.; Shi, Q.; Diao, M.; Liu, H. Ascorbic Acid-Induced Photosynthetic Adaptability of Processing Tomatoes to Salt Stress Probed by Fast OJIP Fluorescence Rise. Front. Plant Sci. 2021, 12, 594400. [Google Scholar] [CrossRef]
- Oukarroum, A.; Bussotti, F.; Goltsev, V.; Kalaji, H.M. Correlation between Reactive Oxygen Species Production and Photochemistry of Photosystems I and II in Lemna gibba L. Plants under Salt Stress. Environ. Exp. Bot. 2015, 109, 80–88. [Google Scholar] [CrossRef]
- Rahim, A.; Çakir, C.; Ozturk, M.; Şahin, B.; Soulaimani, A.; Sibaoueih, M.; Nasser, B.; Eddoha, R.; Essamadi, A.; El Amiri, B. Chemical Characterization and Nutritional Value of Spirulina Platensis Cultivated in Natural Conditions of Chichaoua Region (Morocco). S. Afr. J. Bot. 2021, 141, 235–242. [Google Scholar] [CrossRef]
- Smythers, A.L.; Crislip, J.R.; Slone, D.R.; Flinn, B.B.; Chaffins, J.E.; Camp, K.A.; McFeeley, E.W.; Kolling, D.R.J. Excess Manganese Increases Photosynthetic Activity via Enhanced Reducing Center and Antenna Plasticity in Chlorella Vulgaris. Sci. Rep. 2023, 13, 11301. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, D.; Soni, V. Performance of Chlorophyll a Fluorescence Parameters in Lemna Minor under Heavy Metal Stress Induced by Various Concentration of Copper. Sci. Rep. 2022, 12, 10620. [Google Scholar] [CrossRef]
- Biscéré, T.; Ferrier-Pagès, C.; Gilbert, A.; Pichler, T.; Houlbrèque, F. Evidence for Mitigation of Coral Bleaching by Manganese. Sci. Rep. 2018, 8, 16789. [Google Scholar] [CrossRef]
- Fernandes, R.; Campos, J.; Serra, M.; Fidalgo, J.; Almeida, H.; Casas, A.; Toubarro, D.; Barros, A.I.R.N.A. Exploring the Benefits of Phycocyanin: From Spirulina Cultivation to Its Widespread Applications. Pharmaceuticals 2023, 16, 592. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, T.; Zhao, R.; Zhao, Y.; Duan, Y.; Liu, X.; Luan, G.; Hu, R.; Tang, S.; Ma, X.; et al. Arthrospira Promotes Plant Growth and Soil Properties under High Salinity Environments. Front. Plant Sci. 2023, 14, 1293958. [Google Scholar] [CrossRef] [PubMed]
- Varia, J. Biostimulation with Phycocyanin-Rich Spirulina Extract in Hydroponic Vertical Farming. Sci. Hortic. 2022, 299, 111042. [Google Scholar] [CrossRef]
- Mériot, V.; Roussel, A.; Brunet, N.; Chomerat, N.; Bilien, G.; Le Déan, L.; Berteaux-Lecellier, V.; Coulombier, N.; Lebouvier, N.; Jauffrais, T. Heterocapsa Cf. Bohaiensis (Dinoflagellate): Identification and Response to Nickel and Iron Stress Revealed through Chlorophyll a Fluorescence. Photosynthetica 2024, 62, 27. [Google Scholar] [CrossRef] [PubMed]
- Brestic, M.; Zivcak, M.; Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I. Photosystem II Thermostability in Situ: Environmentally Induced Acclimation and Genotype-Specific Reactions in Triticum aestivum L. Plant Physiol. Biochem. 2012, 57, 93–105. [Google Scholar] [CrossRef]
- Shanker, A.K.; Amirineni, S.; Bhanu, D.; Yadav, S.K.; Jyothilakshmi, N.; Vanaja, M.; Singh, J.; Sarkar, B.; Maheswari, M.; Singh, V.K. High-Resolution Dissection of Photosystem II Electron Transport Reveals Differential Response to Water Deficit and Heat Stress in Isolation and Combination in Pearl Millet [Pennisetum glaucum (L.) R. Br.]. Front. Plant Sci. 2022, 13, 892676. [Google Scholar] [CrossRef]
- Stefanov, M.A.; Rashkov, G.D.; Yotsova, E.K.; Borisova, P.B.; Dobrikova, A.G.; Apostolova, E.L. Different Sensitivity Levels of the Photosynthetic Apparatus in Zea mays L. and Sorghum bicolor L. under Salt Stress. Plants 2021, 10, 1469. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, Q.; Yang, Y.; Xia, Y.; Cai, H.; Feng, X.; Gonçalves, R.J.; Guan, W. Ocean Acidification Enhances the Tolerance of Dinoflagellate Prorocentrum Donghaiense to Nanoplastic-Induced Oxidative Stress by Modulating Photosynthetic Performance. Front. Mar. Sci. 2024, 11, 1494930. [Google Scholar] [CrossRef]
- Tanvet, C.; Camp, E.F.; Sutton, J.; Houlbrèque, F.; Thouzeau, G.; Rodolfo-Metalpa, R. Corals Adapted to Extreme and Fluctuating Seawater pH Increase Calcification Rates and Have Unique Symbiont Communities. Ecol. Evol. 2023, 13, e10099. [Google Scholar] [CrossRef]

| A | μmax | λ | |
|---|---|---|---|
| CTL | 0.80 ± 0.03 ab | 0.09 ± 0.01 ab | 2.25 ± 0.65 a |
| LD | 0.88 ± 0.05 a | 0.09 ± 0.01 a | 2.24 ± 0.37 a |
| MD | 0.89 ± 0.07 a | 0.08 ± 0.01 a | 2.07 ± 0.62 a |
| HD | 0.77 ± 0.03 ab | 0.09 ± 0.00 ab | 2.04 ± 0.48 a |
| ED | 0.18 ± 0.07 b | 0.05 ± 0.02 b | 2.23 ± 0.50 a |
| rETRm | α | Ik | NPQind | |
|---|---|---|---|---|
| CTL | 66.66 ± 0.61 ab | 0.29 ± 0.00 a | 228.15 ± 0.31 ab | 0.29 ± 0.03 a |
| LD | 67.04 ± 3.20 ab | 0.29 ± 0.00 ab | 230.98 ± 14.02 ab | 0.31 ± 0.04 a |
| MD | 69.15 ± 1.14 a | 0.28 ± 0.00 ab | 242.17 ± 8.13 a | 0.36 ± 0.02 a |
| HD | 67.31 ± 1.75 ab | 0.28 ± 0.01 ab | 240.76 ± 6.75 a | 0.38 ± 0.03 a |
| ED | 46.52 ± 1.89 b | 0.25 ± 0.00 b | 188.38 ± 5.95 b | 0.30 ± 0.02 a |
| CTL | LD | MD | HD | ED | |
|---|---|---|---|---|---|
| Basic parameters calculated from the extracted transcient | |||||
| F0 | 13,600 ± 99 ab | 13,730 ± 188 ab | 13,665 ± 211 ab | 13,925 ± 239 a | 12,993 ± 500 b |
| Fm | 42,448 ± 724 ab | 43,966 ± 441 a | 43,586 ± 473 ab | 43,391 ± 536 ab | 34,700 ± 915 b |
| Fj | 24,849 ± 1377 a | 23,278 ± 254 a | 23,256 ± 262 a | 23,830 ± 366 a | 21,327 ± 511 b |
| Vj | 0.39 ± 0.05 a | 0.32 ± 0.00 b | 0.32 ± 0.00 ab | 0.34 ± 0.01 ab | 0.38 ± 0.01 a |
| M0 | 0.83 ± 0.14 a | 0.61 ± 0.02 a | 0.61 ± 0.01 a | 0.65 ± 0.02 a | 0.87 ± 0.05 a |
| Quantum yields and efficiencies | |||||
| φP0 (=Fv/Fm) | 0.68 ± 0.01 ab | 0.69 ± 0.00 a | 0.69 ± 0.00 ab | 0.68 ± 0.01 ab | 0.63 ± 0.02 b |
| ψE0 | 0.61 ± 0.05 a | 0.68 ± 0.00 b | 0.68 ± 0.00 ab | 0.66 ± 0.01 ab | 0.62 ± 0.01 a |
| φE0 | 0.41 ± 0.04 ab | 0.47 ± 0.00 a | 0.47 ± 0.00 ab | 0.45 ± 0.01 ab | 0.39 ± 0.02 b |
| Specific energy fluxes (per RC: QA− reducing PSII reaction center) in ms−1 | |||||
| ABS/RC | 3.11 ± 0.13 ab | 2.81 ± 0.07 ab | 2.79 ± 0.01 a | 2.84 ± 0.05 ab | 3.61 ± 0.24 b |
| Tr0/RC | 2.11 ± 0.07 ab | 1.93 ± 0.04 ab | 1.92 ± 0.00 a | 1.93 ± 0.02 ab | 2.25 ± 0.08 b |
| Et0/RC | 1.29 ± 0.07 a | 1.32 ± 0.02 ab | 1.3 ± 0.01 a | 1.28 ± 0.01 ab | 1.39 ± 0.03 b |
| Di0/RC | 1.00 ± 0.06 ab | 0.88 ± 0.03 ab | 0.88 ± 0.01 a | 0.91 ± 0.03 ab | 1.35 ± 0.16 b |
| Performance index | |||||
| Pi_Abs | 1.10 ± 0.34 ab | 1.70 ± 0.08 a | 1.66 ± 0.06 a | 1.47 ± 0.12 ab | 0.75 ± 0.13 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Verge-Campion, T.; Jauffrais, T.; Lefeuvre, L.; Houlbrèque, F. Commercial Arthrospira platensis Extract Modifies the Photophysiology of Cladocopium goreaui, Coral Endosymbiont Microalgae. Phycology 2025, 5, 50. https://doi.org/10.3390/phycology5030050
Le Verge-Campion T, Jauffrais T, Lefeuvre L, Houlbrèque F. Commercial Arthrospira platensis Extract Modifies the Photophysiology of Cladocopium goreaui, Coral Endosymbiont Microalgae. Phycology. 2025; 5(3):50. https://doi.org/10.3390/phycology5030050
Chicago/Turabian StyleLe Verge-Campion, Thibault, Thierry Jauffrais, Luc Lefeuvre, and Fanny Houlbrèque. 2025. "Commercial Arthrospira platensis Extract Modifies the Photophysiology of Cladocopium goreaui, Coral Endosymbiont Microalgae" Phycology 5, no. 3: 50. https://doi.org/10.3390/phycology5030050
APA StyleLe Verge-Campion, T., Jauffrais, T., Lefeuvre, L., & Houlbrèque, F. (2025). Commercial Arthrospira platensis Extract Modifies the Photophysiology of Cladocopium goreaui, Coral Endosymbiont Microalgae. Phycology, 5(3), 50. https://doi.org/10.3390/phycology5030050






