Abstract
Sustainable plant-based materials are becoming more popular as a substitute for those of animal origin for the encapsulation of compounds. Among different techniques, microencapsulation is widely used to protect bioactives and keep them intact to reach the desired target area. In this work, microencapsulation of oils by spray-drying using alternative vegan materials was proposed to mitigate oxidative degradation of oils. The determination of the best combination and ratio for different vegan wall materials (pectin, inulin, pea protein, and modified corn starch) was first developed using high-oleic sunflower oil enriched with β-carotene. In terms of efficiency, the best wall materials were pectin and inulin (P:I) in a 1:1 ratio, achieving 67.26 ± 0.78%. This ratio also obtained the best morphological results for shape and size studied by SEM (scanning electron microscopy) and DLS (dynamic light scattering). Additionally, the antioxidant activity of the oil enriched with β-carotene was studied, obtaining an IC5O of 0.15 mg/mL. Moreover, when Schizochytrium sp. was used instead of sunflower oil, as a docosahexaenoic acid (DHA)-enriched plant-based oil, the best results were also obtained for the P:I mixture, but at a ratio of 1:5. In all cases, the preservation of fatty acid profiles was achieved, giving insights for the potential use of alternative materials. The synergy between the use of antioxidants and encapsulation provides an effective method to avoid oxidation of edible oils. This work demonstrates the possibility of encapsulating carotenoid-enriched microalgae oil with vegan materials, improving its stability and bioavailability.
1. Introduction
In recent decades, there has been an increase in the consumption and search by consumers for functional foods with improved properties that provide benefits to the body through dietary intake. This is due to a growing awareness of the important role of diet in health and the link between chronic non-communicable diseases and diet [1].
Carotenoids are known for their ability to prevent the oxidation of other compounds. This is possible because they deactivate electronically excited molecules that are involved in the generation of singlet oxygen and other radicals. Carotenoids can stabilize singlet oxygen both chemically and physically. Chemical stabilization consists of the binding between the carotenoid and the free radical. In contrast, in physical stabilization, the excitation energy of singlet oxygen is transferred to the carotenoid, resulting in a low-energy singlet oxygen radical and an excited carotenoid [2].
β-carotene is a secondary metabolite synthesized by plants and has high antioxidant and anticarcinogenic activity. It also acts as a precursor to vitamin A, which is very important for embryonic development, vision and proper growth. In vivo studies have shown that β-carotene reduces the end products of lipid oxidation [3].
Certain bioactive compounds, such as polyunsaturated fatty acids (PUFAs), are unstable and susceptible to oxidation due to the presence of two or more double bonds in their molecular structure. Encapsulation serves as a highly beneficial technique within the food industry to mitigate the oxidation of oils rich in PUFAs, providing protection against oxygen, light, moisture, and heat. Additionally, it assists in preserving the properties of these compounds, thereby extending their shelf life. Ultimately, encapsulation prevents the formation of undesirable volatile compounds responsible for rancidity, which can negatively impact the taste and aroma of the food product [4,5,6]. In addition, encapsulation also enabled their focus release [7]. An example of a microencapsulation technique is the use of spray drying or mist drying, which rapidly achieves microcapsules by adding an emulsion to the equipment. In addition, no solvents are used in this technique. The equipment normally has a nozzle or needle that sprays the emulsion at high temperatures. On the one hand, the solvent (water) evaporates, and on the other, the microcapsules are deposited dry in a particle collector. In this equipment, different parameters, such as inlet and outlet temperatures, pump power, suction and flow must be controlled [8]. The advantages of the production of microcapsules by spray drying rely on reproducibility and scalability, as well as the broad range of products that could be encapsulated, depending on the wall material. This technology is one of the most common and readily available for encapsulating food ingredients, as it is highly versatile, provides good stability, and extends the shelf life of the bioactive ingredient. Moreover, it is a fast and green method in which solvents are not needed, with high yields, low processing requirements, and high efficiency. On the other hand, some disadvantages associated with spray drying have been identified. One principal drawback is the low recovery rate of encapsulated solids, which is attributed to the adhesion of powder onto the cyclone wall, as the particles are of a small size. The process involves the use of heated air; therefore, it is crucial to regulate the outlet temperature of the microcapsules appropriately, since excessive heat may lead to the degradation of the core compounds [9,10,11,12,13,14].
One of the first steps in microencapsulation is the choice of wall materials. The most prominent materials for microencapsulation are carbohydrates, lipids, and proteins, among others [15]. In recent years, however, natural polymers have gained importance, as they are usually biodegradable. In addition, they are categorized as “GRAS” (generally recognized as safe) [16]. Moreover, there is a huge need to look for greener and more environmentally friendly alternatives that are also accepted by all types of consumers, including vegetarians and vegans [17]. For all these reasons, recent studies focus on the search for alternative wall materials to polymers of animal origin, and plant-based polymers are becoming more important. In addition, some plant polysaccharides show better microencapsulation properties compared to other animal sources, such as good emulsification, solubility, and biodegradability [18], making them an option to consider as alternative wall materials [17].
Thus, the encapsulation of edible oils is also a key point in Food Science, as it can avoid the oxidation and spoilage of high-added-value compounds, such as polyunsaturated omega-3 lipids [19]. In this way, Schizochytrium sp. emerges as a sustainable source for lipids with proven bioactivity regarding neurodegenerative diseases [20], due to the high amount of docosahexaenoic acid in triacylglyceride form [21,22]. Therefore, the encapsulation of DHA-enriched oils by spray drying, coupled with the use of β-carotene as an antioxidant, was studied to obtain stable and suitable microcapsules that could be used in functional foods made from vegan materials.
2. Materials and Methods
2.1. Materials
β-carotene was supplied by Sigma-Aldrich (St. Louis, MO, USA). Pectin and inulin were supplied by Sosa Ingredients S.L. (Barcelona, Spain), pea protein was supplied by NaturGreen (Seville, Spain), and modified corn starch was supplied by Sigma-Aldrich (St. Louis, MO, USA). The hexane was supplied by Macron Fine Chemicals (Center Valley, PA, USA). The high-oleic sunflower oil used was Hacendado brand (Valencia, Spain), and DHA-rich Schizochytrium sp. oil was obtained in the lab. The starch, thiosulfate, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) used were supplied by Sigma-Aldrich (St. Louis, MO, USA), and potassium sodium was supplied by Panreac Química S.L.U. (Castellar del Vallès, Spain).
2.2. Spray Drying
Microencapsulation was carried out using a Spray Dryer Buchi B-191 (Buchi Labortechnik AG, Flawil, Switzerland). First, the drying chamber was preheated so that the temperature was uniform throughout the equipment. The process conditions were as follows: air inlet temperature, 185 °C; compressed air pressure, 5 bar; air flow, 400 L/min; suction, 50% and a peristaltic pump, 10%. Oils were enriched with β-carotene at 200 ppm.
Different mixtures of wall materials were evaluated: pectin and inulin (P:I), pea protein and inulin (PP:I), and pea protein and modified corn starch (PP:MCS). For the mixtures, three ratios between the materials were used as follows: 1:5, 5:1, and 1:1. The ratio between wall material and oil was 3:1. To prepare the emulsions, 2 g of oil were weighed and mixed with the wall materials in the appropriate proportion using a high-power dispersion equipment (Ultraturrax IKA, Staufen, Germany). Pectin and inulin were previously hydrated for one day in the refrigerator. The emulsions were maintained under constant magnetic agitation, and the microcapsules obtained were stored in dark conditions.
The equation to determine efficiency is the ratio of microencapsulated oil (total oil-free oil) divided by total oil, expressed as a percentage. For free oil extraction, 100 mg of microcapsules were mixed with 3 mL of hexane in a vortex for 2 min and then centrifuged for 8 min at 4000 rpm. For the total oil extraction, 100 mg of microcapsules were mixed with 2 mL of 40% acetic acid and vortexed for 2 min. The resulting solution was extracted with 3 mL hexane: isopropanol (1:1), shaken again, and centrifuged for 8 min at 4000 rpm. The supernatant was collected and weighed in a vial tared on a precision balance to determine the gravimetrically extracted oil. A second extraction with hexane was performed again and collected in the same vial.
2.3. Scanning Electron Microscope (SEM)
SEM equipment (Hitachi, model TM-1000; Chiyoda, Tokyo, Japan) was used to observe the morphology of the microcapsules. The SEM works with a beam of high-energy electrons incident on the sample, which reacts by giving signals that are detected by the detector, which shows an image of the sample [23,24]. A small sample was taken and placed on a double-sided tape that was glued to the sample loading area to evaluate the morphology of the microcapsules. It was then blown with a plastic bulb to remove the excess sample and placed in the equipment. Representative images were selected in order to determine the size and shape of a significant portion of the sample.
2.4. Dynamic Light Scattering (DLS)
The DLS (Malvern Zetasizer Nano ZS (Malvern, Herrenberg, Germany) equipped with a 633 nm He-Ne laser and operating at an angle of 173°) is a device used for analyzing complex colloidal systems, also known as photon correlation spectroscopy. Using this technique, it was possible to determine the particle size in colloidal suspensions. When particles are suspended in a liquid, such as water, they generate a motion that scatters light in the suspension, causing changes in the refractive index and intensity variations. The measurements were performed through a laser, usually a helium-neon laser and a photon correlator [25]. To make the measurements, 3 mg of the microcapsules were weighed into a test tube and dissolved in 4 mL of distilled water. Then, they were taken for 1 min to the ultrasonic bath to finish dissolving the sample so that the microcapsules remained in suspension. Then, a small sample was taken and added to a cuvette to be measured in the equipment. The software used to collect and analyze the data was Malvern’s Dispersion Technology software version 6.01.
2.5. Determination of Antioxidant Activity (DPPH Method)
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) method is used to determine the antioxidant activity of different substances, which consists of the compound’s ability to stabilize the free radicals released by the compound 2,2-diphenyl-1-picrylhydrazyl. The free radicals are neutralized by H+ donors provided by the antioxidant compound. Thanks to this method, the IC50 of the compound can be calculated, which is the concentration at which oxidation is inhibited at 50% [26,27]. DPPH assays were performed to determine the antioxidant activity of β-carotene using a 0.004% DPPH solution in methanol, and the method of Xia. et al. (2013) was followed with some modifications, measuring in a spectrophotometer at 517 nm. First, a calibration line of β-carotene was prepared from 0.3 to 0.0125 mg/mL in methanol. For the determination of DPPH inhibition, the following equation was used [28], and the IC50 was inferred.
Inhibition DPPH (%) = [1 − (A1 − A2)/A0] × 100
2.6. Characterization of Oils by GC-MS
The starting sunflower oil, as well as the microencapsulated oil, were characterized by gas chromatography mass spectrometry (GC-MS). For this purpose, the oil was derivatized by obtaining fatty acid methyl esters in a basic medium according to ISO TC34/SC 5. 25 mg of the oil was mixed with 200 μL of hexane 200 μL, then 50 μL of a solution of 2N KOH with methanol was added, vortexed for 1 min and allowed to stand for 5 min. Then, 125 mg of NaHSO4∙H2O was added, vortexed, and centrifuged for 5 min at 5000 rpm. 100 μL of the supernatant was collected and mixed with 400 μL of hexane for analysis. The method was performed in duplicate. The gas chromatograph used was an Agilent Technologies (Palo Alto, CA, USA) model 7820A coupled to an Agilent Technologies 5975 MSD series mass detector. Helium was used as carrier gas at a constant column flow rate of 1.5 mL/min and an Agilent Technologies HP-88 capillary column of 100 m × 0.25 mm × 0.20 μm, the injection temperature was 250 °C, and the injection was 1 μL with a 1:10 split. The column was maintained at 175 °C for 10 min after injection, and the temperature was programmed at 3 °C/min to 220 °C and maintained for 20 min more. The injector temperature was 250 °C, and the detector temperature was 230 °C. The mass spectrometer operated at 70 eV with a mass range of 30 to 400 amu. The fatty acids in the sample were identified using the NIST Mass-Spectral Library 2.0, expressing the amounts as percentages of the total fatty acid content.
2.7. Statistical Analysis
All analyses were performed in triplicate. GraphPad Prism 10 software was used for statistical analysis. Statistical analysis was performed using one-way analysis of variance (ANOVA) with a significance level of p ≤ 0.05.
3. Results
3.1. Composition of the Starting Oil
The starting high oleic sunflower oil was analyzed by GC-MS, and the composition of the oil was observed, showing a fatty acid profile (Figure 1) composed of palmitic acid (4.33 ± 0.12%), stearic acid (2.95 ± 0.22%), oleic acid (65.85 ± 0.33%), and linoleic acid (26.85 ± 0.04%).
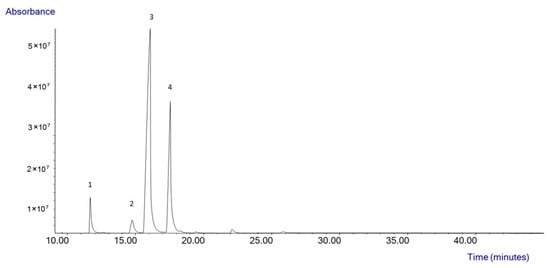
Figure 1.
Composition of the high oleic sunflower oil by GC-MS.
On the other hand, the composition of Schizochytrium sp. oil was also evaluated (Table 1). The main peaks obtained by GC-MS correspond to myristic acid (14:0), palmitic acid (16:0), docosapentaenoic acid (C22:5 n-6, DPA), and DHA (C22:6 n-3). It also had other minority fatty acids, such as stearic acid, oleic acid, linoleic acid, arachidonic acid, and eicosapentaenoic acid (EPA). Saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and PUFA were also reported.

Table 1.
Fatty acid profile of Schizochytrium oil. Results are expressed as a percentage of the total content (relative content). Data is shown as mean ± SD.
3.2. Antioxidant Activity
The β-carotene was selected to enrich the plant-based oils due to its potential biological activity reported in the literature [2]. Therefore, its antioxidant activity was evaluated. The IC50 was determined using the methodology described before (Section 2.5). The β-carotene reported an IC50 of 0.15 mg/mL. Considering that the oil used for microencapsulation was enriched to 200 ppm in this carotenoid (0.2 mg/mL), at least 50% of the oxidation that the oil could undergo was inhibited. This is because the concentration of the carotenoid in the oil (0.2 mg/mL) was higher than its IC50 value (0.15 mg/mL), which means that at least 50% of the potential oxidation was inhibited.
3.3. Encapsulation Efficiency (EE)
Taking into account the different wall materials used, it can be seen in Table 2 that the materials with the highest efficiency were the combination of pectin and inulin (P:I). Microcapsules of pectin and inulin in a 1:1 ratio showed the highest efficiency (67.3 ± 0.8%). In contrast, the use of pea protein (PP) instead of pectin offered efficiencies of around 10% and the worst results were given by the combination of pea protein and modified corn starch (MCS) with an efficiency of approximately 5.5% for all the ratios studied, which means that these microcapsules presented almost 10 times less microencapsulated oil compared to those of pectin and inulin.

Table 2.
Microencapsulation efficiency of β-carotene-enriched sunflower oil.
Based on the results observed, pectin and inulin were chosen as the best alternative wall materials for microencapsulation. Therefore, pectin and inulin were also tested with other plant-based oils, Schizochytrium sp. oil, either enriched or not in carotenoids, as it is represented in Figure 2 [29]. It should be noted that the 5:1 pectin:inulin ratio was not used because, in addition to obtaining the worst results in terms of microencapsulation efficiency among the three ratios (Table 2), this ratio had high viscosity due to its high proportion of pectin, which has excellent thickening properties, and it was not possible to use it in the spray-dryer equipment.
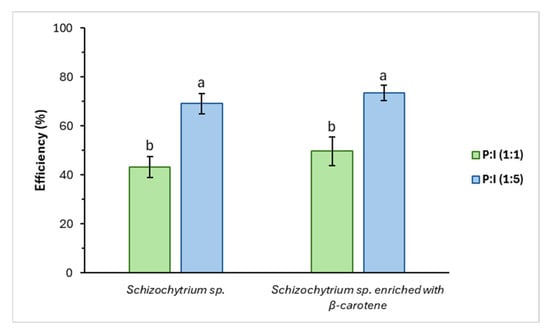
Figure 2.
Efficiency of Schizochytrium sp. oil and Schizochytrium sp. oil with β-carotene, applying the pectin:inulin mixture. a,b Different letters indicate significant differences in efficiency (p < 0.05) for the materials evaluated. Mean and standard deviation values for n = 3.
In this case, a higher EE was obtained with the 1:5 mixture than with 1:1, in contrast to sunflower oil. The encapsulation yield was 73.46 ± 3.19%, the highest EE value compared to those obtained for sunflower oil.
3.4. Morphology of Microcapsules
3.4.1. Scanning Electron Microscopy (SEM)
The microscopic structure of the obtained microcapsules was observed to further compare the wall materials and ratios used. The images were taken using SEM at 1500× magnification, and the figures show a representative picture of the whole sample. Figure 3 compares the different types of wall materials studied (P:I, PP:I, and PP:MCS), using the same ratio (1:5). As seen, the morphology of the microcapsules shows the same trend as the efficiency. The microcapsules made with P:I (Figure 3A) presented two particle sizes within which there was homogeneity, but there were also some deformations. The PP:I (Figure 3B) had a larger size, less homogeneity, and deformations were also observed, and on the other hand, the PP:MCS microcapsules (Figure 3C) were very deformed and had sizes much larger compared to the rest. Thus, microcapsules made with pectin and inulin showed better morphology than when using other wall materials, such as pea protein or corn starch.
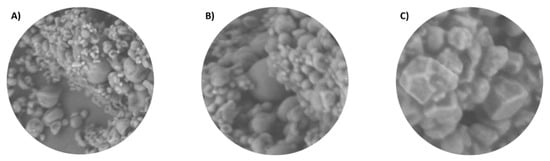
Figure 3.
Representative images analyzed by SEM at 1500× magnification. (A) P:I (1:5). (B) PP:I (1:5). (C) PP:MCS (1:5).
Therefore, in Figure 4, microcapsules made with pectin and inulin at different ratios of 1:5, 5:1, and 1:1 were compared. Accordingly, in all three cases, the microcapsules exhibited little deformation, turgidity, and homogeneity. Comparing the different ratios, the smallest particles were those of the 5:1 ratio (Figure 4B).
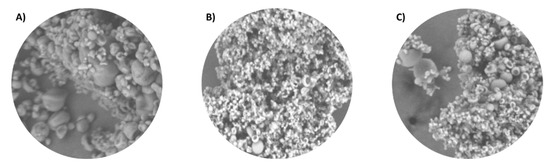
Figure 4.
Representative images analyzed by SEM at 1500× magnification. (A) P:I (1:5). (B) P:I (5:1). (C) P:I (1:1).
Microcapsules with Schizochytrium sp. oil using pectin and inulin at a ratio of 1:5 were also examined in Figure 5 and compared to those of a 1:1 ratio. In this case, both types of microcapsules had similar sizes and morphology.
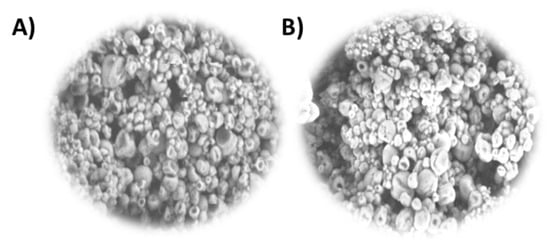
Figure 5.
Schizochytrium sp. oil with β-carotene microcapsules, representative images analyzed by SEM at 1500× magnification. (A) P:I (1:1), (B) P:I (1:5).
3.4.2. Dynamic Light Scattering (DLS)
The size dispersion of the microcapsules made with pectin and inulin using sunflower oil was analyzed by DLS (Figure 6). In accordance with the results obtained by SEM, the smallest sizes were obtained using P:I (5:1). Furthermore, this mixture also represented the worst efficiency, which may be related to the size. The size of the microcapsules made with pectin and inulin was very tiny in all three cases, obtaining capsules smaller than 1 µm. It must be considered that the equipment is only capable of measuring particles smaller than 10 µm.
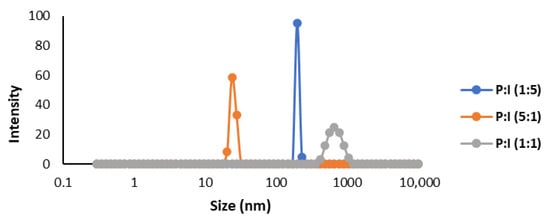
Figure 6.
Size dispersion analyzed by DLS of microcapsules produced by spray drying of sunflower oil enriched in β-carotene.
When analyzing the microcapsules obtained using the microalgal oil (Figure 7), the particle sizes for both the P:I 1:1 and P:I 1:5 mixtures were in agreement with the SEM images, obtaining two defined peaks: one around 1 nm and the other less than 10 µm.
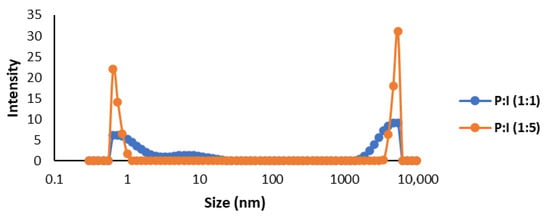
Figure 7.
Size dispersion analyzed by DLS of microcapsules produced by spray drying of Schizochytrium sp. oil with β-carotene.
3.5. Characterization of the Microencapsulated Oil
To study whether microencapsulation modifies the lipid profile of the oil, the oil before and after the microencapsulation process was evaluated by GC-MS. As shown in Figure 8, it was found that there were no significant differences in the composition of the oil. It can therefore be concluded that microencapsulation did not change the oil composition in fatty acids relative content.
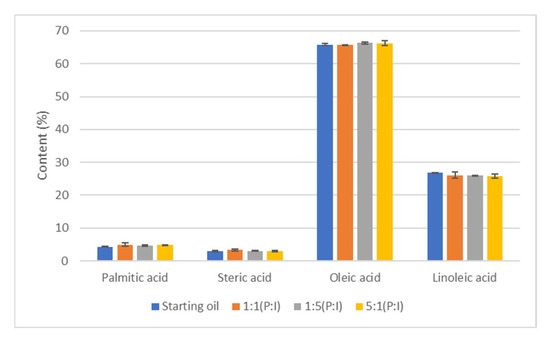
Figure 8.
Analysis of fatty acid composition by GC-MS after microencapsulation.
4. Discussion
Antioxidant compounds such as carotenoids or PUFAs are highly sensitive to light and heat, limiting their applications in food processing and storage conditions. For all these reasons, encapsulation is an interesting option for protecting these compounds [30]. The results obtained in this paper by microencapsulation of edible oils using the spray-dryer method were in accordance with the literature. As expected, oleic acid was the major fatty acid in the starting oil as it was enriched. According to composition, the fatty acid that should be in the greatest quantity in sunflower oil should be linoleic acid, with a content of over 50% [31]. β-carotene is a secondary metabolite synthesized by plants. It also has high antioxidant and anticarcinogenic activity. Additionally, it acts as a precursor to vitamin A, which is very important for embryonic development, vision, and proper growth [32,33,34]. In vivo studies have shown that β-carotene reduces lipid oxidation. For all these advantages, it is interesting to add β-carotene to the oil [3]. The same equivalence was obtained for microalgal oil, mainly composed of four fatty acids: C14:0, C16:0, C22:5 n-6 DPA, and C22:6 n-3 DHA, as it was previously reported [35]. The high number of fatty acids present in both vegan oils was additionally preserved using carotenoids. The addition of antioxidants to oil is widely described in the literature [36,37]. However, the pro-oxidant activity of carotenoids could be explored when working at very high levels. In this paper, the study of β-carotene regarding antioxidant capacity indicated that 200 ppm was enough to avoid the oxidation of lipids. After the encapsulation process, the oil composition remained stable, as reported in Section 3.5.
On the other hand, not only is the composition of the oil that was inside the microcapsules important in terms of nutritional purposes but also the size of the microcapsules in a proper application inside the food matrix. The ideal size is smaller than 100 µm, because if they are larger, they could be detected in the mouth, which could lead to rejection by consumers [38]. Moreover, a compromise solution between size and yield needs to be determined. Microcapsules with very small sizes did not enable the encapsulation of higher amounts of edible oils, affecting the nutritional requirements. In this case, particles smaller than 10 µm were obtained and could then be used in further applications in functional foods [39].
Another key point in the production of microcapsules for extended use in society is the components that are, in fact, creating the microcapsules, which are called cell wall materials. Microcapsules have been produced using different types of proteins and carbohydrates, but a vast number of them are of animal origin, such as gelatin, casein, albumin, or chitosan. This led to a lack of vegan substitutes that should be exploited according to Global Trends [17,40]. In this work, different alternative cell wall materials were studied, with the best results obtained from using pectin and inulin.
Higher results were obtained when using a pectin-inulin mixture and microalgal oil, improving the results obtained for sunflower oil.
Inulin is a polysaccharide found in plants with proven prebiotic activity. Due to its structure, this compound is not digestible. In some studies, inulin has been linked to changes in the composition of the microbiota through the growth of species beneficial to the intestine, such as the Bifidobacterium, Anaerostipes, and Lactobacillus genus, improving metabolic status [41,42,43]. In 1987, inulin was approved by the European Health Committee as a functional ingredient for its potential in fighting chronic diseases and obesity [44]. Other studies have shown that inulin regulates blood glucose and lipid levels. In addition, due to its prebiotic activity, it can improve mineral absorption and relieve constipation [45]. Pectin is another important non-digestible polysaccharide with proven prebiotic activity. For this material, the use of pectin as a carbon source for the growth of probiotic strains, such as Lactobacillus and Bifidobacterium, has been studied in the literature [46]. On the other hand, pectins are key elements for modulating the intestinal microbiota, preventing inflammatory bowel diseases and thus promoting intestinal health [47]. For all these reasons, the combination of both polysaccharides for the formation of the microcapsule wall material is so interesting. Therefore, both inulin and pectin have been effectively used as wall materials in spray drying microencapsulation processes [48]. Their individual and combined applications have shown promising results in terms of encapsulation efficiency, morphological properties, and stability of bioactive compounds.
Microencapsulation of carotenoid-enriched plant-based oils by spray-drying with vegan wall materials, such as inulin and pectin, provides a versatile approach to enhance carotenoid stability and antioxidant activity in food and pharmaceutical products. This process produces protective powder microcapsules that shield carotenoids from oxygen, light, and heat, preserving their functional properties during processing, storage, and transport. By optimizing wall composition and spray-drying conditions, encapsulation efficiency can be increased, enabling fortification of foods and nutraceuticals with vegan-friendly formulations. In food applications, these microcapsules are used to enrich baked goods, beverages, and dairy alternatives, serving as both natural colorants and fat replacers, while the powder format ensures easy handling and protection against oxidation and thermal degradation. In pharmaceuticals, microencapsulated carotenoid oils improve shelf life, allow controlled release, and support standardized dosing in dietary supplements and functional capsules.
In future research, following the investigation line of this work, the toxicity, bioactivity, and bioavailability of microcapsules will be evaluated to analyze if they are able to both resist the digestive system and eventually release the compounds. Equally, the stability of microcapsules during storage should be evaluated. Besides, it is foreseeable that the scale-up of the microencapsulation process through spray-drying with the best mix will be observed (P:I, 1:1). In order to improve the production of liposomes, their production with lower-cost phospholipids should be analyzed. Another objective for future research is the administration of pectin oligosaccharides (POS) instead of pectin. It has been studied that these oligomers can enhance the probiotic effect of pectin because they are smaller in size and do not need to be broken down [46].
Author Contributions
Conceptualization, P.G.-G. and F.J.S.; methodology, M.D. and G.B.; investigation, M.D., G.B. and P.G.-G.; resources, F.J.S.; formal analysis, M.D., G.B. and P.G.-G.; data curation, P.G.-G.; writing—original draft preparation, M.D., G.B. and P.G.-G.; writing—review and editing, P.G.-G.; supervision, P.G.-G. and F.J.S.; project administration, F.J.S.; funding acquisition, F.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Comunidad de Madrid (Spain) through project ALGATEC-CM (P2018/BAA-4532), co-financed by the European Social Fund, and the Ministry of Spain through project POLARALGA (PID2022-143229NB-I00).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors gratefully acknowledge the support from Comunidad de Madrid, through the program Yo Investigo.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| P | Pectin |
| PP | Pea protein |
| I | Inuline |
| MCS | Corn starch |
| SEM | Scanning electron microscopy |
| DLS | Dynamic light scattering |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| GC-MS | Gas chromatography- mass spectrometry |
| EE | Encapsulation efficiency |
| DHA | Docosahexaenoic acid |
| IC | Inhibitory concentration |
| SFA | Saturated fatty acid |
| MUFA | Monounsaturated fatty acid |
| PUFA | Polyunsaturated fatty acid |
References
- Vicentini, A.; Liberatore, L.; Mastrocola, D. Functional Foods: Trends and Development of the Global Market. Ital. J. Food Sci. 2016, 28, 338–351. [Google Scholar]
- Yahia, E.M.; de Jesús Ornelas-Paz, J.; Emanuelli, T.; Jacob-Lopes, E.; Zepka, L.Q.; Cervantes-Paz, B. Chemistry, Stability, and Biological Actions of Carotenoids. In Fruit and Vegetable Phytochemicals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 285–346. [Google Scholar]
- Bogacz-Radomska, L.; Harasym, J. β-Carotene—Properties and production methods. Food Qual. Saf. 2018, 2, 69–74. [Google Scholar] [CrossRef]
- Castejón, N.; Luna, P.; Señoráns, F.J. Microencapsulation by spray drying of omega-3 lipids extracted from oilseeds and microalgae: Effect on polyunsaturated fatty acid composition. LWT 2021, 148, 111789. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Compre-hensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Wang, B.; Adhikari, R.; Adhikari, B. Advances in microencapsulation of polyunsaturated fatty acids (PUFAs)-rich plant oils using complex coacervation: A review. Food Hydrocoll. 2017, 69, 369–381. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Avendaño-Godoy, J.; Santos, J.; Lozano-Castellón, J.; Mardones, C.; von Baer, D.; Luengo, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Gómez-Gaete, C. Encapsulation of Phenolic Compounds from a Grape Cane Pilot-Plant Extract in Hydroxypropyl Beta-Cyclodextrin and Maltodextrin by Spray Drying. Antioxidants 2021, 10, 1130. [Google Scholar] [CrossRef]
- Gu, B.; Linehan, B.; Tseng, Y.C. Optimization of the Büchi B-90 spray drying process using central composite design for preparation of solid dispersions. Int. J. Pharm. 2015, 491, 208–217. [Google Scholar] [CrossRef]
- Parra Huertas, R.A. Food microencapsulation: A review. Rev. Fac. Nac. Agron. Medellín 2010, 63, 5669–5684. [Google Scholar]
- Alexe, P.; Dima, C. Microencapsulation in food products. AgroLife Sci. J. 2014, 3, 9–14. [Google Scholar]
- Ameri, M.; Maa, Y.F. Spray Drying of Biopharmaceuticals: Stability and Process Considerations. Dry. Technol. 2006, 24, 763–768. [Google Scholar] [CrossRef]
- Ghnimi, S.; Budilarto, E.; Kamal-Eldin, A. The New Paradigm for Lipid Oxidation and Insights to Microencapsula-tion of Omega-3 Fatty Acids. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Masum, A.K.M.; Chandrapala, J.; Huppertz, T.; Adhikari, B.; Zisu, B. Influence of drying temperatures and storage pa-rameters on the physicochemical properties of spray-dried infant milk formula powders. Int. Dairy J. 2020, 105, 104696. [Google Scholar] [CrossRef]
- Furuta, T.; Neoh, T.L. Microencapsulation of food bioactive components by spray drying: A review. Dry Technol. 2021, 39, 1800–1831. [Google Scholar] [CrossRef]
- Muhoza, B.; Qi, B.; Harindintwali, J.D.; Farag Koko, M.Y.; Zhang, S.; Li, Y. Combined plant protein modification and complex coacervation as a sustainable strategy to produce coacervates encapsulating bioactives. Food Hydrocoll. 2022, 124, 107239. [Google Scholar] [CrossRef]
- Macías-Cortés, E.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E.; Moreno-Jiménez, M.R.; Medina-Torres, L.; González-Laredo, R.F. Microencapsulation of phenolic compounds: Technologies and novel polymers. Rev. Mex. Ing. Quím. 2019, 19, 491–521. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Peighambardoust, S.H.; Sarabandi, K.; Jafari, S.M. Spray drying encapsulation of bioactive compounds within protein-based carriers; different options and applications. Food Chem. 2021, 359, 129965. [Google Scholar] [CrossRef]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Hennebelle, M.; Villeneuve, P.; Durand, E.; Lecomte, J.; Van Duynhoven, J.; Meynier, A.; Yesiltas, B.; Jacobsen, C.; Berton-Carabin, C. Lipid oxidation in emulsions: New insights from the past two decades. Prog. Lipid Res. 2024, 94, 101275. [Google Scholar] [CrossRef]
- Sahin, D.; Tas, E.; Altindag, U.H. Enhancement of docosahexaenoic acid (DHA) production from Schizochytrium sp. S31 using different growth medium conditions. AMB Express. 2018, 8, 7. [Google Scholar] [CrossRef]
- Metz, J.G.; Kuner, J.; Rosenzweig, B.; Lippmeier, J.C.; Roessler, P.; Zirkle, R. Biochemical characterization of polyunsaturated fatty acid synthesis in Schizochytrium: Release of the products as free fatty acids. Plant Physiol. Biochem. 2009, 47, 472–478. [Google Scholar] [CrossRef]
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.; Beversdorf, D.Q. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 3–13. [Google Scholar] [CrossRef]
- Zhou, W.; Apkarian, R.P.; Wang, Z.L.; Joy, D. Fundamentals of Scanning Electron Microscopy (SEM). In Scanning Microscopy for Nanotechnology; Zhou, W., Wang, Z.L., Eds.; Springer: New York, NY, USA, 2006; pp. 4–40. [Google Scholar]
- Vernon-Parry, K.D. Scanning electron microscopy: An introduction. III-Vs Rev. 2000, 13, 40–44. [Google Scholar] [CrossRef]
- Hoo, C.M.; Starostin, N.; West, P.; Mecartney, M.L. A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J. Nanoparticle Res. 2008, 10, 89–96. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, Characterization, and Antioxidant Activity of Fucoxanthin from the Marine Diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef]
- Raghav, N.; Vashisth, C.; Mor, N.; Arya, P.; Sharma, M.R.; Kaur, R.; Bhatti, S.P.; Kennedy, J.F. Recent advances in cellulose, pectin, carragee-nan and alginate-based oral drug delivery systems. Int. J. Biol. Macromol. 2023, 244, 125357. [Google Scholar] [CrossRef]
- Habtegebriel, H.; Tazart, Z.; Farrugia, C.; Gatt, R.; Valdramidis, V. Storage stability and antioxidant activity of astaxanthin and beta-carotene as affected by the architecture of O/W emulsions of milk proteins. LTW 2024, 209, 116733. [Google Scholar] [CrossRef]
- Gunstone, F. Vegetable Oils in Food Technology: Composition, Properties and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2011; 378p. [Google Scholar]
- Nakonechna, K.; Ilko, V.; Berčíková, M.; Vietoris, V.; Panovská, Z.; Doležal, M. Nutritional, Utility, and Sensory Quality and Safety of Sunflower Oil on the Central European Market. Agriculture 2024, 14, 536. [Google Scholar] [CrossRef]
- Zhao, T.; He, X.; Yan, X.; Xi, H.; Li, Y.; Yang, X. Recent advances in the extraction, synthesis, biological activities, and stabilisation strategies for β-carotene: A review. Int. J. Food Sci. Technol. 2024, 59, 2136–2147. [Google Scholar] [CrossRef]
- Aditi Bhardwaj, R.; Yadav, A.; Swapnil, P.; Meena, M. Characterization of microalgal β-carotene and astaxanthin: Exploring their health-promoting properties under the effect of salinity and light intensity. Biotechnol. Biofuels Bioprod. 2025, 18, 18. [Google Scholar]
- Neylan, K.A.; Johnson, R.B.; Barrows, F.T.; Marancik, D.P.; Hamilton, S.L.; Gardner, L.D. Evaluating a microalga (Schizochytrium sp.) as an alternative to fish oil in fish-free feeds for sablefish (Anoplopoma fimbria). Aquaculture 2024, 578, 740000. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, Z.; McClements, D.J.; Xie, B.; Zheng, R.; Deng, Q.; Chen, Y. Carotenoids encapsulated in natural flaxseed oil body: Different colloidal interfaces induce their behavior and stability disparity. Food Hydrocoll. 2024, 156, 110311. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Cui, B.; Wang, M.; Fu, H.; Wang, Y. Carotenoid-enriched oil preparation and stability analysis during storage: Influence of oils’ chain length and fatty acid saturation. LWT 2021, 151, 112163. [Google Scholar] [CrossRef]
- Capela, P.; Hay, T.K.C.; Shah, N.P. Effect of homogenisation on bead size and survival of encapsulated probiotic bacteria. Food Res. Int. 2007, 40, 1261–1269. [Google Scholar] [CrossRef]
- Villena, M.M.; Hernández, M.M.; Lara, V.G.; Martínez, M.R. Técnicas de microencapsulación: Una propuesta para microencapsular probióticos. Ars. Pharm. 2009, 50, 43–50. [Google Scholar]
- Ma, D.; Yang, B.; Zhao, J.; Yuan, D.; Li, Q. Advances in protein-based microcapsules and their applications: A review. Int. J. Biol. Macromol. 2024, 263, 129742. [Google Scholar] [CrossRef]
- Alonso-Allende, J.; Milagro, F.I.; Aranaz, P. Health Effects and Mechanisms of Inulin Action in Human Metabolism. Nutrients 2024, 16, 2935. [Google Scholar] [CrossRef]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut 2019, 68, 1430–1438. [Google Scholar]
- Rodriguez, J.; Neyrinck, A.M.; Van Kerckhoven, M.; Gianfrancesco, M.A.; Renguet, E.; Bertrand, L.; Cani, P.D.; Lanthier, N.; Cnop, M.; Paquot, N.; et al. Physical activity enhances the improvement of body mass index and metabolism by inulin: A multicenter randomized placebo-controlled trial performed in obese individuals. BMC Med. 2022, 20, 110. [Google Scholar] [CrossRef]
- Talukdar, J.R.; Cooper, M.A.; Lyutvyn, L.; Zeraatkar, D.; Ali, R.; Bierbrier, R.; Janes, S.; Ha, V.; Darling, P.B.; Sievenpiper, J.L.; et al. Effects of inulin-type fructans supplementation on cardiovascular disease risk factors: A protocol for a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2022, 6, e058875. [Google Scholar] [CrossRef]
- Yu, Y.; He, J.; Fu, H.; Mi, Y.; Wu, H.; Gao, Y.; Li, M. Inulin Modulates Gut Microbiota and Increases Short-Chain Fatty Acids Levels to Inhibit Colon Tumorigenesis in Rat Models: A Systematic Review and Meta-Analysis. J. Food Sci. 2025, 90, e70250. [Google Scholar] [CrossRef]
- Alencar JCGde Pinto GTda, S.; Cerqueira e Silva, K.F.; Santos, J.M.S.; Hubinger, M.D.; Bicas, J.L.; Maróstica Júnior, M.R.; Petkowicz, C.L.d.O.; Paulino, B.N. Pectin and pectic oligosaccharides (POS): Recent advances for extraction, production, and its prebiotic potential. Trends Food Sci. Technol. 2025, 155, 104808. [Google Scholar] [CrossRef]
- Santos Donadio, J.L.; Paulo Fabi, J. Comparative analysis of pectin and prebiotics on human microbiota modulation in early life stages and adults. Food Funct. 2024, 15, 6825–6846. [Google Scholar] [CrossRef] [PubMed]
- Rodsuwan, U.; Thumthanaruk, B.; Vatanyoopaisarn, S.; Thisayakorn, K.; Zhong, Q.; Panjawattanangkul, S.; Rungsardthong, V. Microencapsulation of gamma oryzanol using inulin as wall material by spray drying: Optimization of formulation and characterization of microcapsules. J. Food Sci. Technol. 2024, 61, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).