Abstract
Microalgae are photosynthetic organisms with rapid growth and high biochemical diversity, capable of thriving in a variety of environments. Among them, dinoflagellates, particularly symbiotic species like Durusdinium glynnii, have gained attention due to their potential for biotechnological applications, especially in the production of valuable fatty acids. However, the delicate cultivation of dinoflagellates remains a challenge due to their sensitivity to shear stress and complex morphology. In this study, we evaluated the influence of inoculum percentage (10%, 25%, and 50%) on the growth performance and fatty acid profile of D. glynnii during a scale-up process from test tubes to a pilot-scale photobioreactor. Higher inoculum concentrations (50%) promoted faster acclimatization, higher specific growth rates (µmax), and greater final biomass densities, optimizing the cultivation process. Meanwhile, lower inoculum concentrations (10%) favored the accumulation of polyunsaturated fatty acids, particularly DHA (C22:6n3), indicating a trade-off between biomass productivity and fatty acid biosynthesis. Overall, D. glynnii demonstrated robust adaptability, reinforcing its potential as a sustainable source of bioactive compounds. Further studies focusing on cellular and metabolic pathways are needed to better elucidate the mechanisms underlying lipid production and growth in this promising species.
1. Introduction
Microalgae are photosynthesizing organisms characterized by rapid growth and diverse morphological, physiological, and metabolic diversity [1,2]. These organisms can thrive in a variety of environments, including aquatic and terrestrial habitats, industrial and domestic waste, and in symbiosis with other organisms [3]. Their survival and development are influenced by environmental factors such as carbon and light availability, which directly affect their growth and metabolic characteristics. Microalgae have great potential for producing a variety of compounds with applications in different industrial sectors. Additionally, some microalgae produce polyunsaturated fatty acids, which are important for human health and can be used in the production of nutritional supplements and pharmaceuticals [4,5,6].
Among the emerging group of microalgae for pharmaceutical applications, dinoflagellates represent a novel pool of poorly explored organisms. There are approximately 7000 species of dinoflagellates that can be distinguished based on their morphology, metabolism characteristics, and others ecological factors. Among these, approximately half are heterotrophic, while the other half possess plastids, allowing a nutritional range from photoautotrophy to mixotrophy [7,8,9]. Most dinoflagellates are free-living; however, about 10% of the species exhibit parasitic lifestyles, while around 1% engage in mutualistic associations [7]. Mutualistic dinoflagellates are particularly important, as their relationship with reef-building scleractinians underpins the formation and maintenance of coral reef ecosystems [10]. Symbiotic relationships between invertebrates and photosynthetic partners are common in the marine environment, with one of the most well known being the endosymbiosis between dinoflagellates of the family Symbiodiniaceae and cnidarians—including corals, anemones, jellyfish, and hydrocorals. In this endosymbiotic relationship, dinoflagellates provide photosynthetic products to cnidarians while receiving shelter and nutrients from the host [10].
Dinoflagellates have been the focus of many studies due to their ability to produce a wide variety of secondary metabolites, including hormones, allelochemicals, omega-3 fatty acids, essential amino acids, carotenoids, and toxins [11]. These compounds are not essential for cell survival and reproduction; however, they perform a variety of important biological functions. Their biosynthesis can be significantly influenced by culture conditions, including physical (e.g., light intensity, temperature), chemical (e.g., salinity, pH), and nutritional (in terms of nutritional metabolism and nutrient availability) parameters, which collectively modulate the metabolic pathways involved in their production [11,12]. Although dinoflagellates contain various bioactive molecules with great biotechnological and pharmaceutical potential, their cultivation presents several challenges that can negatively affect both biomass production and quality [13]. This process is delicate due to the organisms’ high sensitivity to shear forces. Bioreactors, which are the most common method of cultivation, typically produce a bubble layer and high aeration rates, both of which can inhibit growth. This sensitivity is largely due to the complex cell morphology of dinoflagellates, including the presence of a long, thin flagellum that is easily damaged [14,15,16]. As a result, dinoflagellate cultivation is often the subject of experimental studies aimed at optimizing cultivation parameters, taking both abiotic and biotic factors into account [17,18]
Marine microalgae, particularly dinoflagellates, are promising and sustainable sources of these fatty acids. They can accumulate between 0.1% and 30% lipids in their biomass, making them an attractive option. Moreover, their cultivation does not compete with agricultural land, nor does it require large volumes of freshwater, positioning them as a strategic alternative to meet the growing demand for functional lipid sources in the food and pharmaceutical industries [19,20,21,22]. Despite the extensive diversity of fatty acids already identified and the progress made in research, studies specifically focusing on dinoflagellates are still limited compared to other microalgal groups [23]. However, recent studies have highlighted the significant fatty acid production capabilities of dinoflagellates, pointing to an underexplored biotechnological potential within this group [24,25]. Furthermore, in previous studies, we identified the dinoflagellate Durusdinium glynnii as a promising species for cultivation, characterized by its high-value biomass enriched with fatty acids and antioxidant metabolites. These findings supported its selection for the present investigation, which, to the best of our knowledge, constitutes one of the first systematic evaluations of large-scale cultivation strategies for this species. In this context, the objective of the present work is to assess the impact of inoculum percentage on the growth performance and fatty acid profile of the endosymbiotic dinoflagellate D. glynnii during scale-up.
2. Materials and Methods
2.1. Strain and Culture Conditions
A Durusdinium glynnii strain, clone BMK 211, was kindly provided by the Oceanographic Institute of the University of São Paulo (IO USP). It was maintained at the Live Food Production Laboratory (LAPAVI) in f/2 culture medium [26] with treated seawater at a salinity of 30 PSU, under an irradiance of 150 μmol photons m⁻2 s⁻1, provided by 36 W white LED lamps, at 21 ± 1 °C. The experiments were carried in the same conditions of strain maintaining in terms of salinity, irradiance, and temperature, with aeration using atmospheric air bubbling (0.05 L air L⁻1 min⁻1).
2.2. Scaling-Up Strategies
To evaluate the most effective scaling-up strategies for D. glynnii, the initial inoculations were conducted in 100 mL borosilicate glass tubes containing 50 mL of working volume. Three inoculum percentages—10%, 25%, and 50% of the previous cultivation volume from the previous stage—were tested. The scale-up process consisted of four to six sequential cultivation steps, depending on the inoculum strategy adopted, as detailed below:
- (i)
- Test tubes with 50 mL of working volume;
- (ii)
- Erlenmeyer flasks with working volume ranging from 0.25 L to 2 L;
- (iii)
- 5 L transparent plastic bottles;
- (iv)
- A cylindrical pilot-scale photobioreactor, adapted from a 100 L carboy tank.
In cultivation steps 1 and 2, the f/2 culture medium was employed, while in steps 3 and 4, a more cost-effective inorganic fertilizer (N:P:K 14:10:14, 88% purity), composed of urea, single superphosphate, and potassium oxide, was used as the nutrient base. To ensure consistency in nitrogen availability across scales, the fertilizer solution was diluted to match the nitrogen concentration of the f/2 medium. Additionally, a trace metal solution based on the f/2 formulation was added. The capacity of D. glynnii to assimilate urea as a nitrogen source has been previously demonstrated [27], supporting the feasibility of this substitution in large-scale applications. For step 4, the cultures were conducted without replicates (n = 1), while for the other steps, three independent replicates were conducted (n = 3).
It is noteworthy that, depending on the inoculum volume, the working volume of the reactors at each step was not always fully occupied. In several cases, culture progression occurred within the same vessel—without the need to change to a larger reactor—by simply supplementing with fresh culture medium. This approach allowed for operational flexibility while maintaining appropriate cell densities and growth kinetics. Furthermore, all transfers to subsequent cultivation stages—or additions of fresh medium in the same vessel—were performed on the third day of the exponential growth phase, prior to the onset of the stationary phase. This timing was strategically selected to avoid nutrient and/or light limitations, thereby preserving the physiological activity of the culture and optimizing biomass productivity across the scaling process.
2.3. Growth Analysis
To assess D. glynnii growth during scaling-up, 1.5 mL samples were periodically collected from the experimental units and fixed with 4% formaldehyde for cell counting using a hemocytometer under a binocular light microscope. Based on the average cell density, growth curves were generated for each inoculum percentage, and the specific growth rate (µ, day−1) was calculated [28].
2.4. Biomass Harvesting and Drying
At the end of Step 4, the experimental cultures were centrifugated at 3500× g, freeze-dried at −50 °C and 150 × 10−3 mbar for 48 h, and stored at −20 °C until lipid extraction for the determination of the fatty acids profile.
2.5. Lipid Extraction and Fatty Acid Composition
Total lipid extraction was carried out using the protocol established by Folch et al. [28], modified by Axelsson and Gentili [29]. Briefly, approximately 0.2 g of biomass was homogenized in a 2:1 (v/v) chloroform–methanol mixture using a Vortex mixer for 2 min, followed by extraction through 30 min of sonication in an ultrasonic water bath. Subsequently, a 0.73% sodium chloride solution was added to adjust the final solvent ratio to 2:1:0.8 (chloroform–methanol–water, v/v/v). The mixture was centrifuged at 500× g for 5 min and the organic (lipid-containing) layer was then collected, rinsed once with chloroform, centrifuged again for purification, and subsequently evaporated to dryness under a stream of nitrogen. The fatty acid profile of the lipids extracted from D. glynnii biomass was determined using gas chromatography (model GC-2014, Shimadzu, Kyoto, Japan) after transesterification into their respective methyl esters, as described by O’Fallon et al. [30]. The chromatographic conditions and identification procedures were detailed elsewhere [31].
2.6. Statistical Analysis
The data were subjected to descriptive statistics, including mean, median, and standard deviation. Possible differences between the means (n = 3) of growth parameters and fatty acid methyl esters (FAMEs) were evaluated using one-way analysis of variance (ANOVA), provided that the assumptions (normality and variance homoscedasticity) of this test were met. When statistically significant differences were found at a 5% significance level, Tukey’s post hoc test was applied for mean separation. Linear regression analyses were performed to evaluate the relationship between the major fatty acids identified in the biomass of D. glynnii and the inoculum percentages tested. All statistical analyses and graphical representations were conducted using RStudio software (version 4.3.0).
3. Results
In the present study, Durusdinium glynnii demonstrated robust adaptability and consistent growth performance across all cultivation steps, ranging from initial volumes in test tubes to pilot-scale photobioreactors. Notably, the inoculum concentration played a decisive role in terms of cell growth (Figure 1). The dinoflagellate successfully acclimated to all inoculum strategies tested; however, higher inoculum proportions (25% and 50%) conferred marked advantages over the 10% treatment. These benefits were particularly evident in the reduced acclimatization periods, enhanced specific growth rates, and elevated final cell densities, underscoring the importance of inoculum optimization in scaling up microalgal cultivation for biotechnological applications.
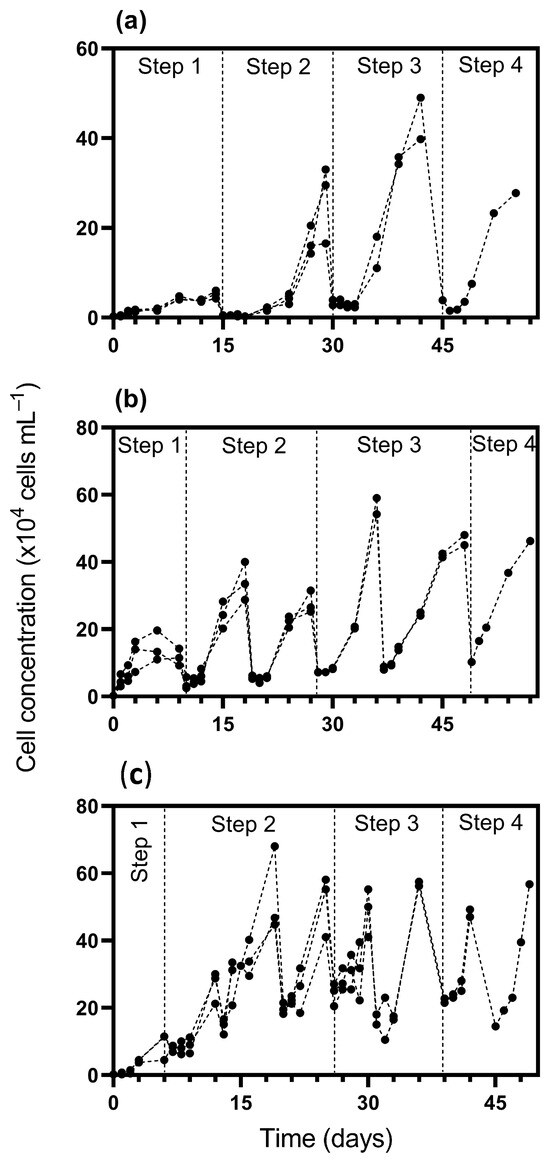
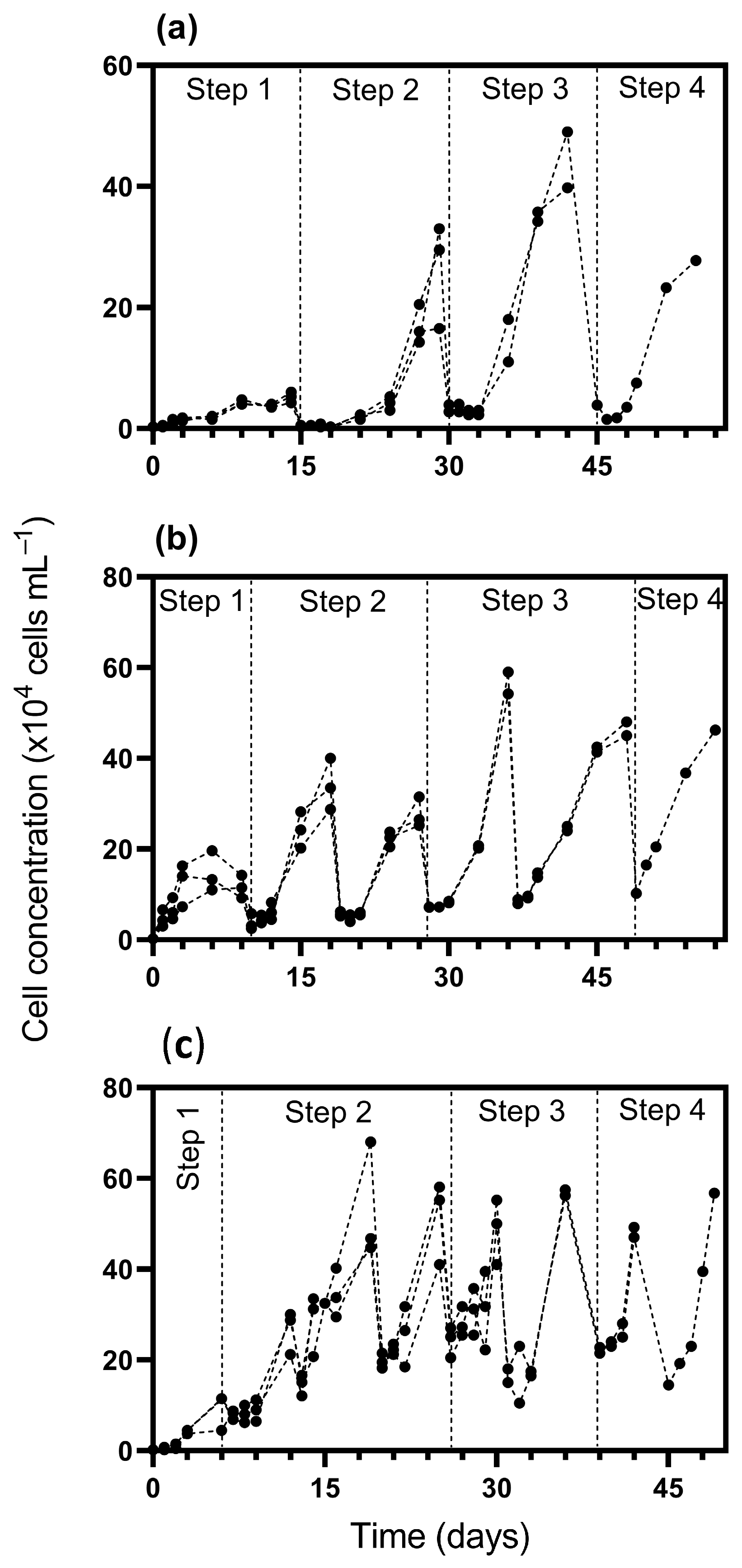
Figure 1.
Cell concentration of Durusdinium glynnii during cultivation using 10% (a), 25% (b), and 50% (c) inoculum percentages as scaling-up strategies. Drops in the growth curves represent dilution events, either due to volume increases within a given cultivation step or during the transition between sequential steps in the scale-up process.
In step 1, the 10% inoculum strategy presented a lower cell density (5.2 × 104 cells mL⁻1), while the strategies with 25% and 50% reached densities of 15 × 104 and 9 × 104 cells mL⁻1, respectively. Regarding the acclimatization period, the 10% strategy required more time (15 days), while the 50% strategy reached the exponential phase more quickly, in only 6 days (Figure 2). In step 2, cultures inoculated with 10% displayed a delayed acclimation, only reaching this phase by day 21, with a cell density of 26.3 × 104 cells mL⁻1. In contrast, cultures with 25% and 50% inoculum transitioned earlier, on days 10 and 6, achieving densities of 27.75 × 104 and 53.16 × 104 cells mL⁻1, respectively.
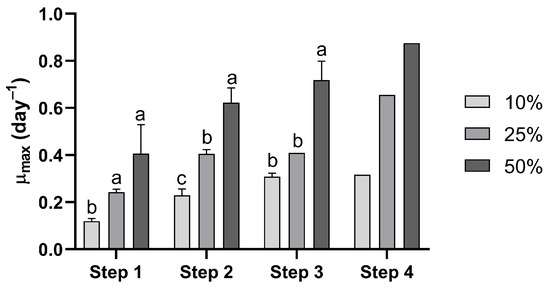
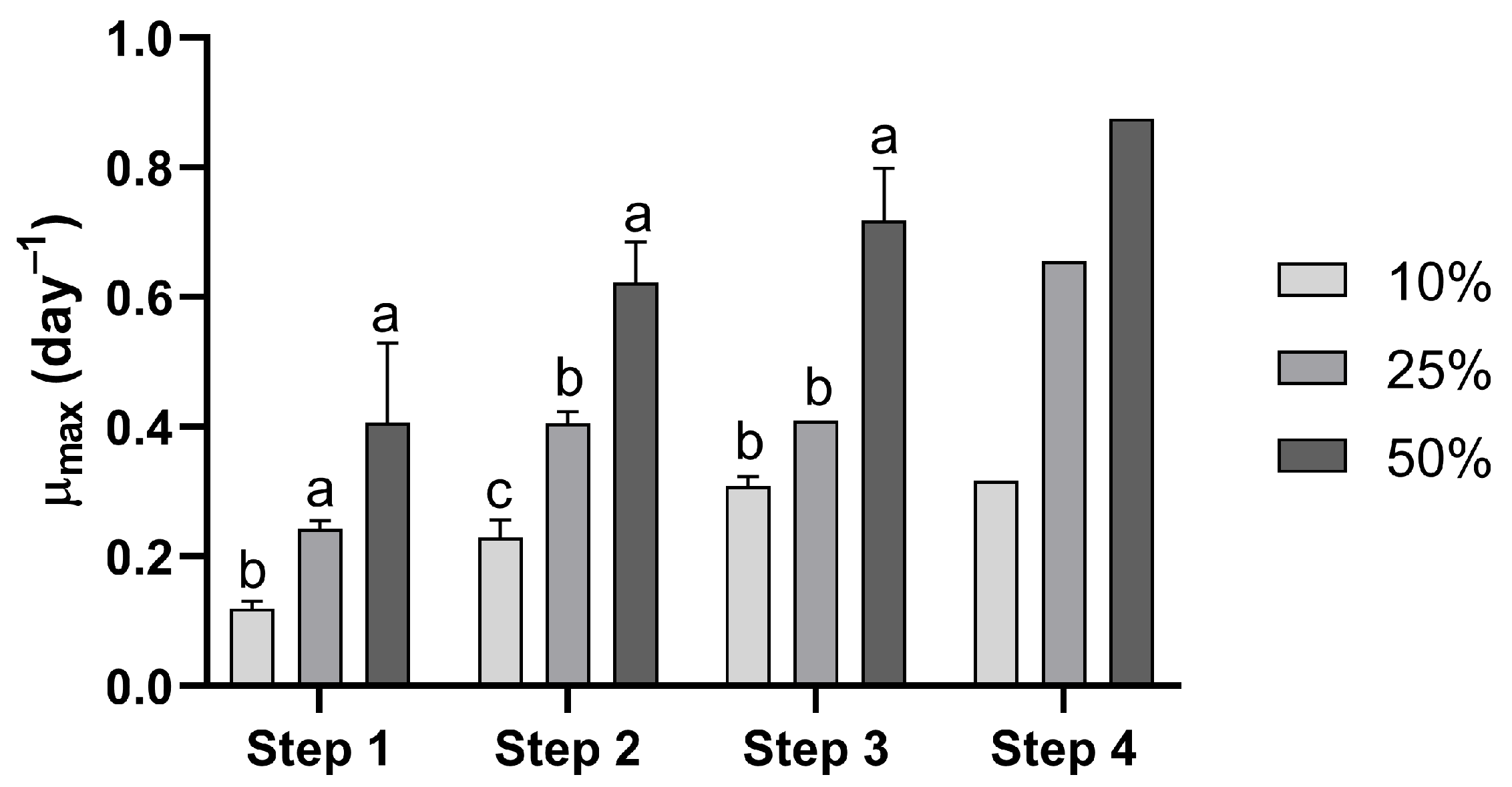
Figure 2.
Specific growth rates of Durusdinium glynnii cultures during the scale-up cultivation using different percentage inoculum strategies. Different letters at steps 1, 2, and 3 indicate significant differences (p < 0.05) between treatments in Tukey’s post hoc test.
By step 3, all strategies showed increased cell densities: 44.37 × 104 cells mL⁻1 for the 10% inoculum, 56.62 × 104 for 25%, and 48.75 × 104 for 50%. The onset of this phase was more synchronized across treatments, occurring on days 30, 28, and 26, respectively. In the final phase (step 4), peak densities were reached at 27.75 × 104, 46.25 × 104, and 56.75 × 104 cells mL⁻1 for the 10%, 25%, and 50% inoculum strategies. Notably, the 10% treatment entered this step on day 45, maintaining a short acclimation span. The 25% culture reached it on day 49, while the 50% strategy showed faster development, beginning exponential growth as early as day 39.
Overall, the 10% inoculum strategy presented a longer adaptation (lag) phase across the four initial steps compared to the 25% and 50% strategies. Using a higher inoculum percentage (i.e., 50%) accelerated the transition to the exponential growth phase, resulting in a higher frequency of volume increases compared to the other strategies. Consequently, step 4 started earlier with the 50% inoculum (39 days) compared to the 10% (45 days) and 25% (49 days) strategies. Additionally, a higher inoculum percentage accelerated the transition between cultivation stages, reducing the time interval between them and shortening the acclimation period across strategies. In terms of biomass concentration, by the end of cultivation in step 4, the cultures reached 0.56, 0.77, and 1.15 g L⁻1 under the 10%, 25%, and 50% inoculum strategies, respectively.
Regarding the maximum specific growth rate (µmax), step 1 showed that the strategy with 10% inoculum differed from the other treatments, presenting a significantly (F = 12.09, p = 0.008) lower µmax compared to the strategies with 25% and 50%, indicating a slower and less efficient start of cultivation. In step 2, significant differences (F = 20.96, p = 0.002) were observed between all inoculum percentages. In step 3, the treatments with 10% and 25% inoculum were statistically similar (F = 61.62, p = 0.0001), differing only from the 50% treatment. Regardless of the cultivation step, the use of a 50% inoculum resulted in higher µmax values compared to the other treatments. Except for step 3, the 10% inoculum strategy led to lower specific growth rates than the 25% treatment (Figure 2).
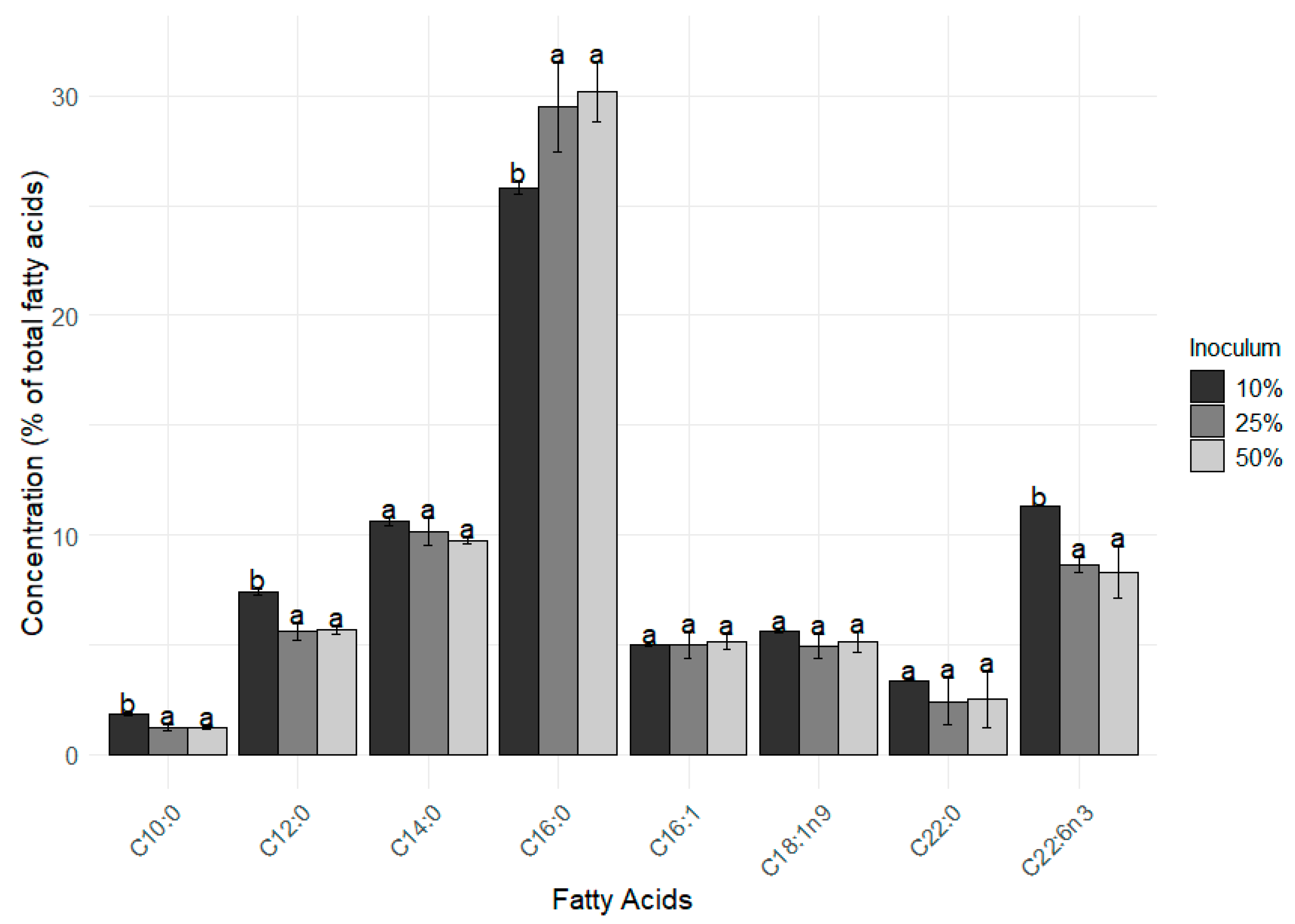
In addition to growth performance, the fatty acid composition was also influenced by different inoculation strategies. The main (% above of 2% of the total FAMEs) FAMEs found in the D. glynnii biomass were C10:0, C12:0, C14:0, C16:0, C16:1, C18:1 n9, C22:0, and C22:6 n3 (Figure 3). The lower inoculum percentage (i.e., 10% strategy) resulted in higher percentages of C10:0, C12:0, and C22:6 n3 in comparison to the 25% and 50% inoculum strategies. On the other hand, values of C16:0 were significantly higher (F = 14.71 p = 0.006) in the 25% and 50% treatments when compared to the 10% one.
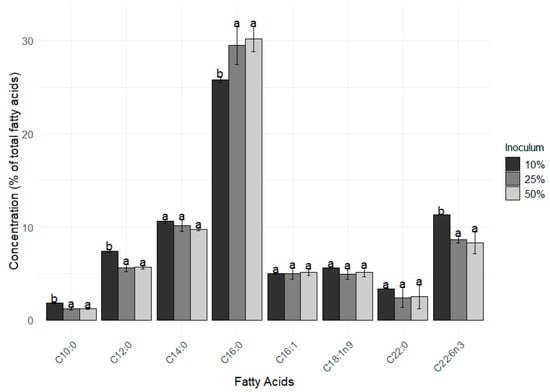
Figure 3.
Main fatty acids methyl esters present in Durusdinium glynnii biomass cultured using different inoculum strategies. Different letters indicate significant differences (p < 0.05) between treatments in Tukey’s post hoc test.
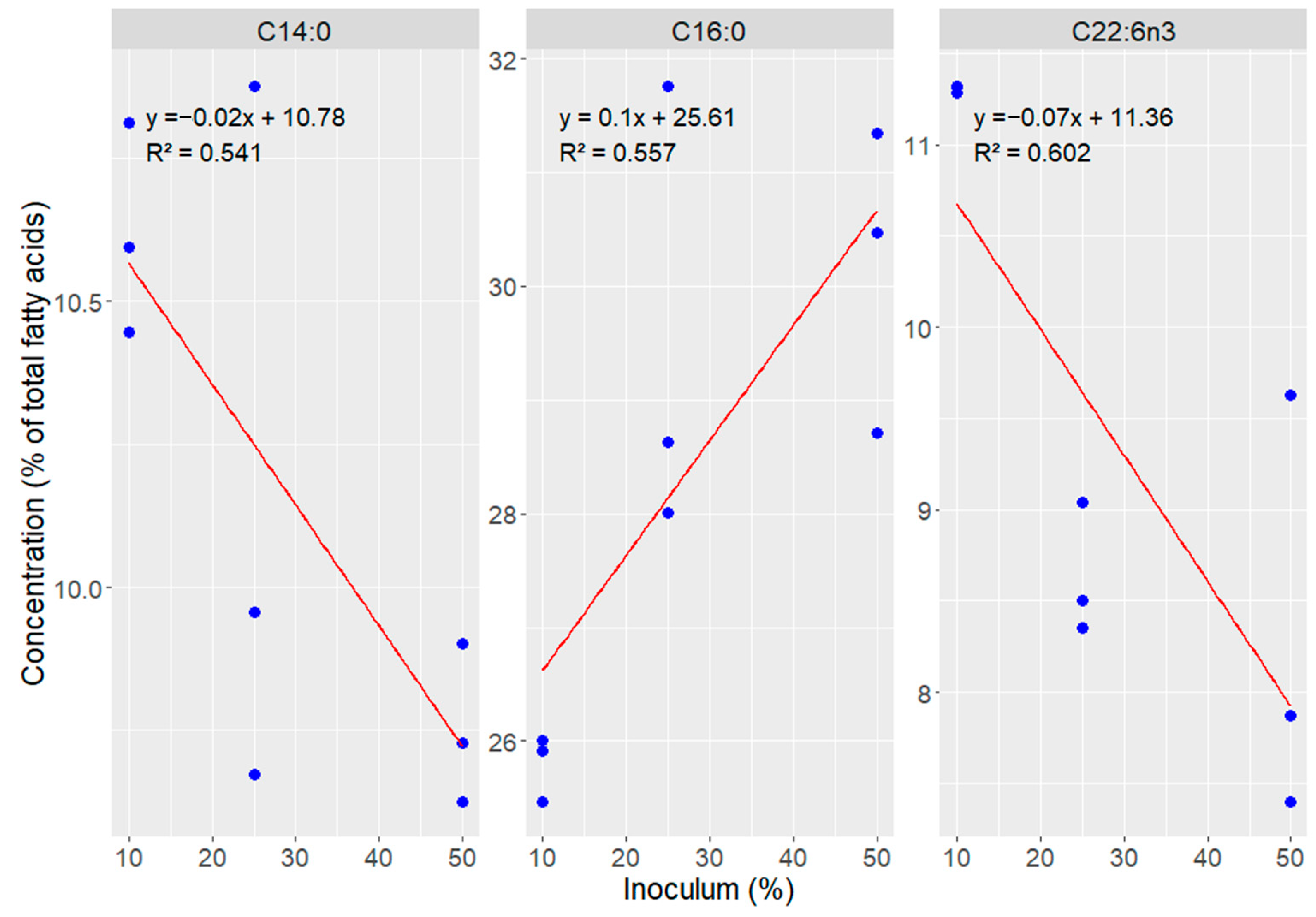
A direct relationship was observed between the increase in inoculum concentration and the increase in the proportion of palmitic acid (C16:0), which ranged from 25.8% with 10% inoculum to 30.17% with 50%. In contrast, polyunsaturated fatty acids showed the opposite trend, with higher levels in the 10% inoculum condition and progressive reduction in concentrations of 25% and 50%, evidencing an inversely proportional effect, clearly observed in the distribution of fatty acids shown in Figure 4.
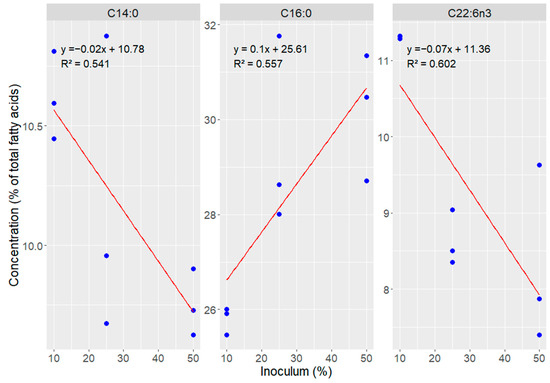
Figure 4.
Correlation between inoculum percentage and fatty acids concentration in Durusdinium glynnii biomass grown using different strategies of scaling-up.
4. Discussion
The fragility of dinoflagellate cells to shear stress in culture systems makes their scaling-up more challenging compared to more robust species. When cultured in a photobioreactor, the dinoflagellate Protoceratium reticulatum achieved promising results when scaled up from 2 L to 15 L [15]. Meanwhile, López-Rosales et al. [32] obtained even better results with Karlodinium veneficum, reaching an 80 L scale, due to factors such as improved structural adaptations, column photobioreactors, and optimized lighting systems. These differences can be attributed to the use of internal light as well as supplementation with carbon dioxide, which is known to enhance microalgal growth rates under controlled cultivation conditions. Prorocentrum micans was also successfully cultivated in 20 L volumes using the initial 2.5% inoculum, with a similar acclimation period as that obtained with the 10% inoculum [33].
While the 50% inoculum strategy demonstrated clear advantages in terms of reduced acclimation time, enhanced specific growth rate, and higher final biomass yield, it is important to acknowledge certain operational considerations. Higher inoculum volumes may lead to faster nutrient consumption and the accumulation of metabolic by-products. Nonetheless, this approach also reduces the lag phase and lowers the risk of bacterial contamination by establishing a dominant microalgal population more rapidly, which can be particularly beneficial in large-scale or non-axenic systems. On the other hand, strategies with lower inoculum concentrations (10% and 25%), even presenting slower growth, proved to be more efficient in the production of compounds of interest, such as polyunsaturated fatty acids.
In the study conducted by Vidyarathna et al. [34] on Karlodinium veneficum, the authors reported μmax values of 0.27 ± 0.01 d⁻1 in short-term cultures (50 generations) under conditions of 29 °C and 1000 ppm pCO2, with an increase to 0.36 ± 0.01 d⁻1 after 200 generations in long-term cultures. The maximum growth rates observed in our experiment were substantially higher than those reported by these authors, reaching up to 0.9 d⁻1 in step 4 using a 50% inoculum. Furthermore, a progressive increase in μmax was observed across the different cultivation stages, even in treatments with lower inoculum levels (10%), which may suggest a physiological adaptation process to the culture environment or a potential selection of more efficient cells throughout generations, despite the relatively short cultivation regime.
Similarly, Aboualaalaa et al. [35] investigated the growth of Gymnodinium catenatum under various environmental conditions and reported μmax values considerably lower than those observed in our experiment. The best results obtained by the authors included a μmax of 0.18 d⁻1 at 24 °C, 0.07 d⁻1 under a salinity of 32, 0.22 d⁻1 with 1764 μM nitrate, and 0.09 d⁻1 with 18.2 μM phosphate. Although these conditions represent the most favorable growth scenarios in the cited study, our cultures—conducted at 21 °C, a salinity of 30, and using inoculation and stepwise growth strategies—showed μmax values equal to or higher in all stages, reaching up to 0.9 d⁻1 in step 4 with a 50% inoculum.
Notable results were obtained in a study that investigated the growth of various dinoflagellate genera cultured under standardized conditions, including a temperature of 24 ± 1 °C, an atmospheric CO2 concentration of 0.03%, a 12:12 h light:dark photoperiod, and a light intensity of 120 μE m⁻2 s⁻1. In this analysis, very low μmax values were reported for certain strains, such as Prorocentrum triestinum (strain b), which exhibited a μmax of only 0.05 d⁻1—significantly lower than that observed in the present study with Durusdinium glynnii, even under the most conservative inoculation strategy (10%). On the other hand, the study also reported higher μmax values for other strains, such as P. triestinum (strain a) and P. minimum (strain a), with maximum growth rates of 0.57 and 0.39 d⁻1, respectively [36]. These values exceeded those observed in the present study during the initial stages (steps 1 and 2) but became comparable or even lower as the inoculum strategy advanced to 50% (step 3), at which point the growth rates of D. glynnii increased significantly.
These findings suggest that while the culture conditions employed by Xu et al. [36] promote robust growth in certain dinoflagellate species, the physiological responses of Durusdinium glynnii demonstrate a greater growth potential when subjected to high inoculum strategies. In a previous study, promising results were obtained, reporting a μmax of 0.41 d⁻1 under optimal cultivation conditions at 25 °C and a salinity of 20. These findings are consistent with those observed in our study, particularly in relation to the 25% inoculum strategy applied in steps 2 and 3, which outperformed the 50% inoculum strategy in step 1 and consistently surpassed the 10% strategy across all steps. However, when Akashiwo sanguinea was cultured at salinity levels above 20, a marked decline in μmax was observed, reaching as low as 0.21 d⁻1 [37].
The fatty acid profile analysis of the dinoflagellate revealed striking differences between Durusdinium glynnii (cultivated under different inoculation strategies: 10%, 25% and 50%) and the endosymbionts Symbiodinium voratum and S. microadriaticum [38]. Among the saturated fatty acids, myristic acid (C14:0) was found in greater quantities in D. glynnii, with levels ranging from 9.75% (1.21% d.w.) to 10.62% (1.33% d.w.). On the other hand, myristic acid levels were considerably higher in the symbionts (5.85% d.w. in S. voratum and 3.3% d.w. in S. microadriaticum). This difference may be related to the regulation of membrane fluidity under different physiological conditions or to the metabolic plasticity of these different endosymbionts.
Stearic acid (C18:0) was detected in smaller proportions in the samples. The levels varied between 1.17% (0.14% d.w.) and 1.62% (0.20% d.w.) in D. glynnii, while the percentages were close those found in S. microadriaticum and S. voratum (0.4% d.w. and 1.08% d.w., respectively). This result may indicate the complementary action of these substances in the stabilization of cell membranes [37]. Palmitic acid (C16:0), widely recognized as the principal structural fatty acid in cellular membranes, was the most abundant component in D. glynnii samples, reaching 30.17% of total fatty acids in the 50% inoculum strategy [39]. This high proportion underscores its fundamental role in maintaining membrane integrity, fluidity, and overall cellular function—especially crucial for microalgae subjected to repeated subculturing and environmental fluctuations during scale-up processes [40].
In D. glynnii, DHA levels reached 11.31% of the total fatty acids (1.41% d.w.) with 10% inoculum, decreasing to 8.63% (1.07% d.w.) and 8.3% (1.03% d.w.) in the 25% and 50% inoculation strategies, respectively. When expressed on a dry weight basis, these values are comparable to those reported for S. voratum (2.0% d.w.) and S. microadriaticum (0.8% d.w.), suggesting that D. glynnii exhibits a similarly favorable DHA profile with potential for biotechnological applications, especially considering the stability of its lipid content across cultivation conditions. This high content of DHA observed in D. glynnii may have been favored by the cultivation conditions at 21 °C, a temperature already associated with increased DHA in previous studies [38]. Nonetheless, other culture parameters—such as light intensity and quality, as well as salinity—are also known to influence DHA biosynthesis and may have contributed to the results observed in this study [41].
In fact, in the present study, Durusdinium glynnii was grown at a temperature of 21 °C, a condition that possibly favored the accumulation of the fatty acid DHA (C22:6n3), essential for maintaining the fluidity of cell membranes at milder temperatures. This behavior is consistent with the other fatty acids found in greater abundance in our cultures—C12:0, C14:0, and C16:0—which also play crucial roles in lipid structure and metabolism. These fatty acids actively participate in both the regulation of membrane fluidity and the formation of lipid reserves, which are especially important in contexts of cellular stress. Such stress may have been induced by the successive subcultures performed during the scaling and inoculum production process, steps necessary for advancing to the subsequent stages of the culture.
5. Conclusions
The results of this study demonstrate that Durusdinium glynnii exhibits high potential for biotechnological applications, combining physiological adaptability and productivity under different inoculation strategies. The use of a higher inoculum concentration (50%) optimized cultivation time, reduced acclimation periods, and increased specific growth rates (µmax) and final cell densities, which are critical factors for efficient scale-up. In contrast, more diluted inoculations (10% and 25%) favored the biosynthesis of polyunsaturated fatty acids, particularly DHA (C22:6n3), highlighting an inversely proportional effect between inoculum concentration and the accumulation of bioactive compounds. Thus, the choice of inoculation strategy should consider the specific cultivation objectives, prioritizing either higher cell productivity or enhanced accumulation of target metabolites, reinforcing D. glynnii as a promising platform for marine biotechnology. However, further studies at the cellular and metabolic levels are necessary to elucidate the mechanisms underlying lipid synthesis pathways and growth processes in this species, aiming to optimize its biotechnological exploitation.
Author Contributions
Conceptualization, C.Y.B.O.; methodology, C.Y.B.O. and A.O.G.; software, B.d.C.S.B.; validation, P.R.d.S., M.E.S.S.L., J.L.d.A. and B.d.C.S.B.; formal analysis, P.R.d.S., M.E.S.S.L., D.W.S.O., J.L.d.A., B.d.C.S.B. and E.S.A.; investigation, P.R.d.S., M.E.S.S.L. and G.T.; resources, A.O.G., G.T. and C.Y.B.O.; data curation, P.R.d.S., M.E.S.S.L., D.W.S.O., J.L.d.A., B.d.C.S.B., W.A.G. and C.Y.B.O.; writing—original draft preparation, P.R.d.S. and M.E.S.S.L.; writing—review and editing, J.L.d.A., E.S.A., W.A.G. and C.Y.B.O.; supervision, C.Y.B.O.; project administration, A.O.G. and C.Y.B.O.; funding acquisition, G.T., A.O.G. and C.Y.B.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—finance code 001, by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)—finance code: 409352/2023-3, and by Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE)—finance code: APQ 1387-3.03/22 and APQ 1285-2.03/22. Moreover, A.O.G. is thankful to the CNPq for the research fellowship PQ 310898/2023-4. Finally, P.R.d.S., B.d.C.S.B. and J.L.d.A. are thankful to the FACEPE for the research fellowships IBPG-0098-5.06/21 and BFP-0131-5.06/22, respectively.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
During the preparation of this manuscript, the authors used ChatGPT-4o solely to enhance manuscript’s language and readability. After using this tool, the authors reviewed and edited the content as needed and took full responsibility for the content of the publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guiry, M.D. How Many Species of Algae Are There? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Sreeharsha, R.V.; Mohan, S.V. Microbial Photosynthesis. In Photosynthetic Microbes: Evolution, Classification, and Structural Physiology; Springer: Singapore, 2024; pp. 3–22. [Google Scholar] [CrossRef]
- Mordret, S.; Romac, S.; Henry, N.; Colin, S.; Carmichael, M.; Berney, C.; Audic, S.; Richter, D.J.; Pochon, X.; de Vargas, C.; et al. The Symbiotic Life of Symbiodinium in the Open Ocean Within a New Species of Calcifying Ciliate (Tiarina sp.). ISME J. 2015, 10, 1424–1436. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garcia, L.; Adjallé, K.; Barnabé, S.; Raghavan, G.S.V. Microalgae Biomass Production for a Biorefinery System: Recent Advances and the Way Towards Sustainability. Renew. Sustain. Energy Rev. 2017, 76, 493–506. [Google Scholar] [CrossRef]
- Oliveira, A.P.F.; Bragotto, A.P.A. Microalgae-Based Products: Food and Public Health. Future Foods 2022, 6, 100157. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the Potential of Microalgae for New Biotechnology Applications and Beyond: A Review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Morse, D.; Tse, S.P.K.; Lo, S.C.L. Exploring Dinoflagellate Biology with High-Throughput Proteomics. Harmful Algae 2018, 75, 16–26. [Google Scholar] [CrossRef]
- Lee, S.K.; Jeong, H.J.; Jang, S.H.; Lee, K.H.; Kang, N.S.; Lee, M.J.; Potvin, E. Mixotrophy in the Newly Described Dinoflagellate Ansanella granifera: Feeding Mechanism, Prey Species, and Effect of Prey Concentration. Algae 2014, 29, 137–152. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeong, H.J.; Kwon, J.E.; You, J.H.; Kim, S.J.; Ok, J.H.; Kang, H.C.; Park, J.Y. First Report of the Photosynthetic Dinoflagellate Heterocapsa minima in the Pacific Ocean: Morphological and Genetic Characterizations and the Nationwide Distribution in Korea. Algae 2019, 34, 7–21. [Google Scholar] [CrossRef]
- Nitschke, M.R.; Rosset, S.L.; Oakley, C.A.; Gardner, S.G.; Camp, E.F.; Suggett, D.J.; Davy, S.K. The Diversity and Ecology of Symbiodiniaceae: A Traits-Based Review. Adv. Mar. Biol. 2022, 92, 55–127. [Google Scholar]
- Camacho, F.G.; Rodríguez, J.G.; Mirón, A.S.; García, M.C.; Belarbi, E.H.; Chisti, Y.; Grima, E.M. Biotechnological significance of toxic marine dinoflagellates. Biotechnol. Adv. 2007, 25, 176–194. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Oliveira, C.D.L.; Müller, M.N.; Santos, E.P.; Dantas, D.M.M.; Gálvez, A.O. A Scientometric Overview of Global Dinoflagellate Research. Publications 2020, 8, 50. [Google Scholar] [CrossRef]
- Assunção, J.; Guedes, A.C.; Malcata, F.X. Biotechnological and Pharmacological Applications of Biotoxins and Other Bioactive Molecules from Dinoflagellates. Mar. Drugs 2017, 15, 393. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.H.; Gibson, C.H. Quantified Small-Scale Turbulence Inhibits a Red Tide Dinoflagellate, Gonyaulax polyedra Stein. Deep Sea Res. I Oceangr. Res. Pap. 1990, 37, 1583–1593. [Google Scholar] [CrossRef]
- Camacho, F.G.; Rodríguez, J.J.G.; Mirón, A.S.; Belarbi, E.H.; Chisti, Y.; Grima, E.M. Photobioreactor Scale-Up for a Shear-Sensitive Dinoflagellate Microalga. Process Biochem. 2011, 46, 936–944. [Google Scholar] [CrossRef]
- Greenhough, H.; Waugh, C.; van Ginkel, R.; Bowater, J.; Kaur, G.; Oakly, J.; Plouviez, M.; Ingebrigtsen, R.A.; Svenson, J.; Selwood, A.I. Mass Cultivation of the Dinoflagellate Alexandrium pacificum for Gonyautoxin-1,4 Production. Sci. Rep. 2025, 15, 7430. [Google Scholar] [CrossRef]
- De Cassia, S.; Brandão, B.; de Abreu, J.L.; Oliveira, D.W.S.; da Silva Campos, C.V.F.; de Aguiar, I.M.T.; de Sena, P.R.; Gálvez, A.O.; Oliveira, C.Y.B. New Findings on the Survival of Durusdinium glynnii Under Different Acclimation Methods to Low Salinities. Microorganisms 2025, 13, 946. [Google Scholar] [CrossRef]
- Molina-Miras, A.; Bueso-Sánchez, A.; Cerón-García, M.C.; Sánchez-Mirón, A.; Contreras-Gómez, A.; García-Camacho, F. Effect of Nitrogen, Phosphorus, and Light Colimitation on Amphidinol Production and Growth in the Marine Dinoflagellate Microalga Amphidinium carterae. Toxins 2022, 14, 594. [Google Scholar] [CrossRef]
- Rathinavel, L.; Ravikumar, S.M.; Jothinathan, D.; Paul, S.J.; Pandey, A.; Mahata, C. Extraction and Enrichment of Fatty Acids from Marine Microalgae. In Marine Molecules from Algae and Cyanobacteria: Extraction, Purification, Toxicology and Applications; Elsevier: Amsterdam, The Netherlands, 2025; Chapter 3; pp. 41–57. [Google Scholar] [CrossRef]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.-Y.; Filaire, E. Microalgae n-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Jacob, A.; Nader, C.; Oliveira, C.D.L.; Matos, Â.P.; Araújo, E.S.; Shabnam, N.; Ashok, B.; Gálvez, A.O. An Overview on Microalgae as Renewable Resources for Meeting Sustainable Development Goals. J. Environ. Manag. 2022, 320, 115854. [Google Scholar] [CrossRef]
- Gui, J.; Chen, S.; Luo, G.; Wu, Z.; Fan, Y.; Yao, L.; Xu, H. Nutrient Deficiency and an Algicidal Bacterium Improved the Lipid Profiles of a Novel Promising Oleaginous Dinoflagellate, Prorocentrum donghaiense, for Biodiesel Production. Appl. Environ. Microbiol. 2021, 87, e0115921. [Google Scholar] [CrossRef]
- Chen, W.; Li, T.; Du, S.; Chen, H.; Wang, Q. Microalgal Polyunsaturated Fatty Acids: Hotspots and Production Techniques. Front. Bioeng. Biotechnol. 2023, 11, 1146881. [Google Scholar] [CrossRef] [PubMed]
- Graeff, J.E.; Leblond, J.D. Composition of Galactolipids, Betaine Lipids and Triglyceride-Associated Fatty Acids of the Symbiotic Dinoflagellate Zooxanthella (Brandtodinium) Nutricula: A Glimpse into Polyunsaturated Fatty Acids Available to Its Polycystine Radiolarian Host. Phycol. Res. 2023, 71, 175–181. [Google Scholar] [CrossRef]
- Didrihsone, E.; Dubencovs, K.; Grube, M.; Shvirksts, K.; Suleiko, A.; Suleiko, A.; Vanags, J. Crypthecodinium cohnii Growth and Omega Fatty Acid Production in Mediums Supplemented with Extract from Recycled Biomass. Mar. Drugs 2022, 20, 68. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L.; Lorenzen, C.J. Yellow-green algae with chlorophyllide c. J. Phycol. 1972, 8, 10–14. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Brandão, B.d.C.S.; Jannuzzi, L.G.d.S.; Oliveira, D.W.S.; Yogui, G.T.; Müller, M.N.; Gálvez, A.O. New insights on the role of nitrogen in the resistance to environmental stress in an endosymbiotic dinoflagellate. Environ. Sci. Pollut. Res. 2023, 30, 82142–82151. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Axelsson, M.; Gentili, F. A single-step method for rapid extraction of total lipids from green microalgae. PLoS ONE 2014, 9, e89643. [Google Scholar] [CrossRef]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Abreu, J.L.; Santos, E.P.; Matos, Â.P.; Tribuzi, G.; Oliveira, C.D.L.; Veras, B.O.; Bezerra, R.S.; Müller, M.N.; Gálvez, A.O. Light induces peridinin and docosahexaenoic acid accumulation in the dinoflagellate Durusdinium glynnii. Appl. Microbiol. Biotechnol. 2022, 106, 6263–6276. [Google Scholar] [CrossRef]
- López-Rosales, L.; García-Camacho, F.; Sánchez-Mirón, A.; Martín Beato, E.; Chisti, Y.; Molina Grima, E. Pilot-scale bubble column photobioreactor culture of a marine dinoflagellate microalga illuminated with light emission diodes. Bioresour. Technol. 2016, 216, 845–855. [Google Scholar] [CrossRef]
- Alizade, A.; Jantschke, A. Dinoflagellates as sustainable cellulose source: Cultivation, extraction, and characterization. Int. J. Biol. Macromol. 2023, 242, 125116. [Google Scholar] [CrossRef] [PubMed]
- Vidyarathna, N.K.; Smith, L.E.; Miller, K.R.; Coyne, K.J.; Cohen, J.H.; Warner, M.E. Short-term and long-term exposure to combined elevated temperature and CO₂ leads to differential growth, toxicity, and fatty acid profiles in the harmful dinoflagellate Karlodinium veneficum. Front. Mar. Sci. 2024, 11, 1305495. [Google Scholar] [CrossRef]
- Aboualaalaa, H.; Leblad, B.R.; Elkbiach, M.L.; Ibghi, M.; Boutaib, R.; Maamour, N.; Savar, V.; Masseret, E.; Abadie, E.; Rolland, J.L.; et al. Effect of temperature, salinity and nutrients on the growth and toxin content of the dinoflagellate Gymnodinium catenatum from the southwestern Mediterranean. Sci. Total Environ. 2024, 945, 174094. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.J.; Wu, K.-C.; Chan, S.C.-Y.; Yau, Y.-H.; Chan, K.-K.; Lee, F.W.-F. Investigation of growth, lipid productivity, and fatty acid profiles in marine bloom-forming dinoflagellates as potential feedstock for biodiesel. J. Mar. Sci. Eng. 2020, 8, 381. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Y.; Song, S.; Li, C.; Tang, Y.Z.; Yu, Z. The effects of major environmental factors and nutrient limitation on growth and encystment of planktonic dinoflagellate Akashiwo sanguinea. Harmful Algae 2015, 46, 62–70. [Google Scholar] [CrossRef]
- Tsirigoti, A.; Tzovenis, I.; Koutsaviti, A.; Economou-Amilli, A.; Ioannou, E.; Melkonian, M. Biofilm cultivation of marine dinoflagellates under different temperatures and nitrogen regimes enhances DHA productivity. J. Appl. Phycol. 2020, 32, 865–880. [Google Scholar] [CrossRef]
- Ali, O.; Szabó, A. Review of eukaryote cellular membrane lipid composition, with special attention to the fatty acids. Int. J. Mol. Sci. 2023, 24, 15693. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K. Fatty acids of microalgae: Diversity and applications. Rev. Environ. Sci. Biotechnol. 2021, 20, 515–547. [Google Scholar] [CrossRef]
- Sharma, T.; Das, N.; Kakkar, P.M.; Mohapatra, R.K.; Pamidimarri, S.; Singh, R.K.; Kumar, M.; Guldhe, A.; Nayak, M. Microalgae as an emerging alternative raw material of docosahexaenoic acid and eicosapentaenoic acid—A review. Crit. Rev. Food Sci. Nutr. 2025, 1–20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).