Abstract
Macroalgal extracts are widely recognised as biostimulants that enhance crop productivity and plant growth under both optimal and stressful conditions. They offer a sustainable approach to mitigating the adverse effects of abiotic stress on crop development. This study investigates the efficacy of macroalgal-based fertilisers in enhancing tomato (Solanum lycopersicum L.) growth, yield, and fruit quality, as sustainable alternatives to chemical fertilisers. Different seaweed species (Sargassum muticum, Ulva ohnoi, Furcellaria lumbricalis, Ascophyllum nodosum, and a commercial A. nodosum extract) were evaluated as foliar treatments. The results showed that while the leaf fresh weight and chlorophyll content were not significantly affected, the fruit morphology and biochemical composition exhibited notable variations. Sargassum muticum-treated fruits displayed the highest °Brix (6.57), indicating superior sugar accumulation, while Ulva ohnoi maintained near-neutral pH levels (avg. 3.94), suggesting balanced acidity. Ascophyllum nodosum extracts induced the highest proline concentrations (peak: 63.77 µmol/g), but also caused extreme acidity (pH 1.39–2.58). Furcellaria lumbricalis enhanced the fruit size (axial length up to 41.4 mm), but reduced the pH sharply (1.69–2.13). The commercial product underperformed in regard to sugar content and flavour complexity. The integrative analysis revealed species-specific flavour profiles: Sargassum yielded sweet, mildly acidic fruits; Ascophyllum produced intensely aromatic, acidic tomatoes; and Ulva resulted in bland flavours. These findings underscore the importance of algal species and extraction methods in tailoring biofertilisers for target fruit qualities. This study advocates for the use of macroalgal fertilisers in sustainable agriculture, but highlights the need for optimised formulations to balance crop yield, taste, and stress adaptation.
1. Introduction
In recent decades, global agriculture has been shifting towards more sustainable and productive practices to address environmental concerns and food security challenges [1]. Climate change and the negative impacts of conventional farming have driven efforts to find alternative, eco-friendly solutions that balance productivity with environmental responsibility [2]. Despite the need to reduce agricultural production or improve its sustainability, the increasing human population demands ever more food. This has created a paradox, wherein the push for sustainability must be balanced with the need to feed a growing global population. In this context, tomato (Solanum lycopersicum L.), a member of the Solanaceae family, and one of the most widely grown and nutritionally rich vegetable crops [3], plays a significant role. Tomatoes, being a staple food in many diets worldwide, are not only important for food security, but also present an opportunity to explore sustainable agricultural practices in regard to their cultivation [4]. Tomatoes are a rich source of essential nutrients, including minerals, carbohydrates, lipids, proteins, and vitamins, making them a valuable component of a balanced diet [5]. Furthermore, the fruits contain significant amounts of carotenoids, such as lutein, lycopene, α- and β-carotene, zeaxanthin, and β-cryptoxanthin, which contribute to their antioxidant properties [6]. Also, tomatoes are regarded as an excellent vegetable that offers physiological and nutritional benefits, due to their low-calorie content, high-fibre content, and the presence of phenols like flavonoids [7]. In addition, the tomato serves as a model dicot plant for research on stress tolerance, fruit development, ecological interactions, and genetics [8]. There has been growing interest in its health benefits and bioactive compounds recently. Studies highlight that nutritional value and flavour are key factors influencing consumer preferences, making producing high-quality tomatoes in large quantities essential to meeting market demand [9].
Tomatoes and their varieties are important for several reasons. Firstly, they are grown in large quantities worldwide, especially in Italy, where a rich diversity of tomato cultivars is celebrated [10]. Tomatoes are widely used in various forms, such as fresh, canned, or processed into sauces, pastes, and other culinary products, making them a versatile ingredient in global cuisine. Additionally, within tomato varieties, the taste can vary significantly, with factors such as the fertilisers used, growing conditions, and agricultural practices influencing their flavour profile. This variation can result in differences in the sugar content (°Brix), acidity, and the presence of compounds like proline, which contribute to the overall taste and quality of the fruit [11,12,13].
While chemical fertilisers enhance plant growth and contribute to global food security, their extensive use poses serious environmental and health risks, including water and soil pollution, nutrient imbalances, soil degradation, and reliance on fossil fuels [14]. To promote sustainable agriculture, adopting eco-friendly alternatives, such as organic fertilisers and bio-based amendments, is essential [15]. In this context, alternative fertilisation methods, particularly using natural resources like macroalgae, have gained significant research interest for their potential to enhance crop productivity, while minimising environmental harm [16,17]. Macroalgae, which are multicellular aquatic photosynthetic organisms, play a crucial role in aquatic habitats and offer a sustainable solution for enhancing agricultural productivity, while promoting environmental sustainability [18]. Thriving in diverse marine ecosystems, macroalgae possess an inherent capacity to assimilate essential nutrients from their surroundings, including nitrogen, phosphorus, potassium, and micronutrients [19]. Moreover, they synthesise an array of bioactive molecules, such as polysaccharides, polyphenols, and plant growth regulators, which exert multifaceted effects on algae physiology and metabolism [20], which algae synthesise for their growth [21,22]. Historically used as mulch in ancient China, it is now recognised as a rich source of nitrogen, phosphorus, potassium, and essential trace elements for plant growth [23]. The application of seaweed extract-based plant biostimulants enhances the nutritional quality of tomato fruit by increasing its bioactive components. By directly supplying essential macro- and micronutrients, seaweed extracts contribute to improved plant nutrition and overall crop quality [24]. Properly processed and applied macroalgal biomass enhances soil fertility and plant growth, while reducing the dependence on synthetic fertilisers, making it a sustainable alternative for modern agriculture. Integrating macroalgae into farming systems promotes circular economy practices by repurposing waste biomass into valuable agricultural inputs, contributing to environmental sustainability [16]. Macroalgae, particularly brown algae, are now widely used in agriculture for the production of fertilisers and biostimulants [25]. These products, obtained through the use of various extraction methods, contain a broad range of bioactive compounds, including phytohormones, polysaccharides, amino acids, and minerals, which promote plant growth, improve plant resistance to abiotic stresses, and enhance nutrient uptake efficiency [26,27,28]. The use of these extracts is expanding in agriculture as part of more sustainable and environmentally friendly practices.
Seaweed-derived biofertilisers were used as sustainable alternatives to chemical fertilisers, promoting seedling development in Lycopersicon esculentum and Capsicum annum [29]. Higher concentrations of seaweed extract significantly enhanced tomato plant growth, including shoot and root length, branch number, and reproductive traits like flower and fruit production. Also, biochemical constituents, such as photosynthetic pigments, proteins, sugars, starch, phenols, lycopene, and vitamin C, increased. Another study highlights the potential of Ulva ohnoi as a sustainable supplement for tomato plant growth, with seaweed powder improving soil properties and plant development [30]. The effectiveness of the foliar application of seaweed sap was investigated as a biostimulant in regard to improving the yield and quality of tomato [31]. In several other studies, seaweeds have been shown to enhance tomato plant growth and productivity, even under salt-stressed conditions [32,33,34]. There is limited information on how seaweed-enriched nutrient solutions affect tomato flavour and chemical composition, and how physiological and biochemical factors influence consumer preferences. Considering these considerations, this study endeavours to investigate the effectiveness of macroalgal fertilisers in enhancing the growth, development, and quality of tomato plants (Solanum lycopersicum var “Frassino”) and their fruits. By evaluating a comprehensive suite of physicochemical parameters, encompassing morphological, physiological, and biochemical attributes, we aim to elucidate the mechanistic underpinnings of the potential benefits of macroalgal fertilisers on tomato cultivation.
2. Materials and Methods
In this study, different seaweed species were collected from different regions across Europe for testing, as fertilised and biostimulant agents. To simplify the references, each species was assigned a specific letter, as follows:
- Sargassum muticum (S) was collected from Venice Lagoon, Italy, at 45°25′42.6″ N 12°19′50.7″ E;
- Ulva ohnoi (U) was gathered from Ganzirri Lake in Sicily, Italy, at coordinates 38°15′46.9″ N 15°37′34.9″ E;
- Furcellaria lumbricalis (F) was sourced from the coast of Saaremaa Island, Estonia, at 58°13′33.0″ N 22°36′12.5″ E;
- Ascophyllum nodosum (A) was obtained from the Icelandic coast at 65°18′12.1″ N 22°14′12.7″ W and was subsequently commercialised, under the name Algafit27, by the company SouthAgro srl (Bari, Italy);
- Additionally, the study included commercial seaweed-based products, such as MC Extra (Valagro® (Chietti, Italy)) derived from Ascophyllum nodosum (X), alongside a control group without fertilisation (Z).
2.1. Preparation of Macroalgal Fertiliser
After sampling, the collected seaweed biomasses were carefully cleaned by washing them with freshwater to remove any impurities. They were then transported in chilled conditions to maintain their biochemical integrity. To preserve the samples for further processing, they were dried in an oven at 50 °C for 72 h and stored in silica gel until extraction. For the preparation of the Liquid Seaweed Fertiliser (LSF), a modified protocol was used, based on [35] and the detailed procedures outlined in [27]. Dried macroalgae were processed by mixing dried seaweed with distilled water (1:20 DW/V ratio). This mixture was heated to 80 °C for three hours to extract the bioactive compounds. Following the extraction, the remaining biomass was removed using a cotton cloth, leaving behind a liquid seaweed extract. This extract, referred to as Seaweed Liquid Fertiliser (SLF), was then prepared with the same concentrations for use in foliar spray water, with a 10% concentration of each extract, including commercial products.
2.2. Tomato Plants and Treatments
This study was conducted on mature, but not yet having produced flowers or fruit, Solanum lycopersicum var. “Frassino” plants, using a randomised complete block design, with five blocks. Each block contained six treatment groups, with five plants per group, totalling 30 plants. The blocks were arranged to minimise environmental variations. Each treatment was applied as a foliar application using a handheld sprayer, ensuring complete leaf coverage. Applications were carried out every seven days, with a total of four treatments over 28 days. At the end of the experiment, the plants’ growth and response to the treatments were measured.
2.3. Measurements of Physical Parameters of the Leaf
After harvest, the fresh weight of the leaves was determined using a digital balance. Individual leaves were carefully collected from each plant, ensuring minimal handling to prevent moisture loss. Each leaf sample was immediately weighed on a precision digital balance to obtain the fresh weight, which was recorded in grams. Five leaves from each treatment group were measured to ensure representative data were collected. The mean leaf weight and the standard deviation were calculated to account for variations across the samples and treatments.
2.4. Measurements of Chlorophyll Content
The chlorophyll content was measured spectrophotometrically to assess the impact of the treatments on the photosynthetic performance. Firstly, fresh leaf samples (0.5 g) were collected from each treatment group, ground using a mortar and pestle, and suspended in 10 mL of 80% acetone to extract the chlorophyll pigments. The suspension was vortexed for 1 min to ensure uniform extraction and then centrifuged at 4000 rpm for 10 min, using an Eppendorf™ 5810R centrifuge (Eppendorf AG, Hamburg, Germany). The supernatant containing the chlorophyll extract was transferred to glass cuvettes, and the absorbance was measured at 632 nm, 649 nm, and 696 nm, using a Thermo Scientific™ Genesys™ 10S UV–Vis spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). These wavelengths were used to determine the chlorophyll a and chlorophyll b concentrations, calculated using standard equations. Chlorophyll a was determined by multiplying the absorbance at 665 nm by 13.95 and subtracting 6.88 times the absorbance at 649 nm, while chlorophyll b was calculated by multiplying the absorbance at 649 nm by 24.96 and subtracting 7.32 times the absorbance at 665 nm. The final chlorophyll concentration was expressed in mg/g of fresh leaf weight. Each treatment was analysed in regard to five replicates, and the mean chlorophyll content, along with the standard deviation, was calculated for statistical accuracy [36].
2.5. Measurements of Physical Parameters of the Fruit
For each group, ten fruits from five different plants (fifty for each group) were weighed individually, using a precision digital balance, to determine their fresh weight. To ensure accuracy, the fruits were weighed immediately after harvesting to prevent moisture loss. Additionally, the axial length (top to bottom) and equatorial width (widest horizontal diameter) of each fruit were measured using digital callipers for precise dimensional analysis. The fruits were positioned on a flat surface for stability, and measurements were taken at the widest and longest points. Each fruit was measured in triplicate, and the average values for the axial length and equatorial width were determined.
2.6. Measurements of Chemical Composition Analysis of the Fruit
To determine the acidity levels of tomato fruits, pH analysis was performed on juice extracted from freshly harvested fruits from each treatment group. Approximately 5 mL of juice was obtained by manually crushing ripe tomato fruits, using a sterile mortar and pestle. The extracted juice was filtered through a fine mesh to remove solid particles and ensure a clear solution. The pH was measured using a calibrated digital pH meter (Hanna Instruments™ HI2211 pH/ORP Meter; Hanna Instruments, Woonsocket, RI, USA) at room temperature. Before each use, the pH meter was calibrated using standard buffer solutions, at pH 4.01 and pH 7.00, to ensure measurement accuracy. Five replicates were conducted for each treatment, and the average pH values were calculated and reported for comparative analysis.
The proline levels were measured using a standard colourimetric method across all the treatments. Fresh leaf samples (approximately 0.5 g each) were collected and homogenised in 10 mL of 3% sulfosalicylic acid, using a mortar and pestle. The homogenate was then filtered to remove solid debris, and the filtrate was mixed with an equal volume of acid–ninhydrin reagent and glacial acetic acid. The mixture was incubated at 100 °C for 1 h to allow colour development. After cooling to room temperature, the reaction mixture was extracted with toluene, and the absorbance of the upper toluene layer was measured at 520 nm, using a Thermo Scientific™ Genesys™ 10S UV–Vis spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The proline concentration was determined using a calibration curve prepared with known proline standards, and the results were expressed in µmol/g of fresh leaf weight. Each treatment was analysed in regard to five replicates, and mean values with standard deviations were calculated for accuracy. The proline content in the tomato samples was quantified using a colourimetric method based on the ninhydrin reaction, with absorbance measured at 546 nm. A standard calibration curve was generated using known amounts of L-proline and their corresponding absorbance values, resulting in a linear regression equation of the form Absorbance = 7.17 × Proline (g) − 0.0272, with an R2 value of 0.96, indicating a high degree of linearity. This calibration equation was then used to determine the proline concentration in the samples by rearranging the formula to: Proline (g) = (Absorbance + 0.0272)/7.17. Absorbance values for each sample were measured in regard to five replicates, and the corresponding proline content was calculated for each. The final proline concentration was expressed in µm/g and averaged across the replicates for each treatment.
The °Brix (soluble solids content correlated with sugar content) was measured using a handheld refractometer to determine the total soluble solids content in the fruit juice. After harvesting, fruits from each treatment group were randomly selected, and the juice was extracted by crushing the fresh fruit. A few drops of the extracted juice were placed onto the refractometer’s prism surface, and the Brix value was recorded at room temperature. The readings, expressed in degrees Brix (°Brix), indicate the percentage of sugar by weight in the solution, providing a reliable measure of fruit sweetness and ripeness. Five replicates were taken for each treatment, and the mean Brix values were calculated and recorded for further analysis.
2.7. Statistical Analysis
A one-way analysis of variance (ANOVA) was performed to determine whether there were any statistically significant differences among the treatment groups for each parameter being studied. The ANOVA was conducted assuming equal variances, and a significance level of α = 0.05 was used.
3. Results
3.1. Physical Parameters of the Leaf
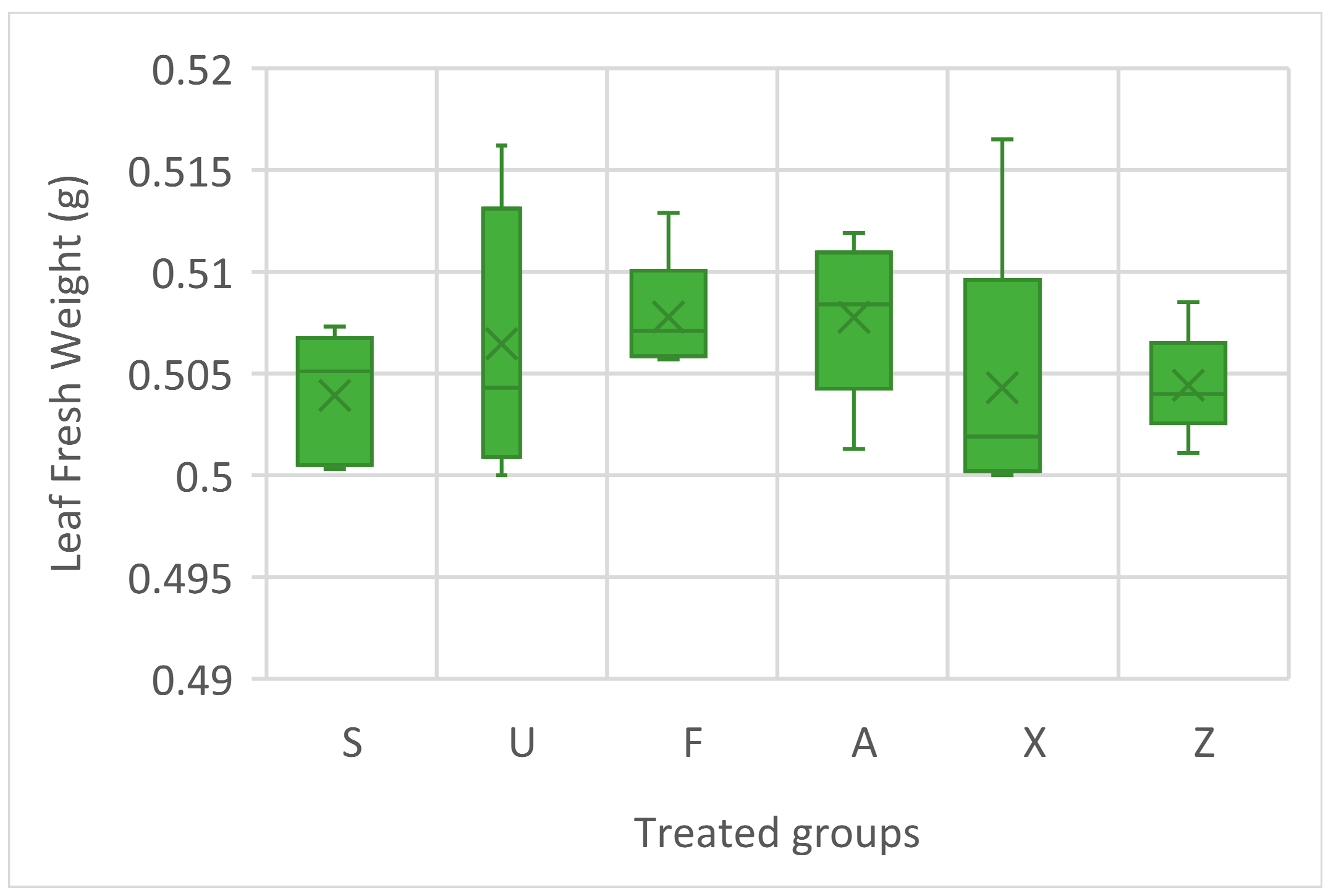
The leaf fresh weights of the treated tomato plants are presented in Figure 1; no statistical difference is evident as a result of the ANOVA; however, the highest median values were observed in the plants treated with Ulva ohnoi (U) and Ascophyllum nodosum (A), both showing slightly elevated fresh weights compared to the other treatments. Furcellaria lumbricalis (F) also showed relatively consistent results, with a narrow range, suggesting uniformity in the response. Sargassum muticum (S), the commercial extract (X), and the untreated control (Z) exhibited lower median leaf fresh weights.
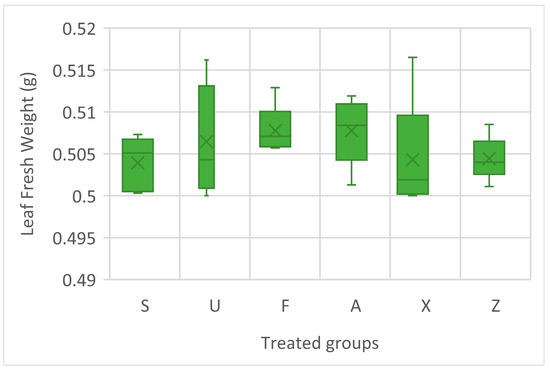
Figure 1.
Leaf fresh weight of tomato plant treatment groups. Notes: Sargassum muticum (S); Ulva ohnoi (U); Furcellaria lumbricalis (F); Ascophyllum nodosum (A); MC Extra, commercial product (X); negative control (Z).
3.2. Chlorophyll Content
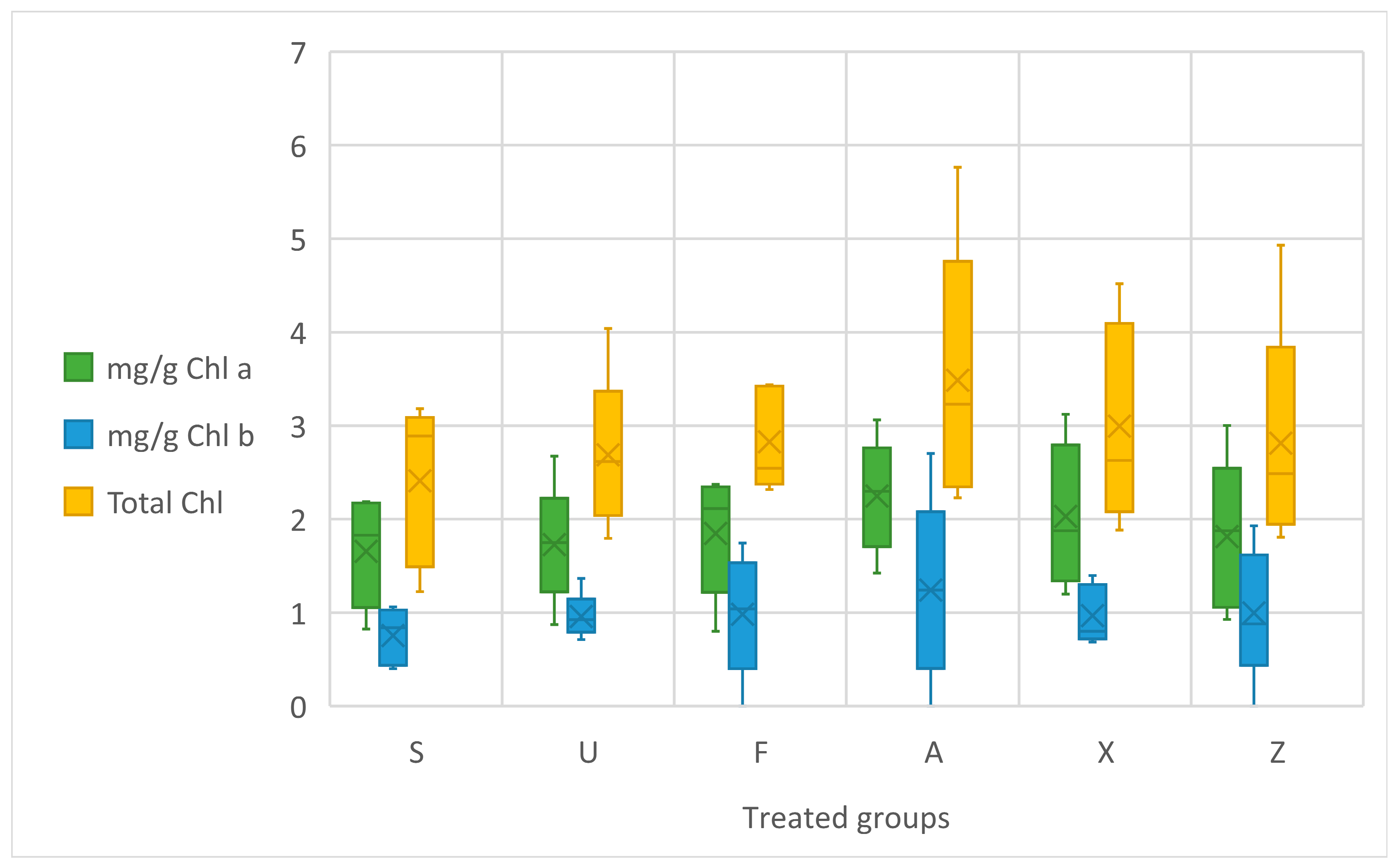
The data on the chlorophyll content (a, b, and total) in treated tomato leaves are shown in Figure 2. No statistical difference is evident as a result of the ANOVA concerning the chlorophyll content. By analysing the data, it is observed that Ascophyllum nodosum (A) exhibited the highest chlorophyll levels, indicating a slight enhancement in photosynthetic potential compared to the negative control (Z). Sargassum muticum (S) and Ulva ohnoi (U) also demonstrated increased chlorophyll content, albeit to a slightly lesser extent than A and the commercial product (X). In contrast, Furcellaria lumbricalis (F) showed only a modest improvement over the untreated control. The control group (Z), without fertilisation, recorded the lowest chlorophyll content.
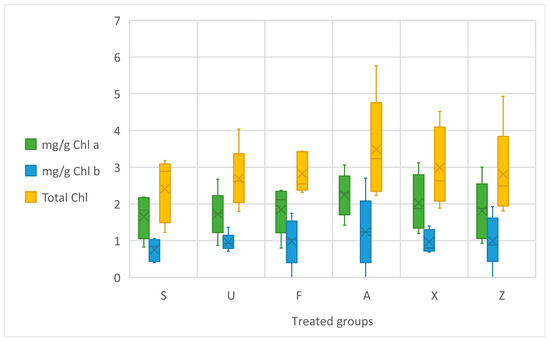
Figure 2.
Chlorophyll leaf content in mg/g Chl a, Chl b, and Total Chl. Notes: Sargassum muticum (S); Ulva ohnoi (U); Furcellaria lumbricalis (F); Ascophyllum nodosum (A); MC Extra, commercial product (X); negative control (Z).
3.3. Physical Parameters of the Fruit
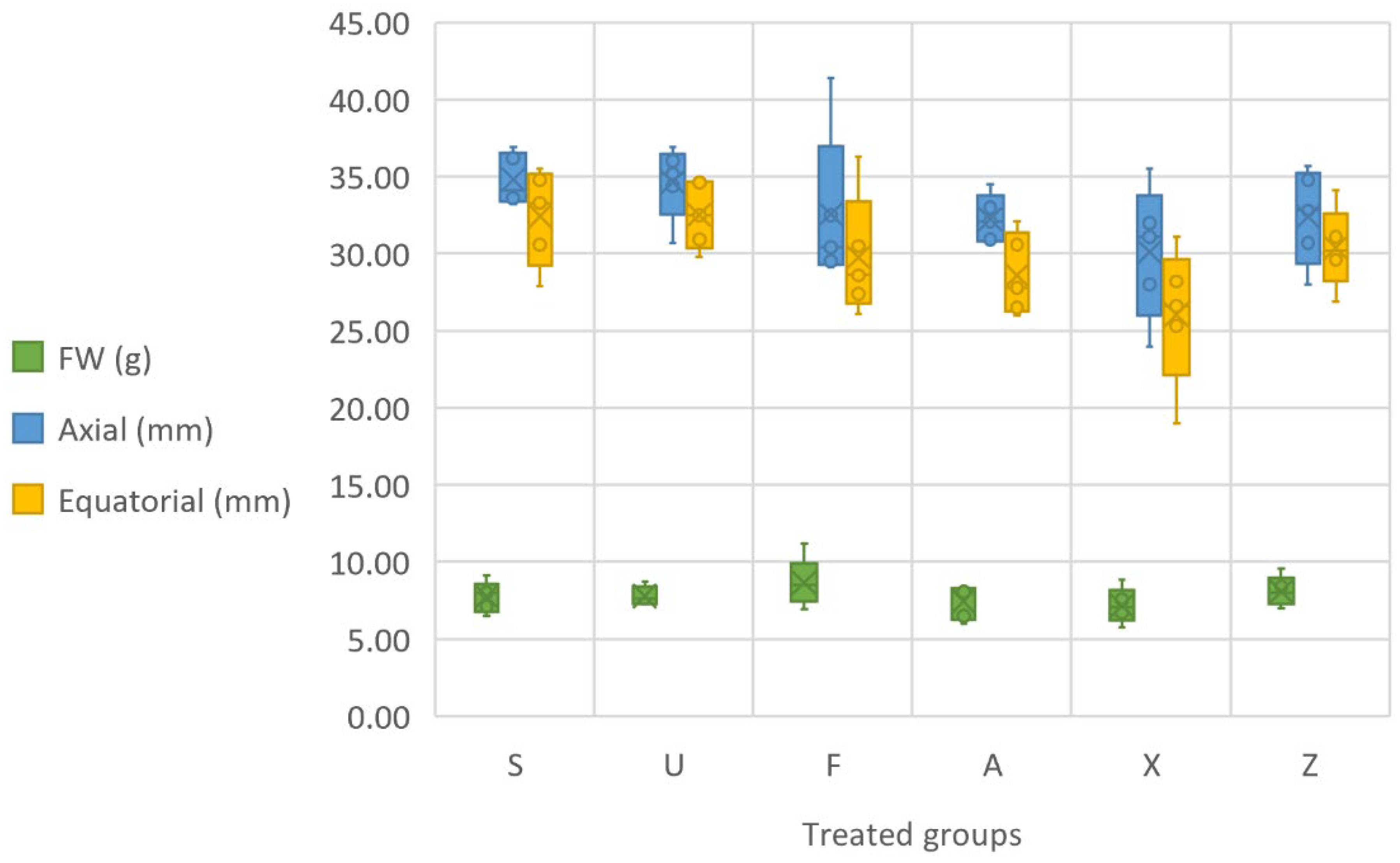
The analysis of the physical parameters of the tomato fruits, including the fresh weight (FW), and the axial and equatorial dimensions (Figure 3), reveal noteworthy trends in regard to the impact of different seaweed treatments. After performing a one-way ANOVA on the fruit weight, it was observed that the variability between the treated groups was significantly different (p < 0.05). Regarding the axial length, the results indicate that there were significant differences between the treated groups (p < 0.05). In particular, the treatments with Furcellaria lumbricalis (F) and Sargassum muticum (S) showed a greater axial length than the control, with some fruits reaching lengths greater than 40 mm. Similarly, the equatorial dimension results from the ANOVA highlighted significant differences between the groups (p < 0.05). The groups treated with Furcellaria lumbricalis and Sargassum muticum showed the largest equatorial dimensions. In terms of fruit fresh weight (FW), the algal treatments generally resulted in varied outcomes. Sargassum muticum showed a range of fresh fruit weights, with the highest value reaching 9.15 g, while the lowest value was 6.49 g. Ulva ohnoi (U) also contributed to an increase in fruit weight, with values ranging from 7.27 g to 8.73 g. The Furcellaria lumbricalis (F) treatments produced some of the highest fruit weights, with a maximum of 11.19 g, which is the largest observed among all the treatments. The Ascophyllum nodosum (A) treatment showed a somewhat modest effect on fruit weight, with values ranging from 6.00 g to 8.41 g. The commercial product (X) demonstrated the lowest fruit weights, with values between 5.75 g and 8.84 g. The control group (Z), which did not receive any fertilisation, presented relatively stable fruit weights, ranging from 7.01 g to 9.55 g. In terms of the axial and equatorial dimensions, the results also show trends that reflect the impact of seaweed-based treatments on tomato fruit development. Sargassum muticum (S) produced fruits with axial lengths ranging from 33.20 mm to 36.90 mm, with equatorial dimensions ranging from 27.90 mm to 35.50 mm. Ulva ohnoi (U) exhibited slightly smaller dimensions overall, with axial lengths ranging from 30.70 mm to 36.90 mm and equatorial dimensions from 29.80 mm to 34.80 mm. Furcellaria lumbricalis (F) had a more varied effect, with axial lengths ranging from 29.10 mm to 41.40 mm, and equatorial dimensions between 26.10 mm and 36.30 mm. The larger axial length and equatorial size observed in some instances (particularly, 41.40 mm in axial length) suggest that Furcellaria lumbricalis (F) can strongly promote fruit size. For Ascophyllum nodosum (A), the axial lengths ranged from 30.00 mm to 34.50 mm, and the equatorial dimensions varied between 26.00 mm and 32.10 mm. The commercial product (X) demonstrated axial lengths ranging from 24.00 mm to 35.50 mm, and equatorial dimensions ranging from 19.00 mm to 31.10 mm. The smaller fruit size observed in some cases, especially with a minimum axial length of 24.00 mm, could indicate that the commercial product may not be as effective in stimulating fruit growth as the other algal extracts. The control group (Z) exhibited axial lengths ranging from 28.00 mm to 35.70 mm, and equatorial dimensions from 26.90 mm to 34.10 mm, with no significant deviation from the treated groups in terms of the overall fruit size.
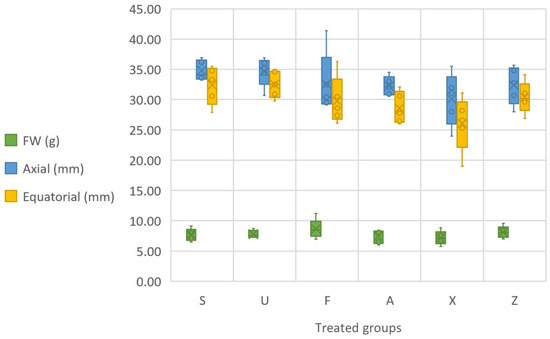
Figure 3.
Physical parameters of the fruit, presented in terms of grams of fresh weight (FW), axial (mm) and equatorial (mm) measurements. Notes: Sargassum muticum (S); Ulva ohnoi (U); Furcellaria lumbricalis (F); Ascophyllum nodosum (A); MC Extra, commercial product (X); negative control (Z).
3.4. Chemical Composition Analysis of the Fruit
3.4.1. Acidity—pH
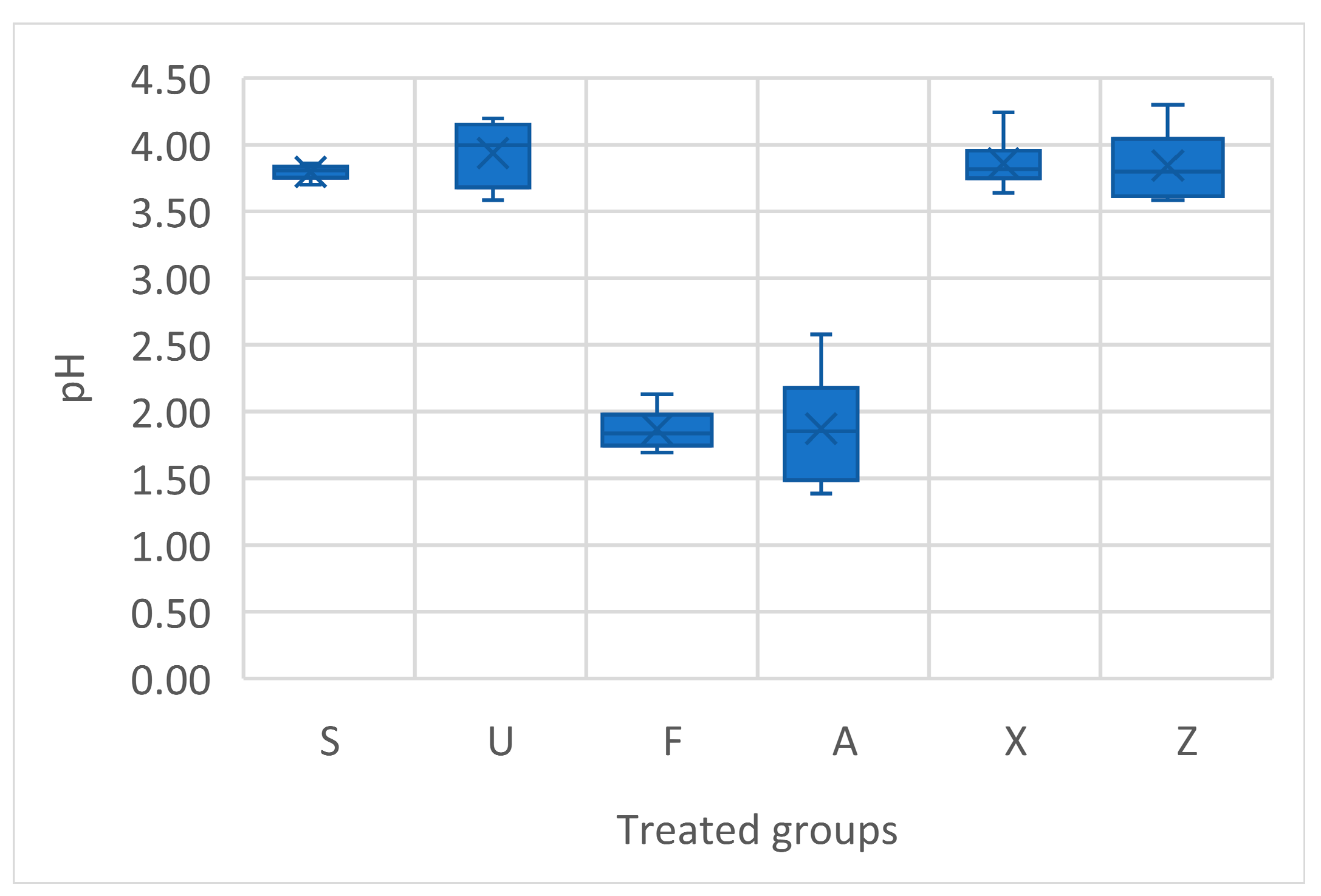
The one-way ANOVA conducted on the pH values among the different treatment groups reveals a highly significant effect of the treatment type on fruit pH (p < 0.0001). The pH values of tomato fruits across the different treatments revealed some significant variations (Figure 4). The Sargassum muticum (S) treatment showed relatively consistent pH values, ranging from 3.70 to 3.86, with an average value of around 3.80. For Ulva ohnoi (U), the pH values were generally higher, with a range from 3.58 to 4.20, and an average of around 3.94. The Furcellaria lumbricalis (F) treatment exhibited a stark contrast, with pH values significantly lower, ranging from 1.69 to 2.13, and a clear tendency towards a much more acidic environment. For Ascophyllum nodosum (A), the pH values were generally low, ranging from 1.39 to 2.58, with an average value of around 1.87. The fruits from plants treated with Ascophyllum nodosum were consistently more acidic, especially compared to Sargassum and Ulva treatments, which is indicative of a potentially strong acidic influence of this seaweed on fruit composition. The commercial product (X) showed a pH range from 3.64 to 4.24, with an average of around 3.84. The control group (Z) group exhibited pH values ranging from 3.58 to 4.30, with an average of 3.84. This indicates that without any seaweed treatment, the pH of the tomato fruit remained in the slightly acidic to neutral range, similar to that observed in regard to the commercial product.
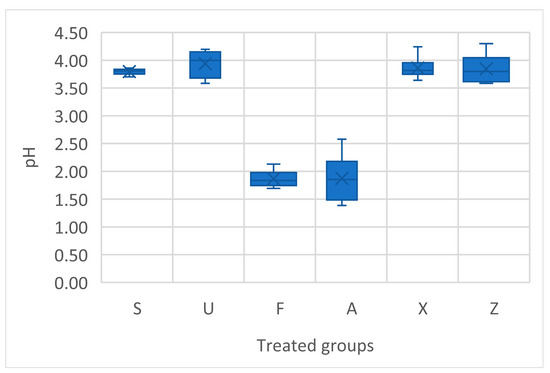
Figure 4.
Acidity measurement in terms of pH of tomato fresh fruits. Notes: Sargassum muticum (S); Ulva ohnoi (U); Furcellaria lumbricalis (F); Ascophyllum nodosum (A); MC Extra, commercial product (X); negative control (Z).
3.4.2. Proline
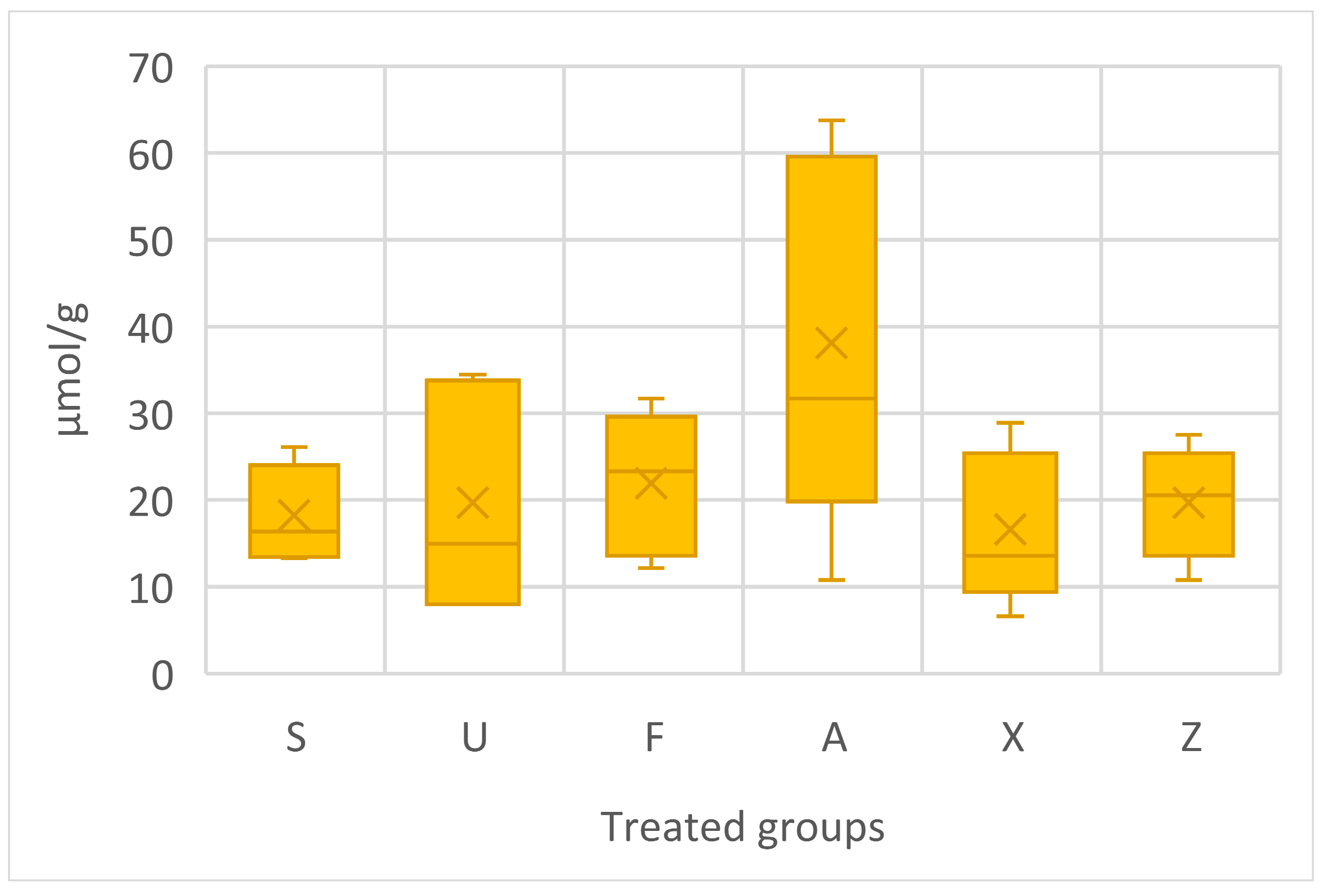
The proline content data are shown in Figure 5. The one-way ANOVA does not show any significant differences; nevertheless, Ascophyllum nodosum emerged as the group with the highest amount of proline content, with consistently high concentrations, particularly the peak value of 63.77 µmol/g. Ulva ohnoi recorded the highest concentration of 34.48 µmol/g. On the other hand, Sargassum muticum and Furcellaria lumbricalis showed more moderate concentrations of proline, with the highest values of 26.11 µmol/g and 31.69 µmol/g, respectively. The commercial product (X) displayed lower concentrations of proline compared to different extraction methods used in terms of Ascophyllum nodosum species, with a maximum of 28.90 µmol/g. The control group, which received no additional fertilisation, still showed comparable concentrations of proline, ranging from 10.77 µmol/g to 27.50 µmol/g.
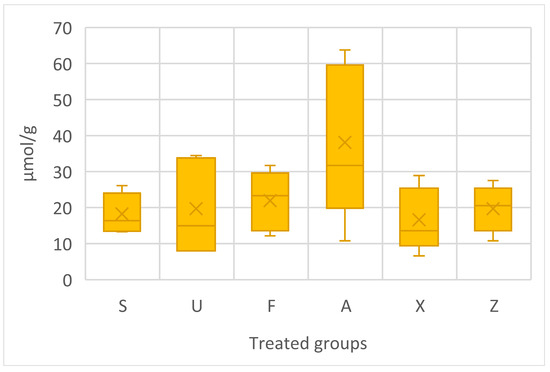
Figure 5.
Proline content in tomato fruits, presented in terms of µmol/g. Notes: Sargassum muticum (S); Ulva ohnoi (U); Furcellaria lumbricalis (F); Ascophyllum nodosum (A); MC Extra, commercial product (X); negative control (Z).
3.4.3. Soluble Solids Content—°Brix
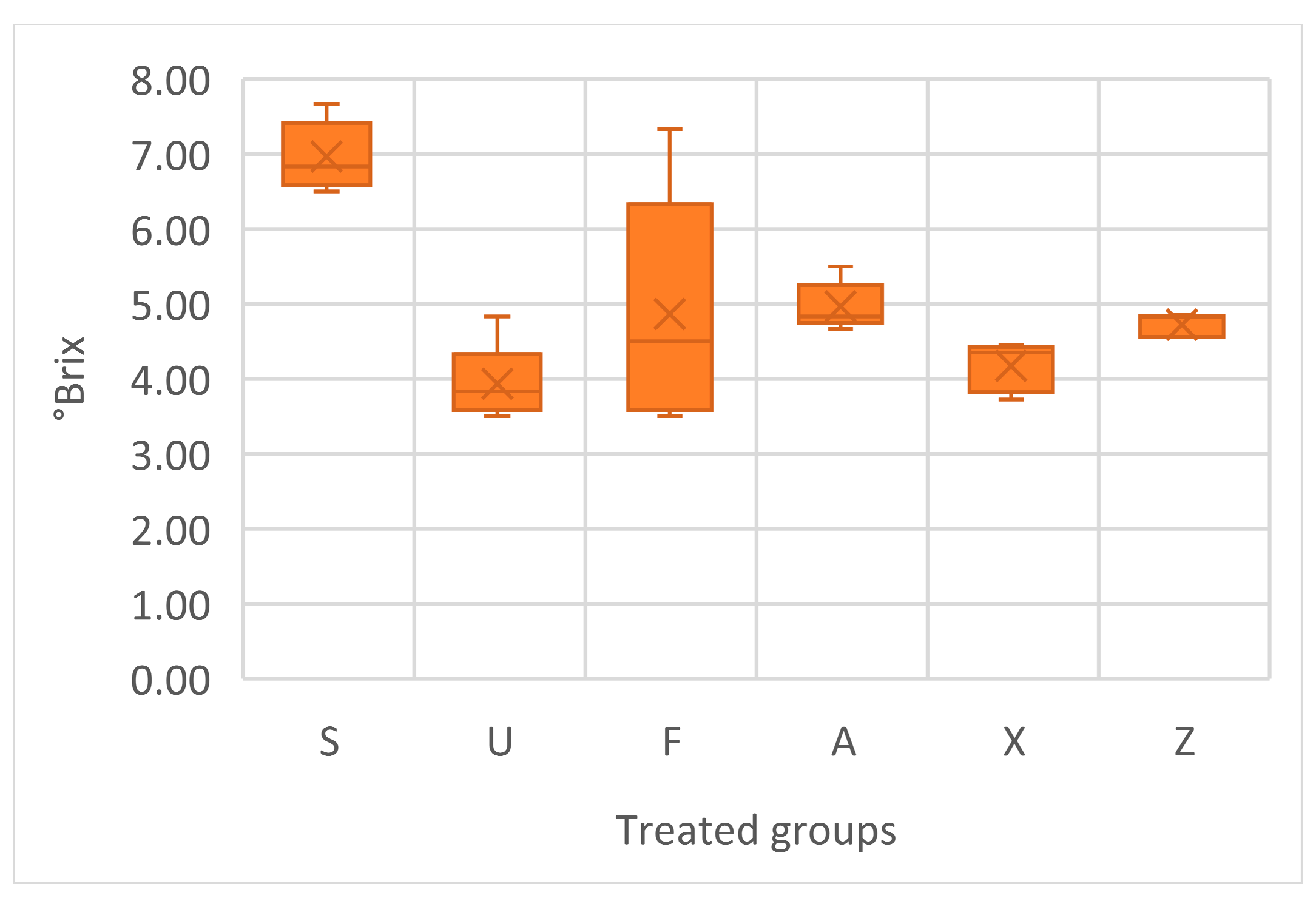
The soluble solids content, measured in °Brix, varied across the treatments (Figure 6). The one-way ANOVA indicates that there are statistically significant differences among the groups in regard to °Brix values (p < 0.005). Sargassum muticum (S) showed the highest average value of 6.57, with a relatively low standard deviation of 0.46, indicating consistent sugar accumulation. The Ulva ohnoi group (U) had the lowest average °Brix at 3.93, with a moderate spread in the data. Furcellaria lumbricalis (F) had a mean of 4.87, but also the highest variability, suggesting the occurrence of heterogeneous responses within this group. Treatments with Ascophyllum nodosum (both A and Z) had similar means, 4.97 and 4.73, respectively. The control group showed an average of 4.17, with moderate consistency.
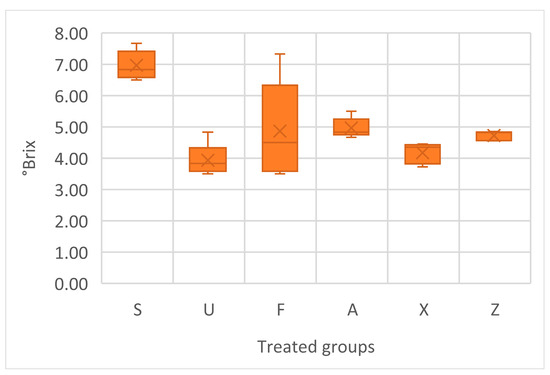
Figure 6.
Soluble solids content in tomato fruits, presented in °Brix. Notes: Sargassum muticum (S); Ulva ohnoi (U); Furcellaria lumbricalis (F); Ascophyllum nodosum (A); MC Extra, commercial product (X); negative control (Z).
4. Discussion
The leaf fresh weight findings provide further support for the potential of certain macroalgal extracts to be used as biostimulants. Ulva ohnoi (U) and Ascophyllum nodosum (A) appeared to be particularly effective, promoting increased leaf biomass, a key indicator of vegetative growth and nutrient uptake efficiency. Notably, the treatment with the commercial product (X) displayed the largest interquartile range, indicating substantial variability in the plant response. The control (Z) presented some of the lowest values, reinforcing the positive effects of seaweed-derived treatments on plant biomass. This aligns with their known biochemical profiles, which include polysaccharides, micronutrients, and plant hormones that enhance water retention and cell expansion [26,37,38]. Interestingly, the commercial product (X), despite being derived from Ascophyllum nodosum, which is widely used in fertiliser and biostimulant formulations [39,40], demonstrated a lower median performance and greater variability. This may reflect differences in the formulation, stability, or application efficiency compared to the raw extract. Furcellaria lumbricalis (F), although not among the top performers, showed a stable and moderate improvement, suggesting that its extract may contribute to growth under consistent conditions. Overall, these results suggest that A. nodosum and U. ohnoi are the most promising candidates for enhancing leaf biomass.
In regard to chlorophyll content, among the treatment groups, both Ascophyllum nodosum extracts (A and its commercial product, X) were particularly effective, exhibiting the highest chlorophyll concentrations. This suggests that Ascophyllum nodosum has a greater ability to promote chlorophyll synthesis, likely due to its high content of bioactive compounds, such as phytohormones and polysaccharides, which are known to enhance plant growth and stress tolerance [40,41]. Sargassum muticum (S), Ulva ohnoi (U), and Furcellaria lumbricalis (F) did not increase the chlorophyll content. This indicates that other macroalgae, while still beneficial, may not be as potent in enhancing the photosynthetic activity as Ascophyllum nodosum. Generally, a trend emerges showing “Ascophyllum nodosum-based” treatments being the most effective for enhancing the chlorophyll content.
One of the most important parameters for tomatoes is the pH. Our results from the analysis of the tomato fruit juice reveal distinct effects of the different seaweed treatments on fruit acidity, a critical factor influencing fruit flavour and consumer preferences. The pH values of the tomato fruits indicate clear differences in the acidity levels, as influenced by the seaweed-based treatments. Ulva ohnoi (U) showed the highest average pH, suggesting a notable reduction in fruit acidity and a potential shift towards a sweeter, more palatable profile. This was followed closely by the commercial product (X), as well as the control (Z) and Sargassum muticum (S), all of which displayed moderately acidic, but comparable, pH levels. In contrast, the Furcellaria lumbricalis (F) and Ascophyllum nodosum (A) extracts produced the lowest pH values, indicating a significant increase in acidity. Among these, A-treated fruits showed a particularly pronounced acidity, which may reflect enhanced organic acid metabolism or stress-related responses in the plants [42]. Interestingly, although both A and X are derived from Ascophyllum nodosum, their divergent effects on fruit pH highlight the impact of formulation differences between different extraction and/or different biomass treatments.
The proline content analysis reveals distinct physiological responses among tomato plants treated with different seaweed extracts. Ascophyllum nodosum (A) produced the highest average proline levels, nearly double those of most other treatments. This suggests the potential induction of mild stress or an enhanced osmoprotective response, often associated with increased biosynthesis of compatible solutes like proline [43]. Furcellaria lumbricalis (F) and Ulva ohnoi (U) also led to moderately elevated proline concentrations, indicating some degree of metabolic stimulation, although with greater variability. In contrast, the commercial product (X) showed the lowest average values among the treatments, even slightly below the control group (Z), suggesting a more limited effect on stress-related metabolic pathways. Interestingly, Sargassum muticum (S) and the control (Z) showed comparable proline levels, which may indicate that Sargassum was relatively neutral in influencing plant osmotic adjustments under the tested conditions.
The °Brix measurements provide valuable insight into the impact of seaweed treatments on fruit sweetness and soluble solids accumulation. Sargassum muticum (S) stood out significantly, producing the highest °Brix values among all the treatment groups, with a consistent trend across the replicates, as confirmed by other authors [44]. Also, Sargassum muticum may enhance sugar metabolism or transport during fruit development, thereby improving the flavour quality of the fruit. In contrast, Ulva ohnoi (U) resulted in the lowest °Brix readings, indicating limited effectiveness in promoting sugar accumulation in tomato fruits under the tested conditions. Interestingly, the commercial seaweed product (X) performed only marginally better than Ulva, with values slightly above 4, and even lower than the untreated control (Z), which had a stable and relatively high mean. The treatments with Furcellaria lumbricalis (F) and Ascophyllum nodosum (A) showed moderate improvements over the control, suggesting a mild stimulatory effect on fruit quality, although not as pronounced as that of S. muticum.
By combining key physicochemical parameters of tomato fruits (pH, proline content, and °Brix) it is possible to define a flavour profile indicative of consumer-relevant sensory attributes. This integrative result allows for a more comprehensive assessment, capturing not only analytical values, but also their implications for the sweetness, acidity balance, and aromatic complexity of fruit. Based on this framework, the flavour profiles of fruits derived from the various macroalgal treatments were critically evaluated. The flavour profile analysis, informed by the integrated values on the °Brix, pH, and proline content, offers a picture of how each seaweed extract influenced tomato fruit quality, as follows:
- Sargassum muticum (S) yielded fruits with a high °Brix and low-to-moderate pH, placing it close to the ideal profile associated with sweetness and balanced acidity. However, the relatively moderate proline levels suggest that while the fruits were sweet and mildly acidic, they lacked the aromatic complexity typical of high-proline samples, resulting in a pleasant but not fully expressive flavour;
- Ulva ohnoi (U) presented high pH values and a low °Brix, resulting in a notably flat flavour. The proline content was moderate, but insufficient to overcome the low sugar and acidity contrast. The resulting taste was perceived as underwhelming, neither sweet nor particularly aromatic, suggesting that this extract may not enhance fruit flavour in its current form;
- Furcellaria lumbricalis (F) offered a markedly different profile. With a very low pH and moderate sugar content, its fruits were sharply acidic. Nonetheless, a fairly high proline level helped introduce a mild aromatic component, giving the fruit a more complex character;
- The Ascophyllum nodosum (A) treatment produced one of the most promising flavour profiles. Despite a very low pH, the extremely high proline content contributed a strong aromatic and umami note, while the sugar levels remained within a moderate range. This combination created a fruit that was intensely flavoured and complex, with a pronounced acidic backbone and a robust aromatic finish;
- The commercial product (X) produced tomatoes that were chemically similar in pH to S and Z; its lower Brix and moderate proline content resulted in a generally bland taste, lacking in both sweetness and aromatic depth.
All these results highlight how the starting material, the algal species, and also the treatment (see the difference between A and X, both deriving from Ascophyllum), are fundamental in order to be able to choose the right extraction method in relation to the chosen product. It is important to note that the foliar application method used in this study significantly reduces the risk of sodium accumulation in the soil, thereby minimizing the potential negative effects associated with prolonged use. Nevertheless, monitoring soil chemistry over extended application periods will be an important consideration for future research.
5. Conclusions
The widespread use of chemical fertilizers has significantly boosted agricultural productivity by directly supplying essential nutrients to plants. However, their prolonged application has raised concerns about their negative impact on soil health and plant growth. Additionally, their continuous use depletes soil organic matter, reduces the water-holding capacity of the soil, and disrupts microbial diversity, ultimately impairing plant development. These challenges highlight the urgent need for sustainable agricultural alternatives that enhance crop productivity and safeguard soil health and ecosystem stability. The observed improvements in both the vegetative and reproductive growth of tomato plants suggest that seaweed extracts contain bioactive compounds capable of activating plant signalling pathways. This activation enhances nutrient uptake and utilization, leading to more efficient growth. The findings in this study highlight the potential of macroalgal treatments in enhancing plant growth, the physiological attributes, and the metabolic activity in tomato plants. It is important to note how not only the macroalgal species influences the final extract and, therefore, also the effects on the product, but also how the treatment and extraction of the fertilizer from the same type of algae are important. In general, no great difference was noted between the groups in regard to the vegetative part and, in particular, it is noted how the Sargassum and Ulva extracts were useful to maintain a pH similar to the control. On the other hand, other extracts lower the pH. The sugar content was higher in Sargassum sp., which holds promise for improving regional crop cultivation along coastal areas; however, large-scale agricultural applications may be limited by resource availability. It is important that the profile of the fertiliser/biostimulant must be carefully chosen based on the profile of the tomato that must be obtained (e.g., sweet, aromatic, balanced acidic of the ripe cherry type). Further research is necessary to explore the potential of macroalgal extracts as biofertilisers, particularly in mitigating environmental stress in essential agricultural crops, thereby contributing to sustainable and resilient farming practices.
Author Contributions
Conceptualization, D.S. and V.R.; methodology, D.S., M.G.D.M., V.R. and G.G.; validation, V.R. and G.G.; investigation, D.S., A.K., M.G.D.M. and V.R.; data curation, D.S., D.P., A.K. and V.R.; writing—original draft, D.S.; writing—review and editing, D.S. and A.K.; visualization, D.S., M.G.D.M., V.R. and G.G.; supervision, D.S., D.P. and G.G.; project administration, D.S. and V.R.; funding acquisition, V.R. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FFABR2021-UniMe (Italian Ministry of University and Research).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article. No additional data are available.
Conflicts of Interest
Author Maria Grazia De Michele and Valentino Russo were employed by the company Promethea Biochem Solutions S.r.l. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ericksen, P.J.; Ingram, J.S.; Liverman, D.M. Food Security and Global Environmental Change: Emerging Challenges. Environ. Sci. Policy 2009, 12, 373–377. [Google Scholar] [CrossRef]
- Zuma, M.; Arthur, G.; Coopoosamy, R.; Naidoo, K. Incorporating Cropping Systems with Eco-Friendly Strategies and Solutions to Mitigate the Effects of Climate Change on Crop Production. J. Agric. Food Res. 2023, 14, 100722. [Google Scholar] [CrossRef]
- Knapp, S.; Peralta, I.E. The Tomato (Solanum lycopersicum L., Solanaceae) and Its Botanical Relatives. In The Tomato Genome; Causse, M., Giovannoni, J., Bouzayen, M., Zouine, M., Eds.; Compendium of Plant Genomes; Springer: Berlin/Heidelberg, Germay, 2016; pp. 7–21. ISBN 978-3-662-53387-1. [Google Scholar]
- Keatinge, J.D.H.; Yang, R.-Y.; Hughes, J.d.A.; Easdown, W.J.; Holmer, R. The Importance of Vegetables in Ensuring Both Food and Nutritional Security in Attainment of the Millennium Development Goals. Food Secur. 2011, 3, 491–501. [Google Scholar] [CrossRef]
- Kumari, R.; Kaur, I.; Bhatnagar, A.K. Effect of Aqueous Extract of Sargassum johnstonii Setchell & Gardner on Growth, Yield and Quality of Lycopersicon esculentum Mill. J. Appl. Phycol. 2011, 23, 623–633. [Google Scholar] [CrossRef]
- Perveen, R.; Suleria, H.A.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Ahmad, S. Tomato (Solanum lycopersicum) Carotenoids and Lycopenes Chemistry; Metabolism, Absorption, Nutrition, and Allied Health Claims—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 919–929. [Google Scholar] [CrossRef]
- Igile, G.O.; Ekpe, O.O.; Essien, N.M.; Bassey, S.C.; Agiang, M.A. Quality Characteristics of Tomato Juice Produced and Preserved with and without Its Seeds. Dannish J. Food Sci. Technol. 2016, 2, 1–9. [Google Scholar]
- Liu, W.; Liu, K.; Chen, D.; Zhang, Z.; Li, B.; El-Mogy, M.M.; Tian, S.; Chen, T. Solanum lycopersicum, a Model Plant for the Studies in Developmental Biology, Stress Biology and Food Science. Foods 2022, 11, 2402. [Google Scholar] [CrossRef]
- Felföldi, Z.; Ranga, F.; Roman, I.A.; Sestras, A.F.; Vodnar, D.C.; Prohens, J.; Sestras, R.E. Analysis of Physico-Chemical and Organoleptic Fruit Parameters Relevant for Tomato Quality. Agronomy 2022, 12, 1232. [Google Scholar] [CrossRef]
- Sardaro, M.L.S.; Marmiroli, M.; Maestri, E.; Marmiroli, N. Genetic Characterization of Italian Tomato Varieties and Their Traceability in Tomato Food Products. Food Sci. Nutr. 2013, 1, 54–62. [Google Scholar] [CrossRef]
- Petro-Turza, M. Flavor of Tomato and Tomato Products. Food Rev. Int. 1986, 2, 309–351. [Google Scholar] [CrossRef]
- Gautier, H.; Diakou-Verdin, V.; Bénard, C.; Reich, M.; Buret, M.; Bourgaud, F.; Poëssel, J.L.; Caris-Veyrat, C.; Génard, M. How Does Tomato Quality (Sugar, Acid, and Nutritional Quality) Vary with Ripening Stage, Temperature, and Irradiance? J. Agric. Food Chem. 2008, 56, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Jiang, P.; Liu, Y.; Miao, X.; Liu, A. Distinct Changes of Taste Quality and Metabolite Profile in Different Tomato Varieties Revealed by LC-MS Metabolomics. Food Chem. 2024, 442, 138456. [Google Scholar] [CrossRef] [PubMed]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health. In Microbiota and Biofertilizers; Dar, G.H., Bhat, R.A., Mehmood, M.A., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 2, pp. 1–20. ISBN 978-3-030-61009-8. [Google Scholar]
- Priya, A.K.; Alagumalai, A.; Balaji, D.; Song, H. Bio-Based Agricultural Products: A Sustainable Alternative to Agrochemicals for Promoting a Circular Economy. RSC Sustain. 2023, 1, 746–762. [Google Scholar] [CrossRef]
- Ammar, E.E.; Aioub, A.A.; Elesawy, A.E.; Karkour, A.M.; Mouhamed, M.S.; Amer, A.A.; El-Shershaby, N.A. Algae as Bio-Fertilizers: Between Current Situation and Future Prospective. Saudi J. Biol. Sci. 2022, 29, 3083–3096. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Macroalgae as a Sustainable Biostimulant for Crop Production According to Techno-Economic and Environmental Criteria. Sustain. Prod. Consum. 2024, 48, 169–180. [Google Scholar] [CrossRef]
- Kholssi, R.; Lougraimzi, H.; Grina, F.; Lorentz, J.F.; Silva, I.; Castaño-Sánchez, O.; Marks, E.A.N. Green Agriculture: A Review of the Application of Micro- and Macroalgae and Their Impact on Crop Production on Soil Quality. J. Soil Sci. Plant Nutr. 2022, 22, 4627–4641. [Google Scholar] [CrossRef]
- Wan, A.H.L.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a Sustainable Aquafeed Ingredient. Rev. Aquac. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Bayat, F.; Afshar, A.; Baghban, N. Algal Cells-Derived Extracellular Vesicles: A Review with Special Emphasis on Their Antimicrobial Effects. Front. Microbiol. 2021, 12, 785716. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, D.; Russo, V.; Manghisi, A.; Di Martino, A.; Morabito, M.; Genovese, G.; Trifilò, P. Screening on the Presence of Plant Growth Regulators in High Biomass Forming Seaweeds from the Ionian Sea (Mediterranean Sea). Sustainability 2022, 14, 3914. [Google Scholar] [CrossRef]
- Rathod, S.G.; Bhushan, S.; Mantri, V.A. Phytohormones and Pheromones in the Phycology Literature: Benchmarking of Data-Set and Developing Critical Tools of Biotechnological Implications for Commercial Aquaculture Industry. Phycology 2023, 4, 1–36. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Cozzolino, E.; Di Mola, I.; Ottaiano, L.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Foliar Application of Plant-Based Biostimulants Improve Yield and Upgrade Qualitative Characteristics of Processing Tomato. Ital. J. Agron. 2021, 16, 1825. [Google Scholar] [CrossRef]
- Song, C.; Lorz, L.R.; Lee, J.; Cho, J.Y. In Vitro Photoprotective, Anti-Inflammatory, Moisturizing, and Antimelanogenic Effects of a Methanolic Extract of Chrysophyllum lucentifolium Cronquist. Plants 2021, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed Extracts as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Prisa, D.; Spagnuolo, D. Evaluation of the Bio-Stimulating Activity of Lake Algae Extracts on Edible Cacti Mammillaria prolifera and Mammillaria glassii. Plants 2022, 11, 3586. [Google Scholar] [CrossRef]
- Spagnuolo, D.; Bressi, V.; Chiofalo, M.T.; Morabito, M.; Espro, C.; Genovese, G.; Iannazzo, D.; Trifilo, P. Using the Aqueous Phase Produced from Hydrothermal Carbonization Process of Brown Seaweed to Improve the Growth of Phaseolus vulgaris. Plants 2023, 12, 2745. [Google Scholar] [CrossRef]
- Fatimah, S.; Daud, N. The Effect of Seaweed Extract (Sargassum sp.) Used as Fertilizer on Plant Growth of Capsicum annum (Chilli) and Lycopersicon esculentum (Tomato). Indones. J. Sci. Technol. 2018, 3, 115–123. [Google Scholar] [CrossRef]
- Espinosa-Antón, A.A.; Zamora-Natera, J.F.; Zarazúa-Villaseñor, P.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Águila Alcántara, E.; Torres-Morán, M.I.; Velasco-Ramírez, A.P.; Hernández-Herrera, R.M. Application of Seaweed Generates Changes in the Substrate and Stimulates the Growth of Tomato Plants. Plants 2023, 12, 1520. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, X.; Chen, B.; Zhang, M.; Ma, J. Seaweed Extract Improved Yields, Leaf Photosynthesis, Ripening Time, and Net Returns of Tomato (Solanum lycopersicum Mill.). ACS Omega 2020, 5, 4242–4249. [Google Scholar] [CrossRef]
- González-González, M.F.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Casarrubias-Castillo, K.; Becerril-Espinosa, A.; Castañeda-Nava, J.J.; Hernández-Herrera, R.M. Physiological, Ecological, and Biochemical Implications in Tomato Plants of Two Plant Biostimulants: Arbuscular Mycorrhizal Fungi and Seaweed Extract. Front. Plant Sci. 2020, 11, 999. [Google Scholar] [CrossRef]
- Yao, G.-F.; Li, C.; Sun, K.-K.; Tang, J.; Huang, Z.-Q.; Yang, F.; Huang, G.-G.; Hu, L.-Y.; Jin, P.; Hu, K.-D. Hydrogen Sulfide Maintained the Good Appearance and Nutrition in Post-Harvest Tomato Fruits by Antagonizing the Effect of Ethylene. Front. Plant Sci. 2020, 11, 584. [Google Scholar] [CrossRef]
- Jalali, P.; Roosta, H.R.; Khodadadi, M.; Torkashvand, A.M.; Jahromi, M.G. Effects of Brown Seaweed Extract, Silicon, and Selenium on Fruit Quality and Yield of Tomato under Different Substrates. PLoS ONE 2022, 17, e0277923. [Google Scholar] [CrossRef] [PubMed]
- Rama Rao, K. Preparation of Liquid Seaweed Fertilizer from Sargassum. In Proceedings of the Seaweed Research and Utilization Association Workshop on Algal Products and Seminar on Phaeophyceae in India, Madras, India, 4–7 June 1990; pp. 4–7. [Google Scholar]
- Parry, C.; Blonquist, J.M.; Bugbee, B. In Situ Measurement of Leaf Chlorophyll Concentration: Analysis of the Optical/Absolute Relationship. Plant Cell Environ. 2014, 37, 2508–2520. [Google Scholar] [CrossRef] [PubMed]
- Mughunth, R.J.; Velmurugan, S.; Mohanalakshmi, M.; Vanitha, K. A Review of Seaweed Extract’s Potential as a Biostimulant to Enhance Growth and Mitigate Stress in Horticulture Crops. Sci. Hortic. 2024, 334, 113312. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant Properties of Seaweed Extracts in Plants: Implications towards Sustainable Crop Production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum Nodosum-Based Biostimulants: Sustainable Applications in Agriculture for the Stimulation of Plant Growth, Stress Tolerance, and Disease Management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- Kumari, S.; Sehrawat, K.D.; Phogat, D.; Sehrawat, A.R.; Chaudhary, R.; Sushkova, S.N.; Voloshina, M.S.; Rajput, V.D.; Shmaraeva, A.N.; Marc, R.A.; et al. Ascophyllum nodosum (L.) Le Jolis, a Pivotal Biostimulant toward Sustainable Agriculture: A Comprehensive Review. Agriculture 2023, 13, 1179. [Google Scholar] [CrossRef]
- Ali, J.; Jan, I.; Ullah, H.; Ahmed, N.; Alam, M.; Ullah, R.; El-Sharnouby, M.; Kesba, H.; Shukry, M.; Sayed, S.; et al. Influence of Ascophyllum nodosum Extract Foliar Spray on the Physiological and Biochemical Attributes of Okra under Drought Stress. Plants 2022, 11, 790. [Google Scholar] [CrossRef]
- Reimer, J.J.; Thiele, B.; Biermann, R.T.; Junker-Frohn, L.V.; Wiese-Klinkenberg, A.; Usadel, B.; Wormit, A. Tomato Leaves under Stress: A Comparison of Stress Response to Mild Abiotic Stress between a Cultivated and a Wild Tomato Species. Plant Mol. Biol. 2021, 107, 177–206. [Google Scholar] [CrossRef]
- Mehta, D.; Vyas, S. Comparative Bio-Accumulation of Osmoprotectants in Saline Stress Tolerating Plants: A Review. Plant Stress 2023, 9, 100177. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Slocum, A.H.; Ceballos, M.L.; Aponte, P.; Bisonó-León, A.G. Beyond the Bloom: Invasive Seaweed Sargassum Spp. as a Catalyst for Sustainable Agriculture and Blue Economy—A Multifaceted Approach to Biodegradable Films, Biostimulants, and Carbon Mitigation. Sustainability 2025, 17, 3498. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).