Abstract
The high protein content of several microalgal species makes them attractive and unconventional candidates for use in the food and pharmaceutical industries. Due to the robust cell walls of microalgae, cell rupture is necessary to improve the extraction of intracellular proteins. Thus, choosing a suitable cell-breaking treatment before protein extraction is a vital downstream processing step. Additionally, it is necessary to use an effective technique for monitoring and measuring the impact of rupture treatments on microalgal cell walls. In our study, Tetradesmus obliquus cells were disrupted using three different mechanical rupture methods: high-pressure homogenization (HPH), ultrasound (US), and ball milling (BM). The ruptured biomass cells were counted, and soluble proteins were extracted and quantified. The cell-counting technique did not detect any differences between intact and damaged cells after BM treatment because the dye (erythrosine B) did not permeate the microalgal biomass accurately. The US treatment promoted the highest yield of total protein extraction (19.95%), while the highest yields in the HPH and BM treatments were 15.68% and 14.11%, respectively. Since the cell breakage method affects protein extraction from microalgal biomass, protein release can be used as a central indicator of the degree of cell disruption.
1. Introduction
Microalgae are a group of organisms with diverse morphological, reproductive, physiological, and ecological characteristics. Microalgae can be used in the cosmetics industry for wastewater treatment, energy generation, and human and animal nutrition; they can survive in areas unsuitable for growing traditional crops; do not compete with food production sites; and are rich in metabolites such as proteins, lipids, vitamins, enzymes, pigments, mineral salts, antioxidants, and antibiotics [1,2]. Short- and long-term feeding tests have demonstrated that some microalgae are safe for human consumption [2,3,4,5,6]. Thus, in human nutrition, microalgae are an unconventional source of green proteins and bioactive peptides [1]. Some of these proteins have nutritional quality comparable to that of proteins of plant origin [7], exhibiting essential amino acid compositions in the quantities recommended by the Food and Agriculture Organization (FAO) [1]. Depending on the species, microalgal protein content can reach 70% [8]. However, approximately 70% of microalgal proteins are intracellularly located and adhere to the cell wall. Thus, the cells must be ruptured to extract or release these components [9].
Cell disruption, as one of the first downstream stages of microalgal biotechnological platform development, promotes the permeabilization or complete breakage of the membranes and walls of cells. Cell wall disruption is necessary for accessing intracellular components since valuable biocomponents, such as lipids and proteins, are located within microalgal cells. Cell breakage enables the release of intracellular contents, which can be further processed, purified, and used in various applications. Rupturing the cell wall also increases the surface area available for extraction processes, enhancing the efficiency of extraction methods through contact between the solvent and the compounds of interest [10]. Consequently, selecting a suitable cell-breaking treatment before protein extraction is crucial for downstream processing. Mechanical cell rupture methods, such as high-pressure homogenization, ball milling, and sonication, have been used [11,12,13,14] because they provide good cell wall disintegration efficiency and do not use chemical reagents. Since downstream steps represent a large part of the operational costs in the production chain, cell lysis technologies must be low-cost and energy-efficient, improving product quality and yield [15,16]. Moreover, an effective technique is necessary for monitoring and measuring the degree of microalgal cell disruption.
This study aimed to (i) investigate the effects of three different disruption methods, namely high-pressure homogenization, ball milling, and ultrasound, on Tetradesmus obliquus cells; (ii) identify the majority of proteins in protein-rich extracts via molecular mass profiling; and (iii) evaluate the color parameters of protein-rich extracts. The latter are useful for studying protein technofunctionalities. The microalgae Tetradesmus spp. (i) are native Brazilian species abundant in aquatic environments; (ii) exhibit sizeable morphological variation within each species; (iii) exhibit a high growth rate; and (iv) contain 30% to 40% protein on a dry-mass basis [13,17]. The literature has also described the great potential of T. obliquus biomass for applications in the food, pharmaceutical, cosmetic, and biofuel industries [13,17,18,19,20,21,22]. Silva et al. [2] reported no toxic effects from the ingestion of T. obliquus biomass by Wistar rats. Analyses of the rats’ livers, spleens, and kidneys indicated that the microalga is a potentially safe food, even at high biomass concentrations (23.2%). Thus, the protein in microalgal biomass is a potential food ingredient. Furthermore, Silva et al. [2] reported that the consumption of T. obliquus reduced the triglyceride content (70%), atherogenic index (80%), and serum glucose concentration (42%) in Wistar rats, even on a balanced diet. Lima et al. [20] reported that lower concentrations (0.5% and 1.0% w/w) of T. obliquus protein concentrate (51.46% w/w) stabilized emulsions after 28 days of storage. Silva et al. [21] observed that T. obliquus protein concentrate (63.14% w/w) could form stable emulsions that were resistant to pH shifts and tolerated high salt concentrations.
2. Materials and Methods
2.1. Tetradesmus obliquus Biomass Processing
Microalgal biomass was a kind gift from the Biofuels Laboratory at Universidade Federal de Viçosa, Brazil. The T. obliquus strain had previously been isolated from freshwater reservoirs and cultivated in BG11 culture media [23,24]. Biomass production was carried out following the protocol established by Vieira et al. [17]. The biomass was produced under photoautotrophic growth conditions in a concrete raceway pond of 4 m3 with a natural photoperiod and light during the summer (Viçosa, Minas Gerais, Brazil). The average temperature in the growing season was approximately 27 °C. The culture media were prepared with macronutrients provided by inorganic fertilizers to increase the protein productivity of T. obliquus through the use of trace elements and without salt stress [13,17,25]. The biomass was collected on the 12th day of cultivation during the stationary growth phase and concentrated using flocculation (Cationic Polyamine, SNF Floerger, Andrézieux, Loire, France) and sedimentation, followed by gravity filtration with a 100 μm polyester membrane, resulting in a microalgal slurry with approximately 5% (w v−1) total solids [17]. The T. obliquus paste was frozen and stored at −10 °C. The frozen biomass was thawed for fractionation, washed with deionized water three times [26] to remove salts, filtered using an organza fabric filter medium to remove impurities, packed in containers, and frozen (GE, São Paulo, São Paulo, Brazil) at −80 °C. The frozen biomass was freeze-dried (LS 3000, Terroni, São Carlos, São Paulo, Brazil), giving rise to dry biomass, reaching a final moisture content of 8 mass %.
The dry biomass was then sheared through a stainless-steel sieve (Tyler 28) with a hole of 0.595 mm. The resulting fine powder, obtained via standardized granulometry, was refrigerated at 8 °C until use.
2.2. Composition of Tetradesmus obliquus Microalgae
The macronutrient composition of the dried Tetradesmus obliquus biomass was characterized in terms of moisture [27], ash [27], and lipids according to the Folch method [28] and in terms of protein according to the Kjeldahl method [27], for which the nitrogen factor was 5.89 [18]. Carbohydrate content was calculated by difference (carbohydrate% = 100% − ash% − lipid% − protein%).
2.3. Separation and Identification of Proteins in the Microalgae Tetradesmus obliquus
- Molecular mass profile
The molecular mass distribution of T. obliquus proteins was determined via SDS-PAGE. Microalgal biomass samples containing known amounts of proteins were kept at 25.0 °C under stirring overnight. The suspensions were processed and subjected to polyacrylamide gel electrophoresis (PAGE) using a 10% gel (0.75 mm) composed of a separation gel (10% acrylamide) and a stacking gel (5% acrylamide) in a 7 × 10 × 0.1 cm Mini-Protean III tank (Bio-Rad, Hercules, CA, USA). The microalgal biomass was initially suspended for solubilization in 1 mL of 25 mM Tris-HCl buffer (pH 7.5), and biomass suspensions were prepared with protein concentrations of 20, 40, and 50 μg/mL. Then, each biomass suspension was (a) mixed with a buffer composed of 10% glycerol, 5% β-mercaptoethanol, 2.3% SDS, and Tris-HCl (pH 6.8), (b) heated at 95 °C for 5 min, (c) centrifuged (Centrifuge 5430, Eppendorf, Hamburg, Germany), and (d) cooled to room temperature (±25 °C). A 21 μL aliquot of each suspension was transferred into the gel channels. The molecular marker (5 μL) used was the prestained standard Precision Plus ProteinTM (Bio-Rad, Hercules, CA, USA), with molecular masses ranging from 10 to 200 kDa.
- Identification by mass spectrometry (MALDI-TOF)
Regions of the gel containing protein bands with the greatest intensity and the best separation were manually cut into cubes of approximately 1.0 mm3. The selected protein bands and a blank were processed via the in-gel digestion technique. Peptide molecular masses were subsequently identified using the methodology of Shevchenko et al. [29]. This protocol included the removal of Coomassie G-250 brilliant blue dye, digestion with the enzyme trypsin, and extraction and concentration of the peptides. Subsequently, 1 μL of the peptide samples was mixed with 3 μL of the ɑ-cyano-4-hydroxycinnamic acid matrix (5 μg/mL in 50% acetonitrile and 0.1% TFA) in the well of a steel plate (MTP Anchor Chip TM 600/384 TF, Bruker Daltonics®, Billerica, MA, USA). MALDI-TOF-MS (laser ionization mass spectrometry) was used to analyze the molecular masses of the peptides originating from protein digestion. External calibration was performed with peptide calibration standard II (Bruker Daltonics®, Billerica, MA, USA) containing the peptides bradykinin (757 Da), angiotensin II (1046 Da), angiotensin I (1296 Da), P substance (1347 Da), bombesin (1619 Da), ACTH clip 1–17 (2093 Da), ACTH clip 18–39 (2465 Da), and somatostatin (3147 Da). The spectrograms were stored to identify the peptides by comparing their molar masses with those deposited in the SwissProt database.
2.4. Biomass Cell Disruption
Three different disruption methods were evaluated:
- Mechanical disruption by ball mill: Freeze-dried biomass (10 g) was mechanically disrupted in a cylindrical ball mill constructed using AISI-304 stainless steel with an internal volume of 0.235 L (Tecnal Equipamentos Científicos, model R-TE-350, Piracicaba, São Paulo, Brazil), following Vieira et al. [17]. The mill balls (0.635 cm in external diameter) and the biomass swung up and down vertically in batches for 25 min, approximately 10 times per second and 617 strokes per minute. The disrupted biomass was kept at 20 °C until use;
- Mechanical disruption by ultrasound: Freeze-dried biomass was mechanically disrupted by ultrasound after resuspension in distilled water (10.0% w/v) following the optimal conditions determined by Silva et al. [21]. The suspensions were mechanically stirred (IKA, RW20 digital, Staufen, Germany) at 25.0 °C overnight and disrupted in a tip sonicator (Sonics, VCX 500, Newtown, CT, USA) at a frequency of 20 kHz and 98% amplitude for 6 min of ultrasonication. The cells were disrupted by cooling in an ice bath to avoid overheating the system. After disruption, the cell suspensions were frozen, freeze-dried (Terroni, LS 3000, São Carlos, São Paulo, Brazil), and stored at 20 °C until use;
- Mechanical disruption by high-pressure homogenization: Microalgal biomass was suspended in distilled water (1.5% w/v) and processed in a homogenizer (Alitec, A100, Pindamonhangaba, São Paulo, Brazil) at 350 bar according to the optimal conditions determined by Shene et al. [30], with modifications. Twenty-five passes of the suspensions in the homogenizer were used, and the suspension was cooled to avoid compound degradation due to an increase in temperature. Based on the results of preliminary tests, we observed that a total of 25 passes promoted greater protein recovery. However, biomass overheating was observed in tests with more than 25 passes. The homogenized samples were collected, frozen, and freeze-dried (Terroni, LS 3000, São Carlos, São Paulo, Brazil) and stored at 20 °C until use.
2.5. Cell Disruption Indicators
- Evaluation of cell disruption level through cell counting
A 100 μL volume of the working solution was added to 100 μL of the disrupted cell suspension in distilled water at a concentration of 0.1% w/v to count the ruptured biomass cells, according to Gminski et al. [31]. The working solution was prepared by diluting 0.1 mL of erythrosine B solution in 5 mL of potassium phosphate buffer. Erythrosine B solution was prepared using an aqueous solution of 10 g/L erythrosine B and a potassium phosphate buffer solution formed with 43.9 g/L dipotassium phosphate (K2HPO4) and 15.52 g/L monopotassium phosphate (KH2PO4).
The working solution and ruptured biomass cells were added, subjected to a mechanical stirrer (IKA, RW20 digital, Staufen, Germany), and rested for 10–15 min. The samples were removed, poured using a pipette, placed on a standard Neubauer hemocytometer (10 μL), and observed under an optical microscope (BX53, Olympus Corporation, Tokyo, Japan) at 100× objective for cell counting. Intact cells appear green and/or magenta, while dead or damaged cells are stained red. The cells were counted in five quadrants with a Neubauer camera. The degree of cell breakage (% of cells) in the disrupted biomass represented the level of physical destruction of the cells and was calculated according to Equation (1).
- Evaluation of cell disruption level through the amount of extracted soluble protein
The effect of microalgal cell disruption on protein extraction was evaluated using the amount of total hydrosoluble protein extracted. The disrupted biomass in each treatment was suspended in distilled water at a concentration of 10.0% w/v and then magnetically stirred for 24 h, following the optimal conditions for protein extraction determined by Silva et al. [21] with modifications. The pH of the microalgal suspension was adjusted to 10, and the suspension was magnetically stirred (Marconi, MA 502/D, Piracicaba, São Paulo, Brazil) at 1500 rpm for 2 h at 25 °C. A pH of 10 was chosen because the proteins of the microalga T. obliquus are more soluble at this pH [21]. According to Afif et al. [18], the further away from the isoelectric point of the T. obliquus proteins (2.5), the greater the solubility of these proteins. The mixture was centrifuged at 5000× g for 5 min (centrifuge 5430, Eppendorf, Hamburg, Germany), after which the supernatant containing the soluble proteins was collected. The residual biomass (pellet) was resuspended in deionized water for a better protein extraction yield, and the pH was adjusted to 10. This extraction procedure was repeated, resulting in 3 sequential extractions.
The soluble protein content in each extraction procedure was quantified according to Lowry’s methodology [32], using bovine serum albumin (fraction V powder, Sigma-Aldrich, St. Louis, MO, USA) to prepare the standard curve. The percentage of protein recovery from the biomass, or the protein yield, was determined as described by [33] using Equation (2). All extractions were performed in triplicate.
where C is the hydrosoluble protein concentration (mg/mL) in each extract and V is the final volume of the protein extract (mL). Pi is the protein content quantified using Lowry’s methodology for microalgal freeze-dried feedstock (mg), which was disrupted using base hydrolysis [17].
The mass of the concentrate of the total extracted protein was determined using gravimetry after freeze-drying (L101, Liobras, São Carlos, São Paulo, Brazil). All extractions were performed in triplicate.
The protein content (%) of each freeze-dried extract was calculated using Equation (3).
The color parameters of each freeze-dried extract were determined on a CR-10 colorimeter (Konica Minolta, Tokyo, Japan). The color was evaluated using the CIELab color space method, which determines yellowness (b), redness (a), and lightness (L*) values.
The freeze-dried extracts were evaluated using scanning electron microscopy (SEM). The samples were covered with gold (15 nm thick) (Quorum, Q150RS, Laughton, UK) and analyzed under an LEO 1430 VP scanning electron microscope (Carl Zeiss, Jena, Germany) at 20 kV.
2.6. Statistical Analysis
The results of the microalgal cell disruption indicators were subjected to analysis of variance (ANOVA), and the Tukey test was used to determine significant differences (p < 0.05) between treatments. The results are presented as means ± standard deviations, and the measurements were performed in triplicate.
3. Results
The proximate composition (w/w) of T. obliquus biomass was 40.29 ± 0.24% proteins, 18.33 ± 0.03% ashes, 7.87 ± 0.10% moisture, 4.23 ± 0.21% lipids, and 29.28% carbohydrates.
The results of the electrophoretic analysis of proteins in homogenized samples of T. obliquus via SDS-PAGE are shown in Figure 1.
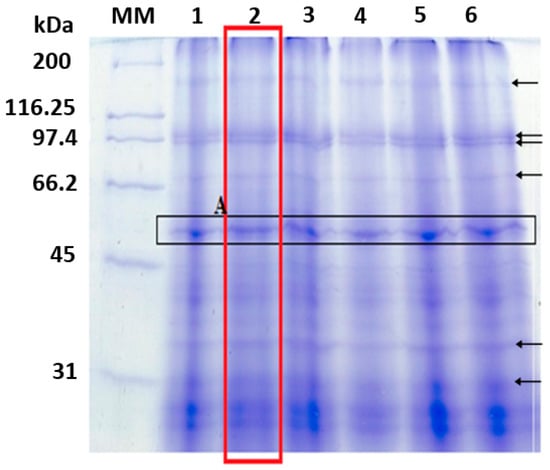
Figure 1.
Electrophoretic analysis of T. obliquus protein extract (PEXT) was performed using a 10% polyacrylamide gel stained with Coomassie brilliant blue G-250. The MM (marker for molecular mass) values for the samples were as follows: band 1 (20 µg/mL of PEXT); band 2 (40 µg/mL of PEXT); band 3 (50 µg/mL of PEXT); band 4 (20 µg/mL of PEXT—repeat); band 5 (40 µg/mL of PEXT—repeat); and band 6 (50 µg/mL of PEXT—repeat).
The extracts with different protein concentrations presented peptide bands with molecular masses varying between 31 and 200 kDa. All the samples exhibited an equal number of bands, but some of them were clearer than others. The best separation between the bands occurred with the sample from line 2 (40 µg/mL of protein extract), and the bands with the highest intensity were located in the molecular mass range between 45 and 66.2 kDa.
Among the proteins identified via electrophoretic analysis, the proteins in letter A of Figure 1 have the highest intensity and best separation. The corresponding band was cut from the gel and digested with trypsin as described in the identification methodology. After tryptic digestion, the molecular mass of the peptides was determined using an ultraflex III MALDI TOF-TOF mass spectrometer (Bruker Daltonics, Billerica, MA, USA). This analysis provides the spectrum shown in Figure 2 with the mass/charge ratio of the peptides from letter A protein.
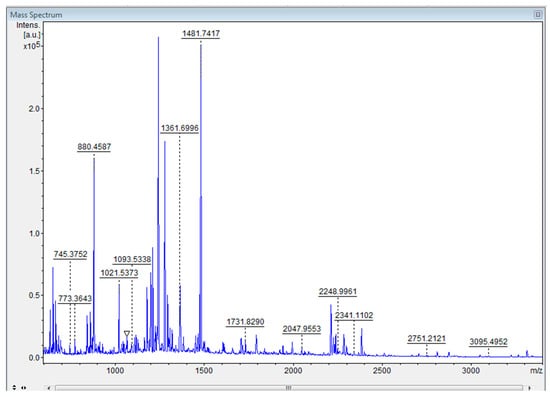
Figure 2.
MALDI-TOF-MS1: Mass spectrogram obtained after tryptic digestion of band A.
The large enzyme ribulose bisphosphate carboxylase (RuBisCO), with a molecular mass of 52.574 kDa, was identified after the masses of peptides arising from protein band digestion were compared with the molecular masses of peptides from protein cleavage available in the SwissProt database; the results also indicated greater homology (score = 80) between the microalga T. obliquus and the microalga Acutodesmus obliquus, which belong to the same genus as the organism studied, Tetradesmus sp. [34].
Figure 3A–D show the morphological photomicrographs of microalgal cells after mechanical disruption, in which Figure 3A represents the control sample, i.e., microalgal cells without mechanical disruption. The control sample showed major cells with a green, intact, ellipsoidal appearance. Ball mill samples presented grouped cells with a primary green color, making it impossible to count the individual cells. High-pressure homogenization yielded green and red cells, reaching 78.51 ± 1.97% of the cells ruptured, while ultrasound samples showed the highest number of disrupted cells (80.17 ± 0.54%).
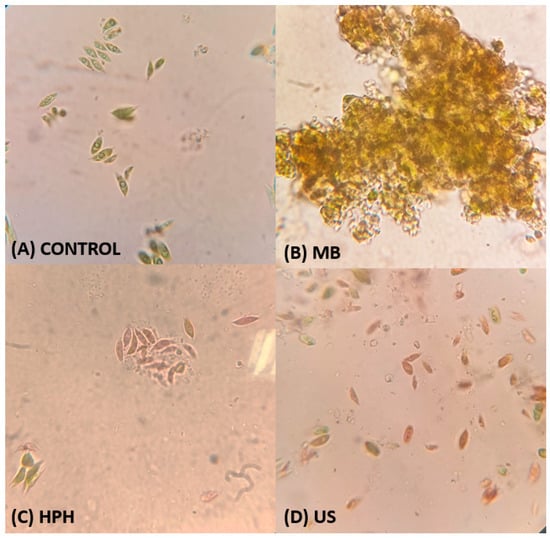
Figure 3.
Optical microscopy images of ruptured T. obliquus cells at 100× magnification. (A) Control sample (reference: without application of treatment for cell disruption). Samples were treated with (B) a ball mill (BM), (C) high-pressure homogenization (HPH), or (D) ultrasound (US). Intact cells appear green and/or magenta, while dead or damaged cells are stained red.
Figure 4 shows protein recovery in each extraction for the ultrasound (US), high-pressure homogenization (HPH), and ball mill (BM) treatments. The highest total protein extraction efficiency was obtained using US disruption (19.95%), while HPH and BM had similar protein recoveries of 15.68% and 14.11%, respectively, with no significant difference between them (p-value ≤ 0.05). The main protein separation occurred in the first extraction stage. The protein content was greatest in the US treatment group (16.02%), and the HPH and BM groups had protein separation percentages of 10.87% and 10.37%, respectively. In the second extraction, 2.47% to 3.22% of the proteins were separated from the biomasses, while in the third extraction, 0.77% to 2.09% of the proteins were separated from the biomasses.
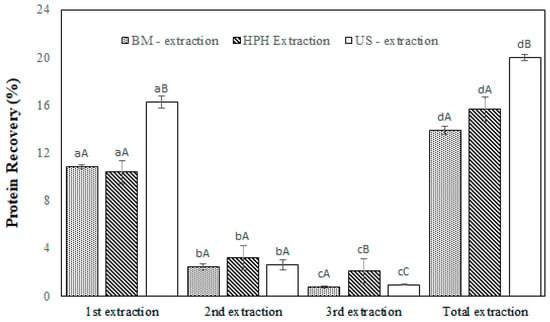
Figure 4.
Protein amount recovered (Equation (3)) after T. obliquus cell breakage as a percentage of total protein content. Disruption treatments of biomass comprised ball mill (BM), high-pressure homogenization (HPH), and ultrasound (US) methods. Different letters indicate a significant difference (p < 0.05). Capital letters indicate differences among cell disruption treatments in the same extraction step. Lowercase letters indicate the difference between each extraction step and the total extraction for the same cell disruption treatment. The results are expressed as the mean, and the bars indicate the standard deviation (n = 3).
Table 1 shows the protein mass yield (%) (g of protein from powder extract/100 g of dried microalgae), % of protein from the extracts (g of protein from powder extract/100 g of extract), the visual appearance of the protein extracts, and colorimetric parameters of the protein extracts. The protein mass yields of the US, HPH, and BM treatments were 20.8%, 17.1%, and 16.1%, respectively. Notably, this value only represents the extraction of aqueous soluble protein at pH 10; therefore, a large portion of insoluble proteins could not be recovered from the biomass. The total protein content (w/w) of the T. obliquus biomass determined in our study using the Kjeldahl method was 40.29 ± 0.24%, but the protein mass yields found in our study were lower than 20.8%. Thus, insoluble proteins were not extracted under the process conditions studied and remained in the residual biomass (pellet) during the centrifugation step; proteins can be released from cells but not fully recovered because they are incorporated into aggregates. Other extraction conditions, such as temperature and pH [35], surfactant [36], and solvent type [9,33], could be used to improve protein extraction yields; however, these conditions were not in the scope of this article. Suarez Garcia et al. [36] reported that 72% of proteins are associated with insoluble cellular structures and can be extracted only using surfactants. In our study, we verified that the content of extracted soluble protein was a reliable indirect index for measuring the extent of cell disruption and comparing different mechanical treatments.

Table 1.
Protein mass yield % (g of protein from powder extract/100 g of dried microalgae), % protein from extracts (g of protein from powder extract/100 g of extract), and color parameters for protein extracts: L* denotes lightness and ranges from 0 (black) to 100 (white); a(+) denotes red, a(−) denotes green, b(+) denotes yellow, and b(−) denotes blue.
All the samples showed lightness with a yellow tone (b+) and red traces (a+). The protein extract obtained from the HPH treatment had the greatest difference in color parameters. Its lightness and yellow color can also be observed in visual images.
Figure 5 shows the microstructures of the microalgal protein powders via SEM analysis, in which various particle types, including irregular particles, elongated particles, and oversized particle agglomerates, adhere to each other. Pores were not observed on the particle surface. BM protein powder (Figure 5A,D) exhibited sheet-like structures with large particle sizes, irregular geometric shapes, and particle sizes larger than those of the HPH (Figure 5B,E) and US (Figure 5C,F) protein powders.
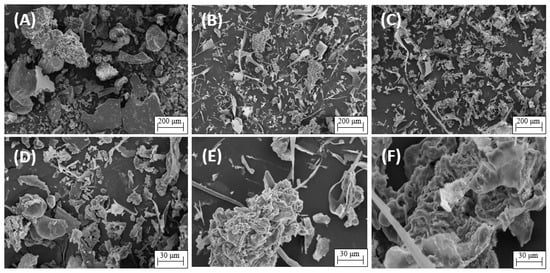
Figure 5.
Scanning electron micrographs of protein extracts obtained via mechanical disruption by ball mill (BM) treatment at magnifications of 200× (A) and 1000× (D); high-pressure homogenization (HPH) at magnifications of 200× (B) and 1000× (E); and ultrasound (US) at magnifications of 200× (C) and 1000× (F).
4. Discussion
4.1. Composition of T. obliquus Biomass
The microalgal biomass contained a high content of proteins (40.29 ± 0.24% w/w), which agrees with previous literature data for Tetradesmus spp. [13,20,21,37]. Thus, T. obliquus biomass is a strategic ingredient for the food industry since it is a potential alternative raw material for obtaining protein isolates and concentrates [20,21] that can be cultivated in large quantities for industrial feed applications in the food industry.
The high ash content (18.33 ± 0.03 w/w) is probably due to the organisms’ absorption of substantial amounts of minerals in culture media [23]. Since minerals can somewhat negatively affect the applicability of biomass and protein concentrates, the mineral content may be reduced. A suggestion is to add washing steps to biomass production to solubilize salts, especially phosphates and calcium [38].
The observed low lipid content (4.23 ± 0.21%), compared with that reported in the literature for T. obliquus [13,17,20], is probably due to the culture conditions and nutrients used [25]. The cultivation process affects the quantity of metabolites, such as lipids and proteins, that accumulate in cells [23,24,25]. Some factors relevant to the cultivation process include culture conditions, temperature, pH, light intensity, and photoperiod to increase biomass and metabolite production; nutrient optimization, which should be adjusted during the different macroalgal growth phases; carbon sources, since CO2 supplementation can increase growth and protein content [25]; and stress induction, as nitrogen starvation can trigger microalgae to accumulate more protein as a survival mechanism [7]. Researchers and cultivators often consider and manipulate these environmental parameters to increase microalgal productivity under controlled conditions. For example, nutrients such as nitrogen and phosphorus at balanced ratios promote protein synthesis to the detriment of the synthesis of other metabolites. Carbohydrates, including fiber and glycogen, were the second major component of the total biomass (29.28%). The total carbohydrate content was obtained using the difference between 100% and the other compound contents (proteins, moisture, ash, and lipids); thus, this value considers the sampling error found in other analyses.
4.2. Identification of Main Proteins in T. obliquus Protein Extract
Protein analysis results were obtained to identify the main protein in the extract. Electrophoretic bands with higher intensities corresponded to proteins with molecular masses between 45 and 66.2 kDa (Figure 1). The greatest intensity was close to 60 kDa. Bands with lower intensity were also observed, as indicated by the arrows in Figure 1, corresponding to molecular masses of 147.61 kDa, 126.53 kDa, 106.52 kDa, 102.72 kDa, and 83.34 kDa. The bands for molecular masses greater than 200 kDa represent protein aggregates. The diversity in protein composition can be explained by microalgae not accumulating distinct proteins as N sources [39].
The large enzyme RuBisCO was identified as the band between 45 and 66.2 kDa with the help of MALDI-TOF MS. MALDI-TOF MS is a mass spectrometric technique widely used in the chemotaxonomy of microorganisms, allowing for the analysis of proteins, peptides, and other biomolecules due to its low ionization energy. Chemotaxonomy is based mainly on differences in the spectral patterns obtained for each organism [40]. Protein identification was considered valid if more than two peptides were matched and if the score was greater than or equal to the significance threshold (p < 0.05) [41].
Among the identified proteins, the SwissProt database indicated greater homology (score = 80) of the microalgae T. obliquus and the microalgae Acutodesmus obliquus than with other organisms listed in the protein ranking. The microalga Acutodesmus obliquus belongs to the same genus as the organism studied, i.e., Tetradesmus sp. [34].
RuBisCO is present in the plastids and chloroplasts of plants, algae, and cyanobacteria. It catalyzes two reactions: the carboxylation of D-ribulose 1,5-bisphosphate, the primary event in carbon dioxide fixation, and the oxidative fragmentation of the pentose substrate in the photorespiration process. Both simultaneous reactions compete for the same active site [42].
4.3. Cell Rupture and Protein Extraction from T. obliquus
- Evaluation of the cell disruption level through cell counting
Optical microscopic examination of cells is a common technique for measuring cell breakage. Figure 3A shows the control sample used in our disruption studies. T. obliquus cells not subjected to any disruption treatment were used; these cells were also referred to as original T. obliquus cells in the present work. The control sample exhibited an ellipsoidal shape, with a concave or linear fusiform contour, and cells were grouped into groups of four or eight cenobia. Even the control sample showed the presence of cells with a reddish color, suggesting that some of the cells may have been damaged or ruptured. This behavior is probably due to cell disintegration and membrane permeabilization of microalgal species with rigid cell walls, such as T. obliquus [37], during freeze-drying [43]. The freeze-drying process causes some damage to the cell membrane, increasing its porosity; however, the cell wall is not ruptured [44,45,46]. It is important to highlight that all disruption methods were carried out with freeze-dried biomass; thus, the potential damage to the cell wall caused by freeze-drying was the same for all the treatments evaluated.
A ball mill (BM) is a simple method for disrupting the cell walls of different microorganisms [47] because it provides good cell wall disintegration efficiency and does not use chemical reagents. The operating conditions of the method were based on previous studies that evaluated the occurrence of cell rupture in microalgae [13,17]. However, as shown in Figure 3B, the cell-counting methodology was not appropriate for determining the level of microalgal cell disruption following BM treatment because the treatment promoted strong agglomeration. In the BM treatment, the microalgal biomass was placed inside a closed chamber in the presence of small steel spheres with a high level of stirring (617 strokes per minute) within the closed chamber to facilitate effective milling. Due to shear force, kinetic energy was transferred to the biomass to break the cells. This behavior hindered dye permeation in the medium, making differentiating intact and damaged cells impossible; moreover, it was impossible to determine the percentage of ruptured cells. In contrast, Bunge et al. [48] observed complete cell disruption of the bacterium Arrhrobacter sp. in stirred BM, in which the enzymes were released without any degradation using small grinding balls. Thus, the type of biomass determines the adequacy of some techniques for analyzing cell breaks.
High-pressure homogenization (HPH) of the microalgal cells revealed significant differences between the treatment and control samples (Figure 3C) and broken fragments (parts with a redder color) of the cell wall. The percentage of ruptured cells reached 78.51 ± 1.97%. In the HPH process, cell disruption is achieved through high-pressure impact (shear forces) of accelerated fluid jet on the homogenizer stationary valve surface and hydrodynamic cavitation from the pressure drop-induced shear stress [15]. After the HPH process, the cells were more disaggregated (Figure 3C); they exhibited more globular, almost swollen shapes, with a possible loss of wall resistance. Broken fragments (lighter parts) of the cell wall can also be observed, increasing the contact surface area and dye permeation.
Additionally, the dye (erythrosin B) permeated into cells that sustained critical damage to their plasma membranes [31], which explains the high level of rupture observed for this method. However, Günerken et al. [15] argue for several disadvantages of the HPH process, such as the release of nonselective intracellular compounds, the difficulty of breaking rigid cell walls, the generation of superfine cell debris, the absence of a mild technique, and the inability to isolate fragile functional compounds. In our study, the suspensions in the homogenizer were cooled to avoid compound degradation due to temperature increase during each pass.
Figure 3D shows that, compared with the BM and HPH treatments, the ultrasound (US) treatment produced the highest number of red cells, suggesting that the US treatment effectively disrupted the microalgal biomass. The percentage of ruptured cells was 80.17 ± 0.54%. Ultrasound is a physical treatment based on bubble cavitation that uses sound waves to propagate pressure fluctuations, induces cavitation, and promotes nonspecific cell surface barrier disruption [49]. The wall structure and size of cells are critical factors affecting cell disruption efficacy during ultrasonic processing. Concerning the wall structure, microalgae with cellulose carbohydrate-based cell walls typically show more resistance against ultrasound than cell walls mainly composed of hydroxyproline-rich glycoproteins [50]. According to Do Carmo Cesário et al. [37], the T. obliquus cell wall is filled predominantly with fibrous material and has three well-defined layers, making breakdown even more challenging.
Spiden et al. [51] used the cell-counting technique to evaluate Saccharomyces cerevisiae cell disruption after HPH treatment. According to the authors, an interval between 10 and 30 min was necessary to perform the cell count, depending on the sample volume and the cell concentration. Due to cell debris, reliable automated cell counting is not always possible. These observations show that cell counting is insufficient for understanding cell disruption, and an effective technique for monitoring and measuring the impact of treatment on microalgal cell disruption is necessary. Thus, the protein extraction yield under different rupture treatments is another parameter for evaluating cell breakage.
- Evaluation of the cell disruption level through the amount of extracted soluble protein
The resistance of cell walls to disruption is a barrier hindering the efficient removal of intracellular components and may interfere with the accuracy of compound quantification [52]. The recovery efficiency of intracellular compounds from microalgae also depends on different factors, such as the type of target compound [17], the specific characteristics of the microalgal strain analyzed [15], and the chosen disruption and recovery methods [26,44]. According to Safi et al. [53], the amount of protein in the aqueous supernatant was appropriate for evaluating the degree of cell disruption in three species of microalgae: M. aeruginosa, C. pyrenoidosa, and C. reinhardtii.
According to Figure 4, an increase in the number of extraction steps positively affected protein recovery, and ultrasound treatment promoted the highest total protein extraction yield (19.95%). This result agrees with the cell disruption behavior observed using optical microscopy after US treatment, in which most cells were red, and the number of disrupted cells (80.17%) was the highest. However, this value is lower than the protein content remaining in the biomass (80.05%) after US treatment. The specific characteristics of the microalgal strains analyzed could explain this behavior. The microalgal cell wall coating components are species-specific and affect disruption efficiency. Do Carmo Cesário et al. [37] observed the presence of proteins in the nucleus and cytoplasmic regions of T. obliquus via histochemistry tests. Thus, mechanical treatments should collapse different parts of T. obliquus microalgal cells to achieve protein extraction.
The US treatment for cell disruption is based on the emission of high-frequency sound waves (up to 15–20 kHz) in liquid. These sound waves create gas bubbles that achieve a critical size, collapse, and release large amounts of energy [54]. Cells adjacent to collapsing cavitation bubbles are broken, while cells located further away from bubble cavitation also experience a smaller local energy flux [55]. Thus, the cell disruption power extends beyond the effect on the cell wall, reaching other microalgal cell organelles. Furthermore, acoustic cavitation occurs by increasing the local temperature [54], and this temperature increase might destroy target compounds, especially proteins. As proteins can be used as techno-functional ingredients in food systems, the protein extraction process must occur without drastic conditions that could make them nonfunctional [9]. González-Fernandez et al. [56] reported temperatures up to 85 °C when 100.7 MJ/kg of energy was supplied to Scenedesmus biomass through US treatment for 15 min. The authors suggested that thermal effects might have accounted for cell disruption of the Scenedesmus biomass. US treatment needs to be optimized to avoid thermal overexposure of biomass; once cell disruption is achieved, additional energy is absorbed or scattered by cell debris [57]. In our study, the cells were disrupted at 40 °C and cooled using an ice bath to avoid overheating the system.
Moreover, increasing the exposure time of cells to US treatment can affect the protein recovery rate. A longer treatment time allows for increased cell disruption due to additional energy input [58]. Delran et al. [11] verified that a low-frequency ultrasound of 20 kHz was adequate for breaking Tetraselmis suecica cells at a power of 120 W and 60 min of ultrasonication, allowing 90% of the total proteins to be extracted. Lupatini et al. [59] studied the US-assisted extraction of algal proteins from Spirulina platensis. The authors found that sonication degraded the cell wall entirely or partially, providing a valuable technique for extracting proteins and carbohydrates. The optimized percentage of protein extracted was 75.76% after 35 min of sonication at 30 °C, 37 Hz, and 100% sonication. In our study, US was the most efficient treatment but failed to reverse more than 20% of the proteins produced by T. obliquus, indicating that additional extraction time is required to completely disrupt the macrostructure of these organisms; thus, additional energy is needed.
As shown in Figure 4, the amounts of protein extracted from T. obliquus following HPH and BM treatment were lower than those following US treatment. The literature on HPH shows that a high working pressure followed by cycling has the most positive effect on cell disruption efficiency [12,14,30,51,53,60]. In our tests, HPH effectively disrupted the T. obliquus cell wall, as demonstrated by optical microscopy (Figure 3C), and disrupted counting (78.51%). The HPH protein extract also showed a remarkable light color (Table 1), indicating potential for food ingredient application. The protein recovery was 15.68% when using a low working pressure of 350 bar due to the homogenizer’s operation limits, the high number of passes of the suspensions in the homogenizer (25), and the pH of the microalgal suspension being 10. Katsimichas et al. [60] reported protein extraction from Chlorella pyrenoidosa after HPH treatment, in which an 800 bar of pressure and a four-pass treatment caused maximization of protein recovery of 382.0 mg proteins/g dry biomass at pH 13. Safi et al. [53] noted that HPH is a more efficient cell disruption technique than manual grinding, ultrasonication, or alkaline treatment for extracting proteins from Nannochloropsis oculata, Chlorella vulgaris, and Hematococcus pluvialis, which are green microalgae with rigid cell walls. Microalgal biomasses at 2% dry weight were disrupted by HPH working with two passes at 2700 bar and a pH of 12. There was a difference in the protein recovery methods used in the studies.
The cell-counting method was not applied to quantify the disruption effect of ball mill treatment since parts of the cellular fragments came together and formed large aggregates; thus, the disruption effects were evaluated using soluble protein extraction data. The yield of protein extracted from T. obliquus following BM treatment was 14.11% (Figure 4), in which the protein showed particles of larger sizes and irregular shapes, including sheet-like structures, as shown in Figure 5. Changes in morphology could significantly affect the functional properties of microalgal protein extracts. The low extraction yields of soluble proteins with BM could be due to the use of dry biomass instead of wet biomass. The energy needed for cell rupture is well utilized when the disintegration rate correlates with a specific energy. Schuller et al. [52] used BM to extract lutein and β-carotene from wet and lyophilized biomass of Tetraselmis sp. The break with wet biomass glass beads best responded to the extractive treatments. However, the literature has also reported promising results for mechanical disruption of BM with dry biomass [13,17].
5. Conclusions
The content of extracted soluble protein was a reliable indirect index for measuring the extent of cell disruption. However, defining an efficient method of protein recovery is still necessary to propose a route to extract proteins from T. obliquus biomass for industrial applications, particularly regarding the recovery of aqueous insoluble proteins. Optical cell counting is not an optimal indicator for monitoring cell disruption, although it is a common technique for determining cell concentration in microbial cultures. The microalgal disruption caused by different mechanical treatments could be evaluated by evaluating the extracted protein content combined with the cell-counting technique. Ultrasound treatment prevented microalgal cells from breaking better than high-pressure homogenization and ball mill treatments. However, the efficiency of the extraction of protein must be improved. Combining conventional techniques, e.g., ultrasonication with high-pressure homogenization, may reduce the energy demand of mechanical disruption methods and provide better protein extraction yields. Additionally, purification steps may be employed for further protein refinement to obtain high purity.
Author Contributions
Conceptualization, C.A.S.d.S., K.V.M.S., J.R.M.J., M.A.M., M.d.O.L. and J.S.d.R.C.; methodology, C.A.S.d.S., K.V.M.S., S.d.R.C., J.R.M.J., R.d.C.S., E.B.d.O., M.A.M., M.d.O.L. and J.S.d.R.C.; formal analysis, C.A.S.d.S., K.V.M.S., S.d.R.C., J.R.M.J., R.d.C.S., M.d.O.L. and J.S.d.R.C.; investigation, K.V.M.S., S.d.R.C., J.R.M.J. and M.d.O.L.; resources, C.A.S.d.S., R.d.C.S., E.B.d.O., M.A.M. and J.S.d.R.C.; data curation, C.A.S.d.S., K.V.M.S., S.d.R.C., M.d.O.L. and J.S.d.R.C.; writing—original draft preparation, C.A.S.d.S., K.V.M.S. and J.S.d.R.C.; writing—review and editing, C.A.S.d.S. and J.S.d.R.C.; visualization, C.A.S.d.S., K.V.M.S., S.d.R.C., J.R.M.J., R.d.C.S., E.B.d.O., M.A.M., M.d.O.L. and J.S.d.R.C.; supervision, C.A.S.d.S., J.R.M.J., R.d.C.S., M.d.O.L. and J.S.d.R.C.; project administration, C.A.S.d.S., E.B.d.O., M.A.M. and J.S.d.R.C.; funding acquisition, C.A.S.d.S., R.d.C.S., E.B.d.O., M.A.M. and J.S.d.R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Brazilian agencies Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Fapemig, APQ-02267-22), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 313641/2021-8), Financiadora de Estudos e Projetos (Finep), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001, as well as by Petroleo Brasileiro S.A. (Petrobras).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors are grateful to Núcleo de Análise de Biomoléculas (NuBioMol) and Laboratório de Biocombustíveis at the Universidade Federal de Viçosa (UFV), Viçosa, Brazil, for providing the facilities for conducting the experiments and data analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Waghmare, A.G.; Salve, M.K.; LeBlanc, J.G.; Arya, S.S. Concentration and Characterization of Microalgae Proteins from Chlorella pyrenoidosa. Bioresour. Bioprocess. 2016, 3, 16. [Google Scholar] [CrossRef]
- Silva, M.E.T.D.; Correa, K.D.P.; Martins, M.A.; Da Matta, S.L.P.; Martino, H.S.D.; Coimbra, J.S.D.R. Food Safety, Hypolipidemic and Hypoglycemic Activities, and in Vivo Protein Quality of Microalga Scenedesmus obliquus in Wistar Rats. J. Funct. Foods 2020, 65, 103711. [Google Scholar] [CrossRef]
- Sengupta, S.; Koley, H.; Dutta, S.; Bhowal, J. Hypocholesterolemic Effect of Spirulina platensis (SP) Fortified Functional Soy Yogurts on Diet-Induced Hypercholesterolemia. J. Funct. Foods 2018, 48, 54–64. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; Niccolai, A.; Tredici, M.R.; Biondi, N.; Rodolfi, L.; Lodovici, M.; D’Ambrosio, M.; Mori, G.; Luceri, C. Safety Evaluations and Lipid-Lowering Activity of an Arthrospira platensis Enriched Diet: A 1-Month Study in Rats. Food Res. Int. 2017, 102, 380–386. [Google Scholar] [CrossRef]
- Niccolai, A.; Bigagli, E.; Biondi, N.; Rodolfi, L.; Cinci, L.; Luceri, C.; Tredici, M.R. In Vitro Toxicity of Microalgal and Cyanobacterial Strains of Interest as Food Source. J. Appl. Phycol. 2017, 29, 199–209. [Google Scholar] [CrossRef]
- Serban, M.-C.; Sahebkar, A.; Dragan, S.; Stoichescu-Hogea, G.; Ursoniu, S.; Andrica, F.; Banach, M. A Systematic Review and Meta-Analysis of the Impact of Spirulina Supplementation on Plasma Lipid Concentrations. Clin. Nutr. 2016, 35, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Hegde, A.S.; Sharma, K.; Parmar, P.; Srivatsan, V. Microalgae as a Sustainable Source of Edible Proteins and Bioactive Peptides—Current Trends and Future Prospects. Food Res. Int. 2022, 157, 111338. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae for Human and Animal Nutrition. In Handbook of Microalgal Culture; Richmond, A., Hu, Q., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 461–503. ISBN 978-0-470-67389-8. [Google Scholar]
- Grossmann, L.; Ebert, S.; Hinrichs, J.; Weiss, J. Effect of Precipitation, Lyophilization, and Organic Solvent Extraction on Preparation of Protein-Rich Powders from the Microalgae Chlorella protothecoides. Algal Res. 2018, 29, 266–276. [Google Scholar] [CrossRef]
- Gouveia, L.; Molnar Jazić, J.; Ferreira, A.; Maletić, S.; Cvetković, D.; Vidović, S.; Vladić, J. Green Approach for the Valorization of Microalgae Tetradesmus obliquus. Sustain. Chem. Pharm. 2021, 24, 100556. [Google Scholar] [CrossRef]
- Delran, P.; Frances, C.; Peydecastaing, J.; Pontalier, P.-Y.; Guihéneuf, F.; Barthe, L. Cell Destruction Level and Metabolites Green-Extraction of Tetraselmis suecica by Low and Intermediate Frequency Ultrasound. Ultrason. Sonochem. 2023, 98, 106492. [Google Scholar] [CrossRef]
- Dias, C.; Nobre, B.P.; Santos, J.A.L.; Lopes Da Silva, T.; Reis, A. Direct Lipid and Carotenoid Extraction from Rhodosporidium toruloides Broth Culture after High Pressure Homogenization Cell Disruption: Strategies, Methodologies, and Yields. Biochem. Eng. J. 2022, 189, 108712. [Google Scholar] [CrossRef]
- Amorim, M.L.; Soares, J.; Vieira, B.B.; Batista-Silva, W.; Martins, M.A. Extraction of Proteins from the Microalga Scenedesmus obliquus BR003 Followed by Lipid Extraction of the Wet Deproteinized Biomass Using Hexane and Ethyl Acetate. Bioresour. Technol. 2020, 307, 123190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Grimi, N.; Marchal, L.; Lebovka, N.; Vorobiev, E. Effect of Ultrasonication, High Pressure Homogenization and Their Combination on Efficiency of Extraction of Bio-Molecules from Microalgae Parachlorella kessleri. Algal Res. 2019, 40, 101524. [Google Scholar] [CrossRef]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell Disruption for Microalgae Biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A. (Ed.) Handbook of Microalgal Culture: Biotechnology and Applied Phycology, 1st ed.; Wiley: Hoboken, NJ, USA, 2003; ISBN 978-0-632-05953-9. [Google Scholar]
- Vieira, B.B.; Soares, J.; Amorim, M.L.; Bittencourt, P.V.Q.; De Cássia Superbi, R.; De Oliveira, E.B.; Dos Reis Coimbra, J.S.; Martins, M.A. Optimized Extraction of Neutral Carbohydrates, Crude Lipids and Photosynthetic Pigments from the Wet Biomass of the Microalga Scenedesmus obliquus BR003. Sep. Purif. Technol. 2021, 269, 118711. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.R.; El Baroty, G.S.; El Baz, F.K.; Abd El Baky, H.H.; Murad, S.A. Scenedesmus obliquus: Antioxidant and Antiviral Activity of Proteins Hydrolyzed by Three Enzymes. J. Gene. Eng. Biotechnol. 2018, 16, 399–408. [Google Scholar] [CrossRef]
- Ferreira, A.; Ribeiro, B.; Ferreira, A.F.; Tavares, M.L.A.; Vladic, J.; Vidović, S.; Cvetkovic, D.; Melkonyan, L.; Avetisova, G.; Goginyan, V.; et al. Scenedesmus obliquus Microalga-based Biorefinery—From Brewery Effluent to Bioactive Compounds, Biofuels and Biofertilizers—Aiming at a Circular Bioeconomy. Biofuels Bioprod. Bioref. 2019, 13, 1169–1186. [Google Scholar] [CrossRef]
- Lima, V.S.; De Oliveira, D.R.B.; Da Silva, C.A.S.; Santana, R.D.C.; Soares, N.D.F.F.; De Oliveira, E.B.; Martins, M.A.; Coimbra, J.S.D.R. Stabilization of Oil–Water Emulsions with Protein Concentrates from the Microalga Tetradesmus obliquus. J. Food Sci. Technol. 2023, 60, 797–808. [Google Scholar] [CrossRef]
- Silva, M.E.T.D.; Leal, M.A.; Resende, M.D.O.; Martins, M.A.; Coimbra, J.S.D.R. Scenedesmus obliquus Protein Concentrate: A Sustainable Alternative Emulsifier for the Food Industry. Algal Res. 2021, 59, 102468. [Google Scholar] [CrossRef]
- Damodaran, S. Protein Stabilization of Emulsions and Foams. J. Food Sci. 2006, 70, R54–R66. [Google Scholar] [CrossRef]
- Covell, L.; Machado, M.; Vaz, M.G.M.V.; Soares, J.; Batista, A.D.; Araújo, W.L.; Martins, M.A.; Nunes-Nesi, A. Alternative Fertilizer-Based Growth Media Support High Lipid Contents without Growth Impairment in Scenedesmus obliquus BR003. Bioprocess. Biosyst. Eng. 2020, 43, 1123–1131. [Google Scholar] [CrossRef]
- Rocha, R.P.; Machado, M.; Vaz, M.G.M.V.; Vinson, C.C.; Leite, M.; Richard, R.; Mendes, L.B.B.; Araujo, W.L.; Caldana, C.; Martins, M.A.; et al. Exploring the Metabolic and Physiological Diversity of Native Microalgal Strains (Chlorophyta) Isolated from Tropical Freshwater Reservoirs. Algal Res. 2017, 28, 139–150. [Google Scholar] [CrossRef]
- Rocha, D.N.; Martins, M.A.; Soares, J.; Vaz, M.G.M.V.; De Oliveira Leite, M.; Covell, L.; Mendes, L.B.B. Combination of Trace Elements and Salt Stress in Different Cultivation Modes Improves the Lipid Productivity of Scenedesmus spp. Bioresour. Technol. 2019, 289, 121644. [Google Scholar] [CrossRef] [PubMed]
- Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Gayen, K.; Bhowmick, T.K. Downstream Processing of Microalgae for Pigments, Protein and Carbohydrate in Industrial Application: A Review. Food Bioprod. Process. 2018, 110, 60–84. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Tomas, H.; Havli, J.; Olsen, J.V.; Mann, M. In-Gel Digestion for Mass Spectrometric Characterization of Proteins and Proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Shene, C.; Monsalve, M.T.; Vergara, D.; Lienqueo, M.E.; Rubilar, M. High Pressure Homogenization of Nannochloropsis oculata for the Extraction of Intracellular Components: Effect of Process Conditions and Culture Age. Eur. J. Lipid Sci. Technol. 2016, 118, 631–639. [Google Scholar] [CrossRef]
- Gminski, R.; Decker, K.; Heinz, C.; Seidel, A.; Könczöl, M.; Goldenberg, E.; Grobéty, B.; Ebner, W.; Gieré, R.; Mersch-Sundermann, V. Genotoxic Effects of Three Selected Black Toner Powders and Their Dimethyl Sulfoxide Extracts in Cultured Human Epithelial A549 Lung Cells in Vitro. Environ. Mol. Mutagen. 2011, 52, 296–309. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.; Farr, A.L.; Randall, R.J. Protein Measurement With The Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Anjos, L.; Estêvão, J.; Infante, C.; Mantecón, L.; Power, D.M. Extracting Protein from Microalgae (Tetraselmis chuii) for Proteome Analysis. MethodsX 2022, 9, 101637. [Google Scholar] [CrossRef]
- Hegewald, E.; Wolf, M. Phylogenetic Relationships of Scenedesmus and Acutodesmus (Chlorophyta, Chlorophyceae) as Inferred from 18S rDNA and ITS-2 Sequence Comparisons. Plant Syst. Evol. 2003, 241, 185–191. [Google Scholar] [CrossRef]
- Gerde, J.A.; Wang, T.; Yao, L.; Jung, S.; Johnson, L.A.; Lamsal, B. Optimizing Protein Isolation from Defatted and Non-Defatted Nannochloropsis Microalgae Biomass. Algal Res. 2013, 2, 145–153. [Google Scholar] [CrossRef]
- Suarez Garcia, E.; Van Leeuwen, J.; Safi, C.; Sijtsma, L.; Eppink, M.H.M.; Wijffels, R.H.; Van Den Berg, C. Selective and Energy Efficient Extraction of Functional Proteins from Microalgae for Food Applications. Bioresour. Technol. 2018, 268, 197–203. [Google Scholar] [CrossRef]
- Do Carmo Cesário, C.; Soares, J.; Cossolin, J.F.S.; Almeida, A.V.M.; Bermudez Sierra, J.J.; De Oliveira Leite, M.; Nunes, M.C.; Serrão, J.E.; Martins, M.A.; Dos Reis Coimbra, J.S. Biochemical and Morphological Characterization of Freshwater Microalga Tetradesmus obliquus (Chlorophyta: Chlorophyceae). Protoplasma 2022, 259, 937–948. [Google Scholar] [CrossRef]
- Martins, P.L.; Reis, A.; Duarte, L.C.; Carvalheiro, F. Effective Fractionation of Microalgae Biomass as an Initial Step for Its Utilization as a Bioenergy Feedstock. Energy Convers. Manag. X 2022, 16, 100317. [Google Scholar] [CrossRef]
- Schwenzfeier, A.; Wierenga, P.A.; Gruppen, H. Isolation and Characterization of Soluble Protein from the Green Microalgae Tetraselmis Sp. Bioresour. Technol. 2011, 102, 9121–9127. [Google Scholar] [CrossRef] [PubMed]
- Mello, R.V.; Meccheri, F.S.; Bagatini, I.L.; Rodrigues-Filho, E.; Vieira, A.A.H. MALDI-TOF MS Based Discrimination of Coccoid Green Microalgae (Selenastraceae, Chlorophyta). Algal Res. 2017, 28, 151–160. [Google Scholar] [CrossRef]
- Irrgang, A.; Weise, C.; Murugaiyan, J.; Roesler, U. Identification of Immunodominant Proteins of the Microalgae Prototheca by Proteomic Analysis. New Microbes New Infect. 2015, 3, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wang, Q.; Xin, Y.; Lu, Y.; Xu, J. Enhancing Photosynthetic Biomass Productivity of Industrial Oleaginous Microalgae by Overexpression of RuBisCO Activase. Algal Res. 2017, 27, 366–375. [Google Scholar] [CrossRef]
- Sang, Y.; Wang, J.; Zhang, Y.; Gao, H.; Ge, S.; Feng, H.; Zhang, Y.; Ren, F.; Wen, P.; Wang, R. Influence of Temperature during Freeze-Drying Process on the Viability of Bifidobacterium longum BB68S. Microorganisms 2023, 11, 181. [Google Scholar] [CrossRef]
- Stirk, W.A.; Bálint, P.; Vambe, M.; Lovász, C.; Molnár, Z.; Van Staden, J.; Ördög, V. Effect of Cell Disruption Methods on the Extraction of Bioactive Metabolites from Microalgal Biomass. J. Biotechnol. 2020, 307, 35–43. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, B.; Rawat, I.; Ramluckan, K.; Bux, F. Efficacy of Drying and Cell Disruption Techniques on Lipid Recovery from Microalgae for Biodiesel Production. Fuel 2014, 128, 46–52. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.-K. Cell Disruption and Lipid Extraction for Microalgal Biorefineries: A Review. Bioresour. Technol. 2017, 244, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, B.; Skill, S.C.; Llewellyn, C.A. A Low Energy Process for the Recovery of Bioproducts from Cyanobacteria Using a Ball Mill. Biochem. Eng. J. 2012, 69, 48–56. [Google Scholar] [CrossRef]
- Bunge, F.; Pietzsch, M.; Müller, R.; Syldatk, C. Mechanical Disruption of Arthrobacter Sp. DSM 3747 in Stirred Ball Mills for the Release of Hydantoin-Cleaving Enzymes. Chem. Eng. Sci. 1992, 47, 225–232. [Google Scholar] [CrossRef]
- Azencott, H.R.; Peter, G.F.; Prausnitz, M.R. Influence of the Cell Wall on Intracellular Delivery to Algal Cells by Electroporation and Sonication. Ultrasound Med. Biol. 2007, 33, 1805–1817. [Google Scholar] [CrossRef]
- Liu, S.; Rouquié, C.; Lavenant, L.; Frappart, M.; Couallier, E. Coupling Bead-Milling and Microfiltration for the Recovery of Lipids and Proteins from Parachlorella kessleri: Impact of the Cell Disruption Conditions on the Separation Performances. Sep. Purif. Technol. 2022, 287, 120570. [Google Scholar] [CrossRef]
- Spiden, E.M.; Scales, P.J.; Kentish, S.E.; Martin, G.J.O. Critical Analysis of Quantitative Indicators of Cell Disruption Applied to Saccharomyces cerevisiae Processed with an Industrial High Pressure Homogenizer. Biochem. Eng. J. 2013, 70, 120–126. [Google Scholar] [CrossRef]
- Schüler, L.M.; Santos, T.; Pereira, H.; Duarte, P.; Katkam, N.G.; Florindo, C.; Schulze, P.S.C.; Barreira, L.; Varela, J.C.S. Improved Production of Lutein and β-Carotene by Thermal and Light Intensity Upshifts in the Marine Microalga Tetraselmis Sp. CTP4. Algal Res. 2020, 45, 101732. [Google Scholar] [CrossRef]
- Safi, C.; Ursu, A.V.; Laroche, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Aqueous Extraction of Proteins from Microalgae: Effect of Different Cell Disruption Methods. Algal Res. 2014, 3, 61–65. [Google Scholar] [CrossRef]
- Corrêa, P.S.; Morais Júnior, W.G.; Martins, A.A.; Caetano, N.S.; Mata, T.M. Microalgae Biomolecules: Extraction, Separation and Purification Methods. Processes 2020, 9, 10. [Google Scholar] [CrossRef]
- Soto-Sierra, L.; Stoykova, P.; Nikolov, Z.L. Extraction and Fractionation of Microalgae-Based Protein Products. Algal Res. 2018, 36, 175–192. [Google Scholar] [CrossRef]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Comparison of Ultrasound and Thermal Pretreatment of Scenedesmus Biomass on Methane Production. Bioresour. Technol. 2012, 110, 610–616. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, D.; Parajuli, K.; Upadhyay, S.; Jiang, Y.; Duan, Z. Comparison of Four Quantitative Techniques for Monitoring Microalgae Disruption by Low-Frequency Ultrasound and Acoustic Energy Efficiency. Environ. Sci. Technol. 2018, 52, 3295–3303. [Google Scholar] [CrossRef] [PubMed]
- Al Hattab, M.A.; Ghaly, A.E. ; Ghaly, A.E. Microalgae Oil Extraction Pre-Treatment Methods: Critical Review and Comparative Analysis. J. Fundam. Renew. Energy Appl. 2015, 5, 1–26. [Google Scholar] [CrossRef]
- Lupatini, A.L.; De Oliveira Bispo, L.; Colla, L.M.; Costa, J.A.V.; Canan, C.; Colla, E. Protein and Carbohydrate Extraction from S. platensis Biomass by Ultrasound and Mechanical Agitation. Food Res. Int. 2017, 99, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Katsimichas, A.; Karveli, I.; Dimopoulos, G.; Giannakourou, M.; Taoukis, P. Kinetics of High Pressure Homogenization Assisted Protein Extraction from Chlorella pyrenoidosa. Innov. Food Sci. Emerg. Technol. 2023, 88, 103438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


