1. Introduction
Many filamentous cyanobacteria exhibit gliding motility on solid surfaces [
1]. In the early 20th century, researchers analyzed the effect of temperature on the velocity of the gliding movement of various filamentous bacteria (for review, see [
2]). They found that the velocity increased in response to an increase in temperature until extremely high temperatures impaired the movement. Since the rate of physical and chemical reactions is generally dependent on temperature, it is natural that the rate of biological processes, such as gliding movement, is affected by temperature.
Arthrospira platensis, used in the present study, is an edible filamentous cyanobacterium utilized as raw material for food and food additives [
3,
4]. Its products are usually commercialized under the name spirulina since this cyanobacterium was formerly classified in the genus
Spirulina. A distinctive feature of
Arthrospira species is that their filaments have a helicoid morphology [
5], whereas most other filamentous cyanobacteria have a straight morphology. The filaments, or trichomes, of
A. platensis exhibit gliding motility on solid media, like many other filamentous cyanobacteria.
Many strains of
A. platensis are maintained in culture collections in the world. We are using one of those strains,
A. platensis NIES-39, collected from Lake Chad in Africa in our biological experiments. The temperatures around Lake Chad vary widely, with daytime temperatures reaching 35–37 °C and night-time temperatures reaching 15–20 °C in summer [
6]. To investigate how this cyanobacterium responds to varying temperatures, we examined the temperature dependence in its migration velocity. We found that the velocity remained almost constant over a wide temperature range. In contrast, straight-trichome mutants isolated from the same strain had strong temperature dependence in migration velocity, like other formerly examined cyanobacteria with straight trichomes. These results indicate the presence of a temperature compensation mechanism in the migration velocity of this cyanobacterium and that the helicoid morphology is involved in the mechanism.
2. Materials and Methods
2.1. Strains
A. platensis NIES-39 was obtained from the Microbial Culture Collection at the National Institute for Environmental Studies (MCC-NIES), Tsukuba, in 2008. The three straight-trichome mutants (str-1, str-2, and str-3) were genetically independent spontaneous mutants found in the subcultures of this strain. These mutants were isolated as follows. In our laboratory, a stock culture of the strain NIES-39 propagated from a single trichome was stored frozen [
7]. In addition to the stock culture, live subcultures were maintained for daily experiments by intermittently transferring a portion of the subculture into a fresh medium every 2–4 weeks. After the subculture was maintained for four years in this way, a trichome with straight morphology was found in 2012 when the trichomes were observed under a dissecting microscope. It was transferred to a fresh medium as a single trichome and propagated. Since its straight-trichome phenotype was stable among its progenies, we named it str-1 for its straight morphology [
8]. The subculture in which str-1 was found was discarded when str-1 was isolated since siblings of str-1 could be present in this culture. After disposing of it, a new subculture was started from the original stock culture of the laboratory. The new subculture was maintained again by intermittently diluting it into a fresh medium every 2–4 weeks. The second straight-trichome mutant, str-2, was found in the subculture in 2016 after four years of maintenance of this subculture. Its single trichome was isolated and propagated as a pure line, and the subculture from which str-2 was obtained was discarded. A new subculture was started again from the original stock culture. The third straight-trichome mutant, str-3, was found and isolated in 2017 from this new subculture in the same way as str-1 and str-2. Since these mutants were obtained from independent subcultures, the mutagenic events that generated them were independent.
2.2. Media and Culture Conditions
A. platensis strains were cultured in the modified SOT medium prepared as described below. The SOT medium contains a high concentration of sodium bicarbonate and is suitable for culturing
Arthrospira species [
9]. In the modified SOT medium, boric acid was omitted from the SOT medium since it was shown to be unnecessary for
A. platensis [
10]. For the modified SOT medium, three kinds of stock solutions were used [
11]. Each stock solution contained reagents in a combination that avoided the formation of insoluble salts (e.g., magnesium phosphate, calcium phosphate, and calcium carbonate) during storage. The three stock solutions for the modified SOT medium were as follows: (1) the basal solution containing, in 1 L, 16.8 g of NaHCO
3, 0.5 g of K
2HPO
4, 2.5 g of NaNO
3, and 1 g of K
2SO
4, (2) the macroelement solution containing, in 100 mL, 5 g of NaCl, 0.4 g of Na
2·EDTA, 0.05 g of FeSO
4·7H
2O, 1 g of MgSO
4·7H
2O, and, 0.2 g of CaCl
2·2H
2O (reagents in this solution were dissolved in this order), and (3) the microelement solution containing, in 100 mL, 218 mg of MnSO
4·5H
2O, 22.2 mg of ZnSO
4·7H
2O, 7.9 mg of CuSO
4·5H
2O, and 2.1 mg of Na
2MoO
4·2H
2O. These stock solutions were autoclaved at 121 °C for 15 min and stored at room temperature. The modified SOT medium was prepared by mixing 1 L of the basal solution, 20 mL of the macroelement solution, and 1 mL of the microelement solution. This medium was stored at room temperature for up to 1 month. In preparing SOT plates, the modified SOT medium was solidified with 1% gellan gum (Fujifilm Wako Chemicals, Osaka, Japan) unless otherwise stated. When using gellan gum, it was autoclaved in deionized water separately from the mineral component of the medium, as in the case of using agar in cyanobacterial culture [
12,
13]. Light and temperature conditions were as previously described unless otherwise stated [
8]. Experiments in this study were performed between the second and tenth hours of the 12 h light period unless otherwise stated.
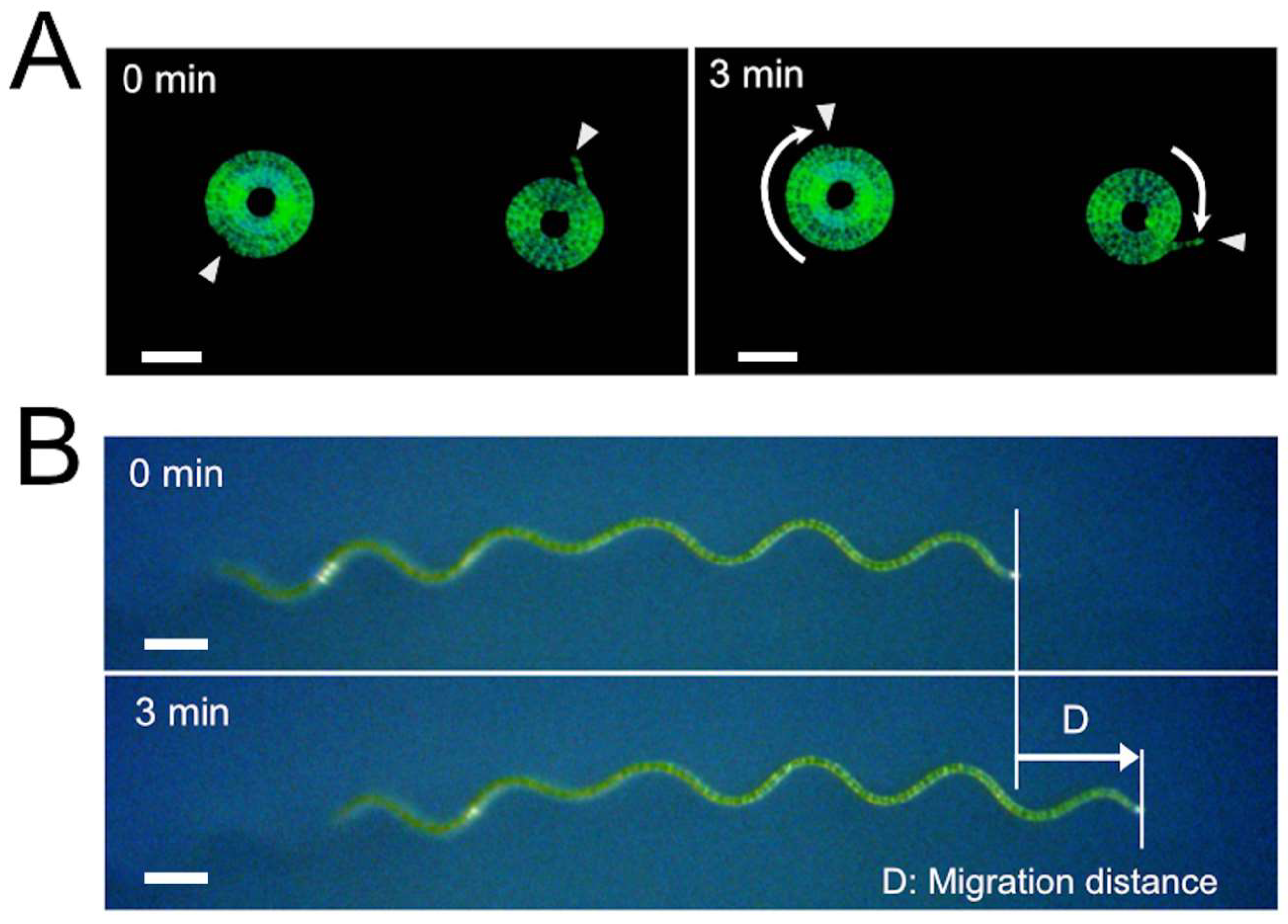
2.3. Photographs and Time-Lapse Movies
Dark-field microscopic photographs were taken using the digital camera WRAYCAM-EL310 (Wraymer, Osaka, Japan) connected to the dissecting microscope S9D (Leica Microsystems, Tokyo, Japan) or the inverted microscope CKX41 (Olympus, Tokyo, Japan). Dark-field images were obtained by illuminating the microscopic samples horizontally from one side with a light source LED-W (Kyowa Optical, Sagamihara, Japan) in a dark room. When making time-lapse movies, time-lapse images were taken by the dissecting microscope S9D equipped with an objective lens 2.0× (Cat. No.10450821, Leica Microsystems) connected with the digital camera WRAYCAM-EL310, using the function of the software Microstudio (Wraymer, Osaka, Japan), which was supplied with the digital camera. The obtained images were assembled using QuickTime Player 7 (Apple Japan, Tokyo, Japan) to make time-lapse movies. Bright-field microscopic images of trichome fragments were taken using the All-in-One Microscope BZ-9000 (Keyence, Osaka, Japan). The Rotterman Contrast illumination to visualize the trajectories of trichomes was performed using the transmitted light base TL3000 Ergo (Leica Microsystems), and microscopic images were obtained with the dissecting microscope S9D connected with the digital camera WRAYCAM-CIX2000 (Wraymer).
2.4. Determination of the Velocity and Trichome Lengths
A. platensis trichomes were placed on SOT plates (9 cm diameter) using a micropipette. The plate with the trichomes was put in a stage-top incubator (C150A; Blast Co., Kawasaki, Japan). The stage-top incubator used in this experiment was an acrylic chamber equipped with a transparent glass heater and its controller. We set the glass heater at the top of the acrylic chamber. Also, a heat insulation material made of polystyrene foam was put around the plate. Since this incubator was equipped with dual temperature sensors that could measure the temperatures of the glass heater and the internal atmosphere, we could keep the temperature around the sample at fixed temperatures. After placing the plate with A. platensis trichomes in this incubator, they were put on the stage of the Free-angle Observation System VHX-S90B (Keyence, Osaka, Japan) and left for 1 h for the temperature to equilibrate. Movement of the trichomes was observed from the top of the chamber using a digital microscope VHX-2000 (Keyence) equipped with a long-distance zoom lens VH-Z50L (Keyence). During the microscopic observation, the samples were illuminated from the top by the Free-angle Observation System VHX-S90B light source, which was equipped with a halogen lamp (JCR12V100W10H; Iwasaki Electric Co., Tokyo, Japan). The photosynthetic photon flux in the samples was 830 µmol/m2/s. In determining the velocity, the migration distance was determined using the measurement function of the digital microscope, and the velocity was calculated by dividing the migration distance by the measurement time. The lengths of the trichomes on the solid medium were determined using either the two-point measurement function or the multiple-point measurement function of the digital microscope VHX-2000, depending on the shapes of the trichomes.
2.5. Fragmentation of Trichomes with Ultrasound
A. platensis trichomes from 20 mL of the culture in the modified SOT medium were recovered on the nylon sieve with 20 µm openings [
8]. Then, they were suspended in 2 mL of fresh medium and sonicated at 25 °C for 5 s with the sonicator (Type 5202 PZT; Ohtake Works Co., Tokyo, Japan) equipped with a microtip probe. The output of the sonicator was set at 10W during sonication. The survival rate after this treatment was 31%, as determined by a colorimetric viability assay [
14] (this value may be helpful when reproducing this experiment using other models of sonicators). Trichome fragments were recovered by centrifugation at 4000×
g for 5 min at 25 °C and washed with 2 mL of the medium. Then, they were cultured for 3 days in 30 mL of the medium under standard culture conditions. Since the generation time of
A. platensis under our culture conditions was approximately 24 h, the number of cells increased up to 8 times after 3 days, resulting in the elongation of the fragmented trichomes.
2.6. Statistical Analysis
All statistical analyses were performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). The Kruskal–Wallis test was used to analyze the data, followed by Mann–Whitney unpaired tests. Spearman’s correlation coefficient (Rs) was determined for evaluating the correlation of the trichome length and the migration velocity.
4. Discussion
It was found that the migration velocity of
A. platensis NIES-39 was not so much affected by temperature as the formerly examined cyanobacterial species [
2]. Notably, no significant difference was detected between 32 °C and 37 °C (
Figure 3). However, when examined with straight-trichome mutants, they showed a strong temperature dependency, drastically increasing migration speed as the temperature increased in this temperature range. If only one mutant strain was examined, then the temperature-dependent change in the migration velocity could be due to another unknown mutation unrelated to the morphological change. However, all the three genetically independent straight mutants showed a strong temperature dependence in migration velocity (
Figure 5 and
Figure 6), like other previously examined filamentous cyanobacteria with straight trichomes. This result indicates that the helical morphology of the wild-type strain is involved in the phenomenon that its migration rate is less affected by temperature.
The rate of physical and chemical reactions is generally affected by temperature; the higher the temperature, the higher the reaction rate. Therefore, reactions in organisms also increase in rate as the temperature increases. However, some reactions in organisms should not change their rates depending on temperature. In such cases, a temperature compensation mechanism may evolve to ensure that the rate does not change as the temperature changes. A well-known example is the temperature compensation of the circadian clock [
15,
16,
17]. The phenomenon newly discovered with
A. platensis is also a kind of temperature compensation in which the migration speed is kept almost constant in a wide temperature range.
Temperature compensation in locomotion speed has been relatively well investigated in ectothermic animals. For example, fish change the composition of their muscle tissue to adapt to low temperatures [
18,
19]. In this case, temperature compensation is achieved through complex mechanisms such as changes in gene expression. In contrast, in
A. platensis, only the helicoid structure appears responsible because the temperature compensation is lost when the morphology becomes straight.
How the helicoid morphology provides temperature compensation is unclear. It has been reported that in
A. platensis, the pitch of the helix changes in a temperature-dependent manner, and an increase in temperature gives rise to a more tightly coiled trichome [
20]. Such a change in the helix structure may be related to the temperature compensation since the alteration in the pitch of the helices may change the relative direction of the propulsive force that the cells are generating. Other physical properties of the trichome, such as its firmness or resistance against bending, might change as the temperature changes. Such a change would also affect the migration velocity. How the helicoid morphology and the physical properties of the trichomes achieve temperature compensation is a subject for further study. Since the temperature compensation in
A. platensis appears to be achieved by a relatively simple physical mechanism, its study might provide insights into the design and control of artificial micromachines [
21].
Results of the experiments in
Figure 7 and
Figure 8 indicated that the expansion of the growth territory is suppressed in the wild-type strain compared to the straight-trichome mutants. The experiment in
Figure 8B showed that, at least on the plate, the movement mode of wild-type
A. platensis has some merit since it reduces the chance of migration to harsher environments.
A. platensis NIES-39 is a strain obtained from Lake Chad, where seasonal and diurnal environmental changes are tremendous [
6]. In the rainy season, the daytime temperature is 35–37 °C and the night-time temperature is 15–20 °C. The lake is surrounded by a barren area of accumulated sodium carbonate complexes called natron. In the dry season, the area of the lake gradually shrinks, leaving dry crusts of minerals behind, resulting in the expanded barren area around the lake. In such an environment, the mode of the movement of the wild-type strain might be advantageous for survival over the uncontrolled fast movement seen in the straight-trichome mutants.
It is worth mentioning that the movement mode of
A. platensis appears to have considerable variations depending on strains since a distinctively different movement mode has been reported in another strain,
A. platensis C005 [
22]. The movement of the wild-type trichomes of this strain comprises frequent random turns and most trichomes twisting around their starting point. In this strain, the longitudinal wavy motion seen in strain NIES-39 is not reported. These differences in the movement mode would reflect the differences in the natural habitats and niches of these strains.
During this investigation, it was found that trichome length affects migration velocity (
Figure 9). The experimental result suggested that the smooth gliding movement was achieved by the coordinated action of a high number of cells that make up the trichome. Filamentous cyanobacteria are thought to have evolved from unicellular cyanobacteria [
23,
24]. The results of our study suggest that the ability to perform efficient gliding movement was acquired only after the evolution of the body structure of the filamentous cyanobacteria, in which multiple cells are connected in tandem so that they can work cooperatively. Therefore, the potential ability to perform efficient gliding movement would have been one of the advantages that the filamentous cyanobacteria acquired when they evolved from unicellular cyanobacteria.