Preliminary Examinations of Phenotypical Changes in Land-Based Long-Term Tumble Culture of Palmaria palmata
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Material
2.2. Cultivation Medium
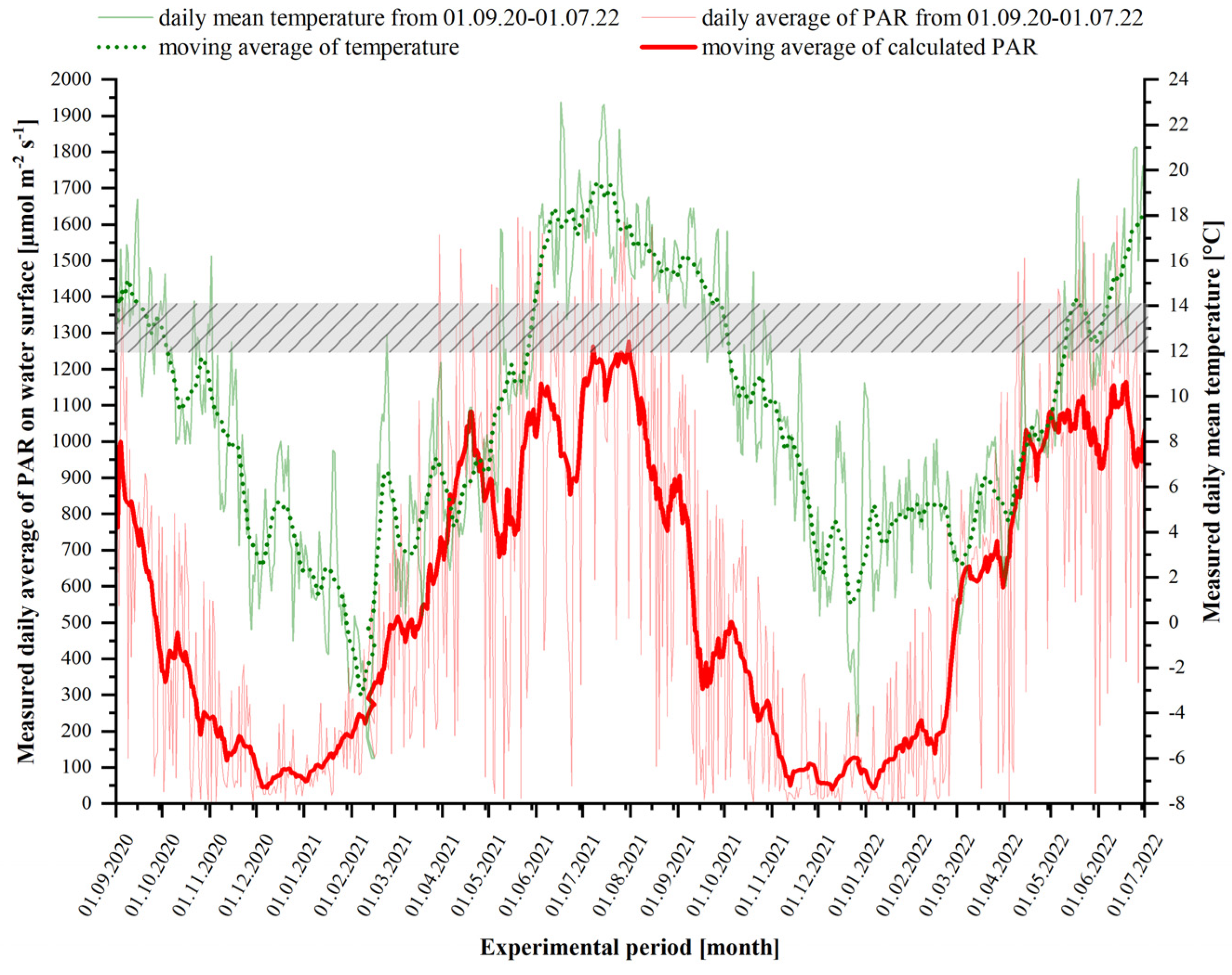
2.3. Experimental Setup Long-Term Cultivation—2000-L Tumble Tanks
2.4. Experimental Setup Batch Tests—2-Liter Beakers
2.5. Microscopy Analysis
2.6. Chlorophyll Fluorescence Analyses
2.7. Statistics
3. Results
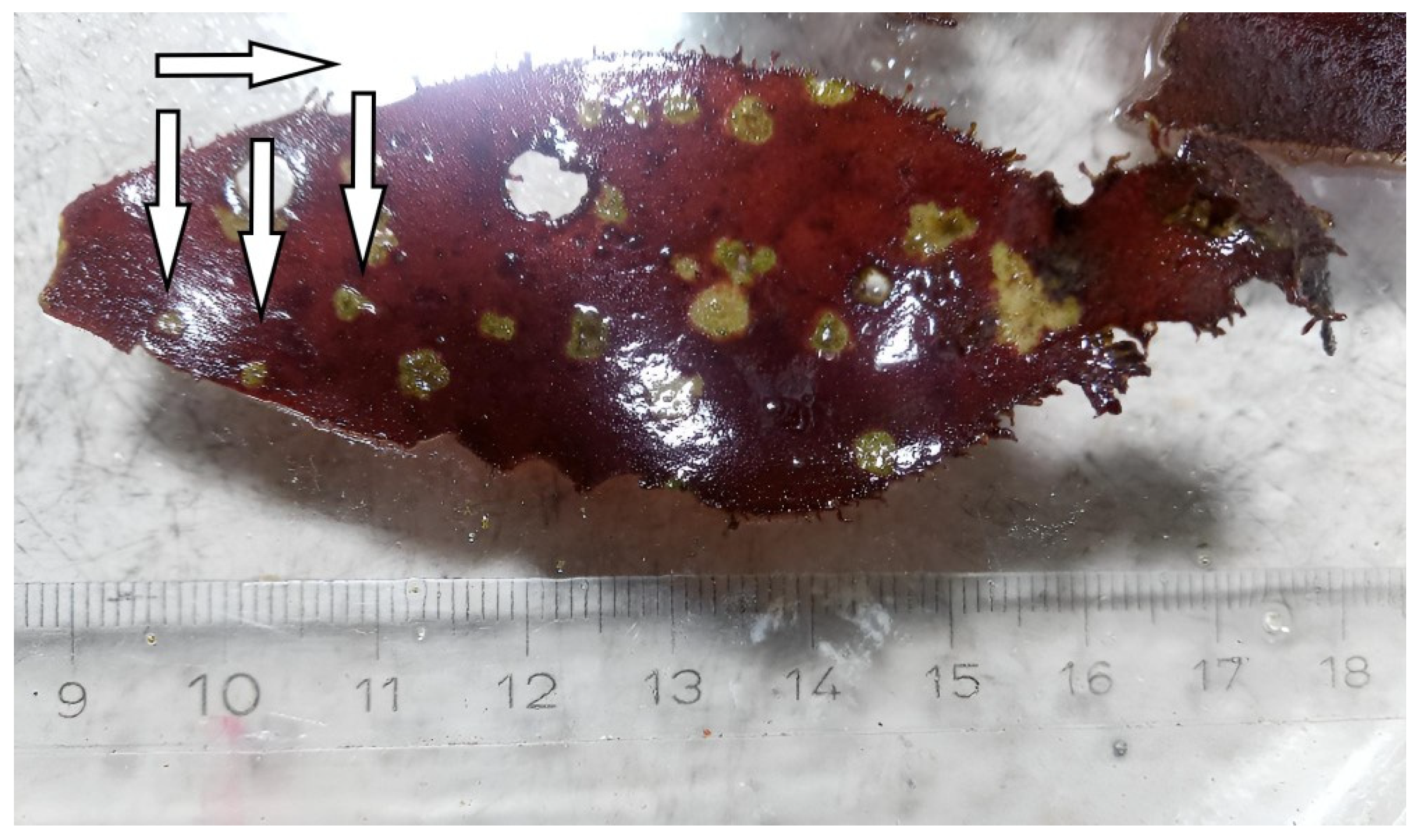
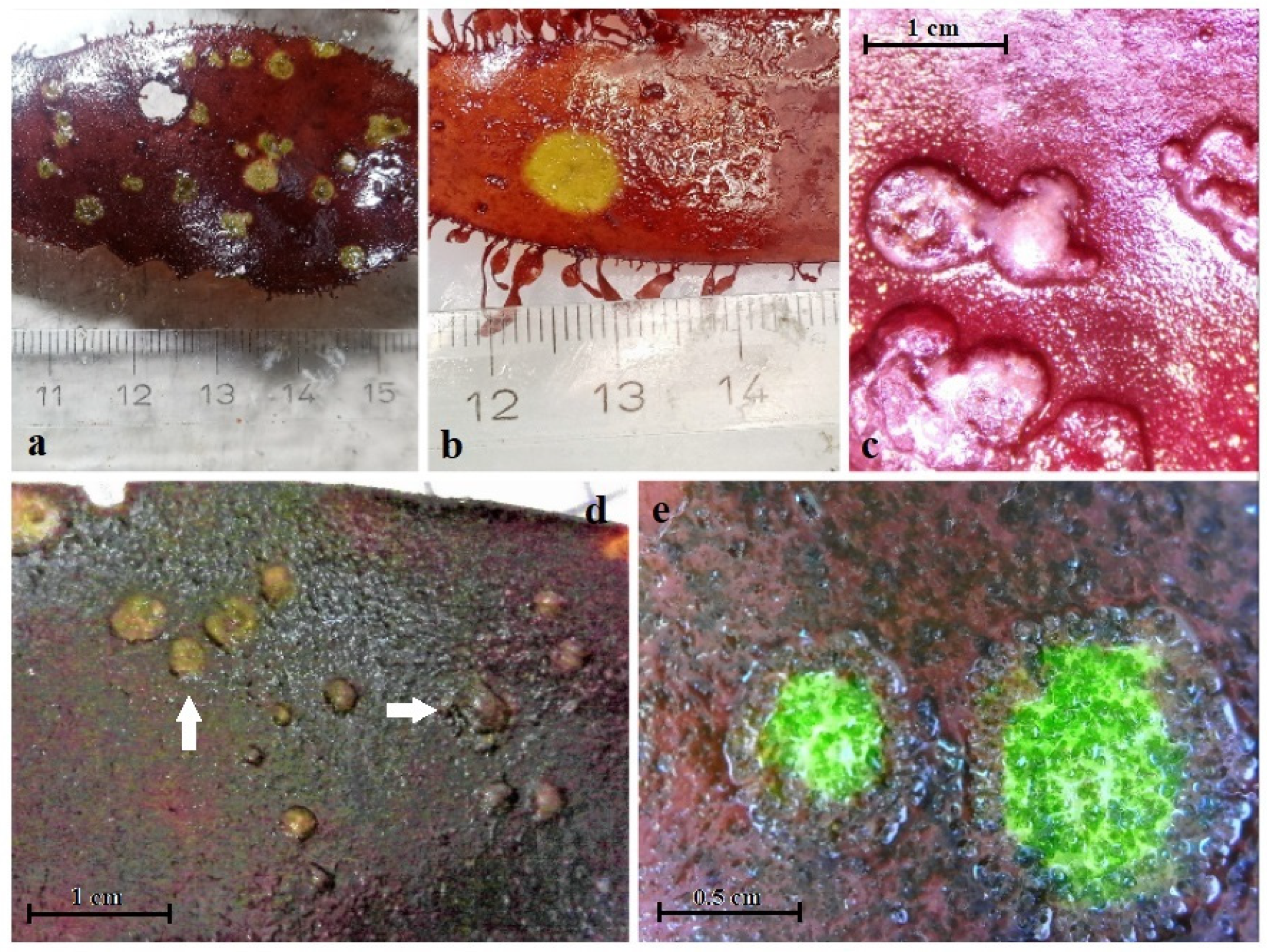
3.1. Temporal Occurrence and Changes in the Phenotype of P. palmata during Long-Term Cultivation
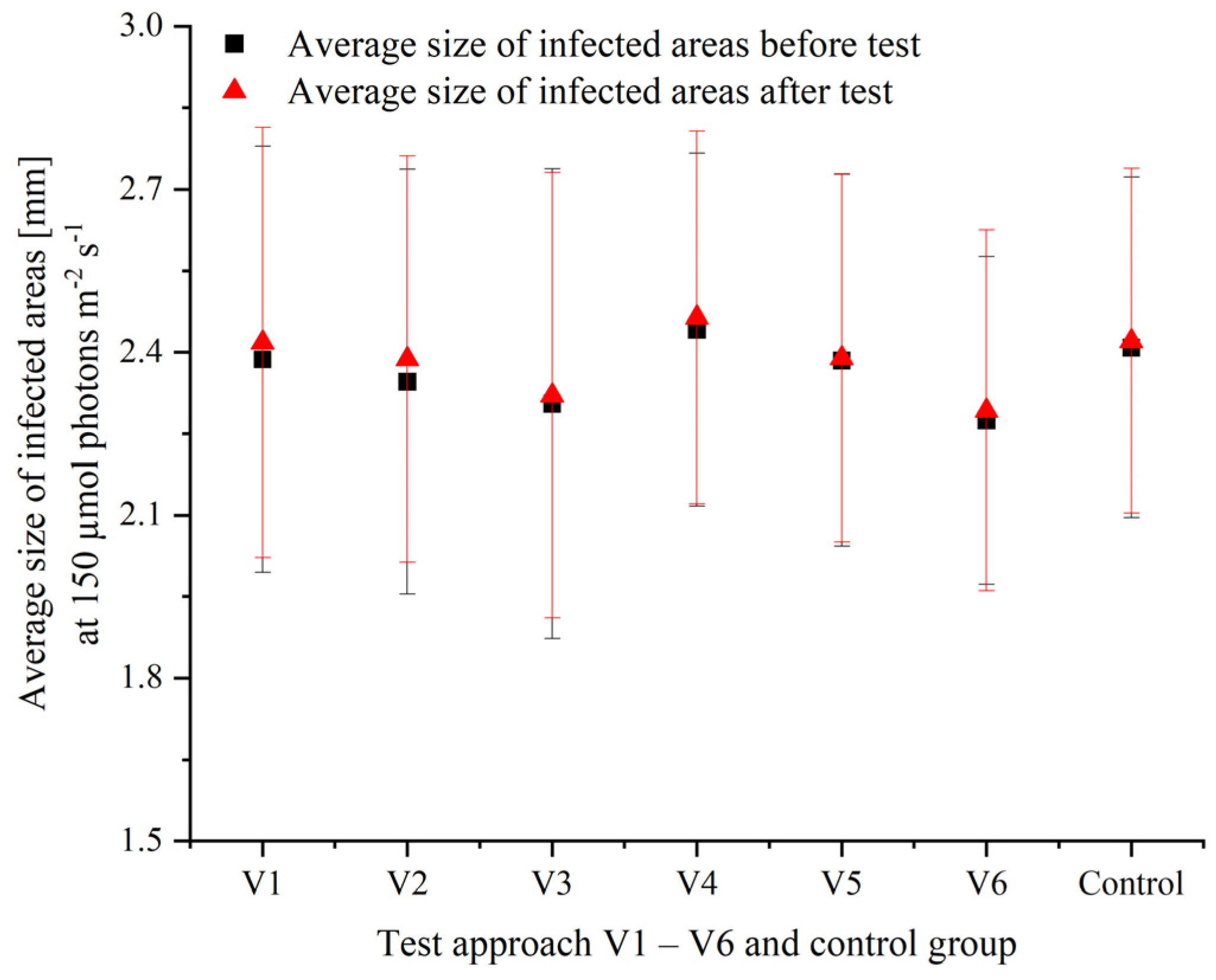
3.2. Effects of Varying Cultivation Conditions on Infection of P. palmata
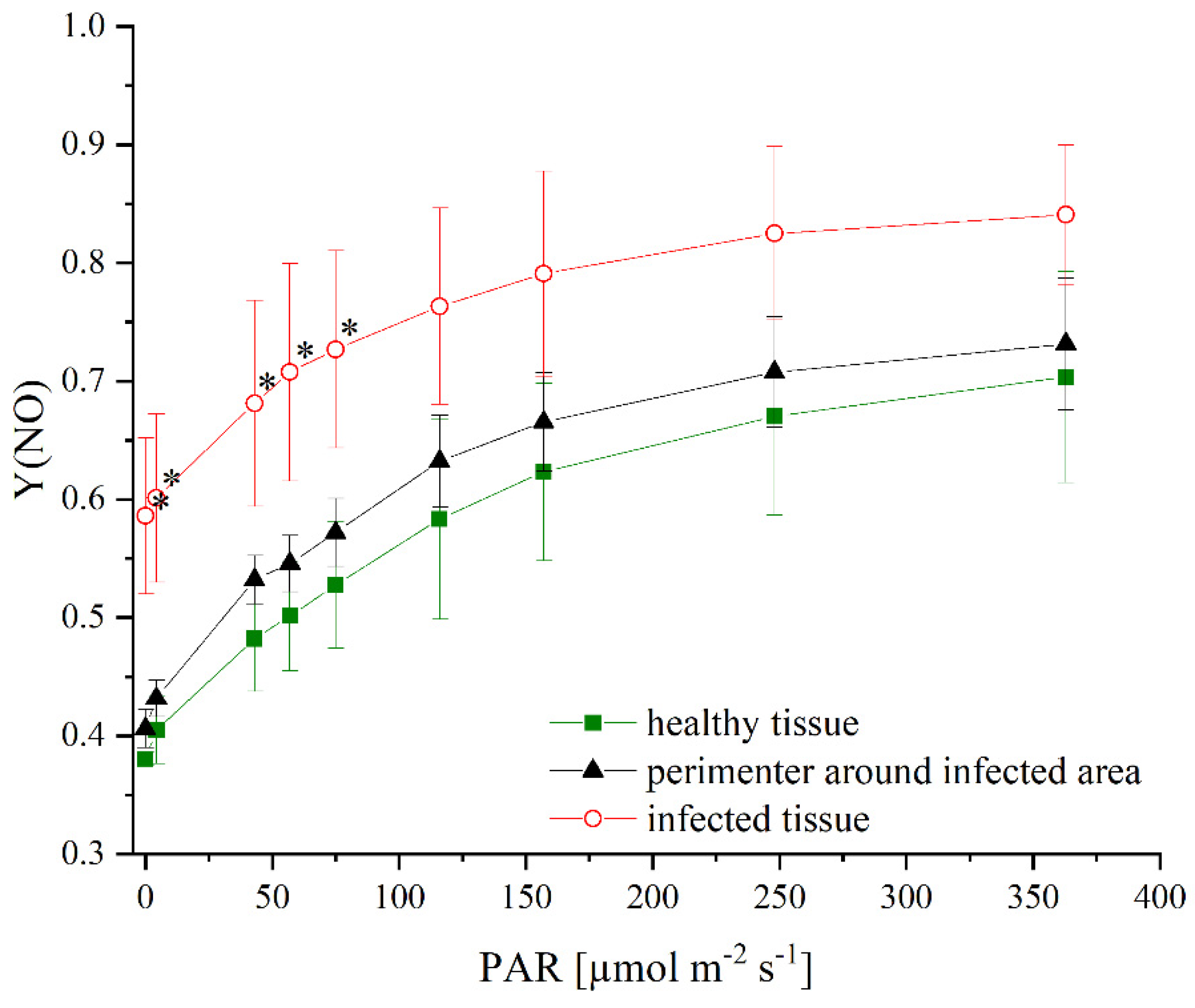
3.3. Effects of Infection on Photosynthetic Activity and Fluorescence Quenching of P. palmata
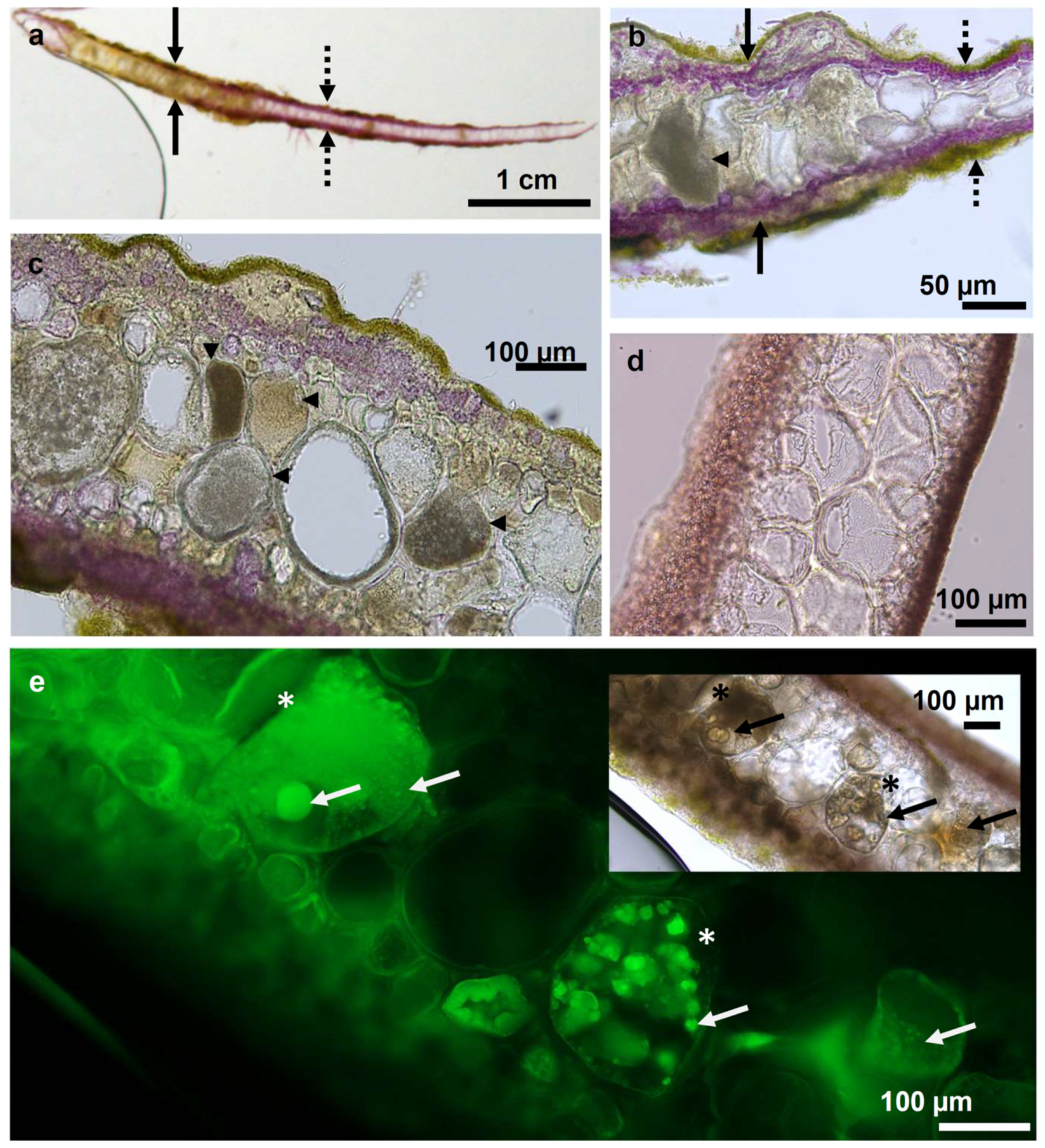
3.4. Preliminary Microscopy Investigation of Diseased P. palmata Thalli
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baghel, R.S.; Suthar, P.; Gajaria, T.K.; Bhattacharya, S.; Anil, A.L.; Reddy, C. Seaweed biorefinery: A sustainable process for valorising the biomass of brown seaweed. J. Clean. Prod. 2020, 263, 121359. [Google Scholar] [CrossRef]
- Chung, I.K.; Beardall, J.; Mehta, S.; Sahoo, D.; Stojkovic, S. Using marine macroalgae for carbon sequestration: A critical appraisal. J. Appl. Phycol. 2011, 23, 877–886. [Google Scholar] [CrossRef]
- Kumar, V.; Zozaya-Valdes, E.; Kjelleberg, S.; Thomas, T.; Egan, S. Multiple opportunistic pathogens can cause a bleaching disease in the red seaweed Delisea pulchra. Environ. Microbiol. 2016, 18, 3962–3975. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Smith, B.; Dyer, P.W.; Harvey, P. Macroalgae-derived biofuel: A review of methods of energy extraction from seaweed biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Frediansyah, A.; Martyasari, N.W.R. Effect of particle size on phytochemical composition and antioxidant properties of Sargassum cristaefolium ethanol extract. Sci. Rep. 2021, 11, 17876. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Broch, O.J.; Forbord, S.; Handå, A.; Skjermo, J.; Reitan, K.I.; Olsen, Y. Assimilation of inorganic nutrients from salmon (Salmo salar) farming by the macroalgae (Saccharina latissima) in an exposed coastal environment: Implications for integrated multi-trophic aquaculture. J. Appl. Phycol. 2014, 26, 1869–1878. [Google Scholar] [CrossRef]
- Pedro, M.; Garvetto, A.; Egan, S.; Gachon, C.M.M. The Reemergence of Phycopathology: When Algal Biology Meets Ecology and Biosecurity. Annu. Rev. Phytopathol. 2023, 61, 231–255. [Google Scholar]
- Wang, G.; Shuai, L.; Li, Y. Phylogenetic analysis of epiphytic marine bacteria on Hole-Rotten diseased sporophytes of Laminaria japonica. J. Appl. Phycol. 2008, 20, 403–409. [Google Scholar] [CrossRef]
- Kim, G.H.; Moon, K.-H.; Kim, J.-Y.; Shim, J.; Klochkova, T.A. A revaluation of algal diseases in Korean Pyropia (Porphyra) sea farms and their economic impact. Algae 2014, 29, 249–265. [Google Scholar] [CrossRef]
- Kim, G.H.; Klochkova, T.A.; Lee, D.J.; Im, S.H. Chloroplast virus causes green-spot disease in cultivated Pyropia of Korea. Algal. Res. 2016, 17, 293–299. [Google Scholar] [CrossRef]
- Cottier-Cook, E.J.; Nagabhatla, N.; Badis, Y.; Campbell, M.; Chopin, T.; Dai, W.; Fang, J.; He, P.; Hewitt, C.; Kim, G.H.; et al. Safeguarding the Future of the Global Seaweed Aquaculture Industry; United Nations University (INWEH) and Scottish Association for Marine Science Policy Brief: Hamilton, ON, Canada, 2016; 12p, ISBN 978-92-808-6080-1. [Google Scholar]
- Hurtado, A.Q.; Cheney, D.P. Propagule production of Eucheuma denticulatum (Burman) Collins et Harvey by tissue culture. Bot. Mar. 2003, 46, 338–341. [Google Scholar] [CrossRef]
- Pedrosa, A.A. Current status of Philippine seaweed industry. In Proceedings of the Regional Scientific Meeting on Attaining Sustainable Development Goals: Philippines Fisheries and Other Aquatic Resources 20/20, SMX Convention Center, Davao City, Philippines, 7 July 2017. [Google Scholar]
- Ward, G.M.; Faisan, J.P., Jr.; Cottier-Cook, E.J.; Gachon, C.; Hurtado, A.Q.; Lim, P.E.; Matoju, I.; Msuya, F.E.; Bass, D.; Brodie, J. A review of reported seaweed diseases and pests in aquaculture in Asia. J. World Aquacul. Soc. 2019, 51, 815–828. [Google Scholar] [CrossRef]
- Zambounis, A.; Strittmatter, M.; Gachon, C.M.M. Chronic stress and disease resistance in the genome model marine seaweed Ectocarpus siliculosus. Aquat Bot. 2013, 104, 147–152. [Google Scholar] [CrossRef]
- Heath, S.E.; Knox, K.; Vale, P.F.; Collins, S. Virus Resistance Is Not Costly in a Marine Alga Evolving under Multiple Environmental Stressors. Viruses 2017, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Vairappan, C.S.; Chung, C.S.; Hurtado, A.Q.; Msuya, F.E.; Bleicher-Lhonneur, G.; Critchley, A. Distribution and symptoms of epiphyte infection in major carrageenophyte-producing farms. J. Appl. Phycol. 2008, 20, 477–483. [Google Scholar] [CrossRef]
- Loureiro, R.; Gachon, C.M.M.; Rebours, C. Seaweed cultivation: Potential and challenges of crop domestication at an unprecedented pace. New Phytol. 2015, 206, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Badis, Y.; Klochkova, T.A.; Strittmatter, M. Novel species of the oomycete Olpidiopsis potentially threaten European red algal cultivation. J. Appl. Phycol. 2019, 31, 1239–1250. [Google Scholar] [CrossRef]
- Grote, B. Recent developments in aquaculture of Palmaria palmata (Linnaeus) (Weber & Mohr 1805): Cultivation and uses. Rev. Aquac. 2019, 11, 25–41. [Google Scholar]
- Le Gall, L.; Pien, S.; Rusig, A.M. Cultivation of Palmaria palmata (Palmariales, Rhodophyta) from isolated spores in semicontrolled conditions. Aquaculture 2004, 229, 181–191. [Google Scholar] [CrossRef]
- Mishra, V.K.; Temelli, F.; Ooraikul, B.; Shacklock, P.F.; Craigie, J.S. Lipids of the red alga, Palmaria palmata. Bot Mar. 1993, 36, 169–174. [Google Scholar] [CrossRef]
- Kerrison, P.D.; Le, H.N.; Twigg, G.C. Decontamination treatments to eliminate problem biota from macroalgal tank cultures of Osmundea pinnatifida, Palmaria palmata and Ulva lactuca. J. Appl. Phycol. 2016, 28, 3423–3434. [Google Scholar] [CrossRef] [PubMed]
- Bidwell, R.G.S.; McLachlan, J.; Lloyd, N.D.H. Tank cultivation of Irish moss, Chondrus crispus Stackh. Bot. Mar. 1985, 28, 87–97. [Google Scholar] [CrossRef]
- Demetropoulos, C.L.; Langdon, C.J. Enhanced production of Pacific dulse (Palmaria mollis) for co-culture with abalone in a land-based system: Effects of stocking density, light, salinity and temperature. Aquaculture 2004, 235, 471–488. [Google Scholar] [CrossRef]
- Berges, J.A.; Franklin, D.J.; Harrison, P.J. Evolution of an artificial seawater medium: Improvements in enriched seawater, artificial water over the last two decades. J. Phycol. 2001, 37, 1138–1145. [Google Scholar] [CrossRef]
- Hanelt, D.; Huppert, K.; Nultsch, W. Photoinhibition of photosynthesis and its recovery in red algae. Bot. Acta. 1992, 105, 278–284. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulated fluorometer. Photosynth. Res. 1996, 10, 51–62. [Google Scholar] [CrossRef]
- Beer, S.; Axelsson, L. Limitations in the use of PAM fluorometry for measuring photosynthetic rates of macroalgae at high irradiances. Eur. J. Phycol. 2004, 39, 1–7. [Google Scholar] [CrossRef]
- Herppich, W.B.; Herppich, M.; Von Willert, D.J. Ecophysiological investigations on plants of the genus Plectranthus (Lamiaceae). Influence of environment and leaf age on CAM gas exchange and leaf water relations in Plectranthus marrubioides. Flora 1998, 193, 99–109. [Google Scholar] [CrossRef]
- Herppich, W.B.; Herppich, M.; Tüffers, A.; Von Willert, D.J.; Midgley, G.F.; Veste, M. Photosynthetic responses to CO2 concentration and photon fluence rates in the CAM-cycling plant Delosperma tradescantioides (Mesembryanthemaceae). New Phytol. 1998, 138, 433–440. [Google Scholar] [CrossRef]
- Hanelt, D. Photosynthesis assessed by chlorophyll fluorescence. In Bioassays, Advanced Methods and Applications; Häder, D.P., Erzinger, G.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 169–198. [Google Scholar]
- Jassby, A.D.; Platt, T. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol. Oceanogr. 1976, 21, 540–547. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Hanelt, D.; Wiencke, C. Exposure to ultraviolet radiation delays photosynthetic recovery in Arctic kelp zoospores. Photosynth. Res. 2006, 88, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the saturation pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Ishikawa, Y.; Saga, N. The Diseases of Economically Valuable Seaweeds and Pathology in Japan; Miyachi, S., Karube, I., Ishida, Y., Eds.; Fuji Technology Press Ltd.: Tokyo, Japan, 1989; pp. 215–218. [Google Scholar]
- Gachon, C.M.M.; Sime-Ngando, T.; Strittmatter, M.; Chambouvet, A.; Kim, G.H. Algal diseases: Spotlight on a black box. Trends. Plant. Sci. 2010, 15, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Blake, R.E.; Duffy, J.E. Influence of environmental stressors and grazer immigration on ecosystem properties of an experimental Eelgrass community. J. Exp. Mar. Biol. Ecol. 2016, 480, 45–53. [Google Scholar] [CrossRef]
- Msuya, F.E.; Matoju, I.; Buriyo, A.; Rusekwa, S.; Shaxson, L.; Le Masson, V.; Nagabhatla, N.; Cottier, E.; De Lombaerde, P. Coping with Climate Change to Safeguard the Seaweed Industry in Eastern Africa: Spotlight on Tanzania; United Nations University Institute on Comparative Regional, Integration Studies Policy Brief: Hamilton, ON, Canada, 2022; ISBN 978-9912-40-076-4. [Google Scholar]
- Behera, D.P.; Ingle, K.N.; Mathew, D.E.; Dhimmar, A.; Sahastrabudhe, H.; Sahu, S.K.; Krishnan, M.G.; Shinde, P.B.; Ganesan, M.; Mantri, V.A. Epiphytism, diseases and grazing in seaweed aquaculture: A comprehensive review. Rev. Aquac. 2022, 14, 1345–1370. [Google Scholar] [CrossRef]
- Apt, K.E. Galls and tumor-like growths on marine macroalgae. Dis. Aquat. Organ. 1988, 4, 211–217. [Google Scholar] [CrossRef]
- McKeown, D.A.; Stevens, K.; Peters, A.F.; Bond, P.; Harper, G.M.; Brownlee, C.; Brown, M.T.; Schroeder, D.C. Phaeoviruses discovered in kelp (Laminariales). ISME J. 2017, 11, 2869–2873. [Google Scholar] [CrossRef]
- Kim, Y.T.; Kim, R.-w.; Shim, E.; Park, H.; Klochkova, T.A.; Kim, G.H. Control of oomycete pathogens during Pyropia farming and processing using calcium propionate. Algae 2023, 38, 71–80. [Google Scholar] [CrossRef]
- Ding, H.; Ma, J. Simultaneous infection by red rot and chytrid diseases in Porphyra yezoensis Ueda. J. Appl. Phyc. 2005, 17, 51–56. [Google Scholar] [CrossRef]
- Badis, Y.; Klochkova, T.A.; Brakel, J.; Arce, P.; Ostrowski, M.; Tringe, S.G.; Kim, G.H.; Gachon, C.M.M. Hidden diversity in the oomycete genus Olpidiopsis is a potential hazard to red algal cultivation and conservation worldwide. Eur. J. Phycol. 2020, 55, 162–171. [Google Scholar] [CrossRef]
- Porter, D.; Farnham, W.F. Mycaureola dilseae, a marine basidiomycete parasite of the red alga, Dilsea carnosa. Trans. Br. Mycol. 1986, 87, 575–582. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Z.; Fan, C.; Cao, S. Study on the pathogenic bacteria of spot decay disease of Undaria pinnatifida in Dailan. J. Fis. Sci. China 1997, 4, 62–65. [Google Scholar]
- Guiry, M.D. The occurrence of the red algal parasite Halosacciocolax lundii Edelstein in Britain. Brit. Phycol. J. 1974, 9, 31–35. [Google Scholar] [CrossRef]
- Hurtado, A.; Critchley, A.; Trespoey, A.; Lhonneur, G.B. Occurrence of Polysiphonia epiphytes in Kappaphycus farms at Calaguas Is., Camarines Norte, Phillippines. J. Appl. Phycol. 2006, 18, 301–306. [Google Scholar] [CrossRef]
- Zozaya-Valdés, E.; Roth-Schulze, A.J.; Egan, S.; Thomas, T. Microbial community function in the bleaching disease of the marine macroalgae Delisea pulchra. Environ. Microbiol. 2017, 19, 3012–3024. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, K.-I.; Park, C.S.; Kakinuma, M.; Amano, H. Effects of varying temperature, salinity and acidity in the treatment of Porphyra infected by red rot disease. Aquac. Sci. 2001, 49, 77–83. [Google Scholar]
- Kambey, C.S.B.; Campbell, I.; Cottier-Cook, E.J. Seaweed aquaculture: A preliminary assessment of biosecurity measures for controlling the ice-ice syndrome and pest outbreaks of a Kappaphycus farm. J. Appl. Phycol. 2021, 33, 3179–3197. [Google Scholar] [CrossRef]
- Kinnby, A.; Gunilla, T.B.; Henrik, P. Climate Change Increases Susceptibility to Grazers in a Foundation Seaweed. Front. Mar. Sci. 2021, 8, 688406. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]


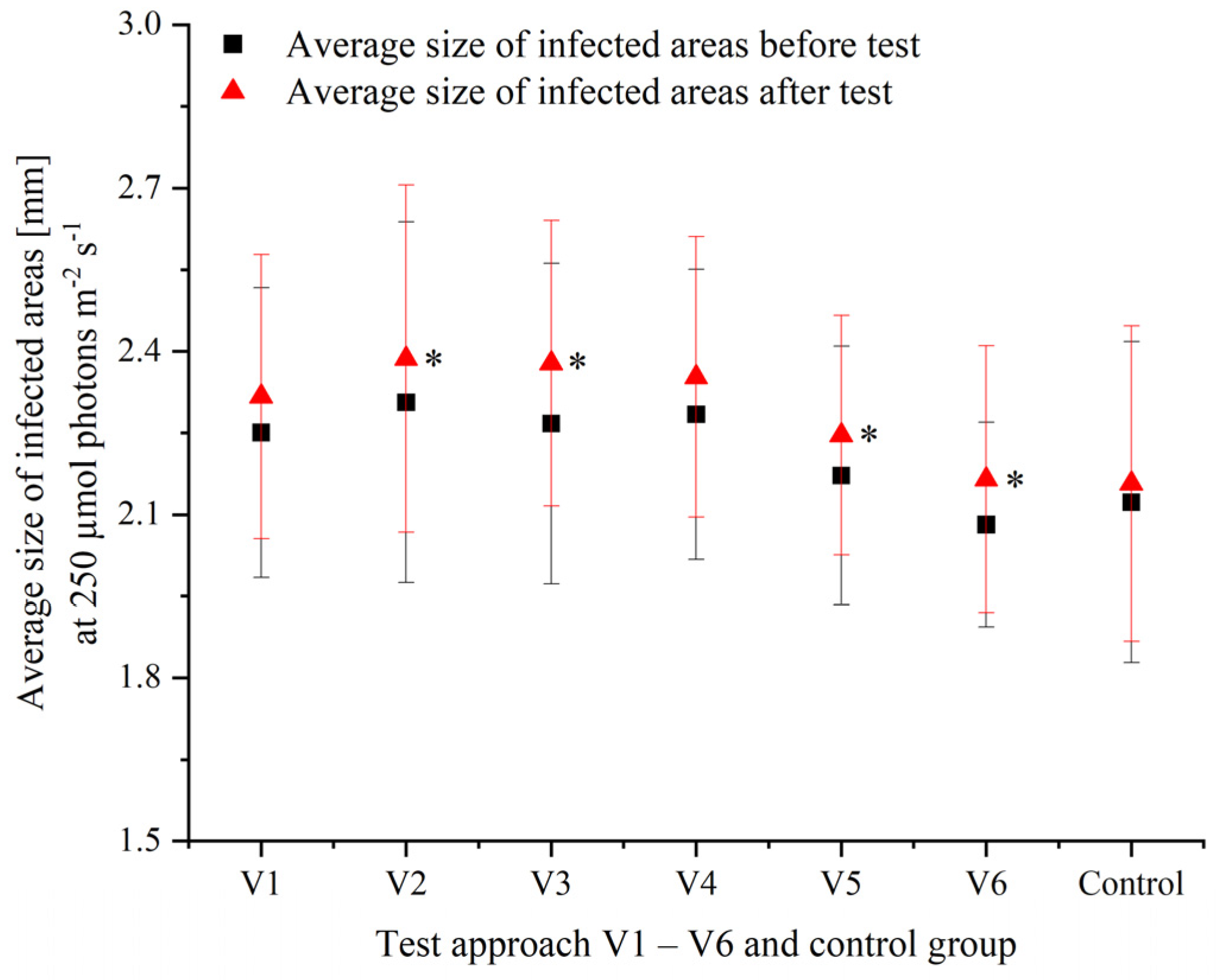


| Tested at PAR of 50, 150, and 250 µmol m−2 s−1 | ph-Level | Biomass Density [g fw L−1] | Nutrients Supply (PE) | ||||
|---|---|---|---|---|---|---|---|
| 7.5 | 8.2 | 9.1 | 2.35 ± 0.33 | 4.85 ± 0.47 | 1 × PE | 3 × PE | |
| control | x | x | x | ||||
| V 1 | x | x | x | ||||
| V 2 | x | x | x | ||||
| V 3 | x | x | x | ||||
| V 4 | x | x | x | ||||
| V 5 | x | x | x | ||||
| V 6 | x | x | x | ||||
| Test Group | Number of infections at PAR 50 | Additional Number of Infections | Number of Infections at PAR 150 | Additional Number of Infections | Number of Infections at PAR 250 | Additional Number of Infections | |||
|---|---|---|---|---|---|---|---|---|---|
| Start | End | Start | End | Start | End | ||||
| V1.1 | 6,8,3 | 7,8,3 | 1 | 5,6,8 | 5,6,8 | 0 | 7,4,5 | 7,4,5 | 0 |
| V1.2 | 4,5,5 | 4,5,5 | 0 | 6,7,6 | 6,7,6 | 0 | 3,5,5 | 4,5,5 | 1 |
| V1.3 | 5,5,6 | 5,6,6 | 1 | 5,9,4 | 5,10,4 | 1 | 7,5,8 | 7,5,8 | 0 |
| V2.1 | 2,8,4 | 2,9,4 | 1 | 8,6,9 | 8,6,9 | 0 | 4,5,8 | 4,5,8 | 0 |
| V2.2 | 4,6,5 | 4,6,5 | 0 | 8,3,2 | 9,3,2 | 1 | 9,6,4 | 9,7,4 | 1 |
| V2.3 | 5,5,8 | 5,6,8 | 1 | 6,4,4 | 6,4,4 | 0 | 4,2,9 | 4,2,9 | 0 |
| V3.1 | 7,8,3 | 7,9,3 | 1 | 6,7,7 | 6,7,7 | 0 | 4,8,5 | 4,8,5 | 0 |
| V3.2 | 5,6,4 | 5,6,5 | 1 | 7,5,9 | 7,5,9 | 0 | 4,5,7 | 4,5,7 | 0 |
| V3.3 | 3,4,3 | 3,4,3 | 0 | 4,6,7 | 4,7,7 | 1 | 4,4,7 | 5,4,7 | 1 |
| V4.1 | 9,4,2 | 10,4,2 | 1 | 5,7,5 | 5,7,5 | 0 | 4,8,5 | 4,8,5 | 0 |
| V4.2 | 4,7,4 | 4,7,4 | 0 | 6,5,8 | 6,5,9 | 1 | 7,3,8 | 7,3,9 | 1 |
| V4.3 | 7,5,8 | 7,6,8 | 1 | 6,7,3 | 6,7,3 | 0 | 5,6,7 | 5,6,7 | 0 |
| V5.1 | 4,7,5 | 4,7,5 | 0 | 10,7,8 | 10,7,8 | 0 | 8,7,8 | 8,7,8 | 0 |
| V5.2 | 7,7,9 | 7,7,9 | 0 | 4,8,5 | 4,8,5 | 0 | 5,7,5 | 5,7,5 | 0 |
| V5.3 | 5,2,7 | 6,2,7 | 1 | 6,6,4 | 6,6,4 | 0 | 3,7,8 | 3,7,8 | 0 |
| V6.1 | 6,3,4 | 6,3,4 | 0 | 4,9,9 | 4,9,9 | 0 | 5,4,6 | 5,4,6 | 0 |
| V6.2 | 8,3,5 | 9,3,6 | 2 | 11,8,6 | 12,8,6 | 1 | 7,6,7 | 7,6,8 | 1 |
| V6.3 | 3,3,6 | 3,3,6 | 0 | 6,9,6 | 6,9,6 | 0 | 5,6,4 | 5,6,4 | 0 |
| Contr. 1 | 4,7,8 | 4,7,8 | 0 | 7,9,8 | 7,9,8 | 0 | 7,4,6 | 7,4,6 | 0 |
| Contr. 2 | 8,7,3 | 8,8,3 | 1 | 4,7,5 | 4,7,5 | 0 | 8,5,6 | 8,5,6 | 0 |
| Contr. 3 | 4,9,5 | 4,9,5 | 0 | 9,4,7 | 9,4,7 | 0 | 5,6,6 | 5,6,6 | 0 |
| Healthy Tissue | |||
|---|---|---|---|
| ETRmax | alpha | EK | |
| Mean value | 23.03 | 0.187 | 123.45 |
| SD | 2.51 | 0.010 | 16.75 |
| Tissue around the infected area | |||
| ETRmax | alpha | EK | |
| Mean value | 21.19 | 0.173 | 123.47 |
| SD | 1.77 | 0.018 | 10.71 |
| Infected tissue | |||
| ETRmax | alpha | EK | |
| Mean value | 12.80 * | 0.138 * | 92.91 * |
| SD | 1.45 | 0.012 | 5.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebök, S.; Strittmatter, M.; Gachon, C.M.M.; Hanelt, D. Preliminary Examinations of Phenotypical Changes in Land-Based Long-Term Tumble Culture of Palmaria palmata. Phycology 2023, 3, 503-519. https://doi.org/10.3390/phycology3040034
Sebök S, Strittmatter M, Gachon CMM, Hanelt D. Preliminary Examinations of Phenotypical Changes in Land-Based Long-Term Tumble Culture of Palmaria palmata. Phycology. 2023; 3(4):503-519. https://doi.org/10.3390/phycology3040034
Chicago/Turabian StyleSebök, Stefan, Martina Strittmatter, Claire M. M. Gachon, and Dieter Hanelt. 2023. "Preliminary Examinations of Phenotypical Changes in Land-Based Long-Term Tumble Culture of Palmaria palmata" Phycology 3, no. 4: 503-519. https://doi.org/10.3390/phycology3040034
APA StyleSebök, S., Strittmatter, M., Gachon, C. M. M., & Hanelt, D. (2023). Preliminary Examinations of Phenotypical Changes in Land-Based Long-Term Tumble Culture of Palmaria palmata. Phycology, 3(4), 503-519. https://doi.org/10.3390/phycology3040034







