Phytohormones and Pheromones in the Phycology Literature: Benchmarking of Data-Set and Developing Critical Tools of Biotechnological Implications for Commercial Aquaculture Industry
Abstract
:1. Introduction
2. Hormones and Pheromones Reported in the Phycology Literature
3. Biosynthetic Pathways
3.1. Hormones
3.1.1. Auxin
3.1.2. Cytokinins
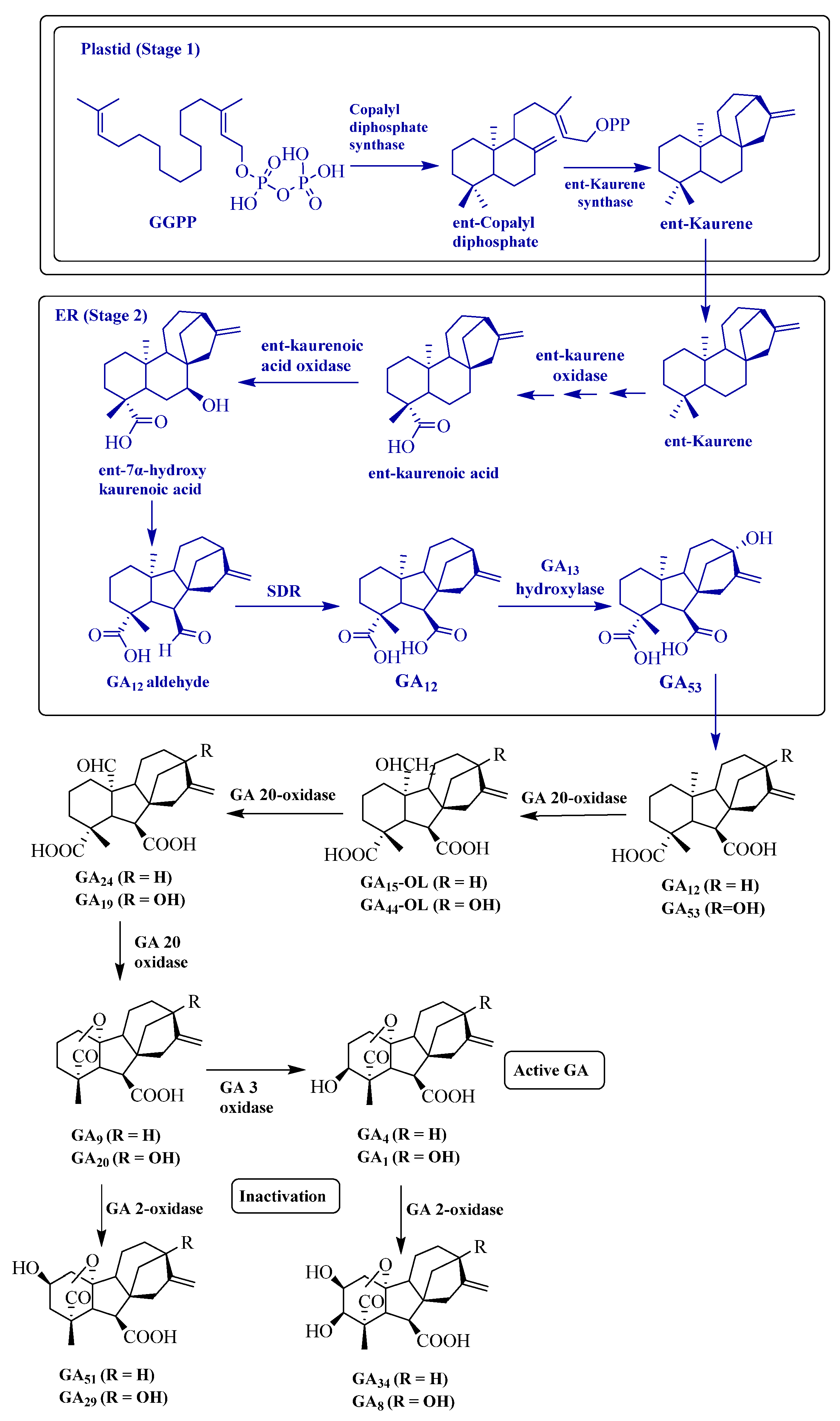
3.1.3. Gibberellins
3.1.4. Abscisic Acid (ABA)
3.1.5. Ethylene
3.2. Pheromones
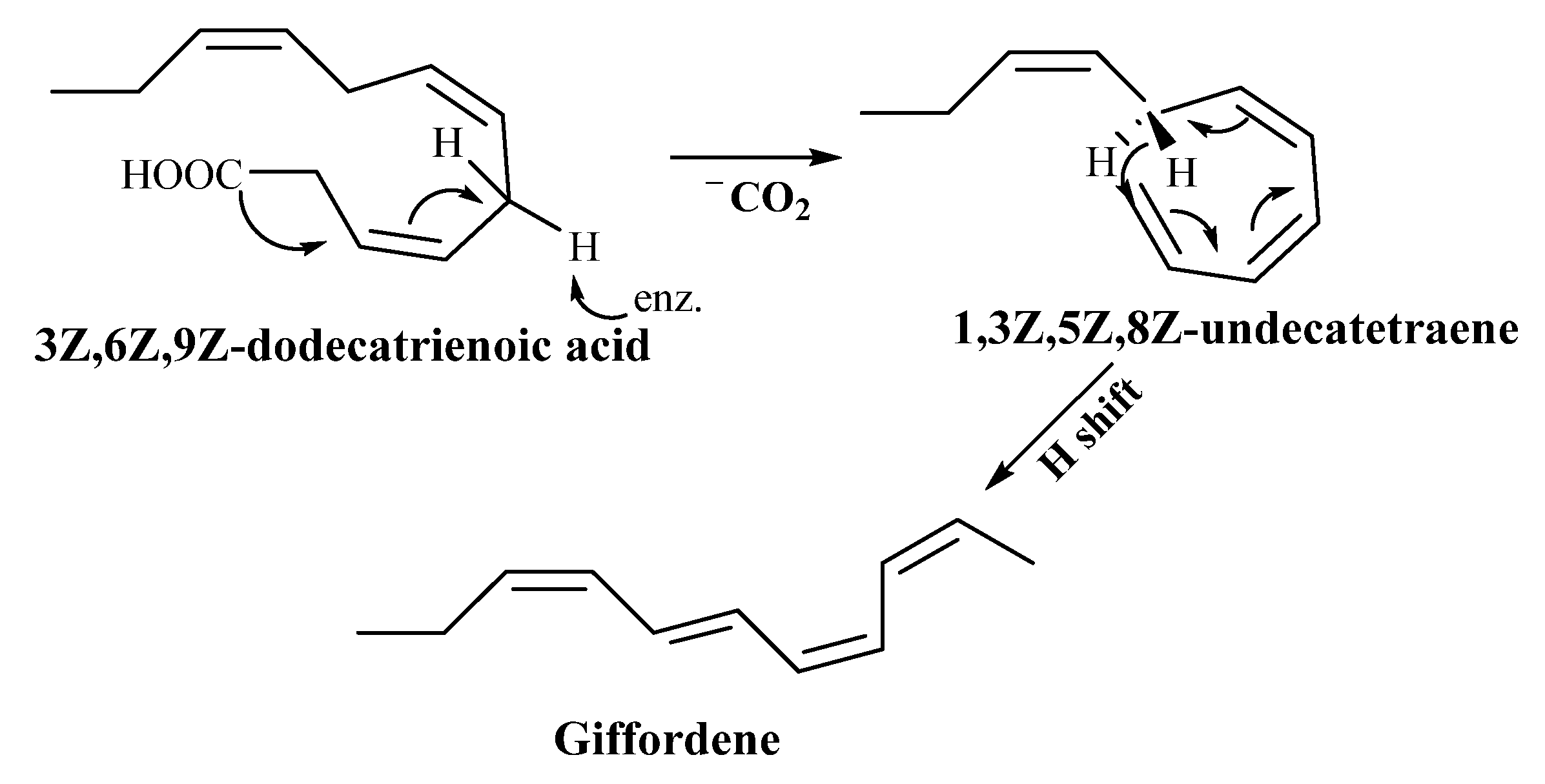
3.2.1. Giffordene
3.2.2. Dictyotene
3.2.3. Cystophorene
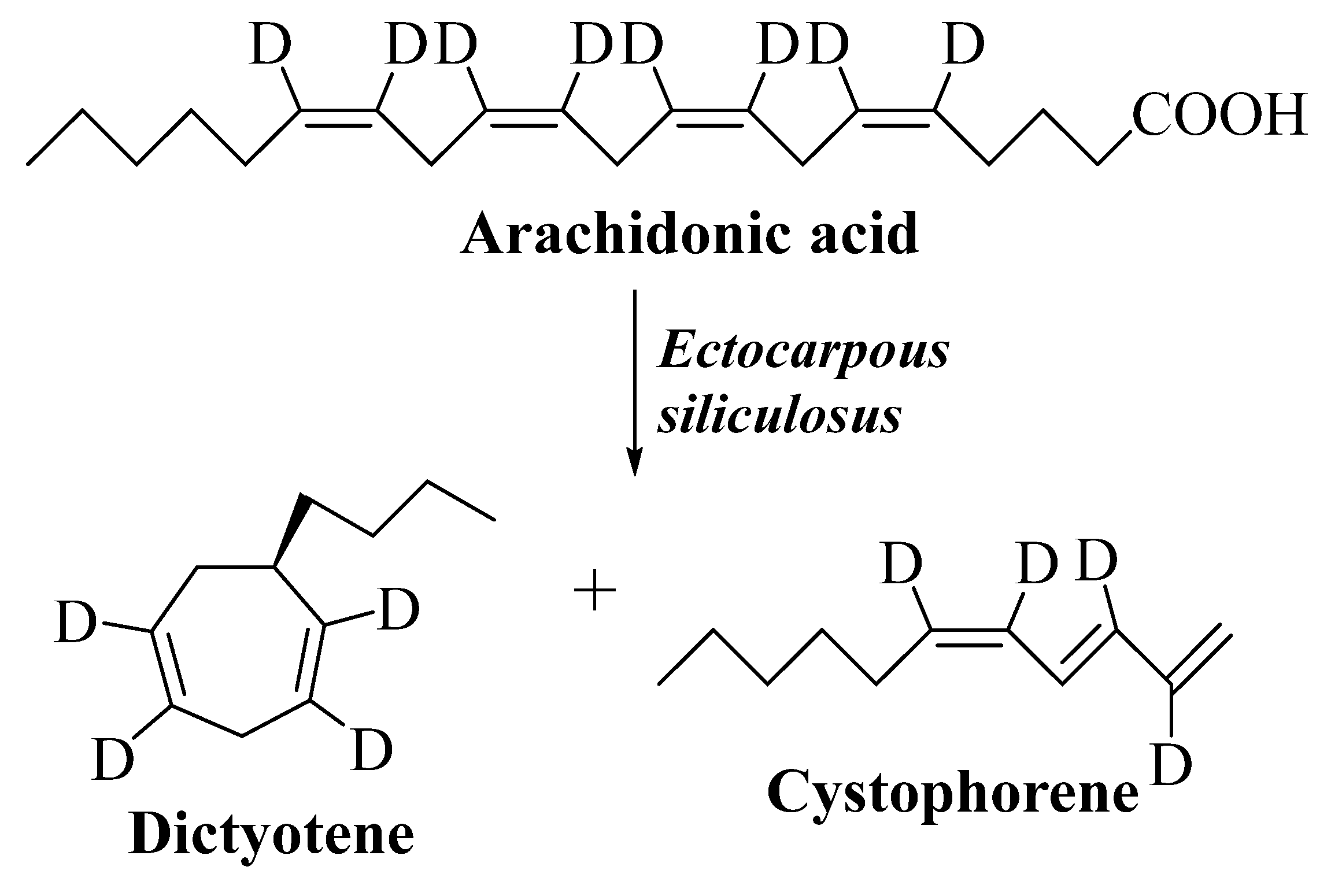
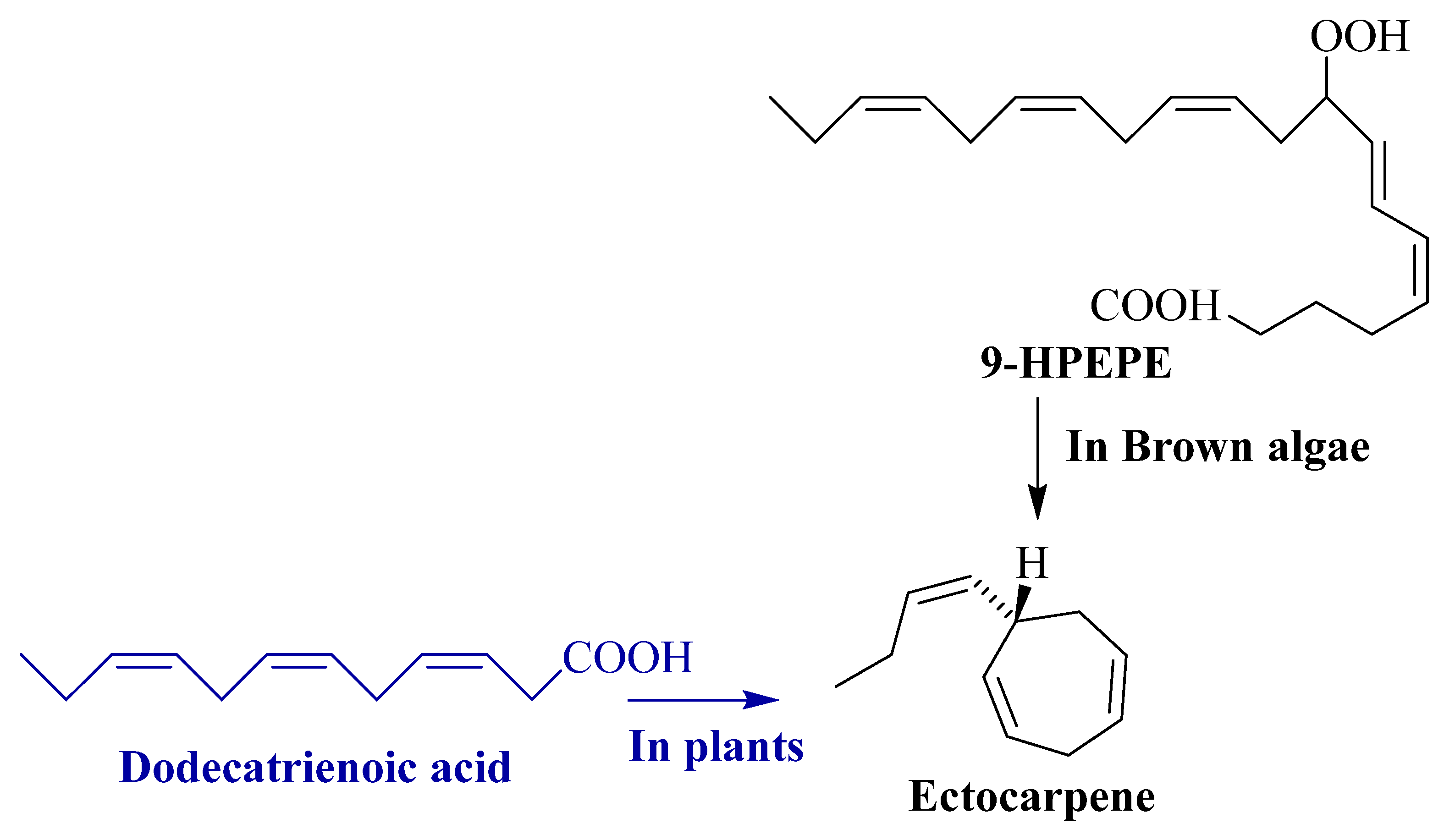
3.2.4. Ectocarpene
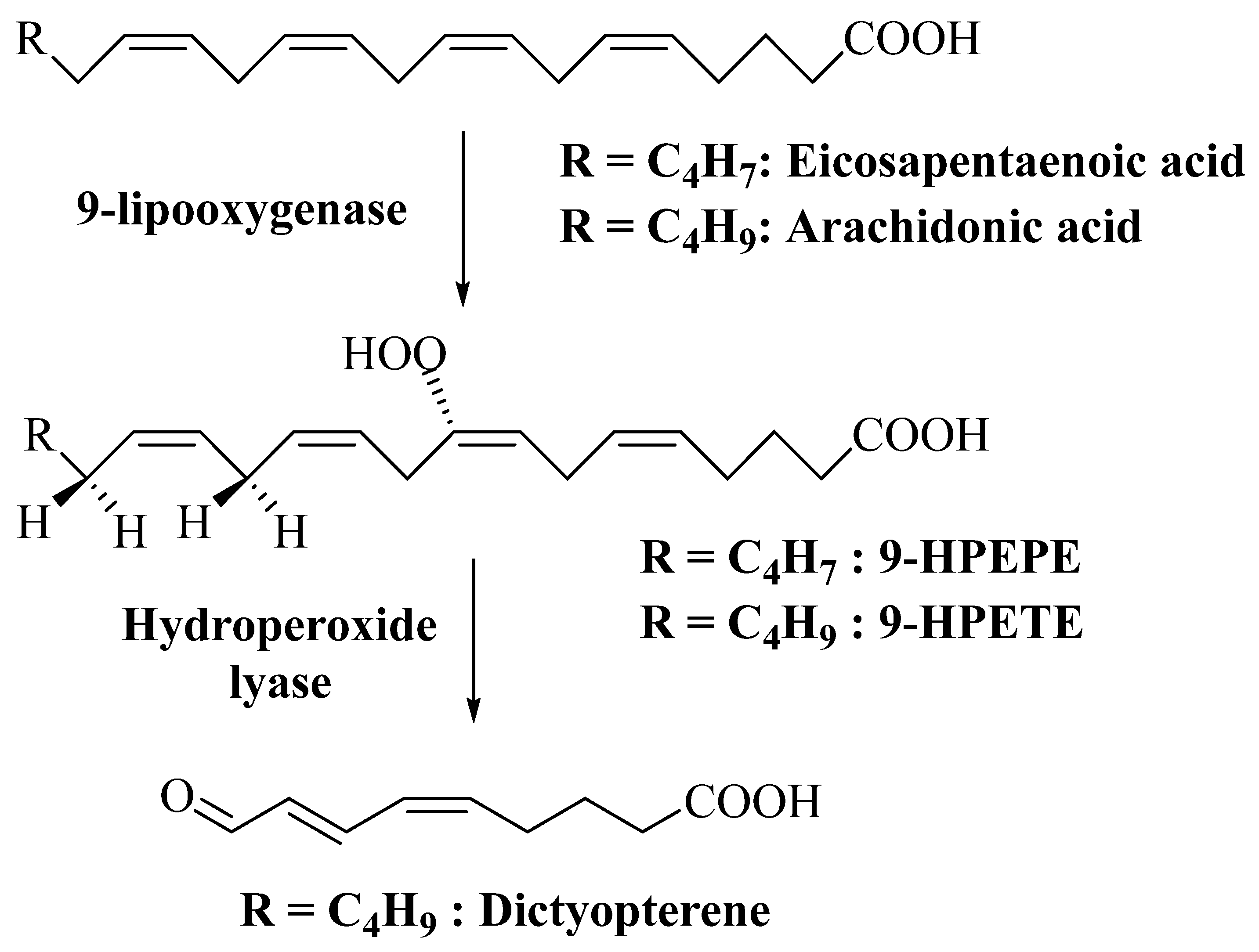
3.2.5. Dictyopterene
4. Role of Hormones in Algae
4.1. Auxin
4.2. Cytokinins
4.3. Gibberellins (GAs)
4.4. Abscisic Acid (ABA)
4.5. Ethylene
4.6. Brassinosteroids (BRs)
4.7. Jasmonic Acid (JA)
4.8. Polyamines (PAs)
4.9. Salicylic Acid (SA)
4.10. Strigolactone (SL)
4.11. Rhodomorphin
5. Role of Pheromones in Algae
5.1. Sporulation Inhibitors
5.2. Swarming Inhibitors
5.3. Ectocarpene
5.4. Dictyotene and C11 Sulfur Compounds
5.5. Ochtodene
5.6. Other Chemoattractants
6. Mode of Action of Hormones in Algae
7. Mode of Action of Pheromones in Algae
8. Methods for Extraction, Identification, and Quantification of Hormones from Algae
9. Methods for Extraction, Identification, and Quantification of Pheromones from Algae
10. Perspectives
11. Way Forward
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoek, C.; Mann, D.; Jahns, H.M. Algae: An Introduction to Phycology; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Koushalya, S.; Vishwakarma, R.; Malik, A. Unraveling the diversity of algae and its biomacromolecules. Microb. Nat. Macromol. 2021, 1, 179–204. [Google Scholar] [CrossRef]
- Rajauria, G.; Cornish, L.; Ometto, F.; Msuya, F.E.; Villa, R. Identification and Selection of Algae for Food, Feed, and Fuel Applications. In Seaweed Sustainability: Food and Non-Food Applications; Academic Press: New York, NY, USA, 2015; pp. 315–345. [Google Scholar] [CrossRef]
- Dittami, S.M.; Heesch, S.; Olsen, J.L.; Collén, J. Transitions between marine and freshwater environments provide new clues about the origins of multicellular plants and algae. J. Phycol. 2017, 53, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Leliaert, F.; Verbruggen, H.; Zechman, F.W. In to the deep: New discoveries at the base of the green plant phylogeny. Bioessays 2011, 33, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.K.; Beardall, J.; Mehta, S.; Sahoo, D.; Stojkovic, S. Using marine macroalgae for carbon sequestration: A critical appraisal. J. Appl. Phycol. 2011, 23, 877–886. [Google Scholar] [CrossRef]
- Druehl, L. Mouritsen, O.G. 2013. Seaweeds, Edible, Available & Sustainable. University of Chicago Press, 283 pp. ISBN 978-0-226-04436-1. J. Phycol. 2013, 49, 1229. [Google Scholar] [CrossRef]
- Cai, J.; Lovatelli, A.; Aguilar-Manjarrez, J.; Cornish, L.; Dabbadie, L.; Desrochers, A.; Diffey, S.; Garrido Gamarro, E.; Geehan, J.; Hurtado, A.; et al. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development. In FAO Fisheries and Aquaculture Circular; FAO: Rome, Italy, 2021; pp. 1–36. [Google Scholar] [CrossRef]
- Hurtado, A.Q. Genetic Resources for Farmed Seaweeds. In Thematic Background Study; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- González-Gloria, K.D.; Rodríguez-Jasso, R.M.; Aparicio, E.; González, M.L.C.; Kostas, E.T.; Ruiz, H.A. Macroalgal biomass in terms of third-generation biorefinery concept: Current status and techno-economic analysis–A review. Bioresour. Technol. 2021, 16, 100863. [Google Scholar] [CrossRef]
- Duerte, C.M.; Bruhn, A.; Krause-Jensen, D. A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustain. 2022, 5, 185–193. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; Volume 441, p. 105. [Google Scholar]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Verkleij, F.N. Seaweed extracts in agriculture and horticulture: A review. Biol. Agric. Hortic. 1992, 8, 309–324. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Shanmugam, M. The effect of potassium-rich biostimulant from seaweed Kappaphycus alvarezii on yield and quality of cane and cane juice of sugarcane var. Co 86032 under plantation and ratoon crops. J. Appl. Phycol. 2017, 29, 3245–3252. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Dangariya, M.; Agarwal, P. Seaweed extracts: Potential biodegradable, environmentally friendly resources for regulating plant defence. Algal Res. 2021, 58, 102363. [Google Scholar] [CrossRef]
- Trivedi, K.; Vijay Anand, K.G.; Kubavat, D.; Patidar, R.; Ghosh, A. Drought alleviatory potential of Kappaphycus seaweed extract and the role of the quaternary ammonium compounds as its constituents towards imparting drought tolerance in Zea mays L. J. Appl. Phycol. 2018, 30, 2001–2015. [Google Scholar] [CrossRef]
- Crouch, I.J.; Van Staden, J. Effect of seaweed concentrate from Ecklonia maxima (Osbeck) Papenfuss on Meloidogyne incognita infestation on tomato. J. Appl. Phycol. 1993, 5, 37–43. [Google Scholar] [CrossRef]
- Banu, A.T.; Ramani, P.S.; Murugan, A. Effect of seaweed coating on quality characteristics and shelf life of tomato (Lycopersicon esculentum mill). Food Sci. Hum. Wellness 2020, 9, 176–183. [Google Scholar] [CrossRef]
- Mlambo, P.Z. Exploring the Fertiliser Potential of Biosolids from Algae Integrated Wastewater Treatment Systems. Ph.D. Thesis, Rhodes University, Grahamstown, South Africa, October 2013. [Google Scholar]
- Bradley, P.M. Plant hormones do have a role in controlling growth and development of algae. J. Phycol. 1991, 27, 317–321. [Google Scholar] [CrossRef]
- Singh, G.; Patel, A.; Tiwari, S.; Gupta, D.; Prasad, S.M. Signaling molecules hydrogen sulfide (H2S) and nitric oxide (NO): Role in microalgae under adverse environmental conditions. Acta Physiol. Plant. 2022, 44, 68. [Google Scholar] [CrossRef]
- Delaux, P.M.; Xie, X.; Timme, R.E.; Puech-Pages, V.; Dunand, C.; Lecompte, E.; Delwiche, C.F.; Yoneyama, K.; Bécard, G.; Séjalon-Delmas, N. Origin of strigolactones in the green lineage. New Phytol. 2012, 195, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Fries, L.; Aberg, S. Morphogenetic effects of phenylacetic and p-OH-phenylacetic acid on the green alga Enteromorpha compressa (L.) Grev. in axenic culture. J. Plant Physiol. 1978, 88, 383–388. [Google Scholar] [CrossRef]
- Zhang, W.; Yamane, H.; Chapman, D.J. The phytohormone profile of the red alga Porphyra perforata. Bot. Mar. 1993, 36, 257–266. [Google Scholar] [CrossRef]
- Provasoli, L. Effect of plant hormones on Ulva. Biol. Bull. 1958, 114, 375–384. [Google Scholar] [CrossRef]
- Imahori, K.; Iwasa, K. Pure culture and chemical regulation of the growth of charophytes. Phycologia 1965, 4, 127–134. [Google Scholar] [CrossRef]
- Jacobs, W.P. Are angiosperm hormones present in, and used as hormones by, algae? In Proceedings of the 12th International Conference on Plant Growth Substances; Bopp, M., Ed.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 249–256. [Google Scholar] [CrossRef]
- Mowat, J.A. A survey of results on the occurrence of auxins and gibberellins in algae. Bot. Mar. 1965, 8, 149–155. [Google Scholar] [CrossRef]
- Stirk, W.A.; Novak, O.; Strnad, M.; van Staden, J. Cytokinins in macroalgae. Plant Growth Regul. 2003, 41, 13–24. [Google Scholar] [CrossRef]
- Dworetzky, B.; Klein, R.M.; Cook, P.W. Effect of growth substances on “Apical Dominance” in Sphacelaria furcigera (Phaeophyta). J. Phycol. 1980, 16, 239–242. [Google Scholar] [CrossRef]
- Prasad, K.; Das, A.K.; Oza, M.D.; Brahmbhatt, H.; Siddhanta, A.K.; Meena, R.; Eswaran, K.; Rajyaguru, M.R.; Ghosh, P.K. Detection and quantification of some plant growth regulators in a seaweed-based foliar spray employing a mass spectrometric technique sans chromatographic separation. J. Agric. Food Chem. 2010, 58, 4594–4601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chapman, D.J.; Phinney, B.O.; Spray, C.R.; Yamane, H.; Takahashi, N. Identification of Cytokinins in Sargassum muticum (Phaeophyta) and Porphyra perforata (Rhodophyta). J. Phycol. 1991, 27, 87–91. [Google Scholar] [CrossRef]
- Borowczak, E.; Kentzer, T.; Potulska-Klein, B. Effect of gibberellin and kinetin on the regeneration ability of Fucus vesiculosus L. Biol. Plant. 1977, 19, 405–412. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Godlewski, M. Studies on the role of gibberellins in the regulation of spermatogenesis in Chara vulgaris L. Acta Soc. Bot. Pol. 1988, 57, 547–553. [Google Scholar] [CrossRef]
- Stirk, W.A.; Novak, O.; Hradecka, V.; Pencík, A.; Rolcík, J.; Strnad, M.; van Staden, J. Endogenous cytokinins, auxins and abscisic acid in Ulva fasciata (Chlorophyta) and Dictyota humifusa (Phaeophyta): Towards understanding their biosynthesis and homoeostasis. Eur. J. Phycol. 2009, 44, 231–240. [Google Scholar] [CrossRef]
- Yokoya, N.S.; Stirk, W.A.; van Staden, J.; Novák, O.; Turečková, V.; Pěnčí, K.A.; Strnad, M. Endogenous cytokinins, auxins, and abscisic acid in red algae from Brazil. J. Phycol. 2010, 46, 1198–1205. [Google Scholar] [CrossRef]
- Tietz, A.; Ruttkowski, U.; Kohler, R.; Kasprik, W. Further investigations on the occurrence and the effects of abscisic acid in algae. Biochem. Physiol. Pflanz. 1989, 184, 259–266. [Google Scholar] [CrossRef]
- Waaland, S.D. Hormonal Coordination of the Processes Leading to Cell Fusion in Algae: A Glycoprotein Hormone from Red Algae. In Proceedings of the 12th International Conference on Plant Growth Substances, Berlin/Heidelberg, Germany, 26–31 August 1985; Bopp, M., Ed.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 257–262. [Google Scholar] [CrossRef]
- Starr, R.C.; Jaenicke, L. Purification and characterization of the hormone initiating sexual morphogenesis in Volvox carteri f. nagariensis Iyengar. Proc. Natl. Acad. Sci. USA 1974, 71, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Driessche, T.V.; Jerebzoff, S.; Jerebzoff-Quintin, S. Phase-shifting effects of indole-3-acetic acid and the synchronization of three circadian metabolic rhythms in Acetabularia. Biol. Rhythm Res. 1988, 19, 81–87. [Google Scholar] [CrossRef]
- Stirk, W.A.; Tarkowska, D.; Turecova, V.; Strnad, M.; Van Staden, J. Abscisic acid, gibberellins and brassinosteroids in Kelpak®, a commercial seaweed extract made from Ecklonia maxima. J. Appl. Phycol. 2014, 26, 561–567. [Google Scholar] [CrossRef]
- Christov, C.; Pouneva, I.; Bozhkova, M.; Toncheva, T.; Fournadzieva, S.; Zafirova, T. Influence of temperature and methyl jasmonate on Scenedesmus incrassulatus. Biol. Plant. 2001, 44, 367–371. [Google Scholar] [CrossRef]
- Bouarab, K.; Adas, F.; Gaquerel, E.; Kloareg, B.; Salaun, J.P.; Potin, P. The innate immunity of a marine red alga involves oxylipins from both the eicosanoid and octadecanoid pathways. Plant Physiol. 2004, 135, 838–1848. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Arad, S.; Heimer, Y.H.; Mizrahi, Y. Polyamine biosynthetic enzymes in the cell cycle of Chlorella: Correlation between ornithine decarboxylase and DNA synthesis at different light intensities. Plant Physiol. 1984, 74, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Theiss, C.; Bohley, P.; Voigt, J. Regulation by polyamines of ornithine decarboxylase activity and cell division in the unicellular green alga Chlamydomonas reinhardtii. Plant Physiol. 2002, 128, 1470–1479. [Google Scholar] [CrossRef]
- Lee, T.M. Investigations of some intertidal green macroalgae to hyposaline stress: Detrimental role of putrescine under extreme hyposaline conditions. Plant Sci. 1998, 138, 1–8. [Google Scholar] [CrossRef]
- Garcia-Jimenez, P.; Just, P.M.; Delgado, A.M.; Robaina, R.R. Transglutaminase activity decrease during acclimation to hyposaline conditions in marine seaweed Grateloupia doryphora (Rhodophyta, Halymeniaceae). J. Plant Physiol. 2007, 164, 367–370. [Google Scholar] [CrossRef]
- Garcia-Jimenez, P.; Rodrigo, M.; Robaina, R.R. Influence of plant growth regulators, polyamines and glycerol interaction on growth and morphogenesis of carposporelings of Grateloupia cultured in vitro. J. Appl. Phycol. 1998, 10, 95–100. [Google Scholar] [CrossRef]
- Marián, F.D.; García-Jiménez, P.; Robaina, R.R. Polyamines in marine macroalgae: Levels of putrescine, spermidine and spermine in the thalli and changes in their concentration during glycerol-induced cell growth in vitro. Physiol. Plant. 2000, 110, 530–534. [Google Scholar]
- Sacramento, A.T.; García-Jimenez, P.; Robaina, R.R. The polyamine spermine induces cystocarp development in the seaweed Grateloupia (Rhodophyta). Plant Growth Regul. 2007, 53, 147–154. [Google Scholar] [CrossRef]
- Guzman-Uriostegui, A.; García-Jimenez, P.; Marian, F.D.; Robledo, D.; Robaina, R.R. Polyamines influence maturation in reproductive structures of Gracilaria cornea (Gracilariales, Rhodophyta). J. Phycol. 2002, 38, 1169–1175. [Google Scholar] [CrossRef]
- Sacramento, A.T.; Garcia-Jimenez, P.; Alcazar, R.; Tiburcio, A.; Robaina, R.R. Influence of polyamines on the sporulation of Grateloupia (Halymeniaceae, Rhodophyta). J. Phycol. 2004, 50, 887–894. [Google Scholar] [CrossRef]
- Zhou, B.; Tang, X.; Wang, Y. Salicylic acid and heat acclimation pretreatment protects Laminaria japonica sporophyte (Phaeophyceae) from heat stress. Chin. J. Oceanol. Limnol. 2010, 28, 924–932. [Google Scholar] [CrossRef]
- Jennings, R.C. Gibberellins as endogenous growth regulators in green and brown algae. Planta 1968, 80, 34–42. [Google Scholar] [CrossRef]
- Abe, H.; Marumo, S. Identification of auxin-active substances as ethyl chlorogenate and indolyl-3-acetic acid in immature seeds of Helianthus annuus. Agric. Biol. Chem. 1972, 36, 42–46. [Google Scholar] [CrossRef]
- Brain, K.R.; Chalopin, M.C.; Turner, T.D.; Blunden, G.; Wildgoose, P.B. Cytokinin activity of commercial aqueous seaweed extract. Plant Sci. Lett. 1973, 1, 241–245. [Google Scholar] [CrossRef]
- Basu, S.; Sun, H.; Brian, L.; Quatrano, R.L.; Muday, G.K. Early embryo development in Fucus distichus is auxin sensitive. Plant Physiol. 2002, 130, 292–302. [Google Scholar] [CrossRef]
- Karlson, P.; Lüscher, M. ‘Pheromones’: A new term for a class of biologically active substances. Nature 1959, 183, 55–56. [Google Scholar] [CrossRef]
- Stratmann, J.; Paputsoglu, G.; Oertel, W. Differentiation of Ulva mutabilis (Chlorophyta) gametangia and gamete release are controlled by extracellular inhibitors. J. Phycol. 1996, 32, 1009–1021. [Google Scholar] [CrossRef]
- Muller, D.G.; Jaenicke, L.; Donike, M.; Akintobi, T. Sex attractant in a brown alga: Chemical structure. Science 1971, 171, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.G.; Gassmann, G. Sexual hormone specificity in Ectocarpus and Laminaria (Phaeophyceae). Nat. Sci. 1980, 67, 462–463. [Google Scholar] [CrossRef]
- Muller, D.G.; Boland, W.; Jaenicke, L.; Gassmann, G. Diversification of chemoreceptors in Ectocarpus, Sphacelaria, and Adenocystis (Phaeophyceae). J. Nat. Sci. 1985, 40, 457–459. [Google Scholar] [CrossRef]
- Hay, M.E.; Piel, J.; Boland, W.; Schnitzler, I. Seaweed sex pheromones and their degradation products frequently suppress amphipod feeding but rarely suppress sea urchin feeding. Chemoecology 1998, 8, 91–98. [Google Scholar] [CrossRef]
- Phillips, J.A.; Clayton, M.N.; Maier, I.; Boland, W.; Muller, D.G. Sexual reproduction in Dictyota diemensis (Dictyotales, Phaeophyta). Phycologia 1990, 29, 367–379. [Google Scholar] [CrossRef]
- Hay, M.E.; Duffy, J.E.; Pfister, C.A.; Fenical, W. Chemical defense against different marine herbivores: Are amphipods insect equivalents? Ecology 1987, 68, 1567–1580. [Google Scholar] [CrossRef]
- Hay, M.E.; Fenical, W. Marine plant-herbivore interactions: The ecology of chemical defense. Annu. Rev. Ecol. Evol. Syst. 1988, 19, 111–145. [Google Scholar] [CrossRef]
- Schnitzler, I.; Boland, W.; Hay, M.E. Organic sulfur compounds from Dictyopteris spp. deter feeding by an herbivorous amphipod (Ampithoe longimana) but not by an herbivorous sea urchin (Arbacia punctulata). J. Chem. Ecol. 1998, 24, 1715–1732. [Google Scholar] [CrossRef]
- Maier, I.; Muller, D.G.; Gassmann, G.; Boland, W.; Marner, F.J.; Jaenicke, L. Pheromone-triggered gamete release in Chorda tomentosa. Nat. Sci. 1984, 71, 48–49. [Google Scholar] [CrossRef]
- Muller, D.G.; Jaenicke, L. Fucoserraten, the female sex attractant of Fucus serratus L. (Phaeophyta). FEBS Lett. 1973, 30, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Maier, I.; Muller, D.G. Sexual pheromones in algae. Biol. Bull. 1986, 170, 145–175. [Google Scholar] [CrossRef]
- Jaenicke, L. One hundred and one years of chemotaxis. Pfeffer, pheromones, and fertilization. Bot. Acta 1988, 101, 149–159. [Google Scholar] [CrossRef]
- Muller, D.G. The role of pheromones in sexual reproduction of brown algae. Plant Biol. 1989, 7, 201–213. [Google Scholar]
- Maier, I. Gamete orientation and induction of gametogenesis by pheromones in algae and plants. Plant Cell Environ. 1993, 16, 891–907. [Google Scholar] [CrossRef]
- Jaenicke, L.; Muellar, D.G.; Moore, R.E. Multifidene and aucantene, C11 hydrocarbons in the male-attracting essential oil from the gynogametes of Cutleria multifida (Phaeophyta). J. Am. Chem. Soc. 1974, 96, 3324–3325. [Google Scholar] [CrossRef]
- McConnell, O.J.; Fenical, W. Ochtodene and ochtodiol: Novel polyhalogenated cyclic monoterpenes from the red seaweed Ochtodes secundiramea. J. Org. Chem. 1978, 43, 4238–4241. [Google Scholar] [CrossRef]
- Chandler, J.W. Local auxin production: A small contribution to a big field. Bioessays 2009, 31, 60–70. [Google Scholar] [CrossRef]
- Kiseleva, A.A.; Tarachovskaya, E.R.; Shishova, M.F. Biosynthesis of phytohormones in algae. J. Plant Physiol. 2012, 59, 595–610. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Mok, M.C.; Martin, R.C.; Mok, D.W. Cytokinins: Biosynthesis metabolism and perception. Vitr. Cell. Dev. Biol. Plant 2000, 36, 102–107. [Google Scholar] [CrossRef]
- Lindner, A.C.; Lang, D.; Seifert, M.; Podlešáková, K.; Novák, O.; Strnad, M.; Reski, R.; von Schwartzenberg, K. Isopentenyltransferase-1 (IPT1) knockout in Physcomitrella together with phylogenetic analyses of IPTs provide insights into evolution of plant cytokinin biosynthesis. J. Exp. Bot. 2014, 65, 2533–2543. [Google Scholar] [CrossRef] [PubMed]
- Ordog, V.; Stirk, W.A.; van Staden, J.; Novak, O.; Strnad, M. Endogenous cytokinins in three genera of microalgae from the Chlorophyta. J. Phycol. 2004, 40, 88–95. [Google Scholar] [CrossRef]
- Von Schwartzenberg, K.; Nunez, M.F.; Blaschke, H.; Dobrev, P.I.; Novak, O.; Motyka, V.; Strnad, M. Cytokinins in the Bryophyte Physcomitrella patens: Analysis of activity, distribution, and cytokinin oxidase/dehydrogenase overexpression reveal the role of extracellular cytokinins. Plant Physiol. 2007, 145, 786–800. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates Incorporated: Sunderland, UK, 2015; p. 761. [Google Scholar]
- Schwender, J.; Seemann, M.; Lichtenthaler, H.K.; Rohmer, M. Biosynthesis of isoprenoids (carotenoids, sterols, prenyl side-chains of chlorophylls and plastoquinone) via a novel pyruvate/glyceraldehyde 3-phosphate non-mevalonate pathway in the green alga Scenedesmus obliquus. Biochem. J. 1996, 316, 73–80. [Google Scholar] [CrossRef]
- Kasahara, H.; Hanada, A.; Kuzuyama, T.; Takagi, M.; Kamiya, Y.; Yamaguchi, S. Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of gibberellins in Arabidopsis. J. Biol. Chem. 2002, 277, 45188–45194. [Google Scholar] [CrossRef]
- Cutler, A.J.; Krochko, J.E. Formation and breakdown of ABA. Trends Plant Sci. 1999, 4, 472–478. [Google Scholar] [CrossRef]
- Hartung, W. The evolution of abscisic acid (ABA) and ABA function in lower plants fungi and lichen. Funct. Plant Biol. 2010, 37, 806–812. [Google Scholar] [CrossRef]
- Seo, M.; Koshiba, T. Complex regulation of ABA biosynthesis in plants. Trends Plant Sci. 2002, 7, 41–48. [Google Scholar] [CrossRef]
- Baroli, I.; Niyogi, K.K. Molecular genetics of xanthophyll–dependent photoprotection in green algae and plants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kondo, N. Ethylene evolution in marine algae and a proteinaceous inhibitor of ethylene biosynthesis from red alga. Plant Cell Physiol. 1976, 17, 1159–1166. [Google Scholar] [CrossRef]
- Plettner, I.N.A.; Steinke, M.; Malin, G. Ethene (ethylene) production in the marine macroalga Ulva (Enteromorpha) intestinalis L. (Chlorophyta, Ulvophyceae): Effect of light-stress and co-production with dimethyl sulphide. Plant Cell Environ. 2005, 28, 1136–1145. [Google Scholar] [CrossRef]
- Maillard, P.; Thepenier, C.; Gudin, C. Determination of an ethylene biosynthesis pathway in the unicellular green alga, Haematococcus pluvialis. Relationship between growth and ethylene production. J. Appl. Phycol. 1993, 5, 93–98. [Google Scholar] [CrossRef]
- Booker, M.A.; DeLong, A. Producing the ethylene signal: Regulation and diversification of ethylene biosynthetic enzymes. Plant Physiol. 2015, 69, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Cembella, A.D. Chemical ecology of eukaryotic microalgae in marine ecosystems. Phycologia 2003, 42, 420–447. [Google Scholar] [CrossRef]
- Frenkel, J.; Vyverman, W.; Pohnert, G. Pheromone signaling during sexual reproduction in algae. Plant J. 2014, 79, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, K.; Boland, W.; Müller, D.G. Pheromones of marine brown algae; a new branch of the eicosanoid metabolism. Angew. Chem. Int. Ed. Engl. 1992, 31, 1246–1248. [Google Scholar] [CrossRef]
- Boland, W.; Jaenicke, L.; Muller, D.G.; Gassmann, G. Giffordene, 2Z, 4Z, 6E, 8Z-undecatetraene, is the odoriferous principle of the marine brown alga Giffordia mitchellae. Experientia 1987, 43, 466–467. [Google Scholar] [CrossRef]
- Muller, D.G.; Schmid, C.E. Qualitative and quantitative determination of pheromone secretion in female gametes of Ectocarpus siliculosus (Phaeophyceae). Biol. Chem. Hoppe-Seyler 1988, 369, 647–653. [Google Scholar] [CrossRef]
- Stratmann, K.; Boland, W.; Müller, D.G. Biosynthesis of pheromones in female gametes of marine brown algae (Phaeophyceae). Tetrahedron 1993, 49, 3755–3766. [Google Scholar] [CrossRef]
- Yannai, S. Dictionary of Food Compounds with CD-ROM: Additives, Flavors, and Ingredients, 1st ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2004; p. 1784. [Google Scholar]
- Rui, F.; Boland, W. Algal pheromone biosynthesis: Stereochemical analysis and mechanistic implications in gametes of Ectocarpus siliculosus. J. Org. Chem. 2010, 75, 3958–3964. [Google Scholar] [CrossRef] [PubMed]
- Pohnert, G.; Boland, W. The oxylipin chemistry of attraction and defense in brown algae and diatoms. Nat. Prod. Rep. 2002, 19, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Boland, W. The chemistry of gamete attraction: Chemical structures, biosynthesis, and (a) biotic degradation of algal pheromones. Proc. Natl. Acad. Sci. USA 1995, 92, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Pohnert, G.; Boland, W. Biosynthesis of the algal pheromone hormosirene by the fresh-water diatom Gomphonema parvulum (Bacillariophyceae). Tetrahedron 1996, 52, 10073–10082. [Google Scholar] [CrossRef]
- Kajiwara, T.; Kodama, K.; Hatanaka, A.; Matsui, K. Volatile Compounds from Japanese Marine Brown Algae. In Bioactive Volatile Compounds from Plants; Teranishi, R., Buttery, R.G., Sugisawa, H., Eds.; American Chemical Society: Washington, DC, USA, 1993; Volume 525, pp. 103–120. [Google Scholar] [CrossRef]
- Moore, R.E. Volatile compounds from marine algae. Acc. Chem. Res. 1977, 10, 40–47. [Google Scholar] [CrossRef]
- Gruen, H.E. Auxins and fungi. Annu. Rev. Plant Physiol. 1959, 10, 405–440. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef]
- Lau, S.; Shao, N.; Bock, R.; Jürgens, G.; De Smet, I. Auxin signaling in algal lineages: Fact or myth? Trends Plant Sci. 2009, 14, 182–188. [Google Scholar] [CrossRef]
- Tarakhovskaya, E.R.; Maslov, Y.I.; Shishova, M.F. Phytohormones in algae. Russ. J. Plant Physiol. 2007, 54, 163–170. [Google Scholar] [CrossRef]
- Yokoya, N.S.; Handro, W. Effects of auxins and cytokinins on tissue culture of Grateloupia dichotoma (Gigartinales, Rhodophyta). Hydrobiologia 1996, 326, 393–400. [Google Scholar] [CrossRef]
- Polevoi, V.V.; Tarakhovskaya, E.R.; Maslov, Y.I.; Polevoi, A.V. Role of auxin in induction of polarity in Fucus vesiculosus zygotes. Russ. J. Dev. Biol. 2003, 34, 360–364. [Google Scholar] [CrossRef]
- Fries, L. Growth regulating effects of phenylacetic acid and p-hydroxyphenylacetic acid on Fucus spiralis L. (Phaeophyceae, Fucales) in axenic culture. Phycologia 1977, 16, 451–455. [Google Scholar] [CrossRef]
- Fries, L.; Iwasaki, H. p-Hydroxyphenylacetic acid and other phenolic compounds as growth stimulators of the red alga Porphyra tenera. Plant Sci. Lett. 1976, 6, 299–307. [Google Scholar] [CrossRef]
- Buggeln, R.G. Morphogenesis and Growth Regulators. In The Biology of Seaweeds; Wiley-Blackwell: Oxford, UK, 1981; pp. 627–660. [Google Scholar]
- Jacobs, W.P.; Falkenstein, K.; Hamilton, R.H. Nature and amount of auxin in algae: IAA from extracts of Caulerpa paspaloides (Siphonales). Plant Physiol. 1985, 78, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, Y.; Sun, X.; Zhang, X.; Xu, N. Comparative transcriptome analysis reveals the promoting effects of IAA on biomass production and branching of Gracilariopsis lemaneiformis. Aquaculture 2022, 548, 737678. [Google Scholar] [CrossRef]
- Mooney, P.A.; Van Staden, J. Seasonal changes in the levels of endogenous cytokinins in Sargassum heterophyllum (Phaeophyceae). Bot. Mar. 1984, 27, 437–442. [Google Scholar] [CrossRef]
- De Nys, R.; Jameson, P.E.; Chin, N.; Brown, M.T.; Sanderson, K.J. The cytokinins as endogenous growth regulators in Macrocystis pyrifera (L.) C. Ag. (Phaeophyceae). Bot. Mar. 1990, 33, 467–475. [Google Scholar] [CrossRef]
- Radley, M. Gibberellin-like Substances in Plants: Leguminous Root Nodules. Nature 1961, 191, 684–685. [Google Scholar] [CrossRef]
- Jacobs, W.P. A search for some angiosperm hormones and their metabolites in Caulerpa paspaloides (Chlorophyta). J. Phycol. 1993, 29, 595–600. [Google Scholar] [CrossRef]
- Taylor, I.B.; Burbidge, A.; Thompson, A.J. Control of abscisic acid synthesis. J. Exp. Bot. 2000, 51, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.; Hartung, W.; Gimmler, H. Abscisic acid content of algae under stress. Bot. Acta 1989, 102, 326–334. [Google Scholar] [CrossRef]
- Nimura, K.; Mizuta, H. Inducible effects of abscisic acid on sporophyte discs from Laminaria japonica Areschoug (Laminariales, Phaeophyceae). J. Appl. Phycol. 2002, 14, 159–163. [Google Scholar] [CrossRef]
- Uji, T.; Matsuda, R.; Takechi, K.; Takano, H.; Mizuta, H.; Takio, S. Ethylene regulation of sexual reproduction in the marine red alga Pyropia yezoensis (Rhodophyta). J. Appl. Phycol. 2016, 28, 3501–3509. [Google Scholar] [CrossRef]
- Uji, T.; Mizuta, H. The role of plant hormones on the reproductive success of red and brown algae. Front. Plant Sci. 2022, 13, 1019334. [Google Scholar] [CrossRef] [PubMed]
- Van de Poel, B.; Cooper, E.D.; Van Der Straeten, D.; Chang, C.; Delwiche, C.F. Transcriptome profiling of the green alga Spirogyra pratensis (Charophyta) suggests an ancestral role for ethylene in cell wall metabolism, photosynthesis, and abiotic stress responses. Plant Physiol. 2016, 172, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Czerpak, R. Physiological and biochemical role of brassinosteroids and their structure-activity relationship in the green alga Chlorella vulgaris Beijerinck (Chlorophyceae). J. Plant Growth Regul. 1998, 17, 131–139. [Google Scholar] [CrossRef]
- Bajguz, A. Effect of brassinosteroids on nucleic acids and protein content in cultured cells of Chlorella vulgaris. Plant Physiol. Biochem. 2000, 38, 209–215. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, W.; Xia, D. Brassinosteroid improves lipid productivity and stress tolerance of Chlorella cells induced by high temperature. J. Appl. Phycol. 2018, 30, 253–260. [Google Scholar] [CrossRef]
- Bajguz, A. Brassinosteroid enhanced the level of abscisic acid in Chlorella vulgaris subjected to short-term heat stress. J. Plant Physiol. 2009, 166, 882–886. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. Intervention of phytohormone pathways by pathogen effectors. Plant Cell 2014, 26, 2285–2309. [Google Scholar] [CrossRef] [PubMed]
- Feys, B.J.; Parker, J.E. Interplay of signaling pathways in plant disease resistance. Trends Genet. 2000, 16, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Wiesemeier, T.; Jahn, K.; Pohnert, G. No evidence for the induction of brown algal chemical defense by the phytohormones jasmonic acid and methyl jasmonate. J. Chem. Ecol. 2008, 34, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Tabor, C.W.; Tabor, H. Polyamines. Annu. Rev. Biochem. 1984, 53, 749–790. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Bo, Y.; Sun, X.; Wang, H.; Guo, H.; Zhou, C.; Ruan, R.; Yan, X.; Cheng, P. Review of the effect of polyamines in microalgae when ingested by shellfish. Algal Res. 2021, 58, 102409. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic acid biosynthesis in plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Faraz, A.; Sami, F.; Siddiqui, H.; Yusuf, M.; Gruszka, D.; Hayat, S. Role of strigolactones: Signalling and crosstalk with other phytohormones. Open Life Sci. 2020, 15, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.J.; Roux, C.; Lopez-Raez, J.A.; Becard, G. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci. 2007, 12, 224–230. [Google Scholar] [CrossRef]
- Stirk, W.A.; Van Staden, J. Plant growth regulators in seaweeds: Occurrence, regulation and functions. Adv. Bot. Res. 2014, 71, 125–159. [Google Scholar] [CrossRef]
- Arioli, T.; Mattner, S.W.; Winberg, P.C. Applications of seaweed extracts in Australian agriculture: Past, present and future. J. Appl. Phycol. 2015, 27, 2007–2015. [Google Scholar] [CrossRef]
- Waaland, S.D.; Watson, B.A. Isolation of a cell-fusion hormone from Griffithsia pacifica Kylin, a red alga. Planta 1980, 149, 493–497. [Google Scholar] [CrossRef]
- Wichard, T.; Oertel, W. Gametogenesis and gamete release of Ulva mutabilis and Ulva lactuca (Chlorophyta): Regulatory effects and chemical characterization of the “swarming inhibitor”. J. Phycol. 2010, 46, 248–259. [Google Scholar] [CrossRef]
- Kamiya, Y. Plant hormones: Versatile regulators of plant growth and development. Annu. Rev. Plant Biol. 2010, 60. [Google Scholar] [CrossRef]
- Jaillais, Y.; Chory, J. Unraveling the paradoxes of plant hormone signaling integration. Nat. Struct. Mol. Biol. 2010, 17, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Friml, J.; Jones, A.R. Endoplasmic reticulum: The rising compartment in auxin biology. Plant Physiol. 2010, 154, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Effendi, Y.; Scherer, G.F. AUXIN BINDING-PROTEIN1 (ABP1), a receptor to regulate auxin transport and early auxin genes in an interlocking system with PIN proteins and the receptor TIR1. Plant Signal. Behav. 2011, 6, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.M.; Kepinski, S.; Rouse, D.; Leyser, O.; Estelle, M. Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 2001, 414, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xu, J. Phytohormones in microalgae: A new opportunity for microalgal biotechnology? Trends Plant Sci. 2015, 20, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Dharmasiri, S.; Jayaweera, T.; Dharmasiri, N. Plant hormone signalling: Current perspectives on perception and mechanisms of action. Ceylon J. Sci. Biol. Sci. 2013, 42, 1–17. [Google Scholar] [CrossRef]
- Lu, Y.; Tarkowská, D.; Turečková, V.; Luo, T.; Xin, Y.; Li, J.; Wang, Q.; Jiao, N.; Strnad, M.; Xu, J. Antagonistic roles of abscisic acid and cytokinin during response to nitrogen depletion in oleaginous microalga Nannochloropsis oceanica expand the evolutionary breadth of phytohormone function. Plant J. 2014, 80, 52–68. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Willige, B.C. Shedding light on gibberellic acid signalling. Curr. Opin. Plant Biol. 2009, 12, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Leivar, P.; Monte, E.; Oka, Y.; Liu, T.; Carle, C.; Castillon, A.; Huq, E.; Quail, P.H. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 2008, 18, 1815–1823. [Google Scholar] [CrossRef]
- Vandenbussche, F.; Fierro, A.C.; Wiedemann, G.; Reski, R.; van der Straeten, D. Evolutionary conservation of plant gibberellin signalling pathway components. BMC Plant Biol. 2007, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.; et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, X.; Weston, D.J.; Chen, J.G. Abscisic acid receptors: Past, present and future. J. Integr. Plant Biol. 2011, 53, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Waadt, R.; Schroeder, J.I. Evolution of abscisic acid synthesis and signaling mechanisms. Curr. Biol. 2011, 21, R346–R355. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Hase, T.; Toyoda, T.; Shinozaki, K.; Okamoto, M. Origin and evolution of genes related to ABA metabolism and its signaling pathways. J. Plant Res. 2011, 124, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.D.; Cho, Y.; Sheen, J. Emerging connections in the ethylene signaling network. Trends Plant Sci. 2009, 14, 270–279. [Google Scholar] [CrossRef]
- Bleecker, A.B. Ethylene perception and signaling: An evolutionary perspective. Trends Plant Sci. 1999, 4, 269–274. [Google Scholar] [CrossRef]
- Mori, I.C.; Ikeda, Y.; Matsuura, T.; Hirayama, T.; Mikami, K. Phytohormones in red seaweeds: A technical review of methods for analysis and a consideration of genomic data. Bot. Mar. 2017, 60, 153–170. [Google Scholar] [CrossRef]
- Moon, J.S.; Kim, G.H. Somatic cell fusion in a red alga Griffithsia monilis is mediated by two different signalling molecules. Phycologia 2017, 56, 130. [Google Scholar]
- Starr, R.C.; Marner, F.J.; Jaenicke, L. Chemoattraction of male gametes by a pheromone produced by female gametes of Chlamydomonas. Proc. Natl. Acad. Sci. USA 1995, 92, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Adair, W.S. Characterization of Chlamydomonas sexual agglutinins. J. Cell Sci. 1985, 1985 (Suppl. S2), 233–260. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Takanashi, S.I. Synthesis of lurlene, the sex pheromone of the green flagellate Chlamydomonas allensworthii. Tetrahedron Lett. 1996, 37, 1821–1824. [Google Scholar] [CrossRef]
- Kirk, D.L.; Kirk, M.M. Heat shock elicits production of sexual inducer in Volvox. Science 1986, 231, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Godl, K.; Hallmann, A.; Rappel, A.; Sumper, M. Pherophorins: A family of extracellular matrix glycoproteins from Volvox structurally related to the sex-inducing pheromone. Planta 1995, 196, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Sumper, M.; Berg, E.; Wenzl, S.; Godl, K. How a sex pheromone might act at a concentration below 10–16 M. EMBO J. 1993, 12, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Amon, P.; Haas, E.; Sumper, M. The sex-inducing pheromone and wounding trigger the same set of genes in the multicellular green alga Volvox. Plant Cell 1998, 10, 781–789. [Google Scholar] [CrossRef]
- Schmid, C.E. Cell-cell-recognition during fertilization in Ectocarpus siliculosus (Phaeophyceae). Hydrobiologia 1993, 260, 437–443. [Google Scholar] [CrossRef]
- Maier, I.; Muller, D.G. Antheridium fine structure and spermatozoid release in Laminaria digitata (Phaeophyceae). Phycologia 1982, 21, 1–8. [Google Scholar] [CrossRef]
- Maier, I.; Muller, D.G.; Schmid, C.; Boland, W.; Jaenicke, L. Pheromone receptor specificity and threshold concentrations for spermatozoid release in Laminaria digitata. Nat. Sci. 1988, 75, 260–263. [Google Scholar] [CrossRef]
- Maier, I.; Muller, D.G. Chemotaxis in Laminaria digitata (Phaeophyceae) I. Analysis of spermatozoid movement. J. Exp. Bot. 1990, 41, 869–876. [Google Scholar] [CrossRef]
- Maier, I.; Wenden, A.; Clayton, M.N. The movement of Hormosira banksii (Fucales, Phaeophyta) spermatozoids in response to sexual pheromone. J. Exp. Bot. 1992, 43, 1651–1657. [Google Scholar] [CrossRef]
- Wirth, D.; Boland, W. Structure and Synthesis of (±)-Caudoxirene, a New Spermatozoid-Releasing and-Attracting Pheromone from the Marine Brown Alga Perithalia caudata (Phaeophyceae, Sporochnales). Helv. Chim. Acta 1990, 73, 916–921. [Google Scholar] [CrossRef]
- Boland, W.; Hoever, F.P.; Kruger, B.W. Application of Molecular Modelling Techniques to Pheromones of the Marine Brown Algae Cutleria multifida and Ectocarpus siliculosus (Phaeophyceae). Metalloproteins as Chemoreceptors? J. Nat. Sci. 1989, 44, 29–837. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; del Pilar Sánchez-Camargo, A.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Górka, B.; Wieczorek, P.P. Simultaneous determination of nine phytohormones in seaweed and algae extracts by HPLC-PDA. J. Chromatogr. B 2017, 1057, 32–39. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, L.; Lu, M.; Chen, G.; Zhang, L. Extraction and analysis of auxins in plants using dispersive liquid−liquid microextraction followed by high-performance liquid chromatography with fluorescence detection. J. Agric. Food Chem. 2010, 58, 2763–2770. [Google Scholar] [CrossRef]
- Gupta, V.; Kumar, M.; Brahmbhatt, H.; Reddy, C.R.K.; Seth, A.; Jha, B. Simultaneous determination of different endogenetic plant growth regulators in common green seaweeds using dispersive liquid–liquid microextraction method. Plant Physiol. Biochem. 2011, 49, 1259–1263. [Google Scholar] [CrossRef]
- Mikami, K.; Mori, I.C.; Matsuura, T.; Ikeda, Y.; Kojima, M.; Sakakibara, H.; Hirayama, T. Comprehensive quantification and genome survey reveal the presence of novel phytohormone action modes in red seaweeds. J. Appl. Phycol. 2016, 28, 2539–2548. [Google Scholar] [CrossRef]
- Benítez García, I.; Dueñas Ledezma, A.K.; Martínez Montaño, E.; Salazar Leyva, J.A.; Carrera, E.; Osuna Ruiz, I. Identification and quantification of plant growth regulators and antioxidant compounds in aqueous extracts of Padina durvillaei and Ulva lactuca. Agronomy 2020, 10, 866. [Google Scholar] [CrossRef]
- Stirk, W.A.; Bálint, P.; Tarkowská, D.; Novák, O.; Maróti, G.; Ljung, K.; Turečková, V.; Strnad, M.; Ördög, V.; van Staden, J. Effect of light on growth and endogenous hormones in Chlorella minutissima (Trebouxiophyceae). Plant Physiol. Biochem. 2014, 79, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Bálint, P.; Tarkowská, D.; Novák, O.; Strnad, M.; Ördög, V.; van Staden, J. Hormone profiles in microalgae: Gibberellins and brassinosteroids. Plant Physiol. Biochem. 2013, 70, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Ördög, V.; Novák, O.; Rolčík, J.; Strnad, M.; Bálint, P.; van Staden, J. Auxin and cytokinin relationships in 24 microalgal strains. J. Phycol. 2013, 49, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Grob, K.; Zürcher, F. Stripping of trace organic substances from water: Equipment and procedure. J. Chromatogr. A 1976, 17, 285–294. [Google Scholar] [CrossRef]
- Boland, W.; Ney, P.; Jaenicke, L.; Gassmann, G. A “Closed-Loop-Stripping” Technique as a Versatile Tool for Metabolic Studies of Volatiles. In Analysis of Volatiles: Method, Application; Schreiber, P., Ed.; Walter de Gruyter GmbH: Berlin, Germany, 1984; pp. 371–380. [Google Scholar]
- Maier, I.; Müller, D.G.; Gassmann, G.; Boland, W.; Jaenicke, L. Sexual pheromones and related egg secretions in Laminariales (Phaeophyta). J. Nat. Sci. 1987, 42, 948–954. [Google Scholar] [CrossRef]
- Maier, I.; Clayton, M.N. Quantitative evaluation of erotactin secretion in eggs of Hormosira banksii (Fucales, Phaeophyceae). Bot. Acta 1993, 106, 344–349. [Google Scholar] [CrossRef]
- Akakabe, Y.; Kajiwara, T. Bioactive volatile compounds from marine algae: Feeding attractants. In Nineteenth International Seaweed Symposium: Proceedings of the 19th International Seaweed Symposium, Kobe, Japan, 26–31 March 2007; Borowitzka, M.A., Critchley, A.T., Kraan, S., Peters, A., Sjøtun, K., Notoya, M., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 211–214. [Google Scholar] [CrossRef]
- Redshaw, E.S.; Hougen, F.W.; Baker, R.J. Distillation technique for isolation of volatile materials for gas chromatographic analysis and its application to coriander seed (Coriandrum sativum). J. Agric. Food Chem. 1971, 19, 1264–1266. [Google Scholar] [CrossRef]
- Derenbach, J.B.; Pesando, D. Investigations into a small fraction of volatile hydrocarbons: III. Two diatom cultures produce ectocarpene, a pheromone of brown algae. Mar. Chem. 1986, 19, 337–341. [Google Scholar] [CrossRef]
- Weber, R.J.; Selander, E.; Sommer, U.; Viant, M.R. A stable-isotope mass spectrometry-based metabolic footprinting approach to analyze exudates from phytoplankton. Mar. Drugs 2013, 11, 4158–4175. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.Q.; Neish, I.; Khan, M.; Norrie, J.; Pereira, L.; Michalak, I.; Shukla, P.S.; Critchley, A.T. Extracts of seaweeds used as biostimulatns on land and sea crops—An efficacious, phyconomic, circular blue economy: With special reference to Ascophyllum (brown) and Kappaphycus (red) seaweeds. In Biostimulants for Crops from Seed Germination to Plant Development: A Practical Approach; Gupta, S., Van Staden, J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 263–288. [Google Scholar] [CrossRef]
- Sati, H.; Chokshi, K.; Soundarya, R.; Ghosh, A.; Mishra, S. Seaweed-based biostimulant improves photosynthesis and effectively enhances growth and biofuel potential of a green microalga Chlorella variabilis. Aquac. Int. 2021, 29, 963–975. [Google Scholar] [CrossRef]
- Pilar, G.J.; Olegario, B.R.; Rafael, R.R. Occurrence of jasmonates during cystocarp development in the red alga Grateloupia imbricata. J. Phycol. 2016, 52, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- García-Jiménez, P.; Robaina, R.R. Effects of ethylene on tetrasporogenesis in Pterocladiella capillacea (Rhodophyta). J. Phycol. 2012, 48, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Uji, T.; Endo, H.; Mizuta, H. Sexual reproduction via a 1-aminocyclopropane-1-carboxylic acid-dependent pathway through redox modulation in the marine red alga Pyropia yezoensis (Rhodophyta). Front. Plant Sci. 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, E.; Correa, J.A.; Contreras-Porcia, L. Role of abscisic acid (ABA) in activating antioxidant tolerance responses to desiccation stress in intertidal seaweed species. Planta 2016, 243, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Yokoya, N.S.; West, J.A.; Luchi, A.E. Effects of plant growth regulators on callus formation, growth and regeneration in axenic tissue cultures of Gracilaria tenuistipitata and Gracilaria perplexa (Gracilariales, Rhodophyta). Phycol. Res. 2004, 52, 244–254. [Google Scholar] [CrossRef]
- Yeong, H.Y.; Phang, S.M.; Reddy, C.R.K.; Khalid, N. Production of clonal planting materials from Gracilaria changii and Kappaphycus alvarezii through tissue culture and culture of G. changii explants in airlift photobioreactors. J. Appl. Phycol. 2014, 26, 729–746. [Google Scholar] [CrossRef]
- Kazi, M.A.; Singh, A.; Grewal, M.; Baraiya, M.; Goswami, S.; Rathore, M.S.; Jaiswar, S.; Mantri, V.A. Comparative evaluation of bio-effectors on survival and regeneration in Gracilaria dura (Rhodophyta). J. Appl. Phycol. 2022, 34, 3127–3139. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Cheney, D.P. Propagule production of Eucheuma denticulatum (Burman) Collins et Harvey by tissue culture. Bot. Mar. 2003, 46, 338–341. [Google Scholar] [CrossRef]
- Aguirre-Lipperheide, M.; Estrada-Rodriyuez, F.J.; Evans, L.V. Facts, problems, and needs in seaweed tissue culture: An appraisal. J. Phycol. 1995, 31, 677–688. [Google Scholar] [CrossRef]
- Patwary, M.U.; Van der Meer, J.P. Construction of backcrossed Gelidium male-sterile and male-fertile lines and their growth comparison. J. Appl. Phycol. 1997, 8, 483–486. [Google Scholar] [CrossRef]
- Luo, L.; Zuo, X.; Guo, L.; Pang, G.; Ma, Z.; Wu, M.; Chen, B. Effects of exogenous hormones on the regeneration of juveniles from Sargassum fusiforme holdfasts. Front. Mar. Sci. 2023, 9, 1072391. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Biter, A.B. Plantlet regeneration of Kappaphycus alvarezii var. adik-adik by tissue culture. J. Appl. Phycol. 2007, 19, 783–786. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Neish, I.C.; Critchley, A.T. Developments in production technology of Kappaphycus in the Philippines: More than four decades of farming. J. Appl. Phycol. 2015, 27, 1945–1961. [Google Scholar] [CrossRef]
- Souza, J.M.; Castro, J.Z.; Critchley, A.T.; Yokoya, N.S. Physiological responses of the red algae Gracilaria caudata (Gracilariales) and Laurencia catarinensis (Ceramiales) following treatment with a commercial extract of the brown alga Ascophyllum nodosum (AMPEP). J. Appl. Phycol. 2019, 31, 1883–1888. [Google Scholar] [CrossRef]
- Dawange, P.; Jaiswar, S. Effects of Ascophyllum marine plant extract powder (AMPEP) on tissue growth, proximate, phenolic contents, and free radical scavenging activities in endemic red seaweed Gracilaria corticata var. cylindrica from India. J. Appl. Phycol. 2020, 32, 4127–4135. [Google Scholar] [CrossRef]
- Panda, D.; Pramanik, K.; Nayak, B.R. Use of sea weed extracts as plant growth regulators for sustainable agriculture. Int. J. Bioresour. Stress Manag. 2012, 3, 404–411. [Google Scholar]
- Kozlova, T.A.; Hardy, B.P.; Krishna, P.; Levin, D.B. Effect of phytohormones on growth and accumulation of pigments and fatty acids in the microalgae Scenedesmus quadricauda. Algal Res. 2017, 27, 325–334. [Google Scholar] [CrossRef]
- Hunt, R.W.; Chinnasamy, S.; Bhatnagar, A.; Das, K.C. Effect of biochemical stimulants on biomass productivity and metabolite content of the microalga, Chlorella sorokiniana. Appl. Biochem. Biotechnol. 2010, 162, 2400–2414. [Google Scholar] [CrossRef]
- Han, X.; Zeng, H.; Bartocci, P.; Fantozzi, F.; Yan, Y. Phytohormones and effects on growth and metabolites of microalgae: A review. Fermentation 2018, 4, 25. [Google Scholar] [CrossRef]
- Motomura, T.; Sakai, Y. The occurrence of flagellated eggs in Laminaria angustata (Phaeophyta, Laminariales). J. Phycol. 1988, 24, 282–285. [Google Scholar] [CrossRef]
- Brawley, S.H.; Johnson, L.E. Gametogenesis, gametes and zygotes: An ecological perspective on sexual reproduction in the algae. Br. Phycol. J. 1992, 27, 233–252. [Google Scholar] [CrossRef]
- Moeys, S.; Frenkel, J.; Lembke, C.; Gillard, J.T.; Devos, V.; Van den Berge, K.; Bouillon, B.; Huysman, M.J.; De Decker, S.; Scharf, J.; et al. A sex-inducing pheromone triggers cell cycle arrest and mate attraction in the diatom Seminavis robusta. Sci. Rep. 2016, 6, 19252. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Jimenez, P.; Montero-Fernández, M.; Robaina, R.R. Analysis of ethylene-induced gene regulation during carposporogenesis in the red seaweed Grateloupia imbricata (Rhodophyta). J. Phycol. 2018, 54, 681–689. [Google Scholar] [CrossRef] [PubMed]
- El Shoubaky, G.A.; Salem, E.A. Effect of abiotic stress on endogenous phytohormones profile in some seaweeds. IJPPR 2016, 8, 124–134. [Google Scholar]
- Kothari, R.; Singh, H.M.; Azam, R.; Goria, K.; Bharti, A.; Singh, A.; Bajar, S.; Pathak, A.; Pandey, A.K.; Tyagi, V.V. Potential avenue of genetic engineered algal derived bioactive compounds: Influencing parameters, challenges and future prospects. Phytochem. Rev. 2023, 22, 935–968. [Google Scholar] [CrossRef]
- Foo, E.; Plett, J.M.; Lopez-Raez, J.A.; Reid, D. The role of plant hormones in plant-microbe symbioses. Front. Plant Sci. 2019, 10, 1391. [Google Scholar] [CrossRef]
- Singh, R.P.; Reddy, C.R.K. Seaweed-microbial interactions: Key functions of seaweed-associated bacteria. FEMS Microbiol. Ecol. 2014, 88, 213–230. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, B.; Ouyang, J.; Li, J.; Wang, Y. Arabidopsis indole synthase, a homolog of tryptophan synthase alpha, is an enzyme involved in the trp-independent indole-containing metabolite biosynthesis. J. Integr. Plant Biol. 2008, 50, 1070–1077. [Google Scholar] [CrossRef]
- Ljung, K.; Hull, A.K.; Kowalczyk, M.; Marchant, A.; Celenza, J.; Cohen, J.D.; Sandberg, G. Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Mol. Biol. 2002, 49, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Piotrowska-Niczyporuk, A. Synergistic effect of auxins and brassinosteroids on the growth and regulation of metabolite content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiol. Biochem. 2013, 71, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Schaller, F.; Schaller, A.; Stintzi, A. Biosynthesis and metabolism of jasmonates. J. Plant Growth Regul. 2004, 23, 179–199. [Google Scholar] [CrossRef]
- Vick, B.A.; Zimmerman, D.C. The biosynthesis of jasmonic acid: A physiological role for plant lipoxygenase. Biochem. Biophys. Res. Commun. 1983, 111, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Hamberg, M.; Hughes, M.A. Fatty acid allene oxides III Albumin-induced cyclization of 12, 13 (S)-epoxy-9 (Z), 11-octadecadienoic acid. Lipids 1988, 23, 469–475. [Google Scholar] [CrossRef]
- Stumpe, M.; Göbel, C.; Faltin, B.; Beike, A.K.; Hause, B.; Himmelsbach, K.; Bode, J.; Kramell, R.; Wasternack, C.; Frank, W.; et al. The moss Physcomitrella patens contains cyclopentenones but no jasmonates: Mutations in allene oxide cyclase lead to reduced fertility and altered sporophyte morphology. New Phytol. 2010, 188, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Gundlach, H.; Zenk, M.H. Biological activity and biosynthesis of pentacyclic oxylipins: The linoleic acid pathway. Phytochemistry 1998, 47, 527–537. [Google Scholar] [CrossRef]
- Collén, J.; Porcel, B.; Carré, W.; Ball, S.G.; Chaparro, C.; Tonon, T.; Barbeyron, T.; Michel, G.; Noel, B.; Valentin, K.; et al. Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proc. Natl. Acad. Sci. USA 2013, 110, 5247–5252. [Google Scholar] [CrossRef]
- Hamberg, M.; Gerwick, W.H. Biosynthesis of vicinal dihydroxy fatty acids in the red alga Gracilariopsis lemaneiformis: Identification of a sodium-dependent 12-lipoxygenase and a hydroperoxide isomerase. Arch. Biochem. Biophys. 1993, 305, 115–122. [Google Scholar] [CrossRef]
- Bagni, N.; Tassoni, A. Biosynthesis, oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids 2001, 20, 301–317. [Google Scholar] [CrossRef]
- Liu, J.H.; Kitashiba, H.; Wang, J.; Ban, Y.; Moriguchi, T. Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnol. 2007, 24, 117–126. [Google Scholar] [CrossRef]
- Kumar, A.; Taylor, M.; Altabella, T.; Tiburcio, A.F. Recent advances in polyamine research. Trends Plant Sci. 1997, 2, 124–130. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Yoneyama, K.; Kisugi, T.; Nomura, T.; Nakatani, Y.; Akiyama, K.; McErlean, C.S. Which are the major players, canonical or non-canonical strigolactones? J. Exp. Bot. 2018, 69, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Chesterfield, R. A Synthetic Biology Toolbox for Examining and Engineering Strigolactone Biosynthesis. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2020. [Google Scholar]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid metabolism in plants: The role of plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Wang, Z.Y. Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu. Rev. Plant Biol. 2010, 61, 681–704. [Google Scholar] [CrossRef]
- de Saint Germain, A.; Ligerot, Y.; Dun, E.A.; Pillot, J.P.; Ross, J.J.; Beveridge, C.A.; Rameau, C. Strigolactones stimulate internode elongation independently of gibberellins. Plant Physiol. 2013, 163, 1012–1025. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Li, S.S.; Han, G.Z. Insights into the origin and evolution of the plant hormone signaling machinery. Plant Physiol. 2015, 167, 872–886. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Yan, S.; Saleh, A.; Wang, W.; Ruble, J.; Oka, N.; Mohan, R.; Spoel, S.H.; Tada, Y.; Zheng, N.; et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 2012, 486, 228–232. [Google Scholar] [CrossRef]
- Kim, G.H.; Fritz, L.A. Signal glycoprotein with α-d-mannosyl residues is involved in the wound-healing response of Antithamnion sparsum (ceramiales, rhodophyta). J. Phycol. 1993, 29, 85–90. [Google Scholar] [CrossRef]
- Riad, N.; Reda Zahi, M.R.; Trovato, E.; Bouzidi, N.; Daghbouche, Y.; Utczás, M.; Mondello, L.; El Hattab, M. Chemical Screening and Antibacterial Activity of Essential Oil and Volatile Fraction of Dictyopteris polypodioides. Microchem. J. 2020, 152, 104415. [Google Scholar] [CrossRef]
- Zhao, B.; Li, J. Regulation of brassinosteroid biosynthesis and inactivation. J. Integr. Plant Biol. 2012, 54, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, S.; Yokota, T. Biosynthesis and metabolism of brassinosteroids. Annu. Rev. Plant Biol. 2003, 54, 137–164. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Borrego, E.; Kolomiets, M.V. Jasmonate biosynthesis, perception and function in plant development and stress responses. In Lipid Metabolism; Baez, R.V., Ed.; InTech: London, UK, 2013; pp. 393–442. [Google Scholar]
- León, J.; Sánchez-Serrano, J.J. Molecular biology of jasmonic acid biosynthesis in plants. Plant Physiol. Biochem. 1999, 37, 373–380. [Google Scholar] [CrossRef]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Bitrián, M.; Zarza, X.; Altabella, T.; Tiburcio, A.F.; Alcázar, R. Polyamines under abiotic stress: Metabolic crossroads and hormonal crosstalks in plants. Metabolites 2012, 2, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, V.V.; Shevyakova, N.I. Polyamines and stress tolerance of plants. Plant Stress 2007, 1, 50–71. [Google Scholar]
- Dempsey, D.M.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic acid biosynthesis and metabolism. Arab. Book Am. Soc. Plant Biol. 2011, 9, e0156. [Google Scholar] [CrossRef]
- Wani, K.I.; Chaudhary, S.; Zehra, A.; Naeem, M.; Aftab, T. Precise Role of Strigolactones and Its Crosstalk Mechanisms in Root Development. In Rhizobiology: Molecular Physiology of Plant Roots; Springer: Cham, Switzerland, 2021; pp. 253–270. [Google Scholar] [CrossRef]

| Category | Hormone Name | Name of Alga | Role | Reference |
|---|---|---|---|---|
| Auxin | Phenylacetic acid (PAA) | Ulva compressa (as Enteromorpha compressa) | Induces the thallus growth | [24] |
| Indole acetic acid (IAA) | Neoporphyra perforata (as Porphyra perforata) | Stimulates the growth and cell division | [25] | |
| Indole acetic acid (IAA) | Ulva lactuca | Induces the filamentous sporelings and promotes their growth | [26] | |
| Indole acetic acid (IAA) | Chara zeylanica | Antagonistic effect, inhibits gibberellic acid and its stimulatory effect on the growth | [27] | |
| Indole-3-acetic acid (IAA) | Caulerpa paspaloides | Enhanced initiation of leaf-like structures | [28] | |
| Indole acetic acid (IAA) | Fucus spiralis | Tissue differentiation | [29] | |
| Indole acetic acid (IAA) | Tretraselmis sps. | |||
| Cytokinin | Isoprenoid and aromatic cytokinins | Cladophora capensis | Growth and morphogenesis | [30] |
| Ulva sp. | ||||
| Caulerpa tongaensis (as Caulerpa filiformis) | ||||
| Halimeda cuneata | ||||
| Brassicophycus sisymbrioides (as Bifurcaria brassicaeformis) | ||||
| Ecklonia maxima | ||||
| Laminaria pallida | ||||
| Macrocystis pyrifera (as Macrocystis angustifolia) | ||||
| Splachnidium rugosum | ||||
| Dictyota sp. | ||||
| Sargassum incisifolium (as Sargassum heterophyllum) | ||||
| Pachymenia orbitosa (as Aeodes orbitosa) | ||||
| Gigartina bracteata (as Gigartina clathrata) | ||||
| Gigartina polycarpa | ||||
| Sarcothalia scutellata | ||||
| Hymenena venosa | ||||
| Hypnea spicifera | ||||
| Mazzaella capensis | ||||
| Nothogenia erinacea | ||||
| Plocamium corallorhiza | ||||
| Carradoeriella virgata | ||||
| Porphyra capensis | ||||
| Sarcothalia stiriata | ||||
| Gelidium vittatum (as Suhria vittata) | ||||
| Amphiroa bowerbankii | ||||
| Amphiroa ephedraea | ||||
| Arthrocardia sp. | ||||
| Cheilosporum sp. | ||||
| Corallina sp. | ||||
| Jania sp. | ||||
| Kinetin | Sphacelaria rigidula (as Sphacelaria furcigera) | Increases the length of lateral branches | [31] | |
| Kinetin | Kappaphycus alvarezii | Promotes cell division | [32] | |
| Zeatin | Kappaphycus alvarezii | |||
| Sargassum tenerrimum | ||||
| Hydropuntia edulis (as Gracilaria edulis) | ||||
| Isopentenyladenine | Sargassum muticum | Early growth of the receptacles | [33] | |
| cis-zeatin riboside | ||||
| Isopentenyladenine | Neoporphyra perforata (as Porphyra perforata) | Helps in maturation of spermatangia and carpogonia | ||
| cis-zeatin riboside | ||||
| Cytokinin | Fucus vesiculosus | Regeneration and morphogenesis | [34] | |
| Gibberellic acid | Gibberellin (GA1/GA3) | Fucus spiralis | Tissue differentiation | [29] |
| Gibberellin (GA4/GA7) | Tretraselmis sps. | |||
| Gibberellin (GA3) | Chara vulgaris | Promotes the number of antheridial filaments and spermatids | [35] | |
| Gibberellin (GA3) | Fucus vesiculosus | Increases adventitious branches | [34] | |
| Abscisic acid | Abscisic acid (ABA) | Ulva lactuca/Ulva linza (as Ulva fasciata) | Releases in extreme conditions and inhibits growth | [36] |
| Dictyota humifusa | ||||
| Abscisic acid (ABA) | Phycocalidia acanthophora (as Porphyra acanthophora (red)) | Releases in stress conditions and inhibits growth | [37] | |
| Phycocalidia acanthophora (as Porphyra acanthophora) | ||||
| Gelidium floridanum | ||||
| Crassiphycus birdiae (as Gracilaria birdiae) | ||||
| Gracilaria cervicornis | ||||
| Gracilariopsis tenuifrons | ||||
| Chondracanthus teedei | ||||
| Hypnea nigrescens | ||||
| Hypnea musciformis (brown) | ||||
| Hypnea musciformis (green) | ||||
| Dunaliella parva | Releases during salinity stress, causes reduction in growth, and in some cases promotes senescence | [38] | ||
| Draparnaldia mutabilis | ||||
| Dunaliella acidophila | Reduction in growth and photosynthesis with increase in pH | |||
| Rhodomorphin | Rhodomorphin | Griffithsia pacifica | Repairs shoot cell and normal regeneration | [39] |
| Anotrichium tenue | ||||
| Antithamnion kylinii | ||||
| Rhodomorphin (as glycoprotein) | Volvox carteri | Sexual hormone | [40] | |
| Ethylene | 1-aminocyclopropane-l-carboxylicacid (ACC) (Ethylene precursor) | Neoporphyra perforata (as Porphyra perforata) | Stimulates cell division and apical cap development | [25] |
| Ethylene | Acetabularia acetabulum (as Acetabularia mediterranea) | Influences the developmental pattern of cap | [41] | |
| Brassinosteroids | Brassinolide (BR) | Ecklonia maxima | Improves stress response to various biotic and abiotic stresses | [42] |
| Castasterone (CS) | ||||
| Jasmonic acid | Jasmonate | Scenedesmus incrassulatus | Provides tolerance to the temperatures and infections stress | [43] |
| Methyl jasmonate | ||||
| Jasmonic acid and its derivatives | Chondrus crispus | Induces defense reaction | [44] | |
| Polyamines | Polyamines | Chlorella vulgaris | Enhances cell division, DNA replication, and autospore release | [45] |
| Polyamines | Chlamydomonas reinhardtii | Enhances cell division | [46] | |
| Putrescine | Ulva lactuca (as Ulva fasciata) | Releases during hyposaline stress causes and decreases the chlorophyll and growth rate | [47] | |
| Spermidine | ||||
| Putrescine | Grateloupia doryphora | Releases during hyposaline shock and helps in physiological performance during acclimation by increasing photosynthetic rate | [48] | |
| Spermidine | ||||
| Spermine | ||||
| Polyamines | Grateloupia doryphora | Enhances cell division, elongation, and morphogenesis | [49,50] | |
| Putrescine | Grateloupia sp. | Induction of cystocarp, development, and release | [51] | |
| Spermidine | ||||
| Spermine | ||||
| Putrescine | Gracilaria cornea (as Crassiphycus corneus) | Promotes cystocarp maturation and liberation and develops cell masses | [52,53] | |
| Salicylic acid | Salicylic acid | Saccharina japonica (as Laminaria japonica) | Imparts thermotolerance | [54] |
| Strigolactone | Strigolactone | Chara coralina | Stimulates rhizoid elongation | [23] |
| Class | Name of Pheromone | Name of Alga | Role | Reference |
|---|---|---|---|---|
| Chlorophyceae | Sporulation inhibitor-1a (Glycoprotein) | Ulva compressa (as Ulva mutabilis) | Suppresses gametogenesis | [60] |
| Swarming inhibitor | Ulva compressa (as Ulva mutabilis) | Inhibits gamete swarming | ||
| Sporulation inhibitor-2 (Non-protein) | Ulva compressa (as Ulva mutabilis) | Suppresses gametogenesis | ||
| Phaeophyceae | Ectocarpene (S(+)l-cis-buten-l-yl-cyclohepta-2,5-diene) | Ectocarpus siliculosus | Releases female gamete and helps in attracting male gametes | [61] |
| Sphacelaria rigidula | Acts as a chemoattractant | [62] | ||
| Adenocystis longissima (as Adenocystis utricularis) | [63] | |||
| Dictyotene (C11 metabolite) | Dictyopteris polypodioides (as Dictyopteris membranacea) | Helps in gamete attraction and acts as a deterrent to mesograzers | [64] | |
| Dictyota dichotoma | ||||
| Dictyota diemensis | ||||
| Sargassum filipendula | ||||
| C11 sulphur metabolite | Dictyopteris polypodioides (as Dictyopteris membranacea) | |||
| Dictyotene (C11 metabolite) | Dictyota dimensis | Acts as a sperm attractant | [65] | |
| Diterpene alcohols | Dictyota dichotoma | Acts as a deterrent to herbivores | [66] | |
| C11 hydrocarbons | Dictyopteris delicatula | [67] | ||
| Thiopyranone (Thiopyran-4-one) | Dictyopteris polypodioides (as Dictyopteris membranacea) | Acts as a deterrent to herbivores | [68] | |
| Dithiepanone (Dithiepan-5-one) | ||||
| Multifidene | Halosiphon tomentosus (as Chorda tomentosa) | Acts as a chemoattractant | [69] | |
| Ectocarpene | ||||
| Dictyopterene | ||||
| Viridiene | ||||
| Fucoserratene (1,3-trans-5-cis-octatriene) | Fucus serratus | Acts as a chemoattractant | [70] | |
| Fucus vesiculosus | [71] | |||
| Fucus spiralis | ||||
| Finavarrene | Ascophyllum nodosum | Acts as a chemoattractant | [71] | |
| Cystophorene | Cystophora siliquosa | Acts as a chemoattractant | [71] | |
| Hormosirene | Hormosira banksii | Acts as a chemoattractant | [71] | |
| Xiphophora chondrophylla | ||||
| Xiphophora gladiate | ||||
| Durvillaea potatorum | ||||
| Durvillaea antarctica | ||||
| Durvillaea willana | ||||
| Colpomenia peregrina | [72] | |||
| Planosiphon complanatus (as Scytosiphon lomentaria) | ||||
| Analipus japonicus | [73] | |||
| Ectocarpene | Ectocarpus flagelliformis (as Ectocarpus fasciculatus) | Acts as a chemoattractant | [71] | |
| Adenocystis longissima (as Adenocystis utricularis) | ||||
| Sphacelaria rigidula | ||||
| Analipus japonicus | [73] | |||
| Dictyopterene C′ | Dictyota dichotoma | Acts as a chemoattractant | [71] | |
| Dictyotene | Dictyota diemensis | Acts as an erotactin | [74] | |
| Desmarcstene | Desmarestia aculeata/Desmarestia menziesii (as Desmarestia aculeata) | Acts as a chemoattractant | [71] | |
| Desmarestia confervoides (as Desmarestia viridis) | ||||
| Cladostephus hirsutus/Cladostephus kuetzingii (as Cladostephus spongiosus) | ||||
| Lamoxirene | Laminariaceae | Acts as a chemoattractant | [71] | |
| Alariaceae | ||||
| Lessoniaceae | ||||
| Pleurophycus gardneri | Acts as an erotactin | [74] | ||
| Agarum clathratum (as Agarum cribrosum) | ||||
| Sachharina gyrata (as Kjellmaniella gyrata) | ||||
| Hedophyllum sessile | ||||
| Cymathaere triplicate | ||||
| Undaria pinnatifida | ||||
| Pterygophora californica | ||||
| Eisenia bicyclis (as Eisenia arborea) | ||||
| Ecklonia biruncinata (as Ecklonia radiata) | ||||
| Macrocystis pyrifera | ||||
| Macrocystis pyrifera (as Macrocystis integrifolia) | ||||
| Nereocystis luetkeana | ||||
| Pelagophycus porra | ||||
| Dictyoneurum reticulatum (as Dictyoneuropsis reticulata) | ||||
| Lessoniopsis littoralis | ||||
| Multifidene cis-4-vinyl-5-cis- buten-l-yl-cyclopentene) | Cutleria multifida | Responsible for chemotaxis of the male microgametes | [75] | |
| Zonaria angustata | Acts as a chemoattractant | [73] | ||
| Viridiene | Microzonia phinneyi (as Syringoderma phinneyi) | [71] | ||
| Caudoxirene | Perithalia caudata | |||
| Giffordene | Feldmannia mirchelliae/Hincksia mitchelliae (as Giffordia mitchelliae) | [72] | ||
| Rhodophyceae | Ochtodene (Monoterpene) | Ochtodes secundiramea | Acts as a deterrent to herbivores | [76] |
| Octodiol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathod, S.G.; Bhushan, S.; Mantri, V.A. Phytohormones and Pheromones in the Phycology Literature: Benchmarking of Data-Set and Developing Critical Tools of Biotechnological Implications for Commercial Aquaculture Industry. Phycology 2024, 4, 1-36. https://doi.org/10.3390/phycology4010001
Rathod SG, Bhushan S, Mantri VA. Phytohormones and Pheromones in the Phycology Literature: Benchmarking of Data-Set and Developing Critical Tools of Biotechnological Implications for Commercial Aquaculture Industry. Phycology. 2024; 4(1):1-36. https://doi.org/10.3390/phycology4010001
Chicago/Turabian StyleRathod, Sachin G., Satej Bhushan, and Vaibhav A. Mantri. 2024. "Phytohormones and Pheromones in the Phycology Literature: Benchmarking of Data-Set and Developing Critical Tools of Biotechnological Implications for Commercial Aquaculture Industry" Phycology 4, no. 1: 1-36. https://doi.org/10.3390/phycology4010001
APA StyleRathod, S. G., Bhushan, S., & Mantri, V. A. (2024). Phytohormones and Pheromones in the Phycology Literature: Benchmarking of Data-Set and Developing Critical Tools of Biotechnological Implications for Commercial Aquaculture Industry. Phycology, 4(1), 1-36. https://doi.org/10.3390/phycology4010001









