Exploration of Microalgae-Activated Sludge Growth Performance in Lab-Scale Photobioreactors under Outdoor Environmental Conditions for Wastewater Biotreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Anaerobically Digested Municipal Wastewater
2.2. Microorganisms and Culture Condition
2.3. Experimental Setup
2.4. Ambient Temperature and Light Intensity for MAS Cultivation
2.5. Analytical Methods
2.6. Statistical Analysis
3. Results and Discussion
3.1. Outdoor Temperature and Light Intensity: Potential for Biomass Growth
3.2. Operational and Environmental Conditions
3.3. Effect of Inoculum Concentrations on Total Biomass Productivity
3.4. Effect of Inoculum Concentrations on Nutrient Removal
3.5. Assessment of Total Coliforms and Escherichia coli Removal
3.6. MAS Inoculum Concentration for Wastewater Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Least Significant Post Hoc Test for Total Biomass Productivity Estimates, and Percentage of Total Dissolved Phosphorus and Nitrogen Uptake
| LSD | Mean Difference (I–J) | Std. Error | Sig. | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Conditions | Lower Bound | Upper Bound | ||||
| con_1 | con_2 | −0.09517 * | 0.02806 | 0.001 | −0.1508 | −0.0395 |
| con_3 | −0.13906 * | 0.02806 | 0.000 | −0.1947 | −0.0834 | |
| con_4 | 0.05206 | 0.02806 | 0.066 | −0.0036 | 0.1077 | |
| con_5 | 0.01828 | 0.02806 | 0.516 | −0.0374 | 0.0739 | |
| con_6 | −0.02783 | 0.02806 | 0.324 | −0.0835 | 0.0278 | |
| con_2 | con_1 | 0.09517 * | 0.02806 | 0.001 | 0.0395 | 0.1508 |
| con_3 | −0.04389 | 0.02806 | 0.121 | −0.0995 | 0.0118 | |
| con_4 | 0.14722 * | 0.02806 | 0.000 | 0.0916 | 0.2029 | |
| con_5 | 0.11344 * | 0.02806 | 0.000 | 0.0578 | 0.1691 | |
| con_6 | 0.06733 * | 0.02806 | 0.018 | 0.0117 | 0.1230 | |
| con_3 | con_1 | 0.13906 * | 0.02806 | 0.000 | 0.0834 | 0.1947 |
| con_2 | 0.04389 | 0.02806 | 0.121 | −0.0118 | 0.0995 | |
| con_4 | 0.19111 * | 0.02806 | 0.000 | 0.1355 | 0.2468 | |
| con_5 | 0.15733 * | 0.02806 | 0.000 | 0.1017 | 0.2130 | |
| con_6 | 0.11122 * | 0.02806 | 0.000 | 0.0556 | 0.1669 | |
| con_4 | con_1 | −0.05206 | 0.02806 | 0.066 | −0.1077 | 0.0036 |
| con_2 | −0.14722 * | 0.02806 | 0.000 | −0.2029 | −0.0916 | |
| con_3 | −0.19111 * | 0.02806 | 0.000 | −0.2468 | −0.1355 | |
| con_5 | −0.03378 | 0.02806 | 0.231 | −0.0894 | 0.0219 | |
| con_6 | −0.07989 * | 0.02806 | 0.005 | −0.1355 | −0.0242 | |
| con_5 | con_1 | −0.01828 | 0.02806 | 0.516 | −0.0739 | 0.0374 |
| con_2 | −0.11344 * | 0.02806 | 0.000 | −0.1691 | −0.0578 | |
| con_3 | −0.15733 * | 0.02806 | 0.000 | −0.2130 | −0.1017 | |
| con_4 | 0.03378 | 0.02806 | 0.231 | −0.0219 | 0.0894 | |
| con_6 | −0.04611 | 0.02806 | 0.103 | −0.1018 | 0.0095 | |
| con_6 | con_1 | 0.02783 | 0.02806 | 0.324 | −0.0278 | 0.0835 |
| con_2 | −0.06733 * | 0.02806 | 0.018 | −0.1230 | −0.0117 | |
| con_3 | −0.11122 * | 0.02806 | 0.000 | −0.1669 | −0.0556 | |
| con_4 | 0.07989 * | 0.02806 | 0.005 | 0.0242 | 0.1355 | |
| con_5 | 0.04611 | 0.02806 | 0.103 | −0.0095 | 0.1018 | |
| LSD | Mean Difference (I–J) | Std. Error | Sig. | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Conditions | Lower Bound | Upper Bound | ||||
| con_1 | con_2 | 0.53267 * | 0.10011 | 0.000 | 0.3145 | 0.7508 |
| con_3 | 0.67200 * | 0.10011 | 0.000 | 0.4539 | 0.8901 | |
| con_4 | 0.09300 | 0.10011 | 0.371 | −0.1251 | 0.3111 | |
| con_5 | −0.12733 | 0.10011 | 0.227 | −0.3455 | 0.0908 | |
| con_6 | −0.21867 * | 0.10011 | 0.050 | −0.4368 | −0.0005 | |
| con_2 | con_1 | −0.53267 * | 0.10011 | 0.000 | −0.7508 | −0.3145 |
| con_3 | 0.13933 | 0.10011 | 0.189 | −0.0788 | 0.3575 | |
| con_4 | −0.43967 * | 0.10011 | 0.001 | −0.6578 | −0.2215 | |
| con_5 | −0.66000 * | 0.10011 | 0.000 | −0.8781 | −0.4419 | |
| con_6 | −0.75133 * | 0.10011 | 0.000 | −0.9695 | −0.5332 | |
| con_3 | con_1 | −0.67200 * | 0.10011 | 0.000 | −0.8901 | −0.4539 |
| con_2 | −0.13933 | 0.10011 | 0.189 | −0.3575 | 0.0788 | |
| con_4 | −0.57900 * | 0.10011 | 0.000 | −0.7971 | −0.3609 | |
| con_5 | −0.79933 * | 0.10011 | 0.000 | −1.0175 | −0.5812 | |
| con_6 | −0.89067 * | 0.10011 | 0.000 | −1.1088 | −0.6725 | |
| con_4 | con_1 | −0.09300 | 0.10011 | 0.371 | −0.3111 | 0.1251 |
| con_2 | 0.43967 * | 0.10011 | 0.001 | 0.2215 | 0.6578 | |
| con_3 | 0.57900 * | 0.10011 | 0.000 | 0.3609 | 0.7971 | |
| con_5 | −0.22033 * | 0.10011 | 0.048 | −0.4385 | −0.0022 | |
| con_6 | −0.31167 * | 0.10011 | 0.009 | −0.5298 | −0.0935 | |
| con_5 | con_1 | 0.12733 | 0.10011 | 0.227 | −0.0908 | 0.3455 |
| con_2 | 0.66000 * | 0.10011 | 0.000 | 0.4419 | 0.8781 | |
| con_3 | 0.79933 * | 0.10011 | 0.000 | 0.5812 | 1.0175 | |
| con_4 | 0.22033 * | 0.10011 | 0.048 | 0.0022 | 0.4385 | |
| con_6 | −0.09133 | 0.10011 | 0.380 | −0.3095 | 0.1268 | |
| con_6 | con_1 | 0.21867 * | 0.10011 | 0.050 | 0.0005 | 0.4368 |
| con_2 | 0.75133 * | 0.10011 | 0.000 | 0.5332 | 0.9695 | |
| con_3 | 0.89067 * | 0.10011 | 0.000 | 0.6725 | 1.1088 | |
| con_4 | 0.31167 * | 0.10011 | 0.009 | 0.0935 | 0.5298 | |
| con_5 | 0.09133 | 0.10011 | 0.380 | −0.1268 | 0.3095 | |
| LSD | Mean Difference (I–J) | Std. Error | Sig. | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Conditions | Lower Bound | Upper Bound | ||||
| con_1 | con_2 | 44.42500 * | 7.76082 | 0.000 | 27.5156 | 61.3344 |
| con_3 | 128.58200 * | 7.76082 | 0.000 | 111.6726 | 145.4914 | |
| con_4 | 1.13100 | 7.76082 | 0.887 | −15.7784 | 18.0404 | |
| con_5 | −0.41200 | 7.76082 | 0.959 | −17.3214 | 16.4974 | |
| con_6 | −7.20667 | 7.76082 | 0.371 | −24.1160 | 9.7027 | |
| con_2 | con_1 | −44.42500* | 7.76082 | 0.000 | −61.3344 | −27.5156 |
| con_3 | 84.15700 * | 7.76082 | 0.000 | 67.2476 | 101.0664 | |
| con_4 | −43.29400 * | 7.76082 | 0.000 | −60.2034 | −26.3846 | |
| con_5 | −44.83700 * | 7.76082 | 0.000 | −61.7464 | −27.9276 | |
| con_6 | −51.63167 * | 7.76082 | 0.000 | −68.5410 | −34.7223 | |
| con_3 | con_1 | −128.58200 * | 7.76082 | 0.000 | −145.4914 | −111.6726 |
| con_2 | −84.15700 * | 7.76082 | 0.000 | −101.0664 | −67.2476 | |
| con_4 | −127.45100 * | 7.76082 | 0.000 | −144.3604 | −110.5416 | |
| con_5 | −128.99400 * | 7.76082 | 0.000 | −145.9034 | −112.0846 | |
| con_6 | −135.78867 * | 7.76082 | 0.000 | −152.6980 | −118.8793 | |
| con_4 | con_1 | −1.13100 | 7.76082 | 0.887 | −18.0404 | 15.7784 |
| con_2 | 43.29400 * | 7.76082 | 0.000 | 26.3846 | 60.2034 | |
| con_3 | 127.45100 * | 7.76082 | 0.000 | 110.5416 | 144.3604 | |
| con_5 | −1.54300 | 7.76082 | 0.846 | −18.4524 | 15.3664 | |
| con_6 | −8.33767 | 7.76082 | 0.304 | −25.2470 | 8.5717 | |
| con_5 | con_1 | 0.41200 | 7.76082 | 0.959 | −16.4974 | 17.3214 |
| con_2 | 44.83700 * | 7.76082 | 0.000 | 27.9276 | 61.7464 | |
| con_3 | 128.99400 * | 7.76082 | 0.000 | 112.0846 | 145.9034 | |
| con_4 | 1.54300 | 7.76082 | 0.846 | −15.3664 | 18.4524 | |
| con_6 | −6.79467 | 7.76082 | 0.398 | −23.7040 | 10.1147 | |
| con_6 | con_1 | 7.20667 | 7.76082 | 0.371 | −9.7027 | 24.1160 |
| con_2 | 51.63167 * | 7.76082 | 0.000 | 34.7223 | 68.5410 | |
| con_3 | 135.78867 * | 7.76082 | 0.000 | 118.8793 | 152.6980 | |
| con_4 | 8.33767 | 7.76082 | 0.304 | −8.5717 | 25.2470 | |
| con_5 | 6.79467 | 7.76082 | 0.398 | −10.1147 | 23.7040 | |
| LSD | Mean Difference (I–J) | Std. Error | Sig. | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Conditions | Lower Bound | Upper Bound | ||||
| con_1 | con_2 | 49.71667 * | 6.95668 | 0.000 | 34.5594 | 64.8740 |
| con_3 | 129.06000 * | 6.95668 | 0.000 | 113.9027 | 144.2173 | |
| con_4 | 22.91333 * | 6.95668 | 0.006 | 7.7560 | 38.0706 | |
| con_5 | 7.83333 | 6.95668 | 0.282 | −7.3240 | 22.9906 | |
| con_6 | 5.57000 | 6.95668 | 0.439 | −9.5873 | 20.7273 | |
| con_2 | con_1 | −49.71667 * | 6.95668 | 0.000 | −64.8740 | −34.5594 |
| con_3 | 79.34333 * | 6.95668 | 0.000 | 64.1860 | 94.5006 | |
| con_4 | −26.80333 * | 6.95668 | 0.002 | −41.9606 | −11.6460 | |
| con_5 | −41.88333 * | 6.95668 | 0.000 | −57.0406 | −26.7260 | |
| con_6 | −44.14667 * | 6.95668 | 0.000 | −59.3040 | −28.9894 | |
| con_3 | con_1 | −129.06000 * | 6.95668 | 0.000 | −144.2173 | −113.9027 |
| con_2 | −79.34333 * | 6.95668 | 0.000 | −94.5006 | −64.1860 | |
| con_4 | −106.14667 * | 6.95668 | 0.000 | −121.3040 | −90.9894 | |
| con_5 | −121.22667 * | 6.95668 | 0.000 | −136.3840 | −106.0694 | |
| con_6 | −123.49000 * | 6.95668 | 0.000 | −138.6473 | −108.3327 | |
| con_4 | con_1 | −22.91333 * | 6.95668 | 0.006 | −38.0706 | −7.7560 |
| con_2 | 26.80333 * | 6.95668 | 0.002 | 11.6460 | 41.9606 | |
| con_3 | 106.14667 * | 6.95668 | 0.000 | 90.9894 | 121.3040 | |
| con_5 | −15.08000 | 6.95668 | 0.051 | −30.2373 | 0.0773 | |
| con_6 | −17.34333 * | 6.95668 | 0.028 | −32.5006 | −2.1860 | |
| con_5 | con_1 | −7.83333 | 6.95668 | 0.282 | −22.9906 | 7.3240 |
| con_2 | 41.88333 * | 6.95668 | 0.000 | 26.7260 | 57.0406 | |
| con_3 | 121.22667 * | 6.95668 | 0.000 | 106.0694 | 136.3840 | |
| con_4 | 15.08000 | 6.95668 | 0.051 | −0.0773 | 30.2373 | |
| con_6 | −2.26333 | 6.95668 | 0.751 | −17.4206 | 12.8940 | |
| con_6 | con_1 | −5.57000 | 6.95668 | 0.439 | −20.7273 | 9.5873 |
| con_2 | 44.14667 * | 6.95668 | 0.000 | 28.9894 | 59.3040 | |
| con_3 | 123.49000 * | 6.95668 | 0.000 | 108.3327 | 138.6473 | |
| con_4 | 17.34333 * | 6.95668 | 0.028 | 2.1860 | 32.5006 | |
| con_5 | 2.26333 | 6.95668 | 0.751 | −12.8940 | 17.4206 | |
References
- Oviedo, J.A.; Muñoz, R.; Donoso-Bravo, A.; Bernard, O.; Casagli, F.; Jeison, D. A half-century of research on microalgae-bacteria for wastewater treatment. Algal Res. 2022, 67, 102828. [Google Scholar] [CrossRef]
- Routley, N. Visualized: The World’s Population at 8 Billion. 2022. Available online: https://www.visualcapitalist.com/visualized-the-worlds-population-at-8-billion/ (accessed on 30 September 2022).
- Aradhana, K.M.; Kumar, M. Microalgal Green and Clean Approach to Mitigate Water Pollution. In Environment Conservation Challenges, Threats I Conservation of Biodiversity; Barwant, M.M., Manam, V.K., Eds.; Scieng Publications: Tamilnadu, India, 2015; Volume 1, pp. 96–104. [Google Scholar]
- Zhu, S.; Huo, S.; Feng, P. Developing Designer Microalgal Consortia: A Suitable Approach to Sustainable Wastewater Treatment. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Alam, M.A., Wang, Z., Eds.; Springer: Singapore, 2019; pp. 569–598. [Google Scholar] [CrossRef]
- Pompei, C.M.E.; Campos, L.C.; Vieira, E.M.; Tucci, A. The impact of micropollutants on native algae and cyanobacteria communities in ecological filters during drinking water treatment. Sci. Total Environ. 2022, 822, 153401. [Google Scholar] [CrossRef]
- Bankole, A.O.; James, A.O.; Odjegba, E.E.; Bankole, A.R.; Emmanuel, B.I.; Fiore, F.A.; Pu, J.H.; Moruzzi, R.B. Factors affecting sanitation coverage in three income levels and potential toward achieving SDG 6.2. Water Policy 2023, 25, 146–176. [Google Scholar] [CrossRef]
- Yan, H.; Lu, R.; Liu, Y.; Cui, X.; Wang, Y.; Yu, Z.; Ruan, R.; Zhang, Q. Development of microalgae-bacteria symbiosis system for enhanced treatment of biogas slurry. Bioresour. Technol. 2022, 354, 127187. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Ho, S.-H. Converting nitrogen and phosphorus wastewater into bioenergy using microalgae-bacteria consortia: A critical review. Bioresour. Technol. 2021, 342, 126056. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Lei, Y.; Li, X.; Nagarajan, D.; Lee, D.-J.; Chang, J.-S. Bioremediation of sulfonamides by a microalgae-bacteria consortium Analysis of pollutants removal efficiency, cellular composition, and bacterial community. Bioresour. Technol. 2022, 351, 126964. [Google Scholar] [CrossRef]
- Farias, S.L.; Ruas, G.; Serejo, M.L.; Boncz, M. Evaluation of the effect of the feeding regime on the removal of metals and pathogens in microalgae–bacterial systems. Water Sci. Technol. 2023, 88, 11–22. [Google Scholar] [CrossRef]
- Serejo, M.L.; Farias, S.L.; Ruas, G.; Paulo, P.L.; Boncz, M.A. Surfactant removal and biomass production in a microalgal-bacterial process: Effect of feeding regime. Water Sci. Technol. 2020, 82, 1176–1183. [Google Scholar] [CrossRef]
- Qiao, S.; Hou, C.; Wang, X.; Zhou, J. Minimizing greenhouse gas emission from wastewater treatment process by integrating activated sludge and microalgae processes. Sci. Total Environ. 2020, 732, 139032. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Nguyen, T.-T.; Binh, Q.A.; Bui, X.-T.; Ngo, H.H.; Vo, H.N.P.; Lin, K.-Y.A.; Vo, T.-D.; Guo, W.; Lin, C.; et al. Co-culture of microalgae-activated sludge for wastewater treatment and biomass production: Exploring their role under different inoculation ratios. Bioresour. Technol. 2020, 314, 123754. [Google Scholar] [CrossRef]
- Su, Y.; Mennerich, A.; Urban, B. Synergistic cooperation between wastewater-born algae and activated sludge for wastewater treatment: Influence of algae and sludge inoculation ratios. Bioresour. Technol. 2012, 105, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.; Brucato, A.; Caputo, G.; Grisafi, F.; Scargiali, F. Inoculum of indigenous microalgae/activated sludge for optimal treatment of municipal wastewaters and biochemical composition of residual biomass for potential applications. J. Water Process. Eng. 2022, 49, 103142. [Google Scholar] [CrossRef]
- Huang, K.-X.; Vadiveloo, A.; Zhou, J.-L.; Yang, L.; Chen, D.-Z.; Gao, F. Integrated culture and harvest systems for improved microalgal biomass production and wastewater treatment. Bioresour. Technol. 2023, 376, 128941. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Tian, J.; Pan, Z.; Chen, Y.; Ming, F.; Wang, R.; Wang, L.; Zhou, H.; Li, J.; et al. Co-cultivation of microalgae-activated sludge for municipal wastewater treatment: Exploring the performance, microbial co-occurrence patterns, microbiota dynamics and function during the startup stage. Bioresour. Technol. 2023, 374, 128733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lei, Z.; Liu, Y. Microalgal-bacterial granular sludge for municipal wastewater treatment: From concept to practice. Bioresour. Technol. 2022, 354, 127201. [Google Scholar] [CrossRef]
- Soroosh, H.; Otterpohl, R.; Hanelt, D. Influence of hydraulic retention time on municipal wastewater treatment using microalgae-bacteria flocs in sequencing batch reactors. Bioresour. Technol. Rep. 2022, 17, 100884. [Google Scholar] [CrossRef]
- Ji, B.; Wang, S.; Silva, M.R.U.; Zhang, M.; Liu, Y. Microalgal-bacterial granular sludge for municipal wastewater treatment under simulated natural diel cycles: Performances-metabolic pathways-microbial community nexus. Algal Res. 2021, 54, 102198. [Google Scholar] [CrossRef]
- Xu, K.; Zou, X.; Xue, Y.; Qu, Y.; Li, Y. The impact of seasonal variations about temperature and photoperiod on the treatment of municipal wastewater by algae-bacteria system in lab-scale. Algal Res. 2021, 54, 102175. [Google Scholar] [CrossRef]
- Liyun, C. Influence of inoculation ratio on the performance and microbial community of bacterial-algal symbiotic system for rural wastewater treatment. Water Environ. Res. 2023, 95, e10838. [Google Scholar] [CrossRef]
- Marazzi, F.; Bellucci, M.; Rossi, S.; Fornaroli, R.; Ficara, E.; Mezzanotte, V. Outdoor pilot trial integrating a sidestream microalgae process for the treatment of centrate under non optimal climate conditions. Algal Res. 2019, 39, 101430. [Google Scholar] [CrossRef]
- Vassalle, L.; García-Galán, M.J.; Aquino, S.F.; Afonso, R.J.d.C.F.; Ferrer, I.; Passos, F.; Mota, C.R. Can high rate algal ponds be used as post-treatment of UASB reactors to remove micropollutants? Chemosphere 2020, 248, 125969. [Google Scholar] [CrossRef] [PubMed]
- Prado, L.d.O.; Bolzani, H.R.; Souza, H.H.d.S.; Ruas, G.; da Silva, G.H.R. Microalgal cultivation in open and closed systems under a tropical climate: A life cycle comparison. J. Clean. Prod. 2023, 422, 138631. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of Photobioreactors for Mass Cultivation of Photosynthetic Organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Von Sperling, M. Wastewater Characteristics, Treatment and Disposal, 1st ed.; IWA Publishing: London, UK, 2007. [Google Scholar]
- Chernicharo, C.A. Biological Wastewater Treatment Series, Anaerobic Reactors, Volume 4. IWA Publishing. 2007. Available online: http://iwaponline.com/ebooks/book-pdf/1100/wio9781780402116.pdf (accessed on 25 September 2023).
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2023. Available online: https://www.algaebase.org (accessed on 20 August 2023).
- Bicudo, C.; Meneses, M. Algae Genera from Brazilian Inland Waters: Key to Identification and Description, 2nd ed.; Editora Rima: São Carlos, Brazil, 2006. [Google Scholar]
- Mathew, M.M.; Khatana, K.; Vats, V.; Dhanker, R.; Kumar, R.; Dahms, H.-U.; Hwang, J.-S. Biological Approaches Integrating Algae and Bacteria for the Degradation of Wastewater Contaminants—A Review. Front. Microbiol. 2022, 12, 801051. [Google Scholar] [CrossRef]
- Alcántara, C.; Domínguez, J.M.; García, D.; Blanco, S.; Pérez, R.; García-Encina, P.A.; Muñoz, R. Evaluation of wastewater treatment in a novel anoxic–aerobic algal–bacterial photobioreactor with biomass recycling through carbon and nitrogen mass balances. Bioresour. Technol. 2015, 191, 173–186. [Google Scholar] [CrossRef]
- Reis, M.; RIbeiro, A. Conversion factors and general equations applied in agricultural and forest meteorology. Agrometeoros 2020, 27, 227–258. [Google Scholar] [CrossRef]
- APHA (American Public Health Association); AWWA (American Water Works Association); WEF (Water Environment Federation). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; Public Health Assoc.: Washington, DC, USA, 2017; ISBN 087-553-047-8. [Google Scholar]
- Sperling, M.V. Principles of Biological Treatment of Wastewater: Introduction to Water Quality and Sewage Treatment, 2nd ed.; DESA, Public University: Belo Horizonte, Minas Gerais, Brazil, 1996; Volume 1, p. 243. [Google Scholar]
- Masojídek, J.; Gómez-Serrano, C.; Ranglová, K.; Cicchi, B.; Bogeat, E.; Manoel, J.A.C.; Zurano, A.S.; Benavides, A.M.S.; Barceló-Villalobos, M.; Carnero, V.A.R.; et al. Photosynthesis Monitoring in Microalgae Cultures Grown on Municipal Wastewater as a Nutrient Source in Large-Scale Outdoor Bioreactors. Biology 2022, 11, 1380. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A Promising Source of Valuable Bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef]
- Carneiro, M.; Cicchi, B.; Maia, I.; Pereira, H.; Zittelli, G.C.; Varela, J.; Malcata, F.X.; Torzillo, G. Effect of temperature on growth, photosynthesis and biochemical composition of Nannochloropsis oceanica, grown outdoors in tubular photobioreactors. Algal Res. 2020, 49, 101923. [Google Scholar] [CrossRef]
- Kliphuis, A.M.J.; de Winter, L.; Vejrazka, C.; Martens, D.E.; Janssen, M.; Wijffels, R.H. Photosynthetic efficiency of Chlorella sorokiniana in a turbulently mixed short light-path photobioreactor. Biotechnol. Prog. 2010, 26, 687–696. [Google Scholar] [CrossRef]
- Coronado-Reyes, J.A.; Salazar-Torres, J.A.; Juárez-Campos, B.; González-Hernández, J.C. Chlorella vulgaris, a microalgae important to be used in Biotechnology: A review. Food Sci. Technol. 2022, 42, e37320. [Google Scholar] [CrossRef]
- Chowdury, K.H.; Nahar, N.; Deb, U.K. The Growth Factors Involved in Microalgae Cultivation for Biofuel Production: A Review. Comput. Water Energy Environ. Eng. 2020, 9, 185–215. [Google Scholar] [CrossRef]
- Yu, H.; Kim, J.; Rhee, C.; Shin, J.; Shin, S.G.; Lee, C. Effects of Different pH Control Strategies on Microalgae Cultivation and Nutrient Removal from Anaerobic Digestion Effluent. Microorganisms 2022, 10, 357. [Google Scholar] [CrossRef]
- Nagabalaji, V.; Maharaja, P.; Nishanthi, R.; Sathish, G.; Suthanthararajan, R.; Srinivasan, S.V. Effect of co-culturing bacteria and microalgae and influence of inoculum ratio during the biological treatment of tannery wastewater. J. Environ. Manag. 2023, 341, 118008. [Google Scholar] [CrossRef]
- Metcalf, E.; Eddy, H.; Tchobanoglous, G. Wastewater Engineering: Treatment and Reuse, 4th ed.; McGraw-Hill Higher Education: Columbus, OH, USA, 2003. [Google Scholar]
- Kumar, A.; Bera, S. Revisiting nitrogen utilization in algae: A review on the process of regulation and assimilation. Bioresour. Technol. Rep. 2020, 12, 100584. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Zhao, Q.; Wei, W.; Sun, Y. Improving high carbon dioxide tolerance and carbon dioxide fixation capability of Chlorella sp. by adaptive laboratory evolution. Bioresour. Technol. 2015, 185, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Ronda, S.R.; Bokka, C.S.; Ketineni, C.; Rijal, B.; Allu, P.R. Aeration effect on Spirulina platensis growth and γ-Linolenic acid production. Braz. J. Microbiol. 2012, 43, 12–20. [Google Scholar] [CrossRef]
- Paquette, A.J.; Vadlamani, A.; Demirkaya, C.; Strous, M.; Siegler, H.D.l.H. Nutrient management and medium reuse for cultivation of a cyanobacterial consortium at high pH and alkalinity. Front. Bioeng. Biotechnol. 2022, 10, 942771. [Google Scholar] [CrossRef]
- Rodero, M.d.R.; Posadas, E.; Toledo-Cervantes, A.; Lebrero, R.; Muñoz, R. Influence of alkalinity and temperature on photosynthetic biogas upgrading efficiency in high rate algal ponds. Algal Res. 2018, 33, 284–290. [Google Scholar] [CrossRef]
- Slompo, N.D.M.; Quartaroli, L.; Fernandes, T.V.; da Silva, G.H.R.; Daniel, L.A. Nutrient and pathogen removal from anaerobically treated black water by microalgae. J. Environ. Manag. 2020, 268, 110693. [Google Scholar] [CrossRef]
- Tricolici, O.; Bumbac, C.; Patroescu, V.; Postolache, C. Dairy wastewater treatment using an activated sludge–microalgae system at different light intensities. Water Sci. Technol. 2013, 69, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D.; Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Lage, S.; Toffolo, A.; Gentili, F.G. Microalgal growth, nitrogen uptake and storage, and dissolved oxygen production in a polyculture based-open pond fed with municipal wastewater in northern Sweden. Chemosphere 2021, 276, 130122. [Google Scholar] [CrossRef] [PubMed]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of Light Intensity and Quality on Growth Rate and Composition of Chlorella vulgaris. Plants 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 1–8. [Google Scholar] [CrossRef]
- Fuentes, J.-L.; Montero, Z.; Cuaresma, M.; Ruiz-Domínguez, M.-C.; Mogedas, B.; Nores, I.G.; del Valle, M.G.; Vílchez, C. Outdoor Large-Scale Cultivation of the Acidophilic Microalga Coccomyxa onubensis in a Vertical Close Photobioreactor for Lutein Production. Processes 2020, 8, 324. [Google Scholar] [CrossRef]
- Mazzelli, A.; Cicci, A.; Di Caprio, F.; Altimari, P.; Toro, L.; Iaquaniello, G.; Pagnanelli, F. Multivariate modeling for microalgae growth in outdoor photobioreactors. Algal Res. 2020, 45, 101663. [Google Scholar] [CrossRef]
- Casagli, F.; Rossi, S.; Steyer, J.P.; Bernard, O.; Ficara, E. Balancing Microalgae and Nitrifiers for Wastewater Treatment: Can Inorganic Carbon Limitation Cause an Environmental Threat? Environ. Sci. Technol. 2021, 55, 3940–3955. [Google Scholar] [CrossRef]
- Amaro, H.M.; Salgado, E.M.; Nunes, O.C.; Pires, J.C.; Esteves, A.F. Microalgae systems environmental agents for wastewater treatment and further potential biomass valorisation. J. Environ. Manag. 2023, 337, 117678. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, D.; Parajuli, K.; Upadhyay, S.; Jiang, Y.; Duan, Z. Comparison of Four Quantitative Techniques for Monitoring Microalgae Disruption by Low-Frequency Ultrasound and Acoustic Energy Efficiency. Environ. Sci. Technol. 2018, 52, 3295–3303. [Google Scholar] [CrossRef]
- Pacheco, M.M.; Hoeltz, M.; Bjerk, T.R.; Souza, M.P.; da Silva, L.F.; Gressler, P.D.; Moraes, M.S.; Lobo, E.A.; Schneider, R.C. Evaluation of microalgae growth in a mixed-type photobioreactor system for the phycoremediation of wastewater. J. Chem. Technol. Biotechnol. 2019, 94, 3102–3110. [Google Scholar] [CrossRef]
- Vella, F.M.; Sardo, A.; Gallo, C.; Landi, S.; Fontana, A.; D’Ippolito, G. Annual outdoor cultivation of the diatom Thalassiosira weissflogii: Productivity, limits and perspectives. Algal Res. 2019, 42, 101553. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, Y.; Osei-Wusu, D.; Liu, T.; Liu, D. Effects of seed age, inoculum density, and culture conditions on growth and hydrocarbon accumulation of Botryococcus braunii SAG807-1 with attached culture. Bioresour. Bioprocess. 2018, 5, 15. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Kligerman, D.C.; Byers, N.; Nasr, L.K.; Cua, C.; Chow, S.; Su, C.; Tang, Y.; Betenbaugh, M.J.; Bouwer, E.J. Effects of inoculum size, light intensity, and dose of anaerobic digestion centrate on growth and productivity of Chlorella and Scenedesmus microalgae and their poly-culture in primary and secondary wastewater. Algal Res. 2016, 19, 278–290. [Google Scholar] [CrossRef]
- Dębowski, M.; Rusanowska, P.; Zieliński, M.; Dudek, M.; Romanowska-Duda, Z. Biomass Production and Nutrient Removal by Chlorella vulgaris from Anaerobic Digestion Effluents. Energies 2018, 11, 1654. [Google Scholar] [CrossRef]
- Yu, H.; Kim, J.; Lee, C. Nutrient removal and microalgal biomass production from different anaerobic digestion effluents with Chlorella species. Sci. Rep. 2019, 9, 612. [Google Scholar] [CrossRef]
- Murray, K.E.; Healy, F.G.; McCord, R.S.; Shields, J.A. Biomass production and nutrient uptake by Neochloris oleoabundans in an open trough system. Appl. Microbiol. Biotechnol. 2010, 90, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.; Dash, S.K.; Sen, R. A biorefinery for valorization of industrial waste-water and flue gas by microalgae for waste mitigation, carbon-dioxide sequestration and algal biomass production. Sci. Total. Environ. 2019, 688, 129–135. [Google Scholar] [CrossRef]
- Çakirsoy, I.; Miyamoto, T.; Ohtake, N. Physiology of microalgae and their application to sustainable agriculture: A mini-review. Front. Plant Sci. 2022, 13, 1005991. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Subramanian, J.; Gopalsamy, J.; Jayasingam, P.; Arumugam, A.; Kannadasan, S.; Sampathkumar, P. The Impact of Using Microalgae as Biofertilizer in Maize (Zea mays L.). Waste Biomass-Valorization 2017, 10, 1101–1110. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Kumaravel, R.; Gopalsamy, J.; Sikder, M.N.A.; Sampathkumar, P. Microalgae as Bio-fertilizers for Rice Growth and Seed Yield Productivity. Waste Biomass-Valorization 2018, 9, 793–800. [Google Scholar] [CrossRef]
- Suleiman, A.K.A.; Lourenço, K.S.; Clark, C.; Luz, R.L.; da Silva, G.H.R.; Vet, L.E.; Cantarella, H.; Fernandes, T.V.; Kuramae, E.E. From toilet to agriculture: Fertilization with microalgal biomass from wastewater impacts the soil and rhizosphere active microbiomes, greenhouse gas emissions and plant growth. Resour. Conserv. Recycl. 2020, 161, 104924. [Google Scholar] [CrossRef]
- Rani, S.; Chowdhury, R.; Tao, W.; Nedbalová, L. Microalga-Mediated Tertiary Treatment of Municipal Wastewater: Removal of Nutrients and Pathogens. Sustainability 2021, 13, 9554. [Google Scholar] [CrossRef]
- Delanka-Pedige, H.M.; Munasinghe-Arachchige, S.P.; Cornelius, J.; Henkanatte-Gedera, S.M.; Tchinda, D.; Zhang, Y.; Nirmalakhandan, N. Pathogen reduction in an algal-based wastewater treatment system employing Galdieria sulphuraria. Algal Res. 2019, 39, 101423. [Google Scholar] [CrossRef]
- Ansa, E.D.O.; Lubberding, H.J.; Gijzen, H.J. The effect of algal biomass on the removal of faecal coliform from domestic wastewater. Appl. Water Sci. 2012, 2, 87–94. [Google Scholar] [CrossRef]
- Liu, L.; Hall, G.; Champagne, P. The role of algae in the removal and inactivation of pathogenic indicator organisms in wastewater stabilization pond systems. Algal Res. 2020, 46, 101777. [Google Scholar] [CrossRef]
- Fallowfield, H.J.; Cromar, N.J.; Evison, L.M. Coliform die-off rate constants in a high rate algal pond and the effect of operational and environmental variables. Water Sci. Technol. 1996, 34, 141–147. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Guidelines for the Safe Use of Wastewater Excreta and Greywater; World Health Organization: Geneva, Switzerland, 2006; Volume 1. [Google Scholar]
- World Bank 2020 Wastewater? From Waste to Resource. Available online: https://www.worldbank.org/en/topic/water/publication/wastewater-initiative (accessed on 20 September 2023).
- Chrispim, M.C.; de Souza, d.M.F.; Scholz, M.; Nolasco, M.A. A Framework for Sustainable Planning and Decision-Making on Resource Recovery from Wastewater: Showcase for São Paulo Megacity. Water 2020, 12, 3466. [Google Scholar] [CrossRef]
- Quijano, G.; Arcila, J.S.; Buitrón, G. Microalgal-bacterial aggregates: Applications and perspectives for wastewater treatment. Biotechnol. Adv. 2017, 35, 772–781. [Google Scholar] [CrossRef]
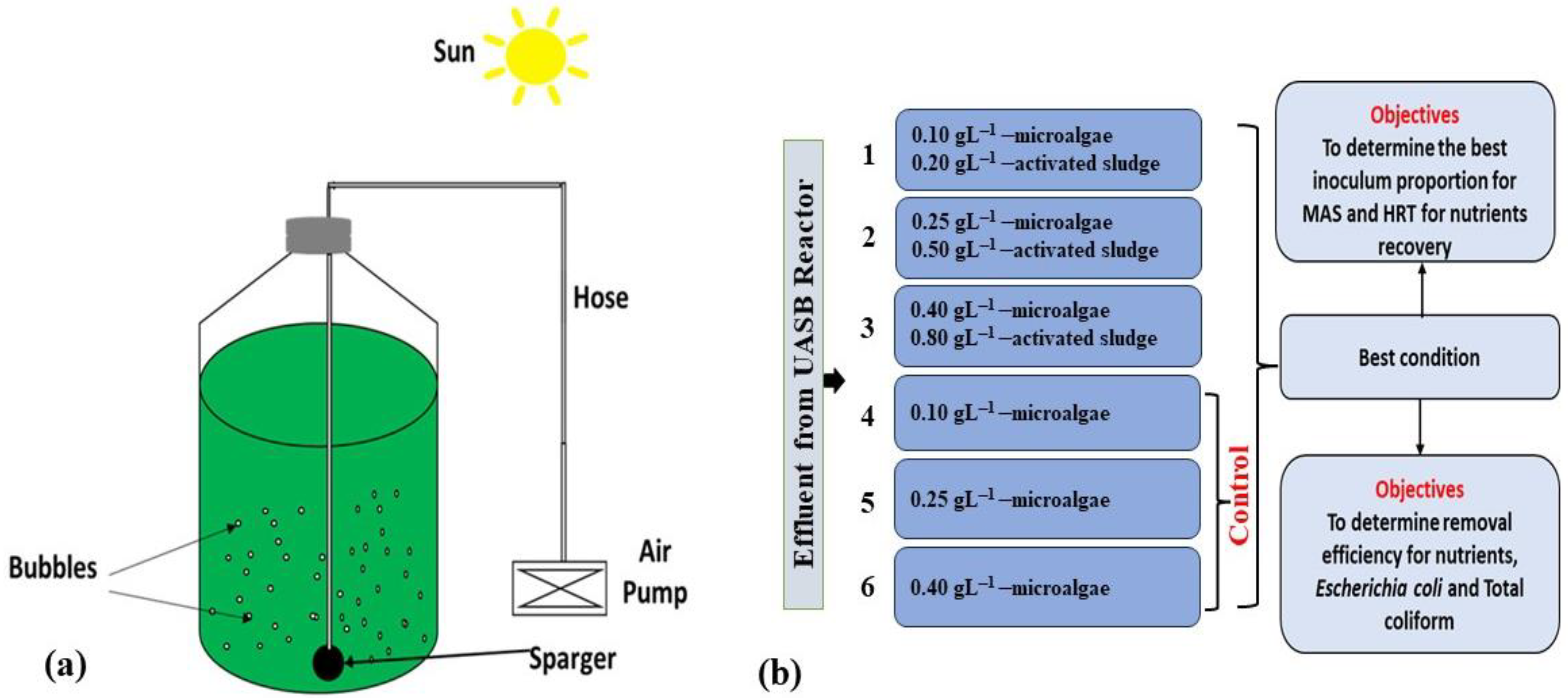
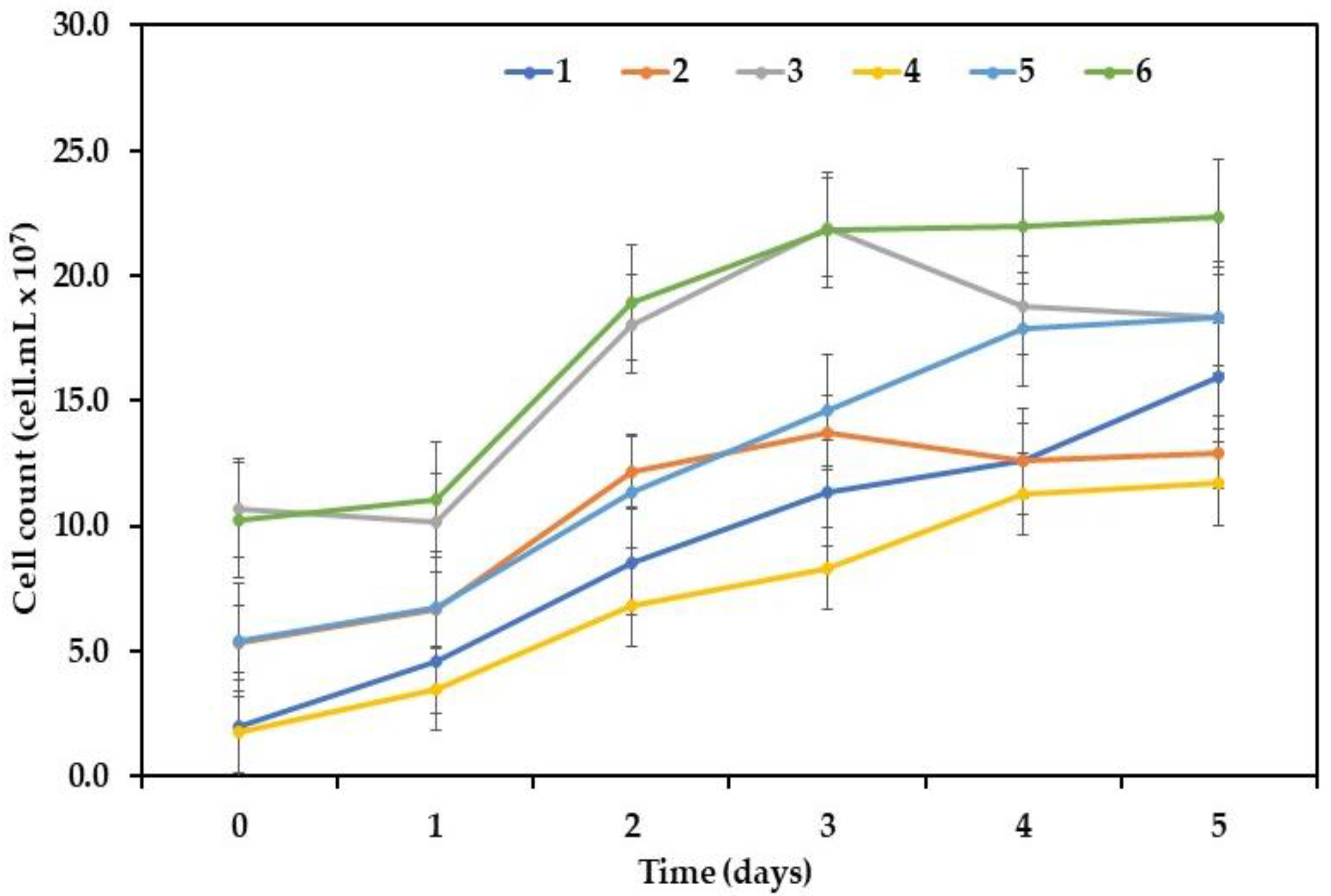
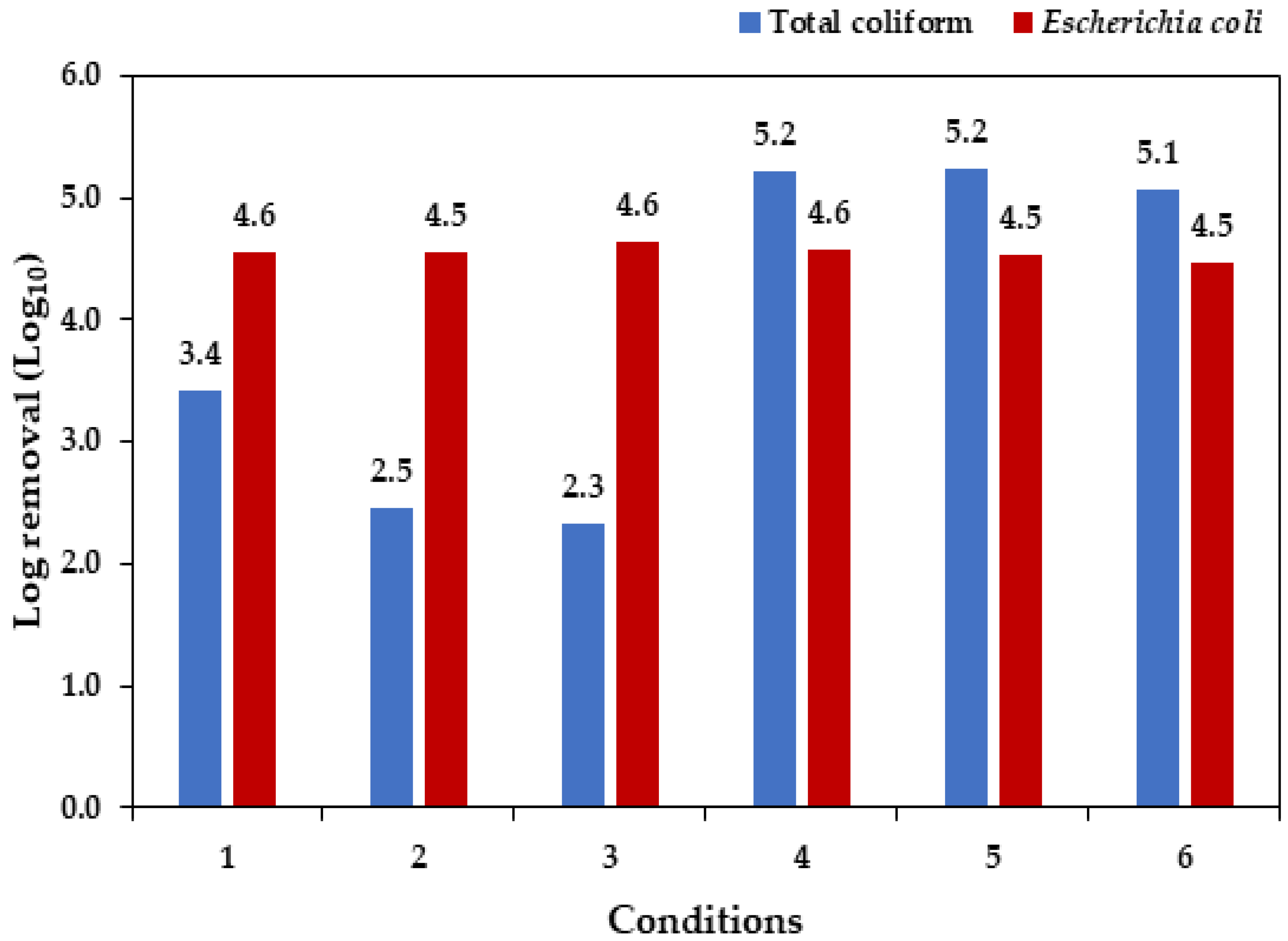
| Parameter | Unit | Average Value |
|---|---|---|
| pH | - | 7.00 ± 0.04 |
| COD | mg L−1 | 119.0 ± 4.70 |
| TDN | mg N L−1 | 58.2 ± 0.60 |
| TDP | mg PO43− L−1 | 6.00 ± 0.20 |
| DO | mg O2 L−1 | 0.59 ± 0.25 |
| TSS | g TSS L−1 | 0.07 ± 0.05 |
| Total Alkalinity | mg CaCO3 L−1 | 339.30 ± 19.50 |
| Volatile Fatty Acid | mg L−1 | 61.30 ± 8.50 |
| Conditions | Volume of Microalgae Inoculated | Volume of Activated Sludge Inoculated | |
|---|---|---|---|
| 1 | Microalgae (0.10 g L−1) + Activated sludge (0.20 g L−1) | 0.118 L | 0.016 L |
| 2 | Microalgae (0.25 g L−1) + Activated sludge (0.50 g L−1) | 0.296 L | 0.039 L |
| 3 | Microalgae (0.40 g L−1) + Activated sludge (0.80 g L−1) | 0.473 L | 0.062 L |
| Controls | |||
| 4 | Microalgae (0.10 g L−1) | 0.118 L | --- |
| 5 | Microalgae (0.25 g L−1) | 0.296 L | --- |
| 6 | Microalgae (0.40 g L−1) | 0.473 L | --- |
| Condition | pH | Total Alkalinity (mg CaCO3 L−1) | ^ Total Biomass Productivity (g TSS L−1 d−1) | ^ Cell Density (OD680nm) | TDP Removal (%) | TDN Removal (%) |
|---|---|---|---|---|---|---|
| 1 | 9.40 ± 1.30 a | 163.70 ± 73.70 | 0.10 ± 0.01 a | 0.84 ± 0.10 a | 85.1 ± 1.04 | 66.1 ± 6.40 |
| 2 | 9.40 ± 1.20 a | 211.40 ± 63.30 | 0.05 ± 0.02 b | 0.31 ± 0.03 b | 40.7 ± 10.30 | 16.4 ± 5.80 |
| 3 | 8.90 ± 0.90 a | 235.30 ± 87.00 | 0.04 ± 0.03 b | 0.17 ± 0.10 b | −43.7 ± 15.70 | −62.90 ± 10.04 |
| Control | ||||||
| 4 | 9.40 ± 1.30 a | 194.80 ± 49.00 | 0.09 ± 0.01 a | 0.75 ± 0.30 a | 83.9 ± 10.40 | 43.20 ± 13.60 |
| 5 | 9.50 ± 1.20 a | 211.80 ± 53.80 | 0.11 ± 0.03 a | 0.97 ± 0.01 a | 85.5 ± 8.80 | 58.3 ± 7.00 |
| 6 | 9.60 ± 1.20 a | 205.30 ± 68.70 | 0.13 ± 0.02 a | 1.10 ± 0.02 a | 92.3 ± 1.20 | 60.6 ± 5.10 |
| Condition/Control | TDP1 | TDP2 | TDP3 | TDP 4 | TDP5 | TDP6 |
|---|---|---|---|---|---|---|
| OD1 | −0.7 | 0.0 | 0.7 | −0.7 | −0.7 | −0.8 |
| OD2 | −0.2 | 0.5 | 0.8 | −0.4 | −0.4 | −0.4 |
| OD3 | 0.0 | 0.6 | 0.7 | −0.2 | −0.1 | −0.2 |
| Control | ||||||
| OD4 | −0.8 | −0.1 | 0.7 | −0.8 | −0.8 | −0.8 |
| OD5 | −0.8 | −0.2 | 0.6 | −0.8 | −0.8 | −0.8 |
| OD6 | −0.8 | −0.2 | 0.6 | −0.8 | −0.8 | −0.8 |
 indicates strong negative relationship between microalgae growth (OD680) and uptake of total dissolved phosphorus (TDP); Neutral:
indicates strong negative relationship between microalgae growth (OD680) and uptake of total dissolved phosphorus (TDP); Neutral:  indicates there is no relationship between microalgae growth (OD680) and uptake of total dissolved phosphorus (TDP); Positive:
indicates there is no relationship between microalgae growth (OD680) and uptake of total dissolved phosphorus (TDP); Positive:  indicates strong possitive relationship and between microalgae growth (OD680) and uptake of total dissolved phosphorus (TDP).
indicates strong possitive relationship and between microalgae growth (OD680) and uptake of total dissolved phosphorus (TDP).| Conditions/ Controls | Day 0 (D0) | Day 5 (D5) | ||
|---|---|---|---|---|
| Total Coliform (CFU 100 mL−1) | Escherichia coli (CFU 100 mL−1) | Total Coliform (CFU 100 mL−1) | Escherichia coli (CFU 100 mL−1) | |
| 1 | 2.79 × 105 | 3.59 × 104 | 1.07 × 102 | 0.00 × 100 |
| 2 | 1.48 × 105 | 3.55 × 104 | 5.23 × 102 | 0.00 × 100 |
| 3 | 1.83 × 105 | 4.33 × 104 | 8.81 × 102 | 0.00 × 100 |
| Controls | ||||
| 4 | 1.64 × 105 | 3.79 × 104 | 0.00 × 100 | 0.00 × 100 |
| 5 | 1.77 × 105 | 3.46 × 104 | 0.00 × 100 | 0.00 × 100 |
| 6 | 1.15 × 105 | 2.91 × 104 | 0.00 × 100 | 0.00 × 100 |
| Parameter | Condition | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Total Alkalinity | 0.0177 | 0.0322 | 0.0191 | 0.0320 | 0.0119 | 0.0054 |
| Productivity | 0.0033 | 0.1475 * | 0.1711 | 0.0015 | 0.0260 | 0.0067 |
| OD680 | 0.0041 | 0.0041 | 0.5139 | 0.0384 | 0.0001 | 0.0001 |
| TDP | 0.0010 | 0.0217 | 0.2012 | 0.0166 | 0.0077 | 0.0002 |
| T. coliform | 0.006 | 0.1240 | 0.0522 | 0.0041 | 0.0002 | 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
James, A.O.; Bankole, A.O.; Pompei, C.M.E.; Dantas, G.A.S.A.; Ruas, G.; Silva, G.H.R. Exploration of Microalgae-Activated Sludge Growth Performance in Lab-Scale Photobioreactors under Outdoor Environmental Conditions for Wastewater Biotreatment. Phycology 2023, 3, 484-502. https://doi.org/10.3390/phycology3040033
James AO, Bankole AO, Pompei CME, Dantas GASA, Ruas G, Silva GHR. Exploration of Microalgae-Activated Sludge Growth Performance in Lab-Scale Photobioreactors under Outdoor Environmental Conditions for Wastewater Biotreatment. Phycology. 2023; 3(4):484-502. https://doi.org/10.3390/phycology3040033
Chicago/Turabian StyleJames, Abraham O., Abayomi O. Bankole, Caroline M. E. Pompei, Gustavo A. S. A. Dantas, Graziele Ruas, and Gustavo H. R. Silva. 2023. "Exploration of Microalgae-Activated Sludge Growth Performance in Lab-Scale Photobioreactors under Outdoor Environmental Conditions for Wastewater Biotreatment" Phycology 3, no. 4: 484-502. https://doi.org/10.3390/phycology3040033
APA StyleJames, A. O., Bankole, A. O., Pompei, C. M. E., Dantas, G. A. S. A., Ruas, G., & Silva, G. H. R. (2023). Exploration of Microalgae-Activated Sludge Growth Performance in Lab-Scale Photobioreactors under Outdoor Environmental Conditions for Wastewater Biotreatment. Phycology, 3(4), 484-502. https://doi.org/10.3390/phycology3040033







