The Effect of Temperature on the Growth of Holopelagic Sargassum Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sargasso Collection
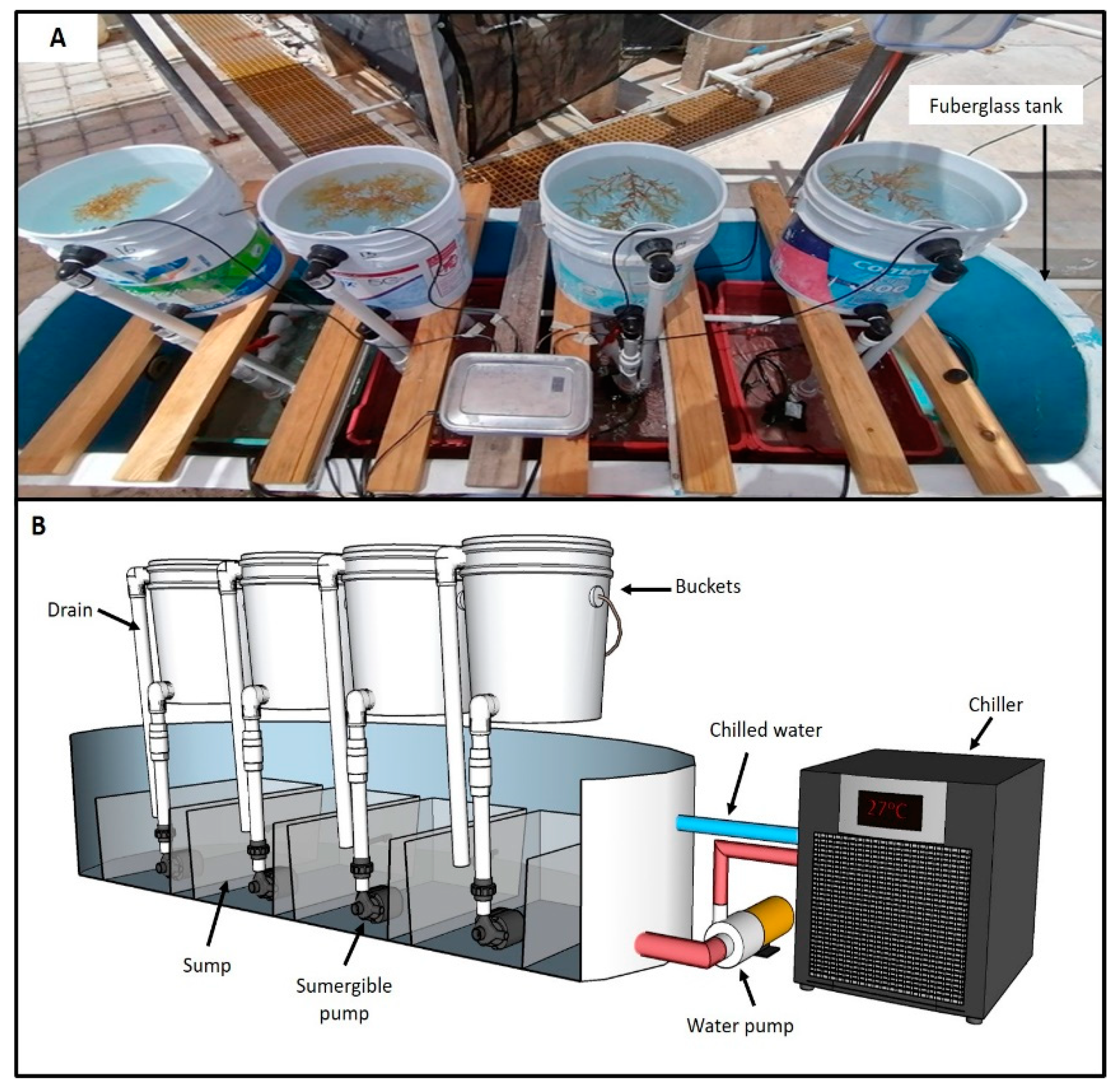
2.2. Description of the Ex Situ Culture System
2.3. Experimental Design
2.4. Statistical Analysis
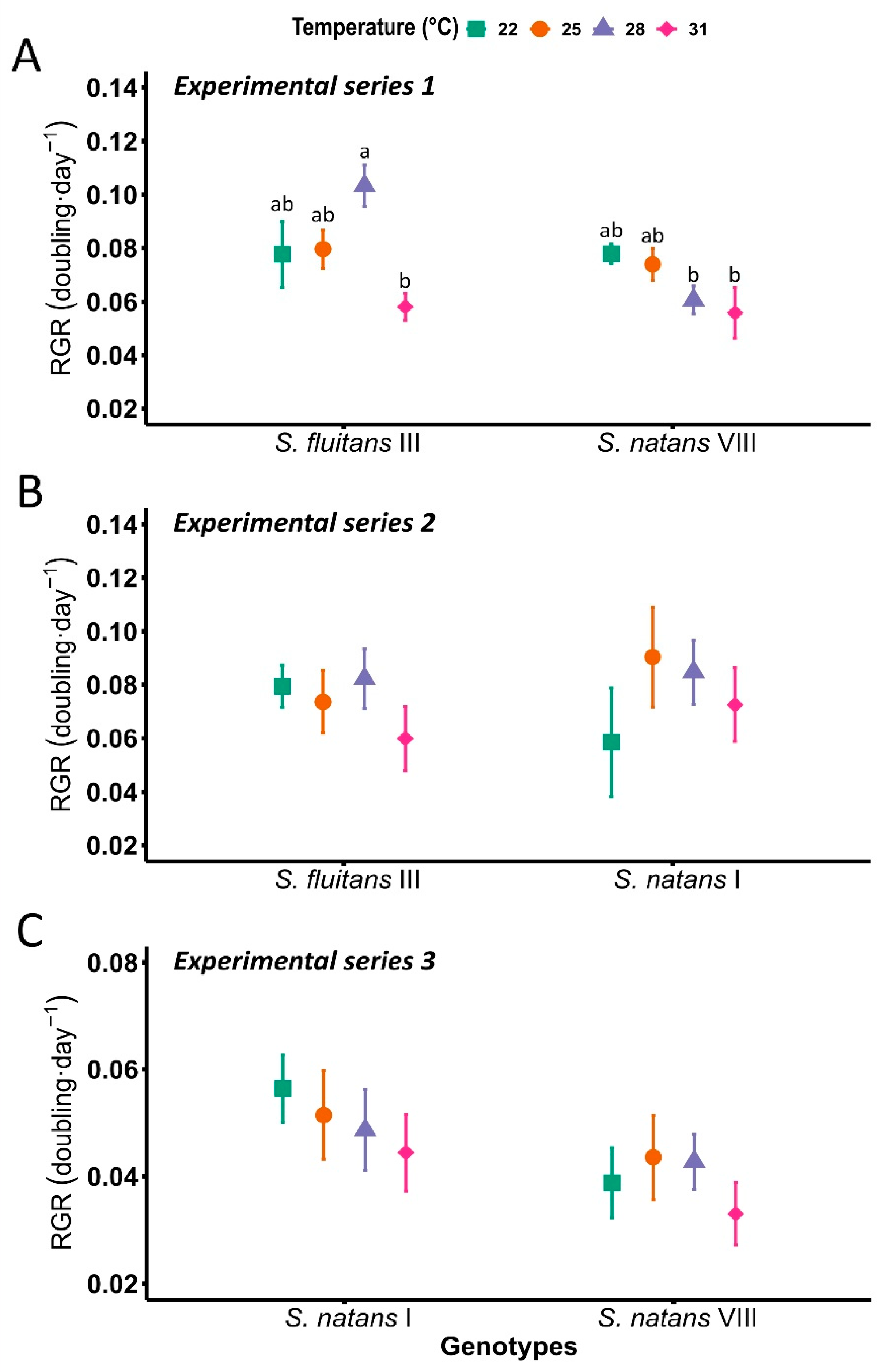
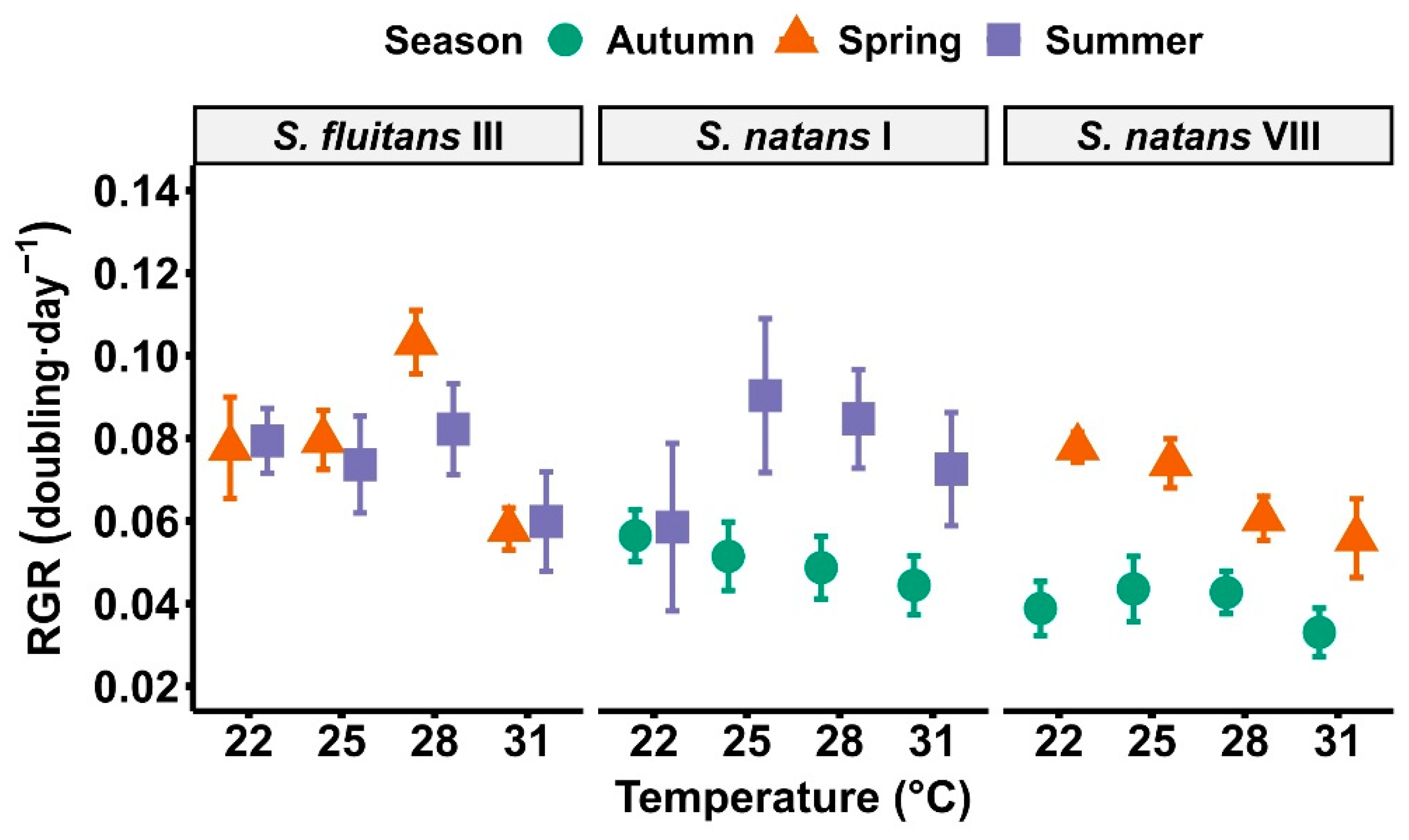
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The great Atlantic Sargassum belt. Science 2019, 365, 83–87. [Google Scholar] [PubMed]
- Skliris, N.; Marsh, R.; Appeaning Addo, K.; Oxenford, H. Physical drivers of pelagic Sargassum bloom interannual variability in the central West Atlantic over 2010–2020. Ocean Dyn. 2022, 72, 383–404. [Google Scholar]
- Schell, J.M.; Goodwin, D.S.; Siuda, A.N. Recent Sargassum inundation events in the Caribbean: Shipboard observations reveal dominance of a previously rare form. Oceanography 2015, 28, 8–11. [Google Scholar]
- Parr, A.E. Quantitative observations on the pelagic Sargassum vegetation of the Western North Atlantic. Bull. Bingham Oceanogr. Collect. 1939, 6, 1–94. [Google Scholar]
- Dibner, S.; Martin, L.; Thibaut, T.; Aurelle, D.; Blanfuné, A.; Whittaker, K.; Cooney, L.; Schell, J.M.; Goodwin, D.S.; Siuda, A.N. Consistent genetic divergence observed among pelagic Sargassum morphotypes in the Western North Atlantic. Mar. Ecol. 2022, 43, e12691. [Google Scholar]
- García-Sánchez, M.; Graham, C.; Vera, E.; Escalante-Mancera, E.; Álvarez-Filip, L.; van Tussenbroek, B.I. Temporal changes in the composition and biomass of beached pelagic Sargassum species in the Mexican Caribbean. Aquat. Bot. 2020, 167, 103275. [Google Scholar]
- Djakouré, S.; Araujo, M.; Hounsou-Gbo, A.; Noriega, C.; Bourlès, B. On the potential causes of the recent pelagic Sargassum blooms events in the tropical North Atlantic Ocean. Biogeosci. Discuss. 2017, 1–20. [Google Scholar] [CrossRef]
- Graba-Landry, A.; Hoey, A.S.; Matley, J.K.; Sheppard-Brennand, H.; Poore, A.G.; Byrne, M.; Dworjanyn, S.A. Ocean warming has greater and more consistent negative effects than ocean acidification on the growth and health of subtropical macroalgae. Mar. Ecol. Prog. Ser. 2018, 595, 55–69. [Google Scholar] [CrossRef]
- Graba-Landry, A.C.; Loffler, Z.; McClure, E.C.; Pratchett, M.S.; Hoey, A.S. Impaired growth and survival of tropical macroalgae (Sargassum spp.) at elevated temperatures. Coral Reefs 2020, 39, 475–486. [Google Scholar] [CrossRef]
- De Wreede, R.E. The phenology of three species of Sargassum (Sargassaceae, Phaeophyta) in Hawaii. Phycologia 1976, 15, 175–183. [Google Scholar] [CrossRef]
- Glenn, E.; Smith, C.; Doty, M. Influence of antecedent water temperatures on standing crop of a Sargassum spp.-Dominated reef flat in Hawaii. Mar. Biol. 1990, 105, 323–328. [Google Scholar]
- Wu, H.; Li, X.; Liu, Y.; Wang, C.; Ji, C.; Xu, J. Increased temperature and nitrogen enrichment inhibit the growth of the golden tide blooming macroalgae Sargassum horneri in the Yellow Sea, China. J. Mar. Sci. Eng. 2022, 10, 1692. [Google Scholar] [CrossRef]
- Bui, H.T.T.; Luu, T.Q.; Fotedar, R. Effects of temperature and pH on the growth of Sargassum linearifolium and S. podacanthum in potassium-fortified inland saline water. Am. J. Appl. Sci. 2018, 15, 186–197. [Google Scholar] [CrossRef]
- Hanisak, M.D.; Samuel, M.A. Growth Rates in Culture of Several Species of Sargassum from Florida, USA; Springer: Berlin/Heidelberg, Germany, 1987; pp. 399–404. [Google Scholar]
- Lapointe, B.E. Phosphorus-limited photosynthesis and growth of Sargassum natans and Sargassum fluitans (Phaeophyceae) in the Western North Atlantic. Deep. Sea Res. Part A Oceanogr. Res. Pap. 1986, 33, 391–399. [Google Scholar]
- Lapointe, B.E.; West, L.E.; Sutton, T.T.; Hu, C. Ryther revisited: Nutrient excretions by fishes enhance productivity of pelagic Sargassum in the Western North Atlantic Ocean. J. Exp. Mar. Biol. Ecol. 2014, 458, 46–56. [Google Scholar]
- Magaña-Gallegos, E.; García-Sánchez, M.; Graham, C.; Olivos-Ortiz, A.; Siuda, A.N.; van Tussenbroek, B.I. Growth rates of pelagic Sargassum species in the Mexican Caribbean. Aquat. Bot. 2022, 185, 103614. [Google Scholar] [CrossRef]
- Brooks, M.T.; Coles, V.J.; Hood, R.R.; Gower, J.F. Factors controlling the seasonal distribution of pelagic Sargassum. Mar. Ecol. Prog. Ser. 2018, 599, 1–18. [Google Scholar]
- Mead, R.; Curnow, R.N.; Hasted, A.M. Statistical Methods in Agriculture and Experimental Biology; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017; ISBN 0203738551. [Google Scholar]
- Posit Team RStudio. Integrated Development Environment for R; Posit Software, PBC: Boston, MA, USA, 2022. [Google Scholar]
- Godínez-Ortega, J.L.; Cuatlán-Cortés, J.V.; López-Bautista, J.M.; van Tussenbroek, B.I. A natural history of floating Sargassum species (Sargasso) from Mexico. In Natural History and Ecology of Mexico and Central America; IntechOpen: London, UK, 2021; p. 35. ISBN 1839684860. [Google Scholar]
- Gagné, J.; Mann, K.; Chapman, A. Seasonal patterns of growth and storage in Laminaria Longicruris in relation to differing patterns of availability of nitrogen in the water. Mar. Biol. 1982, 69, 91–101. [Google Scholar] [CrossRef]
- Lapointe, B.E. A Comparison of nutrient-limited productivity in Sargassum natans from neritic vs. oceanic waters of the Western North Atlantic Ocean. Limnol. Oceanogr. 1995, 40, 625–633. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Hurd, C.L. Seaweed nutrient physiology: Application of concepts to aquaculture and bioremediation. Phycologia 2019, 58, 552–562. [Google Scholar]
- Rodríguez-Martínez, R.E.; Roy, P.D.; Torrescano-Valle, N.; Cabanillas-Terán, N.; Carrillo-Domínguez, S.; Collado-Vides, L.; García-Sánchez, M.; van Tussenbroek, B.I. Element concentrations in pelagic Sargassum along the Mexican Caribbean coast in 2018–2019. PeerJ 2020, 8, e8667. [Google Scholar] [CrossRef] [PubMed]
- Gouvêa, L.P.; Assis, J.; Gurgel, C.F.; Serrão, E.A.; Silveira, T.C.; Santos, R.; Duarte, C.M.; Peres, L.M.; Carvalho, V.F.; Batista, M. Golden carbon of Sargassum forests revealed as an opportunity for climate change mitigation. Sci. Total Environ. 2020, 729, 138745. [Google Scholar] [CrossRef] [PubMed]
- Marsh, R.; Addo, K.A.; Jayson-Quashigah, P.-N.; Oxenford, H.A.; Maxam, A.; Anderson, R.; Skliris, N.; Dash, J.; Tompkins, E.L. Seasonal predictions of holopelagic Sargassum across the Tropical Atlantic accounting for uncertainty in drivers and processes: The SARTRAC ensemble forecast system. Front. Mar. Sci. 2021, 8, 1417. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Golden tides: Problem or golden opportunity? The valorisation of Sargassum from beach inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar] [CrossRef]
- Oxenford, H.A.; Cox, S.-A.; van Tussenbroek, B.I.; Desrochers, A. Challenges of turning the Sargassum crisis into gold: Current constraints and implications for the Caribbean. Phycology 2021, 1, 27–48. [Google Scholar] [CrossRef]
- Lopez Miranda, J.L.; Celis, L.B.; Estévez, M.; Chávez, V.; van Tussenbroek, B.I.; Uribe-Martínez, A.; Cuevas, E.; Rosillo Pantoja, I.; Masia, L.; Cauich-Kantun, C. Commercial potential of pelagic Sargassum spp. in Mexico. Front. Mar. Sci. 2021, 8, 1692. [Google Scholar] [CrossRef]
| Temperature | 22 °C | 25 °C | 28 °C | 31 °C |
|---|---|---|---|---|
| Relative growth rate (doubling · d−1) | ||||
| S. fluitans III | 0.078 ab ± 0.01 | 0.077 ab ± 0.1 | 0.095 a ± 0.01 | 0.058 b ± 0.01 |
| S. natans I | 0.057 ± 0.01 | 0.067 ± 0.01 | 0.063 ± 0.01 | 0.054 ± 0.01 |
| S. natans VIII | 0.058 ± 0.01 | 0.059 ± 0.01 | 0.053 ± 0.01 | 0.045 ± 0.01 |
| Time to double weight (d) | ||||
| S. fluitans III | 12.8 | 13.0 | 10.5 | 17.2 |
| S. natans I | 17.5 | 14.9 | 15.9 | 18.5 |
| S. natans VIII | 17.2 | 16.9 | 18.9 | 22.2 |
| Detached fragments (wet mg) | ||||
| S. fluitans III | 124 ± 48 | 125 ± 48 | 140 ± 48 | 98 ± 48 |
| S. natans I | 163 ± 39 | 139 ± 39 | 192 ± 39 | 104 ± 39 |
| S. natans VIII | 58 ± 27 | 105 ± 27 | 69 ± 27 | 67 ± 27 |
| Increase in internodes (number of new nodes · 10 d−1) | ||||
| S. fluitans III | 3.7 ± 0.3 | 4.1 ± 0.4 | 4.5 ± 0.3 | 3.6 ± 0.4 |
| S. natans I | 1.3 ± 0.4 | 2.0 ± 0.6 | 2.4 ± 0.6 | 1.2 ± 0.4 |
| S. natans VIII | 2.5 ab ± 0.3 | 3.6 a ± 0.4 | 3.4 ab ± 0.3 | 2.1 b ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magaña-Gallegos, E.; Villegas-Muñoz, E.; Salas-Acosta, E.R.; Barba-Santos, M.G.; Silva, R.; van Tussenbroek, B.I. The Effect of Temperature on the Growth of Holopelagic Sargassum Species. Phycology 2023, 3, 138-146. https://doi.org/10.3390/phycology3010009
Magaña-Gallegos E, Villegas-Muñoz E, Salas-Acosta ER, Barba-Santos MG, Silva R, van Tussenbroek BI. The Effect of Temperature on the Growth of Holopelagic Sargassum Species. Phycology. 2023; 3(1):138-146. https://doi.org/10.3390/phycology3010009
Chicago/Turabian StyleMagaña-Gallegos, Edén, Eva Villegas-Muñoz, Evelyn Raquel Salas-Acosta, M. Guadalupe Barba-Santos, Rodolfo Silva, and Brigitta I. van Tussenbroek. 2023. "The Effect of Temperature on the Growth of Holopelagic Sargassum Species" Phycology 3, no. 1: 138-146. https://doi.org/10.3390/phycology3010009
APA StyleMagaña-Gallegos, E., Villegas-Muñoz, E., Salas-Acosta, E. R., Barba-Santos, M. G., Silva, R., & van Tussenbroek, B. I. (2023). The Effect of Temperature on the Growth of Holopelagic Sargassum Species. Phycology, 3(1), 138-146. https://doi.org/10.3390/phycology3010009







